Abstract
Plant diversity effects on ecosystem functioning can potentially interact with global climate by altering fluxes of the radiatively active trace gases nitrous oxide (N2O) and methane (CH4). We studied the effects of grassland species richness (1–16) in combination with application of fertilizer (nitrogen:phosphorus:potassium = 100:43.6:83 kg ha−1 a−1) on N2O and CH4 fluxes in a long-term field experiment. Soil N2O emissions, measured over 2 years using static chambers, decreased with species richness unless fertilizer was added. N2O emissions increased with fertilization and the fraction of legumes in plant communities. Soil CH4 uptake, a process driven by methanotrophic bacteria, decreased with plant species numbers, irrespective of fertilization. Using structural equation models, we related trace gas fluxes to soil moisture, soil inorganic N concentrations, nitrifying and denitrifying enzyme activity, and the abundance of ammonia oxidizers, nitrite oxidizers, and denitrifiers (quantified by real-time PCR of gene fragments amplified from microbial DNA in soil). These analyses indicated that plant species richness increased soil moisture, which in turn increased N cycling-related activities. Enhanced N cycling increased N2O emission and soil CH4 uptake, with the latter possibly caused by removal of inhibitory ammonium by nitrification. The moisture-related indirect effects were surpassed by direct, moisture-independent effects opposite in direction. Microbial gene abundances responded positively to fertilizer but not to plant species richness. The response patterns we found were statistically robust and highlight the potential of plant biodiversity to interact with climatic change through mechanisms unrelated to carbon storage and associated carbon dioxide removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent syntheses of experimental research have shown that effects of plant diversity loss on ecosystem functioning can be of similar magnitude as those of other global changes, including warming and drought (Hooper et al. 2012). However, the effects of plant diversity interact with other global changes, with mechanisms operating at spatial scales ranging from the plot scale to the globe. For example, drivers of global change may affect ecosystem functions directly and indirectly via alterations of plant diversity (Balvanera et al. 2006). Changes in plant diversity can alter ecosystem carbon and nutrient cycling, thereby changing the ecosystem–atmosphere exchange rates of greenhouse gases and thus affecting climatic drivers at the global scale.
Carbon dioxide (CO2) is the dominant anthropogenic greenhouse gas, but nitrous oxide (N2O) and methane (CH4) also are important drivers of anthropogenic climate change. Despite their low atmospheric volume-mixing ratios, these gases account for over 25 % of the radiative forcing increase since pre-industrial times because of their high warming potential relative to CO2 (IPCC 2007). To date, most experimental plant diversity studies have focused on ecosystem functions related to plant primary productivity and the ecosystem’s carbon cycle (e.g., biomass, photosynthesis, leaf area, litter decomposition), which are processes directly relevant to the land–atmosphere exchange of CO2. However, the effects of plant diversity on land–atmosphere fluxes of N2O and CH4 have only been studied in few mesocosm studies (N2O: Abalos et al. 2014; Chang et al. 2014; Sun et al. 2013; N2O and CH4: Niklaus et al. 2006), and relatively little is yet known on responses of the underlying nitrogen (N) cycling processes to plant diversity (Le Roux et al. 2013).
N2O is released from soils as an intermediate or by-product of microbial N cycling (see Cabello et al. 2009 for a comprehensive overview of soil N transformations). Under oxic conditions, some N2O produced as a by-product of nitrification will escape to the atmosphere. Under more anoxic conditions, nitrate (NO3 −) is sequentially reduced to N2O, which generally is lost from soils if it is not further reduced to molecular nitrogen (Firestone and Davidson 1989). Soil N2O emissions are controlled by many factors (Robertson and Tiedje 1987) including the availability of inorganic N, in particular ammonium (NH4 +) and NO3 −. Soil moisture is also an important factor in the release of N2O from the soil because it affects gas diffusivity and oxygen supply. At a low water-filled pore space, oxygen is readily available and soil organic matter is mineralized, yielding NH4 + and promoting nitrification and associated N2O emissions. At a high water-filled pore space, nitrification ceases and NO3 − is consumed by denitrification, which is the dominant source of N2O emissions from soils. At an even higher water-filled pore space, N2O emissions drop because most of the N2O produced is reduced to molecular N.
Soils can be both sources and sinks of atmospheric CH4. On an annual basis, most aerobic soils are a net sink for atmospheric CH4, although periodic emissions can occur when soils are wet. This soil CH4 sink is essentially driven by the consumption of CH4 by methanotrophic bacteria, which use CH4 as a source of carbon and energy (Dunfield 2007). At a very low redox potential (typically found in wetlands), however, methanogenic archaea produce CH4 through several pathways which involve the consumption of organic substrates or CO2 (Conrad 1996). Non-microbial soil CH4 emissions have recently been described (Wang et al. 2013), but the quantitative importance of this process in natural ecosystems remains to be evaluated. Methanogens and methanotrophs often co-occur in soils, with methanotrophs acting as a biofilter that oxidizes the CH4 produced by methanogens in anoxic soil domains. On the other hand, occasional methanogenesis can prime methanotrophic activity, leading to increased uptake of atmospheric CH4 (West and Schmidt 2002). Several links have been established between CH4 oxidation and N transformations, with complex positive and negative correlations demonstrated between the two (Bodelier and Laanbroek 2004). Ammonia (NH3) has been shown to inhibit CH4 oxidation, at least in laboratory cultures (Hanson and Hanson 1996), but on the other hand inorganic N is an essential nutrient for most methanotrophs. In addition, differential stratification of these processes may occur in the soil, with effects that may be restricted to some soil layers and therefore not necessarily generate effects on soil–atmosphere CH4 fluxes (Stiehl-Braun et al. 2011a).
While the fundamental processes involved in soil N2O and CH4 transformations are reasonably well understood, the ecology of the organisms involved in complex field ecosystems is far from being as clear (see Bodelier and Laanbroek 2004; Robertson 1989). Predicting N2O and CH4 fluxes is difficult given the large number of factors and organisms involved, which interact in ways difficult to predict and change in space and time. Nevertheless, we argue that plant diversity has the potential to alter trace gas fluxes via a range of mechanisms that can reasonably be predicted and tested. First, a more species-rich plant community often leads to tighter N cycling, with a relatively lower accumulation of inorganic N (Ewel et al. 1991; Niklaus et al. 2001; Tilman et al. 1996), which in turn could affect nitrification and denitrification and their associated N2O emissions and, possibly, also CH4 dynamics. Second, increased productivity at higher plant diversity may also translate into an improved supply of organic substrates for soil microbial communities, which could increase heterotrophic activity, lead to higher oxygen consumption, and increase the amount of soil volume with a redox potential sufficiently low for denitrification (Sexstone et al. 1985). Third, water use may increase with plant diversity, resulting in reduced soil moisture and increased soil diffusivity (Caldeira et al. 2001; Leimer et al. 2014a; Spehn et al. 2000), which may affect both N transformations and soil CH4 uptake (Ball et al. 1997). Fourth, altered root densities and root architectures may lead to changes in soil aeration, spatio-temporal organic C deposition, or N use patterns. Fifth, atmospheric nitrogen (N2) fixation by legumes will have particularly strong impacts on N cycling and has been identified as strong determinant of ecosystem responses in many plant diversity experiments (Oelmann et al. 2007; Spehn et al. 2002). With respect to CH4, the study by Niklaus et al. (2006) hints at the complexity of interactions at play, with plant diversity effects on CH4 consumption changing from positive to negative when a soil disturbance treatment was applied in the field.
Here, we present the first study investigating the effects of plant species diversity and fertilization, together with their interaction, on fluxes of N2O and CH4 under field conditions. We investigated the dynamics of these trace gases and the underlying mechanisms at several levels. First, we measured in situ flux rates of N2O and CH4; second, we assessed the enzymatic potential of key N transformations functionally linked to these fluxes; third, we quantified the abundance of bacterial nitrifiers and denitrifiers using quantitative PCR of selected functional and ribosomal genes. We further assessed soil environmental conditions (temperature, moisture) and concentrations of inorganic N species. We were particularly interested in determining whether higher plant species richness would lead to more complete N capture and therefore reduced nitrification and denitrification rates and associated N2O emissions. An additional aim was to test whether the plant diversity effects in our experimental system operated via changes in soil moisture and whether there was evidence that nitrification promotes soil CH4 uptake by the removal of potentially inhibiting NH4 +.
Materials and methods
Study site and experimental design
We studied soil–atmosphere trace gas fluxes in a large grassland biodiversity experiment near Jena, Germany (50°55′N, 11°35′W; 130 m a.s.l.). In 2002, experimental grassland communities varying in diversity were established on an Eutric Fluvisol with a texture progressing from sandy loam to silty clay with increasing distance from the adjacent Saale river. In the present study we focused on 78 plots (20 × 20 m) sown with one, two, four, eight, or 16 herbaceous species (see Roscher et al. 2004a for a complete description of the experimental design). Plot community composition was determined by random selection of species from a 60-species pool, with the constraint that species richness and plant functional type richness were as orthogonal as possible. Plant species had previously been assigned to functional types (grasses, small non-legume herbs, tall non-legume herbs, and legumes) based on a cluster analysis combining a large number of morphological and functional traits (Roscher et al. 2004a). Starting in 2005, factorial management treatments were established (Weigelt et al. 2009) in subplots (1.6 × 4 m) located along one side of the large plots. In the present study, we analyzed data from the main plot (“control” subplots) and the subplot that was managed identically except that fertilizer pellets were added after each mowing (“fertilized” subplots), at a rate of 100 kg N ha−1 a−1, 43.6 kg phosphorus (P) ha−1 year−1 and 83 kg potassium (K) ha−1 year−1. In total, the study thus presents a split plot design with 70 plots (unit of replication for plant species composition and thus plant species richness) and 2 × 70 = 140 subplots (unit of replication for fertilizer application).
Soil–atmosphere trace gas fluxes
We measured soil–atmosphere fluxes of N2O and CH4 on 20 July and 17 October 2007 and on 11 March, 29 April, and twice on 6 June 2008 (one daytime and one nighttime measurement; Table 1), using static chambers that had been installed in all control and fertilized subplots that were part of this study. The static chambers were 32 cm in diameter and lowered 14 cm into the ground, leaving 11 cm extending above ground. During flux measurements, air-tight lids were fitted over the static chambers, and headspace samples were collected 5, 20 and 35 min after chamber closure. These samples were injected into pre-evacuated exetainers and analyzed for N2O and CH4 concentrations in the laboratory (Agilent 6890 gas chromatograph equipped with a flame ionization and an electron capture detector; Agilent Technologies Inc., Santa Clara, CA). Concentrations were determined based on calibration against four standard gases with four different concentrations of CH4 and N2O (see Hartmann et al. 2011 for details of the experimental setup). Gas exchange rates were calculated by linear regression of headspace concentrations against time. Concentration changes were linear over time, and regressions explained >95 % of the variation in gas concentration (R 2 > 0.95), unless flux rates were very low.
Soil sampling
In October 2007 and again in June 2008, we collected eight soil cores (diameter 1.5 cm; depth 15 cm) per subplot within 50 cm of the respective static chamber used for the N 2 O and CH4 flux measurements. Samples were pooled by subplot and sieved (mesh size 2 mm). A sub-sample was stored at −18 °C for molecular analyses, while a second subsample was stored at 4 °C for potential nitrifying and denitrifying activity analysis.
Nitrifying and denitrifying enzyme activity
Nitrifying (NEA) and denitrifying (DEA) enzyme activities were determined in laboratory assays in which the transformation rates of NH4 + and NO3 − were quantified under standardized conditions, with no limitations in water availability nor substrate concentration. The goal of this analysis was to quantify potential activity rates in the absence of constraint by substrate availability or environmental conditions that may have existed in the field. NEA was measured at soil pH using the method described in Patra et al. (2005) and was determined as the difference between soil NO3 − concentration before and after a 7-h incubation of 10 g of soil under conditions favoring nitrification (28 °C, addition of 0.2 mg (NH4)2SO4-N g−1 dry soil, soil moisture equivalent to 70 % water-holding capacity). DEA was assessed over a short time by making all the factors affecting the denitrification rate non-limiting (Smith and Tiedje 1979; Tiedje et al. 1989). DEA was determined as the linear production rate of N2O, as measured by gas chromatography (Agilent P200; Agilent Technologies Inc.), during an 8-h incubation of 10 g soil under conditions favoring denitrification (28 °C; 90:10 He-C2H2 atmosphere providing anaerobic conditions and inhibition of N2O-reductase activity; addition of 1 mg glucose-C, 1 mg glutamic acid-C, and 0.1 mg NO3 −-Ng-1 dry soil; soil moisture content equivalent to 100 % water-holding capacity).
Nitrifier and denitrifier abundances
Microbial DNA was extracted from the soil samples using the PowerSoil DNA isolation kit (MO BIO Laboratories, Carlsbad, CA). The abundance of beta-proteobacterial ammonia oxidizers, i.e., known ammonia-oxidizing bacteria (AOB) in soil, was measured by quantitative PCR (qPCR) according to Le Roux et al. (2008), targeting 16S rRNA gene sequences that are specific for this group (Hermansson and Lindgren 2001). As reference, we used 5.72·108–5.72·102 copies of standard DNA (purified AOB 16S rRNA gene PCR product of ATCC19718 Nitrosomonas europaea).
The abundance of copies of the nitrite (NO2 −) oxidoreductase gene (nxrA) from Nitrobacter-like nitrite-oxidizing bacteria (NOB) was quantified according to Attard et al. (2010). Tenfold standard serial dilutions ranging from 107 to 101 nxrA copies of genomic DNA from Nitrobacter hamburgensis X14 (DSMZ 10229) were used.
Denitrifier abundance was estimated by qPCR targeting fragments of the nirK and nirS genes encoding the copper and cd1 nitrite reductases, respectively. Quantitative PCR assays were carried out from soil DNA with Lightcycler 480 (Roche Diagnostics, Meylan, France). For nirK, thermal cycling was performed and a standard curve constructed according to Henry et al. (2004), except that the last step of the PCR cycling profile was performed for 45 cycles at an annealing temperature of 58 °C. nirS fragments were amplified as described by Baudoin et al. (2009), using tenfold serial dilutions of genomic DNA of Pseudomonas aeruginosa PA14 as standards. We checked melting curves and the lengths of the amplified nirK or nirS fragments after running an agarose gel of randomly chosen final PCR products.
For all of the qPCR assays, measurements were performed in triplicate. Melting curve analysis confirmed the specificity of the amplifications, and possible inhibitory effects of co-extracted humic compounds in soil extracts were checked by dilution series, but no inhibition was observed.
Soil moisture and inorganic N concentrations
Soil moisture was determined gravimetrically in all fresh samples collected for molecular analysis. Soil NH4 + and NO3 − concentrations were determined colorimetrically (SAN+; Skalar Analytical B.V., the Netherlands) in fresh soil samples (20 g dry mass equivalent) following extraction with 60 mL of 2 M KCl (30 min) and filtration.
Data analysis
All data were analyzed by analysis of variance based on mixed-effects models reflecting the experimental design (ASReml 3.0; VSN International, Hemel Hempstead, UK). The models included the main design variables plant species richness (as log-linear contrast) and fertilizer application, and their interaction. The specific composition of plant species communities was included as a random term to ensure adequate testing of the species richness effect (note that this random term is equivalent to “plot” since specific plant community compositions are not replicated at the plot level). The Jena Experiment is located on a flood plain adjacent to the Saale river, and spatial, treatment-independent gradients in soil conditions across the site affected the processes we investigated. We therefore fitted the Cartesian coordinates X and Y, which indicate the plot’s position, plus their second-order terms (X ∙ X, Y ∙ Y, X ∙ Y) to account for non-linearity in spatial gradients and for interactions between the two cardinal directions (i.e., allowing for a response surface that may be curved in a direction that does not align with the main coordinate axes). This spatial model explained more variance than fitting the design’s standard blocks which were placed perpendicular to the river.
The different variables necessitated a range of transformations due to their specific distributions. In part, these distributions result from biological processes; however, they also are the result of specific measurement procedures, such as the PCR reaction, which is an exponential process and results in a corresponding distribution of measurement errors. All gene abundances were therefore log-transformed. Soil CH4 uptake rates as well as nitrification and denitrification enzyme activities were analyzed as untransformed values. Finally, soil N2O emissions resembled a log-normal distribution at large values but included very small and negative values due to measurement error, precluding log-transformation (which further would have over-emphasized differences between very small fluxes). We found that taking the fourth square root (with sign reconstruction for negative values) and shifting the abscissa of the first and third quadrant so that the two legs of the function merged with unity slope resulted in a near-perfect normal distribution of residuals. However, the results of the analysis did not change fundamentally when other transformations were chosen.
After testing for the significance of effects of species richness and fertilization, respectively, we used structural equation modeling to explore possible causal relationships between trace gas fluxes, soil environmental variables (water content, NH4 + and NO3 − concentrations), potential nitrifying and denitrifying activities, and gene abundances. The goal was to develop an ecologically sensible, parsimonious model for which the model-implied covariance between variables matched the observed covariance structure of the data. These covariance matrices were then compared using χ2 tests, with significant results indicating a poor fit. Models were fitted by maximum likelihood using the lavaan software (http://lavaan.ugent.be). As with the mixed model analyses, data were first corrected for spatial gradients across the experimental site. Since not all data were present on all dates, these analyses were carried out on temporal averages of all available measurements, the underlying assumption being that effects and correlations were reasonably stable over time. Structural models were constructed under the premises that (1) processes upstream in terms of N transformation influence downstream processes (e.g., nitrification can influence denitrification), but not vice versa; (2) gene abundance can influence microbial community activity, but not vice versa; (3) trace gas fluxes can be influenced by all variables; (4) the experimental treatments can directly influence all variables. In an educated stepwise process, we then searched for the most parsimonious model that explained the observed covariance structure adequately (insignificant χ2 test; P was >0.5 for all models, indicating a very good fit). This was achieved by omitting paths with small, non-significant coefficients.
Results
Soil N2O emissions
Averaged over all sampling dates, soil N2O emissions were left unaffected by plant species richness but they increased slightly with fertilizer application (P = 0.003). When we analyzed sampling dates separately, we found a significant decrease of N2O emissions with diversity on 21 April 2008 (P = 0.02) and significant species richness by fertilizer application interactions on 20 June 2007 (P = 0.01) and 11 March 2008 (P = 0.05), with the same effect being marginally significant on 21 April 2008 (P = 0.07). These interactions resulted from the difference in N2O emissions between fertilized and control subplots being larger at high plant species richness than at low plant species richness.
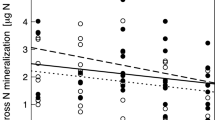
Emissions of N2O were strongly controlled by the presence of legumes. We therefore separately analyzed plots that were sown with and without legumes (Fig. 1). In the absence of legumes, species richness slightly reduced N2O emissions, while N application slightly increased emissions (both effects being marginally significant at P = 0.06). In plots with legumes, N2O emissions were significantly correlated with the fraction of legume biomass recovered in the summer harvest (P = 0.001) and increased with fertilizer application (P = 0.02), but they were unrelated to plant species richness.
Soil nitrous oxide (NO 2 ) emission rates for plots without (left) and with (right) legumes planted. Individual symbols Values for each plot (gray and white circles values for control and fertilized plots, respectively), black pie shape inside symbol fraction of legumes in aboveground plant biomass harvested in June 2007 and 2008. Square symbols and lines Means predicted by the model for both fertilizer treatments, shaded areas corresponding standard errors (SE)
Soil CH4 uptake
Averaged over all sampling dates, soil CH4 uptake rates decreased with plant species richness (P = 0.02), but they remained unaffected by fertilizer application (Fig. 2) and by the abundance of legumes. This effect was remarkably stable over time, with a decrease in CH4 uptake with species richness detected on 18 October 2007 (P = 0.05) and 6 June 2008 (P = 0.002), a marginally significant decrease found on 20 June 2007 (P = 0.08) and non-significant trends in the same direction found on the other sampling dates.
Nitrifying and denitrifying enzyme activities and functional gene abundances
Nitrifying enzyme activity increased with plant species richness (P < 0.05), irrespective of fertilizer application (Table 2). In contrast, DEA increased with fertilizer application (P < 0.001), irrespective of plant species richness (Table 2).
Plant species richness did not affect functional gene abundances, although nxrA showed a marginally significant (P = 0.06) and weak decrease with plant diversity. In contrast, the abundances of AOB, Nitrobacter-like NOB and nirK- and nirS-like denitrifiers increased with fertilizer application (Table 2).
Structural equation models explaining N2O and CH4 emissions
In the absence of sown legumes, N2O emissions were affected negatively by plant species richness, as indicated by a strong direct negative path coefficient (Fig. 3, top). Plant species richness increased soil moisture, resulting in increased potential nitrification (NEA), which in turn stimulated DEA. However, DEA was not significantly linked to N2O emissions. When plant communities contained legumes (Fig. 3, bottom), the percentage of legumes (measured as aboveground biomass fraction harvested in June) dominated the effects on N2O emissions. Since the fraction of legumes in the plant community was negatively correlated with species richness and fertilizer application, these treatments thus reduced emissions by this pathway. However, plant species richness stimulated N2O emissions via increases in soil moisture, which in turn increased NEA (and DEA, but as in the legume-free plots no significant link between DEA and N2O fluxes was detected). Soil NH4 + or NO3 − concentrations had no explanatory power in the analysis and were therefore dropped from all models.
Path diagram with standardized path coefficients showing effects of plant species richness (log-transformed, logSR) and fertilizer (N) on soil moisture (H 2 O), soil microbial nitrification and denitrification enzyme activities (NEA and DEA, respectively), and soil N2O emissions (N 2 O SOURCE), separately for plots without (top) and with (bottom) legumes. Arrows Standardized path coefficients (gray negative; black positive) together with their significance (*P < 0.05, **P < 0.01, ***P < 0.001, n.s. not significant). %LEG Mean fraction of legume aboveground biomass harvested in June 2007 and 2008. χ2 statistics indicate no significant deviation of measured and model-implied covariance structure
While the net effect of plant species richness on soil CH4 oxidation was negative, our path analysis suggested that a positive indirect effect of richness via increased soil moisture and increased NEA was also at play. However, this positive effect was masked by a larger direct negative effect on CH4 uptake (Fig. 4). Fertilizer application stimulated AOB abundance, which positively correlated with nitrification activity. Legume abundance also boosted NEA. Ultimately, NEA had a positive effect on CH4 uptake. Soil NH4 + concentration had no explanatory power in this analysis.
Path diagram showing effects of plant species richness and fertilizer application on soil moisture, nitrification enzyme activity, the abundance of ammonia-oxidizing bacteria (AOB) and soil CH4 uptake (CH 4 SINK). See caption to Fig. 3 for all other abbreviations. Arrows Standardized path coefficients (gray negative, black positive) together with their significance (*P < 0.05, **P < 0.01, ***P < 0.001). χ2 statistics indicate no significant deviation of measured and model-implied covariance structure
Structural equation models linking functional gene abundances with NEA and DEA
Path analysis did not reveal any significant links between potential nitrification and denitrification activities on one hand, and the abundances of AOB, Nitrobacter-like NOB or nirK-like denitrifiers on the other hand (Fig. 5). Due to limited amounts of extracted soil DNA available, nirS abundances could only be determined for both sampling dates in 83 plots, for one sampling date in another 63 plots, and not at all in 10 plots. Analysis of the subset for which both nirS and nirK abundances were available revealed a similar pattern, i.e., no link between nirS and enzyme activities (Online Resource 1).
Path diagram showing relationships between species richness, fertilizer application, nitrifying and denitrifying enzyme activities, and abundances of key microbial functional groups (NOB Nitrobacter-like nitrite oxidizers, nirK-DENITR nirK-like denitrifiers). See captions to Figs. 3 and 4 for all other abbreviations. Arrows Standardized path coefficients (gray negative, black positive) together with their significance (*P < 0.05, **P < 0.01, ***P < 0.001). The correlation between the exogenous variables logSR and N is zero and omitted for clarity. χ2 statistics indicate no significant deviation of measured and model-implied covariance structure
At the level of enzyme activities, the effects of plant species richness were mediated by soil moisture, while the effects of fertilizer application were mediated by a reduced legume fraction in the plant communities. At the level of microbial functional group abundances, N fertilization had a clear positive effect on the abundance of AOB, with cascading effects on the abundance of Nitrobacter-like NOB and nirK-like denitrifiers (Fig. 5). nirS-like denitrifier abundances were affected positively by N fertilization, both directly and indirectly via AOB and Nitrobacter-like NOB (Electronic Supplementary Material 1).
Discussion
Plant species richness decreased soil N2O emissions—at least in the absence of legumes—and decreased soil CH4 uptake. We also detected plant diversity effects on underlying soil microbial processes and the abundances of soil microbial groups related to these processes. However, structural equation modeling suggested that these different effects were linked in a complex way, with net effects on trace gas fluxes emerging from a combination of direct and indirect species richness effects; in this context, “direct” denotes effects not likely related to the dependent variables observed.
Structural equation modeling suggests that the effects on N2O fluxes were at least in part mediated by positive effects of plant species numbers on soil moisture, which in turn led to accelerated inorganic N cycling, as evidenced by increased NEA and DEA. Positive path coefficients to N2O fluxes (Fig. 3) suggest that this increase in N transformation capacity indeed stimulated N2O emissions. At the same time, the structural equation models support a direct negative effect of plant species richness on N2O emissions, which counteracted the positive effects of increased enzyme activities. A possible mechanism underlying this link may stem from disproportionate effects of species richness on soil inorganic N concentrations and the enzymatic potential (in our study: NEA and DEA) to transform this inorganic N. Many studies have shown more efficient soil inorganic N capture and thus lower soil NO3 − concentrations in more species-rich plant communities (Ewel et al. 1991; Niklaus et al. 2001; Tilman et al. 1996). In the Jena experiment, Leimer et al. (2014b) found that soil NO3 − concentrations were reduced at high diversity if no or only few legumes were present, which is compatible with the general trend found in other studies. In our analysis, however, soil inorganic N concentrations were unrelated to the explanatory variables analyzed. One reason for this difference may be that our measurements were not frequent enough to estimate robust average concentrations of these very dynamic parameters. When legumes were present in experimental communities, their fractional contribution to plant community biomass was positively related to nitrification and soil N2O emissions. However, the negative direct effect from species richness to N2O emissions that we identified in the absence of legumes vanished in the structural equation models (Fig. 3). This observation is compatible with the finding by Leimer et al. (2014b) that soil NO3 − concentrations increased with diversity when a large fraction of species were legumes, indicating that a high legume diversity also led to high NO3 − concentrations and that this effect exceeded the higher inorganic N capture efficiency in more diverse plant communities that was otherwise found.
Net soil CH4 uptake decreased persistently with plant species richness. The soil's CH4 balance is determined by the difference between methanogenesis and CH4 oxidation, with the latter dominated by methanotrophic bacteria. We did not observe net CH4 emissions during our study, indicating that CH4 oxidation consistently outweighed methanogenesis; in fact, we think that methanogenesis did not play an important role in the present system and that the observed patterns were thus solely caused by variation in methanotrophic activity. Soil CH4 oxidation is often limited by gas transport rates, which in turn depend on water-filled pore space (Ball et al. 1997). Increased soil moisture at high plant species richness could therefore explain the observed pattern. However, structural equation modeling suggested indirect positive effects of moisture on CH4 oxidation via increased nitrification (Fig. 4). This mechanism is plausible, given that methanotrophy can be inhibited by high NH4 + concentrations; under these conditions, all processes consuming soil NH4 + will indirectly promote CH4 oxidation. Apart from nitrification, NH4 + assimilation by plants and immobilization in microbial biomass would exert such an effect. Path coefficients further suggested a soil moisture-independent direct negative effect of species richness on soil CH4 uptake. This effect persisted even if a (non-significant) link from soil moisture to CH4 uptake was added to the model, suggesting that this effect was unrelated to soil moisture. The potential mechanisms supporting this link remain elusive; however, it also should be noted that path models do not provide strict tests of causalities but rather indicate possible mechanistic links based on correlations. The ecology of soil methanotrophic bacteria consuming atmospheric CH4 is only poorly understood to date, in part due to the lack of success in isolating such organisms from soils (Dunfield 2007). However, it is known from experiments with intact soil cores and with available laboratory cultures that CH4 oxidation is negatively affected by many chemical compounds, including ethylene (Jäckel et al. 2004), some organic acids (Wieczorek et al. 2011), and terpenes (Amaral et al. 1998). One possibility is that the detrimental effect of these substances results from the lack of specificity of methane-monooxygenase, which leads to co-metabolic activity harmful to the bacteria, such as by suicide activation (Mahendra and Alvarez-Cohen 2006; Prior and Dalton 1985). In this light, one possible explanation for the soil moisture-independent reduction of CH4 uptake in plant species-rich communities could be an increased production of such compounds inhibiting CH4 oxidation.
It is surprising that soil inorganic N concentrations were not significantly related to any other variable measured, whereas species richness, fertilization, and legume presence had significant effects on these parameters. This could be due to the fact that soils are spatially heterogeneous, as are the biogeochemical transformations we investigated. For example, denitrification often occurs in the micro-anaerobic parts of soil aggregates (Sexstone et al. 1985), and recent micro-autoradiographic analyses indicate that CH4 assimilation preferentially takes place on aggregate surfaces to which CH4 can readily diffuse (Stiehl-Braun et al. 2011a, b). Directly relating system-level processes, such as land–atmosphere trace gas fluxes, to bulk soil measures of putative drivers, such as to soil inorganic N concentrations or enzymatic activities, disregards the micro-scale character of the interactions involved. When processes respond in a non-linear fashion to substrate availability, different effects will be found when a resource such as NO3 − is homogeneously or heterogeneously distributed. Our measurements, as is typical for ecosystem-level field studies, integrate the small-scale heterogeneity and thus are unable to account for small-scale interactions in hot spots and at hot moments, which show episodic disproportionately high biogeochemical transformation rates (McClain et al. 2003). We consider it likely that the marked effect of legumes is related to such heterogeneity. For example, high local concentrations of NO3 − can occur in the vicinity of legume nodules, possibly creating hot spots of denitrification and N2O emissions. Efficient capture of inorganic N by neighboring plants will not lower inorganic N concentrations at these locations, although it will reduce NO3 − leaching losses. Such localized high concentrations will be difficult to detect in bulk soil measurements. This reasoning is consistent with a previous investigation in which we found nitrification to be only weakly related to NH4 + and denitrification only weakly related to NO3 −, whereas legume presence had comparably large effects (Le Roux et al. 2013). With respect to CH4 fluxes, high bulk soil NH4 + concentrations may not affect CH4 oxidation if they occur in soil space that is distinct from the spatial niche of the active methanotrophs (Hartmann et al. 2011; Stiehl-Braun et al. 2011a).
The quantification of microbial guilds by functional gene abundance promised some insight into the community dynamics underlying N transformations. In our study, different experimental treatments were reflected differently in terms of functional gene abundances and enzyme activities, and no links between the two could be detected in the structural equation models. Soil moisture-mediated effects of plant species could be detected in enzyme activities but not in the community size of the respective microbial guilds. However, fertilizer effects did exert a cascading effect via increased NH4 + oxidizer communities to nitrite oxidizers and denitrifiers. Interestingly, no link from nitrite oxidizer gene abundances to nirK gene abundances was detected (Fig. 5), although the latter is downstream of the first in terms of N transformation processes. This relationship can be understood in the light of NH4 + (and not nitrite) oxidation being the rate-limiting step of nitrification. Probably reflecting differences in ecology of denitrifier groups (Xie et al. 2014), nirS gene abundances were dependent on NH4 + oxidizer 16S genes abundances indirectly via nitrite oxidizer genes. Overall, however, NH4 + oxidizer community size appeared to control denitrifier community size, mostly directly but to some extent also indirectly via intermediate (in terms of N processing) nitrite oxidizer communities. Environmental controls rather than community size have also been detected in contexts other than species richness (e.g., fertilizer and drought effects: Hartmann et al. 2013; land use change: Attard et al. 2011), underlining that gene abundance data reflect biological activities only under specific conditions.
Our study revealed that many effects of plant species richness on microbial processes were mediated by effects on soil moisture. Reports on species richness effects on soil moisture are equivocal (Caldeira et al. 2001; Rosenkranz et al. 2012; Spehn et al. 2000); Caldeira et al. (2001) reported positive effects of species richness on grassland top soil moisture but negative effects at soil depths of >15 cm. In the Jena experiment, Rosenkranz et al. (2012) found similar positive effects on top soil moisture as we did, while Leimer et al. (2014a) detected no such effects. In general, patterns in soil moisture seem to have been caused by decreased evaporative losses under higher species richness due to soil surface shading, with a concomitant increase in water capture from deeper soil layers due to better soil exploration by roots. If processes are soil moisture driven, the depth at which the respective microbial transformations take place is therefore critical. N2O production due to nitrification and denitrification often occurs in the top soil; soil CH4 oxidation generally also dominates in the top soil, whereas methanogenesis often occurs deeper in the soil (Conrad 1996).
The patterns that emerged from our study, in particular from structural equation modeling, were robust and coherent, integrating well into our current understanding of the fundamental ecological processes. With respect to the fluxes of N2O and CH4 per se, one must note that the effects of species richness and fertilization were small compared to the total variation in our data, which commands a cautious interpretation (Low-Décarie et al. 2014). One reason for the large fraction of unexplained variance may be that the between-plant community variation is typically very high in experimental plant diversity experiments, in particular at low species richness. The observed diversity effects thus result from averaging over the large inter-community variation within plant species richness levels. In the Jena experiment, as in many field biodiversity studies, specific species combinations are not replicated in independent plots, so that the diversity-independent composition effect cannot be separated from unrelated (random) variation, with the exception of simple contrasts, such as the presence or absence of specific plant functional types. When studying trace gas dynamics and the underlying microbial processes, the researcher is further confronted with large spatial and temporal variation inherent to the processes. Despite this challenging variability, the present field study is the first to present clear and consistent responses of soil trace gas exchange to plant species richness and fertilization. Controlled experimental studies as the present one complement observational research carried out under more natural conditions but in which it is more difficult to isolate effects of plant diversity from potentially confounding factors (e.g., Keil et al. 2015; Sutton-Grier et al. 2011).
Author contribution statement
PAN and RLB conceived and designed the study with input from MSL and NB. Trace gas flux measurements and soil analyses were carried out by PAN, MSL, and RLB. XLR and FP analyzed functional gene abundances. PAN analyzed the data with input from XLR and RLB. PAN wrote the manuscript with input from all co-authors.
References
Abalos D, de Deyn GB, Kuyper TW, van Groenigen JW (2014) Plant species identity surpasses species richness as a key driver of N2O emissions from grassland. Global Change Biol 20:265–275
Amaral JA, Ekins A, Richards SR, Knowles R (1998) Effect of selected monoterpenes on methane oxidation, denitrification, and aerobic metabolism by bacteria in pure culture. Appl Environ Microb 64:520–525
Attard E, Poly F, Commeaux C, Laurent F, Terada A, Smets BF, Recous S, Le Roux X (2010) Shifts between Nitrospira- and Nitrobacter-like nitrite oxidizers underlie the response of soil potential nitrite oxidation to changes in tillage practices. Environ Microbiol 12:315–326
Attard E, Recous S, Chabbi A, De Berranger C, Guillaumaud N, Labreuche J, Philippot L, Schmid B, Le Roux X (2011) Soil environmental conditions rather than denitrifier abundance and diversity drive potential denitrification after changes in land uses. Global Change Biol 17:1975–1989
Ball BC, Dobbie KE, Parker JP, Smith KA (1997) The influence of gas transport and porosity on methane oxidation in soils. J Geophys Res Atmos 102:23301–23308
Balvanera P, Pfisterer AB, Buchmann N, He JS, Nakashizuka T, Raffaelli D, Schmid B (2006) Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett 9:1146–1156
Baudoin E, Philippot L, Cheneby D, Chapuis-Lardy L, Fromin N, Bru D, Rabary B, Brauman A (2009) Direct seeding mulch-based cropping increases both the activity and the abundance of denitrifier communities in a tropical soil. Soil Biol Biochem 41:1703–1709
Bodelier PLE, Laanbroek HJ (2004) Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol Ecol 47:265–277
Cabello P, Roldán MD, Castillo F, Moreno-Vivián C (2009) Nitrogen cycle. In: Schaechter M (ed) Encyclopedia of microbiology, 3rd edn. Academic Press, New York, pp 299–322
Caldeira MC, Ryel RJ, Lawton JH, Pereira JS (2001) Mechanisms of positive biodiversity-production relationships: insights provided by delta C-13 analysis in experimental Mediterranean grassland plots. Ecol Lett 4:439–443
Chang J, Fan X, Sun HY, Zhang CB, Song CC, Chang SX, Gu BJ, Liu Y, Li D, Wang Y, Ge Y (2014) Plant species richness enhances nitrous oxide emissions in microcosms of constructed wetlands. Ecol Eng 64:108–115
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60:609–640
Dunfield PF (2007) The soil methane sink. In: Gas Greenhouse (ed) Reay D, Hewitt CN, Smith K, Grace J. CAB International, Wallingford, pp 152–170
Ewel JJ, Mazzarino MJ, Berish CW (1991) Tropical soil fertility changes under monocultures and successional communities of different structure. Ecol Appl 1:289–302
Firestone MK, Davidson EA (1989) Microbial basis of NO and N2O production and consumption in soil. In: Andreae MO, Schimel DS (eds) Exchange of trace gases between terrestrial ecosystems and the atmosphere. John Wiley & Sons, Hoboken, pp 7–21
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471
Hartmann AA, Buchmann N, Niklaus PA (2011) A study of soil methane sink regulation in two grasslands exposed to drought and N fertilization. Plant Soil 342:265–275
Hartmann AA, Barnard RL, Marhan S, Niklaus PA (2013) Effects of drought and N-fertilization on N cycling in two grassland soils. Oecologia 171:705–717
Henry S, Baudoin E, Lopez-Gutierrez JC, Martin-Laurent F, Brauman A, Philippot L (2004) Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J Microbiol Methods 59:327–335
Hermansson A, Lindgren PE (2001) Quantification of ammonia-oxidizing bacteria in arable soil by real-time PCR. Appl Environ Microb 67:972–976
Hooper DU, Adair EC, Cardinale BJ, Byrnes JEK, Hungate BA, Matulich KL, Gonzalez A, Duffy JE, Gamfeldt L, O’Connor MI (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–109
IPCC (2007) Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In: Solomon S, Qin D, Manning M et al. (eds) Climate Change 2007: The physical science basis. Cambridge University Press, Cambridge, UK, 996 p
Jäckel U, Schnell S, Conrad R (2004) Microbial ethylene production and inhibition of methanotrophic activity in a deciduous forest soil. Soil Biol Biochem 36:835–840
Keil D, Niklaus PA, von Riedmatten LR, Boeddinghaus RS, Dormann CF, Scherer-Lorenzen M, Kandeler E, Marhan S (2015) Effects of warming and drought on potential N2O emissions and denitrifying bacteria abundance in grasslands with different land-use. FEMS Microbiol Ecol 91:fiv066
Le Roux X, Poly F, Currey P, Commeaux C, Hai B, Nicol GW, Prosser JI, Schloter M, Attard E, Klumpp K (2008) Effects of aboveground grazing on coupling among nitrifier activity, abundance and community structure. ISME J 2:221–232
Le Roux X, Schmid B, Poly F, Barnard RL, Niklaus PA, Guillaumaud N, Habekost M, Oelmann Y, Philippot L, Salles JF, Schloter M, Steinbeiss S, Weigelt A (2013) Soil environmental conditions and microbial build-up mediate the effect of plant diversity on soil nitrifying and denitrifying enzyme activities in temperate grasslands. PLoS One 8:e61069
Leimer S, Kreutziger Y, Rosenkranz S, Bessler H, Engels C, Hildebrandt A, Oelmann Y, Weisser WW, Wirth C, Wilcke W (2014a) Plant diversity effects on the water balance of an experimental grassland. Ecohydrology 7:1378–1391
Leimer S, Wirth C, Oelmann Y, Wilcke W (2014b) Biodiversity effects on nitrate concentrations in soil solution: a Bayesian model. Biogeochemistry 118:141–157
Low-Décarie E, Chivers C, Granados M (2014) Rising complexity and falling explanatory power in ecology. Front Ecol Environ 12:412–418
Mahendra S, Alvarez-Cohen L (2006) Kinetics of 1,4-dioxane biodegradation by monooxygenase-expressing bacteria. Environ Sci Technol 40:5435–5442
McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, McDowell WH, Pinay G (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312
Niklaus PA, Kandeler E, Leadley PW, Schmid B, Tscherko D, Körner C (2001) A link between plant diversity, elevated CO2 and soil nitrate. Oecologia 127:540–548
Niklaus PA, Wardle DA, Tate KR (2006) Effects of plant species diversity and composition on nitrogen cycling and the trace gas balance of soils. Plant Soil 282:83–98
Oelmann Y, Wilcke W, Temperton VM, Buchmann N, Roscher C, Schumacher J, Schulze ED, Weisser WW (2007) Soil and plant nitrogen pools as related to plant diversity in an experimental grassland. SSSA J 71:720–729
Patra AK, Abbadie L, Clays-Josserand A, Degrange V, Grayston SJ, Loiseau P, Louault F, Mahmood S, Nazaret S, Philippot L, Poly E, Prosser JI, Richaume A, Le Roux X (2005) Effects of grazing on microbial functional groups involved in soil N dynamics. Ecol Monogr 75:65–80
Prior SD, Dalton H (1985) Acetylene as a suicide substrate and active-site probe for methane monooxygenase from Methylococcus capsulatus (Bath). FEMS Microbiol Lett 29:105–109
Robertson GP (1989) Nitrification and denitrification in humid tropical ecosystems. In: Proctor J (ed) Mineral nutrients in tropical forest and savanna ecosystems. Blackwell, Cambridge, pp 55–70
Robertson GP, Tiedje JM (1987) Nitrous oxide sources in aerobic soils—nitrification, denitrification and other biological processes. Soil Biol Biochem 19:187–193
Roscher C, Schumacher J, Baade J, Wilcke W, Gleixner G, Weisser WW, Schmid B, Schulze ED (2004) The role of biodiversity for element cycling and trophic interactions: an experimental approach in a grassland community. Basic Appl Ecol 5:107–121
Rosenkranz S, Wilcke W, Eisenhauer N, Oelmann Y (2012) Net ammonification as influenced by plant diversity in experimental grasslands. Soil Biol Biochem 48:78–87
Sexstone AJ, Revsbech NP, Parkin TB, Tiedje JM (1985) Direct measurement of oxygen profiles and denitrification rates in soil aggregates. SSSA J 49:645–651
Smith MS, Tiedje JM (1979) Phases of denitrification following oxygen depletion in soil. Soil Biol Biochem 11:261–267
Spehn EM, Joshi J, Schmid B, Alphei J, Körner C (2000) Plant diversity effects on soil heterotrophic activity in experimental grassland ecosystems. Plant Soil 224:217–230
Spehn EM, Scherer-Lorenzen M, Schmid B, Hector A, Caldeira MC, Dimitrakopoulos PG, Finn JA, Jumpponen A, O’Donnovan G, Pereira JS, Schulze ED, Troumbis AY, Körner C (2002) The role of legumes as a component of biodiversity in a cross-European study of grassland biomass nitrogen. Oikos 98:205–218
Stiehl-Braun PA, Hartmann AA, Kandeler E, Buchmann N, Niklaus PA (2011a) Interactive effects of drought and N fertilization on the spatial distribution of methane assimilation in grassland soils. Global Change Biol 17:2629–2639
Stiehl-Braun PA, Powlson DS, Poulton PR, Niklaus PA (2011b) Effects of N fertilizers and liming on the micro-scale distribution of soil methane assimilation in the long-term Park Grass experiment at Rothamsted. Soil Biol Biochem 43:1034–1041
Sun HY, Zhang CB, Song CB, Chang SX, Gu BJ, Chen ZX, Peng CH, Chang J, Ge Y (2013) The effects of plant diversity on nitrous oxide emissions in hydroponic microcosms. Atmos Environ 77:544–547
Sutton-Grier AE, Wright JP, McGill BM, Richardson C (2011) Environmental conditions influence the plant functional diversity effect on potential denitrification. PLoS One 6:e16584
Tiedje JM, Simkins S, Groffman PM (1989) Perspectives on measurement of denitrification in the field including recommended protocols for acetylene-based methods. Plant Soil 115:261–284
Tilman D, Wedin D, Knops J (1996) Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379:718–720
Wang B, Hou LY, Liu W, Wang ZP (2013) Non-microbial methane emissions from soils. Atmos Environ 80:290–298
Weigelt A, Weisser WW, Buchmann N, Scherer-Lorenzen M (2009) Biodiversity for multifunctional grasslands: equal productivity in high-diversity low-input and low-diversity high-input systems. Biogeosciences 6:1695–1706
West AE, Schmidt SK (2002) Endogenous methanogenesis stimulates oxidation of atmospheric CH4 in alpine tundra soil. Microb Ecol 43:408–415
Wieczorek AS, Drake HL, Kolb S (2011) Organic acids and ethanol inhibit the oxidation of methane by mire methanotrophs. FEMS Microbiol Ecol 77:28–39
Xie Z, Le Roux X, Wang CP, Gu ZK, An M, Nan HY, Chen BZ, Li F, Liu YJ, Du GZ, Feng HY, Ma XJ (2014) Identifying response groups of soil nitrifiers and denitrifiers to grazing and associated soil environmental drivers in Tibetan alpine meadows. Soil Biol Biochem 77:89–99
Acknowledgments
We gratefully acknowledge Ingeborg Schinninger for help with field sampling and laboratory sample preparation, and Nadine Guillamaud (AME platform of UMR5557) for help with enzyme activity measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jennifer Funk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niklaus, P.A., Le Roux, X., Poly, F. et al. Plant species diversity affects soil–atmosphere fluxes of methane and nitrous oxide. Oecologia 181, 919–930 (2016). https://doi.org/10.1007/s00442-016-3611-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3611-8