Abstract
Aims
Zinc (Zn) and phosphorus (P) often interact negatively with each other in soil-plant systems. We investigated the effects of P-Zn interaction on Zn and P accumulation and partitioning in alfalfa.
Methods
Plants were grown in a calcareous soil supplied with different rates of Zn (0, 200, and 800 mg kg−1) and P (0, 20, and 80 mg kg−1). Plant dry mass, Zn and P concentrations in shoots and roots, bulk soil and rhizosheath pH, rhizosheath carboxylates, and DTPA-extractable Zn concentration in the bulk soil were determined.
Results
Phosphorus-Zn interaction significantly affected DTPA-extractable Zn concentration, plant dry mass, accumulation of Zn and P, and partitioning of Zn in alfalfa, but did not affect rhizosheath pH or the amounts of rhizosheath carboxylates. Increasing P rate promoted plant growth at all soil Zn rates and might enhance the plants’ capacity to cope with excessive Zn; it resulted in a lower rhizosheath pH, which likely contributed to greater Zn and P uptake. Zinc deficiency enhanced exudation of citrate, malonate and malate, while the release of tartrate was related with P deficiency.
Conclusions
There are strong P-Zn interactions in calcareous soil-plant system, such interactions significantly affect Zn bioavailability, plant growth, accumulation of Zn and P, and partitioning of Zn in alfalfa. Rational P fertilization should be considered for efficient Zn biofortification on Zn-deficient soils and phytoremediation of Zn-contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is an essential micronutrient and plays critical structural and catalytic roles in a large number of proteins (Palmgren et al. 2008). Zinc deficiency is a major agricultural problem worldwide, because most agricultural soils contain low levels of Zn. Low Zn availability in soil restricts plant growth and reduces crop yield and quality, consequently resulting in malnutrition in humans and livestock (Cakmak et al. 2017; Gupta et al. 2016; Palmgren et al. 2008). Zinc deficiency is more likely to occur at alkaline pH, as the solubility of Zn is less at alkaline pH than under mildly acidic conditions, and Zn solubility is further decreased with increasing carbonate concentration in soil (Duffner et al. 2012; Gupta et al. 2016). On the other hand, plant Zn toxicity can occur in Zn-contaminated soils, resulting from anthropogenic activities such as mining, smelting, production of galvanized materials, combustion of fossil fuels, disposal of sewage sludge, and application of manure and agrochemicals (Broadley et al. 2007). Excess Zn in soil can enhance Zn uptake and accumulation in plants, and induce serious plant growth defects. High Zn concentrations ([Zn]) in plants can be toxic to plants, and also cause health problems in humans and livestock (Broadley et al. 2007; Fukao et al. 2011).
Phosphorus (P) is an essential plant macronutrient which plays important roles in plant growth and metabolism, but its bioavailability in soil is often low, due to the sorption of inorganic phosphate (Pi) to soil particles (Hinsinger 2001). Phosphorus deficiency is common in soil and constrains plant growth and crop yields, therefore, P fertilizers are applied to meet the P demand of plants and to ensure high yields of various crops. However, sources of P, such as rock phosphate and manure, are limited, and fertilizer P use efficiency (PUE) is generally low (Hopkins and Hansen 2019; Johnston et al. 2014). Under P deficiency, a series of morphological, physiological, and biochemical adjustments can enhance plants’ P-acquiring capacity (Lambers and Oliveira 2019). For plants growing in P-deficient calcareous soil, release of protons by roots of can increase P availability in the rhizosphere (Hinsinger et al. 2003). Carboxylates exuded from roots can compete with both inorganic and organic P for binding sites in rhizosphere soil, thus mobilizing sparingly-soluble soil P for uptake by plants (Lambers and Oliveira 2019).
Zinc and P deficiency often co-occur in calcareous soils (Akhtar et al. 2019; Duffner et al. 2012; Schjørring et al. 2019). The availability of Zn in soils depends on a series of factors, including soil texture, water content, pH, organic matter content, bicarbonate content, the availability of competing ions, and activities of plant roots and rhizosphere microflora (Alloway 2009; Cakmak 2008; Duffner et al. 2012). In many calcareous soils, Zn deficiency is mainly caused by low Zn bioavailability, rather than by low total soil [Zn], and high soil pH is often the main factor associated with low Zn bioavailability (Alloway 2009; Cakmak 2008; Wissuwa et al. 2006). Unlike that of Zn, the bioavailability of P peaks at neutral pH and decreases in both the alkaline and acid ranges (Lambers and Oliveira 2019). The solubility of both Zn and P decrease with increasing lime content, and the deficiency of both elements in soil are often linked with high lime content (Akhtar et al. 2019; Hopkins and Hansen 2019).
There are interactions between Zn and P in soil-plant systems, and such interactions can influence the bioavailability of both Zn and P in soil, and the accumulation and partitioning of Zn and P in plants, but the interactive effects of Zn and P are controversial (Akhtar et al. 2019; Duffner et al. 2012; Ova et al. 2015; Pongrac et al. 2019). Zinc and P often interact negatively with each other in soil-plant systems, and a few mechanisms for such interactions have been proposed (Sánchez-Rodríguez et al. 2017). Although Zn and P are retained in soil via different mechanisms and in different amounts by minerals, and the mechanisms and amounts vary between different types of soil, high doses of Pi, in most cases, promote Zn sorption and reduce plant uptake of Zn (Chen et al. 2019). Excessive application of P results in reduced shoot and grain [Zn] and crop yields (Akhtar et al. 2019; Barben et al. 2011; Zhang et al. 2012). It is yet not clear whether ‘P-induced Zn deficiency’ is due to the negative interaction between Zn and P in the soil or in the plant. Precipitation of soluble Pi and Zn (Zn2+) in the soil as Zn3(PO4)2 can make both Zn and P unavailable for plants, precipitation of Zn3(PO4)2 may also occur in roots and restrict the translocation of Zn from roots to shoots and grains (Hayes et al. 2019; Pongrac et al. 2019; Zhang et al. 2012). Other mechanisms involved in P-induced Zn deficiency have also been suggested, including changes in soil pH, inoculation with arbuscular mycorrhizal fungi, and root exudation of carboxylates, which affect Zn and P availability in soil (Chen et al. 2019; Duffner et al. 2012; Pongrac et al. 2019). Exudation of carboxylates such as citrate and malate from roots of some plants can mobilize both Zn and P in calcareous soils (Duffner et al. 2012). Under P deficiency, a considerable amounts of carboxylates are exuded from roots of many plants, and the amounts often decrease with increasing P availability in soil (Lambers and Oliveira 2019). The bioavailability of Zn in soil may decrease with increasing P availability, partly because less Zn would be mobilized by reduced amounts of carboxylates.
Within plants, P-enhanced growth likely causes a ‘dilution’ of the shoot [Zn]; loss of tight control over P uptake by genes encoding high-affinity P transporter proteins under Zn deficiency will result in increased P accumulation and further aggravate Zn deficiency (Huang et al. 2000; Sánchez-Rodríguez et al. 2017; Zhu et al. 2001). Up to now, most studies on Zn-P interactions have been performed under Zn deficiency or low available soil Zn conditions, and investigated the effects and mechanisms of P-fertilizer application on plant Zn uptake and distribution (Dimkpa et al. 2019; Watts-Williams et al. 2014). It is not clear whether Zn-P interactions are different between excessive soil Zn conditions and Zn deficiency, and what mechanisms may be involved in the interactions and contribute to such differences. In addition to Zn biofortification on Zn-deficient soils, Zn-P interactions in the phytoremediation of Zn-contaminated soils also warrant study.
Our previous studies have shown that growth and P uptake of alfalfa (Medicago sativa L.) are responsive to varying soil P supply in calcareous soil; plants can significantly acidify the rhizosheath, and root exudation of carboxylates (mainly tartrate) is greater under less P supply (He et al. 2020). In this study, a pot experiment using calcareous soil was undertaken to investigate Zn-P interactions of alfalfa at three soil Zn rates (i.e. deficient, sufficient, and excessive), and at three rates of P application. The hypotheses were: (i) response of plant growth to soil P rate differ at different soil Zn rates; (ii) root and shoot [Zn] increase with increasing Zn rate but decrease with increasing P rate, and vice versa for root and shoot P concentrations, but total plant contents of both Zn and P increase with increases in both Zn and P rate; (iii) the partitioning of Zn and P to roots and shoots would be affected by both soil Zn and P rates, and a greater proportion of Zn and P would be retained in roots at higher Zn and P rates; (iv) rhizosheath pH would be reduced, and the amounts of rhizosheath carboxylates would be greater at lower Zn and P rates.
Materials and methods
Soil preparation and plant cultivation
The soil used in this study was a loess soil collected from the top 40-cm layer of an undisturbed site (34°51′30″N, 109°19′23″E) at Ansai County, Shaanxi Province on the Loess Plateau, China. The physicochemical properties of the soil are determined and listed in Table 1. Particle size distribution of the soil was determined using a Mastersizer 2000 laser diffraction particle size analyzer (Malvern Instruments, Malvern, UK) (Lamorski et al. 2014). Field capacity was obtained following the indoor cutting-ring weighing method. pH of the soil was measured in a 1:5 soil:water (w:v) suspension using a pH meter (Little 1992). Carbonate content of the soil was determined using a simple titrimetric method described by Bundy and Bremner (1972). Soil organic carbon concentration was analyzed using the potassium dichromate titrimetric method (Mebius 1960). Total N concentration was obtained on a Kjeldahl Auto Analyzer (TECATOR, Sweden) following a sulfuric acid digestion (Purificación et al. 2013). Total P concentration was obtained by the molybdate-blue colorimetric method at 880 nm on a 3900H UV-VIS spectrophotometer (Hitachi Limited, Tokyo, Japan) following a nitric acid and perchloric acid digestion (Kara et al. 1997). Concentrations of total K, Na, Ca, Mg, Fe, Mn, Cu, and Zn were obtained on a PinAAcle 900 atomic absorption spectrometer (PerkinElmer, New York, USA) following an aqua regia digestion (Reimann et al. 2015). Bicarbonate-extractable P was extracted for 16 h in 0.5 M NaHCO3 adjusted to pH 8.5 with NaOH, then determined at 880 nm on a 3900H UV-VIS spectrophotometer by the molybdate-blue colorimetric method after ascorbic acid reduction (Olsen et al. 1954).
The soil was air-dried and passed through a 2-mm nylon fiber sieve; then 1.5 kg soil was spiked with Zn at 0, 200, and 800 mg Zn kg−1 dry soil (hereafter referred to as Zn0, Zn200, and Zn800, respectively), by thoroughly mixing the soil with dry zinc oxide (ZnO) powder (chemical pure, Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) at the designated dose in a sealed polythene bag. The solubility of the ZnO powder was 0.16 mg in 100 mL water at 30 °C. The mixed soil, together with the bag, was then transferred to a pot of 15-cm diameter and 13.5-cm height. For each Zn level, there were three P levels, i.e. 0, 20 and 80 mg P kg−1 dry soil (hereafter referred to as P0, P20, and P80, respectively), with P being supplied as monopotassium phosphate (KH2PO4) solution. There were nine treatments in total, i.e. three Zn and three P treatments in all possible combinations, and each was repeated four times. For all treatments, N was supplied at 50 mg kg−1 as an ammonium-nitrate (NH4NO3) solution, and K was supplied at 100 mg kg−1 as a potassium sulfate (K2SO4) and potassium chloride (KCl) (K2SO4:KCl = 1:2, molar ratio) solution. No other nutrients were added. The soil in all pots was watered to 70% of field capacity with deionized (DI) water every second day and incubated for 15 days. After incubation, the soil in each pot was air dried, passed through a 2-mm nylon fiber mesh, and then mixed thoroughly to ensure homogenous distribution of the added chemicals. The thoroughly mixed soil was then filled back to each pot lined with a plastic bag inside, and watered to 70% of field capacity.
Seeds of alfalfa (Medicago sativa L. cv Golden Empress) were sterilized in 30% (v:v) hydrogen peroxide (H2O2) for 5 min, rinsed thoroughly with DI water, and then left on wet filter paper in Petri dishes kept in the dark at room temperature overnight (He et al. 2017). Thirty seeds were sown in each pot and seedlings were thinned to 20 plants per pot two weeks after sowing, and all 20 plants survived through to harvest. The experiment was carried out from the middle of August to late December in 2018 in a greenhouse at the Institute of Soil and Water Conservation (34°16′33″N, 108°04′13″E), Yangling, Shaanxi, China. During the experiment, the soil in each pot was watered to 70% of field capacity by weighing the pots and supplying DI water every second day, and no drainage was allowed. The temperature in the greenhouse was kept between 15 and 30 °C, and no supplemental lighting was supplied. No significant symptom of stress was observed on the plants during the experiment. There was no pest event, so no effort was made for pest control.
Plant harvest and analyses of rhizosheath carboxylates
Plants were harvest 130 days after sowing and before flowering. Shoots were cut at the soil surface, then the plastic bags were lifted from the pots to separate roots from the soil. For each pot, about 1.0 g fresh fine roots with rhizosheath soil were collected and soaked in a glass beaker containing 20 mL of 0.2 mM CaCl2. Roots were gently stirred in the solution for about 5 min to protect cell integrity and remove the adhering soil as much as possible. The pH of the rhizosheath extract was measured using a pH meter (He et al. 2020). About 1 mL subsample of the rhizosheath extract was filtered into a 1-mL HPLC vial through a 0.22-μm syringe filter, then acidified with one drop of concentrated phosphoric acid to prevent microbial degradation of the carboxylates, and kept in a − 20 °C freezer until HPLC analysis. Analysis of carboxylates in the extracts was performed at 210 nm using a Waters 1525 HPLC equipped with a Waters Symmetry C18 reverse phase column and a Waters 2489 detector (Waters, Milford MA, USA). The mobile phase included 20 mM KH2PO4 (adjusted to pH 2.5 with concentrated H3PO4) flowing at 0.6 mL min−1 and 100% methanol flowing at 0.01 mL min−1. The working standards included tartaric, malic, citric, oxalic, malonic, acetic, and succinic acids. The injection volume and run time of each sample was 10 μL and 16 min, respectively. Succinate was only detected in a few samples, thus not included in the calculation (He et al. 2020). The extracted roots were collected and washed with DI water, and oven-dried at 60 °C. Amounts of rhizosheath carboxylates were expressed in mol per unit root dry mass.
Measurement of plant biomass
Shoots were oven-dried at 60 °C to constant weight to obtain shoot dry mass (SDM). Roots which were not used for carboxylate extraction were washed with tap water and then DI water, oven-dried at 60 °C to constant weight and weighed. Root dry mass (RDM) was the sum of the dry weight of extracted and non-extracted roots. Root mass ratio (RMR) was calculated as the ratio between RDM and total dry mass, which was the sum of SDM and RDM.
Analyses of plant Zn and P concentrations, calculation of Zn and P contents and distributions in plants
For the analyses of [Zn] and [P] in plants, oven-dried plant samples were finely ground, about 0.5 g sub-sample was weighed and then digested in hot concentrated HNO3:HClO4 (4:1, v:v) using a heating block (Zarcinas et al. 1987). Zinc in the solution was analyzed at 213.9 nm on a PinAAcle 900 AAS (Filgueiras et al. 2000), and P in the solution was determined by the vanadate-molybdate yellow colorimetric method at 420 nm (Gupta et al. 1993) using a U-1100 spectrophotometer (Hitachi Limited, Tokyo, Japan). Total content of Zn in plants in each pot was calculated as the sum of shoot Zn content (shoot [Zn] × SDM) and root Zn content (root [Zn] × RDM), and total P content in plants in each pot was calculated in a similar way. Both total Zn and P contents in plants included the Zn and P taken up by plants and those derived from seeds. The distribution of Zn in roots and shoots was calculated as the percentage of Zn content in roots or shoots to the total plant Zn content per pot, and the distribution of P was calculated in a similar way.
Calculation of bioconcentration factor and translocation factor of Zn
Root bioconcentration factor (BCFroot), shoot bioconcentration factor (BCFshoot), and root-to-shoot translocation factor (TFroot-to-shoot) of Zn were calculated by the following equations (Mahdavian et al. 2017; Marques et al. 2013):
Determination of bulk soil pH and DTPA-extractable Zn concentration
After plants were harvested, all bulk soil samples were air-dried and passed through a 2-mm nylon fiber sieve. About 5 g bulk soil sample from each pot was weighed to prepare a 1:5 soil:water (w:v) suspension for pH measurement (Little 1992). Bioavailable Zn in bulk soil was extracted with 5 mM diethylenetriamine-pentaacetic acid (DTPA), and is hereafter referred to as DTPA-extractable Zn; then [Zn] in the extracted solution was determined using the atomic absorption spectrometry (AAS) method at 213.9 nm on a PinAAcle 900 atomic absorption spectrometer (Baker and Amacher 1982).
Statistics
The effects of soil Zn rate, P rate, and their interaction (Zn × P) on all measured and calculated parameters were examined by a two-way ANOVA using the general linear model in the IBM SPSS Statistics 22.0 software package (IBM, Montauk, New York, USA), and P-values of the effects at α = 0.05 were presented. The effects were considered significant when the P-values were less than 0.05, or trending to be significant when the P-values were between 0.05 and 0.10.
Results
The effect of soil Zn rate was significant (P ≤ 0.05) or trending to be significant (0.05 < P ≤ 0.10) for all of the measured and calculated parameters except SDM, RDM, BCFroot of Zn, half of the measured carboxylates, and rhizosheath extract pH (Figs. 1, 2, 3, 4, 5 and 6, Table 2). Similarly, the effect of soil P rate was significant or trending to be significant for all parameters except RMR, shoot [Zn], P and Zn distribution in plants, BCFshoot and TFroot-to-shoot of Zn, all carboxylates but tartrate, and, surprisingly, root [P] (Figs. 1, 2, 3, 4, 5 and 6, Table 2). For the P-Zn interaction, the effect was significant or nearly so for all parameters except the P distribution in plants and the carboxylates (Figs. 1, 2, 3, 4, 5 and 6, Table 2). These data show clearly that alfalfa grown in this calcareous soil had a significant response to both P and Zn rates in soil and a strong P-Zn interaction was observed in this study.
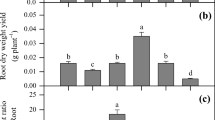
Shoot dry mass (a), root dry mass (b), and root mass ratio (c) of alfalfa grown in the soil with different rates of zinc (Zn) and phosphorus (P) supply in a greenhouse. Zn0, Zn200, and Zn800 represent that Zn was supplied at 0, 200, and 800 mg kg−1 as zinc oxide (ZnO), respectively; P0, P20, and P80 represent that P was supplied at 0, 20, and 80 mg kg−1 as KH2PO4, respectively. Data are presented as means + SE (n = 4). P-values of the effects of Zn, P, and their interaction (Zn × P) obtained from two-way ANOVA at α = 0.05 are given
Shoot zinc concentration ([Zn]) (a) and phosphorus concentration ([P]) (b), and root [Zn] (c) and [P] (d) of alfalfa grown in the soil with different rates of Zn and P supply in a greenhouse. Zn0, Zn200, and Zn800 represent that Zn was supplied at 0, 200, and 800 mg kg−1 as zinc oxide (ZnO), respectively; P0, P20, and P80 represent that P was supplied at 0, 20, and 80 mg kg−1 as KH2PO4, respectively. Data are presented as means + SE (n = 4). P-values of the effects of Zn, P, and their interaction (Zn × P) obtained from two-way ANOVA at α = 0.05 are given
Zinc (Zn) content (a) and phosphorus (P) content (b) in alfalfa grown in the soil with different rates of Zn and P supply in a greenhouse. Zn0, Zn200, and Zn800 represent that Zn was supplied at 0, 200, and 800 mg kg−1 as zinc oxide (ZnO), respectively; P0, P20, and P80 represent that P was supplied at 0, 20, and 80 mg kg−1 as KH2PO4, respectively. Data are presented as means + SE (n = 4). P-values of the effects of Zn, P, and their interaction (Zn × P) obtained from two-way ANOVA at α = 0.05 are given
Distribution of zinc (Zn) (a) and phosphorus (P) (b) in alfalfa grown in the soil with different rates of Zn and P supply in a greenhouse. Zn0, Zn200, and Zn800 represent that Zn was supplied at 0, 200, and 800 mg kg−1 as zinc oxide (ZnO), respectively; P0, P20, and P80 represent that P was supplied at 0, 20, and 80 mg kg−1 as KH2PO4, respectively. Data are presented as means + SE (n = 4). P-values of the effects of Zn, P, and their interaction (Zn × P) obtained from two-way ANOVA at α = 0.05 are given
The amounts of rhizosheath carboxylates, including tartrate, acetate, citrate, malonate, malate, and oxalate of alfalfa grown in the soil with different rates of zinc (Zn) and phosphorus (P) supply in a greenhouse. Zn0, Zn200, and Zn800 represent that Zn was supplied at 0, 200, and 800 mg kg−1 as zinc oxide (ZnO), respectively; P0, P20, and P80 represent that P was supplied at 0, 20, and 80 mg kg−1 as KH2PO4, respectively. Data are presented as means + SE (n = 4, except for some treatments in which certain carboxylate(s) in some sample(s) was not detected)
The DTPA-extractable zinc concentration ([Zn]) in the bulk soil with different rates of Zn and phosphorus (P) supply. Zn0, Zn200, and Zn800 represent that Zn was supplied at 0, 200, and 800 mg kg−1 as zinc oxide (ZnO), respectively; P0, P20, and P80 represent that P was supplied at 0, 20, and 80 mg kg−1 as KH2PO4, respectively. Data are presented as means + SE (n = 4). P-values of the effects of Zn, P, and their interaction (Zn × P) obtained from two-way ANOVA at α = 0.05 are given
Plant biomass
The Zn0 + P20 treatment significantly increased both SDM and RDM, when compared with the Zn0 + P0 treatment, but further increasing P supply to 80 mg kg−1 did not further increase either SDM or RDM (Fig. 1a, b). However, at Zn200 and Zn800, both SDM and RDM increased markedly with increasing soil P rate. At P0 and P20, although not significant, there appeared to be a downward trend for SDM and, especially, RDM, as Zn rate increased. For SDM, there was a positive response to increased Zn rate but only at the highest P rate, where both the Zn200 and Zn800 treatments gave an equivalent response with greater SDM than all other treatments. The same was true for RDM, although Zn appeared to reduce RDM when P was deficient with a significant decrease at P20 and a trend in that same direction at P0. The P-Zn interaction was significant at the P = 0.097 level, with a trend for increasing P to lower the RMR at lower Zn rates, but increase RMR at the highest Zn rate (Fig. 1c). Adding Zn when P was deficient tended to have a greater impact on shoot growth than root growth with the RMR decreasing, but this effect was negated with the higher rate of P with the RMR having about the same ratio.
Shoot and root Zn and P concentrations
For shoot [Zn], although significant, there was not a clear trend for the P-Zn interaction. Similar to shoot [Zn], there was no clear trend for root [Zn] even though the P-Zn interaction was significant (Fig. 2a and c, Table 2). At all P rates, both shoot [Zn] and root [Zn] increased sharply with increasing soil Zn rate, with shoot [Zn] at Zn200 and Zn800 being 1.9–3.9 and 4.2–7.9 times greater than that at Zn0, respectively, and root [Zn] at Zn200 and Zn800 being 5.5–7.8 and 15.6–43.2 times greater than that at Zn0, respectively. Shoot [P] generally increased with increasing P rate, but only at the two lower Zn rates (Fig. 2b). Root [P] increased at the highest P rate for the Zn0 treatment and with a similar trend at Zn200, but an opposite effect occurred at Zn800 (Fig. 2d). Both shoot [P] and root [P] increased with increasing Zn rate at the two lower P rates, but remained relatively stable with increasing Zn rate at the highest P rate.
Accumulation and partitioning of Zn and P in plants
Total plant Zn content increased with increasing P rate at Zn 200; although it also increased when P was added at Zn0 and Zn800, it did not show the same trend as that at Zn200 (Fig. 3a). At the same P rate, increasing Zn rate resulted in an increase in total plant Zn content, especially for P20 at which the value was 3.8 and 17.6 times greater at Zn200 and Zn800 than at Zn0, respectively, and for P80 at which the value was 7.4 and 23.3 times greater at Zn200 and Zn800 than at Zn0, respectively. Total plant P content always increased with increasing P rate (Fig. 3b); at P0 and P20, total plant P content did not vary considerably with Zn rate, but at P80, it increased when Zn was added and peaked at Zn200.
Of the total plant Zn content, more was allocated to roots than to shoots in all cases. For the distribution of Zn, more Zn tended to be partitioned in the roots as Zn rate increased. Although the P-Zn interaction was significant, there was not a clear trend. The distribution of Zn in roots at P0 was greater than at P20 at the Zn0 rate, and greater than at P80 at the Zn200 rate, but it was greater at P20 than at P0 at the Zn800 rate (Fig. 4a). In contrast to Zn, more P was partitioned to the shoots than to the roots in almost all cases, except at P0 and P20 at the Zn0 rate (Fig. 4b). Less P tended to be partitioned to the roots as P rate increased, but there was no impact of Zn or the P-Zn interaction.
Bioconcentration factor and translocation factor of Zn
The BCFroot of Zn was impacted by P, but there was no impact of Zn, and it tended to be impacted by the P-Zn interaction (Tables 2 and 3). The BCFroot was the largest at P0 at the Zn0 rate, similarly the largest at P0 and P20 at the Zn200 rate, but the largest at P20 at the Zn800 rate. The BCFshoot of Zn decreased with Zn rate, with no impact of P at Zn200 or Zn800, but with P20 having a higher value than P0 and P80 at Zn0. The TFroot-to-shoot of Zn declined with increasing Zn rate, but there was no impact of P, and it tended to be affected by the P-Zn interaction (Tables 2 and 3). The TFroot-to-shoot of Zn was the smallest at P0 at the Zn0 rate, similarly the smallest at P0 and P20 at the Zn200 rate, but the smallest at P20 at the Zn800 rate.
The pH of the bulk soil and rhizosheath extract
Bulk soil pH ranged from 8.4 to 8.8, while rhizosheath extract pH ranged from 7.7 to 8.7. In most cases, rhizosheath extract pH was less than bulk soil pH (Tables 2 and 4). Rhizosheath extract pH was significantly affected by soil P rate, and tended to be affected by the P-Zn interaction, but there was no impact of soil Zn rate. Taking an average of three Zn treatments, rhizosheath extract pH was 8.6 at P0, but 0.57 and 0.82 units less at P20 and P80, respectively.
Rhizosheath carboxylates
There were no significant P-Zn interactions for the carboxylates (Fig. 5, Table 2). Only soil Zn rate significantly affected the amount of citrate, malonate, and malate exuded by roots. At Zn0, the average amount of citrate, malonate, and malate across P rates was 0.273, 0.216, and 0.105 mmol g−1 RDM, respectively, and these decreased by 15%, 30% and 33% at Zn200, respectively, and by 35%, 39% and 46% at Zn800, respectively. Soil P rate only markedly affected the amount of tartrate, which was 1.552 mmol g−1 RDM on average across Zn rates at P0, but only 14% and 13% of that amount at P20 and P80, respectively.
DTPA-extractable Zn concentration in the soil
As expected, increasing soil Zn rate dramatically increased DTPA-extractable [Zn], with P having no impact except at the highest Zn rate. Surprisingly, the highest rate of P did not have an inhibitory impact on DTPA-extractable [Zn] (Fig. 6, Table 2). At P0, P20, and P80, DTPA-extractable [Zn] was 258, 93, and 222 times greater at Zn200, respectively, and 728, 358, and 1082 times greater at Zn800, respectively, when compared with Zn0.
Discussion
We studied the effects of Zn-P interaction on Zn and P accumulation and partitioning in alfalfa grown in a calcareous soil supplied with different rates of Zn and P. We tested four hypotheses by analyzing a series of parameters related to plant growth, Zn and P uptake, and translocation within plants, and the pH and amounts of carboxylates in the rhizosheath. The results of the study enhance our understanding of the mechanisms involved in P-Zn interactions in soil-plant systems, and provide a scientific basis for Zn biofortification on Zn-deficient soils, as well as for phytoremediation of Zn-contaminated soils.
Our first hypothesis that response of plant growth to soil P rate would differ under different soil Zn rate was fully supported, as both SDM and RDM were significantly affected by soil P rate and Zn-P interaction. Mean total [Zn] in typical mineral soils is 50 mg kg−1 (Gupta et al. 2016), exchangeable [Zn] typically falls in the range of 0.1–2 mg kg−1 (Broadley et al. 2007). Bioavailable [Zn] in calcareous soils is often too small to support optimal plant growth (Alloway 2009; Cakmak 2008). The calcareous soil we studied had 0.3 mg kg−1 DTPA-extractable Zn and 3.3 mg kg−1 bicarbonate-extractable P, showing that the bioavailability of both Zn and P was small (Alloway 2009; Lambers and Oliveira 2019). The response of plant growth to increasing soil P rate was more sensitive at Zn200 and Zn800 than at Zn0, it is likely that Zn-deficiency at Zn0 limited the positive growth response of plants to increasing soil P rate. At P0 and P20, the downward trend for SDM and, especially, RDM, with increasing Zn rate was likely a result of decreased P availability, and possibly also a result of excessive Zn. At the highest P rate, Zn200 and Zn800 had similar SDM, as well as RDM, and both were greater than that at Zn0, indicating that a Zn rate of 200–800 mg kg−1 is within the sufficient to excessive range for alfalfa. In terms of plant growth, no obvious P-induced Zn deficiency was observed in the present study, most likely because P was not supplied in excess of the plants’ demand (Akhtar et al. 2019; Gupta et al. 2016). It is also likely, because plants grown on calcareous soils are less susceptible to P-induced Zn deficiency than plants grown on non-calcareous soils, as Pi is mainly adsorbed by calcite in calcareous soils rather than being co-adsorbed with Zn on Fe-oxides in non-calcareous soils (Sacristan et al. 2019). Our results suggest that plant growth response to P supply depends on soil Zn level, but increasing P supply within the range of P rate in the present study to the calcareous soil with Zn from deficient to excessive levels always promoted alfalfa growth. The results also suggest that whether soil Zn was deficient, sufficient, or excessive for plant growth also depend on soil P level.
Under P deficiency, shoot growth is reduced more than root growth, and RMR increases (Watts-Williams et al. 2014); a greater RMR is thought to enhance a plant’s P acquisition (Lynch and Ho 2005). However, in the present study and our previous studies on the effects of soil P levels on alfalfa growth (He et al. 2017), no association between greater RMR and less soil P availability was found. Instead, RMR decreased with increasing soil Zn rate, suggesting that greater RMR might enhance a plant’s Zn acquisition under Zn deficiency, while root growth was more sensitive than shoot growth to changes in Zn availability in the soil, and smaller RMR might help plants to cope with excessive Zn (Lambers and Oliveira 2019).
The second hypothesis was that root and shoot [Zn] increase with increasing soil Zn rate but decrease with increasing soil P rate, and vice versa for root and shoot [P], but total plant contents of both Zn and P increase with increases in both soil Zn rate and P rate. This hypothesis was only partly supported. Both shoot [Zn] and root [Zn] increased with increasing Zn rate, but neither of them showed a consistent trend with increasing P rate. Neither shoot [P] nor root [P] increased consistently with increasing P rate, or decreased consistently with increasing Zn rate. The strong P-Zn interaction showed that plant [Zn] and [P] depended on both Zn rate and P rate. In general, total plant contents of both Zn and P increased with increases in both Zn rate and P rate, as a result of the combined effects of P-Zn interaction on plant growth and on plant [Zn] and [P].
Plants with leaf [Zn] (on dry matter (DM) basis) < 10–15 mg kg−1 are considered Zn deficient; most plants require 15–30 mg kg−1 leaf [Zn] for normal growth and maximum yield, while excessive leaf [Zn] (100–700 mg kg−1) results in inhibited growth and reduced yield, but optimal and toxic plant [Zn] vary widely among plant species (Akhtar et al. 2019; Gupta et al. 2016; Hacisalihoglu and Kochian 2003). In the present study, mean shoot [Zn] was 26 mg kg−1 when no Zn was added to the soil, and 92 and 189 mg kg−1 when Zn was supplied at 200 and 800 mg kg−1, respectively. The soil was deficient in Zn when no Zn was supplied, as shoot [Zn] was around the bottom line of the sufficiency range for alfalfa, which has been stated as 21–70 mg kg−1 for new shoot growth on mature alfalfa just prior to flowering (Bryson and Mills 2014). No visible symptom of Zn toxicity was observed during the experiment, although shoot [Zn] might be excessive at Zn200 and Zn800, indicating that alfalfa may have the capacity to cope with excessive tissue [Zn].
Unlike the obvious negative effects of P application to soil on shoot and grain [Zn] of some cereal crops (Akhtar et al. 2019; Zhang et al. 2012), increasing P rate did not significantly reduce either shoot [Zn] or root [Zn] of alfalfa grown in the Zn-deficient soil without external Zn supply in our study. Only shoot [Zn] at Zn800 was considerably reduced at P20, when compared with P0. In contrast, shoot [Zn] at Zn200 was significantly increased at P80, and root [Zn] at Zn800 was markedly increased at P20, when compared with the respective P0. It has been reported in a few studies that Zn is taken up by plants without competition with P (Akhtar et al. 2019). A significant boost of plant growth by increasing P rate may cause a ‘dilution’ of plant [Zn] (Sánchez-Rodríguez et al. 2017), but no such dilution effect was observed in the present study. At the highest Zn rate, it is not clear why shoot and root [Zn] and [P] did not show a consistent trend with increasing P rate. The results of DTPA-extractable [Zn] show that increasing P rate did not reduce Zn bioavailability; instead, increasing P rate resulted in increased Zn bioavailability at Zn800. Our study suggested that no antagonistic P-Zn interaction in the calcareous soil occurred, although some studies have demonstrated that increasing P supply caused decreased leaf [Zn] in crops such as wheat, mainly due to P-Zn interaction in the growth media (Akhtar et al. 2019). The differences in the physicochemical properties of the growth media might account for the different P-Zn interactions on soil Zn availability and plant [Zn] between our study and other studies. Low Zn supply enhances P uptake by roots (Cakmak 2008) and expression of genes encoding P transporters in root cells, thus increasing P accumulation (Huang et al. 2000). However, we did not observe Zn deficiency-induced greater P uptake by alfalfa; instead, increasing Zn rate at P80 from 0 to 200 and 800 mg kg−1 considerably increased P uptake, mainly due to enhanced plant growth in these treatments.
Our third hypothesis that the partitioning of Zn and P to roots and shoots would be affected by both soil Zn and P rates, and that a greater proportion of Zn and P would be retained in roots at higher soil Zn and P rates was partly supported. The partitioning of Zn was affected by Zn rate and P-Zn interaction, but not by P rate, while the partitioning of P was only affected by Zn rate. A greater proportion of Zn was always retained in roots, and this proportion generally increased with increasing Zn rate, although it varied to some degree due to P-Zn interaction. In general, plants have greater root [Zn] than shoot [Zn] and leaf [Zn] (Gupta et al. 2016). After uptake by roots, Zn may be bound to cell walls, or sequestered in the cytoplasm or cellular compartments, such as vacuoles in roots, thus restricting translocation of Zn from roots to shoot, and protecting photosynthetic organs from potential Zn toxicity (Broadley et al. 2007; Gupta et al. 2016; Palmgren et al. 2008).
Formation of Zn phytate and its binding in root cells is the main reason for limited root-to-shoot translocation of Zn (Cakmak 2008), while incorporation of P and sulfur into globular crystals of Zn cysteine and Zn phytate in the root cortex may also limit Zn translocation (Dinh et al. 2015). Deposition of Zn phosphate in the apoplast (Cakmak and Marschner 1987), and Zn complexes in phosphates and silicates in the cytosol of root cells may both contribute to limited Zn translocation to the aerial parts (Neumann and zur Nieden 2001; Sarret et al. 2002). However, the definitive mechanism for the role of P in Zn translocation is yet not clear. Zinc may also be sequestered with phytochelatins, glutathione (GSH), metallothioneins, nicotianamine, and carboxylates such as oxalate, malate, citrate, and tartrate in the intracellular space of root cells without being further translocated upward (Broadley et al. 2007; Gupta et al. 2016; Palmgren et al. 2008). Our results show that high soil Zn rate promoted root-to-shoot translocation of P; in contrast, there are studies suggesting that low soil Zn rate promotes the translocation of P from roots to aerial parts (Cakmak 2008; Dimkpa et al. 2019). The difference in the effect of soil Zn rate on P translocation between our study and other studies is possibly due to the differences in the bioavailability of Zn and P in different soils, and in the uptake capacity and binding sites of Zn and P in plants, as well as in the expression of Zn- and P-transporter proteins between species (Gupta et al. 2016; Hacisalihoglu and Kochian 2003).
Crosstalk of Zn with P can modify the physiological functions of plants (Gupta et al. 2016). Root- mediated changes in rhizosphere chemistry, including rhizosphere pH and release of carboxylates, can affect the bioavailability of both Zn and P (Hacisalihoglu and Kochian 2003). In the present study, the hypothesis that rhizosheath pH would be reduced, and the amounts of rhizosheath carboxylates would be greater at lower soil Zn and P rates was not fully supported. pH of the rhizosheath extract was less than that of bulk soil in most cases, and it declined with increasing P rate. However, neither Zn rate nor P-Zn interaction caused significant changes in rhizosheath extract pH, although both significantly affected bulk soil pH as did P rate. Soil pH is the major determining factor affecting Zn solubility in soil (Broadley et al. 2007; Gupta et al. 2016). Application of P fertilizers in different forms can either significantly increase or decrease soil pH; a lower bulk soil pH caused by P addition in the present study might increase Zn mobility in the calcareous soil (Cakmak 2008). When soil pH is >5.5, Zn bioavailability decreases with increasing pH (Cakmak 2008); theoretically, lowering the rhizosphere pH could promote the mobility of Zn in the calcareous soil, thus increasing the bioavailability of Zn, but definitive evidence is lacking (Cakmak 2008; Hacisalihoglu and Kochian 2003).
We observed no P-Zn interaction on the amounts of carboxylates in the rhizosheath; the hypothesis that the amounts of rhizosheath carboxylates would be greater at lower soil Zn and P rates was partly supported, as the amount of citrate, malonate, and malate were greater at Zn0 than at Zn200 and Zn800, while the amount of tartrate was considerably greater at P0 than at P20 and P80. Under P deficiency, roots of many plants exude carboxylates, thus mobilizing and acquiring soil P (Lambers and Oliveira 2019). In our previous studies, alfalfa grown in calcareous soils released large amounts of carboxylates, including oxalate, malate, citrate, malonate, and tartrate, into the rhizosheath, and tartrate was a major carboxylate released by roots under P deficiency (He et al. 2017, 2020). Zinc is mainly taken up by roots from the soil solution as Zn2+, but chelates of Zn with organic ligands such as carboxylates secreted by roots can also be taken up (Broadley et al. 2007; Hacisalihoglu and Kochian 2003). It seemed that alfalfa can also take up intact ZnO particles (Bandyopadhyay et al. 2015).
In the present study, it is very likely that greater amounts of citrate, malonate, and malate were released to mobilize more Zn under Zn deficiency, while a greater amount of tartrate was released, leading to the mobilization of more P under P deficiency. However, there are studies showing that more citrate is released from roots of alfalfa growing under P-deficient condition than under P-sufficient condition (Lipton et al. 1987; Suriyagoda et al. 2012). In rice (Oryza sativa L.) grown in nutrient solution, both Zn and P deficiency increase the exudation of low-molecular-weight carboxylates, including oxalate and citrate. Although oxalate is quantitatively the most important among all carboxylates detected, citrate mobilizes Zn more effectively, and the exudation rates of citrate correlate with the uptake efficiency of both Zn and P by rice (Hoffland et al. 2006). In white lupin (Lupinus albus L.) grown in nutrient solution, Zn deficiency does not induce exudation of citrate, but P deficiency does. However, when plants were grown in calcareous soils, citrate (∼1.5 mM) mobilized P, but not Zn in one soil, whereas both Zn and P were mobilized by citrate (∼0.5 mM) in another soil (Duffner et al. 2012). The results of our study and Duffner et al. (2012) suggest that the effect of carboxylate exudation on Zn and P mobility are soil-dependent, and the mobilization of Zn and P by carboxylates are controlled by different mechanisms.
Conclusions
We found strong P-Zn interactions in the calcareous soil-plant system; such interactions affected the bioavailability of Zn, as well as plant growth, accumulation of Zn and P, and partitioning of Zn in alfalfa grown in calcareous soil. Increasing soil P rate promoted plant growth at all soil Zn rates, and may enhance the plants’ capacity to cope with excessive Zn. There was no interactive effect of Zn and P on the pH and amounts of carboxylates in the rhizosheath. Roots always lowered the pH of the rhizosheath, and increasing P rate resulted in reduced rhizosheath pH, which should increase the mobility of both Zn and P in the rhizosheath, and contribute to greater root uptake of Zn and P. Exudation of more citrate, malonate, and malate was a response to Zn deficiency, while release of a greater amount of tartrate was related with P deficiency. The results suggest that rational P fertilization should be considered in Zn biofortification on Zn-deficient soils as well as phytoremediation of Zn-contaminated soils. However, some of the findings of the present study may not be applicable in acid soils, where the behaviors of Zn and P in the soil-plant system are different from those in calcareous soils.
Change history
21 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11104-021-04966-1
References
Akhtar M, Yousaf S, Sarwar N, Hussain S (2019) Zinc biofortification of cereals-role of phosphorus and other impediments in alkaline calcareous soils. Environ Geochem Hlth 41:2365–2379
Alloway BJ (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Hlth 31:537–548
Baker DE, Amacher MC (1982) Nickel, copper, zinc, and cadmium. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analyses, part 2, chemical and microbiological properties, 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison, pp 323–336
Bandyopadhyay S, Plascencia-Villa G, Mukherjee A, Rico CM, José-Yacamán M, Peralta-Videa JR, Gardea-Torresdey JL (2015) Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci Total Environ 515:60–69
Barben SA, Hopkins BG, Jolley VD, Webb BL, Nichols BA, Buxton EA (2011) Zinc, manganese and phosphorus interrelationships and their effects on iron and copper in chelator-buffered solution grown russet Burbank potato. J Plant Nutr 34:1144–1163
Bryson GM, Mills HA (2014) Plant analysis handbook IV. MicroMacro Publishing, Athens
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Bundy LG, Bremner JM (1972) A simple titrimetric method for determination of inorganic carbon in soils. Soil Sci Soc Amer Proc 36:273–275
Cakmak I, Marschner H (1987) Mechanism of phosphorus-induced zinc deficiency in cotton. III. Changes in physiological availability of zinc in plants. Physiol Plant 70:13–20
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302:1–17
Cakmak I, McLaughlin MJ, White PJ (2017) Zinc for better crop production and human health. Plant Soil 411:1–4
Chen X-X, Zhang W, Wang Q, Liu Y-M, Liu D-Y, Zou C-Q (2019) Zinc nutrition of wheat in response to application of phosphorus to a calcareous soil and an acid soil. Plant Soil 434:139–150
Dinh NT, Vu DT, Mulligan D, Nguyen AV (2015) Accumulation and distribution of zinc in the leaves and roots of the hyperaccumulator Noccaea caerulescens. Environ Exp Bot 110:85–95
Duffner A, Hoffland E, Temminghoff EJM (2012) Bioavailability of zinc and phosphorus in calcareous soils as affected by citrate exudation. Plant Soil 361:165–175
Dimkpa CO, Singh U, Bindraban PS, Adisa IO, Elmer WH, Gardea-Torresdey JL, White JC (2019) Addition-omission of zinc, copper, and boron nano and bulk oxide particles demonstrate element and size -specific response of soybean to micronutrients exposure. Sci Total Environ 665:606–616
Filgueiras AV, Capelo JL, Lavilla I, Bendicho C (2000) Comparison of ultrasound-assisted extraction and microwave-assisted digestion for determination of magnesium, manganese and zinc in plant samples by flame atomic absorption spectrometry. Talanta 53:433–441
Fukao Y, Ferjani A, Tomioka R, Nagasaki N, Kurata R, Nishimori Y, Fujiwara M, Maeshima M (2011) iTRAQ analysis reveals mechanisms of growth defects due to excess zinc in Arabidopsis. Plant Physiol 155:1893–1907
Gupta AP, Neue HU, Singh VP (1993) Phosphorus determination in rice plants containing variable manganese content by the phospho-molybdo-vanadate (yellow) and phosphomolybdate (blue) colorimetric methods. Commun Soil Sci Plan 24:1309–1318
Gupta N, Ram H, Kumar B (2016) Mechanism of zinc absorption in plants: uptake, transport, translocation and accumulation. Rev Environ Sci Bio 15:89–109
Hacisalihoglu G, Kochian LV (2003) How do some plants tolerate low levels of soil zinc? Mechanisms of zinc efficiency in crop plants. New Phytol 159:341–350
Hayes PE, Pereira CG, Clode PL, Lambers H (2019) Calcium-enhanced phosphorus toxicity in calcifuge and soil-indifferent Proteaceae along the Jurien Bay chronosequence. New Phytol 221:764–777
He H, Peng Q, Wang X, Fan C, Pang J, Lambers H, Zhang X (2017) Growth, morphological and physiological responses of alfalfa (Medicago sativa) to phosphorus supply in two alkaline soils. Plant Soil 416:565–584
He H, Wu M, Guo L, Fan C, Zhang Z, Su R, Peng Q, Pang J, Lambers H (2020) Release of tartrate as a major carboxylate by alfalfa (Medicago sativa L.) under phosphorus deficiency and the effect of soil nitrogen supply. Plant Soil 449:169–178
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hinsinger P, Plassard C, Tang CX, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Hoffland E, Wei C, Wissuwa M (2006) Organic anion exudation by lowland rice (Oryza sativa L.) at zinc and phosphorus deficiency. Plant Soil 283:155–162
Hopkins BG, Hansen NC (2019) Phosphorus management in high-yield systems. J Environ Qual 48:1265–1280
Huang CY, Barker SJ, Langridge P, Smith FW, Graham RD (2000) Zinc deficiency up-regulates expression of high-affinity phosphate transporter genes in both phosphate-sufficient and -deficient barley roots. Plant Physiol 124:415–422
Johnston AE, Poulton PR, Fixen PE, Curtin D (2014) Phosphorus: its efficient use in agriculture. Adv Agron 123:177–228
Kara D, Özsavaşçi C, Alkan M (1997) Investigation of suitable digestion methods for the determination of total phosphorus in soils. Talanta 44:2027–2032
Lambers H, Oliveira RS (2019) Plant physiological ecology, 3rd edn. Springer, Cham
Lamorski K, Bieganowski A, Ryzak M, Sochan A, Slawinski C, Stelmach W (2014) Assessment of the usefulness of particle size distribution measured by laser diffraction for soil water retention modelling. J Plant Nutr Soil Sci 177:803–813
Little IP (1992) The relationship between soil pH measurements in calcium chloride and water suspensions. Aust J Soil Res 30:587–592
Lipton DS, Blanchar RW, Blevins DG (1987) Citrate, malate, and succinate concentration in exudates from P-sufficient and P-stressed Medicago sativa L. seedlings. Plant Physiol 85:315–317
Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269:45–56
Mahdavian K, Ghaderian SM, Torkzadeh-Mahani M (2017) Accumulation and phytoremediation of Pb, Zn, and Ag by plants growing on Koshk lead-zinc mining area, Iran. J Soils Sediments 17:1310–1320
Marques APGC, Moreira H, Franco AR, Rangel AOSS, Castro PML (2013) Inoculating Helianthus annuus (sunflower) grown in zinc and cadmium contaminated soils with plant growth promoting bacteria - effects on phytoremediation strategies. Chemosphere 92:74–83
Mebius LJ (1960) A rapid method for the determination of organic carbon in soil. Anal Chim Acta 22:120–124
Neumann D, Zur Nieden U (2001) Silicon and heavy metal tolerance of higher plants. Phytochemistry 56:685–692
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA circular 939. USDA, Washington, DC
Ova EA, Kutman UB, Ozturk L, Cakmak I (2015) High phosphorus supply reduced zinc concentration of wheat in native soil but not in autoclaved soil or nutrient solution. Plant Soil 393:147–162
Palmgren MG, Clemens S, Williams LE, Kraemer U, Borg S, Schjørring JK, Sanders D (2008) Zinc biofortification of cereals: problems and solutions. Trends Plant Sci 13:464–473
Pongrac P, McNicol JW, Lilly A, Thompson JA, Wright G, Hillier S, White PJ (2019) Mineral element composition of cabbage as affected by soil type and phosphorus and zinc fertilisation. Plant Soil 434:151–165
Purificación SP, Tadeusz M, María JN, Agustín GA, Sławomir W (2013) An overview of the Kjeldahl method of nitrogen determination. Part I. early history, chemistry of the procedure, and titrimetric finish. Crit Rev Anal Chem 43:178–223
Reimann C, Englmaier P, Fabian K, Gough L, Lamothe P, Smith D (2015) Biogeochemical plant-soil interaction: variable element composition in leaves of four plant species collected along a south-north transect at the southern tip of Norway. Sci Total Environ 506:480–495
Sánchez-Rodríguez RA, del Campillo MC, Torrent J (2017) Phosphorus reduces the zinc concentration in cereals pot-grown on calcareous Vertisols from southern Spain. J Sci Food Agr 97:3427–3432
Sacristan D, Gonzalez-Guzman A, Barron V, Torrent J, Del Campillo MC (2019) Phosphorus-induced zinc deficiency in wheat pot-grown on noncalcareous and calcareous soils of different properties. Arch Agron Soil Sci 65:208–223
Sarret G, Saumitou-Laprade P, Bert V, Proux O, Hazemann JL, Traverse AS, Marcus MA, Manceau A (2002) Forms of zinc accumulated in the hyperaccumulator Arabidopsis halleri. Plant Physiol 130:1815–1826
Schjørring JK, Cakmak I, White PJ (2019) Plant nutrition and soil fertility: synergies for acquiring global green growth and sustainable development. Plant Soil 434:1–6
Suriyagoda LDB, Lambers H, Renton M, Ryan MH (2012) Growth, carboxylate exudates and nutrient dynamics in three herbaceous perennial plant species under low, moderate and high phosphorus supply. Plant Soil 358:100–112
Tabatabai MA (1982) Soil enzymes. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analyses, part 2, chemical and microbiological properties, 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison, pp 903–947
Watts-Williams SJ, Turney TW, Patti AF, Cavagnaro TR (2014) Uptake of zinc and phosphorus by plants is affected by zinc fertiliser material and arbuscular mycorrhizas. Plant Soil 376:165–175
Wissuwa M, Ismail AM, Yanagihara S (2006) Effects of zinc deficiency on rice growth and genetic factors contributing to tolerance. Plant Physiol 142:731–741
Zarcinas BA, Cartwright B, Spouncer LR (1987) Nitric-acid digestion and multielement analysis of plant-material by inductively coupled plasma spectrometry. Commun Soil Sci Plan 18:131–146
Zhang Y-Q, Deng Y, Chen R-Y, Cui Z-L, Chen X-P, Yost R, Zhang F-S, Zou C-Q (2012) The reduction in zinc concentration of wheat grain upon increased phosphorus-fertilization and its mitigation by foliar zinc application. Plant Soil 361:143–152
Zhu YG, Smith SE, Smith FA (2001) Zinc (Zn)-phosphorus (P) interactions in two cultivars of spring wheat (Triticum aestivum L.) differing in P uptake efficiency. Ann Bot 88:941–945
Acknowledgments
This work was financially supported by The National Key Research and Development Plan of China (2017YFC0504504), The Natural Science Basic Research Program of Shaanxi Province (2019JM-411), and The National Natural Science Foundation of China (41301570). Rhizosheath carboxylates were analyzed using The Biology Teaching and Research Core Facility at College of Life Sciences, Northwest A&F University. We thank Xiyan Chen for helping the analysis of rhizosheath carboxylates using HPLC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ismail Cakmak.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Tables 3 and 4 are missing in the PDF version of the article. The missing tables have been inserted in the PDF file.
Rights and permissions
About this article
Cite this article
He, H., Wu, M., Su, R. et al. Strong phosphorus (P)-zinc (Zn) interactions in a calcareous soil-alfalfa system suggest that rational P fertilization should be considered for Zn biofortification on Zn-deficient soils and phytoremediation of Zn-contaminated soils. Plant Soil 461, 119–134 (2021). https://doi.org/10.1007/s11104-020-04793-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04793-w