Abstract
Drought is one of the most severe stresses which limit sugarcane production in China. ATP citrate lyase (ACL) is a major enzyme responsible for the production of acetyl-CoA in cytoplasm and plays an important role in plant metabolism and stress response. In this study, sugarcane ACL gene SoACLA-1 was cloned. The plant overexpression vector of SoACLA-1 was built and transformed into sugarcane calli by Agrobacterium-mediated transformation, and PCR analysis confirmed that SoACLA-1 gene had been stably present in the T0, T1, and T2 generations of the transgenic sugarcane. In order to evaluate the drought resistance of the transgenic lines and verify the function of SoACLA-1 gene in the transgenic sugarcane, T1 generation of the SoACLA-1 transgenic sugarcane lines was used as the material to investigate the physiological and biochemical characteristics at 0 day, 3 days, 6 days, and 9 days after water stress and rewatering for 3 days. Comprehensive evaluation of four indicators (chlorophyll, malondialdehyde, proline, soluble sugar) related to drought resistance was done with membership fuzzy function method. The results showed that the drought resistance of five transgenic sugarcane lines from strong to weak, in turn, was RT2 > RT4 > RT3 > RT1 > WT, and the recovery ability after drought, in turn, was RT1 > RT2 > RT4 > RT3 > WT. The T2 generation of the SoACLA-1 transgenic sugarcane lines was used to analyze the physiological and biochemical changes and the expression of drought-related genes under water stress. The results showed that the transgenic sugarcane lines were more tolerant to drought as compared with the wild-type plants. Our findings indicated that SoACLA-1 gene plays an important role as a positive factor in response to water stress, and overexpression of SoACLA-1 can enhance drought tolerance in transgenic sugarcane plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum spp. hybrid) is the most important sugar crop in China and the world. China is the fourth largest sugar-producing country after Brazil, India, and Thailand. Sugarcane sucrose production accounts for more than 90% of China’s total sugar production, and Guangxi has the largest sugarcane planting area and contributes to more than 60% of the total sugar production in China (Li and Yang 2015). Most of the sugarcane planting areas in Guangxi are located on hilly and dry slopes with poor irrigation or rain-fed conditions. In addition, due to the uneven precipitation, the sugarcane production in Guangxi is affected by different degrees of drought every year. Water deficit affects the tillering rate and plant height directly, leading to the decrease of cane and sugar yield. In fact, drought has become one of the most important factors restricting sugarcane production in Guangxi and also an important factor restricting the sustainable development of sugar industry in China. Therefore, improving drought resistance has become one of the key aims in sugarcane breeding and cultivation technology development.
It has been reported that ATP citrate lyase (ACL) plays an important role in the growth and development of plants and is an indispensable enzyme for the development of tissues and organs and the execution of physiological functions (Souter et al. 2002). ACL is a vital way to produce acetyl coenzyme A (acetyl-CoA) in plants (Rangasamy and Ratledge 2000). Acetyl-CoA is a key intermediate product in sugar metabolism and a substrate for fatty acid synthesis (Fatland et al. 2002). The conversion of citric acid and CoA to acetyl-CoA by ACL is accompanied by the hydrolysis of ATP (Sun et al. 2011). Therefore, as an essential key enzyme, ACL functions intermediate the links in the metabolic pathways of physiological and biochemical processes, and it is also a bridge between sugar metabolism and fatty acid production (Nikolau et al. 2003; Sun et al. 2010).
Studies have shown that ACL gene expression is upregulated under water, low light, strong light, or abscisic acid (ABA) stresses, indicating that ACL expression can enhance plant resistance to stress (Choi et al. 1995). The expression of ACL in white lupin (Lupinus albus L.) was responsive for the switch of malate and citrate excretion during root growth and development (Langlade et al. 2002). The expression of ACL in Brassica napus was affected by various stresses and hormones (Tong 2009). The antisense expression of ACL in Arabidopsis thaliana resulted in the change of plant traits and the decrease of oil content in leaves and seeds (Tong 2009). The amount of succinic acid produced by Aspergillus niger increased three times after knocking out the ACL gene, and the content of organic acid also increased significantly (Meijer et al. 2009). Arabidopsis plants with moderately reduced ACL activity have a changed phenotype, with miniaturized organs, smaller cells, abnormal plastids, less wax in epidermis, and more starch and anthocyanin in vegetative tissues (Fatland et al. 2005). Therefore, ACL is beneficial for normal growth and development of plants (Fatland et al. 2005). In addition, studies showed that overexpression of ACL increased the amount of fatty acids and lipids produced in plants of Arabidopsis thaliana (Koziol 2002). Besides, the overexpression of ACL significantly increased the fruits of citrus under mild drought stress and abscisic acid (ABA) treatment (Hu et al. 2015). Moreover, overexpression of ACL increased wax and cutin in Arabidopsis stems, while overexpression of ACL in dandelion increased rubber and triterpene content (Xing et al. 2014). In recent years, the cloning of sugarcane ACL genes and the quantitative study of sugarcane ACL genes under stress have been reported. In our laboratory, sugarcane ACL was first isolated and characterized to have two subunits (SoACLA-1 and SoACLB-1), and ABA and water stress treatment could induce ALC expression in sugarcane (Li et al. 2012; Liu et al. 2014). This suggests that ACL may be involved in the ABA-regulated metabolic process of sugarcane in response to stress and adversity. It has been demonstrated that SoACLA-1 overexpression enhances drought tolerance through improving the transgenic tobacco antioxidant system (Phan et al. 2016). On the basis of the previous studies, it is possible to use ACLA-1 gene to improve sugarcane drought resistance, and it may be involved in the regulation of ABA-responsive metabolic processes in plants.
In this study, SoACLA-1 gene isolated from sugarcane was used to construct a eukaryotic expression vector and was transformed into sugarcane. Three generations of the transgenic sugarcane lines were tested to identify the transformed SoACLA-1 gene by PCR amplification and sequenced to verify if it could be stably present in sugarcane offspring. Simultaneously, the physiological and biochemical roles of SoACLA-1 in the transgenic sugarcane lines were analyzed under water stress, which will improve our understanding of the molecular mechanism of drought tolerance in sugarcane and also provide a reference for evaluating the genetic stability of SoACLA-1 transgenic plants and studying the function of ACL gene in sugarcane.
Material and Methods
Cloning of SoACLA-1 Gene and Vector Construction
RNA was extracted from the leaves of sugarcane variety ROC22 (normal growth) by TrizolA+ reagent (TIANGEN Biotech Co. Ltd., Beijing, China), and the complementary DNA (cDNA) template for RT-PCR amplification was synthesized using cDNA amplification kit (TaKaRa, Dalian, China). Based on the sequence of SoACLA-1 gene found in our laboratory (Li et al. 2012), the upstream and downstream primers were designed (Table S1). The reaction procedure used for PCR experiment was referred to Phan et al. (2016). After PCR amplification, the products were purified and cloned into the PMD18-T vector (TaKaRa, Dalian, China) and sequenced on both strands in Shanghai Bioengineering Institute (Shanghai, China).
The cDNA of SoACLA-1 ligated with GFP was inserted into BamHI and SacI sites of pUBTC vector (Feng et al. 2011; Li et al. 2018). This vector was modified from pCAMBIA3300 vector, which was driven by the maize ubiquitin promoter and terminated by nopaline synthetase (NOS) (provided by Prof. Shu-Zhen Zhang, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, Hainan, China). The plasmid pUBTC contains a bar gene as selectable marker and a gene conferring resistance to phosphinothricin (PPT), which has been widely used in sugarcane genetic transformation.
Resistance Screening of Transformed Materials
PPT was used as a plant selectable marker in the genetic transformation experiment. To determine the appropriate concentration of PPT for selection of transgenic plants, a series of pre-experiments on shoots were carried out. The shoots were incubated in M3 solid media [Murashige and Skoog, a medium (MS) + 1 mg/L 6-Benzylaminopurine (6-BA) + 1 mg/L naphthaleneacetic acid (NAA)] at callus differentiation stage with 6 gradient concentrations of PPT, that is, 0 mg/L, 1.5 mg/L, 2.5 mg/L, 3.5 mg/L, 4.0 mg/L, and 4.5 mg/L, respectively. There were 3 calli cultured in each bottle with three replicates. On the other hand, to determine the PPT sensibility to rooting, the shoots were incubated in the same concentration of PPT in the medium M4 (1/2 MS + 3 mg/L NAA). The calli in all the treatments were subcultured in a 20-day interval under a photoperiod of 16 h/8 h (light/dark) at 25 °C.
Sugarcane Transformation and Generation of Transgenic Plants
The recombinant plasmid pUBTC-SoACLA-1 was introduced into Agrobacterium tumefaciens strain EHA105 and then transferred into wild-type (WT) sugarcane using Agrobacterium-mediated transformation as described by Wang et al. (2005). Fresh sugarcane stem tips were used as explants to induce callus. The calli were transferred to fresh MI medium (MS + 3 mg/L 2,4-D) for 3 days then moved on filter paper and placed on a super clean bench for 30 to 60 min under flow air till the surface got dried and began shrinking. The surface-dried calli were infected by immersing in A. tumefaciens suspension and agitated at 80–90 rpm on a rotary shaker for 30 min and then blotted on a super clean bench. The infected calli were cut into pieces of 0.3–0.5 cm in diameter, placed onto the MI medium containing 150 pM acetosyringone (AS), co-cultured at 23 °C in the dark for 3 days, and then blotted with filter paper. After that, they were incubated onto the M2 medium (MS + 2 mg/L 6-BA + 0.1 mg/L NAA + 0.5 mg/L KT) which was supplemented with 200 mg/L timentin (Sigma) and cultured at 26 °C under illumination at 1500 lx with 14 h/day for selection. After 4 weeks, strong calli were excised with a scalpel and subcultured on the medium with the same composition.
The regenerated shoots were transferred to the selective medium (M3 + 1.5 mg/L PPT) till the shoots grew to 6–8 cm in height and then transferred into the rooting selective medium which was M4 medium containing 1.5 mg/L PPT and 200 mg/L timentin. All the survival plants after selection were transferred to a greenhouse to prepare for next experiment. The T0 generation of the transgenic plants was confirmed by PCR analysis using primers to determine the bar gene.
Drought Tolerance Assay of the T1 Transgenic Sugarcane Plants
To investigate whether overexpression of SoACLA-1 in the transgenic sugarcane enhances the plant tolerance to drought, the PCR-positive transgenic lines were selected with the non-transgenic tissue-cultured plants as WT control for phenotypic observation in T0 generation (Fig. S1). From the T0 generations of the transgenic sugarcane, RT1, RT2, RT3, and RT4 transgenic lines with good agronomic traits were selected for pot (32 cm in diameter and 29 cm in height) planting to obtain the T1 generation with the WT plants as control. The complete randomized pot experiment was conducted in the glasshouse of College of Agriculture, Guangxi University, Nanning, China, and the plants were grown in March 2017. Six pots were used to grow the wild type and each transgenic line with a mixture of soil and sand (3:1). Sugarcane leaves were taken for PCR detection to confirm whether the T1 generation of sugarcane is genetically modified with pUBTC-SoACLA-1 when the plants grew to 6 true leaves. At early elongation stage (August 2017), water stress was applied for both the transgenic and control plants by stopping watering for 0 day, 3 days, 6 days, and 9 days and then rewatering for 3 days (Fig. S2). There were 6 biological repetitions for the treatment. Relative water content (RWC) in soil was measured at different stress times. Simultaneously, the leaf + 1 (top visible dewlap leaf) was sampled for physiological-biochemical analyses to measure the contents of malondialdehyde (MDA), soluble sugar content (SSC), free proline (PRO), and leaf color value [Soil and Plant Analyzer Development (SPAD)], and the activities of superoxide dismutase (SOD) and peroxidase (POD).
Evaluation of Drought Tolerance of Transgenic Sugarcane in T2 Generation
Based on the performance in T1 generation of the SoACLA-1 transgenic sugarcane, RT2 and RT4 lines with better drought tolerance were selected for pot culture to get the T2 generation with the T2 WT plants as control. The pot experiments were also carried out in the glasshouse of College of Agriculture, Guangxi University, and the plants were grown in February 2018. The T2 plants of each line were planted in 6 pots, respectively, totaling 18 pots. Water stress treatment was carried out at the end of elongation stage (October 2018). Water stress treatments and physiological-biochemical measurements were the same as in T1 experiment. Leaf samples were taken after treatment, frozen in liquid nitrogen immediately, and stored at − 80 °C until use for extraction of total RNA and quantitative real-time PCR (qRT-PCR) analysis.
Expression Analysis of SoACLA-1 Gene in Transgenic Sugarcane
Total genomic DNA was extracted from the leaves of T0, T1, and T2 generations of transgenic and wild-type sugarcane plants using a NuClean PlantGen DNA Kit (ComWin Biotech Co. Ltd., Beijing, China) (Zhu et al. 2018). To confirm the presence of bar fragment and SoACLA-1 gene in the transgenic plants, the genomic DNA, bar-specific primers (Table S1), and SoACLA-1-specific primers (Table S1) were used to identify the transgenic plants (Integrated DNA Technologies, www.idtdna.com). Using pUBTC-SoACLA-1 as positive control and WT plant DNA as negative control, PCR was performed in 25 μL total solution containing 50 μg of template DNA (2 μL DNA), 10.5 μL ES Taq Mix (ComWin Biotech Co. Ltd., Beijing, China), 1.6 μL primer (0.5 μM), and 12.9 μL RNase-free water. The PCR procedure used for the bar gene consisted of an initial denaturing at 95 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 50 s, and a final elongation step at 72 °C for 2 min. For SoACLA-1 gene, the PCR procedure was similar to that of bar gene except the annealing temperature was changed to 58 °C for 30 s and a final elongation step at 72 °C for 5 min. The PCR products were identified by gel electrophoresis on 1.0% agarose gel and observed under UV-transmitted illumination. Simultaneously, the PCR products were recovered by gel recovery kit (TIANGEN Biotech Co. Ltd., Beijing, China) and sent for sequencing by Shanghai Bioengineering Co., Ltd., Shanghai, China.
Determination of RWC, MDA, SSC, PRO, SPAD, and Activities of SOD and POD
RWC of soil was determined by oven-drying method (Zhao and Wang 2017). The leaf + 1 samples were used to measure the physiological-biochemical parameters. For MDA content determination, the thiobarbituric acid (TBA)–based colorimetric method was used as described by Heath and Packer (1968). The SSC was determined by the method described by Tang (1999), and the content of free PRO was determined by the method of Bates et al. (1973). POD activity assay was done as described by Li (1990). SOD activity was determined referring to the method described by Giannoplitis and Ries (1977). SPAD values were measured by a chlorophyll analyzer (SPAD-502Plus; Konica Minolta, Japan). All the data were the means from five plants.
qRT-PCR Analysis of Δ1-Pyrroline-5-carboxylatesynthase, Saccharum Drought Responsive 4, Copper/Zinc Superoxide Dismutase, and Dehydrogenase in Transgenic Sugarcane
qRT-PCR was done to determine the expression patterns of the genes Δ1-pyrroline-5-carboxylatesynthase (P5CS), Saccharum drought responsive 4 (SCDR4), copper/zinc superoxide dismutase (Cu/Zn-SOD), and dehydrogenase (dhy) in transgenic sugarcane via SYBR Premix Ex Tap™ II (TaKaRa, Dalian, China). According to the full-length cDNA sequences of the four genes, the specific primers for qRT-PCR were designed as Table S1. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene of sugarcane (accession number EF189713) was used as the internal control gene, and the primers of GAPDH and the protocol of qRT-PCR were the same as those of the study of Zhu et al. (2018). There are three biological replicates with three technical replicates performed in each line. The relative expression level of those genes was calculated using the 2−ΔΔCt formula (Livak and Schmittgen 2001).
Statistical Analysis
The data were analyzed using the IBM SPSS Statistics 21.0 software program and Microsoft Excel software 2010. The statistical differences were compared based on ANOVA at 0.05 and 0.01 levels. The membership function method in fuzzy mathematics is used to comprehensively evaluate drought resistance indexes (Zhang et al. 2011).
Results
Expression Vector Construction
A full-length SoACLA-1 cDNA was isolated from sugarcane leaf according to the gene sequence in NCBI (GenBank accession number JQ292843.1) and verified by sequencing. The entire ORF was 1272 bp in length (Fig. S3a), encoding the SoACLA-1 protein with 423 amino acids. Then, a gene fragment of 1972-bp target band was obtained by PCR amplification, which matched the SoACLA-1 ORF sequence and the fragment of GFP (Fig. S3b). The expression vector pUBTC-SoACLA-1-GFP was constructed through ligation of the SoACLA-1-GFP gene with the vector, transformed to DH5a, and the recombinant plasmid was transformed into EHA105. The result indicated that the putative resultant plasmid was 12,524 bp in length and contained the genes of SoACLA-1 and GFP. The pUBTC vector was then identified by digestion with BamHI/SacI (Fig. S3c). Simultaneously, the correct fusion was further verified by DNA sequencing. Besides, SoACLA-1 harbored in EHA105 was identified by PCR (Fig. S3d), all the bacterial colonies (A. tumefaciens strain) with labeled SoACLA-1-GFP probe, and all the colonies showed positive via PCR with special pair primers (Table S1). The results showed the SoACLA-1 gene had been inserted into the vector plasmid, and the expression vector pUBTC-SoACLA-1-GFP had been successfully constructed.
Determination of PPT Concentration for Transgenic Selection
The tissues from sugarcane variety ROC22 were used for PPT resistance screening. Sugarcane callus tissue culture was carried out in the selective regeneration media containing PPT or without PPT. The results showed that the calli in the regeneration medium without PPT (as control) grew healthily and generally grew vigorous shoots, but the shoots could not grow and survive on 2.5 to 4.5 mg/L PPT medium (Fig. S4a). Therefore, 2.5 mg/L of PPT was selected in the selection medium for transgenic selection. All the roots grew strongly and healthily in the media without PPT; however, the roots grew shorter and weaker in the medium containing 1.5 mg/L PPT, and all the shoots could not develop roots and eventually died in 2.5 mg/L PPT medium as the leaves became yellow (Fig. S4b). Therefore, 2.5 mg/L PPT was used in root differentiation medium for transgene selection (Table S2).
Molecular Analysis of Putative Transgenic Plants
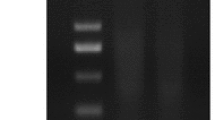
Genomic DNA was extracted from the fresh leaves of putative transgenic plants in 30 days after transplanting. The results showed that there was no such amplification detected in wild-type plants (Fig. 1) while SoACLA-1-GFP gene was amplified by PCR analysis as 18 samples from 30 plants being positive. As shown in Fig. S5, PCR analysis also was used to confirm the presence of bar gene in the transgenic lines. The bar gene was observed in the transgenic plants but not in the non-transgenic plants, and 5 of 30 plants were positive. The results indicated that the SoACLA-1 transgenic sugarcane plants had been obtained successfully, and a total of 22 transgenic plants containing SoACLA-1 and bar genes was obtained.
Physiological Response of T1 Transgenic Plants to Water Stress
The SPAD values in the four transgenic lines and WT in T1 generation decreased gradually in the whole process of water stress, but the decreases were less in the transgenic lines than in the WT in the later stage of water stress. The SPAD values were significantly higher in all the transgenic lines than in the WT at the 9th day of stress and the 3rd day after rewatering except RT2 and RT4 after rewatering (Fig. 2a). The MDA content in all the lines increased during water stress and decreased after rewatering, and it was higher in the WT than in the four transgenic lines. The peak occurred at the 9th day of water stress, and it was found to be significantly lower in the four transgenic lines than in the WT at this time. After rewatering for 3 days, the MDA content showed WT > RT4 > RT1 > RT2 > RT3 and was significantly lower in the RT1, RT2, and RT3 lines than in the WT (Fig. 2b). The POD activity in the transgenic lines and WT increased during water stress except for that in RT1 which showed a decrease at the 6th and 9th days of water stress (Fig. 2c). At the 9th day of stress, the POD activity continued to increase in the RT2, RT3, and RT4 lines and showed to be significantly higher than that in the WT. The POD activity was significantly higher in RT1 and RT3 than in WT in 3 days after rewatering (Fig. 2c). The SOD activity in the transgenic lines and WT increased in the whole process from water stress to rewatering (Fig. 2d). The SOD activity was significantly higher in RT2 than in WT at the 6th day of water stress and after rewatering. In the 3rd and 6th days of water stress, the SOD activity was significantly lower in RT4 than in WT. The SOD activity was significantly higher in RT3 than in WT at the 6th day of water stress. However, there was no significant difference between RT1 and WT in the whole water stress process (Fig. 2d). The free proline content in all the sugarcane lines remained at a low level at the 3rd day of water stress and then began to increase; it was significantly higher in RT2, RT3, and RT4 than in WT at the 6th day of water stress. At the 9th day of water stress, only RT2 had higher proline content than WT. After 3 days of rewatering, the proline content in the four transgenic lines decreased while that in the WT continued to rise, which showed much higher than that in the transgenic lines, and the order was WT > RT2 > RT3 > RT4 > RT1 (Fig. 2e). The soluble sugar content in all the transgenic lines remained basically unchanged when the water stress lasted for 3 days, and that in the RT2 and RT4 lines increased rapidly and was significantly higher than that in the WT at the 6th and 9th days of water stress while there was no significant difference between WT and RT1. At the 9th day of water stress, soluble sugar content is significantly lower in RT3 than in WT (Fig. 2f).
Variation of physiological indexes related to drought resistance of different T1 generation transgenic sugarcane lines under drought stress. There were 6 biological repetitions for the treatment. All the data were the means from five plants. a Leaf color value (SPAD, Soil and Plant Analyzer Development). b Malondialdehyde (MDA) content. c Peroxidase (POD) activity. d Superoxide dismutase (SOD) activity. e Proline content. f Soluble sugar content. Different lowercase letters represent a significant difference between the lines at the P < 0.05 level. RT1, RT2, RT3, and RT4 represent four different transgenic sugarcane lines, and WT represents wild-type sugarcane
The drought resistance and recovery ability after rewatering of the five sugarcane lines were evaluated comprehensively by using the membership function values of four indexes closely related to drought resistance of sugarcane (chlorophyll, malondialdehyde, proline, and soluble sugar). The results showed that the drought resistance of the sugarcane lines was RT2 > RT4 > RT3 > RT1 > WT (Table 1), and the recovery ability after water stress was RT1 > RT2 > RT4 > RT3 > WT (Table 2).
Physiological Response of T2 Transgenic Plants to Water Stress
The transgenic lines RT2, RT4, and WT in T2 generation were selected for the further experiment. It can be seen from Fig. 3a that the SPAD values in the three lines in T2 generation decreased after water stress and increased after rewatering. Although the SPAD values in RT2 and RT4 had no significant difference from the WT at all the stages, they maintained higher levels compared to the WT at the 9th day of stress and the 3rd day of rewatering. The data in Fig. 3b show that there was no significant difference in MDA content between the two transgenic lines and WT at the 6th day of water stress, but it was higher in WT than in RT2 and RT4, and the difference was significant between WT and RT4 at the 9th day of water stress and the 3rd day of rewatering. The POD activity in RT2 and RT4 increased rapidly in 9 days of water stress, which was significantly higher than that in WT, and continued to increase after 3 days of rewatering in WT and RT2 but began to decrease in RT4. At this time, the POD activity showed RT2 > RT4 > WT, and the difference was significant between RT2 and WT (Fig. 3c). The SOD activity increased at the 6th day of water stress in the two transgenic lines, which was also higher than that in the WT, and the difference between RT4 and WT was significant. It continued to increase in WT and RT2 while decreased in RT4 at the 9th day of water stress, and decreased in all the three entries at the 3rd day of rewatering; RT2 showed significantly higher SOD activity than WT at the 9th day of water stress and the 3rd day of rewatering (Fig. 3d). The proline content was at a low level in 6 days of water stress in all the lines, and there was no significant difference between WT and the transgenic lines. After 9 days of water stress, the proline content increased in the transgenic lines, and was significantly higher in RT2 than in WT. At the 3rd day of rewatering, the proline content in all the lines decreased to a low level (Fig. 3e). As presented in Fig. 3f, the soluble sugar content in RT2, RT4, and WT increased first and then decreased in the whole water stress process. That in RT2 and RT4 increased to the highest and was significantly higher than that in WT at the 6th day of water stress, and that in RT2 was still significantly higher than that in WT at the 9th day of stress. After 3 days of rewatering, the soluble sugar content decreased in RT2 and RT4 while increased in WT which was significantly higher than that in RT4.
Variation of physiological indexes related to drought resistance of different T2 generation transgenic sugarcane lines under drought stress. There were 6 biological repetitions for the treatment. All the data were the means from five plants. a Leaf color value (SPAD, Soil and Plant Analyzer Development). b Malondialdehyde (MDA) content. c Peroxidase (POD) activity. d Superoxide dismutase (SOD) activity. e Proline content. f Soluble sugar content. Different lowercase letters represent a significant difference between the lines at the P < 0.05 level. RT1, RT2, RT3, and RT4 represent four different transgenic sugarcane lines, and WT represents wild-type sugarcane
The drought resistance and their recovery ability after rewatering of RT2, RT4, and WT of T2 generation were evaluated by membership function values of chlorophyll, MDA, proline, and soluble sugar. The results showed that the drought resistance of sugarcane was RT4 > RT2 > WT (Table 3), and the recovery ability after water stress was RT2 > RT4 > WT (Table 4).
Expression of Drought-Resistant Genes in T2 Transgenic Plants Under Drought Conditions
The data in Fig. 4a show that the SoACLA-1 gene expression in the three T2 lines reached the highest level at the 6th day of water stress and was significantly higher in RT2 and RT4 than in WT. At the 9th day of water stress, the SoACLA-1 gene expression in all the lines decreased to the lowest level but increased after 3 days of rewatering. The expression of P5CS gene in the three T2 lines was lower at the 6th day of water stress, while increased at the 9th day of water stress, and was significantly higher in RT4 than in WT. After 3 days of rewatering, the expression of P5CS gene in all the lines decreased rapidly to normal level (Fig. 4b). The expression of SCDR4 gene in the three T2 lines increased and reached the highest level in 6 days of water stress and decreased after 9 days of water stress. After 3 days of rewatering, the SCDR4 gene expression increased slightly in RT2 and WT while increased significantly in RT4, and that was significantly higher in RT2 and RT4 than in WT in the whole water stress process and after rewatering (Fig. 4c). The expression of Cu/Zn-SOD gene in RT2 and RT4 increased and showed significantly higher than that in WT at the 6th day of water stress. At the 9th day of water stress, that in RT2 decreased, while that in WT and RT4 increased. At this time, that was significantly higher in RT4 than in WT (Fig. 4d). In the whole process of water stress, the expression of dhy gene in the three T2 lines increased, reached the highest level at the 9th day of water stress, and decreased after 3 days of rewatering (Fig. 4e). That was significantly higher in RT2 and RT4 than in WT at the 6th and 9th days of water stress, and there was a significant difference between RT2 and WT in 3 days after rewatering.
Relative expression of drought-related genes in T2 generation of transgenic sugarcane under drought stress. Different lowercase letters represent a significant difference between the lines at the P < 0.05 level. RT2 and RT4 represent two different transgenic sugarcane strains, and WT represents wild-type sugarcane
The correlations between the physiological-biochemical indices and the expression levels of several drought tolerance–related genes in the transgenic and WT sugarcane of T2 generation under water stress and rewatering were analyzed, and the results are presented in Table 5. As shown in Table 5, the expression of SoACLA-1 gene was significantly positively correlated with the SPAD value and soluble sugar; the expression of P5CS gene was significantly positively correlated with the activity of SOD and highly significantly positively correlated with the content of proline; the expression of SCDR4 gene was significantly positively correlated with the content of soluble sugar; the expression of dhy gene was significantly positively correlated with the activities of SOD, POD and the content of proline, and with the comprehensive drought tolerance; the expression levels of SoACLA-1 gene and several drought-related genes were significantly positively correlated with the comprehensive drought tolerance.
Discussion
ACL can catalyze the production of acetyl-CoA from citric acid in cytoplasm (Fatland et al. 2002). Acetyl-CoA plays an important role in plant growth and development. SoACLA-1 is a small subunit of ACLA gene cloned from sugarcane (Li et al. 2012). Previous studies have shown that SoACLA-1 gene is upregulated by water stress (Li et al. 2012; Liu et al. 2014). Overexpression of SoACLA-1 gene could improve the tolerance of tobacco to water stress (Phan et al. 2016). In this study, the transformation of SoACLA-1 gene in sugarcane by Agrobacterium tumefaciens mediation has been done successfully. The objective fragment was amplified by PCR (Fig. 1). It was verified that the monocotyledon plant expression vector pUBTC-SoACLA-1 could be stable in the transgenic sugarcane. By measuring the physiological and biochemical changes and the expression of stress resistance–related genes in the transgenic sugarcane under water stress, we proved that overexpression of SoACLA-1 gene had a positive effect on the drought tolerance of sugarcane.
Previous studies have found that the effects of water stress on plants are mainly manifested in their physiological and morphological changes, such as slow growth, leaf wilting, and yellowing (Shi 2010). Chlorophyll is the most important pigment in plant, and its content changes directly affect photosynthesis (Dhindsa and Matowe 1981). SPAD value in plants is proportional to chlorophyll (Dhindsa and Matowe 1981). Phan et al. (2016) showed that the SPAD value in the transgenic tobacco with SoACLA-1 gene was significantly higher than that in the wild type under water stress. In this study, the SPAD values were significantly higher in the four transgenic sugarcane lines (RT1–4) than in the WT at the 9th day of water stress (Fig. 2a), and the decrease was greater in the WT than in the four transgenic lines in the whole water stress process (Fig. 2a), which indicated that the chlorophyll content in the transgenic sugarcane was less affected by water stress, reflecting that the drought tolerance of the transgenic sugarcane was stronger than that of the WT. This is consistent with the results of Phan et al. (2016).
Plant cells are attacked by reactive oxygen species (ROS) under water stress. Excessive ROS can induce the peroxidation of unsaturated fatty acids in membrane lipids to produce MDA. Polymerization of MDA with enzyme protein damages would result in membrane degeneration (Li et al. 2010). A large number of studies have shown that overexpression of some drought-resistant genes in plants can reduce MDA content in rice, tobacco, and maize (Phan et al. 2016; Li et al. 2008a, b). In this study, the MDA change rate was lower in the transgenic sugarcane lines than in the WT under water stress and after rewatering for 3 days (Figs. 2b and 3b). It can be inferred that the damage degree of membrane caused by water stress was lighter in the transgenic sugarcane than in the WT, which is consistent with the previous research results.
In order to cope with water stress, plants will activate their own antioxidant system to remove or neutralize excessive ROS, including increasing the activities of antioxidant enzymes such as SOD and POD (Ramiro et al. 2016). The results in the present study showed that the activities of POD and SOD were stronger in the SoACLA-1 transgenic sugarcane lines than in the WT under water stress, especially in the RT2 and RT4 lines (Figs. 2c, d and 3c, d), reflecting the stronger ability of the SoACLA-1 transgenic sugarcane in scavenging peroxide and reactive oxygen species compared with the WT.
Plants not only activate defensive systems but also synthesize and accumulate osmotic protectants such as free proline and soluble sugar to regulate cell fluid concentration and prevent excessive cell dehydration under water stress (Ashraf and Foolad 2007). The results in the present study showed that the contents of free proline and soluble sugar increased in sugarcane after water stress (Fig. 2e, f), which was consistent with the results of Han et al. (2012). The contents of proline and soluble sugar were significantly higher in the RT2 and RT4 lines than in the WT (Fig. 3e, f), indicating that the osmotic regulation ability of the RT2 and RT4 lines was stronger than that of the WT. However, the proline content was significantly higher in the WT than in the transgenic lines after rewatering (Figs. 2e and 3e); this may be due to the fact that the transgenic sugarcane has returned to its normal state, and no high level of osmotic regulation is required at this time, but the WT recovered more slowly and was still at active osmotic regulation stage, indicating that the recovery ability of the SoACLA-1 transgenic sugarcane was stronger than that of the WT to some extent after water stress. The soluble sugar content was significantly higher in the RT2 and RT4 lines than in the WT, especially at the middle and late stages of water stress and rewatering (Figs. 2f and 3f), which indicated that they had stronger drought tolerance.
From the above results, it is inferred that the SoACLA-1 transgenic sugarcane lines, especially RT2 and RT4, are more active than the WT in response to water stress. On the one hand, the RT2 and RT4 lines showed stronger ability to eliminate redundant ROS by increasing the activity of defensive enzymes such as SOD and POD, so they would reduce the membrane lipid peroxidation and the production of malondialdehyde. On the other hand, they can synthesize more osmotic regulators such as proline and soluble sugar to maintain the stability of intracellular environment and protect cell structure. Physiological and biochemical changes in plants were the result of the changes in gene expression (Li 2017). In this study, the results of qRT-PCR analyses showed that the expression of SoACLA-1 gene was significantly positively correlated with the expression levels of several drought-related genes and the comprehensive drought resistance in different sugarcane lines (Table 5). The SoACLA-1 transgenic lines RT2 and RT4 performed better than the WT on both physiological level and transcription level of drought tolerance–related genes, which indicates that the SoACLA-1 transgenic lines have stronger drought tolerance than the WT and the same transgenic sugarcane lines (RT1 and RT3). The difference may be due to the different numbers of integrated DNA copies or different integration sites, which needs further analyses in the future.
Conclusion
In conclusion, overexpression of SoACLA-1 gene could improve the water stress tolerance in the transgenic sugarcane lines by enhancing the antioxidant system to defend against ROS accumulation in cells. Transformation of SoACLA-1 target gene also enhanced the synergy with other drought resistance–related genes at the transcriptional level, which might be useful for further application in the improvement of drought tolerance of sugarcane.
Change history
13 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11105-021-01322-6
References
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant biotic stress resistance. Environ Exp Bot 59(2):206–216
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Choi JK, Yu F, Wurtele ES, Nikolau BJ (1995) Molecular cloning and characterization of the cDNA coding for the biotin-containing subunit of the chloroplastic acetyl-CoA carboxylase. Plant Physiol 109:619–625
Dhindsa R, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defense against lipid peroxidation. J Exp Bot 32(1):79–91
Fatland BL, Ke J, Anderson MD, Mentzen WI, Cui LW, Allred CC, Johnston JL, Nikolau BJ, Wurtele ES (2002) Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis. Plant Physiol 130:740–756
Fatland BL, Nikolau BJ, Wurtele ES (2005) Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-1 citrate lyase in Arabidopsis. Plant Cell 17:182–203
Feng CL, Shen LB, Zhao TT, Wang JG, Xiong GR, Zhang SZ (2011) Genetic transformation of CryIAb gene into sugarcane. Chin J Trop Agric 31(9):21–26
Giannoplitis CN, Ries SK (1977) Superoxide dismutase: I. Occurrence in higher plants. Plant Physiol 59(2):309–314
Han SJ, Luo WG, Zhou JQ, Pei TX, Long CL, Lu GY (2012) The drought resistance evaluation of different sugarcane varieties under drought stress in elongation period. J Anhui Agric Sci 40(35):17044–17047
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 25(1):189–198
Hu XM, Shi CY, Liu X, Jin LF, Liu YZ, Peng SA (2015) Genome-wide identification of citrus ATP-citrate lyase genes and their transcript analysis in fruits reveals their possible role in citrate utilization. Mol Gen Genomics 290:29–38
Koziol C (2002) Overexpression of ATP citrate lyase in Arabidopsis thaliana. Under the direction of the Wurtele Lab, Iowa State University-Department of Botany, Summer 2002 NSF REU report in molecular biotechnology
Langlade NB, Messerli G, Weisskopf L, Plaza S, Tomasi N, Smutny J, Neumann G, Martinoia E, Massonneau A (2002) ATP citrate lyase: cloning, heterologous expression and possible implication in root organic acid metabolism and excretion. Plant, Cell Environ 25(11):1561–1569
Li WW (2017) Functional analysis of foxtail millet autophagy associated gene SiATG8a in regulated plant response to low nitrogen stress. Ph.D. dissertation of Harbin Normal University, Harbin, China
Li YR (1990) A preliminary study on the activity of peroxidase in sugarcane tissues and its relationship with growth and technical maturing. Genomics Appl Biol 9(1):13–18
Li YR, Yang LT (2015) Sugarcane agriculture and sugar industry in China. Sugar Tech 17(1):1–8
Li B, Wei AY, Song CX, Li N, Zhang J (2008a) Heterologous expression of the TsVP gene improves the drought resistance of maize. Plant Biotechnol J 6(2):146–159
Li GW, Zhang MH, Cai WM, Sun WN, Su WA (2008b) Characterization of OsPIP2;7, a water channel protein in rice. Plant Cell Physiol 49(12):1851–1858
Li CN, Srivastava MK, Qian N, Li YR (2010) Mechanism of tolerance to drought in sugarcane plant enhanced by foliage dressing of abscisic acid under water stress. Acta Agron Sin 36(5):863–870
Li CN, Qian N, Tan QL, Manoj KS, Yang LT, Li YR (2012) Cloning and expression analysis of ATP-citrate lyase genes from sugarcane. Acta Agron Sin 38(11):2024–2033
Li J, Phan TT, Li YR, Xing YX, Yang LT (2018) Isolation, transformation and overexpression of sugarcane SoP5CS gene for drought tolerance improvement. Sugar Tech 20(4):464–473
Liu JY, Li YR, Yang LT (2014) Advances in ATP-citrate lyase research. J Southern Agric 45(2):204–208
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 25(4):402–408
Meijer S, Nielsen ML, Olsson L, Nielsen J (2009) Gene deletion of cytosolic ATP: citrate lyase leads to altered organic acid production in Aspergillus niger. J Ind Microbiol Biotechnol 36:1275–1280
Nikolau BJ, Ohlrogge JB, Wurtele ES (2003) Plant biotin-containing carboxylases. Arch Biochem Biophys 414(2):211–222
Phan TT, Li J, Sun B, Liu JY, Zhao WH, Huang C, Yang LT, Li YR (2016) ATP-citrate lyase gene (SoACLA-1), a novel ACLA gene in sugarcane, and its overexpression enhance drought tolerance of transgenic tobacco. Sugar Tech 19(3):258–269
Ramiro DA, Melotto-Passarin DM, Barbosa MD, Santos FD, Gomez SG, Massola Junior NS, Lam E, Carrer H (2016) Expression of Arabidopsis bax inhibitor-1 in transgenic sugarcane confers drought tolerance. Plant Biotechnol J 14(9):1826–1837
Rangasamy D, Ratledge C (2000) Compartmentation of ATP: citrate lyase in plants. Plant Physiol 122(4):1225–1230
Shi XK (2010) Assessment of drought resistance and genetic stability in sugarcane mediated with DREB gene. Dissertation, Fujian Agriculture and Forestry University, pp. 1–3
Souter M, Topping J, Pullen M, Friml J, Palme K, Hackett R, Grierson D, Lindsey K (2002) hydra mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. Plant Cell 14(5):1017–1031
Sun T, Hayakawa K, Bateman KS, Fraser ME (2010) Identification of the citrate-binding site of human ATP-citrate lyase using X-ray crystallography. J Biol Chem 285(35):27418–27428
Sun T, Hayakawa K, Fraser ME (2011) ADP-Mg2+ bound to the ATP-grasp domain of ATP-citrate lyase. Acta Crystallogr 67(10):1168–1172
Tang ZC (1999) A guide to modern plant physiology experiments. Science, Beijing, pp 302–303
Tong J (2009) Cloning and functional research of citrate synthase and ATP-citrate lyase of Brassica napus L. Dissertation, Chinese Academy of Agricultural Sciences, pp 91–95
Wang ZZ, Zhang SZ, Yang BP, Li YR (2005) Trehalose synthase gene transfer mediated by Agrobacterium tumefaciens enhances resistance to osmotic stress in sugarcane. Sugar Tech 7(1):49–54
Xing S, van Deenen N, Magliano P, Frahm L, Forestier E, Nawrath C, Schaller H, Gronover CS, Prufer D, Poirier Y (2014) ATP citrate lyase activity is post-translationally regulated by sink strength and impacts the wax, cutin and rubber biosynthetic pathways. Plant J 79:270–284
Zhang BQ, Yang LT, Li YR (2011) Comparison of physiological and biochemical characteristics eelated to cold resistance in sugarcane under field conditions. Acta Agron Sin 37(3):496–505
Zhao FY, Wang CS (2017) Principles and comparison pf common methods for determining soil water content. Horticult Seed 10:70–73
Zhu K, Yuan D, Zhang XQ, Yang LT, Li YR (2018) The physiological characteristics and associated gene expression of sugar cane inoculated with Leifsonia xyli subsp. xyli. J Phytopathol 166:44–52
Acknowledgments
The present study was supported by the grants from the National Key Research and Development Program of China (2018YFD1000500), Guangxi Special Fund for Scientific Base and Talent (GKAD17195100), Fund for Guangxi Innovation Teams of Modern Agriculture Technology (gjnytxgxcxtd-03-01), and Fund of Guangxi Key Laboratory of Sugarcane Genetic Improvement (16-K-02-01).
Author information
Authors and Affiliations
Contributions
YRL and LTY design the experiment; CH, TTP, and KZ conducted the experiment; CH, BQZ, and KZ analyzed the data; KZ, YXX, and YRL wrote the manuscript; and YXX and YRL revised and finalized the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Standards
The experiments conducted in this study comply with the current laws of China.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Message
An in vivo functional analysis of a sugarcane ATP citrate lyase gene, namely SoACLA-1, has been conducted using a transgenic approach to understand its overexpression role in sugarcane responsive to water stress. Physiological traits and resistance gene expression analysis indicated that overexpression of SoACLA-1 enhances drought tolerance in transgenic lines compared to the wild type.
Supplementary Information
ESM 1
(DOCX 4411 kb)
Rights and permissions
About this article
Cite this article
Zhu, K., Huang, C., Phan, TT. et al. Overexpression of SoACLA-1 Gene Confers Drought Tolerance Improvement in Sugarcane. Plant Mol Biol Rep 39, 489–500 (2021). https://doi.org/10.1007/s11105-020-01263-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-020-01263-6