Abstract
Aims
Popular African upland rice variety Nerica4 performs poorly under phosphorus (P) deficiency; the objective was to identify plant and soil traits likely to improve its P efficiency.
Methods
Field and glasshouse experiments compared P uptake and root parameters between the popular rice genotype Nerica4, and a known P-efficient genotype, DJ123. Glasshouse experiments used fresh field soil, sterilized soil and sterilized soil resupplied with 15% fresh field soil to assess microbial effects.
Results
DJ123 had faster crown root development and higher proportions of fine roots, leading to larger root surface area (RSA). Additionally, it acquired more P per RSA, thus had more efficient roots. Higher root efficiency of DJ123 compared to Nerica4 was detected in fresh field soil, sterile soil, and sterile+resupplied soil, indicating that plant-specific factors rather than soil microbiome effects explained higher root efficiency in DJ123. In non-sterile soils both genotypes were colonized by arbuscular mycorrhizal fungi (AMF), and high expression of an AMF-induced rice P transporter gene (OsPT11) indicated the symbiosis was functional.
Conclusions
We identified plant traits present in DJ123 such as rapid crown root development, higher proportions of fine lateral roots, as well as superior overall root efficiency that make it a promising donor to improve the performance of Nerica4 in P-deficient environments. In addition, Nerica4 appears more susceptible to growth-inhibitory effects of the soil microbiome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P)-deficient soils are a problem for agriculture that is encountered in several parts of the world, including South-east Asia, parts of Central America, China, India, and Africa; this problem is particularly exacerbated in sub-Saharan Africa (SSA) due to low fertilizer inputs (Balasubramanian et al. 2007; Saito et al. 2018) in combination with the prevalence of highly weathered soils such as Oxisols. These soils are known to bind P in forms that are not plant available, making P the essential plant nutrient that is frequently the limiting factor in rice production (Saito et al. 2019). As a food source and thus as a crop, rice (Oryza sativa L.) is consistently gaining popularity in the SSA region (Balasubramanian et al. 2007; Saito et al. 2018). When compared to the other main rice-growing regions of the world, SSA has a high proportion (~38%; Balasubramanian et al. 2007) of upland rice production compared with lowland production. Given that upland rice production relies entirely on rainfall as opposed to irrigation, it is prone to drought stress, and this yield insecurity makes it less likely that fertilizers are applied as the cost vs risk is a concern (Saito et al. 2018). This combination of unique climatic and economic factors in the SSA region means there is a need for rice genotypes that are tolerant of multiple stressors, including drought and nutrient stress, simultaneously.
The upland rice variety Nerica4 has become popular with farmers across the African continent because of its mild drought tolerance, resistance to the parasitic weed striga, and responsiveness to fertilizer application. However, on infertile soils, the performance of Nerica4 is relatively poor (Atakora et al. 2015; Vandamme et al. 2016), which may be attributed in part due to its inability to efficiently acquire P from soil-bound forms (Koide et al. 2013; Mori et al. 2016a). In contrast, the rice gene bank accession DJ123 has been identified as being capable of producing relatively high grain yields on low-P African soils (Vandamme et al. 2016). It has been suggested from field sampling during later growth stages, that DJ123 can be especially efficient in terms of P uptake per unit root biomass or surface area (Mori et al. 2016a). In order to uncover the underlying mechanisms for the greater P uptake efficiency of DJ123, it is necessary to compare in more detail the patterns of root growth and P uptake between DJ123 and the popular variety Nerica4. A comprehensive physiological comparison should reveal whether root attributes in DJ123 exist that can be transferred to Nerica4, to improve its adaptation to nutrient-poor environments such as those in SSA.
Genotypic differences in P uptake by rice can mostly be attributed to differences in root size or root biomass, with genotypic differences in root efficiency (P uptake per unit root biomass or surface area) being less pronounced (Wissuwa and Ae, 2001; Mori et al. 2016a). Unlike most crop species, rice has a complex structure in which five different root classes have been distinguished (Yamauchi et al. 1987; Rebouillat et al. 2009). These are the seminal (primary) root, the embryonic crown roots, the postembryonic (nodal) crown roots, the large L-type lateral roots, and the small S-type lateral roots. These S-type roots can be found branching from all other root type classes, including L-type lateral roots. Due to their small size (diameter of ~0.05 mm) they are easily overlooked in studies characterizing root systems and consequently, little is known regarding genotypic differences in S-type development, despite their large contribution to the total root length of the rice root system (Nestler et al. 2016). Even smaller than S-type roots are root hairs; their potential importance for P uptake has been recognized (Gahoonia and Nielsen 1998), especially in the context of offering a cost-effective way to increase soil exploration (Lynch 2011). Genotypic differences in root hair length and density have recently been reported for rice grown in low-P soil and while differences were small, DJ123 is characterized by longer and more dense root hairs compared to Nerica4 (Nestler and Wissuwa, 2016).
Rice is one of many important cereal crops that can form associations with arbuscular mycorrhizal fungi (AMF) in the soil. The association between rice and AMF is particularly relevant under upland production in ‘typical’ agricultural soils, but the ability for AMF to survive and function under lowland (anoxic) soil conditions is debated (Mbodj et al. 2018, and references therein). There is evidence that AMF can be beneficial for rice yield and nutrition, and particularly for P uptake (Maiti et al. 2011). There is also substantial research on the molecular basis for AMF effects on rice P uptake; the genes, proteins and signaling molecules involved have been relatively well characterized (Paszkowski et al. 2002; Gutjahr et al. 2008). Specifically, the P transporter (PT) protein OsPT11 is located on the peri-arbuscular membrane (the plant-fungal interface) and is responsible for the transport of phosphate (Pi) from the fungus to the plant cytosol (Yang et al. 2012). In non-mycorrhizal rice plants OsPT11 expression is not detected; the expression of gene OsPT11 is therefore a proxy for the mycorrhizal pathway of P uptake while other constitutively expressed PT genes such as OsPT2 represent the direct/root pathway of P uptake (Yang et al. 2012; Chen et al. 2013). Additionally, AMF can improve drought-resistance in rice (Ruíz-Sánchez et al. 2010, 2011) and thus may confer some stress tolerance to rice genotypes growing under both water and P limitation such as in SSA.
In summary, rice plants can achieve greater P uptake and efficiency via three main strategies: (i) producing greater root biomass, (ii) having more efficient roots or, (iii) through association with beneficial soil microorganisms (namely AMF). Thus, the objectives of this study were to evaluate and quantify to what extent these three mechanisms contribute to genotypic differences between Nerica4 and DJ123 in P uptake, in both field- and glasshouse-based experimental systems.
Methods
Field experiment
Experiments were conducted in two upland fields at the Hachimantai experimental station of JIRCAS, located in Tsukuba, Japan. The soil type is a volcanic ash soil (Humic Haplic Andosol) with a pH of 5.8. A low-P field had not been fertilized with P for more than 15 years and was characterized by a very low level of plant available P (5 mg P kg−1 soil, Bray-II). The high-P field had received P fertilizer annually and had a higher P content (14 mg P kg−1 soil, Bray-II). Both fields were part of the same block at the experimental farm. Prior to field preparation a basal dose of NK compound fertilizer was applied to the low-P field at a rate of 50 kg ha−1 of N (urea) and K2O. The high P field received a same dose of compound NPK fertilizer (50 kg N, K2O, P2O5). Seeds of rice (Oryza sativa L.) varieties Nerica4 and DJ123 were sown on 14 June 2018 with 20 seeds per 1.2 m row, and 5 rows per genotype (15 cm distance between rows). Shoots started to emerge on 19 June, which was considered the first day after emergence (DAE). Rows were thinned to 13 remaining plants per row with average distance of 10 cm within rows and weeded twice during the 62-day experimental period.
Plants were sampled five times during this period. At 8 (during thinning), 20 and 62 DAE roots were only sampled to a shallow depth of 7–8 cm, while roots were excavated to a depth of 30 cm at 28 and 48 DAE by gently washing away soil with running water. At all sampling points crown root number was counted and shoots were separated from roots and dried in an oven at 65 °C for a minimum of 3 days, after which shoots were weighed and ground for analysis of plant P content (see below for more detail). For samplings at 8, 20 and 62 DAE roots were discarded after crown root number determination. For excavated plants, roots were soaked in water, soil removed gently, after which roots were placed in water-filled sealed containers and stored in a refrigerator until further analysis (root scanning and P analysis; see below for more detail). A final sampling of roots at 7.5 cm depth intervals was done at 70 DAE. The central 3 rows per plot with a total of 9 plants were excavated in layers using a spade. Four layers were excavated to a maximum depth of 30 cm. Roots were washed out from each layer, dried and weighed to determine the root mass distribution at depth intervals.
Glasshouse experiments
Experiment 1 was conducted in a glasshouse at JIRCAS, Tsukuba, Japan during June and July 2017 with soil obtained from the fields described above. We added the equivalent of 25 kg P ha−1 to high-P soil while filling experimental containers, and both soils received the equivalent of 50 kg ha−1 N-K fertilizer (using container surface area to calculate per ha equivalents). The experimental containers were 22 L (dimensions 32 × 46 cm × 15 cm depth) for harvests at 7 and 14 DAE, and 72 L for harvests at 21 and 28 DAE (dimensions 40 × 60 cm × 30 cm depth). Pre-germinated seeds of rice varieties Nerica4 and DJ123, in which the radicle had emerged by 1–2 mm, were sown with 2 seeds per hole that were later thinned to a single plant. Both genotypes were grown together in the same container in double rows with eight plants per genotype per sampling time. Shoots emerged from the soil 3–4 days after planting, and 4 days after sowing (DAS) was considered day 1 of the experiment (DAE). Plants were sampled in weekly intervals by cutting the container open and gently rinsing roots from soil with running water. Per replicate container an average of three or four plants were sampled per treatment. Plants were then treated and stored as the excavated plants from the field experiment.
Experiment 2 consisted of two overlapping sub-experiments that evaluated the potential effects of AMF on P uptake from low-P soil under sterile and non-sterile conditions, using soil from the low-P field described above. This field soil was previously characterized for the AMF species present, and includes populations of Rhizophagus irregularis, Glomus mosseae and Scutellospora pellucida (Campos-Soriano et al. unpublished). One batch of soil was air dried and sterilized by autoclaving twice at 121 °C for 20 min; the second batch was fresh field soil. Sub-experiment a) evaluated P uptake and colonization by AMF in fresh field soil in weekly intervals starting at 14 DAS and ending at 35 DAS. Sub-experiment b) compared P uptake from fresh soil to sterilized soil and sterilized soil to which 15% fresh field soil had been added (sterile+) at one sampling time at 28 DAS. The experiment was conducted in a heated glasshouse at JIRCAS, Tsukuba, Japan during October and November 2018. Procedures were generally as described for glasshouse experiment 1. Pre-germinated seeds of varieties Nerica4 and DJ123 were sown on 15 October and samples were taken 14, 21, 28, and 35 DAS. For the 14-day growth period plants were grown in 22 L containers, for the 21-day period in 30 L containers and 45 L containers were used for the 28- and 35-day growth periods. Plants were harvested in the same way as for Expt. 1. After washing roots 1.5 cm long sections were cut from across the entire root system at 10 and 16 cm from the crown (~200 mg fresh weight each) and these sub-samples were either stored in 50% ethanol for determination of mycorrhizal colonization, or quickly blotted dry and flash frozen in liquid nitrogen for RNA extraction. The experiment had four replications and per replicate 4 plants were sampled. Root scanning and P analysis was done for 2 plants per replicate, AMF counts were obtained from 6 plants (1–2 per replicate) and qPCR performed on 1 plant per replicate.
Sub-samples of fresh roots stored in 50% EtOH were left at room temperature for at least 24 h, then rinsed and moved into a 10% potassium hydroxide solution and left to clear at room temperature for five days. Cleared roots were rinsed well and moved into a 5% Schaeffer blue ink in a 5% acetic acid (vinegar) solution and heated at 60 °C for 10 min to stain (Vierheilig et al. 1998). Roots were then moved into a 5% vinegar solution and left to de-stain for at least 24 h before viewing under the dissecting microscope. Root mycorrhizal colonization was quantified by the gridline intersection method following Giovanetti and Mosse (1980).
Flash frozen root samples were placed in pre-chilled 2 mL plastic milling tubes containing two 5 mm zirconia beads, and subsequently homogenized using a Mixer Mill MM300 (Qiagen) with components pre-chilled using liquid nitrogen. Total RNA was extracted from the homogenized root samples using an RNeasy Plant Mini kit (Qiagen), including an on-column DNase treatment step (Qiagen). Following quantification of the extracted RNA, cDNA from each sample was synthesized from 500 ng RNA using the PrimeScript RT Reagent Kit (TaKaRa Bio) following the manufacturer’s instructions, then diluted to a final concentration of 10 ng μL−1 with DEPC-treated water before being used in qPCR reactions. The quantification of the mycorrhiza-induced phosphate transporter gene OsPT11 and non-induced OsPT2 and OsPT3 was performed by quantitative real-time PCR (Mini Opticon Real-Time PCR system, BioRad, USA), using SYBR Premix Ex Taq (TaKaRa Bio) with forward and reverse oligo nucleotide sequences designed to target genes of interest (suppl. Table S1). qPCR cycling conditions were as follows: 95 °C for 30 s; 40 cycles of 95 °C for 5 s, 60 °C for 20 s; melt curve. Expression of the PT genes-of-interest were normalized to the m value of the two most stable O. sativa housekeeping genes: GAPDH and Ubi1.
Root scanning
Roots were spread out in a Perspex tray (20 × 25 cm) filled to 1 cm with water for image acquisition with an Epson Perfection V700 photo dual lens scanner with top lighting (scanner settings: 600 dpi, 8-bit grayscale, positive film). Images were then analyzed by the WinRhizo software (Version 2008a, Regent Instruments Inc.) to estimate root surface area (RSA) with ‘Pixel classification’ set to manual with a grey-scale value of 225, which improved the detection of very fine roots. Root systems were separated into the following diameter classes: 0–90 nm, 90–250 nm, 250–400 nm, 400–600 nm, 600–1000 nm (suppl. Fig. S1). Approximately, they correspond to S-type lateral roots (<90 nm), L-type lateral roots (90–250 nm) and crown roots (250–1000 nm). After scans, roots were washed more thoroughly to remove any remaining soil, patted down on paper towels and dried at 65 °C for several days, after which roots were weighed and ground for analysis of plant P content. In addition to the WinRhizo analysis, root samples from experiment 1 were further analyzed using ImageJ. Scaling options against a scanned object of know length were used to manually measure lateral root density and length on up to 10 root sections of 4 replicate root systems per genotype.
Tissue P analysis
Root and shoot samples were digested using a 4:1 HNO3/HClO4 (nitric/perchloric) mixture at 105 °C for 90 min, 140 °C for 90 min, followed by 20 min at 170 °C in order to dehydrate any silica in the digest. The digests were diluted with MiliQ water up 50 mL and filtered through No. 6 Advantec filter paper. The tissue P concentrations were analyzed in a microplate reader at 882 nm wavelength using the molybdenum blue assay following Murphy and Riley (1962).
Statistical analysis
Data were analyzed as two-factorial with P treatment (P) and genotype (G) as treatment variables using the software statistix (version 10). Means separations were done using ANOVA-guided LSDs. For most traits unequal error variances existed due to the much higher means in the high-P treatment. Within treatment separation between genotype group means in the low-P treatment was therefore done based on a separate treatment-specific ANOVA.
Results
Uncovering genotypic differences in root traits and P uptake in the field
A first sampling point at 8 days after emergence (DAE) when seedlings were in the 2–3 leaf stage revealed the early effects of P supply, with a small but significant increase in shoot biomass and shoot P content in the fertilized (high-P) plots (Table 1). The difference in both shoot traits increased further and reached a maximum at 28 DAE when both were reduced by more than 90% in the low-P treatment. Until the final sampling point at 62 DAE, plants in the low-P treatment were able to catch up slightly but shoot biomass and P content remained significantly lower with reductions of 54% and 64%, respectively. The very pronounced difference at 28 DAE was due to an effect of P deficiency on plant development. Plants in the high-P treatment had entered the tillering stage by 20 DAE and apparently reached the mid-tillering stage by 28 DAE, whereas tillering in low-P plants only started at 28 DAE, and in DJ123 only. The increase in tiller number between days 20–28 was mirrored by an increase in crown root number (Table 1). This trait showed the smallest difference between P treatments at 62 DAE, with a reduction of only 20% between the high- and low-P plants, indicating that each tiller produced a higher number of crown roots under P deficiency.
The very large treatment effects at 28 DAE, i.e. more than 10-fold higher mean shoot biomass and P content in the high-P treatment, resulted in very unequal error variances between treatments (data not shown), and this affected the ANOVA by rendering G and PxG effects non-significant (Table 1). While the genotype effect was indeed not significant for the high-P treatment, a separate single-factor analysis within P treatments detected a significant genotype effect in the low-P treatment (Fig. 1). Further, the use of standardized data for these two traits (standardized against the mean and standard deviation within P treatments) detected significant genotype effects for both traits and a significant PxG interaction for shoot P content (Table 1; data in parentheses).
Crown root number (a), shoot P content (b) and shoot biomass (c) of two contrasting rice genotypes grown over a 62-day period in a P-deficient field. Small plots provide a zoom-in for the first 30 days. Different letters above means indicate significant difference (LSD at the P < 0.05 level of significance)
As the objective was to investigate the presence of genotypic differences in root efficiency (RE) under P deficiency, the following analyses focused on the low-P treatment only. The number of crown roots was determined, and genotypic differences were significant at each time point sampled; Nerica4 consistently had fewer crown roots compared to DJ123 (Fig. 1a). For shoot P content and shoot biomass, DJ123 also significantly out-performed Nerica4.
At time points 28 and 48 DAE, entire root systems were excavated from the soil to a depth of 30 cm and after root scanning and determination of RSA, RE was calculated as: plant P uptake / RSA. Washing of roots was apparently successful in removing most soil attached to roots as indicated by low root P concentrations at 28 DAE (0.73 mg P g−1 in DJ123 and 0.76 mg P g−1 in Nerica4, data not shown) and at 48 DAE (0.60 mg P g−1 in DJ123 and 0.84 mg P g−1 in Nerica4, data not shown). We therefore assume that total plant P uptake was not over-estimated using roots excavated from soil. Daily P uptake during the period between 20 and 28 DAE was 4-fold higher in DJ123 compared to Nerica4, with RSA being 2.5-fold greater in DJ123 (Table 2). Because the difference in P uptake was more pronounced than for RSA, significant differences existed for RE, with DJ123 having 82% higher P uptake per root surface area (Table 2). This estimate of RE is an underestimation of true RE because end-point RSA (28 DAE) was used in the calculation, whereas roots at the beginning of the 20–28 DAE period would have been much smaller and therefore more efficient.
For the period between 28 and 48 DAE, daily P uptake increased as root size increased but RE remained remarkably stable in comparison (Table 2). Genotypic differences were also unchanged with DJ123 having higher P uptake, RSA and RE. Having a measurement for RSA at the start and end point of the 28–48 DAE interval further allowed for the computation of a mid-point RSA and a subsequent RE using the mid-point RSA. While genotypic rankings were not affected by this, this approach produces estimates of RE that are about 65% higher compared to the end-point RSA calculation.
To investigate whether higher RE can be associated with root system properties, root distribution at different depth and differences in root fineness were assessed. Very little root biomass was found below 22.5 cm soil depth for both genotypes (Fig. 2), which is already below the plow layer, found at around 15–18 cm depth in this field. Roots were therefore not excavated past a depth of 30 cm. Only minor differences in root distribution over depth were detected between genotypes. Both had more than 80% of their RSA concentrated in the very shallow (<7.5 cm) and shallow (7.5–15 cm) layers. Thus, DJ123 and Nerica4 quite uniformly concentrated roots in the top 15 cm in this field (Fig. 2). The only significant difference was the proportionally higher root biomass of DJ123 in the 15–22.5 cm layer.
Root biomass distribution at 7.5 cm depth intervals in the field experiment at 70 DAE. Size of boxes proportionally represent the biomass measured per layer and genotype. The relative contribution of each layer to total root biomass is given in percent, with ‘*’ indicating contributions were significantly different at P < 0.05 (ns = not significant)
The proportion of total root length (TRL) and RSA contributed by very fine S-type lateral roots (< 90 μm) or by normal L-type lateral rootss (90–250 μm) was investigated. Genotypic differences were small but at 28 DAE, 61% of the TRL of DJ123 was due to S-type lateral roots compared to only 55% for Nerica4 (suppl. Table S2). For RSA the proportions were 23% versus 19%, respectively. Larger diameter crown roots made up a similar proportion in all genotypes (about 16% of TRL and 48% of RSA; data not shown). DJ123 also had a far greater number of crown roots and root tips.
Compared to 28 DAE, the proportion of very fine root length had further increased at 48 DAE, and DJ123 continued to have a higher proportion of fine roots (suppl. Table S2). For RSA on the other hand, the contribution of fine roots and lateral roots decreased, which likely indicates that over time the diameter of newly developed crown roots increased, and that these contributed over-proportionally to RSA.
Exploring relevant traits of entire root systems in more detail (glasshouse expt. 1)
To investigate root architectural differences in more detail, entire root systems were extracted from large boxes filled with P-deficient or P-fertilized low-P field soil, and root scans were analyzed for fine root parameters. The S-type lateral roots were present at a high density of about 14–16 roots cm−1 of main axis length (crown root or L-type lateral); while DJ123 consistently showed slightly higher density, this was only significant at the end of the first week (Table 3).
The length of individual S-type lateral roots depended on whether they initiated from crown or lateral roots. Average S-type length on lateral roots were between 2.3–3.1 mm, and maximum length between 4.5–7.0 mm; however, on crown roots a maximum length of 10 mm could be reached, particularly towards the base of a crown root. Again, DJ123 tended to have longer S-type roots, but the difference was not significant at 2 or 3 weeks after emergence. Typically, no S-type roots were formed until 8–13 mm from the tip. For L-type lateral roots, two opposing patterns emerge: Nerica4 produced roots at higher density, but they tended to be shorter compared to DJ123. As considerable variation exists for these traits, most means were not significantly different except for the higher lateral root growth rates of DJ123 at 2 and 3 weeks.
Effects of mycorrhizal fungi on the rice genotypes (glasshouse expt. 2)
To investigate the potential effects of the natural AMF community on genotype differences in P uptake and root efficiency, a glasshouse experiment was conducted in large 45 L containers filled with P-deficient field soil. This experiment produced plant physiological genotype differences comparable to the earlier field experiment. After very slow early development, plant growth and P uptake accelerated from 21 DAS onwards and a first tiller developed in DJ123 at 28 DAS, while tillering in Nerica4 was further delayed (data not shown). By 28 DAS, DJ123 produced roots with more than double the RSA compared to Nerica4 (Fig. 3a), and this was accompanied by an even more pronounced difference in P uptake (Fig. 3b). As a result, root efficiency was significantly higher in DJ123 at 28 and 35 DAS (Fig. 3c). Root length colonized by AMF was close to zero at 14 DAS and started to develop from 21 DAS; it increased markedly between 21 and 28 DAS, and at 28 and 35 DAS approximately 25–30% of root length was colonized by AMF in both rice genotypes (Fig. 3d).
Root surface area (a), total plant P uptake (b), root efficiency (c) and per cent root length colonized by arbuscular mycorrhizal fungi (d) of two rice genotypes over a 35-day growth period in 45-L containers filled with non-sterile P-deficient field soil. Small plots provide a zoom-in for the 14 and 21 day sampling points. Levels of significance of mean differences are indicated by *, **, ***, which refer to the 0.05, 0.01, and 0.001 levels of significance
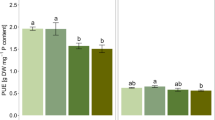
This experiment had two additional treatments, sterilized soil (sterile) and sterilized soil which had 15% (w/w) fresh field soil added (sterile+). Plants grown in both treatment soils were harvested at 28 DAS together with the non-sterile field soil (data presented in Fig. 3). No internal mycorrhizal colonization was detected in any of the root samples from plants grown on sterile soil, whereas root colonization was similar (25–30%) in the non-sterile field soil (non-s) and the sterile+ treatment (Fig. 4a). Plant P uptake was lowest in the fresh non-sterile field soil and highest in the sterile+ soil (Fig. 4b); in all soil treatments, DJ123 had significantly greater P uptake compared to Nerica4 (Fig. 4b). The P uptake of Nerica4 relative to DJ123 was lowest in the non-s soil (16%) and highest in sterile+ (36%). Root efficiency followed a similar pattern as P uptake and again the difference between genotypes was least pronounced in the sterile+ treatment (Fig. 4c).
Analysis of OsPT11 expression in root samples from different soil treatments aligned with the microscopic determination of mycorrhizal colonization: At 14 DAS, OsPT11 expression was largely absent (Fig. 5a), indicating that the symbiosis between rice roots and AMF had not reached a functional (i.e. P-transfer) stage. At 21 DAS, OsPT11 expression was detected, and expression levels had increased dramatically by 28 DAS (Fig. 5a), in line with the increase in % colonization (Fig. 4a). There were no significant genotypic differences in OsPT11 expression.
The expression of the constitutively expressed (direct pathway) P transporter gene OsPT2 was contrasting with OsPT11 expression. There was very strong genotypic difference whereby DJ123 had higher expression than Nerica4 in all the treatments (Fig. 5b), especially at the earliest sampling time point. In DJ123, the expression of OsPT2 decreased significantly with age of the plant (between 14 and 28 DAS), and was lowest at 28 DAS in the sterile+ treatment. A similar pattern was seen but to a lesser extent in Nerica4, as expression was already quite low in Nerica4 at 14 DAS.
Discussion
Physiological differences in root traits between genotypes contribute to improved P uptake efficiency in DJ123
Earlier studies conducted in Japan and Africa had identified DJ123 as being superior to the widely grown African upland variety Nerica4 in acquiring P from low-P soils (Koide et al. 2013; Mori et al. 2016a) and results from all three experiments conducted here confirmed the far greater P uptake of DJ123. The objective of this study was to determine to what extent superior P uptake in DJ123 was associated with root size vs root efficiency (P uptake per unit root size), or AMF colonization. Data from field and glasshouse experiments clearly demonstrate that root size effects were detectable at the seedling stage with DJ123 developing crown roots more rapidly, which resulted in a larger root surface area as early as 14 DAS. However, despite root size differences being very pronounced between DJ123 and Nerica4, they were not sufficiently large to account fully for the better P uptake in DJ123 and additional root efficiency effects were detected with DJ123 having between 35 and 400% higher P uptake per RSA. Similarly higher efficiencies were obtained when calculating P uptake per number of root tips (data not shown) and this was because the ratio of tip number per RSA was not significantly different between genotypes.
While root development appeared to be the more influential factor in the field, it needs to be kept in mind that P deficiency reduced root development in both genotypes (Table 1), and that maintaining root growth under P-limited conditions will depend on the amount of P taken up to sustain biomass accumulation. Using a modeling approach, Wissuwa (2003) showed that any increase in root efficiency would subsequently enhance root growth under P-limitation. As a result, the contribution of root efficiency is likely underestimated when root growth is not restricted by the soil volume to be explored, as in the large pots used here or under field conditions. Thus, rather than being able to properly quantify the contribution of each effect on P uptake, we conclude that the better adaptation to P deficiency in DJ123 is due to the presence of mechanisms leading to better root development and higher root efficiency, and potential underlying traits for both shall be discussed.
Traits potentially associated with better root development
Nerica4 is an upland rice variety and although the precise origin of DJ123 is unknown, it resembles an upland type rice. Neither of the genotypes have the very shallow root system typical of rice developed for irrigated conditions. DJ123 had a higher proportion of roots penetrating the plow layer but it is unlikely that these contributed proportionally to P uptake, as P acquisition was shown to be severely reduced from the sub-soil at this field site (Mori et al. 2016a), presumably due to very low P availability in sub-soil layers.
Investigating possible differences in root classes first and foremost shows more rapid development of crown roots in DJ123. Since all root development starts with the emergence of crown roots (apart from the single seminal root), from which both L-type and S-type roots develop, having roughly twice the number of crown roots can be considered one main cause for the superior P uptake of DJ123 under P deficiency. In the +P control treatment DJ123 had 50% higher crown root number compared to Nerica4 (data not shown) and one may thus assume that both constitutive and P-response factors explain genotypic differences in this important trait. Similar observations have been made for crown root development in lowland rice under Zn deficiency (Nanda and Wissuwa, 2016; Mori et al. 2016b) and while key crown rootless genes (crl1 and crl2) affecting crown root emergence have been identified (Inukai et al. 2001), genotypic differences in crown root number in the field were accompanied by similar expression levels of these genes among genotypes (Nanda et al. 2017).
In addition to the presence of more main root axes from which first or higher order lateral roots can develop, we detected small differences related to root fineness. A higher proportion of the total RSA was contributed by very fine S-type lateral roots in DJ123. S-type roots have only one layer of cortical cells (Rebouillat et al. 2009) and may thus represent an economical way to build a root system capable of exploring a large soil volume, both in terms of P and C required to build roots, as well as in terms of respiratory burden (Lynch and Ho 2005). Having a higher proportion of S-type roots should be the result of either higher S-type density or S-type length. DJ123 typically had around 10% higher density and length and while neither parameter alone reached significance, a multiplication effect should be expected and could explain the superior RSA of DJ123.
Such a multiplication effect would further increase if lateral roots including S-types were covered with root hairs at higher length or density. Nestler and Wissuwa (2016) evaluated both traits in a set of rice genotypes and detected a decrease in root hair density in Nerica4 under P deficiency, with the opposite trend seen in DJ123. With slightly longer root hairs DJ123 may thus possess a second multiplication effect that could substantially increase the root surface area in contact with soil and therefore involved in P uptake. Strictly speaking, root hairs therefore contribute to root size, however, due to the inability to quantify root hairs in root scans, their contribution to total RSA is never taken into account. Thus, the RSA used to calculate RE does not include SA contributed by root hairs, and any difference in root hair properties would have to be discussed as related to contributing to higher RE. Employing a root-soil modeling approach may represent the most efficient way to estimate the contributions of difficult to quantify root fine structures to P uptake.
Traits potentially associated with increased RE
A possible contributing factor for the higher P uptake per quantifiable RSA (RE) of DJ123 are the above-mentioned differences in root hair length and density between DJ123 and Nerica4. Nestler and Wissuwa (2016) estimated that minor increases in root hair length and density may increase total RSA by up to 30%. Since this increase is not quantifiable in root scans due to the small size of hairs, the 30% increase in RSA would lead to an over-estimation of RE by 30%. Estimates for RE differences between both genotypes obtained in field and greenhouse studies ranged between 35 and 400% and root hair differences are therefore likely not the only factor behind higher RE.
Other potential factors leading to higher RE may be related to root exudation; for example, Rakotoson (2014) demonstrated that P uptake of rice was several times larger than the P flux to roots due to mass flow and diffusion, and concluded that rice is able to mobilize large amounts of P through root-induced solubilization. Plant-induced changes in the rhizosphere that increase P availability to the root are typically thought to underly changes in RE, and two mechanisms discussed in the context of RE in rice are the release of organic acids (Kirk et al. 1999; Hoffland et al. 2006), and a modification of soil pH (Kirk et al. 1998). Organic acids such as citrate or malate are exuded from rice roots but quantities released are typically far lower than in other crops like lupin or barley (Tawaraya et al. 2013). At the same time, the total amount of carbon released from rice roots is substantial (Tawaraya et al. 2013) and it remains to be tested whether the effect of that release is related to an unspecific enhancement of soil microbes in the rhizosphere (due to exudation of sugars and amino acids), whether this enhancement is more specific to certain beneficial microbes, or whether some compounds released from rice can increase P solubility directly. Andosols as used here are characterized by a high proportion of the organic soil-P fraction, thus effects of phosphatases or phytases are of interest. However, recent data from Holz and Rakotoson (unpublished) indicated that rhizosphere phosphatase activity per root surface area was equal in Nerica4 and DJ123.
Mycorrhizal fungi colonized the rice genotypes and interacted with the soil microbiome to produce changes in plant P uptake
The association between rice roots and AMF may contribute another aspect of potential root efficiency; the mycorrhizal symbiosis works by increasing the volume of soil explored by fungal external hyphae for uptake of Pi and other inorganic nutrients. The soil used in the glasshouse studies contained AMF propagules that were able to colonize both rice genotypes to a reasonable degree (maximum 30% root length colonized) and consistently. The expression of OsPT11 in roots by 28 DAS further indicated a functional symbiosis and suggested that by that age, P transfer was taking place between plant and colonizing fungi in both genotypes (Karandashov and Bucher 2005). In general, the expression of the mycorrhiza-inducible PT indicates that the colonizing AMF were alive and functional at the time of harvest, while the microscope determination gives a more general indication of the extent of the symbiosis as it includes both live and dead fungus. It is worth noting that the decrease in OsPT2 expression in DJ123 was in line with the increase in OsPT11 expression over the course of the experiment; this pattern may be related to the interplay between the mycorrhizal and direct P uptake pathways, whereby OsPT11 expression compensates for a decrease in OsPT2 expression (Grønlund et al. 2013; Watts-Williams et al. 2015). However, the decrease in OsPT2 expression may also be constitutive, linked with the increasing age of the plant. Furthermore, the expression of OsPT2 was higher in DJ123 than Nerica4 in all treatments, and this could be linked to the greater P uptake phenotype in DJ123 whereby higher OsPT2 expression potentially lead to increased P uptake.
The glasshouse experiment where the rice genotypes were grown in sterilized soil lacking its natural microbiome including AMF, demonstrated that both genotypes were able to maintain P uptake and in the case of DJ123, had higher root efficiency compared to the non-sterilized ‘fresh’ soil. However, plant P uptake and root efficiency both increased further still when plants were grown in sterilized soil supplemented with some (15%) fresh soil. Therefore, we cannot discount soil microbiome effects on plant P uptake and RE based on the sterile soil treatment alone; although, it is difficult to separate direct AMF effects from the effects of the rest of the microbiome (discussed below). That genotypic differences in P uptake were consistent across the different soil sterilization treatments suggests that the superior RE of DJ123 is a genotype-specific factor related to root morphology and/or plant-induced rhizosphere factors.
The increase in plant P uptake and RE with the supplement of 15% fresh field soil demonstrates that there were soil microbiome effects on the physiology of the rice plants. In this supplemented soil treatment, the roots were colonized by AMF and the expression of OsPT11 was similar to that of the non-sterilized soil, which indicated that the symbiosis was functional. However, the effects of the supplemental soil on plant traits were dramatic; the efficiency of root P uptake increased by 30% in DJ123. In Nerica4, the positive effect of fresh soil supplementation was even more pronounced, as its RE more than doubled compared to the other soils. This could be due to more P being supplied by AMF in addition to P from roots; that is, a product of the combined scavenging efforts of AMF external hyphae and the roots.
Clearly the soil microbiome of the supplemented soil is functionally different to the non-sterilized soil. The P-uptake promotion effects of the supplemented sterile soil suggest that there are direct or indirect effects of the altered microbiome on the plants and more so in Nerica4. Directly, there may be a suppressive component (ie., a pathogenic microbe) to the fresh soil that impacts upon the ability for the rice genotypes to take up P (Ashizawa et al. 2010; Spence et al. 2014; Sesma and Osbourn 2004). When reduced to 15% (supplemented soil) or 0% (sterile soil) of the soil volume, the suppression is alleviated, particularly for Nerica4. Indirectly, there may have been soil microbes antagonistic to AMF in the fresh soil, which suppressed P uptake by the mycorrhizal pathway, as has been shown in a wide range of cultivated and natural soils previously (Svenningsen et al. 2018; Cruz-Paredes et al. 2019). Following on from this hypothesis, in the 15% fresh soil supplementation, the AMF were able to function in terms of colonization and OsPT11 expression as though the soil were 100% fresh, however the pathway activity may have been increased due to the elimination of a critical mass of AMF-suppressive microbes. In line with this hypothesis and our findings, Svenningsen et al. (2018) showed that 10–50% fresh suppressive soil could be added back to sterile soil without suppressive effects on AMF P uptake manifesting.
Conclusions and further work
We confirmed that popular African upland rice variety Nerica4 is inefficient in acquiring P from a soil with a low level of plant available P whereas genotype DJ123 is efficient. The higher ability to acquire P in DJ123 was independent of the presence/absence of AMF and must therefore be due to plant-specific traits. DJ123 showed high root vigor, producing a much larger root system that improved its ability to forage for P, and crucially, this was combined with a second main mechanism, being greater root efficiency. Having superior expression of two key complementary traits makes DJ123 a very likely donor to improve adaptation to P deficiency in rice breeding. The most pronounced superiority of DJ123 was in crown root number and the relative ease of phenotyping would allow for selections for this trait to be made in breeding programs. The relative difficulty is assessing root efficiency, which may be caused by a combination of having more extensive S-type development, longer root hairs (Nestler and Wissuwa, 2016) and more extensive rhizosphere modifications will likely mean selection for such traits is going to be indirect, through the choice of a selection environment where breeding lines with the desired trait will perform advantageously (Wissuwa et al. 2016).
To more efficiently exploit useful genetic variation for traits like S-type or root hair length and density it would be crucial to identify the underlying genetic variation and future studies should aim to do so. For RE the basic underlying question as to whether plant root activities affect pH changes or whether root exudation directly affects P availability or through modifications of an altered soil microbiome need to be addressed. It is noteworthy that Nerica4 appears to be more sensitive to changes in the soil microbiome and to what extent this is linked to positive mycorrhizal effects vs growth suppressing properties should be investigated further.
Abbreviations
- AMF:
-
Arbuscular mycorrhizal fungi
- DAE:
-
Days after emergence
- DAS:
-
Days after sowing
- RE:
-
Root efficiency
- RSA:
-
Root surface area
References
Ashizawa T, Takahashi M, Moriwaki J, Hirayae K (2010) Quantification of the rice false smut pathogen Ustilaginoidea virens from soil in Japan using real-time PCR. Eur J Plant Pathol 128:221–232
Atakora WK, Fosu M, Abebrese SO, Asanta M, Wissuwa M (2015) Evaluation of low phosphorus tolerance of rice varieties in northern Ghana. Sust Agric Res 4:109–114. https://doi.org/10.5539/sar.v4n4p109
Balasubramanian V, Sie M, Hijmans RJ, Otsuka K (2007) Increasing Rice production in sub-Saharan Africa: challenges and opportunities. Adv Agron 94:55–133
Chen XW, Wu FY, Li H, Chan WF, Wu C, Wu SC, Wong MH (2013) Phosphate transporters expression in rice (Oryza sativa L.) associated with arbuscular mycorrhizal fungi (AMF) colonization under different levels of arsenate stress. J Exp Bot 87:92-99. doi.org/https://doi.org/10.1016/j.envexpbot.2012.08.002
Cruz-Paredes C, Svenningsen NB, Nybroe O, Kjøller R, Frøslev TG, Jakobsen I (2019) Suppression of arbuscular mycorrhizal fungal activity in a diverse collection of non-cultivated soils. FEMS Microbiol Ecol 95. https://doi.org/10.1093/femsec/fiz020
Gahoonia TS, Nielsen NE (1998) Direct evidence on participation of root hairs in phosphorus (32P) uptake from soil. Plant Soil 198:147–152
Giovannetti M, Mosse B, (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84(3):489–500
Grønlund M, Albrechtsen M, Johansen IE, Hammer EC, Nielsen TH, Jakobsen I (2013) The interplay between P uptake pathways in mycorrhizal peas: a combined physiological and gene-silencing approach. Physiol Plantarum 149:234–248
Gutjahr C, Banba M, Croset V, An K, Miyao A, An G, Hirochika H, Imaizumi-Anraku H, Paszkowski U (2008) Arbuscular mycorrhiza–specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell 20. https://doi.org/10.1105/tpc.108.062414
Hoffland E, Wei C, Wissuwa M (2006) Organic anion exudation by rice (Oryza sativa L.) at zinc and phosphorus deficiency. Plant Soil 283:155–162
Inukai Y, Miwa M, Nagato Y, Kitano H, Yamauchi A (2001) Characterization of rice mutants deficient in the formation of crown roots. Breeding Sci 51:123–129
Karandashov V, Bucher M (2005) Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci 10:22–29
Kirk GJD, George T, Courtois B, Senadhira D (1998) Opportunities to improve phosphorus efficiency and soil fertility in rainfed lowland and upland rice ecosystems. Field Crop Res 56:73–92
Kirk GJD, Santos EE, Findenegg GR (1999) Phosphate solubilization by organic anion excretion from rice (Oryza sativa L.) growing in aerobic soil. Plant Soil 211:11–18
Koide Y, Pariasca Tanaka J, Rose T, Fukuo A, Konisho K, Yanagihara S, Fukuta Y, Wissuwa M (2013) QTLs for phosphorus-deficiency tolerance detected in upland NERICA varieties. Plant Breed. https://doi.org/10.1111/pbr.12052
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156:1041–1049
Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269:45–56
Maiti D, Variar M, Singh RK (2011) Optimizing tillage schedule for maintaining activity of the arbuscular mycorrhizal fungal population in a rainfed upland rice (Oryza sativa L.) agro-ecosystem. Mycorrhiza 21(3):167–171
Mbodj D, Effa-Effa B, Kane A, Manneh B, Gantet P, Laplaze L, Diedhiou AG, Grondin A (2018) Arbuscular mycorrhizal symbiosis in rice: establishment, environmental control and impact on plant growth and resistance to abiotic stresses. Rhizosphere 8:12–26
Mori A, Fukuda T, Vejchasarn P, Nestler J, Pariasca-Tanaka J, Wissuwa M (2016a) The role of root size versus root efficiency in phosphorus (P) acquisition of rice. J Exp Bot. https://doi.org/10.1093/jxb/erv557
Mori A, Kirk GJD, Lee JS, Morete MJ, Nanda AK, Johnson-Beebout SE, Wissuwa M (2016b) Rice genotype differences in tolerance of zinc-deficient soils: evidence for the importance of root-induced changes in the rhizosphere. Front Plant Sci 6. https://doi.org/10.3389/fpls.2015.01160
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nanda AK, Wissuwa M (2016) Rapid crown root development confers tolerance to zinc deficiency in rice. Front Plant Sci 7. https://doi.org/10.3389/fpls.2016.00428
Nanda AK, Puyol V, Wissuwa M (2017) Patterns of stress response and tolerance based on transcriptome profiling of rice crown tissue under Zn deficiency. J Exp Bot. https://doi.org/10.1093/jxb/erx039
Nestler J, Wissuwa M (2016) Superior root hair formation confers root efficiency in some, but not all, rice (Oryza sativa) genotypes upon P deficiency. Front Plant Sci 7:1935. https://doi.org/10.3389/fpls.2016.01935
Nestler J, Keyes SD, Wissuwa M (2016) Artificial growth conditions alter root hair formation on rice (Oryza sativa L.) root types. J Exp Bot 67:3699–3708. https://doi.org/10.1093/jxb/erw115
Paszkowski U, Kroken S, Roux C, Briggs SP (2002) Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A 99:13324–13329
Rakotoson T (2014) Overcoming phosphate deficiency in flooded rice in Madagascar. Ph.D. dissertation, KU Leuven
Rebouillat A, Dievart A, Verdeil JL, Escoute J, Giese G, Breitler JC, Gantet P, Espeout S, Guiderdoni E, Périn C (2009) Molecular genetic of rice root development. Rice 2:15–34
Ruiz-Sánchez M, Aroca R, Muñoz Y, Polón R, Ruiz-Lozano JM (2010) The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J Plant Physiol 167:862–869
Ruíz-Sánchez M, Armada E, Muñoz Y, García de Salamone IE, Aroca R, Ruíz-Lozano JM, Azcón R (2011) Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J Plant Physiol 168:1031–1037
Saito K, Asai H, Zhao D, Laborte AG, Greiner C (2018) Progress in varietal improvement for increasing upland rice productivity in the tropics. Plant Prod Sci 21:145–158
Saito K, Vandamme E, Johnson JM et al (2019) Yield-limiting macronutrients for rice in sub-Saharan Africa. Geoderma 338:546–554
Sesma A, Osbourn AE (2004) The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 431:582–586
Spence C, Alff E, Johnson C, Ramos C, Donofrio N, Sundaresan V, Bais H (2014) Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol 14:130
Svenningsen NB, Watts-Williams SJ, Joner EJ, Battini F, Efthymiou A, Cruz-Paredes C, Nybroe O, Jakobsen I (2018) Suppression of the activity of arbuscular mycorrhizal fungi by the soil microbiota. ISME J 12:1296–1307. https://doi.org/10.1038/s41396-018-0059-3
Tawaraya K, Horie R, Saito A, Shinano T, Wagatsuma T, Saito K, Oikawa A (2013) Metabolite profiling of shoot extracts, root extracts, and root exudates of rice plant under phosphorus deficiency. J Plant Nutr 36:1138–1159
Vandamme E, Wissuwa M, Rose TJ, Dieng I, Drame KN, Fofana M, Senthilkumar K, Venuprasad R, Jallow D, Segda Z, Suriyagoda L, Sirisena D, Kato Y, Saito K (2016) Genotypic variation in grain P loading across diverse rice growing environments and implications for field P balances. Front Plant Sci 7:1435. https://doi.org/10.3389/fpls.2016.01435
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and Vinegar, a Simple Staining Technique for Arbuscular-Mycorrhizal Fungi. Appl Environ Microbiol 64(12):5004–5007
Watts-Williams SJ, Jakobsen I, Cavagnaro TR, Grønlund M (2015) Local and distal effects of arbuscular mycorrhizal colonization on direct pathway Pi uptake and root growth in Medicago truncatula. J Exp Bot 66:4061–4073
Wissuwa M (2003) How do plants achieve tolerance to phosphorus deficiency - small causes with big effects. Plant Physiol 133:1947–1958
Wissuwa M, Ae N (2001) Genotypic variation for tolerance to phosphorus deficiency in Rice and the potential for its exploitation in Rice improvement. Plant Breed 120:43–48
Wissuwa M, Kretzschmar T, Rose TJ (2016) From promise to application: root traits for enhanced nutrient capture in rice breeding. J Exp Bot 67:3605–3615
Yamauchi A, Kono Y, Tatsumi J (1987) Quantitative analysis on root system structures of upland rice and maize. JPN J Crop Sci 56:608–617
Yang S-Y, Grønlund M, Jakobsen I, Grotemeyer MS, Rentsch D, Miyao A, Hirochika H, Kumar CS, Sundaresan V, Salamin N, Catausan S, Mattes N, Heuer S, Paszkowski U (2012) Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the PHOSPHATE TRANSPORTER1 gene family. Plant Cell 24:4236–4251
Acknowledgements
Authors acknowledge the excellent technical assistance of Taro Matsuda in the qPCR analysis. Scans of root images used for the measurements of root properties shown in Table 3 were provided by James DM King. Financial support in the form of a CONACYT – I2T2 scholarship to DG is acknowledged. SJWW acknowledges the University of Adelaide Ramsay Fellowship for support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Andrea Schnepf.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlight
Efficient P uptake by rice is explained by genotypic differences in root traits and rhizosphere effects, even in the presence of an active mycorrhizal symbiosis
Rights and permissions
About this article
Cite this article
Wissuwa, M., Gonzalez, D. & Watts-Williams, S.J. The contribution of plant traits and soil microbes to phosphorus uptake from low-phosphorus soil in upland rice varieties. Plant Soil 448, 523–537 (2020). https://doi.org/10.1007/s11104-020-04453-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04453-z