Abstract
Background
The vast majority of terrestrial plants, including most crops, associate with fungi of the phylum Glomeromycota to form symbiotic associations, known as arbuscular mycorrhizas. Arbuscular mycorrhizas play a pivotal role in the terrestrial cycling of nitrogen (N). Recent advances in mycorrhizal research show that arbuscular mycorrhizal fungi (AMF) can reduce denitrification rates and nitrous oxide (N2O) emissions from soils. The rapid increase in the literature, over the last five years, opens up the opportunity to address mechanisms through which AMF might control denitrification.
Scope
In this review, we classify likely mechanisms through which AMF modify through their hyphae denitrification and N2O emissions into two categories: proximal mechanisms, manifested through direct changes to denitrifiers and distal mechanisms which induce indirect changes to denitrifiers. We distinguish between two types of influences, (i) alterations in the size and activity of denitrifiers and (ii) alterations in the relative availability of two key groups of genes, nitrite reductases (nirK & nirS) and nitrous oxide reductases (nosZ).
Conclusion
Proximal mechanisms could reduce N2O emissions through depleting available soil N and C, metal ions, modifying soil moisture, immobilizing C and N or through altering the denitrifying community, and the relative abundance of genes involved in denitrification. Distal mechanisms could impact denitrification through changing soil pH, organic matter decomposition, improvement in soil aggregation, as well as promoting plant diversity and productivity. There are apparently many likely mechanisms, proximal and distal, through which AMF could alter N2O production, even though their ecological importance for N cycling remains open to question.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O) is a potent greenhouse gas contributing to global warming. N2O has a 300 times higher per unit mass global warming potential compared to carbon dioxide (CO2) and has been labelled the “dominant ozone-depleting substance in the twenty-first century”, responsible for the destruction of the stratospheric ozone layer (Forster et al. 2007; Ravishankara et al. 2009; Griffis et al. 2017). An estimated 60% of the global soil N2O emissions originates from agricultural soils (Syakila and Kroeze 2011; Reay et al. 2012). Grasslands account for two thirds of agricultural land across the world and are responsible for approximately 18% of global N2O emissions (Lee et al. 1997). Arbuscular mycorrhizal fungi (AMF) are the dominant mycorrhizal type in these ecosystems (Smith and Read 2008; Brundrett and Tedersoo 2018). Global N2O emissions and temperature are on the rise and expected to increase further (IPCC 2021). Increases in human population and living standards will lead to an intensification of the food production system, including a heavier use of nitrate (N) fertilizers, which will likely increase losses of N from ecosystems through processes such as N-leaching and denitrification (Springmann et al. 2018), if no technological changes or dedicated mitigation measures are implemented. Springmann et al. (2018) estimated that there could be a 50 to 90% increase in greenhouse gas emissions from agriculture and more intense land use between 2010 and 2050.
N2O is produced in soils in the course of two contrasting processes: denitrification and nitrification (Butterbach-Bahl et al. 2013). However, over two thirds of global soil N2O emissions originate from heterotrophic denitrification (Thompson et al. 2012). Heterotrophic denitrification, to which from now on we will refer to as denitrification, is a biological process in which soil nitrate (NO3−) is reduced to dinitrogen (N2) under anaerobic conditions with the intermediate formation of N2O. The first step of denitrification, nitrate reduction, requires an electron donor, which in most cases is C. The identity of the electron donor determines the energetic costs of the process, which in some cases can be exothermic (i.e. autotrophic denitrification; Park and Yoo 2009). By contrast, nitrification describes a process where soil ammonium ions (NH4+) are reduced to NO−3 under aerobic conditions and can simultaneously generate N2O through nitrifier-denitrification (Butterbach-Bahl et al. 2013). It is therefore relevant to have an in-depth understanding of the environmental factors controlling denitrification. We grouped here the environmental factors controlling denitrification rates (N2O and N2 emissions) into two categories, proximal and distal factors (Groffman et al. 1988; Wallenstein et al. 2006; Robertson and Groffman 2015, see “Classification of proximal and distal controls of denitrification” section). Being a microbially driven process, denitrification depends strongly on ongoing competitive and facultative interactions with other microbes, including plant-associated microbes such as AMF. AMF are a widespread group of soil borne fungi associating symbiotically with the roots of about a 72% of the terrestrial plant species that have been described so far, including many agricultural plants (Brundrett and Tedersoo 2018; Fig. 2). AMF often enhance plant nitrogen (N) and phosphorus (P) uptake and plant yield and simultaneously promote soil aggregation (van der Heijden et al. 2006a, b; Nwaga et al. 2010; Okiobe et al. 2015, Tisdall and Oades 1982; Rillig and Mummey 2006; Leifheit et al. 2014). The direct and indirect AMF effects of AMF on terrestrial ecosystem processes and C cycling have been discussed, for instance in Rillig (2004a, b), but the potential role of AMF on N-cycling and denitrification processes has been less explored.
One of the first studies addressing the role of AMF on N2O emissions was by Cavagnaro et al. (2012), finding negligible alterations on N2O fluxes between mycorrhiza-defective tomato mutants and their wild-type progenitors. There have been several reviews addressing the role of AMF in N related processes (Veresoglou et al. 2012b; Hodge and Storer 2015; Cavagnaro et al. 2015), but none of these studies has addressed AMF-mediated controls of denitrification and related N2O emissions in detail. Recent advances in mycorrhizal research demonstrate that AMF reduce N2O emissions from soils (e.g. Bender et al. 2014; Lazcano et al. 2015; Zhang et al. 2015; Bender et al. 2015; Storer et al. 2018; Teutscherova et al. 2018 but see Okiobe et al. 2019). These new findings are of great relevance because agricultural management practices, such as conventional tillage, have been reported to reduce AMF colonization and abundance in cropping systems, which might contribute to increased N2O emissions (Bender et al. 2014; Bowles et al. 2016). Managing agricultural soils for conserving and augmenting AMF may not only contribute to a greater sustainability in crop production (Rillig et al. 2018), but also to a reduction of N2O emissions. A comprehensive understanding of AMF-mediated mechanisms on environmental controls of denitrification is therefore particularly desirable.
Here, we present a comprehensive review with new insights into how AMF can potentially alter proximal and distal controls of denitrification rates and how they could impact the magnitude of N2O emissions. First, we illustrate the difference between proximal and distal controls (Fig. 1a) and their potential effect on denitrification rates and related N2O emissions. Second, we explain how AMF can influence proximal environmental controls of denitrification such as the denitrifier community structure, soil nitrate, oxygen and metal concentrations, available C and soil pH as well as distal environmental controls such as organic matter and C/N ratio, nitrification, soil moisture and structure and plant diversity, and how this relates to the magnitude of N2O emissions. We briefly discuss the potential of AMF to mitigate climate change via their influence on denitrification rates. We conclude with suggestions for future studies on AMF-mediated mechanisms on denitrification and N2O emissions.
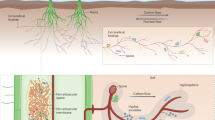
(a) Overview of the proximal (blue links) and distal (red links) mechanisms through which arbuscular mycorrhizal fungi (AMF) alters N2O emission rates (we describe these in detail in the text). Proximal mechanisms induce instantaneous or relatively quick changes to N2O emissions whereas distal mechanisms act at larger temporal scales via modifying the microenvironment for denitrifiers. Next to each of the mechanisms we highlight the most probable direction of the effect on N2O emissions on which we further rationalize in Table S1. Network path lengths and intermediate nodes are not indicative of the strength of the reationships and only serve the purpose of highlighting that most likely the effects of AMF are of high complexity and that we remain unaware of possible interactions between different mechanisms such as C availability and nitrification. We further depict the two types of changes in denitrification which the abovementioned mechanisms can induce and alter N2O emission rates: a change in the population size and activity of denitrifiers which is depicted here as a petri dish with bacterial cultures (b) and changes in the relative abundance and spatial distribution of enzymes (in particular nitrite reductases and nitrous oxide reductases) in the soil matrix which is depicted here for visualization purposes as a two-dimensional set of pores in a light brown color (c)
Classification of proximal and distal controls of denitrification
During biological denitrification, soil bacteria, but also some fungi and archaea convert NO3− to NO2−, then to nitric oxide (NO) gas, and to N2O and finally to N2 in a sequence of reactions (Fig. 1; Fig. S1) (Zumft 1997; Saggar et al. 2013). The reactions are catalyzed by nitrate reductase (Nar); nitrite reductase (Nir); nitric oxide reductase (Nor) and nitrous oxide reductase (Nos), respectively (Zumft 1997). The complete process can be expressed as a net balanced redox reaction, where NO3− gets fully reduced to N2. In order for denitrification to proceed, N must be present in the form of NO3−. The NO3− can be present in soil either through the direct addition of ammonium nitrate fertilizer (NH4NO3) or following nitrification of NH4+.
Denitrifiers are a diverse group of microbes comprising bacteria, fungi and archaea with over 160 variants of nitrite reductase (Wei et al. 2015) and 165 variants of nitrous oxide reductase (Sanford et al. 2012). A significant fraction of denitrifiers, estimated to approximately 1/3 of bacterial denitrifiers, have a truncated denitrification pathway, meaning that they lack the nosZ gene and cannot reduce N2O to N2 (Fig. S1), even though some denitrifiers lacking these genes have been reported to influence denitrification rates from soils (Philippot et al. 2011; Saggar et al. 2013). This means that denitrification gene counts are not particularly informative of potential denitrification activity or potential N2O production across terrestrial habitats (Philippot et al. 2009; Domeignoz-Horta et al. 2015) and complicates predicting N2O emissions from terrestrial systems. It is therefore important to differantiate between instances when mechanisms indiscriminately alter the population sizes and activity of all denitrifiers (i.e. counts of denitrifiers abstracted here as microbial colonies in a Petri dish; Fig. 1b) and mechanisms that alter the relative abundances of denitrifiers with the ability to carry out complete deinitrification (i.e. the endproduct is N2) over those that can only do partial denitrification (i.e. the endproduct is N2O; here depicted as spatial heterogeneity of genes in soil pores; Fig. 1c). In the latter group we include cases where the organisms are present but their physiological activity might be lower, such as nosZ genes at low pH (e.g. Liu et al. 2014).
In many cases hyphae of AMF can induce direct changes to the microbes that carry out denitrification. The list of direct changes includes (i) soil N availability which AMF alter through uptake, (ii) labile C availability which changes in response to hyphodeposition and hyphal senescence, (iii) soil oxygen concentrations which can be determined via hyphal respiration (iv) gene abundance ratios (e.g. nirK vs. nirS; nosZ clade I vs type II) and overall community shifts in the denitrifiers that may alter the efficiency of denitrification and the ratio of the end-products (i.e. N2O over N2) (v) soil moisture which AMF could modify through absorbing and translocated water (e.g. Allen 2007) and (vi) changes in metal availability which AMF hyphae assimilate. We classify here such drivers as proximal controls of denitrification. Proximal mechanisms, thus, unfold through alterations that AM fungal hyphae growing in the soil induce that influence denitrifiers without the involvement of other organisms, but in a way that immediately affects aspects of the soil environment relevant for denitrification. There are, however, many additional controls of denitrification which occur through indirect pathways. We refer to these factors here as distal controls of denitrification and they describe all other mechanisms via which AM in their symbiosis with plants may affect microbes carrying out denitrification; these indirect effects either cascade through or involve other organisms (at a minimum the host plant) and thus typically manifest themselves with a time lag compared to the direct effects. Effects of the AM symbiosis in ecosystems unfold in a hierarchical manner (Rillig 2004a, b; Powell and Rillig 2018). We here distinguish direct effects of the hyphae of AM fungi in the soil on the environment experienced by denitrifiers from indirect effects that involve other organisms (e.g. plants) or processes (e.g. soil aggregation). It should be understood, however, that the parameters we discuss under "proximal effects" can also be affected by the AM symbiosis indirectly, at a higher level of the ecological hierarchy, for example via changing plant productivity or community composition. We summarize our classification into proximal and distal controls of denitrification and our expectations on how they alter denitrification rates and N2O emissions in Fig. 1a. The concept of proximal and distal controls can be highly useful to guide future research to more clearly describe the multifaceted mechanisms that lead to changes in N2O production as we do here for AMF-mediated processes and mechanisms.
A few studies have investigated AMF-mediated effects on denitrification rates and related N2O emissions via changes in environmental controls of denitrification. Most of these studies reported declines in N2O emissions upon addition of AMF (Bender et al. 2014, Storer et al. 2018, see Table 1, Fig. 1a). A decline in denitrification rates suggests that either N2O emissions are lower or that N2 losses decline either with AMF or N2O/N2 ratio, i.e. total denitrification is reduced in the presence of AMF. Quantifying directly N2 emissions from soils is difficult due to technical issues and its high atmospheric background (Butterbach-Bahl et al. 2013). The role of AMF in denitrification and N2 emissions is largely unknown. We will discuss in detail how AMF modifies proximal and distal controls of denitrification and N2O emissions later in the review (Table S1, Fig. 1).
It is not always straightforward to compare denitrification and N2O emission rates between studies (and thus assess the impact of AMF on them), because of occassional incompatibility of methods. The two most common approaches to assay denitrification are via gas traps (i.e. either via static chambers or assaying headspace air in sealed units) to which authors refer as actual rates (Chadwick et al. 2014) and via a denitrification enzyme activity assay, usually described in the literature as denitrification potential rates (Tiedje et al. 1989). The two approaches have several limitations. Actual denitrification rates reflect strongly the soil and environmental settings at plant harvest and combining multiple measurements over time is often necessary to obtain representative denitrification estimates (e.g. Barton et al. 2015). Also, pot-bound plants can deplete N from soil, such that actual denitrification rates are no longer detectable; to address this issue in many experiments with AMF, authors fertilize the soil shortly before initiating the measurements (e.g. Cavagnaro et al. 2012; Bender et al. 2014). The denitrification enzyme activity assay, by contrast, has a range of technical limitations (e.g. Keeney 1986), which lead to an underestimation of potential rates (Watts and Seitzinger 2000) and assumes unrealistically that the enzyme optima are invariable across enzyme variants (i.e. enzymes produced by different organisms to catalyze a given process but maintain a slightly different protein codons) and are effectively captured by the assay. Denitrification potential measurements are occasionally interpreted as a proxy of the size of the denitrifying microbial community (e.g. Groffman et al. 1999), whereas actual rates additionally capture environmental settings, meaning that results are not always comparable. For example, AMF likely increase denitrification potential/ N2O potential emission rates (as shown in the two studies that assayed denitrification potential rates: Okiobe et al. 2019, 2020), but decrease actual denitrification/N2O emission rates (e.g. Bender et al. 2014; Storer et al. 2018). Possibly the best way to address the shortcomings of either of the approaches assaying denitrification is to combine them both in experimental procedures. Despite being less cost effective, combining the approaches could increase considerably the mechanistic resolution of any study. Alternatively, under field settings it should be possible to additionally capture the potential of the soil to support denitrification via assaying actual denitrification iteratively over longer periods (i.e. establish long trajectories of measurements). Unfortunately, the existing studies on AMF addressing denitrification have so far narrowed their focus to one of the two approaches.
AMF as proximal mechanisms of denitrification
AMF-mediated mechanisms acting on available mineral soil N concentrations
Many of the studies addressing the role of AMF on N2O emissions have been short term mechanistic studies under well controlled conditions (e.g. Bender et al. 2014; Storer et al. 2018; Table 1). In such cases, the most pervasive change that AMF induce to denitrification and N2O emissions should have been a decline in the availability of N in the system via N uptake and immobilization (Storer et al. 2018). Denitrification is substrate controlled, meaning that there is usually a good relationship between the availability of NO3− in soil and N2O emissions. AMF assimilate most of their N from soil in the form of NH4+ (Govindarajulu et al. 2005; Tanaka and Yano 2005) and do not directly influence NO3− availability. In doing so, AMF likely slow down nitrification and thus do impact NO3− availability (Veresoglou et al. 2011, 2019). An alternative way through which AMF could influence N availability (and thus denitrification) is via speeding up assimilation (and thus depleting) of inorganic N pools from, soil (Johansen et al. 1992; Hodge and Storer 2015; Atul-Nayyar et al. 2009; Cavagnaro et al. 2012). N-depleting effects of AMF should be more pronounced in controlled pot experiments, the typical settings under which AMF-mediated effects are tested. As a result and particularly in pot studies, a depletion of N from systems with AMF was occasionally observed, resulting in lower N2O production (Bender et al. 2015; Zhang et al. 2015).
AMF-mediated mechanisms acting on soil C availability
The vast majority of denitrifiers are heterotrophs and denitrification in agricultural soils is controlled by labile C availability (Saggar et al. 2013). Any AMF-mediated mechanism that influences C availability in soils therefore has a big impact on N2O emissions. One possibility is that AMF reduce the availability of C in the (myco)rhizosphere. AMF are a major C sink and alter the composition of rhizodeposition (Graham et al. 1981; Jones et al. 2004). Between 4 and 30% of photoassimilated plant C is transferred to AMF hyphae (Drigo and Donn 2017), providing an essential C source for the survival of the fungus and therefore limiting rhizodeposition to the soil (e.g. Marschner et al. 1997; Artursson et al. 2006). Via this mechanism AMF likely reduce the amount of energy available to denitrifiers thereby delaying and reducing N2O emissions (Fig. 1a). AMF-reduced C input into the mycorrhizosphere (the volume of soil influenced by AMF and roots) or hyphosphere (the volume of soil influenced by AMF hyphae) has also been reported to decrease the abundance of N2O-producing bacteria (see “AMF-mediated mechanisms on soil moisture” section for more details, Amora-Lazcano et al. 1998; Nuccio et al. 2013). There is also the possibility, however, that AMF increase C availability. AMF can be a source of C in soil, via hyphodeposition, hyphal grazing, and through turnover of AM fungal hyphae (Jones et al. 2004; Johnson et al. 2005; Kaiser et al. 2015). Additionally, AMF can increase the decomposition of labile plant litter (Cheng et al. 2012), potentially because of a stimulation of associated microbes, thereby enhancing the release of C compounds. However, microbially derived C is efficiently being incorporated into mineral-stabilized soil organic matter, thereby mitigating possible stimulating effects on the microbial community (Sokol and Bradford 2019). In the case that AMF increase the availability of C in the rhizosphere they could also foster higher rates of denitrification in the (hypho)rhizosphere. A final possibility is that we observe both increases and decreases in C availability which depend on the exact environmental settings we observe at each microsite. In that particular case the net effect that AMF induce on denitrification should additionally depend on the degree to which denitrification is possible (e.g. whether microsites foster anoxic conditions; if the availability of N locally is high etc.) at the various microsites in soil. Overall, the magnitude to which AMF modify C availability in soil is unknown and this complicates upscaling gas fluxes in the rhizosphere.
AMF-mediated mechanisms acting on soil oxygen concentration and respiration
Overall, a large number of studies has observed AMF-induced increases in soil respiration (Langley et al. 2005; Hughes et al. 2008; Cavagnaro et al. 2008, 2012; Shi et al. 2010; Nottingham et al. 2010), which have been attributed to heterotrophic respiration of external AMF hyphae (Heinemeyer et al. 2006). For example, Nottingham et al. (2010) found that AMF mycelia respired C at a rate of 1.4 t ha−1 yr−1, which accounted for about 14% of total soil respiration and 26% of root-derived respiration in the forest soil. Zhang et al. (2016), by contrast, reported that in a semiarid step suppressing AMF increased soil respiration by up to 9%, suggesting that the impact of AMF on respiration could also be negative. A greater AMF mycelium respiration may deplete oxygen in soil and favour denitrification and N2O production (Sextone et al. 1985). This effect could stimulate denitrifying N2O producers that are known to thrive under poor oxygen conditions (Groffman et al. 1988). Low soil oxygen concentrations could therefore increase denitrifying enzyme activities while high oxygen concentrations would inhibit this activity (Zumft 1997; Burgin and Groffman 2012). The ecological importance of such likely mechanisms that reduce oxygen concentrations in soil remains, however, questionable and it is quite likely that other distal AMF-mediated mechanisms such as promoting soil aggregation (Rillig and Mummey 2006) are more important than soil oxygen effects per se.
Most of the existing literature addressing how AMF alter soil respiration in experiments has used soils from woody habitats. Propagule denisties of AMF in soil may be comparably lower in woody habitats, at least in the case of ectomycorrhizal dominated temperate ecosystems, but the depth and density of the root systems should be higher (e.g. Zhou et al. 2019), meaning that the results may not be generalizable for herbaceous systems. A particularity of agricultural soils is that in addition to seasonal variation in rooting depth, AMF colonization and abundance are considerably low (Bender et al. 2014; Bowles et al. 2016), meaning that soil respiration could have significant implications for soil C turnover and denitrification. It would be desirable to expand the focus of the existing literature through addressing the influence of AMF on N2 and N2O emissions via AMF-induced change in soil oxygen availability under agricultural soils.
AMF-mediated shifts in the denitrifying community structure
AMF-induced modifications on proximal controls of denitrification such as alterations in C availability and competition intensity for other nutrients may evoke shifts in the denitrifying community. Few studies have shown that AMF may alter denitrifying community composition as well as the nitrifying microbial community (Veresoglou et al. 2011, 2012a; Nuccio et al. 2013). AMF further alter the relative abundance of nitrifying and denitrifying genes in soil but also alter the potential nitrification and denitrification rates in soil (e.g. Storer et al. 2018). For example, AMF reduce the activity of ammonia oxidisers (e.g., ammonia oxidising bacterial-AOB) and thereby lower potential nitrification rates (Veresoglou et al. 2011, 2019). This indicates that they may be capable of outcompeting nitrifiers for NH4+ (Veresoglou et al. 2019). Unfortunately, the degree to which AMF alter the structure of the denitrifying microbial community has not yet been quantitatively estimated. A reduction in potential nitrification rates and abundance of denitrifiers by AMF could then potentially reduce overall denitrification rates and the magnitude of N2O emissions (Veresoglou et al. 2011; Veresoglou et al. 2012a). Additionally, the presence of AMF significantly increased the relative abundance of two out of four denitrifying bacterial groups (Gemmatimonadetes and Deltaproteobacteria) possessing nosZ genes (Nuccio et al. 2013). Any differences in the distribution of the groups of microbial denitrifiers with and without the ability to reduce N2O (i.e. availability of nosZ genes), including alterations resulting from AMF, could as a result potentially (i.e. given suitable environmental conditions) change the proportion of denitrification that is incomplete (i.e. results in N2O emissions) and represent a mechanism of N2O production independent of N availability. Hyphae of AMF have also been reported to alter the abundance of denitrifying key genes nirK and nirS, which are involved in the reduction of NO3− into NO2− and nosZ genes (Bender et al. 2014), but also the community structure of bacteria maintaining nirK genes (Veresoglou et al. 2012a). Many studies have used the abundance of denitrifying genes as a proxy of assayed denitrification (but see concerns raised in Henderson et al. 2010; Philippot et al. 2009, 2013). Bender et al. (2014) found that the abundance of key genes responsible for N2O production (nirK and nirS) was negatively, and for N2O reduction (nosZ) positively correlated to the abundance of AMF, indicating that N2O reduction was mediated by AMF-induced changes in the soil microbial denitrifying community composition. The direct effect of AMF on N2O emissions rates via microbial community shifts and the relative importance of those in relation to associated changes in environmental conditions are poorly understood. If AMF disproportionally promote nosZ genes over nirK and nirS ones, they might also stimulate the N2O-reducing ability of the denitrifiers and accelerate the conversion of N2O to N2, lowering N2O emissions.
AMF can indirectly influence N2O emissions by altering the abundance of N metabolism-related genes, rather than by altering soil chemical properties or the diversity of bacterial communities (Gui et al. 2021). We believe that most microbial shifts in soil (with some notable exceptions for groups such as ammonia oxidizers) occur relatively fast and this is as a result a proximal mechanism rather than a distal one. Further exploring the soil and environmental conditions under which AMF might alter nosZ gene activity thus has the potential to develop into a N2O mitigation technology, whereby soils are managed for conserving propagules of AMF as proposed in Rillig et al. (2016).
AMF-mediated mechanisms on soil moisture
AMF over longer timespans can translocate considerable quantities of water and thus structure the soil microenvironment in relation to soil moisture (e.g. Allen 2007; but see evidence from Püschel et al. 2020 that the water transported is insufficient to meet transpiration demands of the hosts). The easiest way to quantify the effect of soil moisture on denitrification rates is via assessing water filled pore space (WFPS) (Table S1). N2O emission rates increase with increasing WFPS (Groffman et al. 1988; Butterbach-Bahl et al. 2013). The AM symbiosis with plant roots can increase plant water uptake and nutrient use efficiency, thereby changing soil moisture conditions (Augé 2001, 2004). Lazcano et al. (2015) performed a greenhouse experiment to compare the effect of mycorrhizal tomato (76R MYC) and its non-mycorrhizal mutant (rmc) on the N2O emissions under different soil moisture conditions (drought and watered). They found that plant genotype affected the relationship between N2O and WFPS: soil N2O emissions in the 76R MYC treatment were significantly reduced at high soil moisture with WFPS higher than 50% compared to rmc. They reported that the reduction of N2O production was related to an increased photosynthesis rate and stomatal conductance at high soil moisture with 76R MYC, but also to increased plant water use efficiency by AMF colonized roots. This study supports earlier findings from Bender et al. (2014), indicating a reduction in WFPS in response to AMF inoculation. They presumed that the fast water removal in the 76R MYC treatment increased the oxygen availability in the soil and therefore reduced the N2O emissions, because denitrifying enzymes are only active under conditions of low oxygen (Burgin and Groffman 2012). By contrast, Zhang et al. (2015) found that the WFPS of the non-inoculated soil was slightly greater than that of the AMF inoculated soil and there were no differences between the control and AMF treatment during the flooding and draining stages in the rice paddy field. Nevertheless, N2O emissions were significantly reduced in the inoculated soils compared to non-inoculated because AMF reduced NH4+ and NO3−concentrations in the flooded and drained stages, respectively. AMF would lower the rates of N2O emissions during precipitation events (i.e. rainy seasons) that are known to be the hotspot of denitrification compared to dryer seasons. Generally, the influence of AMF on soil water transport to plants seems to be higher in dryer soils as shown in a meta-analysis from Augé et al. (2015). Hence, AMF effects on denitrification via mediating water availability vary depending on moisture availability, but overall seem to render the soil habitats more oxic, particularly at low water availability, thereby reducing N2 and N2O production.
AMF-mediated mechanisms on soil metal availability
Soil metals have been overlooked as proximal controls of denitrification (Wang et al. 2016). Denitrifying enzymes require cofactors to operate, which can be soil metal ions such as copper (Cu), iron (Fe) and molybdenum (Mo) (Zumft 1997). A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's activity. Cofactors are "helper molecules" that assist in biochemical transformations, for example denitrification (Zumft 1997). Mo acts as a cofactor for Nar, Fe for both Nar and Nir while Cu acts as a cofactor for Nir and specifically for Nos because it is the only enzyme with no alternative Cu-cofactor (Saggar et al. 2013; Zumft 1997). Although AMF are usually considered important primarily for N and P uptake (Smith and Read 2008), they can also increase plant acquisition of zinc (Zn), Mo, Fe and Cu. A meta-analysis reported overall positive effect of AMF on Cu, manganese (Mn) and Fe uptake by crops (Lehmann and Rillig 2015). Cu and Fe protein transporters have been identified in the mycorrhizal structures of R. irregularis, a model AMF (Tamayo et al. 2014). We postulate that changes in soil metal concentration by AMF will have potential effects on denitrification enzymes and N2O emission rates. The Nir in some organisms can contain Cu and could therefore be limited by Cu availability (Zumft 1997; Suzuki et al. 2000; Stein 2011). If AMF are taking up Cu, they may reduce the magnitude of N2O production by repressing the activity of the Cu-based Nir. However, a reduction in the availability of Cu can also increase N2O production as the activity of the Nos, which also has a Cu-cofactor, is reduced (Zumft 1997). AMF reduced soil Cu2+ availability via uptake (Lehmann and Rillig 2015) would therefore leave most of the denitrified N in the form of N2O. On the other hand, when the availability of soil Fe (III) is high, N2O production increases (Bengtsson et al. 2002). Recent studies found that the availability of Fe in soils can be positively linked to N2O production (Zhu et al. 2013; Wang et al. 2016). Since AMF can reduce the availability of Fe by taking up Fe2+ and transfering it to their host plants, they have the potential to reduce denitrification rates by altering the activity of Nar and Nir. However, Wang et al. (2016) reported a high N2O production when soil Fe2+ concentration was decreased. This study indicated that the increased production of N2O was regulated by oxido-reduction reactions and the donation of electrons to NO−3 via microbial oxidation of Fe (II). The presence of AMF may therefore alter oxydo-reactions between nitrates and metal ions (Fig. 2). We presume that changes in N2O production could be dependent on the regulation of soil Cu(II)/NO3− or Fe(II)/ NO3− via proximal AMF-reduced soil metal concentration in soils.
Bibliometric coupling (i.e. an analysis of citation tables of related documents) network of the eleven studies (Table 1) that have addressed how arbuscular mycorrhizal fungi alter N2O emissions. The analysis clustered the studies into three compartments (while Zhang et al. 2015 did not cluster to any other study). The red compartment describes studies on crops replicating agricultural settings. The purple compartment includes mechanistic studies, where a quantification of N2O emission rates was coupled to other N-cycling processes such as nitrifcation rates or N-leaching. Studies in the green compartment maintain some more realistic elements and were either carried out with multiple hosts (Jia et al. 2020; Okiobe et al. 2020), addressed more than one soil (Bender et al. 2014) or were set up in soils that had not been sterilized (Okiobe et al. 2019; Okiobe et al. 2020; some other studies such as Bender et al. 2014 did this as well but experimented with congeneric mutants). The width of the links is indicative of how similar the set of references between any given pair of studies has been. Node size is representative of how similar a study is with the other studies in the set. In the insert we depict the shared (i.e. we excluded keywords present in a single study) keywords across the abovementioned set of eleven studies, which should thus present good candidate keywords for indexing purposes in future studies
In summary, by lowering trace metal availability in soils, AMF could contribute to an inhibition of N2O production. This idea needs to be tested with studies focusing on the direct effect of the presence of AMF on metal concentrations in soil and the relation to N2O release.
AMF-mediated mechanisms on distal controls of denitrification rates
AMF-mediated mechanisms acting on soil organic matter and soil C/N ratio
The C/N ratio of mineralized organic matter (OM) likely presents an important distal control on denitrification rates because it considerably influences the availability of soil N (Fig. 1a). AMF enhance decomposition and mineralization of mostly high quality plant litter (with low C/N ratios), and thus stimulate the release of mineral N, but at the same time they could stimulate the non-symbiotic assimilation of newly released mineral N by their host plants (Hodge 2001; Hodge et al. 2001; Hodge and Fitter 2010; Hodge and Storer 2015). Such effects would also alter the composition of the denitrifying microbial community, which consists of mainly heterotrophic bacteria. The fraction of mineralized N that is assimilated by AMF is considerable, possibly spanning up to a 20% of plant derived from N-rich patches (Leigh et al. 2009). AMF-mediated decomposition in organic patches results in higher C/N ratios than when AMF are absent and this eventually increases the amount of N that is mineralized (Atul-Nayyar et al. 2009). In this study, up to 25% of the mineralized N were recovered and translocated to host plants. AMF can firstly influence decomposition rates of OM and secondly the fate of the mineralized N and C substances and out-compete denitrifiers. We can see that distal mechanisms, such as AMF-mediated effects on OM decomposition, are closely linked to proximal mechanisms, such as AMF induced-changes in soil N-nutrient concentration and competition between AMF and denitrifying microbial community for C and N-nutrients in the soil. Therefore, the presence of AMF may reduce the availability of organic C to the denitrifying soil community while decreasing the N2O/N2 product ratio. We do not yet fully understand the trophic and biotic interactions (i.e. competition) between AMF and denitrifying microbial communities and whether these can slow down the cycling of N in the hyphosphere or else how they affect the rates of N2O emissions and the product ratio of denitrification.
Most of the studies looking at the effects of AMF on decomposition of organic matter were conducted in the lab or greenhouse and were short-term studies (Hodge 2001; Leigh et al. 2009). Even though most of the existing literature supports that AMF increase decomposition of organic C, it is precarious to generalize the observation for longer term experiments this differs (Verbruggen et al. 2013). We can, however, make use of these studies to design future experiments that disentangle how changes in organic C would alter the biotic interactions between AMF and denitrifiers. Such studies are of high relevance for gauging how AMF could alter N-cycling and denitrification and can potentially be of a particularly high applied value for modelling purposes given several recent calls to integrate mycorrhizal technology in organic agriculture (Rillig et al. 2016; Zhang et al. 2019).
AMF-mediated mechanisms acting on soil pH
Soil pH plays an important role in the activity of denitrifying enzymes and the regulation of N2O emissions. N2O emissions and the denitrification potential ratio [N2O/(N2O + N2)] decrease with increasing pH values (Šimek and Cooper 2002; Èuhel and Šimek 2011; Samad et al. 2016). The activity of Nos, a relevant enzyme responsible for the reduction of N2O to N2 in soils, could be repressed by acidic pH, even when the denitrifiers are present, and thereby increase N2O emissions (Dannenmann et al. 2008; Èuhel and Šimek 2011; Liu et al. 2014). Bago et al. (1996) reported that the presence of AMF hyphae increased the pH of a growth medium enriched with NO−3 by enhanced NO−3 uptake and releasing OH− into or removing H+ from the medium. Increase in soil pH by AMF was also observed in the rhizosphere of onion (Allium cepa L.) (Bago and Azcón-Aguilar 1997). Any increase in the soil pH could reduce N2O production. Bender et al. (2014) observed pronounced differences between inoculated and non-inocculated AMF tomato plants in soil pH, indicating that an increase in soil pH may have contributed to reduced N2O emissions. Reduced N2O emissions might have also been observed because there is often an increase in copy numbers of nosZ genes (Zumft 1997). There are also studies reporting a soil pH decrease with mycorrhiza. For example, in a compartmentalized pot system; Li et al. (1991) found that AMF reduced the pH of both mycorrhizosphere and hyphosphere soils by up to 1 pH unit (but see Marschner and Baumann 2003 for a report where AMF reduced pH in the rhizosphere). The observed decreases in pH were likely a result of H+ release during NH4 uptake (Li et al. 1991; Villegas and Fortin 2001). An interesting perspective is that acidic soil conditions can reduce AMF diversity and functioning (Graw 1979; Wang et al. 1993; Helgason and Fitter 2009), meaning that any AMF-induced declines in pH could be self-regulated and thus have a small impact on denitrification. In summary, the changes which AMF induce on soil pH and their likely ecological significance have not been resolved and as a result it remains unclear how such changes might alter denitrification. Most of the existing studies do not discriminate between AMF effects on soil pH and those on denitrification (e.g. Bender et al. 2014) which could only get resolved through sophisticated mechanistic experiments.
AMF-mediated mechanisms acting on plant diversity and productivity
Few studies reported that increased plant diversity reduces N2O production via enhanced N removal efficiency and below-ground plant complementary trait effects in soils or changes in denitrifying communities (Niklaus et al. 2016; Abalos et al. 2017; Wenjuan et al. 2017 but see Bremer et al. 2007). AMF, under common growth settings, such as a low in P soil substrates, promote plant productivity and diversity (van der Heijden et al. 2015; Lin et al. 2015). The authors found that mycorrhizal responsiveness (difference in plant biomass between AMF and non-AMF treatments) was the main mechanism driving plant biodiversity and productivity. AMF enhance plant productivity through increased plant N uptake (Nwaga et al. 2010; Veresoglou et al. 2011; Okiobe et al. 2015). AMF-induced increases in plant productivity, through higher nutrient acquisition could simultaneously lower N and C availability in the rhizosphere (see “AMF-mediated mechanisms acting on soil C availability” section) and this could be the reason why researchers occasionally report lower population densities of bacterial denitrifiers (Amora-Lazcano et al. 1998; Bender et al. 2015). Moreover, increased abundance of AMF in the plant community can lead to a stronger competition between plants for soil nutrients, especially N and P and water (van der Heijden et al. 2006a, b; van der Heijden et al. 2015). These distal AMF-mediated effects could therefore reduce the amount of denitrified N2O. Furthermore, AMF promoted plant diversity could also increase plant complementary traits effects (Van der Heijden 2002; Lin et al. 2015). Decrease in N2O emissions have been previously related to increased grassland plant functional traits (specific leaf area and root length density) as well as to grassland plants that produced higher plant biomass and showed larger N uptake (Abalos et al. 2017). However, Okiobe et al. (2020) observed no relationship between AMF and potential N2O emission rates in a controlled-environment study, even though plant diversity related negatively with N2O emission rates. Plant functional group and plant identity could also play an important role in the denitrification process. AMF can form tripartite symbioses with leguminous plants and rhizobia (Larimer et al. 2014), which are known to enhance N2O production via atmospheric fixation of N2 and increased soil NH+4 availability while grasses generally reduce N2O production (Abalos et al. 2014, 2017; Niklaus et al. 2016). Plant species identity and plant-association status with AMF are the most significant independent variables explaining the reduction in potential nitrification rates in nitrogen-limited grassland soils (Veresoglou et al. 2012b), thus reducing substrate (i.e. NO3−) availability for denitrification. Moreover, plant species identity strongly reduced N2O emissions from grassland and, in terms of relative importance, surpassed species richness, which is considered as a key driver of N2O production (Abalos et al. 2014). There is no study reporting on AMF-promoted effects on plant community structure and productivity in relation to denitrification rates.
We postulate that AMF-promoted increases in plant productivity and diversity will reduce N2O emissions. The magnitude of reduction in N2O release would differ considerably with the degree of mutualism between AMF and their individual host (importance of plant species identity) and the availability of symbiotic partners (importance of plant community).
AMF-mediated mechanisms acting on soil structure
AMF promote soil structure and aggregation (Tisdall and Oades 1982; Rillig and Mummey 2006; van der Heijden et al. 2006a, b; Leifheit et al. 2014). AMF-induced changes in soil structure and the resulting soil aeration could strongly influence microbial activity and N2O emissions, because of the anaerobic requirements of denitrification (Sexstone et al. 1985; Khalil et al. 2005; Ball 2013; Balaine et al. 2016). The hyphae of AMF enmesh and entangle soil primary particles, organic material and small aggregates, facilitating macroaggregate formation and thereby increasing the volume of pore space and air porosity between soil particles (Rillig and Mummey 2006). Soil-aggregation-induced modifications in denitrification might represent a distal AMF-mediated mechanism, which decreases N2 and increases N2O production via promoting incomplete over complete denitrification in the soil (Schlüter et al. 2018).
Okiobe et al. (2019) explored alterations in potential N2O emission rates in relation to a manipulation of soil structure and a manipulation of the densities of mycorrhizal propagules in the soil. The authors observed that extraradical hyphal densities of AMF correlated positively with potential N2O emission rates and water stable aggregates and that potential N2O emission rates increased with soil aggregation leading to a synergy of a proximal mechanism (i.e. direct effects of the abundance of AMF) and a distal mechanism (i.e. alterations in soil aggregation; Okiobe et al. 2019).
Mycelial networks of AMF could also improve soil water holding capacity and soil water repellency through enhanced soil aggregation (Rillig et al. 2010; Veresoglou et al. 2012b). We review the likely changes on soil moisture in “AMF-mediated mechanisms acting on soil pH” section.
Additional to the effects on soil aeration and water retention properties, improved soil aggregation by AMF can increase microbial heterogeneity in the soil matrix, which can in turn affect denitrification. A large number of different microenvironments, such as those formed in and between soil aggregates, can promote diverse microbial communities (Rillig and Mummey 2006; Bach et al. 2018). Any increases in spatial soil heterogeneity, driven by AMF, may induce aggregated point patterns in many microbial denitrifiers, and an uneven distribution of microbial N2O producers and N2O reducers. Sey et al. (2008) found that N2O production is different between micro- and macroaggregates. These are two microhabitats, of which the macroaggregates are much more influenced by AMF than microaggregates (Rillig and Mummey 2006). This could result in spatial variability of denitrification where some aggregated soil microhabitats host incomplete and others complete denitrification.
The abiotic interaction between soil aggregation and denitrification rates and the role of AMF therein have not yet been adequately addressed. The existing literature on AMF and denitrification has not separated between direct (i.e. effect of hyphal growth of AMF on mineral N) and indirect effects (i.e. effect of AMF on soil aggregation) on denitrification and N2O emissions (see Tables 1 and S1).
The role of AMF on denitrification rates in global change
The global surface temperature is predicted to increase between 1.8 and 3.6 °C by the year 2100 and incidences of extreme weather events are expected to increase in frequency (IPCC 2021). Changes in climate directly affect beneficial-plant microbes including AMF and denitrification interactions and could exacerbate the rate of N2O emissions via changes in temperature and soil moisture regimes (e.g. drought). Indirect effects include rising atmospheric CO2 concentrations and associated changes in plant water use efficiency, plant biomass production and rhizodeposition of C substrates (Butterbach-Bahl and Dannenmann 2011). In this section, we elaborate why AMF may reduce N2O emissions from soils at increased temperatures and atmospheric CO2 concentration. The changes (i.e. global change) we review here occur at considerably larger temporal scales (i.e. anthropocene) and this is the reason we felt that we should dedicate a separate section to report on them.
Overall, hyphal growth of AMF and root colonization have been reported to positively respond to increased temperature and atmospheric CO2 levels while negatively to drought stress (Augé 2001, 2004; Rillig et al. 1999, 2000, 2002; Fitter et al. 2000; Compant et al. 2010). Increased air temperature can result in increased plant photosynthesis rates, which lead to a greater allocation of C to AMF and thereby fostering root colonization of AMF and hyphal growth (Rillig et al. 2002; Heinemeyer and Fitter 2004). We postulate that an increase in temperature will increase AMF hyphal densities, and thereby reduce N2O emissions through soil N and C limitation in soils (see paragraph 2.4, 3.2).
Similarly, increased levels of atmospheric CO2 increase growth rates of the mycelium of AMF and soil microbial activity, and induce higher rates of plant photosynthesis and oxygen consumption in the rhizosphere (Anderson et al. 2011; Staddon and Fitter 1998; Staddon et al. 1999a, b; Rillig et al. 1999, 2000). Cheng et al. (2012) found that AMF increased organic C decomposition under elevated CO2, but optimized NH4+ acquisition from soil while reducing nitrification rates. We assume that reduction in nitrification rates by AMF (see Veresoglou et al. 2011; Storer et al. 2018) would also reduce denitrification under elevated CO2 enrichment. AMF reduced N2O emissions under current and predicted future warming scenarios, pinpointing the relevance of AM plant symbiosis in climate change mitigation and under future climate change conditions (Zhang et al. 2021).
Drought stress, which is projected to increase in intensity and frequency in many parts of the globe (IPCC 2021) can impair both below- and above-ground plant growth. This may alter the allocation of photosynthates in the rhizosphere as well as within extraradical AM mycelium (e.g. Zhang et al. 2016). The density of extraradical mycorrhizal hyphae and root colonization of AMF declines under drought settings, even though some ubiquitous species of AMF, such as Rhizophagus irregularis, tolerate it well (Augé 2001; Staddon et al. 2004; Stockinger et al. 2009). N2O emissions from soil of mycorrhizal plants (76R MYC) were higher than that of non-mycorrhizal mutants (rmc) in a drought treatment (low water-filled spore space < 50%), but decreased in a watering treatment (high water-filled spore space > 50%), therefore showing lower emissions at high soil moisture and water-filled spore space (Lazcano et al. 2015). This was explained by the fact that photosynthesis rate and stomatal conductance of 76R MYC were reduced by drought stress compared to the watering treatment. However, water use efficiency increased in 76R MYC compared with rmc plants during drought (Lazcano et al. 2015). Zhang et al. (2015) showed that the proximal AMF-mediated effects on soil N concentrations showing reduction in N2O emissions were dependent on the flooding and draining seasons. While drought stress is expected to affect growth of AMF, flooding conditions could be better tolerated by arbuscular mycorrhizal associated plant hosts. Thus, inoculation with AMF may condition a different ecophysiological response of plants in different stress conditions and this would impact N2O production. The proximal and distal AMF-mediated effects on denitrification rates could vary in arid regions compared to temperate or tropical areas, because of variation in climate drivers accompanied by a spatial and temporal heterogeneity of N2O production. Most of the changes we report here hint us that undergoing climate change, via promoting mycorrhizae, should most likely reduce N2O emissions from ecosystems with AMF.
Future perspectives
The existing literature strongly supports that manipulating AMF could modify emission rates of N2O from soils. A shortcoming of most existing studies is that they have been carried out under artificial settings. Often, the microbial community in such studies has been reintroduced following a sterilization of soil and it is thus questionable whether the assayed denitrification and N2O emission rates are representative of more realistic settings. A particular issue, however, is that the vast majority of studies have been carried out with a single species of AMF, usually Rhizophagus irregularis (e.g. Zhang et al. 2015; Storer et al. 2018). Because of pronounced physiological and functional differences across species of AMF, especially at high taxonomic ranks (e.g. Hart and Reader 2002; Sikes et al. 2010), it is likely that the literature is biased towards the contributions (i.e. performance) of a specific set of widely-cultured isolates of AMF. The fact that plants in soil are colonized by diverse communities of AMF and experience, as a result, complementarity in functional benefits (van der Heijden et al. 1998; Maherali and Klironomos 2007; Wagg et al. 2011) further implies that the contribution of AMF on N cycling processes and denitrification is most likely being underestimated in controlled studies. These issues complicate projections on the contribution of AMF on N2O global emissions and hinder bids to integrate AMF-centred management practices for policy purposes. We can overcome these shortcomings through encouraging experimentation under more realistic settings such as directly comparing N2O emissions under different management practices targeting AMF.
An exciting recent development is the appreciation that the relative abundance of nitrous oxide reductase (nosZ) genes of clade I and clade II could determine the frequency at which N2O is produced as an end product of denitrification (Domeignoz-Horta et al. 2015; Jones et al. 2014). The vast majority of bacteria that possess clade I nosZ genes are denitrifiers possessing the full arsenal of genes needed for denitrification. By a considerable proportion of denitrifiers possessing clade II nosZ genes are limited to the specific denitrification process (Graf et al. 2014). As a result, high relative abundances of bacteria possessing clade II nosZ genes factor complete denitrification (i.e. N2 as end product; Jones et al. 2014). AMF, through hyphodeposition, mycelial turnover, and competition for plant C and soil nutrients (Veresoglou et al. 2012a, b), induce systematic community shifts to the microbial community in the rhizosphere. Oligotrophic conditions can promote clade II over clade I nosZ bacteria (Graf et al. 2016; Conthe et al. 2018) and thus AMF-induced declines in plant available C could promote clade II nosZ denitrifiers. The possibility that the ratio of clade I over clade II nosZ gene-holding organisms changes in response to mycorrhiza has, however, not been addressed so far.
It is clear that almost all crops associate with mycorrhizas of the arbuscular type. Temperate woody habitats are dominated by ectomycorrhizas (ECM). ECM have comparably received little attention on how their manipulation modifies N2O emissions (e.g. Ernfors et al. 2011), possibly because N cycling is slower in these systems and growing woody plants is time consuming. This implies that most likely N2O emissions are considerably lower from ECM-dominated systems (Phillips et al. 2013; Tatsumi et al. 2020; Mushinski et al. 2021). Unlike AMF, however, some ectomycorrhizal fungi possess the ability to denitrify (e.g. Prendergast-Miller et al. 2011), which could also have implications to the denitrification capacity of the ecosystems. Linking the study of AMF on denitrification and N2O emissions with that of ECM could uncover many common proximal and distant mechanisms such as immobilizing N in plant and microbial biomass. There is an increasingly large body of literature assessing the relative efficiency of AMF and ECM systems in performing ecosystem processes (e.g. Jones et al. 1998; Averill et al. 2014). Via combining data on N2O emissions from arbuscular mycorrhizal and ECM systems with background ecosystem process rate information, it might be possible to disentangle the relative contributions of different distant and proximal mycorrhizal mechanisms in mitigating N2O emissions.
We foresee that the accumulation of studies on how AMF alter denitrification will eventually lead to future studies pursuing more specialized topics and thus the field will get subdivided. To address current trends in topics, we carried out a bibliometric coupling analysis (Martin 1964) where studies are analyzed in relation to how similar their reference tables are. We observed in our analysis three main clusters of studies (Fig. 2): studies aiming at a higher mechanistic resolution which integrated assays of alternative N-cycling processes (cluster in purple), studies effectively reproducing agricultural settings (cluster in red) and a set of diverse studies using more settings more reminicent of natural habitats (cluster in green).
Conclusion
We synthesize recent advances in the various routes by which AMF may influence terrestrial denitrification rates in soils. Contrary to the conspicuous ways that AMF can alter denitrification, it remains open to question if and to what degree those are of an ecological significance, meaning that they have strong effects on ecosystems. An obvious way to move forward in relation to the topic is to address individual mechanistic constituents (i.e. individual proximal and distant mechanisms) of how AMF suppress N2O emissions, the end product N2O/N2 ratio and increased N2 losses. Via combining such estimates it should be possible to develop novel process based models which address the ecological importance of AMF in denitrification. Alternatively, to assess ecological significance, research should move to embrace realistic settings and directly compare process rates. We introduce a novel classification of mechanisms via which AMF alter N2O emissions and propose ways to better integrate AMF in ongoing efforts to mitigate greenhouse gas emissions and global change.
References
Abalos D, de Deyn GB, Kuyper TW, van Groenigen JW (2014) Plant species identity surpasses species richness as a key driver of N2O emissions from grassland. Global Change Biol 20:265–275
Abalos D, de Deyn GB, van Groenigen JW (2017) What plant functional traits can reduce nitrous oxide emissions from intensively managed grasslands? Global Change Biol 24:e248–e258
Allen MF (2007) Mycorrhizal fungi: highways for water and nutrients in arid doils. Vedose Zone J 6:291–297
Amora-Lazcano E, Vázquez MM, Azcón R (1998) Response of nitrogen transforming microorganisms to arbuscular mycorrhizal fungi. Biol Fert Soils 27:65–70
Anderson TH, Heinemeyer O, Weigel HJ (2011) Changes in the fungal to-bacterial respiratory ratio and microbial biomass in agriculturally managed soils under free-air CO2 enrichment (FACE) -a six-year survey of a field study. Soil Biol Biochem 43:895–904
Artursson V, Finlay RD, Jansson JK (2006) Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ Microbiol 8:1–10
Atul-Nayyar A, Hamel C, Hanson K, Germida J (2009) The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza 19:239–246
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Augé RM (2004) Arbuscular mycorrhizae and soil/plant water relations. Can J Soil Sci 84:373–381
Augé RM, Saxton AM, Toler HD (2015) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta -analysis. Mycorrhiza 25:13–24
Averill C, Turner BL, Finzi AC (2014) Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505:543–545
Bach EM, Williams RJ, Hargreavesa SK, Yanga F, Hofmockela KS (2018) Greatest soil microbial diversity found in micro-habitats. Soil Biol Biochem 118:217–226
Bago B, Azcón-Aguilar C (1997) Changes in the rhizospheric pH induced by arbuscular mycorrhiza formation in onion (Allium cepa L.). Zeitschrift für Pflanzenernährung und Bodenkunde 160:333–339
Bago B, Vierheilig H, Piché Y, Azcón-Aguilar C (1996) Nitrate depletion and pH changes induced by the extraradical mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown in monoxenic culture. New Phytol 133:273–280
Balaine N, Clough TJ, Beare MH, Thomas SM, Meenken ED (2016) Soil Gas Diffusivity Controls N2O and N2 Emissions and their Ratio. Soil Sci Soc Am J 80:529–540
Ball BC (2013) Soil structure and greenhouse gas emissions: a synthesis of 20 years of experimentation. Eur J Soil Sci 64:357–373
Barton L, Wolf B, Rowlings D, Scheer C, Kiese R, Grace P, Stefanova K, Butterbach-Bahl K (2015) Sampling frequency affects estimates of annual nitrous oxide fluxes. Sci Rep 5:15912
Bender SF, Plantenga F, Neftel A, Jocher M, Oberholzer HR, Kohl L, Giles M, Daniell TJ, Van der Heijden MGA (2014) Symbiotic relationships between soil Fungi and plants reduce N2O emissions from soil. ISME J 8:1336–1345
Bender SF, Conen F, Van der Heijden MGA (2015) Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biol Biochem 80:283–292
Bengtsson G, Fronæus S, Bengtsson-Kloo L (2002) The kinetics and mechanism of oxidation of hydroxylamine by iron(iii). J Chem Soc Dalton Trans 1:2548–2552
Bowles TM, Jackson LE, Loeher M, Cavagnaro TR (2016) Ecological intensification and arbuscular mycorrhizas: a meta-analysis of tillage and cover crop effects. J Appl Ecol 17:1785–1793
Bremer C, Braker G, Matthies D, Reuter A, Engels C, Conrad R (2007) Impact of plant functional group, plant species and sampling time on the composition of nirK-type denitrifier communities in soil. App Environ Microbiol 73:6876–6884
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol 220:1108–1115
Burgin AJ, Groffman PM (2012) Soil O2 controls denitrification rates and N2O yield in a riparian wetland. J Geophys Res 117:G01010
Butterbach-Bahl K, Dannenmann M (2011) Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr Opin Environ Sustain 3:389–395
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister BS (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Phil Trans R Soc B 368:20130122
Cavagnaro TR, Langley AJ, Jackson LE, Smukler SM, Koch GW (2008) Growth, nutrition, and soil respiration of a mycorrhiza-defective tomato mutant and its mycorrhizal wild-type progenitor. Funct Plant Biol 35:228–235
Cavagnaro TR, Barrios-Masias FH, Jackson LE (2012) Arbuscular mycorrhizas and their role in plant growth, nitrogen interception and soil gas efflux in an organic production system. Plant Soil 353:181–194
Cavagnaro RT, Bender SF, Asghari HR, van der Heijden MGA (2015) The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci 20:283–290
Chadwick DR, Cardenas L, Misselbrook TH, Smith KA, Rees RM, Watson CJ, Mcgeough KL, William JR, Clou JM, Thorman RE, Dhanoe MS (2014) Optimizing chamber methods for measuring nitrous oxide emissions from plot-based agricultural experiments. Eur J Soil Sci 65:295–307
Cheng L, Booker FL, Tu C, Burkey KO, Zhou L, Shew HD, Rufty TW, Hu S (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084–1087
Compant S, van der Heijden MGA, Sessitsch A (2010) Climate change effects on beneficial plant-microorganism interaction. FEMS Microbiol Ecol 73:197–214
Conthe M, Wittorf L, Kuenen GK, Kleerebezem R, van Loosdrecht MCM, Hallin S (2018) Life on N2O: deciphering the ecophysiology of N2O respiring bacterial communities in a continuous culture. ISME J 12:1142–1153
Dannenmann M, Butterbach-Bahl K, Gasche R, Willibald G, Papen H (2008) Dinitrogen emissions and the N2:N2O emission ratio of a Rendzic Leptosol as influenced by pH and forest thinning. Soil Biol Biochemi 40:2317–2323
Davidson EA, Keller M, Erickson HE, Verchot LV, Veldkamp E (2000) Testing a conceptual model of soil emissions of nitrous and nitric oxides. Bioscience 50:667–680
Domeignoz-Horta LA, Spor A, Bru D, Breuil MC, Bizouard F, Léonard J, Philippot L (2015) The diversity of the N2O reducers matters for the N2O:N2 denitrification end-product ratio across an annual and perennial cropping system. Front Microbiol 6:971
Drigo B, Donn S (2017) Trading carbon between arbuscular mycorrhizal fungi and their hyphae-associated microbes. In: Johnson NC, Gehring C, Jansa J (Eds). Mycorrhizal Mediation of Soil. Elsevier, pp 395–412
Ernfors M, Rütting T, Klemedtsson L (2011) Increased nitrous oxide emissions from a drained organic forest soil after exclusion of ectomycorrhizal mycelia. Plant Soil 343:161–170
Èuhel J, Šimek M (2011) Proximal and distal control by pH of denitrification rate in a pasture soil. Agric Ecosyst Environ 141:230–233
Fitter AH, Heinemeyer A, Staddon PL (2000) The impact of elevated CO2 and global climate change on arbuscular mycorrhizas: a mycocentric approach. New Phytol 147:179–187
Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW, Haywood J, Lean J, Lowe DC, Myhre G, Nganga J, Prinn R, Raga G, Schulz M, Van Dorland R (2007) Changes in Atmospheric Constituents and in Radiative Forcing. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
Govindarajulu M, Pfeffer PE, Jin HR, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Graf DRH, Jones CM, Hallin S (2014) Intergenomic comparisons highlight modularity of the denitrification pathway and underpin the importance of community structure for N2O emissions. PLoS ONE 9:e114118
Graf DRH, Zhao M, Jones CM, Hallin S (2016) Soil type overrides plant effect on genetic and enzymatic N2O production potential in arable soils. Soil Biol Biochem 100:125–128
Graham JH, Leonard RT, Menge JA (1981) Membrane-mediated decrease in root exudation responsible for phosphorus inhibition of vesicular-arbuscular mycorrhiza formation. Plant Physiol 68:548–552
Graw D (1979) The influence of soil pH on the efficiency of vesicular-arbuscular mycorrhiza. New Phytol 82:687–695
Griffis TJ, Chen Z, Baker JM, Wood JD, Millet DB, Lee X, Venterea RT, Turner PA (2017) Nitrous oxide emissions are enhanced in a warmer and wetter world. Proc Natl Acad Sci U S A 114:12081–12085
Groffman PM, Tiedje JM, Robertson GP, Christensen S (1988) Dentrification at different temporal and geographical scales: proximal and distal controls. In: Wilson JR (ed) Advances in nitrogen cycling in agricultural ecosystems. CAB International, Walling ford, pp 174–192
Groffman PM, Holland E, Myrold DD, Robertson GP, Zou X (1999) Denitrification. In: Robertson GP, Bledsoe CS, Coleman DC, Sollins P (eds) Standard soil methods for long term ecological research. Oxford University Press, New York, pp 272–288
Gui H, Gao Y, Wang Z, Shi L, Yan K, Xu J (2021) Arbuscular mycorrhizal fungi potentially regulate N2O emissions from agricultural soils via altered expression of denitrification genes. Sci Total Environ 774:145133
Hart MM, Reader RJ (2002) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153:335–344
Heinemeyer A, Fitter AH (2004) Impact of temperature on the arbuscular mycorrhizal (AM) symbiosis: growth responses of the host plant and its AM fungal partner. J Exp Bot 55:525–534
Heinemeyer A, Ineson P, Ostle N, Fitter AH (2006) Respiration of the external mycelium in the arbuscular mycorrhizal symbiosis shows strong dependence on recent photosynthates and acclimation to temperature. New Phytol 171:159–170
Helgason T, Fitter AH (2009) Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). J Exp Bot 60:2465–2480
Henderson SL, Dandie CE, Pattern CL, Zebarth BJ, Burton DL, Trevors JT, Goyer C (2010) Changes in denitrifier abundance, denitrification gene mRNA levels, nitrous oxide emissions, and denitrification in anoxic soil microcosms amended with glucose and plant residues. Appl Environ Microbiol 76:2155–2164
Hino T, Matsumoto Y, Nagano S, Sugimoto H, Fukumori Y, Murata T, Iwata S, Shiro Y (2010) Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science 330:1666–1670
Hodge A (2001) Arbuscular mycorrhizal fungi influence decomposition of, but not plant nutrient capture from, glycine patches in soil. New Phytol 151:725–734
Hodge A, Fitter AH (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci U S A 107:13754–13759
Hodge A, Storer K (2015) Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386:1–19
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299
Hughes JK, Hodge A, Fitter AH, Atkin OK (2008) Mycorrhizal respiration: implications for global scaling relationships. Trends Plant Sci 13:583–588
IPCC (2021) Climate Change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. In: Masson-Delmotte V., et al., (Eds.) Cambridge University Press
Jia Y, van der Heijden MGA, Wagg C, Feng G, Walder F (2020) Symbiotic soil fungi enhance resistance and resilience of an experimental grassland to drought and nitrogen deposition. J Ecol 109:3171–3181
Johansen A, Jakobsen I, Jensen ES (1992) Hyphal transport of 15N-labeled nitrogen by a vesicular-arbuscular mycorrhizal fungus and its effect on depletion of inorganic soil-N. New Phytol 122:281–288
Johnson D, Krsek M, Wellington EMH, Stott A, Cole L, Bardgett RD, Read DJ, Leake JR (2005) Soil invertebrates disrupt carbon flow through fungal networks. Nature 309:1047
Jones MD, Durall DM, Tinker PB (1998) A comparison of arbuscular and ectomycorrhizal Eucalyptus coccifera: growth response, phosphorus uptake efficiency and external hyphal production. New Phytol 140:125–134
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480
Jones CM, Spor A, Brennan FP, Breuil MC, Bru D, Lemanceau P, Griffiths B, Hallin S, Philippot L (2014) Recently identified microbial guild mediates soil N2O sink capacity. Nat Clim Change 4:801–805
Kaiser C, Kilburn MR, Clode PL, Fuchslueger L, Koranda M, Cliff JB, Solaiman ZM, Murphy DV (2015) Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytol 205:1537–1551
Keeney DR (1986) Critique of the acetylene blockage technique for field measurement of denitrification. In: Hauck RD, Weaver RW (eds) Field measurement of dinitrogen fixation and denitrification, vol 18. SSSA Special Publications
Khalil K, Renault P, Mary B (2005) Effects of transient anaerobic conditions in the presence of acetylene on subsequent aerobic respiration and N2O emission by soil aggregates. Soil Biol Biochem 37:1333–1342
Krause HM, Thonar C, Eschenbach W, Well R, Paul Mader P, Behrens S, Kappler A, Gattinger A (2017) Long term farming systems affect soils potential for N2O production and reduction processes under denitrifying conditions. Soil Biol Biochem 114:31–41
Langley JA, Johnson NC, Koch GW (2005) Mycorrhizal status influences the rate but not the temperature sensitivity of soil respiration. Plant Soil 277:335–344
Larimer AL, Clay K, Bever JD (2014) Synergism and context dependency of interactions between Arbuscular mycorrhizal fungi and Rhizobia with a prairie legume. Ecology 95:1045–1054
Lazcano C, Barrios-Masias FH, Jackson LE (2015) Arbuscular mycorrhizal effects on plant water relations and soil greenhouse gas emissions under changing moisture regimes. Soil Biol Biochem 74:184–192
Lee DS, Bouwman AF, Asman WAH, Dentener FJ, van der Hoek KW, Olivier JGJ (1997) Emissions of nitric oxide, nitrous oxide and ammonia from grasslands on a global scale. In: Jarvis SC, Pains BF (eds) Gaseous nitrogen emissions from grasslands. CAB International, Wallingford, pp 353–371
Lehmann A, Rillig MC (2015) Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops - A meta-analysis. Soil Biol Biochem 81:147–158
Leifheit EF, Veresoglou SD, Lehmann A, Morris EK, Rillig MC (2014) Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation- a meta-analysis. Plant Soil 374:523–537
Leigh J, Hodge A, Fitter AH (2009) Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol 181:199–207
Li XL, George E, Marschner H (1991) Phosphorus depletion and pH decrease at the root-soil and hyphaesoil interfaces of VA mycorrhizal white clover fertilized with ammonium. New Phytol 119:397–404
Lin G, McCormack ML, Guo D (2015) Arbuscular mycorrhizal fungal effects on plant competition and community structure. J Ecol 103:1224–1232
Liu B, Frostegård Å, Bakken LR (2014) Impaired reduction of N2O to N2 in acid soils is due to a posttranscriptional interference with the expression of nosZ. Mbio 5:e01383–e1414
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748
Marschner P, Baumann K (2003) Changes in bacterial community structure induced by mycorrhizal colonisation in split-root maize. Plant Soil 251:279–289
Marschner P, Crowley DE, Higashi M (1997) Root exudation and physiological status of a root-colonizing fluorescent pseudomonad in mycorrhizal and non-mycorrhizal pepper (Capsicum annuum L.). Plant Soil 189:11–20
Martin J (1964) Bibliometric coupling. J Doc 20:236–236
Mushinski RM, Rayns ZC, Raff JD, Craig ME, Pusede SE, Rusch DB, White JR, Phillips RP (2021) Nitrogen cycling microbiomes are structured by plant mycorrhizal associations with consequences for nitrogen oxide fluxes in forests. Glob Chang Biol 27:1068–1082
Niklaus PA, Le Roux X, Poly F, Buchmann N, Lorenzen MS, Weigelt A, Barnard RL (2016) Plant species diversity affects soil-atmosphere fluxes of methane and nitrous oxide. Oecologia 181:919–930
Nottingham AT, Turner BL, Winter K, van der Heijden MGA, Tanner EVJ (2010) Arbuscular mycorrhizal mycelial respiration in a moist tropical forest. New Phytol 186:957–967
Nuccio EE, Hodge A, Pett-Ridge J, Herman DJ, Weber PK, Firestone MK (2013) An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ Microbiol 15:1870–1881
Nwaga D, Jansa J, Abossolo AM, Frossard E (2010) The potential of soil beneficial micro-organisms for slash-and-burn agriculture in the humid forest zone of sub Saharan Africa. In: Dion P (ed) Soil biology and agriculture in the tropics. Springer Heidelberg Dordrecht, London, New York, pp 80–107
Okiobe ST, Abossolo AM, Bougnom BP, Boyomo O, Nwaga D (2015) Improvement of arbuscular mycorrhizal fungi inoculum production by nutrient solution concentration and soil texture variation. Int J Agron Agric Res 6:7–20
Okiobe ST, Augustin J, Mansour I, Veresoglou SD (2019) Disentangling direct and indirect effects of mycorrhiza on nitrous oxide activity and denitrification. Soil Biol Biochem 134:142–151
Okiobe ST, Rillig MC, Mola M, Augustin J, Parolly G, Veresoglou SD (2020) Arbuscular mycorrhiza has little influence on N2O potential emissions compared to plant diversity in experimental plant communities. FEMS Microbiol Ecol 96:fiz208
Park JY, Yoo YJ (2009) Biological nitrate removal in industrial wastewater treatment: which electron donor we can choose. Appl Microbiol Biotechnol 82:415–429
Philippot L, Cuhel J, Saby NP, Cheneby D, Chronakova A, Bru D, Arrouays D, Martin F, Laurent F, Simek M (2009) Mapping field-scale spatial patterns of size and activity of the denitrifier community. Environ Microbiol 11:1518–1526
Philippot L, Andert J, Jones CM, Bru DI, Hallin S (2011) Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil. Glob Change Biol 17:1497–1504
Philippot L, Spor A, Hénault C, Bru D, Bizouard F, Jones CM, Sarr A, Maron PA (2013) Loss in microbial diversity affects nitrogen cycling in soil. ISME J 7:1609–1619
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol 199:41–51
Powell JR, Rillig MC (2018) Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol 220:1059–1075
Prendergast-Miller MT, Baggs EM, Johnson D (2011) Nitrous oxide production by the ectomycorrhizal fungi Paxillus involutus and Tylospora fibrillosa. FEMS Microbiol Let 316:31–35
Püschel D, Bitterlich M, Rydlová J, Jansa J (2020) Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: a Gordian knot of roots and hyphae. Mycorrhiza 30:299–313
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125
Reay DS, Davidson EA, Smith KA, Smith P, Melillo JM, Dentener F, Crutzen PJ (2012) Global agriculture and nitrous oxide emissions. Nat Clim Chang 2:140–416
Rillig MC (2004a) Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Lett 7:740–754
Rillig MC (2004b) Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Let 7:740–754
Rillig MC, Mummey DL (2006) Mycorrhizas and soil structure. New Phytol 171:41–53
Rillig MC, Field CB, Allen MF (1999) Soil biota responses to long-term atmospheric CO2 enrichment in two California annual grasslands. Oecologia 119:572–577
Rillig MC, Hernandez GY, Newton PCD (2000) Arbuscular mycorrhizae respond to elevated atmospheric CO2 after long-term exposure: evidence from a CO2 spring in New Zealand supports the resource balance model. Ecol Let 3:475–478
Rillig MC, Wright SF, Shaw MR, Field CB (2002) Artificial climate warming positively affects arbuscular mycorrhizae but decreases soil aggregate water stability in an annual grassland. Oikos 97:52–58
Rillig MC, Mardatin NF, Leifheit EF, Antunes PM (2010) Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates. Soil Biol Biochem 42:1189–1191
Rillig MC, Sosa-Hernandez MA, Roy J, Aguilar-Trigueros CA, Valyi K, Lehmann A (2016) Towards an integrated mycorrhizal technology: harnessing mycorrhizae for sustainable intensification in agriculture. Frontiers Plant Sci 7:1625
Rillig MC, Aguilar-Trigueros CA, Camenzind T, Cavagnaro T, Degrune F, Hohmann P, Lammel D, Mansour I, Roy J, van der Heijden MGA, Yang G (2018) Why farmers should manage the arbuscular mycorrhizal symbiosis - a response to Ryan and Graham. New Phytol 222:1171–1175
Robertson GP, Groffman PM (2015) Nitrogen transformations. In: Paul EA (ed) Soil microbiology, ecology and biochemistry, 4th edn. Academic Press, Burlington, pp 421–446
Saggar S, Jha N, Deslippe J, Bolan NS, Luo J, Giltrap DL, Kim DG, Zaman M, Tilman RW (2013) Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci Tot Environ 465:173–195
Samad MS, Bakken LR, Nadeem S, Clough TJ, de Klein CAM, Richards KG, Lanigan GJ, Morales SE (2016) High resolution denitrification kinetics in pasture soils link N2O emissions to pH, and denitrification to C Mineralization. PLoS ONE 11:0151713
Sanford RA, Wagner DD, Wu Q, Chee-Sanford JC, Thomas SH, Cruz-García C, Rodríguez G, Massol-Deyá A, Krishnani KK, Ritalahti KM, Nissen S, Konstantinidis KT, Löffleret FE (2012) Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc Natl Acad Sci U S A 109:19709–19714
Schlüter S, Henjes S, Zawallich J, Bergaust L, Horn M, Ippisch O, Vogel HJ, Dörsch P (2018) Denitrification in soil aggregate analogues-effect of aggregate size and oxygen diffusion. Front Environ Sci 6:17
Sexstone AJ, Revsbech NP, Parkin TB, Tiedje JM (1985) Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci Soc Am J 49:645–651
Sey BS, Manceur AM, Wahlen JK, Gregorich EG, Rochette P (2008) Small-scale heterogeneity in carbon dioxide, nitrous oxide and methane production from aggregates of a cultivated sandy-loam soil. Soil Biol Biochem 40:2468–2473
Shi ZY, Zhang XF, Wang FY (2010) Influence of mycorrhizal fungi on soil respiration. Ecol Environ Sci l9:233–238
Sikes BA, Powell JR, Rillig MC (2010) Deciphering the relative contributions of multiple functions within plant-microbe symbioses. Ecology 91:1591–1597
Šimek M, Cooper JE (2002) The influence of soil pH on denitrification: progress towards the understanding of this interaction over the last 50 years. Eur J Soil Sci 53:345–354
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, Boston
Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A (2003) Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur J Soil Sci 54:779–791
Sokol NW, Bradford MA (2019) Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat Geosci 12:46–53
Springmann M, Clark M, Mason-D’croz D, Wiebe K, Bodirsky BL, Lassaletta L, de Vries W, Vermeulen SJ, Herrero M, Carlson KM, Jonell M, Troell M, Declerck F, Gordon LJ, Zurayk R, Scarborough P, Rayner M, Loken B, Fanzo J, Godfray HCJ, Tilman D, Rockström J, Willett W (2018) Options for keeping the food system within environmental limits. Nature 562:519–525
Staddon PL, Fitter AH (1998) Does elevated atmospheric carbon dioxide affect arbuscular mycorrhizas? Trends Ecol Evol 13:455–458
Staddon PL, Fitter AH, Graves JD (1999a) Effect of elevated atmospheric CO2 on mycorrhizal colonization, external mycorrhizal colonization on photosynthesis and biomass hyphal production and phosphorus inflow in Plantago Lanceolata lanceolata and Trifolium repens in the association with the arbuscular mycorrhizal fungus Glomus mosseae. Glob Chang Biol 5:347–358
Staddon PL, Fitter AH, Robinson D (1999b) Effects of mycorrhizal colonization and elevated atmospheric carbon dioxide on carbon fixation and below-ground carbon partitioning in Plantago lanceolata. J Exp Bot 50:853–860
Staddon PL, Gregersen R, Jakobsen I (2004) The response of two Glomus mycorrhizal fungi and a fine endophyte to elevated atmospheric CO2, soil warming and drought. Glob Chang Biol 10:1909–1921
Stein LY (2011) Surveying N2O-Producing Pathways in Bacteria. Methods Enzymol 486:131–152
Stockinger H, Walker C, Schüßler A (2009) ‘Glomus intraradices DAOM197198 ’, a model fungus in arbuscular mycorrhiza research, is not Glomus intraradices. New Phytol 183:1176–1187
Storer K, Coggan A, Ineson P, Hodge A (2018) Arbuscular mycorrhizal fungi reduce nitrous oxide emissions from N2O hotspots. New Phytol 220:1285–1295
Suzuki S, Kataoka K, Yamaguchi K (2000) Metal coordination and mechanism of multicopper nitrite reductase. Acc Chem Res 33:728–735
Syakila A, Kroeze C (2011) The global nitrogen budget revisited. Greenhouse Gas Meas Manage 1:17–26
Tamayo E, Gómez-Gallego T, Azcón-Aguilar C, Nuria Ferrol N (2014) Genome-wide analysis of copper, iron and zinc transporters in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front Plant Sci 5:547
Tanaka Y, Yano K (2005) Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant Cell Environ 28:1247–1254
Tatsumi C, Taniguchi T, Du S, Yamanaka N, Tateno R (2020) Soil nitrogen cycling is determined by the competition between mycorrhiza and ammonia-oxidizing prokaryotes. Ecology 101:e02963
Teutscherova N, Vazquez E, Arango J, Arevalo A, Benito M, Pulleman M (2018) Native arbuscular mycorrhizal fungi increase the abundance of ammonia-oxidizing bacteria, but suppress nitrous oxide emissions shortly after urea application. Geoderma 338:493–501
Teutscherova N, Vazquez E, Arangoc J, Arevalo A, Benito M, Pulleman M (2019) Native arbuscular mycorrhizal fungi increase the abundance of ammonia-oxidizing bacteria, but suppress nitrous oxide emissions shortly after urea application. Geoderma 338:493–501
Thompson AJ, Giannopoulos G, Pretty J, Baggs EM, Richardson DJ (2012) Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philos Trans R Soc Lond B Biol Sci 367:1157–1168
Tiedje JM, Simkins S, Groffman PM (1989) Perspectives on measurement of denitrification in the field including recommended protocols for acetylene based methods. Plant Soil 115:261–284
Tisdall JM, Oades JM (1982) Organic matter and water-stable aggregates in soils. J Soil Sci 33:141–163
Van der Heijden MGA (2002) Arbuscular mycorrhizal fungi as a determinant of plant diversity: in Search of Underlying Mechanisms and General Principles. van der Heijden MGA , Sanders IR (eds). In Mycorrhizal Ecology. pp 243–256
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:72–75
Van der Heijden MGA, Bakker R, Verwaal J, Scheublin TR, Rutten M, van Logtestijn R, Staehelin C (2006a) Symbiotic bacteria as a determinant of plant community structure and plant productivity in dune grassland. FEMS Microbiol Ecol 56:178–187
van der Heijden MGA, Streitwolf-Engel R, Riedl R, Siegrist S, Neudecker A, Ineichen K, Boller T, Wiemken A, Sanders IR (2006b) The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol 172:739–752
van der Heijden MGA, De Bruin S, Luckerhoff L, van Logtestijn RSP, Schlaeppi K (2015) A widespread plant-fungal-bacteria symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J 10:389–399
Verbruggen E, Veresoglou SD, Anderson IC, Caruso T, Hammer EC, Kohler J, Rillig MC (2013) Arbuscular mycorrhizal fungi – short-term liability but long-term benefits for soil carbon storage. New Phytol 197:366–368
Veresoglou SD, Robin S, Mamolos AP, Veresoglou DS (2011) Plant species identity and arbuscular mycorrhizal status modulate potential nitrification rates in nitrogen-limited grassland soils. J Ecol 99:1339–1349
Veresoglou SD, Shaw LJ, Hooker JE, Sen R (2012a) Arbuscular mycorrhizal modulation of diazotrophic and denitrifying microbial communities in the (mycor)rhizosphere of Plantago lanceolata. Soil Biol Biochem 53:78–81
Veresoglou SD, Chen B, Rillig MC (2012b) Arbuscular mycorrhiza and soil nitrogen cycling. Soil Biol Biochem 46:53–62
Veresoglou SD, Verbruggen E, Makarova O, Mansour I, Sen R, Rillig MC (2019) Arbuscular mycorrhizal fungi alter the community structure of ammonia oxidizers at high fertility via competition for soil NH4+. Microb Ecol 78:147–158
Villegas J, Fortin JA (2001) Phosphorus solubilization and pH changes as a result of the interactions between soil bacteria and arbuscular mycorrhizal fungi on a medium containing NH+4 as nitrogen source. Can J Bot 79:865–870
Wagg C, Jansa J, Schmid B, van der Heijden MGA (2011) Belowground biodiversity effects of plant symbionts support aboveground productivity. Ecol Lett 14:1001–1009
Wallenstein MD, Myrold DD, Firestone M, Voytek M (2006) Environmental controls on denitrification communities and denitrification rates: insights from molecular methods. Ecol Appl 16:2143–2152
Wang GM, Stribley DP, Tinker PB, Walker C (1993) Effects of pH on arbuscular mycorrhiza I. Field observations on the long-term liming experiments at Rothamsted and Woburn. New Phytol 124:465–472
Wang M, Hu R, Zhao J, Kuzyakov Y, Liu S (2016) Iron oxidation affects nitrous oxide emissions via donating electrons to denitrification in paddy soils. Geoderma 271:173–180
Watts SH, Seitzinger SP (2000) Denitrication rates in organic and mineral soils from riparian sites: a comparison of N2 flux and acetylene inhibition methods. Soil Biol Biochem 32:1383–1392
Wei W, Isobe K, Nishizawa T, Zhu L, Shiratori Y, Ohte N, Koba K, Otsuka S, Senoo K (2015) Higher diversity and abundance of denitrifying microorganisms in environments than considered previously. ISME J 9:1954–1965
Wenjuan H, Mengmeng S, Jie C, Yuan R, Ronghua X, Chongbang Z, Ying G (2017) Plant species diversity reduces NO but not CH emissions from constructed wetlands under high nitrogen levels. Environ Sci Pollut Res 24:5938–6584
Zhang X, Wang L, Ma F, Shan D (2015) Effects of arbuscular mycorrhizal fungi on N2O emissions from rice paddies. Water Air Soil Pollut 226:222–232
Zhang B, Li S, Chen S, Ren T, Yang Z, Zhao H, Liang Y, Han X (2016) Arbuscular mycorrhizal fungi regulate soil respiration and its response to precipitation change in a semiarid steppe. Sci Rep 6:19990
Zhang S, Lehmann A, Zheng W, You Z, Rillig MC (2019) Arbuscular mycorrhizal fungi increase grain yields: a meta-analysis. New Phytol 222:543–555
Zhang S, You Z, Guo X, Yun W, Xia Y, Rillig MC (2020) Suitability of mycorrhiza-defective rice and its progenitor for studies on the control of nitrogen loss in paddy fields via arbuscular mycorrhiza. Frontiers Microbiol 11:186
Zhang H, Powell JR, Power SA, Churchill AC, Plett JM, Macdonald CA, Jacob V, Kim GW, Pendall E, Tissue DT, Catunda KLM, Igwenagu C, Carrillo Y, Moore BD, Anderson IC (2021) Arbuscular mycorrhizal fungal-mediated reductions in N2O emissions were not impacted by experimental warming for two common pasture species. Pedobiologia 87–88:150744
Zhou Y, Watts SE, Boutton TW, Archer SR (2019) Root density distribution and biomass allocation of co-occurring woody plants on contrasting soils in a subtropical savanna parkland. Plant Soil 438:263–279
Zhu X, Silva LCR, Doane TA, Horwath WR (2013) Iron: the forgotten driver of nitrous oxide production in agricultural soil. PLoS ONE 8:60146
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616
Acknowledgements
The manuscript benefitted considerably from the constructive comments from four anonymous reviewers.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Katharina Pawlowski.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Okiobe, S.T., Pirhofer-Walzl, K., Leifheit, E.F. et al. Proximal and distal mechanisms through which arbuscular mycorrhizal associations alter terrestrial denitrification. Plant Soil 476, 315–336 (2022). https://doi.org/10.1007/s11104-022-05534-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05534-x