Abstract
Aims
Grass fungal endophyte symbioses are widespread in the Qinghai-Tibetan Plateau grasslands. The symbioses with fungal endophytes would likely have an important role in affecting ecosystem functioning (e.g., carbon and nutrients cycling, and primary productivity) It is necessary to understand the role of fungal endophytes in the litter decomposition as well as the nutrient transition of ecosystem in which endophyte host species are abundant.
Methods
Taking Festuca sinensis, Stipa purpurea and Achnatherum inebrians as study objects, litterbag method was used to compare the litter decomposition of these species with / without endophyte (E+ and E-). The changes in litter weight, total nitrogen, lignin and cellulose contents and their residual rate during the decomposition process were estimated in this study. The microbial biomass carbon and nitrogen of soil under litters were also compared.
Results
The litter from E+ F. sinensis and S. purpurea decomposed more quickly along with the cellulose compared with E-. The contents and residual rates of nitrogen and lignin in the F. sinensis and S. purpurea litters had no apparent trend of change. The microbial biomass nitrogen of soil under the E+ F. sinensis and S. purpurea litters was higher than that of the E- litters. Alternatively, the rates of decomposition and degradation of lignin were lower in the E+ A. inebrians litter than those of the E- litter. The endophyte decreased the microbial biomass carbon of soil under A. inebrians litter.
Conclusions
Endophytes will affect litter decomposition of host plants. Different grass-endophyte symbioses had different decomposition rates. Our results will enhance the knowledge of the role of endophytes in ecological progress of grassland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For the past few years, many studies have been conducted to explain the relationship between Epichloë endophytes, which were formerly classified in the genus Neotyphodium, and their hosts grasses in the subfamily Pooideae or to explore the diversity of symbionts in different ecosystems (Chen et al. 2019; Clay and Holah 1999; Leuchtmann et al. 2014; Rodriguez et al. 2008; Saikkonen et al. 2000; Siegel et al. 1987). Studies have shown that these endophytes confer the ability to tolerate stress tolerance to host species and play a significant role in the survival of some plants in high-stress environments, such as those subjected to drought, salt, cold, waterlogging, insects and diseases (Kuldau and Bacon 2008; Monnet et al. 2001; Schardl et al. 2004; Song et al. 2015a, b). In addition, the fungal endophytes have also been proven to affect belowground components and processes (Omacini et al. 2012). Therefore, the studies focused on the fungal endophyte are vital to understanding key ecosystems processes.
Litter decomposition is a fundamental ecosystem process that drives nutrient and carbon cycling at the local, regional, and global scales (Aerts 1997; Bradford et al. 2017). Litter decomposition rate can be affected by both abiotic and biotic factors (Couteaux et al. 1995; Zhou et al. 2008). These factors include climate (e.g., temperature and soil moisture), litter quality (e.g., N, lignin and cellulose contents) and the nature and abundance of decomposing organisms (e.g., bacteria, fungi and soil animals) (Couteaux et al. 1995; Porre et al. 2020). In the same environment, the original litter quality can affect the abundance, composition and activity of microorganism related to litter decomposition and then adjust the rate of decomposition and nutrient cycling (Austin et al. 2014; Berg et al. 1993; Chomel et al. 2016). Climatic condition is also a primary factor determining litter decomposition (Anderson 1991; Fierer et al. 2005). The Qinghai-Tibetan Plateau is characterized by low temperatures, limited precipitation and low oxygen concentrations at high altitudes (Zhang et al. 2015), thus their alpine grasslands are very fragile, and leading to be highly sensitive to climatic and ecological variations (Hope 2014; Tang et al. 2021). Furthermore, the alpine grasslands of the Qinghai-Tibetan Plateau have been affected by anthropogenic factors and have degenerated seriously in recent decades, not only decreased the herbage yields but also altered the soil microorganism activity and the soil nutrient cycling (Dong and Sherman 2015; Li et al. 2018; Wang et al. 2015). Many studies suggest that altered nutrient cycling may be a key response in the alpine ecosystems in response to climate and grazing perturbations (Jonasson et al. 1993; Schmidt et al. 2002; Xu et al. 2010). Hence, the studies of nutrient dynamics (like litter decomposition) or factors that might influence these processes in the Qinghai-Tibetan Plateau are essential.
Although endophytes only occur in aerial plant tissues, the effects on root-feeding herbivores and soil-dwelling organisms suggest that endophyte byproducts could be exuded by the roots of infected plants, which would affect the soil microflora (Bernard et al. 1997; Latch 1993; Omacini et al. 2004). It has been proposed that foliar endophytes of grasses could influence the decomposition of litter by altering its quality or the microenvironment for decomposition (Bernard et al. 1997; Casas et al. 2011; Clay 1997) found that fungal endophytes in Lolium multiflorum increased the activity of soil fungi and affected the metabolic diversity of soil microbial community. However, an increase in microbial diversity does not always accelerate decomposition rates. For example, part of the fungal endophytes could change their role from endophytes to saprophytes to become one of the decomposers (Purahong and Hyde 2011). Thus, the rate of decomposition might be increased by the higher diversity of decomposers. However, studies have reported that there is a competitive relationship between fungal endophytes and secondary saprophytic decomposing fungi (Dowson et al. 1988). So the endophytes may prevent the colonization of these new saprotrophic invaders resulting in lower decomposition rates (Fukasawa et al. 2009; Purahong and Hyde 2011). In addition, Franzluebbers et al. (1999) also concluded that infection by fungal endophytes in Festuca arundinacea could decrease the soil microbial mass and soil respiration and also partly inhibit soil microbial activity. Therefore, the responses of the decomposing microorganism to fungal endophytes will vary with the difference of endophyte species, host species and growing environments. Fungal endophytes not only affect the decomposer community; they also affect the litter quality that is the part of abiotic component (Lemons et al. 2005; Omacini et al. 2004). Fungal endophytes alter the metabolism of their host plants, causing changes in litter components, such as endophytic alkaloids and the contents of elements (Lyons et al. 1990; Schmidt et al. 1982; Song et al. 2015a). These changes directly affect the rates of decomposition or indirectly affect microbial decomposers and the decomposition microenvironment and may alter the degradation of litter (Lemons et al. 2005; Omacini et al. 2004; Purahong and Hyde 2011). As a whole, the effects of fungal endophytes on the decomposition of plant litter have been proven to be variable with negative, neutral, or positive results depending on the host species, fungal and plant genotypes, or ecological conditions (Gundel et al. 2016; Lemons et al. 2005; Mikola et al. 2016; Omacini et al. 2004; Siegrist et al. 2010). Fungal endophytes have a wide distribution in the Qinghai-Tibetan Plateau (Bao and Li 2016; Tian et al. 2020; Yao et al. 2015). However, the role of fungal endophytes in litter decomposition of host grasses in alpine grassland of the Qinghai-Tibetan Plateau remains unknown. It is necessary for revealing the role of endophyte in the nutrient transition of ecosystem in the Qinghai-Tibetan Plateau which is more sensitive to the global climate change.
Festuca sinensis, Stipa purpurea and Achnatherum inebrians are perennial bunchgrasses that are widespread throughout the Qinghai-Tibetan Plateau. They can grow well in severe alpine environments, thus, playing an important role in the preservation and stabilization of landscape diversity and heterogeneity (Ma and Sun 2018; Tian et al. 2020; Yao et al. 2015). These species are frequently symbiotic with Epichloë endophytes, which may increase their ability to resist or tolerate pathogenic fungi, cold, drought, heavy metals, and root hemiparasites among others (Bao et al. 2020; Li et al. 2007; Wang et al. 2017; Zhang et al. 2010; Zhou et al. 2015). As reported, the fungal endophyte species infected by these three grasses could be E. sinensis for F. sinensis (Tian et al. 2020), E. inebrians or E. chisosa for S. purpurea (Bao and Li 2016; Bao et al. 2020), and E. inebrians or E. gansuensis for A. inebrians (Chen et al. 2015; Li et al. 2004a). However, it is still unknown whether Epichloë endophytes will affect the rate of decomposition of litter of these three host grasses in the habitat of Qinghai-Tibetan Plateau.
Based on the evidence above, we hypothesized that (1) fungal endophytes will affect the quality of host grass litter by changing the degradation of its components to affect the rate of decomposition of the grass litter; (2) the microenvironment for decomposition will be altered by the presence of fungal endophytes; and (3) different grass-endophyte symbioses will have different rates of litter decomposition. The main goal of this study was to determine how the decomposition of litter from the host plant was affected by fungal endophytes and the related potential mechanism. Our work provide insight into the role of grass fungal endophytes in nutrient cycling and ecological protection in alpine grasslands.
Materials and methods
Origin of plant litter
The study site was located at an alpine grassland in Haiyan County (N 37°04′, E 100°52′), Qinghai Province of China. Mean elevation of the area is 3,200 m. This area has a typical plateau continental climate, with a mean annual solar radiation of 2,580 h, mean annual temperature of 0.4 to 3.4 °C, and annual precipitation of 277.8 to 499.5 mm (most of which falls between May and September). The vegetation is typical of an alpine grassland, with Kobresia and Elymus species serving as the dominant plants in our study area. Other companion species included F. sinensis, Poa pratensis, Melissitus ruthenica, K. humilis, Carex atrofusca and S. purpurea.
We collected natural F. sinensis, S. purpurea and A. inebrians plants with mature reproductive tillers from the study site in October 2017. 30 plants of each species were collected. The endophyte infection status of the plants was determined by the microscopic examination of stained sheaths (Li et al. 2004a). The endophyte infection rates of F. sinensis, S. purpurea and A. inebrians were 90.00%, 86.67% and 100.00%. The seeds of plants infected with endophytes (E+) were divided into two groups. One group was treated with a 100-fold dilution of the fungicide thiophanate-methyl for 1.5 h and then rinsed with distilled water to obtain seeds without endophyte (E-). Simultaneously, the other group was treated with distilled water (Chen et al. 2019). The status of their endophyte infection was confirmed using the seed staining method (Li et al. 2004a). E+ seeds of three species were 100% infected, and E- F. sinensis, S. purpurea and A. inebrians were 4.00%, 4.00% and 10.00%. E+ and E- seeds of the three species were planted separately in a greenhouse. Seeds of each species were sown in 100 pots (50 pots for E+ and 50 pots for E- plants), with three plants being reserved finally in each pot. The temperature and light cycle in the greenhouse was adjusted to 25:18 °C and a 14:10 h light : dark cycle. The leaves of E+ and E- plants were collected after four weeks of germination, and the status of their endophyte infection was confirmed using the leaf staining method (Li et al. 2004a. The inappropriate plants (E+ in E- pots or E- in E+ pots) were removed.
Litter decomposition experiment
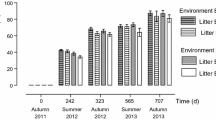
We collected litter from newly senesced E+ and E- F. sinensis, S. purpurea and A. inebrians at the end of growing season in October 2018. All the plant materials were air dried in the laboratory. To observe the long-term impact of endophytes on the rates of decomposition, litter bags (10 × 10 cm) composed of nylon mesh (1 × 1 mm openings) were used (Chuan et al. 2018). Air dried litter was cut into pieces of approximately 2 cm, and 5 g was inserted into each litter bag. A total of 18 litter bags of each type of litter (108 bags in total) with E+ and E- F. sinensis, S. purpurea and A. inebrians were placed at a study plot in the alpine grassland study site in December 2018. The study plot was enclosed in 2016 to prevent disturbance by livestock. After the manual removal of aboveground vegetation, the litter bag sets were laid on the soil surface and fixed using plastic sticks. Three randomly selected litter bags of each type of litter were retrieved after 0, 3, 6, 8, 10, and 12 months (which represented Winter 2018, spring, summer, summer-autumn, autumn-winter and winter 2019). Soil samples that were 10 cm deep were collected under each litter bag at 6 months using a soil auger with a diameter of 5 cm. The soil samples were sieved through a 1 cm mesh to remove stones and stored at 4 °C to determine the soil microbial biomass carbon and nitrogen.
The litter in bags was collected at each time point, gently washed in deionized water, and dried at 70 °C for 48 h. Soil and little stones were manually removed, and the litter was weighed. Dried samples were ground to powder to measure the contents of total nitrogen (N), lignin and cellulose. The content of total N in litter was determined using a Nitrogen Analyzer System (Kjeltec 2300 Auto System II, Foss Tecator AB, Höganäs, Sweden). The contents of lignin and cellulose were determined after methanol-chloroform extractions and hydrolysis (Rowland and Roberts 1994). The soil microbial biomass carbon was estimated using the chloroform-fumigation-extraction method (FE) described by Vance et al. (1987). The soil microbial biomass nitrogen was estimated using the chloroform-fumigation-incubation method (FI) described by Horwath and Paul (1994).
Data analysis
We fitted the data for loss of litter mass to a negative exponential model (Olson 1963):
where X0 is the initial litter mass; Xt is the residual litter mass at time t expressed as a proportion of the initial dry mass; and k is the decay constant expressed in year−1.
The element residual rate (R) was calculated using the following formula:
where Ct is element content (mg·g−1) in the residual litter mass at t time; Xt is the residual litter mass (g) at t time; C0 is the initial element content (mg·g−1); and X0 is the initial litter mass.
Data analyses were performed with SPSS 22.0 for Windows (IBM, Inc., Armonk, NY, USA). A repeated measures analysis of variance (ANOVA) with Fisher’s LSD test was utilized to estimate the effect of species, endophyte infection and time on the plant litter weight, total N content and residual rate, lignin content and residual rate, cellulose content and residual rate in the litter samples. The determination of significance of the difference between E+ and E- plants in all of the parameters was conducted using an independent t-test. Statistical significance was defined at the 95% confidence level. The means are reported with their standard errors.
Results
The weights of the F. sinensis, S. purpurea and A. inebrians litters declined over time; the litters of E+ F. sinensis and S. purpurea had a higher rate of decomposition than those of E-, but the speed of decomposition of E+ A. inebrians litter was slower than that of the E- (Fig. 1; Table 1). The range of k-values was 0.613~0.836, and the time at which 95% of the tissue that decomposed was calculated from the k-value indicating that it was 3.67~4.98 years (Table 1). The weight of litter was significantly lower in E+ F. sinensis than that of the E- plants at 6, 8 and 10 months. A lower level was observed in the weight of E+ S. purpurea litter from 6 to 12 months when compared with the E- plants. In contrast, E+ A. inebrians had significantly higher weights of litter compared with the E- plant from 8 to 12 months (Fig. 1, P < 0.05). There was a significant interaction effect between endophyte and species over time (Table 2).
After one year of decomposition in the field, the total N content in the F. sinensis, S. purpurea and A. inebrians litters increased gradually over time (Fig. 2a–c; Table 2). The total N content of E+ F. sinensis litter changed from increasing to stable during the period of decomposition. However, the E- litter had an increased N content throughout the time. Thus, the E+ F. sinensis litter had a significantly higher content of N at 6 and 8 months but a significantly lower N content at 12 months compared with that of E- F. sinensis (Fig. 2a). The total N content in E+ litter of S. purpurea was significantly higher than that in the E- litter at 6, 10 and 12 months (Fig. 2b). The total N content in E+ A. inebrians was higher than that of E- between the decomposition time, and it was significantly different at 8 and 12 months (Fig. 2c, P < 0.05). There was a significant interaction effect on the content of N in the litter between endophyte and species over time (Table 2).
The changes in content of lignin in F. sinensis, S. purpurea and A. inebrians litters declined from quick to slow during the decomposition process (Fig. 2d–f). In addition, the contents of lignin in all that litters were lower than 1% from 8 months. There was no significant difference in the content of lignin in litter between E+ and E- F. sinensis during the process of decomposition (Fig. 2d, P > 0.05). In contrast, the E+ litter from S. purpurea and A. inebrians had a significantly higher content of lignin compared with the E- litters at 3 and 6 months (Fig. 2e, f). Species, endophyte and the time of decomposition had a significant effect on the contents of lignin in litters, but there was no significant interaction effect for these three factors (Table 2). The contents of cellulose of F. sinensis, S. purpurea and A. inebrians litters clearly decreased over the period of decomposition (Fig. 2g–i). Similarly with the content of lignin, the change in speed of decomposition of cellulose in litter changed from fast to slow over the course of experiment. The content of cellulose in the E+ F. sinensis litter was higher than that of E- at 12 months, and there was no significant difference between the E+ and E- litters before 12 months (Fig. 2g). However, the E+ S. purpurea and A. inebrians litters had lower contents of cellulose compared with those of E- during the period of decomposition, and the difference between E+ and E- was significant for S. purpurea during 3, 8 and 10 months and 8, 10 and 12 months for A. inebrians (Fig. 2h, i). There was no significant interaction effect on the content of cellulose in litter for species, endophyte and time (Table 2).
The residual rate of N in F. sinensis litter decreased with the progression of time. There was no significant difference between the E+ and E- F. sinensis litter during the whole decomposition process (Fig. 3a). The residual rate of N in S. purpurea and A. inebrians litters had no discernable trend during the decomposition (Fig. 3b, c). In addition, the difference between E+ and E- litters was not significant during most of the decomposition stage. Species and decomposition time had a significant effect on the residual rate of lignin in the litters, respectively, but there was no significant interaction effect for species, endophyte and time (Table 3). The residual rate of lignin had a similar trend of variation with the content of lignin in F. sinensis, S. purpurea and A. inebrians litters, which decreased gradually with the progression of time (Fig. 3a–c). The litter from E+ F. sinensis had a significantly lower residual rate of lignin at 6 months, and E+ A. inebrians litters had a significantly higher residual rate of lignin at 3 months. With the exception of that, the difference between E+ and E- litters of the three species was not significant during the time of decomposition (Fig. 3d–f). The time had a significant effect on the residual rate of lignin in litters, and the interaction effect induced by species, endophyte and time was significant (P = 0.006, Table 3). Alternatively, the residual rate of cellulose in the litters of F. sinensis, S. purpurea and A. inebrians also declined as the decomposition time was prolonged (Fig. 3g–i). The residual rate of cellulose in E+ F. sinensis litter was lower than that in the E- litter at 6 and 8 months (Fig. 3g, P < 0.05). The E+ S. purpurea litter had a lower residual rate of cellulose during the process of decomposition, and the difference was significant at 8 and 10 months (Fig. 3h). However, the residual rate of cellulose in A. inebrians litter was not significantly different among the whole period of decomposition (Fig. 3i). The residual rate of cellulose in litter was significantly influenced by endophyte and time separately, but the interaction effect of species, endophyte and time was not significant (Table 3).
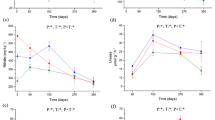
The microbial biomass carbon of soil under the E+ and E- F. sinensis litters did not differ significantly (P < 0.05, Fig. 4a). However, the microbial biomass carbon of soil under the E+ S. purpurea and A. inebrians litters was significantly lower than that of the E- litters. The species did not result in a significant effect on microbial biomass carbon, but the effect of endophyte was significant (Table 4). There was no significant interaction effect for these two factors. The microbial biomass nitrogen of soil in the E+ F. sinensis and S. purpurea litters was higher when compared with that of E- (P < 0.05, Fig. 4b), but the difference between E+ and E- A. inebrians litters was not significant. Similarly, the interaction effect of species and endophyte was not significant (P > 0.05, Table 4).
Effects of endophyte on the microbial biomass carbon (a) and microbial biomass nitrogen (b) of soil under different grass litters. F.s: Festuca sinensis; S.p: Stipa purpurea; A.i: Achnatherum inebrians. E+: with endophyte; E-: without endophyte. *P < 0.05 between the E+ and E- litters simultaneously
Discussion
As hypothesized, we found that the fungal endophyte affects the decomposition of litter of their host grasses F. sinensis, S. purpurea and A. inebrians. Different species varied in their response to fungal endophyte infection in the rate of decomposition of litter and related factors, such as litter quality and the surrounding microbial mass.
Grasses infected with fungal endophytes can release allelochemicals and alter the area of soil in which they grow (Malinowski et al. 1999; Petroski et al. 1990). A review meta-analysis showed that an inhibitory effect of fungal endophyte on host litter decomposition (Omacini et al. 2012). At that time, Siegrist et al. (2010) suggested that fungal alkaloids would be a key inhibitory factor on decomposers and eventually, driving the observed low rate of decomposition. Omacini et al. (2004) found that the rates of decomposition in E+ litters were 18% lower on average than that in the E- litters in both a garden microcosm experiment and a greenhouse experiment. Lemons et al. (2005) also found that the rates of decomposition were 6% slower in E+ litter than in E- litter in an agricultural field experiment. In this study, the speed of decomposition in E+ A. inebrians litter was slower than that in the E- litter (Fig. 1c), which is similar to previous results (Lemons et al. 2005; Omacini et al. 2004). Other studies demonstrated that the enhanced accumulation of soil carbon in pastures planted with F. arundinacea that were highly infected with endophytes compared with those that had low levels of infection could be attributed to the reduction in decomposition rates of E+ litters (Franzluebbers et al. 1999; Osono 2006; Schomberg et al. 2000).
However, in contrast with A. inebrians, the rate of decomposition of litter in F. sinensis and S. purpurea with endophytes was quicker and the period of decomposition was shorter compared with the litters that lacked endophytes (Fig. 1a, b; Table 1). These results are consistent with a previous study that found that the E. uncinatum fungal endophyte increased the rate of litter decomposition of Schedonorus pratensis when both E+ and E- litter bags were incubated in a common garden (Gundel et al. 2016). Grass endophytes can utilize simple sugars, such as glucose, sucrose, and xylose, as a sole carbon source in tissues that had recently died (White et al. 1991). Thus, endophytes within the tissue have an advantage in their ability to utilize these readily available components, while compared to the fungi in the soil under the dead plant. Such an ecological advantage suggests that grass infected by endophytes will decompose at a quicker rate (Gundel et al. 2016; Osono 2006).
Litter quality becomes a more effective determinant of the rate of decomposition than the climate (Aerts 1997; Meentemeyer 1978). At the ecosystem scale, litter quality is most often related to the chemical characteristics of the litter, for example, carbon : nitrogen ratios and/or lignin content (Aber et al. 1990; Aerts 1997). In this study, the content of nitrogen in the A. inebrians, F. sinensis and S. purpurea litters increased with the progression of time (Fig. 2a). The content of nitrogen or phosphorus in litter would gradually increase during the decomposition of litter because of the lower speed of release of nutrient elements compared with the rate of loss of litter mass (Gallardo and Merino 1993). This could also be reflected in the change in residual rate of nitrogen, which decreased with the progression of time of decomposition (Fig. 3a). However, the residual rates of total nitrogen in the litters of three species had no significant difference between the E+ and E- plants during most of the time of decomposition. Lemons et al. (2005) and Omacini et al. (2004) found there was no significant difference in the total content of N between E+ and E- Lolium litters. In this study, the total nitrogen content appeared to have a different status in varied host species with the progression of time. Thus, the content of nitrogen in litter might not play a decisive role in the relative progress of the process of decomposition (Gundel et al. 2016).
In addition to changes in the mineral content, endophytes have been associated with changes in plant structural parameters, such as the content of fibers and lignin, that could also be linked with the decomposition of litter (Gundel et al. 2017; Rogers et al. 2011; Soto-Barajas et al. 2016). In this study, there was no visible effect from fungal endophytes on the original contents of lignin and cellulose of host grass. However, the degradation of lignin and cellulose varied between E+ and E- symbiont for different species. First, S. purpurea had a higher content of lignin in the E+ litter compared with that of E- during the process of decomposition, but the residual rates of lignin did not differ significantly (Figs. 2e and 3e). This may be owing to the higher speed of rate of decomposition of the E+ litter. The content and residual rate of lignin in the E+ litter from A. inebrians were higher than those of the E- litter to some degree during the process of decomposition (Figs. 2f and 3f). Thus, the slower rate of decomposition of the E+ A. inebrians litter compared with that of E- may be partly attributed to the slower decomposition of lignin. Secondly, the content and residual rate of cellulose in the E+ S. purpurea litter were lower than those in the E- litter to some degree during the year of decomposition (Fig. 2h, 3h). Thus, the lower concentration of cellulose in the lower mass weight of E+ litter could result from the faster degradation of cellulose compared with that of the E- S. purpurea litter. The lower residual rate of cellulose in E+ F. sinensis compared with that of E- could also induce the quicker decomposition of E+ litter (Fig. 3g). However, the lower concentration of cellulose in E+ A. inebrians litter was mostly caused by the slower decomposition of the E+ litter based on the lack of a significant difference in the residual rate of cellulose between the E+ and E- litters (Fig. 2i). These results suggest that the effect of fungal endophytes on the decomposition of litter could be partly attributed to the influence of lignin degradation for A. inebrians and the influence of cellulose degradation for F. sinensis and S. purpurea.
Soil microbial biomass is the main driving force in the decomposition of organic materials and is frequently used as an early indicator of changes in the chemical and physical properties of soil (Baaru et al. 2007; Brookes 1995). The quicker rate of decomposition of organic materials related to the higher values of soil microbial biomass carbon that resulted from the ready supply of nutrients that provide for microbial growth (Baaru et al. 2007). Grasses infected with fungal endophytes can release allelochemicals and alter the area of soil in which they grow (Malinowski et al. 1999; Petroski et al. 1990). In this study, the lower rate of decomposition of A. inebrians litter was associated with a lower amount of soil microbial biomass carbon. This result is consistent with those of previous studies (Baaru et al. 2007; Franzluebbers et al. 1999; Lemons et al. 2005). Thus, the exit of fungal endophytes in litter could decrease the soil microbial biomass by decreasing the speed of decomposition of litter by A. inebrians. This could be related to the secondary metabolites synthesized by the endophyte in A. inebrians, which altered the composition of the soil microbial community (Malinowski et al. 1998; Ponce et al. 2009; Siegel et al. 1990). However, other studies have found that the endophyte E. occultans (formerly Neotyphodium occultans) in Italian ryegrass (L. multiflorum) increased the activity of soil fungal community (Casas et al. 2011). The value of microbial biomass nitrogen in soil can represent the dynamic balance during the process of the mineralization and immobilization of nutrients through the reproduction and death of microorganisms (Li et al. 2004b). Thus, the higher microbial biomass nitrogen of the soil under E+ F. sinensis and S. purpurea could be attributed to the promotion of endophytes on microbial activity. This could be one indirect explanation for the quicker decomposition of E+ F. sinensis and S. purpurea litters compared with that of the E- litters. Therefore, different endophyte species may induce various changes in the soil microbial community, from amount to vitality, during the decomposition of litter.
Fungal endophytes can alter the contents of amino acids, water soluble carbohydrates, lipids, organic acids or chlorogenic acid in the host plant, which result in different chemical constitutions between the E+ and E- plants (Rasmussen et al. 2007, 2008). These differences may directly influence the decomposition of litter by the host plant or indirectly change soil physicochemical properties and microbial community (Gundel et al. 2017; Omacini et al. 2004). Our results suggest that the endophyte of A. inebrians could decrease the degradation of lignin in A. inebrians litter, reducing the microbial mass in soil, to slow the decomposition of host grass litter. We found that the endophyte of S. purpurea accelerated the degradation of cellulose in S. purpurea litter, enhanced the microbial activity of soil, and finally promoted the rate of decomposition of this litter. The fact that the promotion of rate of decomposition by the F. sinensis endophyte may be owing to the higher microbial biomass of nitrogen in the soil under E+ litter, whose litter quality did not differ significantly between E+ and E- during the process of decomposition. Accordingly, the endophytes in A. inebrians, F. sinensis and S. purpurea played different roles in the decomposition of litter from their host, resulting in varied litter quality and environment for decomposition. Our results on decomposition further demonstrate a role for endophyte–plant mutualisms in ecosystem processes under field conditions. However, using seeds treated with fungicide to kill Epichloë endophytes in this study, it is likely that other non-systemic fungi are being also removed (Zabalgogeazcoa et al. 2013). To fully understand the mechanism by which one endophyte affects the decomposition of litter, a more solid conclusion must await further examination on the effects of endophytes on litter quality or microbial community composition and the effect of environmental conditions in the future.
Conclusions
In conclusion, different symbioses of grass-endophytes had different litter decomposition rates and processes. Also, the impact of different symbioses to the number of soil microorganism is various. The endophytes in F. sinensis and S. purpurea promoted the rate of decomposition of host and the degradation of cellulose in litter, and they also increased the microbial biomass nitrogen of soil under litter. While the endophyte in A. inebrians delayed the decomposition of host litter and the degradation of lignin degradation in the litter, it also decreased the microbial biomass carbon of soil under litter. Therefore, F. sinensis and S. purpurea endophyte symbioses are suggested to be applied in alpine grasslands in the Qinghai-Tibetan Plateau for accelerating the nutrient flow and improving the vegetation coverage of degraded grassland. However, the mechanism of different fungal endophytes modifying the decomposition of host grass and the role of endophytes playing in the microbial community needs additional study.
References
Aber JD, Melillo JM, McClaugherty CA (1990) Predicting long-term patterns of mass loss, nitrogen dynamics, and soil organic matter formation from initial fine litter chemistry in temperate forest ecosystems. Can J Bot 68:2201–2208
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Anderson J (1991) The effects of climate change on decomposition processes in grassland and coniferous forests. Ecol Appl 1:326–347
Austin AT, Vivanco L, González-Arzac A, Pérez LI (2014) There’s no place like home? An exploration of the mechanisms behind plant litter–decomposer affinity in terrestrial ecosystems. New Phytol 204:307–314
Baaru M, Mungendi D, Bationo A, Verchot L, Waceke W (2007) Soil microbial biomass carbon and nitrogen as influenced by organic and inorganic inputs at Kabete, Kenya. Advances in integrated soil fertility management in sub-Saharan Africa: challenges and opportunities. Springer, Berlin
Bao GS, Li CJ (2016) Isolation and identification of endophytes infecting Stipa purpurea, a dominant grass in meadows of the Qinghai-Tibet Plateau. Acta Prataculturae Sin 25:32–42
Bao GS, Song ML, Wang YQ, Saikkonen K, Li CJ (2020) Does Epichloë endophyte enhance host tolerance to root hemiparasite? Microb Ecol 82:35–48
Berg B, Berg M, Bottner P, Box E, Breymeyer A, De Anta RC, Couteaux M, Escudero A, Gallardo A, Kratz W (1993) Litter mass loss rates in pine forests of Europe and Eastern United States: some relationships with climate and litter quality. Biogeochemistry 20:127–159
Bernard E, Gwinn K, Pless C, Williver C (1997) Soil invertebrate species diversity and abundance in endophyte-infected tall fescue pastures. Neotyphodium/grass interactions. Springer, Berlin
Bradford MA, Veen GC, Bonis A, Bradford EM, Classen AT, Cornelissen JHC, Crowther TW, Jonathan R, Freschet GT, Kardol P (2017) A test of the hierarchical model of litter decomposition. Nat Ecol Evol 1:1836–1845
Brookes P (1995) The use of microbial parameters in monitoring soil pollution by heavy metals. Biol Fertil Soils 19:269–279
Casas C, Omacini M, Montecchia MS, Correa OS (2011) Soil microbial community responses to the fungal endophyte Neotyphodium in Italian ryegrass. Plant Soil 340:347–355
Chen L, Li XZ, Li CJ, Swoboda GA, Young CA, Sugawara K, Leuchtmann A, Schardl CL (2015) Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia 107:863–873
Chen TX, Li CJ, White JF, Nan ZB (2019) Effect of the fungal endophyte Epichloë bromicola on polyamines in wild barley (Hordeum brevisubulatum) under salt stress. Plant Soil 436:29–48
Chomel M, Guittonny-Larchevêque M, Fernandez C, Gallet C, DesRochers A, Pare D, Jackson BG, Baldy V (2016) Plant secondary metabolites: a key driver of litter decomposition and soil nutrient cycling. J Ecol 104:1527–1541
Chuan XZ, Carlyle CN, Bork EW, Chang SX, Hewins DB (2018) Long-term grazing accelerated litter decomposition in northern temperate grasslands. Ecosystems 21:1321–1334
Clay K (1997) Consequences of endophyte-infected grasses on plant biodiversity. Neotyphodium/grass interactions. Springer, Berlin
Clay K, Holah J (1999) Fungal endophyte symbiosis and plant diversity in successional fields. Science 285:1742–1744
Couteaux M-M, Bottner P, Berg B (1995) Litter decomposition, climate and liter quality. Trends Ecol Evol 10:63–66
Dong SK, Sherman R (2015) Enhancing the resilience of coupled human and natural systems of alpine rangelands on the Qinghai-Tibetan Plateau. Rangel J 37:i–iii
Dowson C, Rayner A, Boddy L (1988) Inoculation of mycelial cord-forming basidiomycetes into woodland soil and litter II. Resource capture and persistence. New Phytol 109:343–349
Fierer N, Craine JM, McLauchlan K, Schimel JP (2005) Litter quality and the temperature sensitivity of decomposition. Ecology 86:320–326
Franzluebbers A, Nazih N, Stuedemann J, Fuhrmann J, Schomberg H, Hartel P (1999) Soil carbon and nitrogen pools under low-and high-endophyte-infected tall fescue. Soil Sci Soc Am J 63:1687–1694
Fukasawa Y, Osono T, Takeda H (2009) Effects of attack of saprobic fungi on twig litter decomposition by endophytic fungi. Ecol Res 24:1067
Gallardo A, Merino J (1993) Leaf decomposition in two Mediterranean ecosystems of southwest Spain: influence of substrate quality. Ecology 74:152–161
Gundel PE, Helander M, Garibaldi LA, Vázquez-de-Aldana BR, Zabalgogeazcoa I, Saikkonen K (2016) Role of foliar fungal endophytes in litter decomposition among species and population origins. Fungal Ecol 21:50–56
Gundel PE, Helander M, Garibaldi LA, Vázquez-de-Aldana B, Zabalgogeazcoa I, Saikkonen K (2017) Direct and indirect effects of the fungal endophyte Epichloë uncinatum on litter decomposition of the host grass, Schedonorus pratensis. Plant Ecol 218:1107–1115
Hope G (2014) The sensitivity of the high mountain ecosystems of New Guinea to climatic change and anthropogenic impact. Arct Antarct Alp Res 46:777–786
Horwath W, Paul E (1994) Microbial biomass. Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties 5, 753-773
Jonasson S, Havström M, Jensen M, Callaghan TV (1993) In situ mineralization of nitrogen and phosphorus of arctic soils after perturbations simulating climate change. Oecologia 95:179–186
Kuldau G, Bacon C (2008) Clavicipitaceous endophytes: their ability to enhance resistance of grasses to multiple stresses. Biol Control 46:57–71
Latch GC (1993) Physiological interactions of endophytic fungi and their hosts. Biotic stress tolerance imparted to grasses by endophytes. Agric, Ecosyst Environ 44:143–156
Lemons A, Clay K, Rudgers JA (2005) Connecting plant-microbial interactions above and belowground: a fungal endophyte affects decomposition. Oecologia 145:595–604
Leuchtmann A, Schardl CL, White JF, Tadych M (2014) Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 106:202–215
Li CJ, Nan ZB, Paul VH, Dapprich PD, Liu Y (2004a) A new Neotyphodium species symbiotic with drunken horse grass (Achnatherum inebrians) in China. Mycotaxon 90:141–147
Li SQ, Ren SJ, Li SX (2004b) Seasonal change of soil microbial biomass and the relationship between soil microbial biomass and soil moisture and temperature. Plant Nutr Fertil Sci 10:18–23
Li CJ, Gao JH, Nan ZB (2007) Interactions of Neotyphodium gansuense, Achnatherum inebrians, and plant-pathogenic fungi. Mycol Res 111:1220–1227
Li W, Wang JL, Zhang XL, Shi SL, Cao WX (2018) Effect of degradation and rebuilding of artificial grasslands on soil respiration and carbon and nitrogen pools on an alpine meadow of the Qinghai-Tibetan Plateau. Ecol Eng 111:134–142
Lyons PC, Evans JJ, Bacon CW (1990) Effects of the fungal endophyte Acremonium coenophialum on nitrogen accumulation and metabolism in tall fescue. Plant Physiol 92:726–732
Ma BB, Sun J (2018) Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. BMC Ecol 18:10
Malinowski DP, Alloush GA, Belesky DP (1998) Evidence for chemical changes on the root surface of tall fescue in response to infection with the fungal endophyte Neotyphodium coenophialum. Plant Soil 205:1–12
Malinowski D, Belesky D, Fedders J (1999) Endophyte infection may affect the competitive ability of tall fescue grown with red clover. J Agron Crop Sci 183:91–101
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472
Mikola J, Helander M, Saikkonen K (2016) No effects of Epichloë endophyte infection on nitrogen cycling in meadow fescue (Schedonorus pratensis) grassland. Plant Soil 405:257–264
Monnet F, Vaillant N, Hitmi A, Coudret A, Sallanon H (2001) Endophytic Neotyphodium lolii induced tolerance to Zn stress in Lolium perenne. Physiol Plant 113:557–563
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Omacini M, Chaneton EJ, Ghersa CM, Otero P (2004) Do foliar endophytes affect grass litter decomposition? A microcosm approach using Lolium multiflorum. Oikos 104:581–590
Omacini M, Semmartin M, Pérez LI, Gundel PE (2012) Grass-endophyte symbiosis: a neglected aboveground interaction with multiple belowground consequences. Appl Soil Ecol 61:273–279
Osono T (2006) Role of endophytic fungi in grass litter decomposition. NZGA: Res Pract Ser 13:103–105
Petroski RJ, Dornbos DL Jr, Powell RG (1990) Germination and growth inhibition of annual ryegrass (Lolium multiflorum L.) and alfalfa (Medicago sativa L.) by loline alkaloids and synthetic N-acylloline derivatives. J Agric Food Chem 38:1716–1718
Ponce MA, Bompadre MJ, Scervino JM, Ocampo JA, Chaneton EJ, Godeas AM (2009) Flavonoids, benzoic acids and cinnamic acids isolated from shoots and roots of Italian rye grass (Lolium multiflorum Lam.) with and without endophyte association and arbuscular mycorrhizal fungus. Biochem Syst Ecol 37:245–253
Porre RJ, van der Werf W, De Deyn GB, Stomph TJ, Hoffland E (2020) Is litter decomposition enhanced in species mixtures? A meta-analysis. Soil Biol Biochem :107791
Purahong W, Hyde KD (2011) Effects of fungal endophytes on grass and non-grass litter decomposition rates. Fungal Divers 47:1–7
Rasmussen S, Parsons AJ, Bassett S, Christensen MJ, Hume DE, Johnson LJ, Johnson RD, Simpson WR, Stacke C, Voisey CR (2007) High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytol 173:787–797
Rasmussen S, Parsons AJ, Fraser K, Xue H, Newman JA (2008) Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiol 146:1440–1453
Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim Y-O, Redman RS (2008) Stress tolerance in plants via habitat-adapted symbiosis. ISME J 2:404–416
Rogers JK, Morton BC, Mosali J (2011) Plant and endophyte effect on fiber, N, and P concentrations in tall fescue. Int J Agron 2011:7
Rowland AP, Roberts JD (1994) Lignin and cellulose fractionation in decomposition studies using acid-detergent fibre methods. Commun Soil Sci Plant Anal 25:269–277
Saikkonen K, Ahlholm J, Helander M, Lehtimäki S, Niemeläinen O (2000) Endophytic fungi in wild and cultivated grasses in Finland. Ecography 23:360–366
Schardl CL, Leuchtmann A, Spiering MJ (2004) Symbioses of grasses with seedborne fungal endophytes. Annu Rev Plant Biol 55:315–340
Schmidt SP, Hoveland CS, Clark EM, Davis ND, Smith LA, Grimes HW, Holliman JL (1982) Association of an endophytic fungus with fescue toxicity in steers fed Kentucky 31 tall fescue seed or hay. J Anim Sci 55:1259–1263
Schmidt IK, Jonasson S, Shaver GR, Michelsen A, Nordin A (2002) Mineralization and distribution of nutrients in plants and microbes in four tundra ecosystems-responses to warming. Plant Soil 242:93–106
Schomberg HH, Stuedemann JA, Franzluebbers AJ, Wilkinson SR (2000) Spatial distribution of extractable phosphorus, potassium, and magnesium as influenced by fertilizer and tall fescue endophyte status. Agron J 92:981–986
Siegel MR, Latch GCM, Johnson MC (1987) Fungal endophytes of grasses. Annu Rev Phytopathol 25:293–315
Siegel MR, Latch GCM, Bush LP, Fannin FF, Rowan DD, Tapper BA, Bacon CW, Johnson MC (1990) Fungal endophyte-infected grasses: alkaloid accumulation and aphid response. J Chem Ecol 16:3301–3315
Siegrist JA, McCulley RL, Bush LP, Phillips TD (2010) Alkaloids may not be responsible for endophyte-associated reductions in tall fescue decomposition rates. Funct Ecol 24:460–468
Song ML, Chai Q, Li XZ, Yao X, Li CJ, Christensen MJ, Nan ZB (2015a) An asexual Epichloë endophyte modifies the nutrient stoichiometry of wild barley (Hordeum brevisubulatum) under salt stress. Plant Soil 387:153–165
Song ML, Li XZ, Saikkonen K, Li CJ, Nan ZB (2015b) An asexual Epichloë endophyte enhances waterlogging tolerance of Hordeum brevisubulatum. Fungal Ecol 13:44–52
Soto-Barajas MC, Zabalgogeazcoa I, Gómez-Fuertes J, González-Blanco V, Vázquez-de-Aldana BR (2016) Epichloë endophytes affect the nutrient and fiber content of Lolium perenne regardless of plant genotype. Plant Soil 405:265–277
Tang R, DeLuca TH, Cai Y, Sun S, Luo J (2021) Long-term decomposition dynamics of broadleaf litters across a climatic gradient on the Qinghai-Tibetan Plateau, China. Plant Soil 465:403–414
Tian P, Xu WB, Li CJ, Song H, Wang MN, Schardl CL, Nan ZB (2020) Phylogenetic relationship and taxonomy of a hybrid Epichloë species symbiotic with Festuca sinensis. Mycol Prog 19:1069–1081
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wang P, Lassoie JP, Morreale SJ, Dong S (2015) A critical review of socioeconomic and natural factors in ecological degradation on the Qinghai-Tibetan Plateau, China. Rangeland J 37:1–9
Wang JJ, Zhou YP, Lin WH, Li MM, Wang MN, Wang ZG, Kuang Y, Tian P (2017) Effect of an Epichloë endophyte on adaptability to water stress in Festuca sinensis. Fungal Ecol 30:39–47
White JF, Breen JP, Morgan-Jones G (1991) Substrate utilization in selected Acremonium, Atkinsonella and Balansia species. Mycologia 83:601-610
Xu GP, Chao ZG, Wang SP, Hu YG, Zhang ZH, Duan JC, Chang XF, Su AL, Luo CY, Li YN, Du MY (2010) Temperature sensitivity of nutrient release from dung along elevation gradient on the Qinghai-Tibetan plateau. Nutr Cycl Agroecosyst 87:49–57
Yao X, Christensen MJ, Bao GS, Zhang CP, Li XZ, Li CJ, Nan ZB (2015) A toxic endophyte-infected grass helps reverse degradation and loss of biodiversity of over-grazed grasslands in northwest China. Sci Rep 5:18527
Zabalgogeazcoa I, Gundel PE, Helander M, Saikkonen K (2013) Non-systemic fungal endophytes in Festuca rubra plants infected by Epichloë festucae in subarctic habitats. Fungal Divers 60:25–32
Zhang XX, Li CJ, Nan ZB (2010) Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense. J Hazard Mater 175:703–709
Zhang XL, Wang SJ, Zhang JM, Wang G, Tang XY (2015) Temporal and spatial variability in precipitation trends in the Southeast Tibetan Plateau during 1961–2012. Clim Past Discuss 11:447–487
Zhou GY, Guan LL, Wei XH, Tang XL, Liu SG, Liu JX, Zhang DQ, Yan JH (2008) Factors influencing leaf litter decomposition: an intersite decomposition experiment across China. Plant Soil 311:61–72
Zhou LY, Li CJ, Zhang XX, Johnson R, Bao GS, Yao X, Chai Q (2015) Effects of cold shocked Epichloë infected Festuca sinensis on ergot alkaloid accumulation. Fungal Ecol 14:99–104
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31700098, 32060398), the Natural Science Foundation of Qinghai Province (2019-ZJ-967Q) and Qinghai Provincial Key Laboratory of Forage Germplasm utilization on Qinghai-Tibetan Plateau (2020-ZJ-Y03). The authors would like to thank Liu Yan, Yin Yali, and Zhao Wen for their help with the collection and analysis of samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Birgit Mitter.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, M., Wang, Y., Wang, H. et al. Effects of Epichloë endophytes on litter decomposition--depending on different host species. Plant Soil 471, 715–728 (2022). https://doi.org/10.1007/s11104-021-05235-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05235-x