Abstract
Purpose
Phosphorus (P) is one of the plant nutrients most frequently deficient in soils. Under such condition, adoption of P-efficient crops is desirable to maintain agricultural production and avoid heavy reliance on fertilizer application. Previous studies reported significant genotypic difference in internal P use efficiency (PUE) in rice, but key physiological processes remain poorly understood. We aimed at revealing novel key factors that affect PUE.
Methods
Rice seedlings were cultivated with different nitrogen (N) sources, and PUE and root traits were characterized. In addition, genotypes that differ in P efficiency were grown under different P supply and growth, gene expression and nutrient uptake were analyzed.
Results
Addition of nitrate to P-deficient plants improved PUE compared with ammonium-only plants. Maximum root length of P-inefficient plants was significantly shorter in the presence of ammonium compared with nitrate-only plants under low P supply, while the difference was absent in P-efficient plants. Under low P supply, P-efficient genotypes had lower ratio of ammonium/nitrate accumulation in root and AMT1;1/NRT1.1B expression (encoding an ammonium and nitrate transporter, respectively) than P-inefficient plants, suggesting that PUE positively correlates with the ability to use nitrate. The ability to use nitrate also positively correlated with root efficiency in the field under low P supply, suggesting that nitrate use may positively modulate both internal P utilization and P uptake.
Conclusion
This study provides physiological evidence that N metabolism is linked with PUE and suggests that strengthening the ability to use nitrate may improve P use, hence contributing to the crop production in P-impoverished soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants require various essential nutrients for their growth, most of which are absorbed by roots from the rhizosphere. Due to the sessile nature of plants, optimal soil nutrient availability is an important key to vigorous growth and successful seed setting. However, the plant-available nutrient content of agricultural soils is often not in the optimal range, causing plants to suffer from nutrient deficiencies or toxicities (Marschner 1995; Rakotoson et al. 2022). In response to such nutrient imbalances plants can alter their nutrient uptake capacity, internal distribution and metabolism (Xu et al. 2012; Ueda et al. 2021). Among the essential plant nutrients, the demand for phosphorus (P) is relatively large, since P is indispensable as building blocks for nucleic acids, lipids and other metabolites including intermediate metabolites for carbon fixation (Heuer et al. 2017). Despite its abundance, most P in soil is present in forms of low plant availability due to adsorption to soil particles or formation of insoluble salts (Nishigaki et al. 2021). Lynch (2011) estimated that 50% of worldwide soils are potentially deficient in plant-accessible P, which severely limits the productivity of plants in these regions (Andrianary et al. 2021; Rakotoson et al. 2022). Notably, P deficits in agricultural soils occur mainly in developing countries with sub-Saharan Africa being particularly affected (MacDonald et al. 2011). Amendment of these soils with P fertilizers is indeed a measure to increase agricultural productivity, but small-scale farmers in these regions often cannot afford adequate fertilizers due to limited financial resources (Niang et al. 2017; Vandamme et al. 2018). In addition, high-grade rock phosphate reserves, from which P fertilizers are produced, are expected to be depleted in the future (Vinod and Heuer 2012), and their concentration in few geographical regions is a matter of concern at present (Cooper et al. 2011). Increasing crop productivity on P-deficient soils without relying heavily on application of P fertilizers is therefore an important objective globally.
Utilization of P is determined by two factors, namely, P acquisition efficiency (PAE) and internal P use efficiency (PUE) (Rose et al. 2011; López-Arredondo et al. 2014). Thus, one countermeasure to cope with the shortage of P is to develop crop varieties with improved PAE. PAE is affected by root size and root efficiency (RE), which is defined as the amount of P taken up per root surface area. Previous studies with rice reported large genotypic differences in both root size and RE (Mori et al. 2016; Wissuwa et al. 2020), suggesting the potential to improve PAE in modern cultivars exists. Recently, rhizosphere processes were also shown to play a potentially pivotal role in P acquisition of rice. In highly weathered acidic soils with pH-dependent charge, an increase in soil pH will solubilize P and therefore increase its availability for uptake (Barrow 2017). Such rise in rhizosphere pH is expected if rice takes up excess anion over cations, as would be the case if the uptake of nitrate (NO3−) exceeds that of ammonium (NH4+) (Kuppe et al. 2022). An alternative way to improve P utilization is to develop crop varieties with high PUE. Since PUE is defined as the inverse of tissue P concentration, plants with high PUE produce more biomass per unit of absorbed P (Rose et al. 2011). Contrary to PAE that has been the focus of many previous studies, the mechanisms for PUE have been explored to a lesser extent. Modern improved rice varieties tend to have low PUE, suggesting the potential for genetic improvement (Wissuwa et al. 2015). One difficulty with screening for PUE is that plants with higher P uptake tend to have lower PUE, since increasing P uptake decreases the P deficiency stress of a plant (Rose et al. 2016). This confounding effect likely hampers the development of varieties with high PUE, since most screening systems neglect the differences of total P uptake in different accessions, under which condition genotypic differences in PUE cannot be appropriately evaluated (Rose et al. 2016). Overcoming such a confounding effect by supplying the same low quantity of P to each plant, Wissuwa et al. (2015) observed wide variation in PUE among rice accessions. In a genome-wide association study it was further shown that PUE is controlled by many small-effect loci, suggesting that several genetic and physiological mechanisms are involved in PUE (Wissuwa et al. 2015).
The analysis of the metabolome in four rice accessions contrasting in PUE revealed that certain metabolites (e.g. threonine and benzoate) were enriched in leaves of P-efficient genotypes under low P supply, suggesting that these metabolites could serve as markers to select P-efficient plants (Watanabe et al. 2020). Further investigation of changes in main leaf P pools showed that P-efficient rice accessions have lower investment of P in the lipid-P pool (Hayes et al. 2021). It has also been shown that P-efficient rice genotypes preferentially allocate P to roots (Adem et al. 2020; Hayes et al. 2021), as well as having characteristic root transcriptome (Prodhan et al. 2022), suggesting that roots also play important roles in determining PUE.
Nitrogen (N) is another important essential element for plant growth with a strong impact on crop productivity (Erisman et al. 2008; Saito et al. 2019). Under normal physiological conditions, plants take up N either as the ammonium or nitrate ion from soils. Nitrate taken up is converted to ammonium enzymatically via nitrate reductase and nitrite reductase, after which ammonium is incorporated into glutamate to produce glutamine with the help of glutamine synthetase (Xu et al. 2012). Whether N is taken up mainly as nitrate or ammonium affects many physiological aspects of plants, such as root morphology, metabolite contents and gene expression patterns (Patterson et al. 2010; Meier et al. 2020; Tian et al. 2021). Nitrate also plays a role as a signaling molecule (Crawford 1995), bridging N and P signaling pathways (Kiba et al. 2018; Maeda et al. 2018; Hu et al. 2019; Medici et al. 2019; Ueda et al. 2020a) and enabling coordinated modulation of N and P uptake and adaptation to diverse nutrient conditions. In the presence of nitrate, rice SPX4 interacts with a nitrate transporter NRT1.1B and is subject to degradation via a proteasomal pathway. The degradation of SPX4 releases NIN-LIKE PROTEIN (NLP) 3 and PHOSPHATE STARVATION RESPONSE (PHR) 2 that were originally bound with SPX4, and free NLP3 and PHR2 activate the transcription of nitrate-related and P deficiency-inducible genes, respectively (Hu et al. 2019). A second link between nitrate and P deficiency signaling is via the nitrate-inducible NIGT1 family transcription factors that repress the expression of SPX family genes in Arabidopsis. Since SPX proteins negatively regulate P starvation response, their repression would actually enhance P starvation responses and P uptake (Ueda et al. 2020a). In rice and Arabidopsis, these molecular linkages affect PAE in the presence of nitrate by promoting P starvation responses and thereby the expression of P transporter genes that enhance P uptake (Hu et al. 2019; Medici et al. 2019; Ueda et al. 2020a). These recently discovered links between nitrate and the P starvation response may affect various processes related to P uptake, redistribution and utilization but since experiments were conducted in mutants or genetically engineered plants, it is unknown to what extent natural genotypic variation in preference for nitrate exists and could be exploited for crop improvement under P deficiency, and how such differences may affect PAE and PUE.
In this study, our objectives were to 1) examine to what extent the N form supplied would affect PUE, 2) test whether genotypic differences in preference for nitrate or ammonium exist, and 3) examine the potential relationship between N form taken up and P efficiency (i.e. PUE and PAE). To these aims, we used groups of rice genotypes with contrasting PUE and PAE, and characterized their gene expression pattern and physiological parameters in relation to N form supplied under P replete and deficient levels.
Materials and methods
Plant materials and growth conditions
Five rice genotypes used in the current study were previously shown to contrast in PUE, as well as RE in the field with IR64 and Taichung Native being P-inefficient, while DJ123, Mudgo, and Yodanya are P-efficient (Wissuwa et al. 2015; Mori et al. 2016; Adem et al. 2020; Watanabe et al. 2020; Hayes et al. 2021). Seeds were sterilized with 5% NaClO solution for 5 min, rinsed with tap water and germinated in a petri dish on moist tissue paper at 28 °C in darkness. Germinated seeds were transferred to a tray with a stainless steel mesh bottom in contact with 8 L of deionized water below, and grown for 3 d in darkness. Afterwards, seedlings were grown in natural light in a glasshouse with Yoshida nutrient solution (Yoshida et al. 1976); initially with very diluted solution (0.1 × Ca, 0.15 × Fe, 0.05 × K, 0.05 × Mg, and 0.05 × micronutrient (Mn, B, Cu, Zn and Mo)). The solution was refreshed every 2–3 d until transplanting. After further 9 d, 2 seedlings of the same genotype were transferred to non-transparent 1-L bottle containing 0.2 × P-free Yoshida nutrient solution. After 3 d, 800 µg and 400 µg of P (as NaH2PO4), corresponding to 400 and 200 µg of P per plant, was added to each bottle of the high P and low P treatments, respectively. Until 43–44 d after germination, the concentration of nutrient solution was increased gradually (Fig. S1). P was periodically added to each bottle to a total amount of 8,000 µg P (high P) or 800 µg P (low P). The full strength (1x) P-free Yoshida solution contained 1.42 mM NH4NO3, 0.5 mM K2SO4, 1 mM CaCl2,1 mM MgSO4, 36 µM Fe-EDTA, 9 µM MnCl2, 18.5 µM H3BO3, 0.16 µM CuSO4, 1.5 µM ZnSO4 and 0.07 µM (NH4)6Mo7O24. Ammonium-only plants received the same molarity of (NH4)2SO4 instead of NH4NO3. Nitrate-only plants received twice the molar ratio of KNO3 instead of NH4NO3. pH of the solution was adjusted to 5.5–6.0 twice a week with potassium silicate solution (for NH4NO3- and ammonium-only plants) or with hydrochloric acid (for nitrate-only plants).
Plants were harvested 46–48 d after germination. Shoot and root dry weight were measured after drying at 70 °C for > 3 d. For gene expression analysis, whole root samples were flash-frozen by liquid N and stored at -80 °C until the analysis.
N uptake analysis
Plants at 41 d after germination were used for the analysis. Prior to the N uptake experiment, plants were preincubated with fresh 1 × Yoshida solution containing 2 mM MES-KOH (pH 5.7) for 2 d to stabilize pH, since pH changes may affect the rate of ammonium and nitrate uptake (Fried et al. 1965). The preincubation solution contained 0 and 100 µM P for low and high P treatment, respectively. For the uptake analysis, roots were briefly washed by 0.1 mM CaSO4 and immediately soaked into 1 × Yoshida nutrient solution containing 2 mM MES-KOH (pH 5.7), where 20% of either of NO3− or NH4+ of NH4NO3 was replaced with 15NO3− or 15NH4+ (Shoko Science Co., Ltd). The P concentration of the solution was 0 and 100 µM for low and high P treatment, respectively. After 1 h, roots were washed with 0.1 mM CaSO4 and immediately separated from shoot. Samples were dried at 70 °C for > 3 d, pulverized into a fine powder, and subjected to isotope ratio mass spectrometer (Delta V Advantage, Thermo Fisher Scientific) connected with an elemental analyser (Flash2000, Thermo Fisher Scientific).
Analysis of mineral content
Dried shoot and root samples were cut into small pieces with scissors. Around 100 mg of dried samples were subjected to wet-acid digestion using 8 mL of HNO3 and HClO4 mixture (3:1 [v/v]) as previously described (Wang et al. 2017). P concentration in the digest was analyzed by the molybdenum-blue method using a standard curve (Murphy and Riley 1962). Total iron (Fe) was extracted from pulverized dried whole root samples with 1 M sodium dithionite solution with gentle shaking at 450 rpm for 2 min at room temperature by a bead beater (Hartmann and Asch 2018). The supernatant was obtained after centrifugation at 16,000 g for 5 min and an aliquot of 100 µL was mixed with the assay solution (120 mM ascorbic acid, 8.5 mM 2,2’-bipyridyl, 70 mM acetate buffer [pH 5.0]) (Waters and Troupe 2012). The mixture was incubated at 37 °C for 1 h and the absorbance at 520 nm was recorded. Standard curve was prepared using a dilution series of Fe standard solution (Fujifilm WAKO Pure Chemical). N concentrations were measured from pulverized dry samples during the mass spectrometry analysis.
Gene expression analysis
Frozen whole root samples were ground into a fine powder with chilled mortar and pestle. Total RNA was extracted using the ISOSPIN Plant RNA kit (Nippon Genetics) according to the manufacturer’s instructions including DNase treatment. cDNA was synthesized using the PrimeScript RT Master Mix (Perfect Real Time) (Takara Bio Inc.). Quantitative real-time PCR (qPCR) was carried out using the CFX96 Real-Time PCR Detection System (Bio-Rad) and TB Green Premix Ex Taq II (Tli RNaseH Plus) (Takara Bio Inc.). Quantification was based on the standard curve method as previously described using OsC3H38 as the internal standard (Adem et al. 2020). Since the presence of polymorphisms may affect the efficiency of qPCR, gene-specific primers (Table S1) were designed following two criteria: 1) no polymorphism in the annealing site, and 2) no more than 3 bp of indels in the amplified fragment. Polymorphisms were searched for on the RiceVarMap website (http://ricevarmap.ncpgr.cn/, last accessed in February 2022) (Zhao et al. 2015).
Data analysis
The ANOVA was conducted to compare genotype means while differences between group means (i.e. high- and low-PUE groups) were tested by the Wilcoxon’s rank sum test at α = 0.05.
Results
Effect of N source on biomass production, P concentration, PUE and root elongation
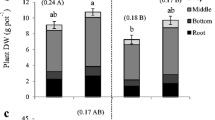
To reveal the effect of N sources on PUE, P-inefficient indica cultivar Taichung Native (Wissuwa et al. 2015) was grown in the presence of both nitrate and ammonium ions (1:1 ratio, as NH4NO3) or sole ammonium ((NH4)2SO4). N sources did not affect shoot biomass production (Fig. 1a,b), but the presence of nitrate increased root biomass significantly under the low P condition (Fig. 1c) and this resulted in lower root P concentrations (Fig. 1d,e). PUE based on total plant biomass was positively affected by the presence of nitrate and this was more pronounced in the low P treatment (Fig. 1f).
Effect of different N sources on biomass production and internal P use efficiency. a Representative Taichung Native plants grown with (NH4)2SO4 as sole N source (NH4+) or with NH4NO3, under high or low P supply. All the photos are in the same scale. The scale bar indicates 20 cm. b, c Dry weight of shoot (b) and root (c). d, e Concentration of total P in shoot (d) and root (e). f Internal P use efficiency. For b-f, data represent mean ± S.D. (n = 4). Significant differences between the two N conditions are indicated using asterisks (*, P < 0.05; **, P < 0.001; two-tailed Student’s t-test)
The N-form supplied furthermore affected root growth patterns, especially maximum root length. All genotypes had longest roots when supplied with nitrate only and the addition of ammonium had an inhibitory effect. However, it was only P-inefficient IR64 and Taichung Native which significantly reduced root length in the ammonium-nitrate treatment compared with nitrate-only treatment, which made the contrast between the groups more evident under this condition (Fig. 2a,b).
Examination of ammonium and nitrate-related phenotypes. a Roots of plants grown with (NH4)2SO4 (left panel), NH4NO3 (middle panel) or KNO3 (right panel) as N source under low P supply. Scale bar indicates 10 cm. b Maximum root length of plants grown under the same condition as a. Data represent mean ± S.D. (n = 4). Significant differences were determined among 3 conditions in each genotype, followed by Tukey’s HSD test, and are indicated by different lowercase letters
Gene expression patterns of P response and N-related genes
To further investigate potential link between N use and PUE, we carried out gene expression analyses in roots. P deficiency-inducible marker genes IPS1 and PT6 were strongly up-regulated by the low P condition (Fig. 3a) but more so in the P-inefficient group (IR64 and Taichung Native) compared to the P-efficient group (Mudgo, Yodanya, and DJ123). This indicated that higher PUE was not simply caused by a stronger general P deficiency response.
Expression of P and N signaling-related genes in roots. The expression of P-inducible marker genes (a) and genes involved in N-P signaling (b) are shown for 5 genotypes under high and low P supply. Data represent mean ± S.D. (n = 4). Significant differences among 5 genotypes in each P condition were determined using one-way ANOVA, followed by Tukey’s HSD test, and are indicated by different lowercase letters. Significant differences between the P-efficient and –inefficient group were determined by Wilcoxon’s rank sum test, and the resultant P values are shown in red letters if P < 0.05
Next, we tested the expression of genes mediating N-P interaction; the transcription factor NLP3, NIGT1 and a suppressor of P deficiency signaling SPX4. Gene expression analysis in roots revealed that, even though SPX4 showed slightly higher expression in P-efficient group, this pattern was absent in another set of genotypes, suggesting that these previously established molecular links are likely not the determinant for PUE (Fig. 3b; Fig. S2).
Ammonium is mainly taken up by three members of the AMT1 family (AMT1;1, AMT1;2 and AMT1;3) at the high-affinity range (Konishi and Ma 2021). Nitrate uptake is suggested to be mediated by NRT1.1A, NRT1.1B, NRT2.3, NPF2.4 and NRT1.5A under various physiological conditions (Wang et al. 2020a). Among the AMT1 family genes, the expression pattern of AMT1;1 and AMT1;2 was very similar across samples (R = 0.94), and both exhibited significantly lower expression in P-efficient genotypes compared to P-inefficient ones (P < 0.01) (Fig. 4a). The expression of N deficiency-inducible AMT1;3 (Ferreira et al. 2015; Konishi and Ma 2021) did not show a contrast between P-efficient and -inefficient genotypes (Fig. 4a). On the other hand, one of the examined nitrate transporters, NRT1.1B, showed higher expression in P-efficient genotypes even under high P supply, and this trend remained under low P supply (Fig. 4b). The other major nitrate transporter genes that contribute to nitrate uptake (NRT1.1A, NRT2.3, NPF2.4, and NRT1.5A) did not exhibit significant genotypic differences under low P supply (Fig. 4b).
Expression of N uptake-related genes in roots. The expression of genes involved in the uptake of ammonium (a) and nitrate (b) are shown for 5 genotypes under high and low P supply. Data represent mean ± S.D. (n = 4). Significant differences among 5 genotypes in each P condition were determined using one-way ANOVA, followed by Tukey’s HSD test, and are indicated by different lowercase letters. Significant differences between the P-efficient and –inefficient group were determined by Wilcoxon’s rank sum test, and the resultant P values are shown in red letters if P < 0.05
Since plants cannot directly utilize nitrate and ammonium ions, uptake of these ions should be followed by assimilation into amino acids (Fig. S3a). The expression of two nitrate assimilation-related genes NR1 (encoding a nitrate reductase) and NIR (encoding a nitrite reductase), which are involved in reduction of nitrate to ammonium, were significantly higher in the P-efficient group (P < 0.01), whereas ammonium assimilation-related genes, GS1;2 (encoding a cytosolic glutamine synthetase) and NADH-GOGAT (encoding a NADH-dependent glutamate synthase), had significantly lower expression in the P-efficient group (P < 0.01 and < 0.05, respectively) (Fig. S3b).
Uptake analysis of N sources
N uptake patterns were examined using 15 N-labeled ammonium or nitrate. Under low P supply, the accumulation of ammonium-derived N in roots was significantly lower in the P-efficient group (Fig. 5a), whereas nitrate-derived N accumulation in roots was significantly higher in the P-efficient genotypes (Fig. 5b). Similar trend was observed for the uptake of these ions on whole-plant basis, where P-efficient genotypes absorbed more nitrate than P-inefficient genotypes (Fig. S4). As a result, the ratio of ammonium/nitrate uptake in roots was consistently higher in P-inefficient genotypes (Fig. 5c). Accumulation of ammonium-derived N in root exhibited a positive correlation with the expression of AMT1;1 and this correlation was very strong (R = 0.97, P < 0.01) under low P supply (Fig. 5e). Accumulation of nitrate-derived N showed a positive correlation with the expression of NRT1.1B, irrespective of P supply (R = 0.96, P < 0.001) (Fig. 5f). Similar results were observed in an additional experiment using 8 genotypes (Fig. S5), suggesting that the expression of AMT1;1 is a good indicator for ammonium uptake in roots under low P supply, while expression of NRT1.1B predicts nitrate uptake in roots irrespective of the P conditions. Consistent with above data, the ratio of AMT1;1/NRT1.1B expression was also high in the P-efficient group, showing a positive correlation with the actual uptake ratio, especially under the low P supply (R = 0.61 for high and 0.86 for low P supply) (Fig. 5d).
N source uptake patterns in P-inefficient and –efficient genotypes. a,b Enrichment of absorbed 15N in root. The value for ammonium-derived N (a) and nitrate-derived N (b) are shown. c Ratio of ammonium/nitrate uptake in roots. d Ratio of AMT1;1/NRT1.1B expression in roots. e,f Correlation between the expression of AMT1;1 and ammonium-derived N (e) and between the expression of NRT1.1B and nitrate-derived N (f) in root. Asterisks indicate significant correlation (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Data represent mean ± S.D. (n = 4). In a-d, significant differences among 5 genotypes in each P condition were determined using one-way ANOVA, followed by Tukey’s HSD test, and are indicated by different lowercase letters. Significant differences between the P-efficient and –inefficient group were determined by Wilcoxon’s rank sum test, and the resultant P values are shown in red letters if P < 0.05
Correlation of N uptake pattern with root efficiency
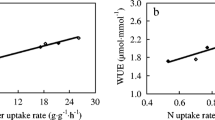
In addition to the effect of N form provided on PUE, we also investigated a possible association with PAE. The 5 genotypes used in the current study had previously been grown in a P-deficient field and their RE had been estimated (Mori et al. 2016). Accumulation of nitrate-derived N measured here in low P supply was closely correlated (R = 0.91, P < 0.05) with the field RE estimate (Fig. 6) but no association was detected based on nitrate uptake in high P supply.
Correlation of nitrate uptake and root efficiency. The data for root efficiency (Mori et al. 2016) was plotted against the values for nitrate uptake (Data from Fig. 5b). Plot was made separately for high (left panel) and low P (right panel) conditions. Correlation coefficients and resultant P values are indicated
Discussion
The two most important plant nutrients are N and P and recent studies have indicated cross-talk between N and P nutrition exists that affects how plants fine-tune their response to the relative scarcity of either nutrient (Kiba et al. 2018; Maeda et al. 2018; Hu et al. 2019; Medici et al. 2019; Ueda et al. 2020a). Previously suggested physiological factors for P efficiency involve P translocation (recycling) and substitution of phospholipid by sulfolipids or galactolipids (Adem et al. 2020; Hayes et al. 2021; Prodhan et al. 2022), which are regulated as part of the P deficiency response pathway (Liu et al. 2010). The magnitude of the P deficiency response is affected by the availability of nitrate (Medici et al. 2019; Ueda et al. 2020a), suggesting a potential link between N use and P deficiency. A second line of evidence for the link between P efficiency and N metabolism in rice was provided by the study of Watanabe et al. (2020) that detected signature N-related metabolites in leaves of P efficient but not inefficient rice genotypes. Here our objective was to determine whether the N form supplied affected the efficiency of P use and related parameters in rice genotypes with contrasting P efficiency.
Genotypic differences in preference for N form
Clear genotypic differences were observed for the the capacity to use nitrate by roots, with two P inefficient varieties (IR64 and Taichung native) accumulating almost 3 times as much ammonium than nitrate, while the ratio was 1.5–2 for P efficient ones. We assume that this differential N use pattern is not confounded by genotypic differences in the speed of nutrient depletion: in our experiment, total N uptake per plant was typically < 8 µmol/ h and < 0.8 µmol/h under high and low P supply, respectively. These values correspond to < 0.6 mmol and < 0.06 mmol total N uptake over 3 d, which is far less than the total amount of N contained in the hydroponic solution (2.8 mmol total N in 1 L of solution). The differences in use of N form were matched by the relative gene expression ratio of ammonium versus nitrate transporters in both groups (Fig. 5d). Under low P supply the higher ammonium to nitrate uptake ratio in roots of the P inefficient group was not caused by differences in ammonia uptake between groups but by significantly higher nitrate uptake in P efficient genotypes (Fig. 5a,b). This higher nitrate uptake was associated with higher expression of the nitrate transporter NRT1.1B in the P efficient group, whereas the inefficient group showed higher expression of ammonium transporters. Previous studies have suggested the presence of within- and inter-species differences in ammonium and nitrate preference (Falkengren-Grerup 1995; Ueda et al. 2020b). It was reported that plants adapted to acidic soils tend to prefer ammonium, suggesting that the preference may reflect the growth environment of the variety (Falkengren-Grerup 1995). Besides, the availability of N form under different growth environment may also affect the preference; under lowland conditions, where the redox potential of soil is low, ammonium is more prevalent, while nitrate is more prevalent under upland conditions (Wang et al. 1993; Crawford and Glass 1998). Upland-adapted plants may have acquired the ability to use nitrate more than lowland-adapted plants, since rapid uptake of nitrate before leaching may benefit plants. In addition, genetic engineering of N signaling pathway and N transporters leads to altered levels of ammonium and nitrate uptake, substantially shifting the preference of N forms (Wang et al. 2020b; Zhang et al. 2022). Considering more frequent occurrence of P deficiency (Ponnamperuma 1972) and more availability of nitrate under upland conditions, our current finding could be more relevant with improvement of plants under P-deficient upland conditions. It is currently unknown if the higher preference for nitrate in P-efficient plants reflects adaptation of these genotypes to specific growth environment or is caused by characteristic N metabolism patterns.
The positive effect of nitrate on PUE and potential underlying mechanisms
The positive effect of nitrate on PUE was predominantly detected in roots and two potentially underlying processes shall be explored: 1) different energy costs for the uptake and assimilation of N forms, and 2) effects of N forms on root elongation. The energy requirements for the uptake and assimilation of ammonium and nitrate differ in amount and in which parts of plants the energy is consumed. The assimilation of nitrate to ammonium requires ~ 10 ATP molecules, while the assimilation of ammonium to glutamine only consumes ~ 2 ATP molecules (Rubio-Asensio and Bloom 2017). However, it should be noted that while nitrate assimilation occurs predominantly in shoot, especially in leaf blades (Rao et al. 1979), most of the absorbed ammonium is assimilated into amino acids in roots (Taylor and Bloom 1998; Hachiya and Sakakibara 2017). In addition, ammonium uptake should be accompanied by active proton extrusion and by plasmamembrane-localized H+-ATPases to maintain proton gradient across the membrane (Zeng et al. 2012; Zhang et al. 2021). Since one molecule of ATP is used to extrude 0.8–1 molecule of H+ (Briskin and Hanson 1992), the additional cost associated with the uptake process of ammonium by roots will at least be one extra ATP.
Thus, ammonium is energetically cheaper than nitrate but costs associated with ammonium are incurred in roots whereas those for nitrate are incurred in shoots. To what extent energy costs of N assimilation are affecting plant productivity under P deficiency is not known. Accumulation of carbohydrates in leaves of P deficient plants is a well-documented observation (Cai et al. 2012; Meng et al. 2020) that may indicate energy for nitrate assimilation in leaves may not be a limitation. However, it has been shown that starch also accumulated in roots of rice under P deficiency (Wissuwa et al. 2005) and that poses the question to what extent energy is limiting under P deficiency and whether higher energy costs associated with nitrate assimilation are inconsequential under such conditions. Further studies are needed to clarify these points. The issue of costs could be seen from a different angle. When P limits plant growth directly, costs in terms of P invested in a process may be of greater relevance and in that regard the high enzyme activity required to assimilate ammonium in roots may be a disadvantage as higher enzyme activity may require higher tissue P concentrations to maintain ribosome activity. In contrast, nitrate can be stored in vacuoles (Martinoia et al. 1981; De Angeli et al. 2006) and therefore its assimilation only incurs a cost when N is needed for further plant growth. This would be consistent with the observation that P-efficient genotypes produce “cheaper” roots, with lower content of P, Fe and N (Fig. S6) (Hayes et al. 2021).
Alternatively, preference for ammonium over nitrate may directly affect root growth and this may lead to reduced PUE in ammonium-fed rice. Previous investigations in hydroponically- and soil-grown rice plants showed that maximum root length was reduced with increasing ammonium supply (Kawata et al. 1977; Hirano et al. 2008). Inhibition of root elongation by ammonium was not caused by the ammonium ion directly, but rather by the accumulation of downstream assimilatory products (Hirano et al. 2008). The negative effect of ammonium on maximum root length was confirmed here and this effect was more pronounced in the P-inefficient group. In particular, this group was more sensitive with dual NH4NO3 supply, which already reduced maximum root length by 30—38% compared to 10—15% in the P-efficient group (Fig. 2b). Their higher AMT1;1 and AMT1;2 as well as GS1;2 and NADH-GOGAT gene expression levels furthermore confirmed a highly active ammonium uptake and assimilation machinery in roots, and this relative preference for ammonium likely caused the reduced root elongation in P-inefficient genotypes.
If differences in root elongation are caused by differences in individual cell elongation, as in the case of Arabidopsis (Li et al. 2010), the resulting root is expected to have required less P per unit root length and this would explain the higher PUE observed in roots of the P-efficient group (Fig. 1f). A carry over effect of higher root PUE is that with the same amount of limiting P invested the plant can produce a larger total root system and the exploration of larger soil volumes with ensuing uptake of additional P has been identified as one main driving factor to overcome P-limitations for growth (Wissuwa et al. 2020; Gonzalez et al. 2021).
Preferential nitrate uptake and P solubilization
In highly weathered acidic soils with pH-dependent charge, an increase in soil pH will increase the P concentration in solution and hence its plant availability (Barrow 2017). Preferential uptake of nitrate over ammonium will result in a rhizosphere pH increase and recent modeling by Kuppe et al. (2022) has shown that this can substantially improve P uptake in rice. The same study also suggested that the presence of short fine laterals enhance the uptake of solubilized P by extending the effective uptake zone around their parent roots. A relative preference for nitrate would therefore not only favor root elongation with its positive effect on P uptake but could furthermore improve RE. We investigated this possibility by associating the nitrate uptake data from Fig. 5b with estimates of RE, obtained for the same genotypes but grown on a highly P-fixing volcanic ash soil (Mori et al. 2016). Interestingly, a highly positive correlation between both traits (r = 0.91) was only detected under low P supply. This observation is in accordance with the fact that an increase in pH results in higher availability of phosphate in volcanic ash soil (Takahashi and Dahlgren 2016). This would suggest that genotypic differences in the preference for nitrate uptake should be explored further in the context of rhizosphere P solubilization.
Similar to the volcanic ash soil used by Mori et al. (2016), the effect of nitrate preference and subsequent pH changes on RE should be also pronounced in acidic soils, since phosphate strongly bound with iron and aluminum can be released by a small pH increases (Price 2006). This has important implications for rice breeding since the highly weathered P-deficient soils typically encountered in sub-Saharan Africa are usually acidic (Nishigaki et al. 2019). The present study presents evidence that genotypes differ for the preference for nitrate over ammonium uptake and suggests this could lead to better root elongation and higher RE. To what extent variation for this trait can be detected and exploited in the larger rice gene pool should be investigated.
Abbreviations
- PAE:
-
P acquisition efficiency
- PUE:
-
Internal P use efficiency
- RE:
-
Root efficiency
References
Adem GD, Ueda Y, Hayes PE, Wissuwa M (2020) Genetic and physiological traits for internal phosphorus utilization efficiency in rice. PLoS ONE 15:e0241842. https://doi.org/10.1371/journal.pone.0241842
Andrianary BH, Tsujimoto Y, Rakotonindrina H et al (2021) Phosphorus application affects lowland rice yields by changing phenological development and cold stress degrees in the central highlands of Madagascar. F Crop Res 271:108256. https://doi.org/10.1016/j.fcr.2021.108256
Barrow NJ (2017) The effects of pH on phosphate uptake from the soil. Plant Soil 410:401–410. https://doi.org/10.1007/s11104-016-3008-9
Briskin DP, Hanson JB (1992) How does the plant plasma membrane H+-ATPase pump protons? J Exp Bot 43:269–289. https://doi.org/10.1093/jxb/43.3.269
Cai J, Chen L, Qu H et al (2012) Alteration of nutrient allocation and transporter genes expression in rice under N, P, K, and Mg deficiencies. Acta Physiol Plant 34:939–946. https://doi.org/10.1007/s11738-011-0890-x
Cooper J, Lombardi R, Boardman D, Carliell-Marquet C (2011) The future distribution and production of global phosphate rock reserves. Resour Conserv Recycl 57:78–86. https://doi.org/10.1016/j.resconrec.2011.09.009
Crawford NM (1995) Nitrate: nutrient and signal for plant growth. Plant Cell 7:859–868. https://doi.org/10.1105/tpc.7.7.859
Crawford NM, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci 3:389–395. https://doi.org/10.1016/S1360-1385(98)01311-9
De Angeli A, Monachello D, Ephritikhine G et al (2006) The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442:939–942. https://doi.org/10.1038/nature05013
Erisman JW, Sutton MA, Galloway J et al (2008) How a century of ammonia synthesis changed the world. Nat Geosci 1:636–639. https://doi.org/10.1038/ngeo325
Falkengren-Grerup U (1995) Interspecies differences in the preference of ammonium and nitrate in vascular plants. Oecologia 102:305–311. https://doi.org/10.1007/BF00329797
Ferreira LM, de Souza VM, Tavares OCH et al (2015) OsAMT1.3 expression alters rice ammonium uptake kinetics and root morphology. Plant Biotechnol Rep 9:221–229. https://doi.org/10.1007/s11816-015-0359-2
Fried M, Zsoldos F, Vose PB, Shatokhin IL (1965) Characterizing the nitrate and ammonium uptake process of rice roots by use of 15N labelled NH4NO3. Physiol Plant 18:313–320. https://doi.org/10.1111/j.1399-3054.1965.tb06894.x
Gonzalez D, Postma J, Wissuwa M (2021) Cost-benefit analysis of the upland-rice root architecture in relation to phosphate : 3D simulations highlight the importance of S-type lateral roots for reducing the pay-off time. Front Plant Sci 12:641835. https://doi.org/10.3389/fpls.2021.641835
Hachiya T, Sakakibara H (2017) Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J Exp Bot 68:2501–2512. https://doi.org/10.1093/jxb/erw449
Hartmann J, Asch F (2018) Micro-method to determine iron concentrations in plant tissues using 2,2′ bipyridine. J Plant Nutr Soil Sci 181:357–363. https://doi.org/10.1002/jpln.201700433
Hayes PE, Adem GD, Pariasca-Tanaka J, Wissuwa M (2021) Leaf phosphorus fractionation in rice to understand internal phosphorus-use efficiency. Ann Bot 129:287–302. https://doi.org/10.1093/aob/mcab138
Heuer S, Gaxiola R, Schilling R et al (2017) Improving phosphorus use efficiency: a complex trait with emerging opportunities. Plant J 90:868–885. https://doi.org/10.1111/tpj.13423
Hirano T, Satoh Y, Ohki A et al (2008) Inhibition of ammonium assimilation restores elongation of seminal rice roots repressed by high levels of exogenous ammonium. Physiol Plant 134:183–190. https://doi.org/10.1111/j.1399-3054.2008.01117.x
Hu B, Jiang Z, Wang W et al (2019) Nitrate–NRT1.1B–SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat Plants 5:401–413. https://doi.org/10.1038/s41477-019-0384-1
Kawata S, Maruyama S, Soejima M (1977) Root formation in rice plant and levels of nitrogen supply. Japanese J Crop Sci 46:193–198. https://doi.org/10.1626/jcs.46.193
Kiba T, Inaba J, Kudo T et al (2018) Repression of nitrogen starvation responses by members of the arabidopsis GARP-type transcription factor NIGT1/HRS1 subfamily. Plant Cell 30:925–945. https://doi.org/10.1105/tpc.17.00810
Konishi N, Ma JF (2021) Three polarly localized ammonium transporter 1 members are cooperatively responsible for ammonium uptake in rice under low ammonium condition. New Phytol 232:1778–1792. https://doi.org/10.1111/nph.17679
Kuppe CW, Kirk GJD, Wissuwa M, Postma JA (2022) Rice increases phosphorus uptake in strongly sorbing soils by intra-root facilitation. Plant Cell Environ 45:884–899. https://doi.org/10.1111/pce.14285
Li Q, Li BH, Kronzucker HJ, Shi WM (2010) Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity. Plant Cell Environ 33:1529–1542. https://doi.org/10.1111/j.1365-3040.2010.02162.x
Liu F, Wang Z, Ren H et al (2010) OsSPX1 suppresses the function of OsPHR2 in the regulation of expression of OsPT2 and phosphate homeostasis in shoots of rice. Plant J 62:508–517. https://doi.org/10.1111/j.1365-313X.2010.04170.x
López-Arredondo DL, Leyva-González MA, González-Morales SI et al (2014) Phosphate nutrition: Improving low-phosphate tolerance in crops. Annu Rev Plant Biol 65:95–123. https://doi.org/10.1146/annurev-arplant-050213-035949
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol 156:1041–1049. https://doi.org/10.1104/pp.111.175414
MacDonald GK, Bennett EM, Potter PA, Ramankutty N (2011) Agronomic phosphorus imbalances across the world’s croplands. Proc Natl Acad Sci U S A 108:3086–3091. https://doi.org/10.1073/pnas.1010808108
Maeda Y, Konishi M, Kiba T et al (2018) A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat Commun 9:1376. https://doi.org/10.1038/s41467-018-03832-6
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Elsevier Ltd
Martinoia E, Heck U, Wiemken A (1981) Vacuoles as storage compartments for nitrate in barley leaves. Nature 289:292–294. https://doi.org/10.1038/289292a0
Medici A, Szponarski W, Dangeville P et al (2019) Identification of molecular integrators shows that nitrogen actively controls the phosphate starvation response in plants. Plant Cell 31:1171–1184. https://doi.org/10.1105/tpc.18.00656
Meier M, Liu Y, Lay KS et al (2020) Auxin-mediated root branching is determined by the form of available nitrogen. Nat Plants 6:1136–1145. https://doi.org/10.1038/s41477-020-00756-2
Meng Q, Zhang W, Hu X et al (2020) Two ADP-glucose pyrophosphorylase subunits, OsAGPL1 and OsAGPS1, modulate phosphorus homeostasis in rice. Plant J 104:1269–1284. https://doi.org/10.1111/tpj.14998
Mori A, Fukuda T, Vejchasarn P et al (2016) The role of root size versus root efficiency in phosphorus acquisition in rice. J Exp Bot 67:1179–1189. https://doi.org/10.1093/jxb/erv557
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Niang A, Becker M, Ewert F et al (2017) Variability and determinants of yields in rice production systems of West Africa. F Crop Res 207:1–12. https://doi.org/10.1016/j.fcr.2017.02.014
Nishigaki T, Tsujimoto Y, Rinasoa S et al (2019) Phosphorus uptake of rice plants is affected by phosphorus forms and physicochemical properties of tropical weathered soils. Plant Soil 435:27–38. https://doi.org/10.1007/s11104-018-3869-1
Nishigaki T, Tsujimoto Y, Rakotoson T et al (2021) Soil phosphorus retention can predict responses of phosphorus uptake and yield of rice plants to P fertilizer application in flooded weathered soils in the central highlands of Madagascar. Geoderma 402:115326. https://doi.org/10.1016/j.geoderma.2021.115326
Patterson K, Cakmak T, Cooper A et al (2010) Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ 33:1486–1501. https://doi.org/10.1111/j.1365-3040.2010.02158.x
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96. https://doi.org/10.1016/S0065-2113(08)60633-1
Price G (2006) Australian Soil Fertility Manual, 3rd edn. Fertilizer Industry Federation of Australia and CSIRO Publishing, Melbourne
Prodhan MA, Pariasca-Tanaka J, Ueda Y et al (2022) Comparative transcriptome analysis reveals a rapid response to phosphorus deficiency in a phosphorus-efficient rice genotype. Sci Rep 12:9460. https://doi.org/10.1038/s41598-022-13709-w
Rakotoson T, Tsujimoto Y, Nishigaki T (2022) Phosphorus management strategies to increase lowland rice yields in Sub-Saharan Africa. F Crop Res 275:108370. https://doi.org/10.1016/j.fcr.2021.108370
Rao EVSP, Prasad R, Abrol YP (1979) Nitrate assimilation in rice grown under lowland conditions. Physiol Plant 47:139–143. https://doi.org/10.1111/j.1399-3054.1979.tb03205.x
Rose TJ, Rose MT, Pariasca-Tanaka J et al (2011) The frustration with utilization: why have improvements in internal phosphorus utilization efficiency in crops remained so elusive? Front Plant Sci 2:73. https://doi.org/10.3389/fpls.2011.00073
Rose TJ, Mori A, Julia CC, Wissuwa M (2016) Screening for internal phosphorus utilisation efficiency: comparison of genotypes at equal shoot P content is critical. Plant Soil 401:79–91. https://doi.org/10.1007/s11104-015-2565-7
Rubio-Asensio JS, Bloom AJ (2017) Inorganic nitrogen form: a major player in wheat and Arabidopsis responses to elevated CO2. J Exp Bot 68:2611–2625. https://doi.org/10.1093/jxb/erw465
Saito K, Vandamme E, Johnson JM et al (2019) Yield-limiting macronutrients for rice in sub-Saharan Africa. Geoderma 338:546–554. https://doi.org/10.1016/j.geoderma.2018.11.036
Takahashi T, Dahlgren RA (2016) Nature, properties and function of aluminum-humus complexes in volcanic soils. Geoderma 263:110–121. https://doi.org/10.1016/j.geoderma.2015.08.032
Taylor AR, Bloom AJ (1998) Ammonium, nitrate, and proton fluxes along the maize root. Plant, Cell Environ 21:1255–1263. https://doi.org/10.1046/j.1365-3040.1998.00357.x
Tian WH, Ye JY, Cui MQ et al (2021) A transcription factor STOP1-centered pathway coordinates ammonium and phosphate acquisition in Arabidopsis. Mol Plant 14:1554–1568. https://doi.org/10.1016/j.molp.2021.06.024
Ueda Y, Kiba T, Yanagisawa S (2020a) Nitrate-inducible NIGT1 proteins modulate phosphate uptake and starvation signalling via transcriptional regulation of SPX genes. Plant J 102:448–466. https://doi.org/10.1111/tpj.14637
Ueda Y, Ohtsuki N, Kadota K et al (2020b) Gene regulatory network and its constituent transcription factors that control nitrogen deficiency responses in rice. New Phytol 227:1434–1452. https://doi.org/10.1111/nph.16627
Ueda Y, Sakuraba Y, Yanagisawa S (2021) Environmental control of phosphorus acquisition: a piece of the molecular framework underlying nutritional homeostasis. Plant Cell Physiol 62:573–581. https://doi.org/10.1093/pcp/pcab010
Vandamme E, Ahouanton K, Mwakasege L et al (2018) Phosphorus micro-dosing as an entry point to sustainable intensification of rice systems in sub-Saharan Africa. F Crop Res 222:39–49. https://doi.org/10.1016/j.fcr.2018.02.016
Vinod KK, Heuer S (2012) Approaches towards nitrogen- and phosphorus-efficient rice. AoB Plants 2012:pls028. https://doi.org/10.1093/aobpla/pls028
Wang MY, Siddiqi MY, Ruth TJ, Glass ADM (1993) Ammonium uptake by rice roots. I. Fluxes and subcellular distribution of 13NH4+. Plant Physiol 103:1249–1258. https://doi.org/10.1104/pp.103.4.1249
Wang F, Morrison King JD, Rose T et al (2017) Can natural variation in grain P concentrations be exploited in rice breeding to lower fertilizer requirements? PLoS ONE 12:e0179484. https://doi.org/10.1371/journal.pone.0179484
Wang P, Yamaji N, Inoue K et al (2020a) Plastic transport systems of rice for mineral elements in response to diverse soil environmental changes. New Phytol 226:156–169. https://doi.org/10.1111/nph.16335
Wang S, Chen A, Xie K et al (2020b) Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc Natl Acad Sci U S A 117:16649–16659. https://doi.org/10.1073/pnas.2000926117
Watanabe M, Walther D, Ueda Y et al (2020) Metabolomic markers and physiological adaptations for high phosphate utilization efficiency in rice. Plant Cell Environ 43:2066–2079. https://doi.org/10.1111/pce.13777
Waters BM, Troupe GC (2012) Natural variation in iron use efficiency and mineral remobilization in cucumber (Cucumis sativus). Plant Soil 352:185–197. https://doi.org/10.1007/s11104-011-0988-3
Wissuwa M, Gamat G, Ismail AM (2005) Is root growth under phosphorus deficiency affected by source or sink limitations? J Exp Bot 56:1943–1950. https://doi.org/10.1093/jxb/eri189
Wissuwa M, Kondo K, Fukuda T et al (2015) Unmasking novel loci for internal phosphorus utilization efficiency in rice germplasm through genome-wide association analysis. PLoS ONE 10:e0124215. https://doi.org/10.1371/journal.pone.0124215
Wissuwa M, Gonzalez D, Watts-Williams SJ (2020) The contribution of plant traits and soil microbes to phosphorus uptake from low-phosphorus soil in upland rice varieties. Plant Soil 448:523–537. https://doi.org/10.1007/s11104-020-04453-z
Xu G, Fan X, Miller AJ (2012) Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol 63:153–182. https://doi.org/10.1146/annurev-arplant-042811-105532
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice, Third edit. The International Rice Research Institute, Manila
Zeng H, Liu G, Kinoshita T et al (2012) Stimulation of phosphorus uptake by ammonium nutrition involves plasma membrane H+ATPase in rice roots. Plant Soil 357:205–214. https://doi.org/10.1007/s11104-012-1136-4
Zhang M, Wang Y, Chen X et al (2021) Plasma membrane H+-ATPase overexpression increases rice yield via simultaneous enhancement of nutrient uptake and photosynthesis. Nat Commun 12:735. https://doi.org/10.1038/s41467-021-20964-4
Zhang Z-S, Xia J-Q, Alfatih A et al (2022) Rice NIN-LIKE PROTEIN 3 modulates nitrogen use efficiency and grain yield under nitrate-sufficient conditions. Plant Cell Environ 45:1526–1536. https://doi.org/10.1111/pce.14294
Zhao H, Yao W, Ouyang Y et al (2015) RiceVarMap: A comprehensive database of rice genomic variations. Nucleic Acids Res 43:D1018–D1022. https://doi.org/10.1093/nar/gku894
Acknowledgements
The authors thank Dr. K. Ikazaki and N. Sekine for the technical support in the mass spectrometry analyses. The authors thank M. Yonemoto and M. Matsuyama for the assistance in element analyses.
Funding
This study was funded by JIRCAS research program “Resilient crops”.
Author information
Authors and Affiliations
Contributions
Conceptualization, investigation, formal analysis, visualization, writing-original manuscript: YU
Resources, supervision, methodology, writing-review & editing: MW
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Responsible Editor: Ad C. Borstlap.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ueda, Y., Wissuwa, M. Physiological evidence that nitrate use positively correlates with internal phosphorus utilization efficiency and phosphorus uptake efficiency in rice (Oryza sativa L.). Plant Soil 481, 547–561 (2022). https://doi.org/10.1007/s11104-022-05655-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05655-3