Abstract
Aims
Iron (Fe) is an essential micronutrient, and plant available Fe is often limited in alkaline soils. Fe deficiency chlorosis decreases plant growth and yield. Identification of germplasm with high and low Fe use efficiency will allow studies to better understand the genetic components for breeding Fe efficient varieties.
Methods
A screen using cucumber (Cucumis sativus) seedlings identified varieties that maintained contrasting levels of chlorophyll under Fe deficiency or limitation. A time course of mineral dynamics in cotyledons was conducted.
Results
The variety Ashley had the highest chlorophyll under Fe deficiency and per unit Fe in the leaf, while the variety Miniature White had the lowest. Ashley also maintained higher chlorophyll when challenged with low Fe or bicarbonate, accumulated greater quantities of Fe, and had higher root ferric reductase activity. Cotyledons accumulated minerals for the first several days, then Fe, P, K, and Cu were remobilized. The Fe use efficient and inefficient varieties remobilized Fe and P on different timescales.
Conclusions
Our results suggest that this screen can identify varieties for systems level studies that could elucidate factors needed for Fe use efficiency and remobilization of minerals. The time course indicated that cotyledon Fe stores did not contribute to seedling Fe use efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron (Fe) is an essential plant and human nutrient that is required for a number of crucial biochemical processes and enzymes for numerous redox reactions. The majority of humans depend on plants as their primary source of Fe and other mineral elements. Thus, understanding how to improve the Fe density of plants is an important goal. Uptake of Fe from the rhizosphere is a prerequisite step that must occur before other processes that lead to distribution to other plant parts, such as leaves or seeds. A substantial percentage of seed Fe can be supplied from previously taken up pools that are remobilized from leaves or other vegetative tissues (Waters and Grusak 2008; Waters et al. 2009). Other mineral elements can also be remobilized from vegetative or seed covering tissues (Garnett and Graham 2005; Gregersen et al. 2008; Masclaux-Daubresse et al. 2010; Naeve and Shibles 2005; Sunarpi and Anderson 1996; Waters and Sankaran 2011). Likewise, cotyledons and endosperm tissues can be important sources of remobilized minerals for developing seedling growth, such as S (Sunarpi and Anderson 1995), Zn (Sudia and Green 1972), Fe (Ambler and Brown 1974; Tiffin et al. 1973), N and P (Bewley and Black 1985; Dalling and Bhalla 1984; Mayer and Poljakoff-Mayber 1982). An increased understanding of genes involved in remobilization from seed reserves or vegetative tissues could lead to strategies to increase internal Fe use efficiency.
Availability of Fe is low in alkaline soils, which make up approximately 30% of the earth’s soils (Chen and Barak 1982). Many alkaline soils also have high bicarbonate concentration (Mengel 1994; Rogovska et al. 2007), which can inhibit Fe uptake (Lucena et al. 2007; Mengel 1994). Leaf chlorosis is a classical Fe deficiency sign, and negatively impacts yield in certain crops, such as soybean in the upper Midwest USA (O'Rourke et al. 2007; Wang et al. 2008). Under low Fe availability conditions, plants increase Fe uptake gene expression and protein activity (Morrissey and Guerinot 2009), which in dicot species includes ferric reductases (Jeong and Connolly 2009), iron transporters (Vert et al. 2002), and H+-ATPases (Santi and Schmidt 2009).
Cucumber (Cucumis sativus) has been used for studying Fe deficiency responses for a number of years (Alcantara et al. 1991; Rabotti and Zocchi 1995; Romera and Alcántara 1994; Romera et al. 1992; Zocchi and Cocucci 1990), including interactions between Fe deficiency and ethylene (Lucena et al. 2006; Romera and Alcántara 1994, 2004; Romera et al. 1999; Waters et al. 2007), and bicarbonate (Lucena et al. 2007). The primary iron uptake response genes for ferric reductase FRO1, iron transporter IRT1 (Waters et al. 2007), and H+-ATPase HA1 (Santi et al. 2005) have been identified from cucumber. More recently, the sequencing of the cucumber genome (Huang et al. 2009) has opened the possibility for additional genomic scale studies such as proteomic responses of roots to Fe deficiency (Donnini et al. 2010; Li and Schmidt 2010). The rapid growth and relatively large size of cucumber organs offers advantages over Arabidopsis thaliana for certain lines of inquiry.
In this work, we utilized a natural variation strategy to identify two cucumber varieties with substantial differences in Fe use efficiency and accumulation. Our objective was to characterize differences in uptake, partitioning, and remobilization of Fe to provide the basis for genomic scale studies to understand the underlying genetic differences between these two varieties. Using a developmental time course of mineral content in cotyledons, we have shown that cucumber cotyledons do not have adequate Fe stores for seedling development, but begin accumulating Fe in the first days after germination.
Materials and methods
Cucumber seeds of non-hybrid varieties were obtained from commercial suppliers. All seeds of a variety were from a single supplier and production lot. The varieties and sources were Ashley, Chicago Pickling, SMR-58, Bush Pickle, Marketer, Homemade Pickles, True Lemon (Jung Seeds Co., Randolph, Wisconsin, USA), H-19 Little Leaf, Miniature White (Johnny’s Selected Seeds, Winslow, Maine, USA), Marketmore 76, Lemon, and Straight Eight (Burpee & Co., Warminster, Pennsylvania, USA).
Seeds were imbibed in germination paper soaked with 0.2 mM CaSO4 for 5 days, then held by foam plugs in lids of hydroponic containers, 4 plants per container. The containers held 750 ml of a nutrient solution of the following composition: 0.8 mM KNO3, 0.4 mM Ca(NO3)2, 0.3 mM NH4H2PO4, 0.2 mM MgSO4, 25 μM CaCl2, 25 μM H3BO3, 2 μM MnCl2, 2 μM ZnSO4, 0.5 μM CuSO4, 0.5 μM Na2MoO4 and 1 mM MES buffer (pH 5.5). The + Fe solution contained 1 or 10 μM Fe(III)-EDDHA (Sprint 138, Becker-Underwood, Ames, IA, USA); Fe was omitted from the -Fe solution. For treatments with bicarbonate, the solution was 10 mM KCl, 2.0 mM Ca(NO3)2, 0.3 mM NH4H2PO4, 1.5 mM MgSO4, 25 μM CaCl2, 25 μM H3BO3, 2 μM MnCl2, 2 μM ZnSO4, 0.5 μM CuSO4, 0.5 μM Na2MoO4 and 1 mM HEPES buffer (pH 7.0), with 10 mM KHCO3. Plants were grown in a growth chamber with lighting provided by a mixture of incandescent and fluorescent sources at 250 μmol m−2 s−1 for a photoperiod of 16 h (on at 06:00 and off at 22:00).
For the variety screen, plants of each variety were grown for 4 days in complete solution (including 10 μM Fe(III)EDDHA), then transferred to complete or -Fe solution for 7 days, after which chlorophyll concentrations were determined by a handheld SPAD meter (Minolta, Japan). The first true leaf was collected for mineral analysis.
For bicarbonate experiments, Ashley and Miniature White seedlings were grown for 4 days in complete solutions, then transferred to 1 μM or 10 μM Fe solution with or without bicarbonate for 8 days, after which SPAD and root ferric reductase activity measurements were taken prior to collection of cotyledons and leaves for mineral analysis. For the time course experiment, Ashley and Miniature White seedlings were grown for 8 days in complete (10 μM) solution, then transferred to complete or -Fe solution until 30 days after planting, with solutions changed every 2–3 days. At 0, 4, 8, 12, 16, 19, 23, and 26 days after planting, SPAD measurements were taken on leaves of 6 randomly selected plants from + Fe and -Fe treatments, with an additional + Fe sampling at 30 days. Cotyledons were collected at each time point from 5 harvested plants, and leaves were collected at 23 days.
Ferric reductase activity was measured on individual roots, using 25 ml of an assay solution of 0.1 mM ferrozine (Sigma-Aldrich, St. Louis, Missouri, USA), 0.1 mM Fe(III)-EDTA and 5 mM of MES buffer (pH 5.5) (Fisher Scientific, Fair Lawn, New Jersey, USA). Reduced Fe was calculated using absorbance at 562 nm with the extinction coefficient of 28.6 mM−1 cm−1.
For mineral analysis, plant tissues were dried at 60°C in a drying oven for at least 48 h, weighed, and digested in concentrated nitric acid/hydrogen peroxide with stepwise heating for 1–2 h at 100, 125, 150, then 160°C to dryness. Residues were resuspended in 1% HNO3. For the variety screen and bicarbonate experiments, Fe concentrations were determined using the 2,2’-bipyridyl method. In brief, a fresh working solution containing 1 ml of 1.2 M L-ascorbic acid, 4 ml of 12.8 mM 2,2’-bipyridyl and 10 mL of 1 M acetate buffer (pH 5.0), was prepared and 150 μl of the working solution was combined with 50 μl of sample in a 96-well microplate. The concentration of Fe was calculated by absorbance at 520 nm after calibration using a standard curve. The method was validated by ICP-MS. For the time course experiments, Fe and other mineral concentrations were determined by digestions as described above followed by ICP-MS. Mineral contents were determined by multiplying mineral concentrations by tissue DW. For the cotyledon time course, averaged tissue DW was calculated for two growth phases (0–12 days and 12–30 days) by fitting a linear regression to the observed data (Online Resource 1).
Results
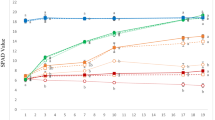
We determined Fe use efficiency by two criteria, comparison of chlorophyll at Fe replete (10 μM) and deficient conditions (Fig. 1a), and by chlorophyll density per Fe density (Fig. 1b). There was striking natural variation in Fe efficiency, which in this case we define as the ability to maintain chlorophyll under low Fe or Fe deficiency. At the extremes were Ashley, which under Fe deficiency had 72% as much chlorophyll as when Fe replete, and Miniature White (MW), which under Fe deficiency had only 28% the chlorophyll level as when Fe replete (Fig. 1a). Under Fe replete conditions, Fe concentration and SPAD values were positively correlated (Fig. 1b). Ashley was among the darkest green varieties and had the highest Fe concentration under Fe replete conditions, with MW among the lowest for both of these values. Under Fe deficiency, there was a narrow range of Fe concentrations in the leaves, but a wide range of SPAD values, with Ashley maintaining the highest chlorophyll and MW having the lowest chlorophyll. Thus, in our screen we determined that Ashley and MW were the varieties with the greatest diversity in Fe efficiency, and these lines were selected for further study.
Screen for Fe use efficiency. a Chlorophyll levels of first true leaf as measured by Minolta SPAD chlorophyll meter in + Fe and -Fe grown cucumber plants of varieties indicated. Values ± s.e. b Correlations of first leaf Fe concentration with first leaf chlorophyll SPAD value for plants grown in + Fe or -Fe media. Correlation coefficients (R2) for linear curves are shown. Points representing Ashley are indicated by A, points representing Miniature White are indicated by MW. Values ± s.e
We then tested whether the differences in Fe efficiency would be present under Fe challenged (not Fe deficient) conditions. In preliminary experiments, we determined that a treatment of 1 μM FeEDDHA was sufficient to increase root ferric reductase activity relative to 10 μM Fe without greatly decreasing leaf chlorophyll levels. Under low Fe treatment or in the presence of bicarbonate for 7 days, Ashley had consistently higher chlorophyll levels in leaves (Fig. 2). Both varieties had higher root ferric reductase activity at low Fe than at replete Fe, with similar activity levels (Fig. 3). However, when challenged with bicarbonate, Ashley had consistently higher ferric reductase activity than MW. Ashley also maintained higher Fe concentration in cotyledons (Fig. 4a) and leaves (Fig. 4b) under low and normal Fe, and slightly higher Fe concentration under low Fe with bicarbonate. These higher concentrations translated into higher overall accumulation, implying a higher uptake rate, as Ashley had a higher Fe content under all treatments (Fig. 4c). Most of the difference between the two lines could be accounted for in the cotyledons.
Ashley and Miniature White (MW) chlorophyll under control and Fe limitation. (a) Photograph of representative cucumber plants after 7 days in complete (10 μM Fe), 1 μM Fe, 1 μM Fe + 10 mM bicarbonate (bic), or 10 μM Fe + bicarbonate solution. (b) Quantification of chlorophyll in first leaf of plants grown as in (a). Values represent combined data from 3 experiments (n = 12) ± s.e. asterisk indicates statistical significance (P < 0.05)
Ashley and Miniature White (MW) leaf and cotyledon Fe concentration and content. (a) Cotyledon Fe concentration after 7 days in complete (10 μM Fe), 1 μM Fe, 1 μM Fe + 10 mM bicarbonate (BIC), or 10 μM Fe + bicarbonate solution. (b) Leaf Fe concentration after 7 days growth on treatments as described in (a). c Total Fe content in cotyledons and first leaf after 7 days growth on treatments as described in (a). asterisk indicates statistical significance (P < 0.05) between varieties. In panel (c) lower asterisk is for cotyledons, upper asterisk is for leaf
Based on these results, we hypothesized that Ashley may be more Fe efficient in Fe acquisition from the nutrient solution because it accumulates greater quantities of Fe into cotyledons that could then be utilized as sources for the growing leaves. To test this, we carried out a time course over the first month of seedling growth to quantify leaf chlorophyll and changes in Fe and other minerals in cotyledons of Ashley and MW. In seedlings grown on 10 μM Fe for the first 8 days, then transferred to complete or -Fe solution, leaves of both lines had decreased chlorophyll relative to + Fe plants after 8 days on -Fe treatments, and at later time points MW became substantially more chlorotic than Ashley (Table 1). For example, at 23 days, -Fe Ashley leaf 4 had 28% of the chlorophyll concentration of + Fe leaf 4, while -Fe MW leaf 4 had only 7% the chlorophyll of + Fe MW leaf 4. The mineral concentrations of leaves of Ashley and MW were determined for samples from 23 days (Fig. 5). Ashley had higher leaf Fe than MW in the + Fe treatment, with both lines having similar low concentrations at -Fe, consistent with the initial variety screen (Fig. 1). Leaf S and Ca concentrations were similar between lines and were not greatly changed by Fe treatment. Leaf Mg, P, and Mn concentrations were also similar between lines, and increased in leaves of -Fe plants. Leaf K was higher in MW in complete solution, but increased in both lines under -Fe. Zinc increased more in MW than in Ashley in the -Fe treatment. Most strikingly, Cu increased by 2.1-fold in Ashley under -Fe, but by 3.5-fold in Fe deficient MW.
Cotyledons grew rapidly for the first 12 days, then reached a plateau (Online Resource 1). Fe deficiency did not affect DW of the cotyledons. The average DW was multiplied by concentration of each mineral in cotyledons at each time point to calculate mineral content over the time course. All minerals rapidly increased in content over the first several days (Fig. 6, Online Resource 2). Differences in remobilization of four minerals (P, Fe, K, and Cu) were seen between the two varieties. Phosphorus increased in + Fe Ashley at most time points, before decreasing at the last time point; in contrast, P increased in both treatments of MW until 12 days, after which it decreased at each time point (Fig. 6a), indicating remobilization. However, in the -Fe treatment of Ashley, P decreased from 16 days onwards. Potassium exhibited a similar pattern of accumulation in both lines, followed by decreasing content for the final three time points. Fe deficient MW began remobilizing K sooner, after 12 days onwards, while K fluctuated in -Fe Ashley cotyledons (Fig. 6b). Copper increased in cotyledons of both lines until 12 days, after which it rapidly decreased for the next two time points before leveling off. Ashley -Fe cotyledons had similar Cu to the + Fe. MW Cu decreased at similar rates in both treatments until 19 days, after which Cu increased and remained at levels substantially higher than those of + Fe MW (Fig. 6c). In + Fe Ashley, Fe content increased rapidly through 12 days, then more slowly before leveling off and decreasing at the last time point, however, dynamics of Fe in + Fe MW was similar to those of P, and remobilization began at 16 days. After removal of Fe from the nutrient solution, Ashley maintained similar Fe content throughout the remainder of the experiment, while MW had a slight decrease in content at each following time point. Other mineral contents (Mg, Ca, Zn, Mn, S) did not show clear remobilization, and except for S there were no differences in content between the lines or Fe treatments (Online Resource 2).
Mineral content over time in cotyledons of Ashley and Miniature White (MW). Plants were grown until 8 days on + Fe solution then changed to + Fe or -Fe solution. Contents were calculated by multiplying average mineral concentration (n = 6) by average mineral DW. a Cotyledon P content time course over seedling development. b Cotyledon K content time course over seedling development. c Cotyledon Cu content time course over seedling development. d Cotyledon Fe content time course over seedling development
Discussion
Screening natural variation in traits, such as mineral homeostasis, among varieties of a species of plant can allow identification of extreme phenotypes that can used to understand differences in physiology or that can be associated with the underlying genetic composition. We have used a simple screen to identify two cucumber varieties with greatly contrasting efficiency for utilization and accumulation of Fe. Borrowing from the concepts of nitrogen use efficiency (NUE) (Hirel et al. 2007; Masclaux-Daubresse et al. 2010; Moll et al. 1982), similar methods of calculation can be used to estimate relative Fe use efficiency. Two measures of NUE for grain, e.g. wheat, are to compare the amount of protein produced per unit N available to the plant (low N vs. high N), or per unit N accumulated in the plant (N content). In our case, instead of protein, we can consider the chlorophyll level at normal and low Fe, and the chlorophyll level per unit Fe in a leaf. By both of these measures, Ashley was the most Fe use efficient variety in our screen, having the highest chlorophyll at both -Fe and normal Fe supply, while MW had among the lowest chlorophyll in + Fe and the lowest chlorophyll levels in -Fe (Fig. 1). The differences in Fe use efficiency between Ashley and MW were also present when plants were Fe challenged by low (1 μM) Fe and with bicarbonate (Fig. 2). Similarly, during the time course experiment, MW became substantially more chlorotic than Ashley when Fe was withheld (Table 1). Another measure of NUE is N uptake efficiency, which is defined as N content relative to N supply. If this concept is used for Fe use efficiency, Ashley is more Fe uptake efficient than MW, as Ashley had a higher Fe content at 1 μM Fe, 10 μM Fe, and 1 μM Fe + bicarbonate (Fig. 3). Although root ferric reductase was typically higher in Fe challenged Ashley roots than MW, it was not higher by a large margin, and under normal Fe supply, ferric reductase activity was at equal, baseline levels in both varieties, while under this treatment Fe accumulation differences were the greatest (Fig. 4). This suggests that ferric reductase activity cannot account for the entire difference in Fe uptake efficiency, and other factors that control Fe uptake and accumulation must also be different. Thus, future studies of these varieties may elucidate key genes to target for crop improvement of Fe use efficiency. Additionally, this result suggests that screens for Fe efficient varieties that rely on root ferric reductase activity (Gogorcena et al. 2004) may not identify the most Fe efficient varieties.
We had hypothesized that part of the difference in Fe efficiency between these varieties might result from more efficient utilization of cotyledon Fe in Ashley as compared to MW. Previous results from soybean indicated that 59Fe in cotyledons of seeds (that were radiolabeled during seed development) declined rapidly over the first 18 days of seedling growth when grown without Fe (Tiffin et al. 1973), and also declined to a lesser extent when grown with 10 μM Fe. However, additional Fe was taken up into cotyledons, resulting in an increase of 1.5 (Ambler and Brown 1974) to 2-fold (Tiffin et al. 1973) in total. Our results in cucumber were quite different, in that we observed that during early seedling growth (0–12 days), both Ashley and MW cotyledons accumulated approximately 10-fold the starting amount of Fe. When Fe was withheld beginning at 8 days, Ashley cotyledons remained at a similar content for the rest of the experiment, while Fe content in MW cotyledons declined only slightly (Fig. 6). As such, differences in cotyledon Fe pool utilization between Ashley and MW cannot explain the differences in Fe use efficiency between these varieties. On the other hand, our results indicated that + Fe Ashley accumulated much greater amounts of Fe and did not remobilize this Fe until late in the time course, while MW accumulated much less Fe and that it began to be remobilized after 16 days of treatment. This difference indicates a different Fe partitioning strategy between these varieties and offers a potential to compare these lines to discover the underlying molecular genetic mechanisms.
There are fundamental differences in cucumber and soybean cotyledon composition and growth that may explain why soybean cotyledons remobilized Fe differently than cucumber. Cucumber cotyledons initially had only very small amounts of Fe (2.3 μg and 1.1 μg per pair for Ashley and MW, respectively), while soybean had 4–14 μg per cotyledon (Ambler and Brown 1974; Tiffin et al. 1973), and enough Fe was present in soybean seeds to support seedling growth to the first trifoliate leaf (Ambler and Brown 1974). Another difference is that while cucumber cotyledons rapidly grew and increased in DW (Online Resource 1), soybean cotyledons had their greatest mass initially and decreased in size at each time point (Tiffin et al. 1973). Similarly, soybean cotyledons remobilized S (Sunarpi and Anderson 1995) and Zn (Sudia and Green 1972), whereas cucumber cotyledons did not. Thus, it appears that there are key differences between soybean and cucumber that may apply to early seedling growth of other crop species, and continued study of both species will contribute to understanding of roles of cotyledon in Fe supply.
The regulators of senescence processes and senescence related genes and proteins are of intense interest (Buchanan-Wollaston et al. 2003; Guo and Gan 2005; Lim and Nam 2005; Lim et al. 2003; Munne-Bosch and Alegre 2004; Van der Graaff et al. 2006; Yoshida 2003). One of the key features of leaf senescence is remobilization of nutrients. Cucumber cotyledons have previously been used as a model system for studying senescence processes (Becker et al. 1978; Delorme et al. 2000; Graham et al. 1992; Kim and Smith 1994; McLaughlin and Smith 1995; Prakash et al. 2001; Yamauchi et al. 2002). Major nutrients such as lipids and total protein were shown to decrease over the first week of germination (Becker et al. 1978). In this work we have shown that certain mineral nutrients (Fe, Cu, P, and K) are also remobilized from cucumber cotyledons, but after several days of accumulation rather than from stored sources (Fig. 6). Nitrogen deficiency stress is known to result in premature senescence (Crafts-Brandner et al. 1998). Fe deficiency may also accelerate senescence (Sperotto et al. 2008, 2007). In our experiments, K was remobilized earlier time points from MW, and P remobilization occurred at earlier time points in Ashley when Fe was withheld. Fe deficiency did not have a noticeable effect on remobilization of Cu at 12–19 days, but MW cotyledons increased in Cu at later time points. This may be because of increased Cu uptake, which was also reflected in leaf Cu concentration (Fig. 5). Fe deficient plants have previously been shown to take up additional Cu (Chaignon et al. 2002; Delhaize et al. 1993; Suzuki et al. 2006; Welch et al. 1993), as has the Arabidopsis ysl1ysl3 mutant, which has phenotypic similarities to Fe deficient plants (Waters et al. 2006; Waters and Grusak 2008).
In recent years, much senescence related research has been carried out in Arabidopsis thaliana (Ay et al. 2009; Balazadeh et al. 2010; Breeze et al. 2011; Evans et al. 2010; Keech et al. 2010; Miao and Zentgraf 2010; Wagstaff et al. 2009; Zhou et al. 2011), in part because of well-developed resources such as genome and cDNA sequences and microarray capabilities. Several mineral nutrients (Cu, Fe, K, N, P, S, and Zn) had decreased concentration (Himelblau and Amasino 2001) and/or content (Waters and Grusak 2008) in Arabidopsis leaves as they aged and senesced. Systems approaches have described a number of genes that have altered expression during the senescence stage of development (Breeze et al. 2011; Buchanan-Wollaston et al. 2005; Liu et al. 2011; Van der Graaff et al. 2006), some of which are likely to be crucial for mineral remobilization from leaves or cotyledons. However, simultaneous transcriptional profiling and nutrient remobilization studies have not been conducted to tightly correlate these events. Cucumber cotyledons offer a potentially ideal model system for this type of experiment, since one cotyledon leaf of a plant could be subjected to mineral analysis while the second one is harvested for RNA isolation. ESTs from senescing cucumber cotyledons have been described (Kim 2004), and with the completion of the cucumber genome (Huang et al. 2009) genome-wide transcriptional profiling experiments are now feasible.
In conclusion, our simple screen for Fe use efficiency has allowed us to identify cucumber varieties with distinct differences in internal use and uptake efficiency of Fe. Within cucumber, Ashley and MW had differences in Fe and P remobilization, suggesting that cucumber could be an excellent model for systems level studies of remobilization of these minerals during senescence.
References
Alcantara E, de la Guardia MD, Romero FJ (1991) Plasmalemma redox activity and H+ extrusion in roots of Fe-deficient cucumber plants. Plant Physiol 96:1034–1037
Ambler JE, Brown JC (1974) Iron supply in soybean seedlings. Agron J 66:476–478
Ay N, Irmler K, Fischer A, Uhlemann R, Reuter G, Humbeck K (2009) Epigenetic programming via histone methylation at WRKY53 controls leaf senescence in Arabidopsis thaliana. Plant J 58:333–346
Balazadeh S, Siddiqui H, Allu AD, Matallana-Ramirez LP, Caldana C, Mehrnia M, Zanor MI, Kohler B, Mueller-Roeber B (2010) A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J 62:250–264
Becker WM, Leaver CJ, Weir EM, Riezman H (1978) Regulation of glyoxysomal enzymes during germination of cucumber. 1. Developmental-changes in cotyledonary protein, RNA, and enzyme activities during germination. Plant Physiol 62:542–549
Bewley JD, Black M (1985) Mobilization of stored seed reserves. In: Seeds: physiology and development of germination. Plenum Press, New York, pp 253–303
Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C, Kiddle S, Kim YS, Penfold CA, Jenkins D, Zhang CJ, Morris K, Jenner C, Jackson S, Thomas B, Tabrett A, Legaie R, Moore JD, Wild DL, Ott S, Rand D, Beynon J, Denby K, Mead A, Buchanan-Wollaston V (2011) High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 23:873–894
Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D (2003) The molecular analysis of leaf senescence—a genomics approach. Plant Biotechnol J 1:3–22
Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, Leaver CJ (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J 42:567–585
Chaignon V, Di Malta D, Hinsinger P (2002) Fe-deficiency increases Cu acquisition by wheat cropped in a Cu-contaminated vineyard soil. New Phytol 154:121–130
Chen Y, Barak P (1982) Iron nutrition of plants in calcareous soils. Adv Agron 35:217–240
Crafts-Brandner SJ, Holzer R, Feller U (1998) Influence of nitrogen deficiency on senescence and the amounts of RNA and proteins in wheat leaves. Physiol Plant 102:192–200
Dalling MJ, Bhalla PL (1984) Mobilization of nitrogen and phosphorus from endosperm. In: Seed physiology. Academic Press Australia, North Ryde, NSW pp 163–199
Delhaize E, Randall PJ, Wallace PA, Pinkerton A (1993) Screening Arabidopsis for mutants in mineral nutrition. Plant Soil 155(156):131–134
Delorme VGR, McCabe PF, Kim DJ, Leaver CJ (2000) A matrix metalloproteinase gene is expressed at the boundary of senescence and programmed cell death in cucumber. Plant Physiol 123:917–927
Donnini S, Prinsi B, Negri AS, Vigani G, Espen L, Zocchi G (2010) Proteomic characterization of iron deficiency responses in Cucumis sativus L. roots. BMC Plant Biol 10
Evans IM, Rus AM, Belanger EM, Kimoto M, Brusslan JA (2010) Dismantling of Arabidopsis thaliana mesophyll cell chloroplasts during natural leaf senescence. Plant Biol 12:1–12
Garnett TP, Graham RD (2005) Distribution and remobilization of iron and copper in wheat. Ann Bot 95:817–826
Gogorcena Y, Abadia J, Abadía A (2004) A new technique for screening iron-efficient genotypes in peach rootstocks: elicitation of root ferric chelate reductase by manipulation of external iron concentrations. J Plant Nutr 27:1701–1715
Graham IA, Leaver CJ, Smith SM (1992) Induction of malate synthase gene-expression in senescent and detached organs of cucumber. Plant Cell 4:349–357
Gregersen PL, Holm PB, Krupinska K (2008) Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol 10:37–49
Guo YF, Gan SS (2005) Leaf senescence: signals, execution, and regulation. In: Current topics in developmental biology, vol 71. pp 83-+
Himelblau E, Amasino RM (2001) Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J Plant Physiol 158:1317–1323
Hirel B, Le Gouis J, Ney B, Gallais A (2007) The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J Exp Bot 58:2369–2387
Huang S, Li R, Zhang Z, Li L, Gu X, Fan W, Lucas W, Wang X, Xie B, Ni P, Ren Y, Zhu H, Li J, Lin K, Jin W, Fei Z, Li G, Staub J, Kilian A, van der Vossen E, Wu Y, Guo J, He J, Jia Z, Tian G, Lu Y, Ruan J, Quian W, Wang M, Huang Q, Li B, Xuan Z, Cao J, Asan WuZ, Zhang J, Cai Q, Bai Y, Zhao B, Han Y, Ying L, Li X, Wang S, Shi Q, Liu S, Cho W, Kim J, Xu Y, Heller-Uszynska K, Miao H, Cheng Z, Zhang S, Wu J, Yang Y, Kang H, Man L, Liang H, Ren X, Shi Z, Wen M, Jian M, Yang H, Zhang G, Yang Z, Chen R, Ma L, Liu H, Zhou Y, Zhao Y, Fang X, Fang L, Li Y, Liu D, Zheng H, Zhang Y, Qin N, Li Z, Yang G, Yang S, Bolund L, Kristiansen K, Li S, Zhang X, Wang J, Sun R, Zhang B, Jiang S, Du Y (2009) The genome of the cucumber, Cucumis sativus L. Nat Genet 41:1275–1283
Jeong J, Connolly EL (2009) Iron uptake mechanisms in plants: functions of the FRO family of ferric reductases. Plant Sci 176:709–714
Keech O, Pesquet E, Gutierrez L, Ahad A, Bellini C, Smith SM, Gardestrom P (2010) Leaf senescence is accompanied by an early disruption of the microtubule network in Arabidopsis. Plant Physiol 154:1710–1720
Kim DJ (2004) A study of cotyledon senescence in cucumber (Cucumis sativus L.) based on expressed sequence tags and gene expression. J Plant Biol 47:244–253
Kim DJ, Smith SM (1994) Expression of a single-gene encoding microbody NAD-malate dehydrogenase during glyoxysome and peroxisome development in cucumber. Plant Mol Biol 26:1833–1841
Li WF, Schmidt W (2010) A lysine-63-linked ubiquitin chain-forming conjugase, UBC13, promotes the developmental responses to iron deficiency in Arabidopsis roots. Plant J 62:330–343
Lim PO, Nam HG (2005) The molecular and genetic control of leaf senescence and longevity in Arabidopsis. In: Current topics in developmental biology, vol 67. pp 49–83
Lim PO, Woo HR, Nam HG (2003) Molecular genetics of leaf senescence in Arabidopsis. Trends Plant Sci 8:272–278
Liu XC, Li ZH, Jiang ZQ, Zhao Y, Peng JY, Jin JP, Guo HW, Luo JC (2011) LSD: a leaf senescence database. Nucleic Acids Res 39:D1103–D1107
Lucena C, Waters BM, Romera FJ, Garcia MJ, Morales M, Alcantara E, Perez-Vicente R (2006) Ethylene could influence ferric reductase, iron transporter, and H+−ATPase gene expression by affecting FER (or FER-like) gene activity. J Exp Bot 57:4145–4154
Lucena C, Romera FJ, Rojas CL, Garcia M, Alcantara E, Perez-Vicente R (2007) Bicarbonate blocks the expression of several genes involved in the physiological responses to Fe deficiency of strategy I plants. Funct Plant Biol 34:1002–1009
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105:1141–1157
Mayer AM, Poljakoff-Mayber A (1982) Metabolism of germinating seeds. In: The germination of seeds. Pergamon Press, New York, pp 85–141
McLaughlin JC, Smith SM (1995) Glyoxylate cycle enzyme-synthesis during the irreversible phase of senescence of cucumber cotyledons. J Plant Physiol 146:133–138
Mengel K (1994) Iron availability in plant-tissues - iron chlorosis on calcareous soils. Plant Soil 165:275–283
Miao Y, Zentgraf U (2010) A HECT E3 ubiquitin ligase negatively regulates Arabidopsis leaf senescence through degradation of the transcription factor WRKY53. Plant J 63:179–188
Moll RH, Kamprath EJ, Jackson WA (1982) Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron J 74:562–564
Morrissey J, Guerinot ML (2009) Iron uptake and transport in plants: the good, the bad, and the ionome. Chem Rev 109:4553–4567
Munne-Bosch S, Alegre L (2004) Die and let live: leaf senescence contributes to plant survival under drought stress. Funct Plant Biol 31:203–216
Naeve SL, Shibles RM (2005) Distribution and mobilization of sulfur during soybean reproduction. Crop Sci 45:2540–2551
O'Rourke JA, Charlson DV, Gonzalez DO, Vodkin LO, Graham MA, Cianzio SR, Grusak MA, Shoemaker RC (2007) Microarray analysis of iron deficiency chlorosis in near-isogenic soybean lines. BMC Genomics 8
Prakash JSS, Baig MA, Mohanty P (2001) Senescence induced structural reorganization of thylakoid membranes in Cucumis sativus cotyledons; LHC II involvement in reorganization of thylakoid membranes. Photosynth Res 68:153–161
Rabotti GPDN, Zocchi G (1995) Metabolic implications in the biochemical responses to iron deficiency in cucumber (Cucumis sativus L.) roots. Plant Physiol 107:1195–1199
Rogovska NP, Blackmer AM, Mallarino AP (2007) Relationships between soybean yield, soil pH, and soil carbonate concentration. Soil Sci Soc Am J 71:1251–1256
Romera FJ, Alcantara E (2004) Ethylene involvement in the regulation of Fe-deficiency stress responses by strategy I plants. Funct Plant Biol 31:315–328
Romera FJ, Alcántara E (1994) Iron-deficiency stress responses in cucumber (Cucumis sativus L.) roots: a possible role for ethylene? Plant Physiol 105:1133–1138
Romera FJ, Alcántara E, de la Guardia MD (1992) Role of roots and shoots in the regulation of the Fe efficiency responses in sunflower and cucumber. Physiol Plant 85:141–146
Romera FJ, Alcantara E, De la Guardia MD (1999) Ethylene production by Fe-deficient roots and its involvement in the regulation of Fe-deficiency stress responses by strategy I plants. Ann Bot 83:51–55
Santi S, Schmidt W (2009) Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol 183:1072–1084
Santi S, Cesco S, Varanini Z, Pinton R (2005) Two plasma membrane H+-ATPase genes are differentially expressed in iron-deficient cucumber plants. Plant Physiol Biochem 43:287–292
Sperotto RA, Ricachenevsky FK, Fett JP (2007) Iron deficiency in rice shoots: identification of novel induced genes using RDA and possible relation to leaf senescence. Plant Cell Rep 26:1399–1411
Sperotto RA, Boff T, Duarte GL, Fett JP (2008) Increased senescence-associated gene expression and lipid peroxidation induced by iron deficiency in rice roots. Plant Cell Rep 27:183–195
Sudia TW, Green DG (1972) The translocation of Zn65 and Cs134 between seed generations in soybean (Glycine max (L.) Merr.). Plant Soil 37:695–697
Sunarpi, Anderson JW (1995) Mobilization of sulfur in soybean cotyledons during germination. Physiol Plant 94:143–150
Sunarpi, Anderson JW (1996) Effect of sulfur nutrition on the redistribution of sulfur in vegetative soybean plants. Plant Physiol 112:623–631
Suzuki M, Takahashi M, Tsukamoto T, Watanabe S, Matsuhashi S, Yazaki J, Kishimoto N, Kikuchi S, Nakanishi H, Mori S, Nishizawa NK (2006) Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. Plant J 48:85–97
Tiffin LO, Chaney RL, Ambler JE (1973) Translocation of iron from soybean cotyledons. Plant Physiol 52:393–396
Van der Graaff E, Schwacke R, Schneider A, Desimone M, Flugge UI, Kunze R (2006) Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol 141:776–792
Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233
Wagstaff C, Yang TJW, Stead AD, Buchanan-Wollaston V, Roberts JA (2009) A molecular and structural characterization of senescing Arabidopsis siliques and comparison of transcriptional profiles with senescing petals and leaves. Plant J 57:690–705
Wang J, McClean PE, Lee R, Goos RJ, Helms T (2008) Association mapping of iron deficiency chlorosis loci in soybean (Glycine max L. Merr.) advanced breeding lines. Theor Appl Genet 116:777–787
Waters BM, Grusak MA (2008) Whole-plant mineral partitioning throughout the life cycle in Arabidopsis thaliana ecotypes Columbia, Landsberg erecta, Cape Verde Islands, and the mutant line ysl1ysl3. New Phytol 177:389–405
Waters BM, Sankaran RP (2011) Moving micronutrients from the soil to the seeds: genes and physiological processes from a biofortification perspective. Plant Sci 180:562–574
Waters BM, Chu HH, DiDonato RJ, Roberts LA, Eisley RB, Lahner B, Salt DE, Walker EL (2006) Mutations in Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol 141:1446–1458
Waters BM, Lucena C, Romera FJ, Jester GG, Wynn AN, Rojas CL, Alcantara E, Perez-Vicente R (2007) Ethylene involvement in the regulation of the H+-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants. Plant Physiol Biochem 45:293–301
Waters BM, Uauy C, Dubcovsky J, Grusak MA (2009) Wheat (Triticum aestivum) NAM proteins regulate the translocation of iron, zinc, and nitrogen compounds from vegetative tissues to grain. J Exp Bot 60:4263–4274
Welch RM, Norvell WA, Schaefer SC, Shaff JE, Kochian LV (1993) Induction of iron(III) and copper(II) reduction in pea (pisum sativum L.) roots by Fe and Cu staus: does the root-cell plasmalemma Fe(III)-chelate reductase perform a general role in regulating cation uptake? Planta 1993:555–561
Yamauchi Y, Sugimoto T, Sueyoshi K, Oji Y, Tanaka K (2002) Appearance of endopeptidases during the senescence of cucumber leaves. Plant Sci 162:615–619
Yoshida S (2003) Molecular regulation of leaf senescence. Curr Opin Plant Biol 6:79–84
Zhou X, Jiang YJ, Yu DQ (2011) WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Mol Cells 31:303–313
Zocchi G, Cocucci S (1990) Fe uptake mechanism in Fe-efficient cucumber roots. Plant Physiol 92:908–911
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jian Feng Ma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Observed and average DW of Ashley and Miniature White cotyledons. (a) Ashley cotyledon DW for + Fe (closed circles) and -Fe treatments (open circles) over time course (n = 6 per data point). Average DW estimates (triangles) include both treatments. (b) Miniature White (MW) cotyledon DW as described for (a). (JPEG 118 kb)
Online Resource 2
Mineral content over time in cotyledons of Ashely and Miniature White. Plants were grown until 8 days on + Fe solution then changed to + Fe or -Fe solution. Contents were calculated by multiplying average mineral concentration (n = 6) by average mineral DW. (JPEG 148 kb)
Rights and permissions
About this article
Cite this article
Waters, B.M., Troupe, G.C. Natural variation in iron use efficiency and mineral remobilization in cucumber (Cucumis sativus). Plant Soil 352, 185–197 (2012). https://doi.org/10.1007/s11104-011-0988-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0988-3