Abstract
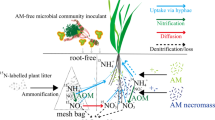
Arbuscular mycorrhizal fungi (AMF) establish mutualistic relationships with the majority of terrestrial plants, increasing plant uptake of soil nitrogen (N) in exchange for photosynthates. And may influence soil ammonia (NH3) volatilization and nitrous oxide (N2O) emissions directly by improving plant N uptake, and/or indirectly by modifying soil bacterial community composition for the soil C availability increasing. However, the effects of AMF on soil NH3 volatilization and N2O emissions and their underlying mechanisms remain unclear. We carried out two independent experiments using contrasting methods, one with a compartmental box device (in 2016) and the other with growth pot experiment (in 2020) to examine functional relationships between AMF and soil NH3 volatilization and N2O emissions under varying N input. The presence of AMF significantly reduced soil NH3 volatilization and N2O emissions while enhancing plant biomass and plant N acquisition, and reducing soil NH4+ and NO3−, even with high N input. The presence of AMF also significantly reduced the relative abundance within the bacterial orders Sphingomonadales and Rhizobiales. Sphingomonadales correlated significantly and positively with soil NH3 volatilization in 2016 and N2O emissions, whereas Rhizobiales correlated positively with soil N2O emissions. High N input significantly increased soil NH3 volatilization and N2O emissions with increasing relative abundance of Sphingomonadales and Rhizobiales. These findings demonstrate the contribution of AMF in regulating NH3 and N2O emission by improving plant N uptake and altering soil bacterial communities. They also suggest that altering the rhizosphere microbiome might offer additional potential for restoration of N-enriched agroecosystems.







Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Data Availability
The data underlying this article are available in the article and in its online Supplementary Material.
References
Bai M, Suter H, Lam SK, Davies R, Flesch TK, Chen DL (2018) Gaseous emissions from an intensive vegetable farm measured with slant-path FTIR technique. Agr Forest Meteorol 258:50–55. https://doi.org/10.1016/j.agrformet.2018.03.001
Huang S, Lv WS, Bloszies S, Shi QH, Pan XH, Zeng YJ (2016) Effects of fertilizer management practices on yield-scaled ammonia emissions from croplands in China: a meta-analysis. Field Crop Res 192:118–125. https://doi.org/10.1016/j.fcr.2016.04.023
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125. https://doi.org/10.1126/science.1176985
Shcherbak I, Millar N, Robertson GP (2014) Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc Natl Acad Sci USA 111:9199–9204. https://doi.org/10.1073/pnas.1322434111
Degaspari IAM, Soares JR, Montezano ZF, Del Grosso SJ, Vitti AC, Rossetto R, Cantarella H (2020) Nitrogen sources and application rates affect emissions of N2O and NH3 in sugarcane. Nutr Cycl Agroecosys 116:329–344. https://doi.org/10.1007/s10705-019-10045-w
Paulot F, Jacob DJ, Pinder RW, Bash JO, Travis K, Henze DK (2014) Ammonia emissions in the United States, European Union, and China derived by high-resolution inversion of ammonium wet deposition data: Interpretation with a new agricultural emissions inventory (MASAGE_NH3). J Geophys Res-Atmos 119:4343–4364. https://doi.org/10.1002/2013JD021130
Gilliam FS (1987) The chemistry of wet deposition for a tallgrass prairie ecosystem: inputs and interactions with plant canopies. Biogeochemistry 4:203–217. https://doi.org/10.1007/BF02187366
Lau N, Charlson RJ (1977) On the discrepancy between background atmospheric ammonia gas measurements and the existence of acid sulfates as a dominant atmospheric aerosol. Atmos Environ 11:475–478. https://doi.org/10.1016/0004-6981(77)90010-5
Sun B, Bai ZH, Bao LJ, Xue LX, Zhang SW, Wei YX, Zhang ZY, Zhuang GQ, Zhuang XL (2020) Bacillus subtilis biofertilizer mitigating agricultural ammonia emission and shifting soil nitrogen cycling microbiomes. Environ Int 144:105989. https://doi.org/10.1016/j.envint.2020.105989
Meng L, Ding WX, Cai ZC (2005) Long-term application of organic manure and nitrogen fertilizer on N2O emissions, soil quality and crop production in a sandy loam soil. Soil Biol Biochem 37:2037–2045. https://doi.org/10.1016/j.soilbio.2005.03.007
Prosser JI, Hink L, Gubry Rangin C, Nicol GW (2020) Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies. Global Change Biol 26:103–118. https://doi.org/10.1111/gcb.14877
Zhu X, Burger M, Doane TA, Horwath WR (2013) Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc Natl Acad Sci USA 110:6328–6333. https://doi.org/10.1073/pnas.1219993110
Frey SD (2019) Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu Rev Ecol Evol S 50:237–259. https://doi.org/10.1146/annurev-ecolsys-110617-062331
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299. https://doi.org/10.1038/35095041
Bender SF, Plantenga F, Neftel A, Jocher M, Oberholzer H, Köhl L, Giles M, Daniell TJ, Van Der Heijden MG (2014) Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil. ISME J 8:1336–1345. https://doi.org/10.1038/ismej.2013.224
Zhang X, Wang L, Ma F, Shan D (2015) Effects of arbuscular mycorrhizal fungi on N2O emissions from rice paddies. Water Air Soil Poll 226:1–10. https://doi.org/10.1007/s11270-015-2493-4
Lazcano C, Barrios-Masias FH, Jackson LE (2014) Arbuscular mycorrhizal effects on plant water relations and soil greenhouse gas emissions under changing moisture regimes. Soil Biol Biochem 74:184–192. https://doi.org/10.1016/j.soilbio.2014.03.010
Storer K, Coggan A, Ineson P, Hodge A (2018) Arbuscular mycorrhizal fungi reduce nitrous oxide emissions from N2O hotspots. New Phytol 220:1285–1295. https://doi.org/10.1111/nph.14931
Okiobe ST, Augustin J, Mansour I, Veresoglou SD (2019) Disentangling direct and indirect effects of mycorrhiza on nitrous oxide activity and denitrification. Soil Biol Biochem 134:142–151. https://doi.org/10.1016/j.soilbio.2019.03.025
Cavagnaro TR, Barrios-Masias FH, Jackson LE (2012) Arbuscular mycorrhizas and their role in plant growth, nitrogen interception and soil gas efflux in an organic production system. Plant Soil 353:181–194. https://doi.org/10.1007/s11104-011-1021-6
Okiobe ST, Rillig MC, Mola M, Augustin J, Parolly G, Veresoglou SD (2020) Arbuscular mycorrhiza has little influence on N2O potential emissions compared to plant diversity in experimental plant communities. FEMS Microbiol Ecol 96:z208. https://doi.org/10.1093/femsec/fiz208
Shen YW, Zhu B (2021) Arbuscular mycorrhizal fungi reduce soil nitrous oxide emission. Geoderma 402:115179. https://doi.org/10.1016/j.geoderma.2021.115179
Gui H, Gao Y, Wang ZH, Shi LL, Yan K, Xu JC (2021) Arbuscular mycorrhizal fungi potentially regulate N2O emissions from agricultural soils via altered expression of denitrification genes. Sci Total Environ 774:145133. https://doi.org/10.1016/j.scitotenv.2021.145133
Pellegrino E, Öpik M, Bonari E, Ercoli L (2015) Responses of wheat to arbuscular mycorrhizal fungi: a meta-analysis of field studies from 1975 to 2013. Soil Biol Biochem 84:210–217. https://doi.org/10.1016/j.soilbio.2015.02.020
Ruzicka DR, Barrios-Masias FH, Hausmann NT, Jackson LE, Schachtman DP (2010) Tomato root transcriptome response to a nitrogen-enriched soil patch. BMC Plant Biol 10:1–19. https://doi.org/10.1186/1471-2229-10-75
Scheublin TR, Van Der Heijden MG (2006) Arbuscular mycorrhizal fungi colonize nonfixing root nodules of several legume species. New Phytol 172:732–738. https://doi.org/10.1111/j.1469-8137.2006.01858.x
Hodge A, Fitter AH (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci USA 107:13754–13759. https://doi.org/10.1073/pnas.1005874107
Leigh J, Hodge A, Fitter AH (2009) Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol 181:199–207. https://doi.org/10.1111/j.1469-8137.2008.02630.x
Govindarajulu M, Pfeffer PE, Jin HR, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823. https://doi.org/10.1038/nature03610
Herman DJ, Firestone MK, Nuccio E, Hodge A (2012) Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol Ecol 80:236–247. https://doi.org/10.1111/j.1574-6941.2011.01292.x
Thompson KA, Bent E, Abalos D, Wagner-Riddle C, Dunfield KE (2016) Soil microbial communities as potential regulators of in situ N2O fluxes in annual and perennial cropping systems. Soil Biol Biochem 103:262–273. https://doi.org/10.1016/j.soilbio.2016.08.030
Veresoglou SD, Verbruggen E, Makarova O, Mansour I, Sen R, Rillig MC (2019) Arbuscular mycorrhizal fungi alter the community structure of ammonia oxidizers at high fertility via competition for soil NH4+. Microb Ecol 78:147–158. https://doi.org/10.1007/s00248-018-1281-2
Mandal S, Donner E, Vasileiadis S, Skinner W, Smith E, Lombi E (2018) The effect of biochar feedstock, pyrolysis temperature, and application rate on the reduction of ammonia volatilisation from biochar-amended soil. Sci Total Environ 627:942–950. https://doi.org/10.1016/j.scitotenv.2018.01.312
Zhou YC, Hu B, Zhang WM, Zhang Y, Zhang YP, Zhang TQ (2020) Nitrous oxide emission from stormwater biofilters in alternating dry and wet weather. Environ Res 191:110137. https://doi.org/10.1016/j.envres.2020.110137
Liang JF, An J, Gao JQ, Zhang XY, Song MH, Yu FH (2019) Interactive effects of biochar and AMF on plant growth and greenhouse gas emissions from wetland microcosms. Geoderma 346:11–17. https://doi.org/10.1016/j.geoderma.2019.03.033
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet-sieving and decanting. Trans Br Mycol Soc 46:235–244. https://doi.org/10.1016/S0007-1536(63)80079-0
Xu J, Liu SJ, Song SR, Guo HL, Tang JJ, Yong JWH, Ma YD, Chen X (2018) Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biol Biochem 120:181–190. https://doi.org/10.1016/j.soilbio.2018.02.010
Chen BD, Christie P, Li XL (2001) A modified glass bead compartment cultivation system for studies on nutrient and trace metal uptake by arbuscular mycorrhiza. Chemosphere 42:185–192. https://doi.org/10.1016/S0045-6535(00)00124-7
Nômmik H (1973) The effect of pellet size on the ammonia loss from urea applied to forest soil. Plant Soil 39:309–318. https://doi.org/10.1007/BF00014798
Li XY, Cheng SL, Fang HJ, Yu GR, Dang XS, Xu MJ, Wang L, Si GY, Geng J, He S (2015) The contrasting effects of deposited NH4+ and NO3− on soil CO2, CH4 and N2O fluxes in a subtropical plantation, southern China. Ecol Eng 85:317–327. https://doi.org/10.1016/j.ecoleng.2015.10.003
Cabrera ML, Beare MH (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Am J 57:1007–1012. https://doi.org/10.2136/sssaj1993.03615995005700040021x
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:118–158. https://doi.org/10.1016/S0007-1536(70)80110-3
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Walder F, Niemann H, Natarajan M, Lehmann MF, Boller T, Wiemken A (2012) Mycorrhizal networks: common goods of plants shared under unequal terms of trade. Plant Physiol 159:789–797. https://doi.org/10.1104/pp.112.195727
Wang SS, Chen AQ, Xie K, Yang XF, Luo ZZ, Chen JD, Zeng DC, Ren YH, Yang CF, Wang LX, Feng HM, López-Arredondo DL, Herrera-Estrella LR, Xu GH (2020) Functional analysis of the OsNPF4. 5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc Natl Acad Sci USA 117:16649–16659. https://doi.org/10.1073/pnas.2000926117
Guo JJ, Ling N, Chen H, Zhu C, Kong YL, Wang M, Shen QR, Guo SW (2017) Distinct drivers of activity, abundance, diversity and composition of ammonia-oxidizers: evidence from a long-term field experiment. Soil Biol Biochem 115:403–414. https://doi.org/10.1016/j.soilbio.2017.09.007
Sun HJ, Zhang Y, Yang YT, Chen YD, Jeyakumar P, Shao QL, Zhou YF, Ma M, Zhu RQ, Qian QW, Fan YR, Xiang SJ, Zhai NN, Li YF, Zhao QF, Wang HL (2021) Effect of biofertilizer and wheat straw biochar application on nitrous oxide emission and ammonia volatilization from paddy soil. Environ Pollut 275:116640. https://doi.org/10.1016/j.envpol.2021.116640
Chen YL, Chen BD, Hu YJ, Li T, Zhang X, Hao ZP, Wang YS (2013) Direct and indirect influence of arbuscular mycorrhizal fungi on abundance and community structure of ammonia oxidizing bacteria and archaea in soil microcosms. Pedobiologia 56:205–212. https://doi.org/10.1016/j.pedobi.2013.07.003
Veresoglou SD, Sen R, Mamolos AP, Veresoglou DS (2011) Plant species identity and arbuscular mycorrhizal status modulate potential nitrification rates in nitrogen-limited grassland soils. J Ecol 99:1339–1349. https://doi.org/10.1111/j.1365-2745.2011.01863.x
Castellano-Hinojosa A, Correa-Galeote D, González-López J, Bedmar EJ (2020) Effect of nitrogen fertilisers on nitrous oxide emission, nitrifier and denitrifier abundance and bacterial diversity in closed ecological systems. Appl Soil Ecol 145:103380. https://doi.org/10.1016/j.apsoil.2019.103380
Qiu YP, Jiang Y, Guo L, Zhang L, Burkey KO, Zobel RW, Reberg-Horton SC, Shew HD, Hu SJ (2019) Shifts in the composition and activities of denitrifiers dominate CO2 stimulation of N2O emissions. Environ Sci Technol 53:11204–11213. https://doi.org/10.1021/acs.est.9b02983
Fang WS, Yan DD, Wang XL, Huang B, Wang XN, Liu J, Liu XM, Li Y, Ouyang CB, Wang QX, Cao AC (2018) Responses of nitrogen-cycling microorganisms to dazomet fumigation. Front Microbiol 9:2529. https://doi.org/10.3389/fmicb.2018.02529
Chen MM, Pan H, Sun MJ, He W, Wei M, Lou YH, Wang H, Yang QG, Feng HJ, Zhuge YP (2021) Nitrosospira cluster 3 lineage of AOB and nirK of Rhizobiales respectively dominated N2O emissions from nitrification and denitrification in organic and chemical N fertilizer treated soils. Ecol Indic 127:107722. https://doi.org/10.1016/j.ecolind.2021.107722
Pan H, Ying SS, Liu HY, Zeng LZ, Zhang QC, Liu YM, Xu JM, Li Y, Di HJ (2018) Microbial pathways for nitrous oxide emissions from sheep urine and dung in a typical steppe grassland. Biol Fert Soils 54:717–730. https://doi.org/10.1007/s00374-018-1297-2
Gilliam FS (2006) Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J Ecol 94:1176–1191. https://doi.org/10.1111/j.1365-2745.2006.01155.x
Han YF, Feng JG, Han MG, Zhu B (2020) Responses of arbuscular mycorrhizal fungi to nitrogen addition: A meta-analysis. Global Change Biol 26:7229–7241. https://doi.org/10.1111/gcb.15369
Johnson NC, Wilson GWT, Wilson JA, Miller RM, Bowker MA (2015) Mycorrhizal phenotypes and the law of the minimum. New Phytol 205:1473–1484. https://doi.org/10.1111/nph.13172
Treseder KK, Allen EB, Egerton-Warburton LM, Hart MM, Klironomos JN, Maherali H, Tedersoo L (2018) Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: a trait-based predictive framework. J Ecol 106:480–489. https://doi.org/10.1111/1365-2745.12919
Tu C, Booker FL, Watson DM, Chen X, Rufty TW, Shi W, Hu SJ (2006) Mycorrhizal mediation of plant N acquisition and residue decomposition: impact of mineral N inputs. Global Change Biol 12:793–803. https://doi.org/10.1111/j.1365-2486.2006.01149.x
Ju XT, Xing GX, Chen XP, Zhang SL, Zhang LJ, Liu XJ, Cui ZL, Yin B, Christie P, Zhu ZL, Zhang FS (2009) Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc Natl Acad Sci USA 106(9):3041–3046. https://doi.org/10.1073/pnas.0813417106
Chen XP, Cui ZL, Fan MS, Vitousek P, Zhao M, Ma WQ, Wang ZL, Zhang WJ, Yan XY, Yang JC, Deng XP, Gao Q, Zhang Q, Guo SW, Ren J, Li SQ, Ye YL, Wang ZH, Huang JL, Tang QY, Sun YX, Peng XL, Zhang JW, He MR, Zhu YJ, Xue JY, Wang GL, Wu L, An N, Wu LQ, Ma L, Zhang WF, Zhang FS (2014) Producing more grain with lower environmental costs. Nature 514(7523):486–489. https://doi.org/10.1038/nature13609
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010. https://doi.org/10.1126/science.1182570
Zhang XL, Wang Q, Gilliam FS, Bai WM, Han XG, Li LH (2012) Effect of nitrogen fertilization on net nitrogen mineralization in a grassland soil, northern China. Grass Forage Sci 67:219–230. https://doi.org/10.1111/j.1365-2494.2011.00836.x
Abbott LK, Robson AD (1991) Factors influencing the occurrence of vesicular-arbuscular mycorrhizas. Agric Ecosyst Environ 35:121–150. https://doi.org/10.1016/0167-8809(91)90048-3
Langeroodi ARS, Mancinelli R, Radicetti E (2021) Contribution of biochar and arbuscular mycorrhizal fungi to sustainable cultivation of sunflower under semi-arid environment. Field Crop Res 273:108292. https://doi.org/10.1016/j.fcr.2021.108292
Li SP, Bi YL, Chen PZ, Chen SL, Zhang YX, Kong WP, Wang J (2013) Effects of AMF on soil improvement and maize growth in mining area under drought stress. Acta Ecol Sin 33:4181–4188. https://doi.org/10.5846/stxb201209041249
Oyewole BO, Olawuyi OJ, Odebode AC, Abiala MA (2017) Influence of Arbuscular mycorrhiza fungi (AMF) on drought tolerance and charcoal rot disease of cowpea. Biotechnol Rep 14:8–15. https://doi.org/10.1016/j.btre.2017.02.004
Funding
This research was partially supported by Natural Science Foundation of Henan Province of China (No. 182300410013), Science and Technology Innovation Fund of Henan Agricultural University (No. 30500712), and the National Key Research and Development Program of China (No. 2018YFD0200605).
Author information
Authors and Affiliations
Contributions
X. Z designed this study. Y. Z. and M. T conducted the experiments, and T. H. and X. Z. wrote the first draft. T. H. improved the figures and tables. T. H., J. D., S. Y., M. T., C. Z., F. S G., Q. Y, and C. L edited the draft and provided editorial advice. All authors contributed to the writing.
Corresponding author
Ethics declarations
Consent to Participate
Informed consent was obtained from all individual participants included in this study.
Competing Interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, T., Zhang, X., Du, J. et al. Arbuscular Mycorrhizal Fungi Shift Soil Bacterial Community Composition and Reduce Soil Ammonia Volatilization and Nitrous Oxide Emissions. Microb Ecol 85, 951–964 (2023). https://doi.org/10.1007/s00248-023-02172-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-023-02172-3