Abstract
Aims
Phosphorus (P)-induced zinc (Zn) deficiency is one of the most commonly studied antagonistic interactions in plant nutrition. However, there are many controversial reports about P–Zn interaction, possibly related to growth conditions. In this study, the effects of P supply on the root uptake and tissue concentrations of Zn as well as the development of Zn deficiency were investigated in wheat (Triticum aestivum) grown in different media.
Methods
Plants were grown under greenhouse and growth chamber conditions in native soil, autoclaved soil and nutrient solution with different P and Zn supplies. In the soil experiment, the shoot biomass and grain yield were measured whereas in the nutrient solution experiment, the root and shoot biomass were determined. Development of Zn deficiency symptoms was examined. Concentrations of Zn, P and other elements were measured in harvested tissues. Mycorrhizal colonization of roots was scored in soil-grown plants. Root uptake of stable Zn isotope (70Zn) was investigated at different P rates in a separate nutrient solution experiment.
Results
Higher P rates caused substantial decreases in shoot and grain Zn concentrations in native soil but not in autoclaved soil. Treatment of native soil with increasing P significantly reduced mycorrhizal colonization. At low Zn, P applications aggravated Zn deficiency symptoms in both soil and solution culture. In solution culture, root and shoot Zn concentrations were not lowered by higher P rates. Root uptake of 70Zn from nutrient solution was even depressed at low P.
Conclusions
The negative effect of increasing P supply on root Zn uptake and tissue Zn concentrations in wheat is mycorrhiza-dependent and may completely disappear in a mycorrhiza-free environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As early as 1970s, phosphorus (P) and zinc (Zn) were documented to interact with each other in plant mineral nutrition (Marschner and Schropp 1977; Loneragan et al. 1979; Warnock 1970). Although this interaction is commonly referred to as “P-induced Zn deficiency”, it is more complex than this term implies (Marschner 2012). In soil-grown plants, higher levels of P often reduce the Zn concentrations in both vegetative tissues and seeds. If the Zn availability is low, this effect can cause or exacerbate Zn deficiency. Under both controlled and field conditions, higher soil P applications were associated with lower tissue Zn concentrations in wheat (Triticum aestivum) (Thompson 1990; Zhu et al. 2001; Zhang et al. 2012) as well as numerous other crops (Singh et al. 1988; Li et al. 2003; Broadley et al. 2010). Intriguingly, the negative effect of higher P supply on tissue Zn levels was not observed in solution culture studies (Cakmak and Marschner 1986; Nichols et al. 2012).
Zinc deficiency is also associated with elevated shoot P levels and can cause P toxicity at high P supplies (Marschner 2012). The basipetal transport of P, which normally acts as a feedback mechanism regulating P uptake, was shown to be specifically inhibited by Zn deficiency in cotton (Gossypium hirsutum) (Marschner and Cakmak 1986). Zinc deficiency also induces the expression of genes encoding P uptake transporters as demonstrated in barley (Hordeum vulgare) (Huang et al. 2000). Elevations in shoot P concentrations due to Zn deficiency can in turn aggravate Zn deficiency. In corn (Zea mays), high levels of P can immobilize Zn in roots and nodes by increasing the Zn-binding properties of the cell wall (Dwivedi et al. 1975; Youngdahl et al. 1977). Supporting these findings, reduced translocation of Zn from root to shoot tissues was reported to contribute to P-induced Zn deficiency in bean (Phaseolus vulgaris) (Singh et al. 1988). Elevated tissue P levels may reduce the water-soluble Zn concentration and thus lower the physiological availability of Zn (Rahimi and Schropp 1984; Cakmak and Marschner 1987). Moreover, P toxicity as an additional stress factor accentuates symptoms and worsens the condition of Zn-deficient plants (Loneragan et al. 1982; Cakmak and Marschner 1986; Webb and Loneragan 1988).
Increased root-to-shoot biomass ratio due to impaired shoot growth and relatively less affected or even stimulated root growth is a typical response of plants to P deficiency (Anuradha and Narayanan 1991; Cakmak et al. 1994; Watts-Williams et al. 2013). Under P-limited conditions, improvements in P nutritional status may “dilute” Zn in the above-ground parts of plants by promoting the shoot growth while reducing the root growth and thus the Zn acquisition capacity (Marschner 2012). However, in the literature, results related to P-induced Zn deficiency could rarely be explained by the dilution effect alone (Gianquinto et al. 2000). In most studies, although the dilution effect was taken into account as a possibly contributing factor, the negative effects of higher P supply on tissue Zn concentrations and Zn deficiency symptoms could not be simply ascribed to dilution (Lambert et al. 1979; Loneragan et al. 1979; Singh et al. 1988; Li et al. 2003; Zhang et al. 2012).
Chemical interactions between Zn and P species in the growth medium have also been discussed as a possible reason of P-induced Zn deficiency in plants (Verma and Minhas 1987; Marschner 2012). Loneragan et al. (1979) documented that high P applications depressed the Zn uptake of clover (Trifolium subterraneum) from ferruginous sand but not from siliceous sand, suggesting an interaction between P and Zn dependent on soil chemistry. It was also shown that phosphate could form complexes with Zn on colloid surfaces in acidic soils rich in Fe and Al oxides and thus boost Zn adsorption to soil particles (Perez-Novo et al. 2011). However, the practical relevance of such P-Zn interactions in soils is not clear. In a field study on wheat, where higher P applications significantly reduced grain Zn levels, the DTPA-extractable Zn concentration of the soil did not vary with P applications (Zhang et al. 2012).
Arbuscular mycorrhiza (AM), which enables the host plant to extract P from soil beyond the rhizosphere depletion zone, is also known to contribute to the Zn (and Cu) uptake of the plant significantly (Kothari et al. 1991; Li et al. 1991; Tinker et al. 1992; Smith and Read 2008; Ortas 2012). A meta-analysis conducted on 104 research articles including 263 trials showed that AM positively contributes to shoot and seed concentrations of Zn in different plants (Lehmann et al. 2014). The well-documented suppression of mycorrhizal colonization by high P could therefore adversely affect the Zn nutritional status of plants (Lambert et al. 1979; Loneragan and Webb 1993; Marschner 2012; Watts-Williams et al. 2013) and has been a common issue in research on P–Zn interaction. A pot study on soybean (Glycine max) and corn grown in steam-pasteurized soil revealed that higher levels of P reduced the shoot Zn and Cu concentrations when the soil was inoculated with AM fungi whereas this effect was less pronounced or absent in non-inoculated soil (Lambert et al. 1979). Similarly, in a pot experiment with wheat, the Zn uptake and tissue Zn concentration were reduced by P application in the presence of AM colonization but unaffected in its absence (Ryan and Angus 2003). The negative effect of P applications on the grain Zn concentration of wheat was also documented in several field studies (Ryan and Angus 2003; Ryan et al. 2008; Zhang et al. 2012). Phosphorus application impaired the root AM colonization, which correlated well with the grain Zn concentration (Ryan and Angus 2003; Ryan et al. 2008). Reductions in grain Zn by P applications may have adverse impacts on human Zn nutrition in countries where cereals are main source of daily calorie intake (Cakmak 2008). Zinc deficiency is one of the well-documented micronutrient deficiencies occurring in human populations with serious health complications (Krebs 2013).
Despite extensive research on P–Zn interaction in wheat, it has still not been clarified which of these mechanisms is primarily responsible for the observed negative effects of high P applications on the tissue Zn levels. An improved understanding of this phenomenon may also have important implications for Zn biofortification efforts in cereals. In order to test the hypothesis that P-induced Zn deficiency is mycorrhiza-dependent, P effects on tissue Zn concentrations were investigated both in autoclaved vs. non-autoclaved soil. The same interaction was also examined in solution culture, which is free from AM activity. In addition, the effect of increasing P treatments on the root uptake and root-to-shoot translocation of Zn in the absence of AM was studied in solution culture by using the stable 70Zn isotope. To our knowledge, this is the first study where the P–Zn interaction was examined in soil- and hydroponically-grown wheat at the same time.
Materials and methods
In this study, one soil and one solution culture experiment were conducted simultaneously under greenhouse conditions to investigate the Zn–P interaction in bread wheat (Triticum aestivum cv. Adana99). Then, a second solution culture experiment was performed in a growth chamber to further investigate the effect of P on root Zn uptake in solution culture under controlled conditions.
Greenhouse and growth chamber conditions
The greenhouse used in this study is a Venlo-type greenhouse located in Istanbul at the following geographic coordinates: 40° 53′ 25″ N, 29° 22′ 47″ E. It has computerized climate control (heating and evaporative cooling) and supplemental lighting. During this study, the daytime temperature was kept at 25 ± 4 °C and the night time temperature at 20 ± 4 °C in the greenhouse.
In the growth chamber, during the daytime (16 h), the photosynthetic flux density was 400 μmol m−2 s−1, the temperature was 24 °C, and the relative humidity was 60 %. During the night, the temperature was 22 °C, and the relative humidity was 70 %.
Soil experiment
For studying the Zn–P interaction under both Zn-deficient and Zn-sufficient soil conditions, a Zn-deficient soil of Central Anatolian origin was used in this experiment. It was a calcareous (18 % CaCO3) and alkaline (pH 8.0 in dH2O) clay loam with low organic matter (1.5 %). The diethylenetriaminepentaacetic acid (DTPA)-extractable Zn concentration of the untreated soil was 0.13 mg kg−1 according to the method described by Lindsay and Norvell (1978).
One half of the experimental soil was autoclaved at 121 °C for 2 h in order to eliminate the native mycorrhizal fungi and then air-dried. Pots were filled with 3.1 kg of either non-autoclaved or autoclaved soil. Before the seeds were sown, the following mineral nutrients were added to each pot as concentrated solutions and uniformly incorporated into the soil my mixing thorougly (per kg air-dry soil): 300 mg N in the form of Ca(NO3)2∙4H2O; 25 mg S in the form of K2SO4; 15 mg (low), 60 mg (medium) or 180 mg (high) P in the form of Ca(H2PO4)2; and 0.2 mg (low) or 5.0 mg (high) Zn in the form of ZnSO4∙7H2O. The soil experiment had a fully factorial design with 2 sets of 5 pot replicates per treatment, and the pots were completely randomized in the greenhouse. Seven plants were grown in each pot. Throughout the experiment, the pots were watered daily with deionized water. The pots were supplied with additional 100 mg N in the form of Ca(NO3)2∙4H2O 52 days after sowing (DAS).
At the beginning of the experiment, samples were taken from the fertilized soils (both non-autoclaved and autoclaved), and their DTPA-extractable Zn, iron (Fe), copper (Cu) and manganese (Mn) concentrations were determined. The extractable Zn concentrations of the treated soils ranged from 0.17 to 0.20 mg kg−1 in the low-Zn group and from 2.32 to 2.44 mg kg−1 in the high-Zn group. Neither autoclaving nor P applications had any effect on the extractable Zn concentration. None of the treatments affected the extractable concentrations of Fe and Cu, which ranged from 2.83 to 3.04 mg kg−1 and from 0.62 to 0.65 mg kg−1, respectively. However, autoclaving greatly enhanced the extractable Mn concentration (from 1.22 to 6.52 mg kg−1 on average), and higher P applications reduced the extractable Mn by up to 20 %.
At booting (66 DAS), plants in the first set of pots were harvested, and samples from their roots were used to assess mycorrhizal colonization as described below. For analyzing the shoot dry matter production and shoot mineral concentrations in developing plants, two plants were harvested at anthesis (81 DAS) from each pot in the second set by cutting them off at the soil level. The remaining five plants were harvested at maturity. Harvested shoots were dried for 2 days at 65 °C, weighed and analyzed for mineral nutrient concentrations as described in “Digestion and mineral analyses”.
Solution culture experiments
Seeds were germinated in perlite moistened with a saturated CaSO4 solution for 5 days at room temperature. Then, seedlings were transferred to 3-L pots containing a nutrient solution with the following composition: 2 mM Ca(NO3)2∙4H2O, 0.7 mM K2SO4, 0.75 mM MgSO4∙7H2O, 0.1 mM KCl, 100 μM Fe-EDTA, 1 μM H3BO3, 1 μM MnSO4∙H2O, 0.2 μM CuSO4∙5H2O and 0.01 μM (NH4)6Mo7O24.4H2O. Different levels of P in the form of Ca(H2PO4)2 and Zn in the form of ZnSO4∙7H2O were added to the nutrient solutions, depending on the treatment. Nutrient solutions were continuously aerated and refreshed three times a week. Both solution culture experiments had fully factorial designs with four pot replicates per treatment, and the pots were completely randomized.
In the first solution culture experiment, 20 plants were grown in each pot. The plants were supplied with 20 μM (low), 100 μM (medium) or 500 μM (high) P and 0.01 μM (low), 0.1 μM (medium) or 1 μM (high) Zn. When the plants were 34 days old, their shoots and roots were harvested.
The second solution culture experiment was the 70Zn uptake experiment. Here, ten plants were grown in each pot. They were supplied with 20 μM (low), 100 μM (medium) or 500 μM (high) P and either 0.01 μM (low) or 1 μM (high) Zn. When they were 19 days old, the 70Zn uptake study was performed on two randomly selected plants from each pot. They were transferred to Erlenmeyer flasks filled with 300 ml of dilute (1:10) nutrient solution and kept there for 30 min. Then, they were transferred to another set of flasks containing 2 μM 70Zn plus all macronutrients at standard levels but no other micronutrients in 300 ml. In order to study the rate of 70Zn depletion from the solution, 3 ml samples were taken from the flasks at 0 h (just before transferring the plants to the flasks), 1 h, 2 h, 3 h, 5 h and finally 7 h. After the last sampling, the plants were once again transferred to new flasks filled with 300 ml of dilute (1:10) nutrient solution. They were kept there for 30 min and then put back to their original pots, where they were grown for another day. So, the plants had 24 h to translocate the 70Zn they took up from the root to the shoot. The experiment was then terminated by harvesting the shoots and roots separately.
In both solution culture experiments, the harvested roots were washed thoroughly by sequentially dipping them in dH2O, 1 mM CaCl2, 1 mM EDTA and finally again dH2O. All shoot and root samples were dried for 2 days at 65 °C, weighed and analyzed for mineral nutrient concentrations as described in “Digestion and mineral analyses”.
Measurement of mycorrhizal colonization
In harvested roots, mycorrhizal colonization was evaluated according to the “slide” method described by Giovanetti and Mosse (1980). The roots were washed with dH2O in order to remove the soil particles. About 3 cm-long root tips were cut and preserved in ethanol until further processing. First, the root tips were immersed in 10 % (w/v) KOH and kept at 65 °C for 1 h. Then, the KOH solution was discarded, and the samples were treated with 10 % HCl (v/v) at 65 °C for 15 min. After discarding the HCl solution, the root tips were treated with 0.05 % (w/v) trypan blue solution at 65 °C for 25 min. The processed root samples were preserved in lactic acid until microscopic evaluation of mycorrhizal colonization. For each sample, ten pieces of root tips were arranged under the microscope. Root tips were used to focus on a standard part of the root system. Presence or absence of colonization was recorded for each of the 10 pieces and thus each sample was given a score between 0 (no sign of infection) and 10 (heavily infected).
Digestion and mineral analyses
All dried shoot or grain samples were ground to fine powders in an agate vibrating cup mill (Pulverisette 9; Fritsch GmbH; Germany). Ground samples (~0.2 g) were digested in a closed-vessel microwave system (MarsExpress; CEM Corp; Matthews, NC, USA) with 2 ml of 30 % H2O2 and 5 ml of 65 % HNO3.
In soil DTPA extracts, digested plant samples and nutrient solution samples containing 70Zn as the only Zn isotope, the concentrations of mineral nutrients including Cu, Fe, Mg, Mn, P and Zn were measured by inductively coupled plasma optical emission spectrometry (ICP-OES; Vista-Pro Axial; Varian Pty Ltd; Mulgrave, Australia). For measuring the 70Zn concentration in digested plant samples, inductively coupled plasma mass spectrometry (ICP-MS; Agilent; CA, USA) was used. Measurements were checked by using certified standard reference materials obtained from the National Institute of Standards and Technology (Gaithersburg, MD, USA).
Statistical analyses
The JMP software (Version 11.0.0) was used for statistical analyses. Analysis of variance (ANOVA) was conducted to evaluate the significance of the effects of the treatments and their interactions. When effects were significant according to ANOVA, significant differences between means were determined by Tukey’s honestly significant difference (HSD) test (P < 0.05).
Results
The shoot dry weights of 81-day-old wheat plants grown in soil culture were significantly affected by soil sterilization as well as Zn and P applications, but no significant interactions were observed (Table 1). On average, soil sterilization and high soil Zn increased the shoot biomass by 14 % and 7 %, respectively. With respect to the low-P treatment, the medium-P treatment enhanced the shoot biomass production by 50 %, but the high-P application did not provide any additional benefit. As can be seen in Fig. 1, the general condition and visual appearance of the plants varied greatly according to the treatments at this stage. Under non-autoclaved soil conditions (Fig. 1a), when the Zn supply was low, increasing P supply resulted in reduced plant height, delayed development and caused more severe leaf symptoms including chlorosis and necrosis. In contrast, when the Zn supply was high, higher P applications increased plant height and overall vigor. Similar trends were also observed in autoclaved soil (Fig. 1b), but the effects were less pronounced. In terms of grain yield, the interactive effects of soil Zn and P applications were significant at maturity (Table 1). They markedly augmented the positive effects of each other on grain yield. At low P, the grain yield did not respond to high Zn, whereas at high P, plants produced 50 % more grains by weight in response to high Zn.
In roots of 66-day-old wheat plants grown in non-autoclaved soil, the extent of mycorrhizal colonization depended only on the P supply (Fig. 2). With each increase in P rate, a dramatic decrease was observed in the mycorrhizal colonization score under both the low-Zn and high-Zn conditions. In plants grown in autoclaved soil, mycorrhizal colonization was not detectable at booting (data not shown). The shoot Zn concentration at anthesis was significantly affected by the triple interaction of the soil sterilization, Zn and P treatments (Table 2). In non-autoclaved soil, the low-P plants had markedly higher shoot Zn concentrations than the medium-P and high-P plants at both Zn supply levels. This effect of P supply on the shoot Zn concentration disappeared completely in autoclaved soil where the shoot Zn concentrations of the high-Zn plants were 4–5 times as high as those of the low-Zn plants, irrespective of the P level. In terms of the shoot P concentration, the interaction of soil sterilization and P application appeared to have the most important effect. Higher soil P applications resulted in remarkably higher shoot P concentrations in any case. Autoclaving the soil reduced the shoot P concentration by 35 % at the low P level, but did not have any significant effect on it at the medium and high P levels.
Effects of Zn (low: 0.2 mg kg−1; high: 5 mg kg−1) and P (low: 15 mg kg−1; medium: 60 mg kg−1; high: 180 mg kg−1) applications on the mycorrhiza infection scores of 66-day-old bread wheat (Triticum aestivum cv. Adana99) plants grown in non-autoclaved soil under greenhouse conditions. Plotted values are means and standard deviations of 5 pot replicates. Different letters above columns indicate significantly different means according to Tukey’s HSD test (p < 0.05)
In parallel with the shoot P concentration, the shoot Mg concentration decreased significantly with decreasing soil P supply in developing wheat plants (Table 2). Similarly, the lowest shoot Cu concentrations were measured in the low-P plants. The shoot Mn levels were dramatically altered by the interactive effects of the treatments. Both autoclaving soil and higher P application resulted in elevated shoot Mn concentrations. However, the effects of P on shoot Mn were particularly prominent in non-autoclaved soil. At high Zn, the shoot Mn levels were lower, and the effects of soil autoclaving and P supply were weaker than at low Zn.
The grain Zn concentrations varied significantly in response to the treatments (Table 3). In non-autoclaved soil, the grain Zn concentration decreased markedly with increasing soil P supply at both Zn supply levels, whereas in autoclaved soil, the P supply did not have any clear effect on the grain Zn. The grain P concentration did not show a clear response to the Zn supply. While higher P supply enhanced the grain P significantly, the effects of soil autoclaving depended on the P supply. At high P, soil autoclaving did not affect the grain P concentration, but at medium and low P, autoclaving caused significant decreases in the grain P, and these were particularly pronounced at low P. In terms of grain concentrations, Mg and Cu exhibited similar trends. The grain concentrations of both were enhanced by higher P supply and unaffected by Zn supply. Autoclaving the soil reduced their grain concentrations markedly in the low-P treatment. Finally, all treatments had prominent effects on the grain Mn concentration. Higher Zn supply decreased the grain Mn whereas soil autoclaving and higher P application increased it significantly.
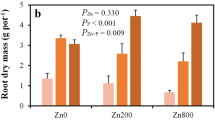
The Zn–P interaction was also studied in solution culture with varied Zn and P supply. In the low-Zn treatment, the plants appeared smaller at higher P levels and showed more severe leaf symptoms including chlorosis and necrosis (Fig. 3). The medium P level increased the shoot dry weight of the low-Zn plants when compared to the low P level, but a further increase in P supply tended to decrease the dry shoot biomass (Table 4). In contrast, at the medium and high Zn levels, the plants produced significantly more shoot biomass with the medium or high P supply than with the low P supply (Fig. 3; Table 4). In terms of root dry weight, both lower Zn and lower P supplies were associated with higher values. Consequently, the root-to-shoot ratio increased dramatically with decreasing Zn and/or P availability. The root-to-shoot ratio of the low-Zn-low-P plants was over three times as high as that of the high-Zn-high-P plants.
In this solution culture experiment, the shoot Zn concentration increased greatly with increasing Zn supply, but did not vary according to the P supply (Table 5). The shoot P concentrations were elevated significantly not only by higher P supplies but also by the low Zn level. The extent of the “low-Zn effect” on the shoot P concentration depended on the P supply and was most prominent at high P. At low Zn, the shoot Mg and Cu concentrations were not affected by the P level and significantly higher than at higher Zn levels. However, at medium and high Zn, the low-P treatment was associated with markedly lower Mg and Cu concentrations in shoots as compared to the medium-P and high-P treatments. Significantly elevated Mn concentrations were measured in shoots under both the low-Zn and low-P conditions.
In the roots of hydroponically grown wheat plants, the Zn concentration was almost tripled with each increase in Zn supply, and the P concentration was doubled with each increase in P supply (Table 6). The Zn and P treatments did not have any effects on the root concentrations of each other. Significantly reduced Mg concentrations were measured in the roots of both the low-Zn and low-P plants. The low-P treatment also lowered the root Cu concentration. However, in contrast to Mg, Cu accumulated to higher concentrations in the roots of the low-Zn plants as compared to the medium-Zn and high-Zn plants. Increasing P supply had a lowering effect on the root Mn concentrations in all Zn treatments, and this effect was particularly pronounced in the high-Zn treatment.
In the next solution culture experiment, which was conducted to study 70Zn uptake, the shoot and root dry weights of 20-day-old wheat plants were affected by only the P treatment (Table 7). The shoot dry weights exhibited a tendency to increase with increasing P supply whereas the root dry weights decreased slightly. Consequently, the low-P plants had greater root-to-shoot ratios than the medium-P and high-P plants. As shown in Fig. 4a, the cumulative 70Zn uptake per unit root biomass values obtained for the low-Zn plants were significantly higher than those obtained for the high-Zn plants at all time points during the 7-h uptake experiment. In the low-Zn group, the low-P plants absorbed only about half as much 70Zn as the medium-P and high-P plants. The same negative effect of the low-P treatment on the 70Zn uptake per unit root biomass was also observed in the high-Zn treatment as a trend though the differences were statistically not significant. One day after the end of the uptake experiment, both the shoot and the root 70Zn concentrations measured in the low-Zn plants were markedly higher than those measured in the high-Zn plants (Fig. 4b, c). While the 70Zn concentrations in the high-Zn plants were unaffected by the P nutritional status, higher P supplies were associated with significantly higher 70Zn concentrations in the low-Zn plants.
a Cumulative 70Zn uptake of 19-day-old, and b shoot and c root 70Zn concentrations of 20-day-old bread wheat (Triticum aestivum cv. Adana99) plants grown hydroponically in a growth chamber at different Zn (low: 0.01 μM; high: 1 μM) and P (low: 20 μM; medium: 100 μM; high: 500 μM) levels. Different letters indicate significantly different means according to Tukey’s HSD test (p < 0.05)
Discussion
In the soil experiment, the positive responses of the shoot dry weight to higher Zn and P applications show that both nutrients were limiting plant growth at low levels under given growth conditions (Table 1). Although the interaction of Zn and P did not significantly affect the shoot dry weight, the grain yield showed a significant response to this interaction. The lack of a yield response to higher Zn application at low P indicates that P was the primary limiting nutrient under this condition. Clear responses to Zn were observed at higher P levels, where Zn and P applications augmented the effects of each other on grain yield. The P–Zn interaction had also some remarkable effects on the general condition of developing plants, particularly in non-autoclaved soil (Fig. 1a). In wheat, the typical leaf symptoms of Zn deficiency are whitish necrotic patches observed on middle-aged leaves (Cakmak et al. 1997; Sharma et al. 2004; Kutman et al. 2010). Moreover, Zn deficiency is often associated with delayed development in cereals (Cakmak et al. 1997; Neue et al. 1998; Genc et al. 2002). At low Zn, higher P supply aggravated these symptoms, indicating P-induced Zn deficiency. The clearer expression of this interaction in native soil indicates mycorrhizal involvement (Fig. 1).
Soil sterilization by autoclaving or other techniques soil can stimulate plant growth significantly, possibly by eliminating soil-borne pathogens and competing microorganisms (Meredith and Anderson 1992; Endlweber and Scheu 2006; Mahmood et al. 2014), unless it causes Mn toxicity by killing the Mn-oxidizing bacteria that transform plant-available Mn2+ into unavailable higher oxides (Boyd 1971; Williams-Linera and Ewel 1984). Here, irrespective of the Zn and P treatments, autoclaving had a modest positive effect on shoot growth but no significant effect on yield (Table 1), indicating that the non-autoclaved soil was not a significant source of biotic stress.
As a rule, applications of P fertilizers reduce mycorrhizal colonization of plant roots (Jackson et al. 2002; Ryan et al. 2008; Marschner 2012) although at extremely P-deficient conditions, low levels of P application may also have a positive impact (Bolan et al. 1984). The effects of Zn additions to soil on mycorrhizal colonization of roots can be negative, positive or neutral, depending on the conditions (Cavagnaro 2008). In many studies dealing with non-toxic levels of Zn fertilizers, the mycorrhizal colonization was unaffected by Zn treatments (McIlveen and Cole 1979; Ortas et al. 2002; Subramanian et al. 2009). Here, in agreement with the literature, the mycorrhizal colonization in non-autoclaved soil was drastically suppressed by higher levels of P but unaffected by Zn fertilization (Fig. 2), which was necessary for preventing Zn deficiency (Fig. 1) and maximizing grain yield (Table 1). It is also well-documented that sterilization of soil by autoclaving eliminates native AM (Smith and Smith 1981; Endlweber and Scheu 2006). Therefore, any indirect effects of P fertilization mediated by changes in mycorrhizal activity should disappear in autoclaved soil.
In the soil study, the shoot P concentrations measured in 81-day-old plants supplied with low P were below the P adequacy range, which is reported as ~0.2–0.5 % dry weight for wheat at this developmental stage (Table 2; Reuter and Robinson 1997). It is well established that AM can significantly contribute to P uptake of plants, especially under P-deficient conditions (Smith and Read 2008; Marschner 2012). The marked decrease in the shoot P concentrations of the low-P plants as a result of soil autoclaving can be explained by the elimination of mycorrhizal activity (Fig. 2; Table 2). Dilution effect on its own cannot account for this decrease because the plants grown in non-autoclaved soil had a 50 % higher shoot P concentration but only a 13–19 % lower shoot biomass than those grown in autoclaved soil (Tables 1 and 2). The apparent benefit of AM on the grain P levels of the low-P plants were even greater since the grain yields of mycorrhizal plants were at least as high as the plants grown in sterilized soil and thus, dilution effect was out of question (Tables 1 and 3). Reportedly, the AM contribution to P uptake decreases with increasing soil P supply and direct root uptake becomes more important (Nagy et al. 2009). In agreement, the effect of soil sterilization on the shoot and grain P concentrations started to fade at the medium P and disappeared at the high P supply (Tables 2 and 3).
Wheat is considered Zn-deficient if the shoot Zn concentration is lower than 10–15 mg/kg dry weight (Dang et al. 1993; Cakmak et al. 1996; Reuter and Robinson 1997). Accordingly, in the soil experiment, all plants grown at low Zn were either marginally low in Zn or Zn-deficient whereas all grown at high Zn were Zn-sufficient (Table 2). At low P, where the mycorrhizal activity is particularly high in non-autoclaved soil (Fig. 2), soil autoclaving dramatically reduced both shoot and grain Zn concentrations (Tables 2 and 3). The diminishing effect of soil autoclaving on tissue Zn concentrations at higher P levels points out the critical role of AM in Zn uptake (Table 2 and 3). It is well documented that AM colonization of roots enhances plant Zn uptake under non-toxic conditions (Kothari et al. 1991; Ryan and Angus 2003; Watts-Williams et al. 2013). In non-autoclaved soil, the P-induced decreases in tissue Zn concentrations were remarkable at both the low and high Zn supply. Consideration of the large biomass and yield responses to higher P applications (Table 1) might lead to the deception that dilution was responsible for this effect. However, although similar biomass responses to higher P applications were observed in both non-autoclaved and autoclaved soil conditions, the negative effect of higher P on shoot and grain Zn concentrations was significantly weaker (at low Zn) or totally absent (at high Zn) in autoclaved soil (Tables 1, 2, and 3). Thus, in agreement with several previous studies on cereals (Li et al. 2003; Ryan et al. 2008; Zhang et al. 2012), the P–Zn interaction cannot be simply explained by dilution. These observations provide strong evidence that the P effects on tissue Zn concentrations of soil-grown plants are mediated primarily by AM. This may also explain why P-induced Zn deficiency was not observed in soil-grown canola, which is a non-mycorrhizal species (Lu et al. 1998).
In the solution culture experiment, both low Zn and low P impaired the shoot growth, stimulated the root growth, and thereby led to markedly elevated root-to-shoot ratios (Table 4) in accordance with the literature (Anuradha and Narayanan 1991; Cakmak et al. 1994; Erenoglu et al. 2011). High P supply visibly worsened the general condition of the low-Zn plants in solution culture (Fig. 3) as it did also in non-autoclaved soil culture (Fig. 1). However, the physiological reasons behind this apparently similar effect were different in these two systems. In non-autoclaved soil, higher P significantly lowered tissue Zn concentrations (Tables 2 and 3) most probably by suppressing AM (Fig. 2) whereas in AM-free solution culture, in agreement with some previous reports (Cakmak and Marschner 1986; Nichols et al. 2012), higher P did not decrease shoot or root Zn concentrations at any Zn level (Tables 5 and 6). Instead, Zn-deficiency-induced P toxicity was responsible for the deteriorated condition of the plants at low Zn and high P in this solution culture experiment (Fig. 3; Table 5). Here, the shoot P concentration exceeded 2 % dry weight, which is well above the P toxicity thresholds reported in the literature (Loneragan et al. 1982; Reuter and Robinson 1997). The excess P in the tissues may, in addition to causing direct toxicity, also have decreased the physiological availability of Zn (Cakmak and Marschner 1987). In the soil study, P toxicity was not observed in Zn-deficient plants grown with high P (Table 2), presumably because plant-available P in the alkaline and calcareous soil used in this study was never as abundant as it was in the nutrient solution.
It was very obvious that the level of P supply had a marked effect on the tissue Mg concentrations in both the soil and solution culture experiments (Tables 2, 3, 5, and 6). Phosphorus deficiency reduced the shoot Mg concentrations to ~0.14 % dry weight (Tables 2 and 5), which is the critical level for Mg deficiency in wheat (Reuter and Robinson 1997). That P deficiency could induce Mg deficiency and P fertilization enhanced tissue Mg concentrations was documented in several field and solution culture studies on various species including grapevine (Vitis vinifera), squash (Cucurbita pepo), tall fescue (Festuca arundinacea) and wheat (Skinner and Matthews 1990; Reinbott and Blevis 1994, 1999). As mycorrhizal colonization of roots can contribute significantly to Cu uptake of plants (Kothari et al. 1991; Lambert and Weidensaul 1991; Li et al. 1991), Cu can be expected to respond to the sterilization and P treatments, which affect mycorrhizal activity in the soil study. Accordingly, the reduction of the grain Cu concentration by soil sterilization at low P (Table 3) may be due to the elimination of mycorrhizal activity. However, tissue Cu concentrations were in many cases not reduced but enhanced by higher P supply (Tables 2, 3, 5, and 6), suggesting that factors other than AM are involved in the effects of P supply on Cu in wheat.
Arbuscular mycorrhiza is known to attenuate Mn uptake of plants by stimulating Mn-oxidizing and suppressing Mn-reducing bacteria and thus lowering the bioavailability of Mn in the soil (Kothari et al. 1991; Arines et al. 1992; Ryan and Angus 2003; Nogueira et al. 2004). This, in addition to the 5-fold increase in the DTPA-extractable Mn concentration of the soil upon autoclaving (see Materials and methods for details), explains why shoot Mn concentrations were significantly enhanced by soil sterilization as well as higher P supply in non-autoclaved soil (Table 2). In contrast, the shoot and root Mn concentrations decreased with increasing P supply in solution culture (Tables 5 and 6), probably due to dilution (Table 4). The positive response of the grain Mn concentration to higher P supply, which is observed in not only non-autoclaved but also sterilized soil (Table 3), may be related to the possible role of phytate as a storage compound for Mn in wheat grain (Rodrigues-Filho et al. 2005).
The effect of P supply on 70Zn uptake of wheat was studied on young, hydroponically-grown plants, which were not significantly different from each other with respect to biomass but just starting to show growth responses to Zn and P treatments (Table 7), in order the minimize the potential impact of growth differences on mineral uptake. Zinc starvation was reported to increase Zn uptake rates in wheat by de-repression of the uptake system (Rengel et al. 1998; Erenoglu et al. 2011). Accordingly, plants pre-cultured with low Zn absorbed remarkably higher levels of 70Zn than plants pre-cultured with high Zn (Fig. 4). Most importantly, low-P plants were inefficient in 70Zn uptake when compared to P-sufficient plants. This means that in the absence of AM, P-deficiency may impair direct Zn uptake by plant roots.
The main findings of this study are summarized in Fig. 5. In non-autoclaved soil, higher P supply reduces tissue Zn concentrations of wheat in both Zn-deficient and Zn-sufficient conditions, primarily by suppressing mycorrhizal activity. This effect disappears in autoclaved soil, where AM fungi have been eliminated. Solution culture conditions are in this respect representative for autoclaved soil. All these results indicate that the well-documented negative effect of high P on tissue Zn concentration is mycorrhiza-dependent. By avoiding excessive P fertilization and implementing AM-stimulating agronomic practices, P-induced losses to Zn concentrations may be minimized. The AM-mediated P–Zn interaction in wheat should also be considered in biofortification of cereal grains with Zn.
Effects of P supply on the shoot Zn concentrations of bread wheat plants (Triticum aestivum cv. Adana99) grown in non-autoclaved soil (with natural mycorrhiza), autoclaved soil (without mycorrhiza) and nutrient solution (without mycorrhiza) under low-Zn and high-Zn conditions (values were compiled from Tables 2 and 5)
References
Anuradha M, Narayanan A (1991) Promotion of root elongation by phosphorus deficiency. Plant Soil 136:273–275
Arines J, Porto ME, Vilarino A (1992) Effect of manganese on vesicular-arbuscular mycorrhizal development in red clover plants and on soil Mn-oxidizing bacteria. Mycorrhiza 1:127–131
Bolan NS, Robson AD, Barrow NJ (1984) Increasing phosphorus supply can increase the infection of plant roots by vesicular-arbuscular mycorrhizal fungi. Soil Biol Biochem 16:419–420
Boyd HW (1971) Manganese toxicity to peanuts in autoclaved soil. Plant Soil 34:133–144
Broadley MR, Lochlainn SO, Hammond JP, Bowen HC, Cakmak I, Eker S, Erdem H, King GJ, White PJ (2010) Soil zinc (Zn) concentration varies widely within Brassica oleracea L. and is affected by soil Zn and phosphorus (P) levels. J Hortic Sci Biotechnol 85:375–380
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302:1–17
Cakmak I, Marschner H (1986) Mechanism of phosphorus-induced zinc deficiency in cotton. I. Zinc deficiency-enhanced uptake rate of phosphorus. Physiol Plant 68:483–490
Cakmak I, Marschner H (1987) Mechanism of phosphorus induced zinc deficiency in cotton. III. Changes in physiological availability of zinc in plants. Physiol Plant 70:13–20
Cakmak I, Hengeler C, Marschner H (1994) Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J Exp Bot 45:1245–1250
Cakmak I, Yilmaz A, Kalayci M, Ekiz H, Torun B, Erenoglu B, Braun HJ (1996) Zinc deficiency as a critical problem in wheat production in Central Anatolia. Plant Soil 180:165–172
Cakmak I, Ekiz H, Yilmaz A, Torun B, Koleli N, Gultekin I, Alkan Ai Eker S (1997) Differential response of rye, triticale, bread and durum wheats to zinc deficiency in calcareous soils. Plant Soil 188:1–10
Cavagnaro TR (2008) The role of arbuscular mycorrhizas in improving plant zinc nutrition under low soil zinc concentrations: a review. Plant Soil 304:315–325
Dang YP, Edwards DG, Dalal RC, Tiller KG (1993) Identification of an index tissue to predict zinc status of wheat. Plant Soil 154:161–167
Dwivedi RS, Randhawa NS, Bansal RL (1975) Phosphorus-zinc interaction I. Sites of immobilization of zinc in maize at high level of phosphorus. Plant Soil 43:639–648
Endlweber K, Scheu S (2006) Establishing arbuscular mycorrhiza-free soil: a comparison of six methods and their effects on nutrient mobilization. Appl Soil Ecol 34:276–279
Erenoglu EB, Kutman UB, Ceylan Y, Yildiz B, Cakmak I (2011) Improved nitrogen nutrition enhances root uptake, root-to-shoot translocation and remobilization of zinc (65Zn) in wheat. New Phytol 189:438–448
Genc Y, McDonald GK, Graham RD (2002) A soil-based method to screen for zinc efficiency in seedlings and its ability to predict yield responses to zinc deficiency in mature plants. Aust J Agric Res 53:409–421
Gianquinto G, Abu-Rayyan A, Tola LD, Piccotino D, Pezzarossa B (2000) Interaction effects of phosphorus and zinc on photosynthesis, growth and yield of dwarf bean grown in two environments. Plant Soil 220:219–228
Giovanetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Huang C, Barker SJ, Langridge P, Smith FW, Graham RD (2000) Zinc deficiency up-regulates expression of high-affinity phosphate transporter genes in both phosphate-sufficient and -deficient barley roots. Plant Physiol 124:415–422
Jackson LE, Miller D, Smith SE (2002) Arbuscular mycorrhizal colonization and growth of wild and cultivated lettuce in response to nitrogen and phosphorus. Sci Hortic 94:205–218
Kothari SK, Marschner H, Romheld V (1991) Contribution of the VA mycorrhizal hyphae in acquisition of phosphorus and zinc by maize grown in a calcareous soil. Plant Soil 131:177–185
Krebs NF (2013) Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab 62:19–29
Kutman UB, Yildiz B, Ozturk L, Cakmak I (2010) Biofortification of durum wheat with zinc through soil and foliar applications of nitrogen. Cereal Chem 87:1–9
Lambert DH, Weidensaul TC (1991) Element uptake by mycorrhizal soybean from sewage-sludge treated soil. Soil Sci Soc Am J 55:393–398
Lambert DH, Baker DE, Cole H (1979) The role of mycorrhizae in the interactions of phosphorus with zinc, copper, and other elements. Soil Sci Soc Am J 43:976–980
Lehmann A, Veresoglou SD, Leifheit EF, Rillig MC (2014) Arbuscular mycorrhizal influence on zinc nutrition in crop plants-a meta-analysis. Soil Biol Biochem 69:123–131
Li XL, Marschner H, George E (1991) Acquisition of phosphorus and copper by VA-mycorrhizal hyphae and root-to-shoot transport in white clover. Plant Soil 136:49–57
Li HY, Zhu YG, Smith SE, Smith FA (2003) Phosphorus–zinc interactions in two barley cultivars differing in phosphorus and zinc efficiencies. J Plant Nutr 26:1085–1099
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci Soc Am 42:421–428
Loneragan JF, Webb MJ (1993) Interactions between zinc and other nutrients affecting the growth of plants. In: Robson AD (ed) Zinc in soils and plants. Springer, the Netherlands, pp 119–134
Loneragan JF, Grove TS, Robson AD, Snowball K (1979) Phosphorus toxicity as a factor in zinc-phosphorus interactions in plants. Soil Sci Soc Am J 43:966–972
Loneragan JF, Grunes DL, Welch RM, Aduayi A, Tengah A, Lazar VA, Cary EE (1982) Phosphorus accumulation and toxicity in leaves in relation to zinc supply. Soil Sci Soc Am J 46:345–352
Lu Z, Grewal HS, Graham RD (1998) Dry matter production and uptake of zinc and phosphorus in two oilseed rape genotypes under differential rates of zinc and phosphorus supply. J Plant Nutr 21:25–38
Mahmood T, Mehnaz S, Fleischmann F, Ali R, Hashmi ZH, Iqbal Z (2014) Soil sterilization effects on root growth and formation of rhizosheaths in wheat seedlings. Pedobiologia 57:123–130
Marschner P (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, Elsevier, USA
Marschner H, Cakmak I (1986) Mechanism of phosphorus-induced zinc deficiency in cotton. II. Evidence for impaired shoot control of phosphorus uptake and translocation under zinc deficiency. Physiol Plant 68:491–496
Marschner H, Schropp A (1977) Vergleichende Untersuchungen über die Empfindlichkeit von 6 Unterlagensorten der Weinrebe gegenüber Phosphat-induziertem Zink-Mangel. Vitis 16:79–88
McIlveen WD, Cole H (1979) Influence of zinc on development of the endomycorrhizal fungus Glomus mosseae and its mediation of phosphorus uptake by Glycine max ‘Amsot 71’. Agric Environ 4:245–256
Meredith JA, Anderson RC (1992) The influence of varied microbial substrate conditiond on the growth and mycorrhizal colonization of little bluestem [Schizachyrium scoparium (Michx.) Nash]. New Phytol 121:235–242
Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M (2009) Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol 181:950–959
Neue HU, Quijano C, Senadhira D, Setter T (1998) Strategies for dealing with micronutrient disorders and salinity in lowland rice systems. Field Crop Res 56:139–155
Nichols BJRA, Hopkins GB, Jolley VD, Webb BL, Greenwood BG, Buck R (2012) Phosphorus and zinc interactions and their relationships with other nutrients in maize grown in chelator-buffered nutrient solution. J Plant Nutr 35:123–141
Nogueira MA, Magalhaes GC, Cardoso EJBN (2004) Manganese toxicity in mycorrhizal and phosphorus-fertilized soybean plants. J Plant Nutr 27:141–156
Ortas I (2012) The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crop Res 125:35–48
Ortas I, Ortakci D, Kaya Z, Cinar A, Onelge N (2002) Mycorrhizal dependency of sour orange in relation to phosphorus and zinc nutrition. J Plant Nutr 25:1263–1279
Perez-Novo C, Bermudez-Couso A, Lopez-Periago E, Fernandez-Calvino D, Arias-Estevez M (2011) Zinc adsorption in acid soils influence of phosphate. Geoderma 162:358–364
Rahimi A, Schropp A (1984) Carboanhydraseaktivität und extrahierbares Zink als Masstab fur die Zink-Versorgung von Pflanzen. Z Pflanzenernähr Bodenkd 147:572–583
Reinbott EM, Blevins DG (1999) Phosphorus nutritional effects on root hydraulic conductance, xylem water flow and flux of magnesium and calcium in squash plants. Plant Soil 209:263–273
Reinbott EM, Blevis DG (1994) Phosphorus and temperature effects on magnesium, calcium, and potassium in wheat and tall fescue leaves. Agron J 86:523–529
Rengel Z, Romheld V, Marschner H (1998) Uptake of zinc and iron by wheat genotypes differing in tolerance to zinc deficiency. J Plant Physiol 152:433–438
Reuter DJ, Robinson JB (1997) Plant analysis: an interpretation manual, 2nd edn. CSIRO Publishing, Australia
Rodrigues-Filho UP, Vaz S, Felicissimo MP, Scarpellini M, Cardoso DR, Vinhas RCJ, Landers R, Schneider JF, McGarvey BR, Andersen ML, Skibsted LH (2005) Heterometallic manganese/zinc-phytate complex as a model compound for metal storage in wheat grains. J Inorg Biochem 99:1973–1982
Ryan MH, Angus JF (2003) Arbuscular mycorrhizae in wheat and field pea crops on a low P soil: increased Zn-uptake but no increase in P-uptake or yield. Plant Soil 250:225–239
Ryan MH, McInerney JK, Record IR, Angus JF (2008) Zinc bioavailability in wheat grain in relation to phosphorus fertilizer, crop sequence and mycorrhizal fungi. J Sci Food Agric 88:1208–1216
Sharma PN, Kumar P, Tewari RK (2004) Early signs of oxidative stress in wheat plants subjected to zinc deficiency. J Plant Nutr 27:451–463
Singh JP, Karamanos RE, Stewart JWB (1988) The mechanism of phosphorus-induced zinc deficiency in bean (Phaseolus vulgaris L.). Can J Soil Sci 68:345–358
Skinner PW, Matthews MA (1990) A novel interaction of magnesium translocation with the supply of phosphorus to roots of grapevine (Vitis vinifera L.). Plant Cell Environ 13:821–826
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic, Amsterdam
Smith FA, Smith SE (1981) Mycorrhizal colonization and growth of Trifolium subterraneum: use of sterilized soil as a control treatment. New Phytol 88:299–309
Subramanian KS, Tenshia V, Jayalakshmi K, Ramachandran V (2009) Role of arbuscular mycorrhizal fungus (Glomus intraradices)—(fungus aided) in zinc nutrition of maize. J Agric Biotechnol Sustain Dev 1:029–038
Thompson JP (1990) Soil sterilization methods to show VA-mycorrhizae aid P and Zn nutrition of wheat in vertisols. Soil Biol Biochem 22:229–240
Tinker PB, Jones MD, Durall DM (1992) A functional comparison of ecto- and endomycorrhizas. In: Read DJ, Lewis DH, Fitter AH, Alexander IJ (eds) Mycorrhizas in ecosystems. C.A.B... International, Wellingford, pp 303–310
Verma TS, Minhas RS (1987) Zinc and phosphorus interaction in a wheat-maize cropping system. Fertil Res 13:77–86
Warnock RE (1970) Micronutrient uptake and mobility within corn plants (Zea mays L.) in relation to phosphorus-induced zinc deficiency. Soil Sci Soc Am J 34:765–769
Watts-Williams SJ, Patti AF, Cavagnaro TR (2013) Arbuscular mycrorrhizas are beneficial under both deficient and toxic soil zinc conditions. Plant Soil 371:299–312
Webb MJ, Loneragan JF (1988) Effect of zinc deficiency on growth, phosphorus concentration, and phosphorus toxicity of wheat plants. Soil Sci Soc Am J 52:1676–1680
Williams-Linera G, Ewel JJ (1984) Effect of acutoclave sterilization of a tropical andept on seed germination and seedling growth. Plant Soil 82:263–268
Youngdahl LJ, Svec LV, Liebhardt WC, Teel MR (1977) Changes in the zinc-65 distribution in corn root tissue with a phosphorus variable. Crop Sci 17:66–69
Zhang YQ, Deng Y, Chen RY, Cui ZL, Chen XP, Yost R, Zhang FS, Zou CQ (2012) The reduction in zinc concentration of wheat grain upon increased phosphorus-fertilization and its mitigation by foliar zinc application. Plant Soil 361:143–152
Zhu YG, Smith SE, Smith FA (2001) Zinc (Zn)-phosphorus (P) interactions in two cultivars of spring wheat (Triticum aestivum L.) differing in P uptake efficiency. Ann Bot 88:941–945
Acknowledgments
This study was financially supported by the HarvestPlus Program (www.harvestplus.org) and the sponsors of the HarvestPlus Global Zinc Fertilizer Project (www.harvestzinc.org) including Mosaic Company, K + S Kali GmbH, International Zinc Association, Omex Agrifluids, International Fertilizer Industry Association and International Plant Nutrition Institute. We would like also to thank Prof. Dr. Ibrahim Ortas and his team at the Cukurova University in Adana, Turkey, for the advice and contribution to measurement of mycorrhizal colonization of roots.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: N. Jim Barrow.
Rights and permissions
About this article
Cite this article
Ova, E.A., Kutman, U.B., Ozturk, L. et al. High phosphorus supply reduced zinc concentration of wheat in native soil but not in autoclaved soil or nutrient solution. Plant Soil 393, 147–162 (2015). https://doi.org/10.1007/s11104-015-2483-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2483-8