Abstract
Background and aims
Plant growth and photosynthetic ability have been frequently demonstrated to increase with nitrogen (N) supply. N can also promote changes in root water absorption and shoot water status via mechanisms that remain poorly understood. This study aims at investigating the effects of N supply on water absorption.
Methods
A hydroponic experiment with two independent rice varieties (cv. ‘Shangyou63’ hybrid indica and cv. ‘Yangdao6’ conventional indica, China) supplied by three distinct N levels was performed in a greenhouse. Physiological characteristics were analyzed after a few weeks.
Results
Compared to low N supply (20 mg·L−1), exposure to high N supply (100 mg·L−1) increased the light-saturated photosynthetic rate (A) and water use efficiency (WUE) by 17 % and 22 %, respectively, in Shanyou63 and by 43 % and 26 %, respectively, in Yangdao6. The leaf water potential was significantly decreased in Shanyou63 but not in Yangdao6. There were increases in the rate of water uptake and the root hydraulic conductance (L r ) under high N supply in both rice cultivars; these changes were accompanied by increased transcription levels of aquaporins (AQPs), decreased aerenchyma formation and root porosity, and decreased root lignin content. Under high N supply, Yangdao6 also exhibited much higher AQP activity, lower aerenchyma and root porosity compared with those of Shanyou63, indicating that Yangdao6 had an increased ability to absorb water compared with that of Shanyou63.
Conclusions
The enhanced expression of AQPs and decreased root aerenchyma and lignin contributed to increased water absorption ability under high N supply. In addition, the responses of each of the two rice cultivars (hybrid and conventional) to N supply is related to their water uptake ability, resulting from root porosity and an increase in AQP activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water and nitrogen are major limiting factors for plant growth and production (Evans 1983; Tezara et al. 1999; Lawlor 2002; Passioura 2007). Water and N uptake are generally associated with higher plants; for example, it has been demonstrated that nitrate uptake is positively related to water uptake (Guo et al. 2002; Gorska et al. 2008a). Decreased hydraulic conductance as well as water deficits will reduce the leaf elongation rate, leaf potential, stomatal conductance (gs), and nutrient uptake (Tanguilig et al. 1987; Saliendra et al. 1995).
Numerous studies have reported that water uptake into roots and transport to the shoot are often affected by root/rhizosphere water potential and oxygen deficiency, transpiration rate, and biotic or abiotic perturbation (Steudle 2000a; Maurel et al. 2008; Vandeleur et al. 2009). Root hydraulic conductance (L r ), an important parameter demonstrating water transport ability through root xylem, is plant species-dependent, which is mainly affected by the root morphology, anatomy and aquaporin (AQP) activity (Bramley et al. 2009). The formation of aerenchyma and the impermeable barrier to oxygen potential poses a large resistance to radial flow, which inevitably decreases the L r (Colmer 2003). Inhibition of the root cortical cell hydraulic conductivity (L c ) could account for the decrease in L r of wheat, while L c may be related to AQP activity under the regulation of cytoplasmic pH, free Ca2+, phosphorylation and H2O2 (Bramley et al. 2010; Bramley and Tyerman 2010). Vandeleur et al. (2005) reported that the water permeability of root plasma membrane vesicles was also strongly inhibited by reductions in the pH.
Low N can significantly reduce the effective leaf area as a result of reductions in the leaf size, chlorophyll content and CO2 assimilation, leading to a subsequent decrease in the crop yield (Bennett et al. 1989; Dordas and Sioulas 2008). Conversely, increased N supply can improve the water use efficiency (WUE) and plant productivity (Lajtha and Whitford 1989; Green and Mitchell 1992; Livingston et al. 1999; Hobbie and Colpaert 2004). Many studies have focused on the effect of N on stomatal conductance or mesophyll conductance. For example, increasing the photosynthetic rate (A) following increased N investment in Rubisco with little or no effect on stomatal conductance (gs) (Wang et al. 1998; Welander and Ottosson 2000) or with an increase in mesophyll conductance (gm) (Warren and Adams 2006; Flexas et al. 2008) lead to an increase in WUE. Other work has also shown that A and gs were directly proportional to the plant hydraulic conductance, suggesting that changes in the plant hydraulic conductance may affect the plant carbon gain (Hubbard et al. 2001). Whether N affected the plant hydraulic conductance by altering characteristics of the roots or leaves remains unclear, although changes induced by N nutrition to each of these attributes can potentially affect L r .
Previous studies have shown that low N can decrease the plant hydraulic conductance (Radin and Boyer 1982; Radin 1983). Low phosphorus (P) and low potassium (K) also showed effects that paralleled those of low N (Dordas and Sioulas 2008; Oddo et al. 2011). Plants grown on low P had a reduced leaf water potential, transpiration rate, and L r , leading to reductions in the leaf area and shoot dry weight. The decrease in L r was caused by a reduced radial conductivity, which could result from decreased root cell membrane permeability and from changes in apoplastic or symplastic pathways (Augé and Stodola 1990; Cui and Nobel 1992). Recent findings have suggested that the N availability potentially affected L r via changes in AQP expression (Ishikawa-Sakurai et al. 2014). Another study reported that there was a rapid increase in L r associated with the addition of NO3 −, but similar effects were not observed for other anions, such as SO4 2− and H2PO3 −. This response could be attributed to changes in root cell membrane hydraulic conductance by NO3 −, which was related to a hydraulic signal and AQP activity but not to AQP expression or de novo protein synthesis (Gorska et al. 2008a, b). It is possible that the rapid increase in L r in the presence of increased NO3 − availability is an important trait that enhances the ability of plants to compete for the NO3 − in soil (Gloser et al. 2007). Although these studies provided some explanation for the increase in L r observed when the plant is supplied with N, how N uptake is affected by water uptake and the relationship between these two factors are still not completely understood.
Based on our previous cultivar selection experiment, we chose the two rice cultivars of ‘Shanyou63’ (hybrid indica, China) and ‘Yangdao6’ (conventional indica, China), which were widely planted in China and showed different responses of water uptake ability and photosynthesis to nitrogen forms and/or concentrations (Yang et al. 2012; Li et al. 2013). Our previous studies had shown that under well watered conditions, physiological effects of moderate N supply were independent of the applied N form (Yang et al. 2012). In consideration of the field environment, lowland rice roots are actually exposed to a mixed nitrate and ammonium supply in the rhizosphere (Briones et al. 2003; Kirk and Kronzucker 2005). In addition, aggravating water shortage, conventional flood irrigation methods should be replaced with intermittent irrigation or dry cultivation in rice production. There will be an increase in nitrate content in these conditions. So, in the present study, we supplied even concentration of nitrate and ammonium, and assessed growth, leaf gas exchange, WUE, L r , and factors affecting water absorption under various N supplies in two rice cultivars of Shanyou63 and Yangdao6. We discuss the effects of N on root anatomy and water transport pathways as well as the contribution of AQP to water uptake; we also comment on the differences between these two cultivars.
Materials and methods
Plant materials and growth conditions
Rice seeds (Oryza sativa L., cv. “Shanyou63” hybrid indica China, and “Yangdao6” conventional indica China) were sterilized in 10 % H2O2 for 30 min and transferred to moist filter paper for germination. When the seedlings developed an average of 2.5 visible leaves in distilled water, they were transplanted into 6-L rectangular containers (30 × 20 × 10 cm) with quarter-strength nutrient solution (for composition, see below) for 2 days. Then, the seedlings were transferred to half-strength nutrient solution for 3 days. Later, the seedlings were supplied with full-strength nutrient solution for 4 days. Next, the seedlings were supplied with low-N (20 mg·L−1), moderate-N (40 mg·L−1) and high-N (100 mg·L−1) nutrient solutions. The nutrient solutions included the following components: macronutrients (mg·L−1): N as equal moles of (NH4)2SO4 and Ca(NO3)2, 10 P as KH2PO4, 40 K as K2SO4 and KH2PO4, 40 Mg as MgSO4·7H2O, 57 Ca as CaCl2·2H2O and micronutrients (mg·L−1): 2.0 Fe as Fe-EDTA (FeSO4·7H2O), 0.5 Mn as MnCl2·4H2O, 0.2 B as H3BO3, 0.05 Mo as (NH4)6Mo7O24·4H2O, 0.01 Zn as ZnSO4·7H2O, 0.01 Cu as CuSO4·5H2O, and 2.8 Si as Na2SiO3·9H2O. A nitrification inhibitor dicyandiamide (DCD, 1 mg·L−1) was added to each nutrient solution to prevent ammonium oxidation. The nutrient solutions were changed every 3 days, and the pH was adjusted to 5.50 ± 0.05 daily with 0.1 M HCl and 0.1 M NaOH. Each treatment included five replicates in a completely randomized design. The spatial arrangement of the treatments was randomized to avoid edge effects in the greenhouse.
Plants were grown in the greenhouse at a temperature of 25 ± 5 °C. The maximum light intensity at midday was more than 1,500 μmol·m−2·s−1 at the leaf level. Physiological and biochemical parameters were measured at tillering stage about 35–45 days after transplanting.
Biomass measurements
Plant samples were harvested and separated into root, stem, and leaf fractions at tillering stage. All samples were oven-dried at 105 °C for 30 min and then at 70 °C to a constant weight. The leaf area was photographed and then analyzed using Image-Pro Plus 6.0 software.
Gas exchange measurements
At tillering stage, the photosynthetic rate of the newly expanded leaves was measured using a Li-Cor 6400 portable photosynthesis open system with red-blue light from 09:00 to 11:00 AM. During the measurements, the leaf temperature was maintained at 28 °C, and the photosynthetic photo flux density (PPFD) was 1,500 μmol photons·m−2·s−1. In addition, the CO2 concentration in the cuvette was adjusted to the atmospheric concentration of CO2 (390 μmol·mol−1), and the relative humidity was maintained at 40 %. After equilibration to a steady state, the gas exchange parameters, including light-saturated photosynthetic rate (A), stomatal conductance (gs), intercellular CO2 concentration (Ci) and transpiration rate (Tr), were recorded. More details about measurement can be found in our previous studies or other studies (Long et al. 1996; Long and Bernacchi 2003; Li et al. 2009). Water use efficiency (WUE) was calculated as the ratio of A to Tr (A/Tr), and the stomata limitation (Ls) was calculated from the equation of Ls = (A i -A)/A i (Farquhar and Sharkey 1982), where A i is the light-saturated photosynthetic rate when Ci was 390 μmol·mol−1.
Leaf water potential measurements
The leaf water potential was determined at 00:00–04:00 AM at tillering stage (Han et al. 2013). Newly expanded leaves were sampled, immediately followed by cutting into small pieces and spread evenly on the bottom of the sample cup to determine water potential using a WP4 Dewpoint Potential Meter (Decagon devices, Pullman, WA, USA).
Nitrogen absorption measurements
At tillering stage, each rice plant was transferred to a medium containing 20, 40 or 100 mg·L−1 N as equal moles of NO3 − and NH4 + for 8 h. Nitrogen absorption was assessed by the amount of NO3 − and NH4 + remaining in the medium after the measurement period. The levels of NO3 − and NH4 + were determined using an Auto Analyzer 3 digital colorimeter (BRAN+LuEBBE). The reagents used for NO3 − determination were 1 % sulfanilamide (C6H8N2O2S), 0.05 % N-(1-naphthyl) ethylenediamine dihydrochloride (NEDD), 10 % (V/V) H3PO4, 4 % NaOH, 0.3 % (V/V) H3PO4, 0.001 % CuSO4·5H2O, 0.01 % ZnSO4·7H2O and 0.2 % hydrazine sulphate (N2H4·H2SO4). The reagents used for NH4 + determination were 4 % tri-sodium citrate (C6H5Na3O7·2H2O), 4 % sodium salicylate (Na2C7H5O3), 0.1 % sodium nitroprusside {Na2[Fe(CN)5NO]·2H2O}, 4 % NaOH and 0.3 % dichloro-isocyanuric acid sodium salt dehydrate (DCI).
Water uptake rate and AQP activity measurements
The water uptake by intact plants with or without the addition of 0.1 mM HgCl2 to hydroponic solutions was determined by the depletion of nutrient solution (by weight) between 10:30 to 11:30 AM (1 h) at tillering stage. The contribution of Hg-sensitive AQP to the water uptake was calculated from the difference in the depletion of nutrient solution between the control and HgCl2-treated plants, and the water uptake of HgCl2-treated plants was generally regarded as an apoplastic contribution. The water uptake per leaf area (LWU) was calculated from the ratio of total water uptake to total leaf area per plant.
Root hydraulic conductance measurements
Hydraulic conductance was measured using a high-pressure flow meter (HPFM, Decagon Devices) at 10:00–12:00 AM at tillering stage. The HPFM was attached to the detached root with an Omnifit connector. Positive pressure (P i ) was applied at a constant rate of 3–7 kpa·s−1, allowing the slope of the relationship between flow (F) and pressure (P i ) to be obtained as a transient measurement (K). After measuring K, the root was oven-dried to a constant weight (W). The root hydraulic conductance (L r ; kg·s−1·g−1·MPa−1) was calculated as the following: L r = K/W; for method details, see Yang et al. (2012).
Root anatomy observations
At tillering stage, the root aerenchyma tissue was studied using fresh free-hand sections (Lux et al. 2005). The sections were cut from 6–7 cm segments above the root tip with a razor blade, transferred to a drop of water on a microscope slide and then observed using an Olympus BX51 light microscope. Images were recorded using a high-sensitivity CCD color camera system.
Lignin measurements
At tillering stage, the lignin content of the roots was estimated using the acetyl bromide procedure (Iiyama and Wallis 1990; Vander et al. 1998). The cell walls were prepared from frozen rice roots by grinding in liquid nitrogen, followed by three sequential extractions with 95 % ethanol, ethanol: n-hexane (1:2) twice and then distilled water. Lyophilized and vacuum oven-dried cell walls (20 mg) were transported into glass tubes with 2.5 ml acetyl bromide reagent [25 %(v/v) acetyl bromide in acetic acid] and then incubated in a 70 °C water bath for 30 min. Ice-cooled samples were then added to 2 ml of 2 M NaOH, 5 ml of acetic acid, and 0.2 ml of 7.5 M hydroxylamine hydrochloride, and the volume was adjusted with acetic acid to 20 ml. The absorbance of the resulting solution was measured using an ultraviolet (UV) spectrophotometer at 280 nm. The lignin content was calculated using the molar extinction coefficient of 17 L·g−1·cm−1 (Hatfield and Fukushima 2005).
Root porosity measurements
At tillering stage, the porosity of the roots was determined according to previous methods (Visser et al. 2000; Fan et al. 2003). Briefly, 0.8 g of fresh roots was cut into approximately 1–2 cm length segments and weighed (RFW, root fresh weight). A 25 ml Pyrex pycnometer flask filled with degassed water was weighed before (W1) and after (W2) the addition of these segments. Next, the root segments were transferred to a vial, which was also filled with degassed water and vacuum-infiltrated until no air bubbles appeared. These vacuum-infiltrated root segments were retransferred to the above degassed water-filled pycnometer, and the pycnometer was weighed again (W3). The root porosity was calculated as the following:
RNA extraction and semi-quantitative RT-PCR
At tillering stage, tissues of the rice roots and newly expanded leaves were sampled and quickly frozen in liquid nitrogen at 10:00–11:00 AM, then ground in a pre-cooled mortar with a pestle. RNA was extracted from the frozen tissue powder with TRIZOL reagent (Invitrogen). For reverse transcription-polymerase chain reaction (RT-PCR), first-strand cDNA was synthesized using M-MLV reverse transcriptase (Promega, Madison, WI, USA). The PCR conditions used were 95 °C for 3 min followed by 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s. The PCR products were subjected to agarose gel electrophoresis and stained with ethidium bromide. The signal intensity of the stained bands was imaged using a charge-coupled device (CCD) camera. All of the primers and cycle numbers used for PCR are listed in Table 1.
Statistical analysis of the data
Differences among treatments were analyzed with the SAS 9.0 statistical software package, using the analysis of variance (ANOVA) program. Significant differences (P < 0.05) among treatments are indicated in the tables and figures by different letters.
Results
Effects of the various N supplies on plant growth
The shoot dry mass was increased with increasing N supply in both cultivars, while the root dry mass was decreased, resulting in a reduced root/shoot ratio under high N supply (Table 2). Compared with low N supply, the root/shoot ratios under high N supply were decreased by 56 % and 57 % in Shanyou63 and Yangdao6, respectively. The leaf area was increased significantly in Shanyou63, but there was no difference between moderate-N-supplied plants and high-N-supplied plants in Yangdao6.
Effects of the various N supplies on photosynthetic parameters
Light-saturated photosynthetic rate (A) was increased significantly under high N supply in both rice cultivars. Under low N and moderate N supplies, A was lower in Yangdao6 than in Shanyou63, while there was no significant difference between the two cultivars under high N supplies (Table 3). This finding indicated that, compared with the low N supply, the increase in A under high N supply was more pronounced in Yangdao6 than in Shanyou63.
Stomatal conductance (gs) showed no significant difference under the three N supplies in Shanyou63, while it was increased in Yangdao6 supplied with high N (Table 3). The intercellular CO2 concentration (Ci) was decreased from 319 to 303 μmol mol−1 in Shanyou63 and was approximately 310 μmol mol−1 under all three N supplies in Yangdao6. The leaf transpiration rate (Tr) showed no significant differences in Shanyou63, while an increase was observed in Yangdao6. A comparison of the two cultivars revealed that the Tr was lower in Yangdao6 than in Shanyou63, particularly under low N and moderate N supplies. WUE was increased with increasing N supplies in both cultivars, and this effect was comparable under all three of the N supplies between both cultivars (Table 3 and Fig. 1). Yangdao6 had lower stomatal limitation (Ls) than that of Shanyou63 under high N supply.
The relationship between WUE and the water uptake rate (a), WUE and the N uptake rate (b). Filled squares and open squares represent Shanyou63 and Yangdao6, respectively. The lines represent linear regression, and the regression equations are (a) y = 0.0249x + 1.5876, R 2 = 0.9817, P = 0.000; (b) y = 1.0995x + 1.1019, R 2 = 0.8789, P = 0.006
Effects of the various N supplies on plant water status and related parameters
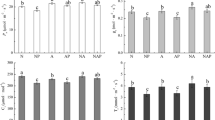
The root hydraulic conductance (L r ) was also increased under high N supply in both rice cultivars (Fig. 2). Compared with the low N supply, a 168 % increase was observed in Shanyou63 and a 324 % increase was observed in Yangdao6 under high N supplies. There was no difference in the low-N-supplied plants between the two cultivars, but higher levels of L r were observed in Yangdao6 compared with Shanyou63 under both moderate and high N supplies.
Effects of various N supplies on the root hydraulic conductance of rice seedlings. Plant growth conditions are the same as those shown in Table 2. Data are represented as the mean of five replicates, and the bars indicate the SD. Significant differences (P < 0.05) are indicated by different letters
The leaf water potential (ψleaf) was decreased significantly in Shanyou63 supplied with high N, but no significant difference was observed in Yangdao6. Under low N supply, ψleaf was similar between the two cultivars, but it was lower in Shanyou63 than in Yangdao6 under both moderate and high N supplies (Fig. 3).
Effects of various N supplies on the leaf water potential of newly expanded leaves in rice seedlings. Plant growth conditions and data representations are the same as those shown in Fig. 2
The root water uptake rate was increased with increases in the N supply in both rice cultivars (Table 4). Compared with low N supply, this parameter was increased by 292 % and 255 %, respectively, in Shanyou63 and Yangdao6 under high N supply. Under low or moderate N supply, the root water uptake rate was similar in both cultivars, while under high N supply, it was higher in Yangdao6 than in Shanyou63.
The addition of 0.1 mM HgCl2 significantly suppressed the root water uptake rate in both cultivars, but it also increased with an increased N supply. Under high N supply, Yangdao6 had an increased water uptake rate than that of Shanyou63 in response to HgCl2 treatment. When the HgCl2 percentage of inhibition was calculated, it was shown that the magnitude of the decrease was greater and this effect was more pronounced in Yangdao6 than in Shanyou63 (Table 4). Compared with moderate N supply, there was a significant increase in the water uptake per leaf area (LWU) under high N supply in Yangdao6, but not in Shanyou63 (Table 4).
Effects of the various N supplies on root aerenchyma, lignin and porosity
To study the effects of root anatomy and morphology on the water uptake, the aerenchyma tissue was observed, and the root porosity and lignin content were also measured. The transverse freehand sections from the root showed evidence of aerenchyma and fine intercellular spaces (Fig. 4). High N visibly decreased the formation of aerenchyma in both rice cultivars. In addition, Yangdao6 had less aerenchyma than did Shangyou63 under high N supply.
Root porosity was also significantly decreased in both cultivars, but there was no difference between moderate and high N supplies in Shanyou63 (Fig. 5). Compared with a low N supply, root porosity was decreased by 19 % and 56 % in Shanyou63 and Yangdao6, respectively, under high N supply.
Effects of various N supplies on the root porosity of rice seedlings. Plant growth conditions and data representations are the same as those shown in Fig. 2
The lignin content of the roots exhibited a similar tendency to that of the root porosity. Compared with low N supply, the former parameter was decreased by 24 % and 26 % under high N supply in Shanyou63 and Yangdao6, respectively. There was no difference in the lignin content between two cultivars at each N supply (Fig. 6).
Effects of various N supplies on the root lignin content of rice seedlings. Plant growth conditions and data representations are the same as those shown in Fig. 2
Effects of the various N supplies on the expression of the leaf and root AQP genes
The mRNA levels of the plasma membrane intrinsic proteins (PIP2s) genes were analyzed by reverse transcription-PCR (RT-PCR) in the leaves and roots. Three replicates rendered qualitatively similar results. Representative examples are shown in Figs. 7 and 8. The expression levels of OsPIP2;4 increased with increasing N supplies in the leaves of both rice cultivars. The leaf mRNA levels of OsPIP2;1, OsPIP2;2 and OsPIP2;3 were increased in Yangdao6, while there was no difference in Shanyou63. The root mRNA levels of OsPIP2;1 and particularly OsPIP2;4, OsPIP2;5 were significantly increased with increasing N supplies in both rice cultivars. The other PIPs expression in roots was nearly the same among the various N supplies.
Effects of various N supplies on leaf AQP expression in rice seedlings. Total RNA fractions were prepared from the roots and newly expanded leaves as described in the Materials and Methods section. The mRNA levels were analyzed by semi-quantitative RT-PCR using specific primers as shown in Table 1. The PCR products were separated on agarose gel and imaged after ethidium bromide staining. Photographic images are shown at the bottom of each graph. The numbers in parentheses indicate the number of PCR cycles. SY and YD represent Shanyou63, Yangdao6; L, M and H represent low-N, moderate-N and high-N, respectively. The OsActin (ACT) gene was used for an internal standard
Effects of various N supplies on root AQP expression in rice seedlings. The procedure and data representations are the same as those shown in Fig. 7
Discussion
Water absorption, photosynthetic rate and WUE are increased under high N supply
Plant growth or crop productivity is always correlated with the N supply, and high N supply can improve photosynthesis and the shoot dry weight but inhibit root growth, resulting in a decreased root/shoot ratio (Linkohr et al. 2002; Tian et al. 2005; Li et al. 2009). In the present study, we observed similar patterns in both rice cultivars, with an increased shoot weight and lower root/shoot ratio under high N supply (Table 2). Analysis of the gas exchange parameters illustrated that the photosynthetic rate (A) was increased with increasing N supply in both rice cultivars, but the magnitude of the increase varied. Compared with low N supply, A was increased by 17 % and 43 % in Shanyou63 and Yangdao6, respectively, under high N supply. This finding may have been caused by a lower stomatal limitation (Ls) in Yangdao6 compared with Shanyou63 under high N supply (Table 3).
Compared with low N treatment, the instantaneous WUE was increased by 22 % and 26 % under high N supply in Shanyou63 and Yangdao6, respectively; these findings are consistent with those obtained in previous studies (Guehl et al. 1995; Livingston et al. 1999; Hobbie and Colpaert 2004). Livingston et al. (1999) indicated that the higher WUE of white spruce with increased N supply was due to a decrease in gs. In our study, there was an increase in A, while gs was constant or slightly increased with increasing N supply, particularly in Yangdao6 (Table 3). Thus, we suggest that gs was not responsible for the higher WUE in rice. We found a strong positive correlation not only between WUE and the N uptake rate but also between WUE and the water uptake rate (Fig. 1), indicating that the N uptake or water uptake might be a crucial factor influencing changes in WUE.
It is well known that the roots are the most important factor in water uptake for plant water relations. The root hydraulic conductivity (L r ) reflects the ability of the root to absorb water and may vary in response to external or internal factors, such as the water status due to drought or salinity or the demand for water from the shoot due to transpiration, the nutritional state, changes in the anatomy and structure of the root and AQP activity (Steudle 2000a). The decreased L r temporarily decreased the xylem hydrostatic potential and consequently the leaf water potential (Else et al. 2001), which significantly affected plant water relations.
The present study showed that with an increasing N supply, L r was increased (Fig. 2), which was accompanied by increased water absorption (Table 4). There was a significant positive correlation (R 2 = 0.75) between water absorption and the hydraulic conductance. The leaf water potential was also affected by the various N supplies, while each of the two cultivars showed a distinct response (Fig. 3). Yangdao6 had an increased leaf water potential compared with that of Shanyou63 under high N supply; these results suggested that Yangdao6 had a more favourable water status due to the higher L r .
Root anatomy, morphology and AQPs are responsible for the ability to absorb water
In the root cylinder, there are three parallel pathways for water movement, i.e., the apoplastic, symplastic and transcellular pathways, with the latter two representing cell-to-cell pathways (Steudle 2000b). The apoplastic pathway resistance is strongly associated with the formation of the aerenchyma (Yang et al. 2012) and suberized and/or lignified cell walls (North and Nobel 1995; Hose et al. 2001). Many studies have indicated that N-, P- or sulfur (S)-deficiency induces aerenchyma formation in the root (Drew et al. 1989; Bouranis et al. 2003; Fan et al. 2007; Postma and Lynch 2011) and an increase in the lignin and/or suberin of the root or stem (Entry et al. 1998; Fritz et al. 2006; Pitre et al. 2007). The formed aerenchyma and lignin will inevitably inhibit L r and water uptake via the apoplastic pathway. Taken together, our results illustrated that with an increasing N supply, aerenchyma formation, porosity and lignin were significantly decreased in the root (Figs. 4, 5 and 6), thereby improving the apoplastic water uptake (Table 4). Both porosity and lignin showed significant negative correlations with the water uptake rate, which was consistent with the results of previous studies (Yang et al. 2012). Under high N supply, the aerenchyma and root porosity of Yangdao6 were significantly lower than those of Shanyou63, which might underlie the increased ability to absorb water in Yangdao6.
In the cell-to-cell pathway, the water molecules need to diffuse through the plasma membrane into the cytoplasm. During this process, the AQPs embedded in the membrane play an important role in water transport (Steudle and Peterson 1998; Steudle 2001; Kaldenhoff and Fischer 2006). Under well-watered conditions, the contribution of AQP to L r was approximately 35 %–75 % (Maggio and Joly 1995; Lu and Neumann 1999; Wan and Zwiazek 1999; Barrowclough et al. 2000; North and Nobel 2000; Martre et al. 2001). Hacke et al. (2010) showed that seven AQPs were upregulated in the stem xylem of hybrid poplar under high N conditions. It is also possible that N or P deficiency may have decreased the activity and/ or content of AQPs, further reducing the L r (Carvajal et al. 1996; Clarkson et al. 2000). In the present study, Hg-sensitive AQP activity was determined by the discrepancy in the water uptake rate with and without the addition of 0.1 mM HgCl2, an AQP inhibitor (Javot and Maurel 2002; Wan et al. 2004; Beaudette et al. 2007). This finding showed that independent of the N supply and compared with the control (without HgCl2), the water uptake rates in all HgCl2 treatments decreased in both rice cultivars (Table 4). The decreased percentage represents the Hg-sensitive AQP activity, which was higher under high N supply, demonstrating that the high-N-supplied plants had an increased ability to take up water via the cell-to-cell pathway. We also investigated the transcription levels of OsPIPs that is a subfamily of AQPs using RT-PCR. The root mRNA expression levels of OsPIP2;1, OsPIP2;4 and OsPIP2;5 were significantly increased with increasing N concentration in both rice cultivars, which may also result in an increased ability to take up water via the cell-to-cell pathway of high-N plants. Upon comparison of the two rice cultivars, the mRNA expression levels of Shanyou63 were similar or slightly higher than those of Yangdao6, but the contribution of Hg-sensitive AQPs to water uptake was higher in Yangdao6 than in Shanyou63 regardless of the N supply (Table 4); these findings suggest that the root AQP activity plays a more important role in water uptake in Yangdao6 than in Shanyou63, which might account for the increased ability to take up water in Yangdao6 than in Shanyou63 under high N supply.
Differential response of water absorption under the various N supplies among the rice cultivars
Although the ability to take up water and the L r both increased with the N supply, they were significantly higher in Yangdao6 than in Shanyou63 under high N supply, and the WUE was lower in Shanyou63 (Table 3). As previously described, this effect was caused by differences in the aerenchyma, root porosity and root AQP activity between Yangdao6 and Shanyou63. In addition, although the root/shoot ratio decreased with the N supply, and proportion of the decrease was consistent from Shanyou63 to Yangdao6 (Table 2), the transpiration rate (Tr) in Shanyou63 was higher than that in Yangdao6, suggesting that Shanyou63 had a much higher water loss than that of Yangdao6. In addition, Shanyou63 had a reduced ability to absorb water than that of Yangdao6 under high N supply. When we calculated the total water uptake per leaf area, the results also showed the significant lower values in Shanyou63 (Table 4). This indicates relative water absorption ability on the base of leaf area under high N supply was lower in Shanyou63 compared with Yangdao6. Some studies reported that the decrease in leaf water potential was correlated with the changes in structure of cell or tissue of plants (Morgan 1986; Shangguan et al. 2000) or osmolytes, such as proline (Nayyar 2003). The changed leaf structure and/or osmolytes of Shanyou63 under high N condition might be the potential candidate for the decreased leaf water potential. Moreover, the mRNA expression levels in the leaves of Yangdao6 were higher than those of Shanyou63, particularly OsPIP2;3 and OsPIP2;6 under high N supply. Parent et al. (2009) showed that water-deprived maize plants with high AQP expression and activity had an increased rate of recovery of leaf water potential after re-watering. Higher AQP expression in the leaf may be used for fine water regulation, which decreases water loss and increases WUE, thereby contributing to the maintenance of a favorable plant water status. In this study we provided even concentrations of nitrate and ammonium, we do not explore which N form may dominate the physiological effects observed here. But, in our previous studies there were no significant differences in L r , root xylem sap flow rate, aerenchyma formation and root porosity between the nitrate fed plants and ammonium fed plants under non-water stress condition (Yang et al. 2012). The differences in nitrogen form effects on water relations were mainly observed under water stress condition. By varying nitrate and ammonium concentrations, the contribution of the different N forms to the physiological effects will be explored in a future study.
It can be concluded that under high N supply, decreased aerenchyma, porosity and lignin content of the roots, as well as increased AQP expression, were responsible for the high water absorption rate. Compared with the characteristics of Shanyou63, the greatly reduced aerenchyma and porosity of the root, higher hydraulic conductivity, and higher AQP activity in Yangdao6 resulted in increased WUE and water potential. These findings indicated that increasing N fertilization might increase the water absorption, WUE and production of rice plants via changes in the root properties.
References
Augé R, Stodola A (1990) An apparent increase in symplastic water contributes to greater turgor in mycorrhizal roots of droughted rosa plants. HortSci 25:1096–1096
Barrowclough DE, Peterson CA, Steudle E (2000) Radial hydraulic conductivity along developing onion roots. J Exp Bot 51:547–557
Beaudette PC, Chlup M, Yee J, Emery RJN (2007) Relationships of root conductivity and aquaporin gene expression in Pisum sativum: diurnal patterns and the response to HgCl2 and ABA. J Exp Bot 58:1291–1300
Bennett JM, Mutti LSM, Rao PSC, Jones JW (1989) Interactive effects of nitrogen and water stresses on biomass accumulation, nitrogen uptake, and seed yield of maize. Field Crop Res 19:297–311
Bouranis DL, Chorianopoulou SN, Siyiannis VF, Protonotarios VE, Hawkesford MJ (2003) Aerenchyma formation in roots of maize during sulphate starvation. Planta 217:382–391
Bramley H, Tyerman SD (2010) Root water transport under waterlogged conditions and the roles of aquaporins. In: Mancuso S, Shabala S (eds) Waterlogging signalling and tolerance in plants. Springer, Berlin, pp 151–180
Bramley H, Turner NC, Turner DW, Tyerman SD (2009) Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiol 150:348–364
Bramley H, Turner NC, Turner DW, Tyerman SD (2010) The contrasting influence of short-term hypoxia on the hydraulic properties of cells and roots of wheat and lupin. Funct Plant Biol 37:183–193
Briones AM Jr, Okabe S, Umemiya Y, Ramsing NB, Reichardt W, Okuyama H (2003) Ammonia-oxidizing bacteria on root biofilms and their possible contribution to N use efficiency of different rice cultivars. Plant Soil 250:335–348
Carvajal M, Cooke D, Clarkson D (1996) Responses of wheat plants to nutrient deprivation may involve the regulation of water-channel function. Planta 199:372–381
Clarkson DT, Carvajal M, Henzler T, Waterhouse RN, Smyth AJ, Cooke DT, Steudle E (2000) Root hydraulic conductance: diurnal aquaporin expression and the effects of nutrient stress. J Exp Bot 51:61–70
Colmer T (2003) Long‐distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Cui M, Nobel PS (1992) Nutrient status, water uptake and gas exchange for three desert succulents infected with mycorrhizal fungi. New Phytol 122:643–649
Dordas CA, Sioulas C (2008) Safflower yield, chlorophyll content, photosynthesis, and water use efficiency response to nitrogen fertilization under rainfed conditions. Ind Crop Prod 27:75–85
Drew MC, He CJ, Morgan PW (1989) Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen- or phosphate-starvation in adventitious roots of Zea mays L. Plant Physiol 91:266–271
Else MA, Coupland D, Dutton L, Jackson MB (2001) Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol Plantarum 111:46–54
Entry JA, Runion GB, Prior SA, Mitchell RJ, Rogers HH (1998) Influence of CO2 enrichment and nitrogen fertilization on tissue chemistry and carbon allocation in longleaf pine seedlings. In: Box J (ed) Root demographics and their efficiencies in sustainable agriculture, grasslands and forest ecosystems. Springer, Netherlands, pp 3–18
Evans JR (1983) Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.). Plant Physiol 72:297–302
Fan M, Zhu J, Richards C, Brown KM, Lynch JP (2003) Physiological roles for aerenchyma in phosphorus-stressed roots. Funct Plant Biol 30:493–506
Fan M, Bai R, Zhao X, Zhang J (2007) Aerenchyma formed under phosphorus deficiency contributes to the reduced root hydraulic conductivity in maize roots. J Integr Plant Biol 49:598–604
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Ann Rev Plant Physiol 33:317–345
Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmes J, Medrano H (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31:602–621
Fritz C, Palacios-Rojas N, Feil R, Stitt M (2006) Regulation of secondary metabolism by the carbon-nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J 46:533–548
Gloser V, Zwieniecki MA, Orians CM, Holbrook NM (2007) Dynamic changes in root hydraulic properties in response to nitrate availability. J Exp Bot 58:2409–2415
Gorska A, Ye Q, Holbrook NM, Zwieniecki MA (2008a) Nitrate control of root hydraulic properties in plants: translating local information to whole plant response. Plant Physiol 148:1159–1167
Gorska A, Zwieniecka A, Holbrook NM, Zwieniecki MA (2008b) Nitrate induction of root hydraulic conductivity in maize is not correlated with aquaporin expression. Planta 228:989–998
Green T, Mitchell R (1992) Effects of nitrogen on the response of loblolly pine to water stress I. Photosynthesis and stomatal conductance. New Phytol 122:627–633
Guehl J, Fort C, Ferhi A (1995) Differential response of leaf conductance, carbon isotope discrimination and water-use efficiency to nitrogen deficiency in maritime pine and pedunculate oak plants. New Phytol 131:149–157
Guo S, Brück H, Sattelmacher B (2002) Effects of supplied nitrogen form on growth and water uptake of French bean (Phaseolus vulgaris L.) plants. Plant Soil 239:267–275
Hacke UG, Plavcová L, Almeida-Rodriguez A, King-Jones S, Zhou W, Cooke JEK (2010) Influence of nitrogen fertilization on xylem traits and aquaporin expression in stems of hybrid poplar. Tree Physiol 30:1016–1025
Han Y, Wang Y, Jiang H, Wang M, Korpelainen H, Li C (2013) Reciprocal grafting separates the roles of the root and shoot in sex-related drought responses in Populus cathayana males and females. Plant Cell Environ 36:356–364
Hatfield R, Fukushima RS (2005) Can lignin be accurately measured? This paper was originally presented at the Lignin and Forage Digestibility Symposium, 2003 CSSA Annual Meeting, Denver, CO. Crop Sci 45:832–839
Hobbie EA, Colpaert JV (2004) Nitrogen availability and mycorrhizal colonization influence water use efficiency and carbon isotope patterns in Pinus sylvestris. New Phytol 164:515–525
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W (2001) The exodermis: a variable apoplastic barrier. J Exp Bot 52:2245–2264
Hubbard R, Ryan M, Stiller V, Sperry J (2001) Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ 24:113–121
Iiyama K, Wallis AFA (1990) Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. J Sci Food Agr 51:145–161
Ishikawa-Sakurai J, Hayashi H, Murai-Hatano M (2014) Nitrogen availability affects hydraulic conductivity of rice roots, possibly through changes in aquaporin gene expression. Plant Soil 379:289–300
Javot H, Maurel C (2002) The role of aquaporins in root water uptake. Ann Bot 90:301–313
Kaldenhoff R, Fischer M (2006) Functional aquaporin diversity in plants. BBA Biomembranes 1758:1134–1141
Kirk GJD, Kronzucker HJ (2005) The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: a modelling study. Ann Bot 96:639–646
Lajtha K, Whitford W (1989) The effect of water and nitrogen amendments on photosynthesis, leaf demography, and resource-use efficiency in Larrea tridentata, a desert evergreen shrub. Oecologia 80:341–348
Lawlor DW (2002) Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. J Exp Bot 53:773–787
Li Y, Gao Y, Xu X, Shen Q, Guo S (2009) Light-saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration. J Exp Bot 60:2351–2360
Li Y, Ren B, Ding L, Shen Q, Peng S, Guo S (2013) Does chloroplast size influence photosynthetic nitrogen use efficiency? PloS one 8(4):e62036. doi:10.1371/journal.pone.0062036
Linkohr BI, Williamson LC, Fitter AH, Leyser H (2002) Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J 29:751–760
Livingston N, Guy R, Sun Z, Ethier G (1999) The effects of nitrogen stress on the stable carbon isotope composition, productivity and water use efficiency of white spruce (Picea glauca (Moench) Voss) seedlings. Plant Cell Environ 22:281–289
Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54:2393–2401
Long SP, Farage PK, Garcia RL (1996) Measurement of leaf and canopy photosynthetic CO2 exchange in the field. J Exp Bot 47:1629–1642
Lu Z, Neumann PM (1999) Water stress inhibits hydraulic conductance and leaf growth in rice seedlings but not the transport of water via mercury-sensitive water channels in the root. Plant Physiol 120:143–152
Lux A, Morita S, Abe J, Ito K (2005) An improved method for clearing and staining free-hand sections and whole-mount samples. Ann Bot 96:989–996
Maggio A, Joly RJ (1995) Effects of mercuric chloride on the hydraulic conductivity of tomato root systems (evidence for a channel-mediated water pathway). Plant Physiol 109:331–335
Martre P, North GB, Nobel PS (2001) Hydraulic conductance and mercury-sensitive water transport for roots of Opuntia acanthocarpa in relation to soil drying and rewetting. Plant Physiol 126:352–362
Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
Morgan JA (1986) The effects of N nutrition on the water relations and gas exchange characteristics of wheat (Triticum aestivum L.). Plant Physiol 80:52–58
Nayyar H (2003) Accumulation of osmolytes and osmotic adjustment in water-stressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ Exp Bot 50:253–264
North GB, Nobel PS (1995) Hydraulic conductivity of concentric root tissues of Agave deserti Engelm. under wet and drying conditions. New Phytol 130:47–57
North GB, Nobel PS (2000) Heterogeneity in water availability alters cellular development and hydraulic conductivity along roots of a desert succulent. Ann Bot 85:247–255
Oddo E, Inzerillo S, La Bella F, Grisafi F, Salleo S, Nardini A (2011) Short-term effects of potassium fertilization on the hydraulic conductance of Laurus nobilis L. Tree Physiol 31:131–138
Parent B, Hachez C, Redondo E, Simonneau T, Chaumont F, Tardieu F (2009) Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiol 149:2000–2012
Passioura J (2007) The drought environment: physical, biological and agricultural perspectives. J Exp Bot 58:113–117
Pitre F, Cooke JK, Mackay J (2007) Short-term effects of nitrogen availability on wood formation and fibre properties in hybrid poplar. Trees 21:249–259
Postma JA, Lynch JP (2011) Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiol 156:1190–1201
Radin JW (1983) Control of plant growth by nitrogen: differences between cereals and broadleaf species. Plant Cell Environ 6:65–68
Radin JW, Boyer JS (1982) Control of leaf expansion by nitrogen nutrition in sunflower plants role of hydraulic conductivity and turgor. Plant Physiol 69:771–775
Saliendra NZ, Sperry JS, Comstock JP (1995) Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis. Planta 196:357–366
Shangguan ZP, Shao MA, Dyckmans J (2000) Nitrogen nutrition and water stress effects on leaf photosynthetic gas exchange and water use efficiency in winter wheat. Environ Exp Bot 44:141–149
Steudle E (2000a) Water uptake by plant roots: an integration of views. Plant Soil 226:45–56
Steudle E (2000b) Water uptake by roots: effects of water deficit. J Exp Bot 51:1531–1542
Steudle E (2001) The cohesion-tension mechanism and the acquisition of water by plant roots. Annu Rev Plant Biol 52:847–875
Steudle E, Peterson CA (1998) How does water get through roots? J Exp Bot 49:775–788
Tanguilig V, Yambao E, O’toole J, De Datta S (1987) Water stress effects on leaf elongation, leaf water potential, transpiration, and nutrient uptake of rice, maize, and soybean. Plant Soil 103:155–168
Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401:914–917
Tian Q, Chen F, Zhang F, Mi G (2005) Possible involvement of cytokinin in nitrate-mediated root growth in maize. Plant Soil 277:185–196
Vandeleur RK, Niemietz C, Tilbrook J, Tyerman SD (2005) Roles of aquaporins in root responses to irrigation. Plant Soil 274:141–161
Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD (2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol 149:445–460
Vander P, Vårum KM, Domard A, Eddine El Gueddari N, Moerschbacher BM (1998) Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reactions in wheat leaves. Plant Physiol 118:1353–1359
Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ (2000) Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ 23:1237–1245
Wan X, Zwiazek JJ (1999) Mercuric chloride effects on root water transport in aspen seedlings. Plant Physiol 121:939–946
Wan X, Steudle E, Hartung W (2004) Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. J Exp Bot 55:411–422
Wang JR, Hawkins CDB, Letchford T (1998) Photosynthesis, water and nitrogen use efficiencies of four paper birch (Betula papyrifera) populations grown under different soil moisture and nutrient regimes. Forest Ecol Manag 112:233–244
Warren CR, Adams MA (2006) Internal conductance does not scale with photosynthetic capacity: implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant Cell Environ 29:192–201
Welander NT, Ottosson B (2000) The influence of low light, drought and fertilization on transpiration and growth in young seedlings of Quercus robur L. Forest Ecol Manag 127:139–151
Yang X, Li Y, Ren B, Ding L, Gao C, Shen Q, Guo S (2012) Drought-induced root aerenchyma formation restricts water uptake in rice seedlings supplied with nitrate. Plant Cell Physiol 53:495–504
Acknowledgments
This work was supported by the National Basic Research Program of China (2013CB127403), the National Natural Science Foundation of China (31172020 and 31272236), Jiangsu Postdoctoral Science Foundation (1402148C) and China Postdoctoral Science Foundation (2015M571768).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ad C. Borstlap.
Rights and permissions
About this article
Cite this article
Ren, B., Wang, M., Chen, Y. et al. Water absorption is affected by the nitrogen supply to rice plants. Plant Soil 396, 397–410 (2015). https://doi.org/10.1007/s11104-015-2603-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2603-5