Abstract
ATP-citrate lyase catalyzes cracking citrate to produce acetyl CoA in the cytoplasm and plays an important role in normal growth and development of plants. In this study, SoACLA-1 gene in sugarcane was cloned and characterized. The amino acid sequence of SoACLA-1 showed high homology with other ACLAs families, and they were clustered together in the phylogenetic tree. On transcript level, SoACLA-1 expressed strongly in root and stalk and responded to polyethylene glycol 6000, drought and ABA stresses. The results indicated that SoACLA-1 possibly involves in stress-responsive metabolic process. PCR analysis was performed to confirm the putative transgenic type in T1 tobacco plants. Overexpression of SoACLA-1 enhanced the tolerance of tobacco plants to drought. The sense transgenic tobacco plants were healthier, had longer roots and higher contents of relative water content and chlorophyll and activities of superoxide dismutase, peroxidase and catalase, but lower ion leakage and contents of malondialdehyde (MDA) and H2O2 than the wild-type and antisense transgenic plants under drought stress. In conclusion, SoACLA-1 plays an important role as a positive factor in drought stress response pathways and can be utilized for transformation to improve crop drought tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought is one of the most severe stresses which influence growth, development, productivity and quality of crops (Farooq et al. 2009). It will be important to improve plant drought tolerance via genetic engineering that transforms a gene with drought tolerance function. The gene-modified plants adapt and respond to drought stress by improving transcriptional levels of a large number of genes and synthesis of proteins functioning in physiological, biochemical and metabolic adaptations (Seki et al. 2001, 2002; Yamaguchi-Shinozaki et al. 2003; Hu et al. 2006). In biosynthesis pathways, ATP-citrate lyase is an important enzyme that catalyzes converting citrate into oxaloacetate and acetyl coenzyme A (acetyl CoA) in plants, and acetyl CoA as an essential key plays intermediate links in metabolic pathways with at least five subcellular units of physiological and biochemical processes (Rangasamy and Ratledge 2000; Nikolau et al. 2003). The expression of plant ATP-citrate lyase is reported to be induced by pathogen stress (Suh 2001).
In eukaryotic cell, acetyl CoA was generated through three different mechanisms and played an important role in regulating chromosome structure and gene expression. In cells, acetyl CoA is incorporated into TCA cycle and then transported to the mitochondria for the ATP generation and the synthesis of carbon for complete oxidation. In the nucleus, acetyl CoA is the substrate for histone and transcription factor of acetylation. In addition, in the cytoplasm, acetyl CoA carboxylase and ACL convert acetate and citric acid into acetyl CoA, which involved in the synthesis of a number of substances that regulate the physiological activity (Souter et al. 2002; Shalit et al. 2003). On the other hand, in the cytosol, acetyl CoA plays a crucial role in the biosynthesis, which is important for growth, development and responses to adverse environments (Clouse 2002).
ACL is mainly a cytosolic enzyme, which is along with the hydrolysis of ATP and presents in animals and plants. Animal ACL has one polypeptide (ACLY), which is a homotetramer of 110- to 120-kDa subunits (Elshourbagy et al. 1992). Recent studies indicated that expression or activity of ACLY in animals plays crucial roles in tumor cell growth, survival in fetal growth and development, and metabolic disorders (Chypre et al. 2012; Zaidi et al. 2012). Plant ACL has two distinct subunits, ACLA (45–46 kDa) and ACLB (65 kDa) (Fatland et al. 2002). In 2002, for the first time ACL has been characterized in Arabidopsis and lupin at genome level by Fatland et al. (2002) and Langlade et al. (2002). In lupin, each subunit ACLA or ACLB was encoded by one gene. In contrast, in Arabidopsis thaliana, each subunit was encoded by small genes. For example, ACLA subunit is encoded by ACLA-1, ACLA-2 and ACLA-3 small genes, and the ACLB subunit is encoded by ACLB-1 and ACLB-2 genes (Fatland et al. 2002).
Recently, ACL isolated from plants has been characterized on molecular level. Reverse genetic analysis demonstrated that ACL activity decrease could induce phenotype disorders. Therefore, ACL is beneficial for normal growth and development of plants (Fatland et al. 2005). In contrast, overexpression of ACL increased the amount of fatty acids and lipids produced in plants of Arabidopsis thaliana (Koziol 2002). Moreover, overexpression of ACL also activated the wax, cutin and rubber biosynthesis pathways (Xing et al. 2014). In addition, in lupin, expression of ACL was responsive for the switch of maltase and citrate excretion during the cluster root growth and development (Langlade et al. 2002). The expression of ACL significantly increased in fruits of the citrus under mild drought stress and ABA treatment (Hu et al. 2015). However, ACL gene enhancing drought tolerance by regulating reactive oxygen species (ROS) as a scavenger through effective antioxidant system is yet to be revealed in plants.
As a most important sugar and energy crop in the world, sugarcane production is affected by multiple stresses, such as drought and extreme temperatures. Therefore, improvement of sugarcane tolerance to abiotic stress is necessary. In our laboratory, sugarcane ACL gene was reported to have two subunits (SoACLA-1 and SoACLB-1), and the treatments with abscisic acid (ABA), water stress and water stress plus ABA induced the expression of ACL (Li et al. 2012; Liu et al. 2014). In this study, we characterized SoACLA-1 gene and demonstrated that its over expression enhances drought tolerance through improving the antioxidant system as well as the possibility of utilization of ACLA-1 gene to improve sugarcane drought resistance, and it may be involved in the regulation of ABA-responsive metabolic processes in the plants.
Materials and Methods
Plant Material and ABA, PEG and Drought Treatments
Sugarcane (Saccharum spp. hybrids) variety GT21, which was planted in greenhouse at Sugarcane Research Institute, Guangxi University in Nanning, China was used in this study. The single-bud seedcanes were initially grown with sand culture in a glasshouse condition (Niu et al. 2013). After 4 weeks, the young plants were transplanted into plastic pots (21 cm in diameter and 19 cm high) containing Hoagland nutrient solution. The solution of each pot was changed once every 3 days in order to remove the deposition of toxic metabolites and root debris. Plants of 3 months old were used for studying target gene expression in leaf under diverse abiotic stresses. For expression of SoACLA-1 in different organs, leaf and root were collected and immediately frozen in liquid nitrogen for next experiment. For ABA treatment, leaves were sprayed with 100 μM ABA, while they were sprayed with water for the control. For PEG stress treatment, plants were transferred to pots containing 15 % PEG 6000 and incubated for different durations, while those without 15 % PEG 6000 were used as the controls. For drought treatment, the plants were taken out from pots and placed on filter paper to dry (Li et al. 2012), and those without treatment were used as the controls. At 0, 6, 12, 24, 48 and 72 h after treatment, respectively, leaf samples were taken, frozen in liquid nitrogen immediately and stored at −80 °C until use for extraction of total RNA and qRT-PCR analysis.
Cloning and Phylogenetic Analysis
Total RNA was extracted from plant tissues using Trizol-A+ (Tiangen, Beijing, China), which was reversely transcribed into cDNA template for PCR amplification. The reverse transcription product with an oligo (dT) 16 primer and MLV reverse transcriptase (TaKaRa, Dalian, China) were used in PCR to amplify the target gene SoACLA-1 open reading frame according to the Takara (SYBR Premix Ex Taq II) manufacturer’s instruction. Based on the sequence of SoACLA-1 found in our laboratory (Li et al. 2012), the cDNA of SoACLA-1 gene was obtained using cDNA amplification kit (TaKaRa, Dalian, China), and a pair of specific primers (Table S1) was designed to amplify the full-length cDNA using Primer Premier 5.0 software. The following reaction procedure was used for PCR experiment: 10 min at 95 °C for denaturing, 35 cycles for 30 s at 95 °C, for 30 s at 58 °C, for 2 min at 72 °C and 10 min at 72 °C. The amplified product was cloned into the pMD18-T vector and transformed into Escherichia coli strain DH5α before sequencing.
The evolutionary relationship between SoACLA-1 and other ACL family genes was analyzed using BLAST (http://ncbi.nlm.nih.gov/blast) in the NCBI database. The molecular weight and isoelectric point (pI) of protein were predicted using ExPASy (http://expasy.org/tools/). And then, MEGA 6.0 (http://www.mega software.net) was used to construct a phylogenetic tree of SoACLA-1 and ACLAs members from other plant species (Tamura et al. 2013).
qRT-PCR Analysis Expression of SoACLA-1 in Sugarcane
Quantitative real-time PCR (qRT-PCR) method was utilized to determine the expression patterns of SoACLA-1 in sugarcane via SYBR Premix Ex Tap™ II (TaKaRa, Dalian, China). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene of sugarcane (accession number EF189713) (Table S1) was used as an internal control to quantify the relative transcript level of SoACLA-1 gene in sugarcane. Specific primers of SoACLA-1 gene (Table S1) were also designed based on the cDNA sequence, and reverse transcription was performed for qRT-PCR. The reaction system was prepared in a total volume of 20 μL as follows: 12.5 μL SYBR Premix Ex Tap™ II, 2 μL cDNA, 1 μL of each 10 μM primer and 8.5 μL ddH2O at 42 °C. The relative level of gene expression was calculated using the 2−ΔΔCt formula (Livak and Schmittgen 2001).
Tobacco Transformation and Generation of Transgenic Plants
The seeds of tobacco (Nicotiana tabacum L.) variety K346 were utilized in this study. The cDNA of SoACLA-1 containing the entire ORF was amplified by its special primer (Table S1) and then was inserted into the BamH I and Smal I sites of pRI 101-AN vector (site in bold italics, Table S1). The recombinant plasmid pRI 101-AN-SoACLA-1 constructed under control of the CaMV35S promoter and NOS terminator was introduced into Agrobacterium tumefaciens strain EHA 105 and then transferred into wild-type tobacco using an Agrobacterium-mediated leaf disk transformation as described by Horsch et al. (1985). The seeds of Nicotiana tabacum were sterilized with 70 % ethanol for 30 s, then incubated for 5 min in 0.001 % HgCl2 and rinsed with sterile distilled water for four times before they were sown on germination medium M1 containing MS (Murashige and Skoog 1962) supplemented with 30 g L−1 sucrose and 0.7 % agar (pH 5.7). After inflection, the explants were co-cultivated in MS with salts suitable for growth containing 100 mg L−1 of kanamycin and 200 ng L−1 timentin. The T1 generation of the transgenic plants was confirmed by PCR analysis using primers to determine NPTII gene (Table S1) before drought stress treatment. Simultaneously, seeds of the transgenic T1 plants were harvested for further experiment.
RT-PCR Analysis Expression of SoACLA-1 in Transgenic Tobacco
The expression of SoACLA-1 in the T1 transgenic tobacco plant was detected by RT-PCR method. The gene was identified using special primers (Table S1) and then confirmed by 1 % agarose gel electrophoresis. The reaction of RT-PCR experiment was carried out in the total volume 20 μL for 35 cycles according to TaKaRa DNA polymerase introduction (Pang et al. 2007). Actin transcript was used as an internal control to quantify the expression level of SoACLA-1 gene between WT and transgenic line under drought treatment. The RT-PCR experiment was replicated with three times using independent cDNA samples.
PCR Analysis Expression of SoACLA-1 in Transgenic Tobacco
Total DNA was extracted from the leaves of T1 and T2 generations of in vitro tobacco transgenic and wild-type plants using a modified CTAB (cetyl trimethyl ammonium bromide) extraction method (Rogers and Bendich 1985). To confirm the presence of NPTII fragment (722 bp of NPTII) and SoACLA-1 gene (1272 bp) in the transgenic plants, the genomic DNA and NPTII-specific primers (Table S1) and SoACLA-1-specific primers (Table S1) were used to identify transgenic plants (Integrated DNA Technologies, www.idtdna.com). PCR was performed in 25 μL total solution containing 50 μg of template DNA (μL DNA), 2.5 μL 10× Ex Taq Buffer, 1.6 μL 2.5 mM dNTP Mixture, 1 μL primer (0.5 μM) and 0.125 μL 5U μL−1 Takara Ex Taq (TaKaRa, Dalian, China). The PCR procedure used for the NPTII gene consisted of an initial denaturing at 95 °C for 10 min, followed by 35 cycles of 95 °C for 30 s, 68 °C for 30 s, 72 °C for 90 s and a final 7-min elongation step at 72 °C. For the SoACLA-1 gene, PCR was similar to the PCR program of NPTII gene except the annealing temperature was changed to 58 °C for 30 s. The PCR products were identified by gel electrophoresis on 1.0 % agarose gel and observed under UV transmitted illumination.
Drought Tolerance Assay of the WT and Transgenic Tobacco Plants
For drought tolerance assay, the WT and transgenic tobacco plants were acclimated under a 16-h light/8-h dark cycle at 25 °C for 1 week before moving to pots in greenhouse with a mixture of soil and sand (3:1). The seedlings were regularly watered for 4 weeks before withholding water for 20 days. To examine the root morphology, the seedlings were placed vertically so that the root tips pointed downwards and then the root size of the SoACLA-1-transgenic tobacco plant was measured. On the other hand, whole leaves were collected from the drought treatment to determine the expression of SoACLA-1 in the transgenic plants. Simultaneously, the samples were used for physiological–biochemical analyses to measure root length, relative water content (RWC), ion leakage (IL), contents of malondialdehyde (MDA), H2O2, chlorophyll and activities of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD).
Analysis of Contents of RWC, MDA, IL and Activities of SOD, POD and CAT
To measure the RWC in tobacco, total leaves were collected from the WT and transgenic plants at 0, 5, 10, 15 and 20 days since withholding water. The fresh weight (FW) of tobacco leaves was recorded immediately after leaf was cut out. The turgid weight (TW) was recorded after soaking the leaves for 4 h in distilled water at room temperature under constant light. The leaves were recorded after dried for 24 h at 80 °C for dry weight (DW) measurement. RWC was calculated from the equation: RWC (%) = [(FW − DW)/(TW − DW)] × 100 [30] (Barrs and Weatherley 1962).
To measure IL content, the leaves after treatment were cut into strips and incubated in 10 mL distilled water at room temperature for 12 h. The initial conductivity (C1) was measured with a DDBJ-350 conductivity meter (Shanghai Precision and Scientific Instrument Co., Ltd., Shanghai, China) followed by boiling the samples for 30 min to measure the complete ion leakage (IL). The leaves were then cooled to room temperature to measure the electrolyte conductivity (C2). Ion leakage (IL) was calculated according to the equation: IL (%) = C1/C2 × 100 (Jiang and Zhang 2001).
To determine MDA content, the thiobarbituric acid (TBA)-based colorimetric method was utilized as described by Heath and Packer (1968).
The activities of SOD, POD and CAT were measured by using SOD, POD and CAT Detection Kit (Jiancheng, Nanjing, China) according to the manufacturer’s instruction. The three antioxidant enzymes were extracted from about 0.5 g of samples which were ground using a mortar and pestle in ice bath and homogenized in 6 mL extraction buffer containing 0.05 M phosphate buffer (pH 7.8) and 1 % polyvinylpyrrolidone. The homogenate was centrifuged at 10,000×g for 10 min at 4 °C, and the resulted supernatant was used for enzyme assays (Polle et al. 1994). The activities of SOD, POD and CAT were detected with a spectrophotometer.
Measurement of Chlorophyll and H2O2
Chlorophyll content was measured as described by Krause and Weis (1991). H2O2 content was detected as described by Jiang and Zhang (2001).
Statistical Analysis
The data were analyzed using the IBM SPSS Statistics 19 software program and Excel Micro software 2007. The statistical differences were compared based on Duncan’s test with setting the critical P values at 0.05 and 0.01, respectively, and each experiment had three replicates.
Results
Phylogenetic Tree Analysis of SoACLA-1 in Sugarcane Plants
The phylogenetic tree was constructed using the full-length amino acid sequences of ACL family members via MEGA 6.0 software. To analyze the evolutionary interrelationship of SoACLA-1 in different plants from ACL family, it was described in the neighbor-joining tree developed based on an alignment of the complete protein sequences of plants. The results showed that SoACLA-1 was clustered closely with Zea mays, Oryza sativa and Sorghum bicolor, suggesting that SoACLA-1 belongs to ACLA family (Fig. 1). Alignment of the SoACLA-1 amino acid sequences revealed that SoACLA-1 protein is highly homologous with the monocot plants of ACL family Zea mays (ZmACLA-NP 001141736.1) and Oryza sativa (OsACLA-3-NP001067052.1) and dicot species such as Arabidopsis thaliana (AtACLA-1-AEE28627.1).
Phylogenetic tree of SoACLA-1 and ACL family proteins. The phylogenetic tree was constructed using the neighbor-joining method with MEGA6.0 software. The numeric values in each node display the percentage of homology in ACLs family members. The accession number of protein is listed in the bracket. Abbreviations are used as follows: At Arabidopsis thaliana, Bd Brachypodium distachyon, Cs Camellia sinensis, Hs Homo sapiens, Os Oryza sativa, Rc Ricinus communis, Sb Sorghum bicolor, Sm Selaginella moellendorffii, So Saccharum officinarum, Vv Vitis vinifera, Zm Zea mays
Expression Patterns of SoACLA-1 Gene in Sugarcane
Sugarcane variety GT21 was used to investigate SoACLA-1 gene expression in root, stalk and leaf under normal conditions. As shown in Fig. 2, the expression of SoACLA-1 was the highest in root and the lowest in leaf. The relative expression of SoACLA-1 in the root is 1.8-fold of that in the stalk and 2.77-fold of that in the leaf, respectively, which suggested that the expression of SoACLA-1 may play an important role in sugarcane root development.
Expressions of SoACLA-1 Under Various Abiotic Stresses
Sugarcane leaves were used to investigate the expression of SoCLA-1 under various treatments. Under drought stress, the expression of SoACLA-1 increased gradually, reached its maximum at 24 h, which was 5.72-fold of that at 0 h, and then declined gradually (Fig. 3a). Under 15 % PEG stress, the expression level of SoACLA-1 was sensitive at early stage, reached the peak at 12 h, which was 36.84-fold of that at 0 h, and then declined sharply to a lower level at 72 h (Fig. 3b). For ABA treatment, the expression level of SoACLA-1 increased gradually at 6 and 12 h, but showed certain decrease at 24 h before reached the highest level at 48 h which was 12.91-fold of that at 0 h (Fig. 3c). These results suggested that SoACLA-1 expression possibly plays a role under abiotic stresses and might involve in the stress responses.
Molecular Analysis of Putative Transgenic Tobacco Plants
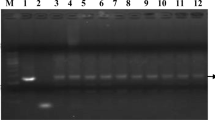
The transgenic plants were first grown on selection plate containing of kanamycin (100 mg L−1) and timentin (200 mg L−1). A total of 20 tobacco plants survived after screening, and they were selected as the putative transgenic plants. Twelve plants were tested for detecting both NPTII and SoACLA-1 genes using PCR with the transgenic-specific primers (Table S1) (Fig. 4a, b). The result of PCR analysis demonstrated that the co-transformation frequency of NPTII and SoACLA-1 genes was approximately 60 %.
Putative transgenic tobacco plants detected by PCR. a PCR for identification of NPTII gene. Lanes from left to right: M, DL 2000 marker; 1, positive control of plasmid; 2, non-transgenic of tobacco; 3–12, putative transgenic tobacco. b PCR for identification GFP gene. Lanes from left to right: M, DL 2000 marker; 1, non-transgenic of tobacco; 2, positive control of plasmid; 3–12, putative transgenic tobacco
Overexpression of SoACLA-1 Enhanced Seedling Growth in Tobacco Transgenic Plant
To evaluate the effect of SoACLA-1 on drought tolerance of the transgenic tobacco plants, the phenotypes of the SoACLA-1 transgenic tobacco plants were characterized (Fig. 5). As shown in Fig. 5a, the sense transgenic plants were slightly bigger than the WT and antisense transgenic plants. The roots of sense transgenic plants were significantly longer than those of the two controls (P < 0.01 with the WT and P > 0.05 with the antisense transgenic plants) (Fig. 5b). The simultaneous expression of SoACLA-1 was verified by RT-PCR analysis (Fig. 5c). These results suggest that SoACLA-1 gene may involve in regulation of shoot and root growth.
Overexpression of SoACLA-1 enhanced root growth of the SoACLA-1 sense transgenic (ST) plants compared with the WT and antisense transgenic (AT) plants in tobacco. a Morphology of tobacco plants; b root length in tobacco plants; c expressions of SoACLA-1 in the transgenic plants detected with RT-PCR analysis. Data represent means ± SD of three replicates. Symbols * and ** indicate significant difference between lines at 0.05 and 0.01 levels, respectively
Overexpression of SoACLA-1 Improves Drought Tolerance in Transgenic Tobacco
To assess the effect of SoACLA-1 expression on the drought tolerance of the transgenic lines, the morphology of the WT, ST and AT plants was compared before and after 20 days of withholding water (Fig. 6a, b). PCR analysis found that totally 12 plants were with SoACLA-1 and NPTII genes, which confirmed the presence two genes in the transgenic planes (Fig. 6c). As shown in Fig. 6a, b, all the leaves in the WT and antisense transgenic plants were severely curled and wilted, while almost all the leaves of the sense transgenic plants were still green and completely expanded. After 20 days of drought stress, the survival rate of the WT, sense transgenic and antisense transgenic lines was 36.67, 56.67 and 33.37 %, respectively (Fig. 5c). These results indicated that the over expression of SoACLA-1 might have improved the drought tolerance of the transgenic plants.
SoACLA-1 sense transgenic (ST), antisense transgenic (AT) and WT plants grown under normal (a) and drought stress (b) conditions. The survival rate (c) was determined after the seedlings were withheld from water for 20 days and then re-watered for 3 days. Data represent means ± SD of three replicates. Symbol * indicates significant difference between the WT and the sense, antisense transgenic lines (*P < 0.05; **P < 0.01)
Overexpression SoACLA-1 Increases RWC While Decreasing IL and MDA Under Drought Stress
RWC is regarded as an important index to evaluate drought resistance of plants under drought stress. In this study, RWC was higher in the ST tobacco than in the WT and AT tobacco, respectively, in all the measurements (Fig. 7a). After 20 days of drought treatment, the water loss was less in the ST plants (65.99 %) than in the WT (38.11 %) and AT plants (34.64 %). The results demonstrated that the stress resistance of SoACLA-1 ST tobacco had been improved.
RWC (a), IL (b) and MDA (c) in the SoACLA-1 transgenic and WT tobacco plants under drought stress. Six-leaf tobacco seedlings were used for deficit water treatment for 20 days. After treatment, the leaves were collected to assay RWC, IL and MDA. All the data are means ± SD calculated from three replicates. Symbol * indicates significant difference between the WT and the sense, antisense transgenic lines (*P < 0.05; **P < 0.01). Three biological experiments were performed, which produced similar results
IL and MDA contents were important indicators of membrane injury under stress. In this study, the IL content in the ST plants (28.15 %) was significantly lower than that in the WT (50.16 %) and AT plants (48.28 %) after they had been subjected to 20 days of drought stress (Fig. 7b). The MDA content in the ST plants (5.35 μmol g−1 FW) also was significantly lower in comparison with AT (9.01 μmol g−1 FW) and WT (9.18 μmol g−1 FW) (Fig. 7c).
These results indicated that the ST tobacco line had stronger drought tolerance than the WT and AT plants after 20 days of drought treatment.
Overexpression of SoACLA-1 Improved SOD, POD and CAT Activities Under Drought Stress
Activities of SOD, POD and CAT play an important role in ROS scavenging and cellular ROS level regulation. In this study, the activities of SOD and CAT were higher in the ST plants than in the WT and AT plants although the difference was not significant at 0 day (Fig. 8a, c), while POD activity was significantly higher all the time in the ST plants than in the WT and AT plants (Fig. 8b). These results suggested that the overexpression of SoACLA-1 plays a critical role in decreasing ROS accumulation by increasing the activities of the three key enzymes.
SOD (a), POD (b) and CAT (c) activities in the SoACLA-1 transgenic and WT tobacco plants under drought stress. Six-leaf tobacco seedlings were used for deficit water treatment for 20 days. After treatment, the leaves were collected to assay SOD, POD and CAT. All the data are means ± SD calculated from three replicates. Symbol * indicates significant difference between the WT and the sense, antisense transgenic lines (*P < 0.05; **P < 0.01). Three biological experiments were performed, which produced similar results
Chlorophyll and H2O2 Contents in SoACLA-1 Transgenic and WT Plants Under Drought Stress
H2O2 accumulation is one of the main reaction oxygen species, so it is an important indicator of ROS level in plants under drought stress. In this study, after drought stress treatment, the H2O2 content increased in both the transgenic and WT plants. However, the WT and AT plants had higher H2O2 accumulation than the ST plants under drought stress from 5 to 20 days (Fig. 9a).
H2O2 accumulation and total chlorophyll content SPAD in SoACLA-1 transgenic and WT tobacco plants under drought stress. Six-leaf tobacco plants were deprived of water for 20 days. Tobacco leaves were collected to determine H2O2 content. Chlorophyll content SPAD was measured using SPAD502 chlorophyll content analyzer, each of the different leaves was measured for three times, and the average was taken. Data are means ± SD calculated from three replicates. Symbols * and ** are tagged as the significant difference between the WT and the tobacco transgenic lines (*P < 0.05; **P < 0.01). Three biological experiments were performed, which produced similar results
As shown in Fig. 9b, under normal condition (data at 0 day), the chlorophyll content in the transgenic tobacco plants was not significantly different with the WT tobacco plants. However, after drought stress, the chlorophyll content showed relatively stable in the ST plants while it decreased more rapidly in the WT and AT plants, so that in the ST plants it was significantly higher than in the WT and AT plants, and it showed the lowest in the AT plants. These results suggested that overexpression of SoACLA-1 reduced H2O2 and kept relatively stable chlorophyll content under drought stress.
Discussion
Although ACL is an important cytosolic enzyme in plants and was first discovered more than 40 years ago (Nelson and Rinne 1975), the molecular characterization has been reported in Arabidopsis thaliana, lupin, sugarcane and citrus in the recent decade (Fatland et al. 2002; Langlade et al. 2002; Li et al. 2012; Hu et al. 2013). In this study, SoACLA-1 gene is a small gene of ACLA subunit cloned from sugarcane. Sequence analysis showed that SoACLA-1 has a high similarity of amino acid sequence with ACLs from other species, such as Zea mays, Sorghum bicolor or Oryza sativa. SoACLA-1 also displayed an evolutionary conservation and close relationship with ACLA subfamily that plays important roles in growth, development and protection of plants. These results allow us to deduce that SoACLA-1 belongs to ACLA subunit family.
Plants respond to abiotic stresses such as drought, low temperature and high salt through a number of physiological and developmental changes. Many plant genes have been suggested to suffer under drought stresses condition (Shinozaki and Yamaguchi-Shinozaki 1997). They can protect the stability of cell protein structure by osmotic adjustment to prevent the enzyme denatured inactivation and adverse stress damage on plants (Sinha and Hofmann 2002; Oliver et al. 2007). Some previous studies have reported that ACL may be involved in response to abiotic stress and ABA treatment (Li et al. 2012; Hu et al. 2013). However, there is no report about the relationship between overexpression of ACL gene and drought tolerance in tobacco and sugarcane. In the present study, expression of SoACLA-1 was detected in sugarcane root, stalk and leaf and found that the gene expression had significant tissue specificity. The SoACLA-1 gene expressed differentially in parts of sugarcane, and the expression was higher in root and stalk than leaf. The expression was upregulated by the stresses of PEG, drought and ABA. Further investigation demonstrated that SoACLA-1 transcripts were induced by PEG and drought treatments. Endogenous and exogenous ABA might be involved in the expression of SoACLA-1 gene in plants.
Transgenic plants have been successfully produced with microprojectile transformation in sugarcane (Bower and Birch 1992), Sorghum (Liu and Godwin 2012), Arabidopsis thaliana (Zhang et al. 2010) and tobacco (Hu et al. 2013) with NPTII as the marker gene. Agrobacterium-mediated transformation of sorghum was proven efficient with NPTII marker gene (Gurel et al. 2009). In this study, the result showed that the SoACLA-1 transgenic plants were successfully obtained through PCR and RT-PCR to detect the SoACLA-1 gene and selective marker (NPTII) gene.
To assess the effect of overexpression of SoACLA-1 in the transgenic tobacco plants, phenotypic traits were carefully monitored in this study. The results demonstrated that SoACLA-1 overexpression improved the root growth in the transgenic plants, which could benefit the uptake of water and nutrients under abiotic stress conditions. Numerous studies indicated that drought stress induced a rapid ROS accumulation and damaged the cell membrane (Polle 2001; Mittler et al. 2004). However, the correlation between SoACLA-1 and ROS under drought stress is still not known. According to previous studies, exogenous ABA and abiotic stress induced the activity of antioxidant defense system enzymes in plants and strengthened ROS scavenging (Li et al. 2010). Therefore, plant antioxidant system can be improved, and simultaneously, IL, MDA and H2O2 contents in plants decreased to protect the plants from oxidative damage under abiotic stress condition. Although numerous evidence showed that the overexpression of some transgenic genes can decrease IL, H2O2 and MDA contents in some plant species, such as rice (Li et al. 2008) and wheat (Zhang et al. 2011), but overexpression of SoACLA-1 gene has not been reported yet. IL and MDA are indicators reflecting the level of membrane injury under stress (Moore and Roberts 1998). In the present study, we found that MDA, IL and H2O2 contents were the highest in the AT plants than in the WT plants and the lowest in the ST plants of tobacco. These results suggested that the AT and WT tobacco plants had higher membrane damage than the ST plants under drought stress. When IL, MDA and H2O2 are used to evaluate ROS-mediated injuries in plant cell, the complex antioxidant system also includes three enzymes, SOD, POD and CAT, which play important roles to scavenge ROS and protect cell from damage of oxidative stress (Jaleel et al. 2009). The activities of these enzymes were described in some reports that SOD provides the first line of defense in plant cell against ROS and then the coordinated action of CAT and POD in scavenging ROS (Blokhina et al. 2003). In the present study, the activities of the three antioxidant enzymes were not different in the SoACLA-1 transgenic and WT plants under normal condition, and this can be explained that ROS production exists at low level and oxidative stress was not significant, which is absolutely consistent with previous research (Miller et al. 2010). The activities of the three enzymes were enhanced more in the sense transgenic plants than in the WT and antisense transgenic plants under drought stress, indicating that the overexpression of SoACLA-1 may be involved in adjusting the antioxidant enzymes under water stress, which displayed that the ST plants were less damaged than the AT and WT plants because of the improved antioxidant system and scavenging ROS more effectively.
Chlorophyll plays an important role to increase the scale of the photosynthetic reaction capability (Dhindsa and Matowe 1981). In this experiment, chlorophyll content showed higher in the ST than in the AT and WT tobacco plants under drought stress, implying that the ST plants could more efficiently enhance the photosynthetic capacity to regulate the osmotic pressure of the plant cells under drought stress.
In conclusion, a novel ACL gene in sugarcane, SoACLA-1, was cloned and characterized in this study. The overexpression of SoACLA-1 gene improved the drought tolerance of the transgenic tobacco plants by enhancing the antioxidant system to defend against ROS accumulation in the cells. Transformation of SoACLA-1 target gene might be utilized for improvement of sugarcane drought stress.
References
Barrs, H.D., and P.E. Weatherley. 1962. A reexamination of the relative turgidity technique for estimating water deficit in leaves. Australian Journal of Biological Sciences 15: 413–428.
Blokhina, O., E. Virolainen, and K.V. Fagerstedt. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Annals of Botany 91: 179–194.
Bower, R., and R.G. Birch. 1992. Transgenic sugarcane plants via microprojectile bombardment. Plant Journal 2(3): 409–416.
Chypre, M., N. Zaidi, and K. Smans. 2012. ATP-citrate lyase: A mini-review. Biochemical and Biophysical Research Communications 422: 1–4.
Clouse, S.D. 2002. Arabidopsis mutants reveal multiple roles for sterols in plant development. The Plant Cell 14: 1995–2000.
Dhindsa, R., and W. Matowe. 1981. Drought tolerance in two mosses: Correlated with enzymatic defense against lipid peroxidation. Journal of Experimental Botany 32(1): 79–91.
Elshourbagy, N.A., J.C. Near, P.J. Kmetz, T.N. Wells, P.H. Groot, B.A. Saxty, S.A. Hughes, M. Franklin, and I.S. Gloger. 1992. Cloning and expression of a human ATP-citrate lyase cDNA. European Journal of Biochemistry 204: 491–499.
Farooq, M., A. Wahid, N. Kobayashi, D. Fujita, and S.M.A. Basra. 2009. Plant drought stress: Effects, mechanisms and management. Agronomy for Sustainable Development 29: 185–212.
Fatland, B.L., J. Ke, M.D. Anderson, W.I. Mentzen, L.W. Cui, C.C. Allred, J.L. Johnston, B.J. Nikolau, and E.S. Wurtele. 2002. Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis. Plant Physiology 130: 740–756.
Fatland, B.L., B.J. Nikolau, and E.S. Wurtele. 2005. Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-citrate lyase in Arabidopsis. The Plant Cell 17: 182–203.
Gurel, S., E. Gurel, R. Kaur, J. Wong, L. Meng, H.Q. Tan, and P.G. Lemaux. 2009. Efficient, reproducible Agrobacterium-mediated transformation of sorghum using heat treatment of immature embryos. Plant Cell Reports 28(3): 429–444.
Heath, R.L., and L. Packer. 1968. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives in Biochemistry and Biophysics 125: 189–198.
Horsch, R.B., J.E. Fry, N.L. Hoffmann, D. Eichholtz, S.C. Rogers, and R.T. Fraley. 1985. A simple and general method for transferring genes into plants. Science 227: 1229–1231.
Hu, H.H., M.Q. Dai, J.L. Yao, B.Z. Xiao, X.H. Li, Q.F. Zhang, and L.Z. Xiong. 2006. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proceedings of the National Academy of Sciences of the United States of America 103: 12987–12992.
Hu, W., C. Huang, X. Deng, S. Zhou, L. Chen, Y. Li, C. Wang, Z. Ma, Q. Yuan, Y. Wang, R. Cai, X. Liang, G. Yang, and G. He. 2013. TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant, Cell and Environment 36(8): 1449–1464.
Hu, X.M., C.Y. Shi, X. Liu, L.F. Jin, Y.Z. Liu, and S.A. Peng. 2015. Genome-wide identification of citrus ATP-citrate lyase genes and their transcript analysis in fruits reveals their possible role in citrate utilization. Molecular Genetics and Genomics 290: 29–38.
Jaleel, C.A., K. Riadh, R. Gopi, P. Manivannan, J. Inès, H.J. Al-Juburi, C.X. Zhao, H.B. Shao, and R. Panneerselvam. 2009. Antioxidant defense response: Physiological plasticity in higher plants under abiotic constraints. Acta Physiologiae Plantarum 31: 427–436.
Jiang, M.Y., and J.H. Zhang. 2001. Effect of abscisic acid on active oxygen species, antioxidative defense system and oxidative damage in leaves of maize seedlings. Plant and Cell Physiology 42: 1265–1273.
Koziol, C. 2002. Overexpression of ATP citrate lyase in Arabidopsis thaliana. Under the direction of the Wurtele Lab, Iowa State University—Department of Botany, Summer 2002 NSF REU Report in Molecular Biotechnology.
Krause, G., and E. Weis. 1991. Chlorophyll fluorescence and photosynthesis: The basics. Annual Review of Plant Physiology and Plant Molecular Biology 42(1): 313–349.
Langlade, N.B., G. Messerli, L. Weisskopf, S. Plaza, N. Tomasi, J. Smutny, G. Neumann, E. Martinoia, and A. Massonneau. 2002. ATP citrate lyase: Cloning, heterologous expression and possible implication in root organic acid metabolism and excretion. Plant, Cell and Environment 25: 1561–1569.
Li, C.N., N. Qian, Q.L. Tan, K.S. Manoj, L.T. Yang, and Y.R. Li. 2012. Cloning and expression analysis of ATP-citrate lyase genes from sugarcane. Acta Agronamica Sinica 38: 2024–2033.
Li, C.N., M.K. Srivastava, Q. Nong, and Y.R. Li. 2010. Mechanism of tolerance to drought in sugarcane plant enhanced by foliage dressing of abscisic acid under water stress. Acta Agronamica Sinica 36(5): 863–870.
Li, G.W., M.H. Zhang, W.M. Cai, W.N. Sun, and W.A. Su. 2008. Characterization of OsPIP2;7, a water channel protein in rice. Plant and Cell Physiology 49: 1851–1858.
Liu, G., and I.D. Godwin. 2012. Highly efficient sorghum transformation. Plant Cell Reports 31: 999–1007.
Liu, J.Y., Y.R. Li, and L.T. Yang. 2014. Advances in ATP-citrate lyase research. Journal of Southern Agriculture 45(2): 204–208.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25: 402–408.
Miller, G., N. Suzuki, S. Ciftci-Yilmaz, and R. Mittler. 2010. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant, Cell and Environment 33: 453–457.
Mittler, R., S. Vanderauwera, M. Gollery, and F. Van Breusegem. 2004. Reactive oxygen gene network of plants. Trends in Plant Science 9: 490–498.
Moore, K., and L.J. Roberts. 1998. Measurement of lipid peroxidation. Free Radical Research 28: 659–671.
Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15(3): 473–497.
Nelson, D.R., and R.W. Rinne. 1975. Citrate cleavage enzymes from developing soybean cotyledons incorporation of citrate carbon into fatty acids. Plant Physiology 55: 69–72.
Nikolau, B.J., J.B. Ohlrogge, and E.S. Wurtele. 2003. Plant biotincontaining carboxylases. Archives of Biochemistry and Biophysics 414: 211–222.
Niu, J.Q., A.Q. Wang, J.L. Huang, H. Zhu, Y.R. Li, and L.T. Yang. 2013. Cloning and expression analysis of a soluble acid invertase Gene (SoSAI1) of Sugarcane. Scientia Agricultura Sinica 46(24): 5248–5260.
Oliver, S.N., E.S. Dennis, and R. Dolferus. 2007. ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant and Cell Physiology 48: 1319–1330.
Pang, X.B., X.G. Mao, R.L. Jing, J.F. Shi, T. Gao, X.P. Chang, and Y.F. Li. 2007. Analysis of gene expression profile responses to water stress in wheat (Triticum aestivum L.) seedlings. Acta Agronomica Sinica 33: 333–336.
Polle, A. 2001. Dissection the superoxide dismutase–ascorbate–glutathione pathway by metabolic modeling: Computer analysis as a step towards flux analysis. Plant Physiology 126: 445–462.
Polle, A., T. Otter, and F. Seifert. 1994. Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abies L.). Plant Physiology 106: 53–60.
Rangasamy, D., and C. Ratledge. 2000. Compartmentation of ATP: Citrate lyase in plants. Plant Physiology 122: 1225–1230.
Rogers, S., and A. Bendich. 1985. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Molecular Biology 5(2): 69–76.
Seki, M., M. Narusaka, H. Abe, M. Kasuga, K. Yamaguchi-Shinozaki, P. Carninci, Y. Hayashizaki, and K. Shinozaki. 2001. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. The Plant Cell 13: 61–72.
Seki, M., M. Narusaka, J. Ishida, T. Nanjo, M. Fujita, Y. Oono, A. Kamiya, M. Nakajima, A. Enju, T. Sakurai, M. Satou, K. Akiyama, T. Taji, K. Yamaguchi-Shinozaki, P. Carninci, J. Kawai, Y. Hayashizaki, and K. Shinozaki. 2002. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold, and high-salinity stresses using a full-length cDNA microarray. Plant Journal 31: 279–292.
Shalit, M., I. Guterman, H. Volpin, E. Bar, T. Tamari, N. Menda, Z. Adam, D. Zamir, A. Vainstein, D. Weiss, E. Pichersky, and E. Lewinsohn. 2003. Volatile ester formation in roses: Identification of an acetyl-coenzyme A geraniol/citronellol acetyltransferase in developing rose petals. Plant Physiology 131: 1868–1876.
Shinozaki, K., and K. Yamaguchi-Shinozaki. 1997. Gene expression and signal transduction in water-stress response. Plant Physiology 115: 327–334.
Shinozaki, K., K. Yamaguchi-Shinozaki, and M. Seki. 2003. Regulatory network of gene expression in the drought and cold stress responses. Current Opinion in Plant Biology 6: 410–417.
Sinha, A.K., and M.G. Hofmann. 2002. Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiology 128: 1480–1489.
Souter, M., J. Topping, M. Pullen, J. Friml, K. Palme, R. Hackett, D. Grierson, and K. Lindsey. 2002. Hydra mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. The Plant Cell 14: 1017–1031.
Suh, M.C. 2001. Pathogen-induced expression of plant ATP:citrate lyase. FEBS Letters 488: 211–212.
Tamura, K., G. Stecher, D. Peterson, A. Filipski, and S. Kumar. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30(12): 2725–2729.
Xing, S., N. van Deenen, P. Magliano, L. Frahm, E. Forestier, C. Nawrath, H. Schaller, C.S. Gronover, D. Prüfer, and Y. Poirier. 2014. ATP citrate lyase activity is post-translationally regulated by sink strength and impacts the wax, cutin and rubber biosynthetic pathways. Plant Journal 79: 270–284.
Zaidi, N., J.V. Swinnen, and K. Smans. 2012. ATP-citrate lyase: A key player in cancer metabolism. Cancer Research 72: 3709–3714.
Zhang, H., X. Mao, R. Jing, X. Chang, and H. Xie. 2011. Characterization of a common wheat (Triticum aestivum L.) TaSnRK2.7 gene involved in abiotic stress responses. Journal of Experimental Botany 62(3): 975–988.
Zhang, H., X. Mao, C. Wang, and R. Jing. 2010. Overexpression of a common wheat gene TaSnRK2.8 enhances tolerance to drought, salt and low temperature in Arabidopsis. PLoS One 5(12): e16041.
Acknowledgments
This work was supported by the grants from the National High Technology Research and Development Program (“863” Program) of China (2013AA102604), Natural Science Foundation of China (31360293), International Scientific Cooperation Program of China (2013DFA31600), Guangxi Special Funds for Bagui Scholars and Distinguished Experts (2013) and Guangxi Natural Science Foundation (2011GXNSFF018002, 2012GXNSFDA053011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material. ξ
Rights and permissions
About this article
Cite this article
Phan, TT., Li, J., Sun, B. et al. ATP-Citrate Lyase Gene (SoACLA-1), a Novel ACLA Gene in Sugarcane, and Its Overexpression Enhance Drought Tolerance of Transgenic Tobacco. Sugar Tech 19, 258–269 (2017). https://doi.org/10.1007/s12355-016-0464-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-016-0464-8