Abstract
Morpho-physiological and biochemical responses of Arabidopsis thaliana (accession N1438) to bicarbonate-induced iron deficiency were investigated. Plants were grown in cabinet under controlled conditions, in a nutrient solution containing 5 μM Fe, added or not with 10 mM NaHCO3. After 30 days, bicarbonate-treated plants displayed significantly lower biomass, leaf number and leaf surface area as compared to control plants, and slight yellowing of their younger leaves was observed. Potassium (K+) content was not modified by bicarbonate treatment in roots, whereas it was significantly diminished in shoots. Their content in ferrous iron (Fe2+) and in leaf total chlorophylls was noticeably lower than in control plants. Root Fe(III)-chelate reductase and phosphoenolpyruvate carboxylase (PEPC) activities were significantly enhanced, but leaf ribulose 1.5-bisphosphate carboxylase (Rubisco) activity was decreased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron chlorosis induced by bicarbonate is very common in Tunisia where most soils are calcareous (Gharsalli and Hajji 2002). In such soils, bicarbonate (HCO3 −) ions are present at high concentrations, which make iron (Fe) not easily available to roots (Rabhi et al. 2007). To overcome this constraint, plants have evolved specific adaptive mechanisms for iron acquisition, classified into two distinct “strategies”. The response to Fe deficiency of dicotyledonous and non-graminaceous monocotyledonous plants involves a series of morpho-physiological and biochemical changes (Zaharieva et al. 2004) depicted as “strategy I”. The main morphological changes are the increase of lateral root formation and the emergence of root hairs and transfer cells, increasing in this way the root surface and consequently Fe uptake (Schmidt et al. 2000). The strategy I response includes also a proton excretion by roots, which lowers the rhizosphere pH, and an increase in the capacity to reduce Fe3+ (less soluble) to Fe2+ (more soluble) by a Fe(III)-chelate reductase (FCR) (Kim and Guerinot 2007). The reduction step, prior to Fe2+ uptake, has been shown to be critical for Fe uptake from Fe-deficient soil (Kim and Guerinot 2007). In addition, Fe deficiency results in an accumulation of organic acids in roots, mainly malate and citrate (Abadía et al. 2000), accompanied with an increase in activity of phosphoenolpyruvate carboxylase (PEPC) and several other enzymes involved in tricaboxylic acid cycle (López-Millán et al. 2000; Rabotti et al. 1995). PEPC catalyses the carboxylation of phosphoenolpyruvate (PEP) to oxaloacetate. The latter is reduced to malate via the cytosolic enzyme, malate dehydrogenase, then malate is transported to mitochondria through the malate-oxaloacetate shuttle and converted into citrate by citrate synthase (Andaluz et al. 2002). The role of organic acid accumulation in Fe deficiency response is mainly related to Fe transport to shoots as Fe citrate in xylem sap (Stephan 2002). This work was aimed at studying morpho-physiological and biochemical changes involved in A. thaliana (accession N1438) responses to bicarbonate-induced Fe deficiency, in particular root PEPC and Fe(III)-chelate reductase activities.
Materials and methods
Plant material and growth conditions
Seeds of A. thaliana N1438 were sown in pots containing a mixture of sand and peat (1V:2V). After germination, seedlings were grown in a growth chamber under controlled conditions (Gibeaut et al. 1997): 12 h daily light, 150 μmol m−2 s−1 photon flux density; 22/18°C day/night temperature regime, and 60/80% day/night relative humidity regime. They were irrigated with distilled water for 8 days then with a complete nutrient solution containing 5 μM Fe-EDTA (Gay and Hauck 1994). After 3 weeks, they were transferred into 300 ml plastic pots and acclimated over 1 week. Then, two treatments were started. In the first one, seedlings were cultivated in the same nutrient solution and considered as control. In the second treatment, 10 mM bicarbonate was added to the medium. The medium was weekly renewed and its pH buffered at 7.7 with NaOH (1 N). The plants were harvested after 30 days of treatment.
Chlorophyll concentration
The chlorophyll content of young leaves was determined according to Torrecillas et al. (1984). Small discs (100 mg FW) from young leaf lamina were incubated in 5 ml 80% acetone in darkness at 4°C over 3 days. Then the contents in chlorophyll a, chlorophyll b, and total chlorophylls were determined at 649 and 665 nm (Strain and Svec 1966).
Ferrous iron and potassium contents
Cations were extracted from desiccated tissues with HCl 1 N. Bivalent iron (Fe2+) content was determined using 1-10-O-phenanthroline according to Kaytal and Sharma (1980). Potassium was assayed by flame photometry (Eppendorf) with butane-air flame.
Electrolyte leakage
Electrolyte leakage was determined as described by Dionisio-Sese and Tobita (1998). Leaf samples (approximately 200 mg FW) were submerged into 10 ml distilled water and kept at 32°C over 2 h. Then, the initial electrical conductivity of the medium (EC1) was measured. After the conductivity measurement, the leaf tissues were killed by autoclaving at 121°C for 20 min to release all electrolytes, cooled to 25°C, and then the final electrical conductivity (EC2) was measured. The electrolyte leakage (EL) was calculated as EL = 100 EC1/EC2.
Root Fe(III)-reductase activity
Fe(III)-chelate reductase activity was estimated as in vivo reduction of Fe(III)–ethylenediaminetetraacetic acid (EDTA) by intact plant roots. The formation of the red Fe(II)-bathophenanthrolinedisulphonate (BPDS) complex was followed by measuring its absorbance at 535 nm (Chaney et al. 1972). The reaction was performed for 30 min with BPDS (0.3 mM) and Fe(III)–Na–EDTA (0.1 mM) in full-strength nutrient solution, buffered with 10 mM MES–KOH (pH 5.5).
Enzyme extraction and assay
Fresh leaf or root samples were ground in a mortar with 100 mM Tris–bicine (pH 8.0) containing 1 mM EDTA, 5% glycerol (v/v), 5 mM MgCl2, 1% mercaptoethanol (v/v), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 5% polyvinylpyrrolidone (PVP) (w/v of sample FW). After centrifugation at 12,000×g for 20 min at 4°C, the supernatant was collected and enzyme activities were measured immediately.
The activities of phosphoenolpyruvate carboxylase (PEPC; EC 4.1.1.31) and Rubisco (Rubisco E.C.4.1.1.39) were assayed according to Ouerghi et al. (2000). PEPC reaction mixture contained 100 mM Tris–bicine (pH 8.0), 5 mM MgCl2, 1 mM DTT, 5 mM NaHCO3, 0.2 mM NADH, 4 mM phosphoenolpyruvate, 5 enzyme units of malate dehydrogenase (MDH). The crude extract (100 μl) was added to the reaction medium then the activity was monitored at 340 nm for 15 min.
Rubisco activity was assayed in a reaction medium containing 100 mM Tris–bicine (pH 8.0), 10 mM MgCl2, 0.2 mM EDTA, 5 mM dithiothreitol (DTT), 40 mM NaHCO3, 4 mM ATP, 0.2 mM NADH, 0.2 mM ribulose 1, 5-biphosphate (RuBP), and one enzyme unit of 3-phosphoglycerate kinase (PGK) and glyceraldehyde 3-phosphate dehydrogenase (3-PGADH). The reaction was initiated by adding 0.2 mM RuBP, and activity was assayed spectrophotometrically at 340 nm for 10 min at 30°C (Sato et al. 1980). Enzyme activities were expressed as μmol h−1 g−1.
Statistical analysis
Means were compared using Student’s t test at P = 0.05.
Results
Plant aspect and growth
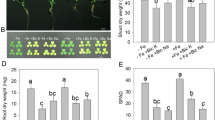
One month after bicarbonate treatment was started, yellowish green colouration of young leaves revealed incipient chlorosis. A marked effect of bicarbonate-induced iron deficiency was observed for shoot biomass production, which was reduced to 68% of control (Table 1). This response was accompanied by a diminution of total leaf area, mainly associated with a reduction of the leaf number (Table 1). The harmful effect of bicarbonate on biomass was less pronounced in roots than in leaves.
Physiological effects
Shoot K+ and Fe2+ contents were significantly modified by bicarbonate treatment, amounting only 30 and 42%, respectively, of control (Fig. 1a, b). In roots, no significant effect on K+ and Fe2+ contents was observed. Although HCO3 −-induced chlorosis visual symptoms were not severe, significant reduction in chlorophyll content was found in treated plants as compared to control plants (−37% for chlorophyll a and −42% for chlorophyll b) (Table 2). Physiological damage was revealed by a significant electrolyte leakage in leaves of plants subjected to bicarbonate treatment. Such a damage, that indicates a detrimental effect of the stress on membrane integrity, was not found in roots (Fig. 2).
K+ (a) and Fe2+ (b) contents in shoots and roots of A. thaliana plants grown over 1 month on a complete nutrient solution containing (+HCO3 −) or not (−HCO3 −) 10 mM bicarbonate. Bars are means of ten replicates ± SE. Those labelled by the same letter are not statistically different according to Student’s test at P < 0.05
Electrolyte leakage from leaves and roots of A. thaliana plants grown over 1 month on a complete nutrient solution containing (+HCO3 −) or not (−HCO3 −) 10 mM bicarbonate. Bars are means of ten replicates ± SE. Those labelled by the same letter are not statistically different according to Student’s test at P < 0.05
Biochemical effects and responses
Root medium acidification
During the last week before harvest, the nutrient solution pH was daily measured over 6 days (Fig. 3a). Roots of bicarbonate-treated plants did not decrease the pH of the medium, which, on the contrary, increased up to the third day of measurement and then stabilized at about 8.4. In control plants, however, roots slightly acidified the medium.
pH values of the culture media during the last 6 days of treatment (a) and Fe-chelate reductase activity in roots (b) of A. thaliana plants grown over 1 month on a complete nutrient solution containing (+HCO3 −) or not (−HCO3 −) 10 mM bicarbonate. Bars are means of six replicates ± SE. Those labelled by the same letter are not statistically different according to Student’s test at P < 0.05
Enzyme activity
In roots of bicarbonate-treated plants, the Fe-chelate reductase activity was doubled as compared to control (Fig. 3b), and that of PEPC was augmented by 38% (Fig. 4a). In leaves, Rubisco activity was reduced to 30% of control (Fig. 4b).
PEPC (a) and Rubisco (b) activities of A. thaliana plants grown over 1 month on a complete nutrient solution containing (+HCO3 −) or not (−HCO3 −) 10 mM bicarbonate. Bars are means of three replicates ± SE. Those labelled by the same letter are not statistically different according to Student’s test at P < 0.05
Discussion
Bicarbonate-induced iron deficiency severely inhibited shoot growth of A. thaliana, expressed as biomass production or as leaf number and leaf surface area. Roots, however, were much less sensitive to the treatment than shoots, showing no significant variation between control and treated plants. Similar discrepancy between shoot and root growth sensitivity to bicarbonate has been described for olive tree (De La Guardia and Alcantara 2002). Despite the large decrease in chlorophylls, bicarbonate-treated plants exhibited only slight leaf chlorosis. The impact of iron deficiency, either direct or induced by bicarbonate, on chlorophyll formation has been reported in several studies. For example, bicarbonate presence in the medium significantly reduced chlorophyll concentration in sunflower leaves (Gharsalli and Hajji 1992) as well as in two peach rootstocks (Molassiotis et al. 2006). These findings are explained by the primordial role of Fe2+ on the formation of the chlorophyll precursors ∂-aminolevulinic acid and protochlorophyllide (Marschner 1995).
Significant decrease in Fe2+ content occurred in shoots of bicarbonate-treated plants, but not in roots. Probably, iron transport from roots to shoots was limited in bicarbonate-treated plants, which reduced their shoot growth. Similarly, Gharsalli and Hajji (2002) observed that iron uptake by peach roots was not significantly decreased in the presence of HCO3 −, but that transport towards aerial organs was strongly inhibited. This behaviour may be explained by iron immobilization within root apoplast (Zribi and Gharsalli 2002). Potassium transport to shoots was also restricted by bicarbonate treatment. According to Tagliavini and Rombolà (2001), this cation plays a major role in Fe assimilation under iron deficiency conditions, by increasing root plasma membrane H+-ATPase activity. Hence, the reduction observed in shoot growth of treated plants and in new leaf production could be attributed to Fe and K+ shortages within shoot apex tissues and by their effect on photosynthesis and growing capacity. Indeed, K+ nutrition plays an important role in the activation of many enzymes required for photosynthetic processes. According to Mozaffari et al. (2004), low K+ nutrition decreases the activity of such enzymes, including Rubisco. Our results showed, indeed, that bicarbonate-induced Fe deficiency diminished photosynthetic competence by lowering RuBP carboxylation capacity through a reduction of leaf Rubisco activation. In sugar beet, Winder and Nishio (1995) showed that the rate of fully activated CO2 fixation by RuBP carboxylase was reduced by more than 50% in severely Fe-deficient leaves. The decrease in Rubisco activation measured by these authors was probably the result of a low [ATP]/[ADP] ratio in Fe deficient leaves, which is known to inhibit Rubisco activase (Streusand and Portis 1987). In the same way, Arulanantham et al. (1990) reported that a 50% decline in Rubisco activity under severe Fe deficiency was due to a reduction of RuBP substrate levels by about 70%, which limited photosynthesis in Fe-deficient sugar beet leaves.
Iron uptake efficiency of strategy I plants have been largely attributed to the adaptive biochemical mechanisms developed at root cell plasma membrane. Acidification capacity and Fe(III)-chelate reduction by Fe-chelate reductase have been shown to be stimulated in response to Fe deficiency (Schmidt 2006; Zaharieva et al. 2004). We did not observe root acidification under bicarbonate constraint, probably because of the bicarbonate buffer power. However, Fe-chelate reductase activity was significantly increased in bicarbonate-treated plants, suggesting that A. thaliana was able to reduce and thus to absorb Fe despite the presence of HCO3 − ions. Similar stimulation of root Fe-chelate reductase under bicarbonate-induced Fe deficiency has been described by Molassiotis et al. (2006) for peach tree. Several lines of evidence support the role of PEPC in plant response to Fe deficiency. The increased activity of this enzyme that we observed upon bicarbonate treatment was perhaps a response to root medium high pH (Andaluz et al. 2002). PEPC activity appears to be a suitable metabolic marker of the Fe nutritional status in plants and would poise the root cells for an efficient Fe acquisition. The activation of this enzyme has been related to organic acid synthesis (López-Millán et al. 2000) and to the need for cytoplasmic pH homeostasis in Fe-deficient tissues (Rabotti et al. 1995).
References
Abadía J, López-Millán AF, Rombolà A, et al. (2000) Organic acids and Fe deficiency: a review. Plant Soil 241:75–86
Andaluz S, López-Millán AF, Peleato ML et al (2002) Increases in phosphoenol pyruvate carboxylase activity in iron-deficient sugar beet roots: analysis of spatial localization and post-translational modification. Plant Soil 241:43–48. doi:10.1023/A:1016000216252
Arulanantham AR, Rao IM, Terry N (1990) Limiting factors in photosynthesis. IV. Regeneration of ribulose 1.5-bisphosphate limits photosynthesis at low photochemical capacity. Plant Physiol 93:1465–1475. doi:10.1104/pp.93.4.1466
Chaney RL, Brown JC, Tiffin LO (1972) Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol 50:208–213. doi:10.1104/pp.50.2.208
De La Guardia MD, Alcantara E (2002) A comparison of ferric-chelate reductase and chlorophyll and growth ratios as indices of selection of quince, pear and olive genotypes under iron deficiency stress. Plant Soil 241:49–56. doi:10.1023/A:1016083512158
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9. doi:10.1016/S0168-9452(98)00025-9
Gay AP, Hauck B (1994) Acclimation of Lolium temulentum to enhanced carbon dioxide concentration. J Exp Bot 45:1133–1141. doi:10.1093/jxb/45.8.1133
Gharsalli M, Hajji M (1992) Effets d’une déficience ferrique sur l’activité de la pompe à protons de la membrane plasmique et la réduction de fer chez le tournesol. C R Acad Sci Paris 315:559–565
Gharsalli M, Hajji M (2002) Comparison of physiological responses of peach and almond seedlings to iron deficiency. J Plant Nutr 25:1139–1154. doi:10.1081/PLN-120003945
Gibeaut DM, Hulett J, Cramer GR et al (1997) Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol 115:317–319. doi:10.1104/pp.115.2.317
Kaytal JC, Sharma BD (1980) A new technique of plant analysis to resolve iron chlorosis. Plant Soil 55:105–119. doi:10.1007/BF02149714
Kim SA, Guerinot ML (2007) Mining iron: iron uptake and transport in plants. FEBS Lett 581:2273–2280. doi:10.1016/j.febslet.2007.04.043
López-Millán AF, Morales F, Andaluz S et al (2000) Responses of sugar beet roots to iron deficiency. Changes in carbon assimilation and oxygen use. Plant Physiol 124:885–897. doi:10.1104/pp.124.2.885
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Molassiotis A, Tanou G, Diamantidis G et al (2006) Effect of 4-month Fe deficiency exposure on Fe reduction mechanism, photosynthetic gas exchange, chlorophyll fluorescence and antioxidant defense in two peach rootstocks differing in Fe deficiency tolerance. J Plant Physiol 163:176–185. doi:10.1016/j.jplph.2004.11.016
Mozaffari M, Oosterhuis DM, McConnell JS, Slaton NA, Evans EE, Bibi AC, Gonias ED, Bourland FM, Kennedy C (2004) Effect of potassium fertilization on yield, petiole K, and reflective properties of cotton. Summaries of Arkansas cotton research. AAES Res Ser 533:85–88
Ouerghi Z, Cornic G, Roudani M et al (2000) Effect of NaCl on photosynthesis of two wheat species (Triticum durum and T. aestivum) differing in their sensitivity to salt stress. J Plant Physiol 156:335–340
Rabhi M, Barhoumi Z, Ksouri R et al (2007) Interactive effects of salinity and iron deficiency in Medicago ciliaris. C R Biologies 330:779–788. doi:10.1016/j.crvi.2007.08.007
Rabotti G, De Nisi P, Zocchi G (1995) Metabolic implications in the biochemical responses to iron deficiency in cucumber (Cucumis sativus L.) roots. Plant Physiol 107:1195–1199
Sato FK, Nishida K, Yamada Y (1980) Activities of carboxylation enzymes and products of 14CO2 fixation in photoautotrophically cultured cells. Plant Sci Lett 20:91–97. doi:10.1016/0304-4211(80)90027-9
Schmidt W (2006) Iron stress response in roots of strategy I plants. In: Barton LL, Abadía J (eds) Iron nutrition in plants and rhizospheric microorganisms. Springer, The Netherlands
Schmidt W, Tittel J, Schikora A (2000) Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol 122:1109–1118. doi:10.1104/pp.122.4.1109
Stephan UW (2002) Intra- and intercellular iron trafficking and subcellular compartmentation within roots. Plant Soil 241:19–25. doi:10.1023/A:1016086608846
Strain HH, Svec WA (1966) Extraction, separation, estimation and isolation of chlorophylls. In: Vernon LP, Seeley GR (eds) The chlorophylls. Academic Press, New York, pp 21–66
Streusand VJ, Portis AR (1987) Rubisco activase mediates ATP- dependent activation of ribulose bisphosphate carboxylase. Plant Physiol 85:152–154. doi:10.1104/pp.85.1.152
Tagliavini M, Rombolà AD (2001) Iron deficiency and chlorosis in orchard and vineyard ecosystems. Eur J Agron 15:72–92. doi:10.1016/S1161-0301(01)00125-3
Torrecillas A, Leon A, Del Amor F et al (1984) Determinacion rapida de clorofila en discos foliares de limonero. Fruits 39:617–622
Winder TL, Nishio JN (1995) Early iron deficiency stress response in leaves of sugar beet. Plant Physiol 108:1487–1494
Zaharieva TB, Gogorcena Y, Abadia J (2004) Dynamics of metabolic responses to iron deficiency in sugar beet roots. Plant Sci 166:1045–1050. doi:10.1016/j.plantsci.2003.12.017
Zribi K, Gharsalli M (2002) Effect of bicarbonate on growth and iron nutrition of pea. J Plant Nutr 25:2143–2149. doi:10.1081/PLN-120014066
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Aniol.
Rights and permissions
About this article
Cite this article
Msilini, N., Attia, H., Bouraoui, N. et al. Responses of Arabidopsis thaliana to bicarbonate-induced iron deficiency. Acta Physiol Plant 31, 849–853 (2009). https://doi.org/10.1007/s11738-009-0318-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-009-0318-z