Abstract
Background and aims
Although phosphorus (P) application is known to affect the zinc (Zn) nutrition of crops, the underlying mechanisms and effects of soil type are unclear.
Methods
A greenhouse pot experiment was conducted with wheat, two soils (calcareous and acid), and nine P fertilizer rates (0, 50, 100, 200, 400, 1000, 2000, 3000, and 5000 mg P2O5 kg−1 soil).
Results
The effects of P application on the Zn content of shoots and roots in wheat and on the levels of available Zn in soil differed on the two soils. The wheat dry weight on both soils was highest with 2000 mg P2O5 kg−1. Total Zn accumulation was reduced above 2000 mg P2O5 kg−1 on the acid soil and above 100 mg P2O5 kg−1 on the calcareous soil. Available soil Zn declined when the Bray-P concentration reached about 34 mg kg−1 in the acid soil and when the Olsen-P concentration exceeded 200 mg kg−1 in the calcareous soil. Shoot Zn concentrations were negatively related to available soil P on the two soils.
Conclusion
The negative effects of increasing P application rates on Zn accumulation by wheat differed between the two soils. The effects showed no close relationship to available soil Zn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Millions of hectares of cropland around the world are affected by low concentrations of available soil zinc (Zn), and approximately one-third of the global human population suffers from an inadequate Zn intake (Alloway 2009; Brown et al. 2004). Thus, Zn deficiency is a widespread nutritional problem for both crops and humans. As one of the “big three” cereal crops worldwide, wheat and its products have a strong impact on human nutrition (Shewry 2009). They provide about 22 and 20% of the daily human Zn intake in rural and urban regions of China, respectively (Ma et al. 2008). Also in developed countries such as the UK, wheat and other cereals are significant sources of human Zn nutrition, contributing 25% of the daily Zn intake (11% from bread) (Henderson et al. 2003). Thus, human Zn nutrition can be improved by enhancing Zn density in wheat grains.
Grain yields depend on an adequate supply of phosphorus (P) (George et al. 2016; Nesme et al. 2014), but excessive P application can reduce Zn uptake by crop plants (Imran et al. 2016; Wang et al. 2008; Zhang et al. 2012, 2015). Gao et al. (2011) found that P fertilizer application at a rate of 80 mg P kg−1 soil decreased shoots Zn concentration in pot-grown durum wheat to 12.8 mg kg−1. In another study, 20 kg P ha−1 applied as triple superphosphate, decreased the grain Zn concentration of wheat by 33–39% (Ryan et al. 2008).
There is more than one mechanism involved in the interaction. Biswapati and Mandal (1990) found that the decrease of Zn concentration in shoots and roots of rice in response to P application was related to a decrease in available Zn on a Haplustalf soil with pH 6.6. Saeed (1977) reported that the availability of soil Zn was not affected by interactions between Zn and P in three calcareous soils with pH 8.0. He also reported that P application increased Zn availability in calcareous soils by increasing its desorption under low levels of P application. Available soil Zn content as affected by P application may play an important role in the negative effect of P on Zn nutrient in crops. In his book on soil nutrient bioavailability, Barber (1995) concluded that Zn availability in soil is affected by soil pH. In other two studies, Zn adsorption increased (and the concentration of available Zn decreased) with P application on acid soils because of the abundance of iron and aluminium oxides (Pérez-Novo et al. 2011; Stanton et al. 1970). Another study, however, reported that increases in P application did not influence Zn solubility when the soil pH was <5 (Agbenin 1998) and that Zn adsorption changed little with increasing P application rates up to 2000 mg P kg−1 on a sandy loam Orthic Acrisol and up to 40 mg P kg−1 on an Alfisol, an Oxisol, and a Vertisol with soil pH >7 (Agbenin 1998; Rupa and Tomar 1999).
The effect of P application on the Zn nutrition of crops can be partially attributed to the effect of P on the uptake of Zn by roots (Yang et al. 2011; Zhang et al. 2016). For example, Zhang et al. (2016) reported that P application decreased Zn uptake by wheat under field conditions. In a pot experiment, the total amount of Zn (the product of concentration and weight) in roots and shoots of maize was positively correlated with root biomass, which was affected by P application, and by soil properties as well (Zhang et al. 2017a). In a field experiment conducted on a calcareous soil, the biomass of wheat roots increased when P application was increased from a low to a moderate rate, until it plateaued when the rate exceeded 50 kg P ha−1 (Teng et al. 2013). In a pot experiment with an acid soil, the biomass of wheat roots increased as the P application increased from 0 to 160 mg kg−1, but then decreased at higher application rates (Rupa et al. 2003). These results suggest that the involvement of root biomass in how P application affects Zn uptake warrants additional research.
P application also affects Zn concentrations in shoots. Zhang et al. (2015) reported that accumulation of Zn in wheat shoots on calcareous soil increased as the P application rate increased up to a threshold; at P application rates above that threshold, Zn accumulation decreased. Yang et al. (2011), in contrast, found that Zn accumulation in shoots of wheat growing in an acid medium decreased as the P application rate increased.
The papers cited in the previous paragraphs show that Zn uptake by crop plants can be influenced by the rate of P fertilizer application, depending on soil acidity. Acid soils accounts for nearly 21 and 50% of arable soils in China and worldwide, respectively (Kochian et al. 2004). On the other hand, calcareous soils with low Zn availability dominate on the North China Plain (Xiang et al. 1995). Although it is known that the high pH of calcareous soils and the low pH and high iron-aluminum oxide concentration of acid soils limit crop Zn nutrition (Saeed and Fox 1979; Pérez-Novo et al. 2011), there is a lack of knowledge on how P application affects Zn accumulation by wheat on typical acid and calcareous soils.
This study had two objectives. The first objective was to investigate how P application affects the growth and Zn accumulation of wheat growing on a typical acid soil and a typical calcareous soil from China. The second objective was to clarify to what extent P application effects on wheat Zn accumulation are related to available soil Zn concentrations.
Materials and methods
Experimental design
A pot experiment with wheat (Triticum aestivum L., cv. Liangxing 99) was conducted in a greenhouse at China Agriculture University. Two soils were used: an acid purple Regosol (according to WRB classification) with low pH from Sichuan Basin and a calcareous Fluvisol (according to WRB classification) with high pH from the North China Plain. The soils were air-dried and passed through a 3-mm plastic sieve before they were filled into the pots. Information about soil properties is presented in Table 1.
Both soils were treated with nine levels of P (0, 50, 100, 200, 400, 1000, 2000, 3000, and 5000 mg P2O5 kg−1 soil) in the form of Ca(H2PO4)2·H2O. Before sowing, the following nitrogen (N), potassium (K), and zinc (Zn) fertilizers were mixed into the soil: 150 mg N kg−1 soil as urea, 150 mg K2O kg−1 soil as K2SO4, and 10 mg Zn kg−1 soil as ZnSO4·7H2O. Each pot (5 L) received 4 kg of soil. Each P level was represented by four replicate pots, which were arranged on a greenhouse bench in a random design. Fifteen germinated seeds were sown in each pot, and the seedlings were thinned to 10 per pot after 1 week. Pots were irrigated with tap water as needed.
Sample collection and nutrient analysis
At the stem elongation stage (65 days after sowing), the shoots and roots of the wheat plants were harvested, and soil samples were collected. The shoots were separated from the roots using stainless steel scissors. Then the roots were collected by gently shaking off the soil adhering to the root system and individually collecting visible small roots that remained detached in the soil after the shaking. After the plant samples were washed with tap water and then with deionized water, they were dried at 60-65°C to a constant weight and weighed. The dried shoots and roots samples were ground with a stainless-steel grinder for P and Zn analysis. All plant samples were digested with HNO3-H2O2 (6 ml HNO3 and 2 ml H2O2) in a microwave-accelerated reaction system (CEM, Matthews, NC, USA), and the P and Zn concentrations in the digesting solutions were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES, OPTIMA 3300 DV, Perkin-Elmer, USA). Standard materials for P and Zn (IPE126) were obtained from Wageningen Evaluation Programs for Analytical Laboratories (WEPAL, Netherlands).
The collected soil samples were air dried and passed through a 1 mm plastic sieve to determine pH, available P and Zn. Soil pH (1:2.5 w/v in water) was determined using a pH meter (PB-10, Sartorius, GER) which was sensitive to 0.01 pH units. Olsen-P, Bray-P, and CaCl2-extractable soil P (CaCl2-P) concentrations were measured by the molybdovanado phosphatase method based on extraction with 0.5 mol L−1 NaHCO3 (Olsen 1954), 0.03 mol L−1 ammonium fluoride and 0.025 mol L−1 HCl (Bray and Kurtz 1945), and 0.01 mol L−1 CaCl2 (Schofield 1955), respectively. Bray-P and CaCl2-P concentrations were determined to indicate the available P concentration in the acid soil, while Olsen-P and CaCl2-P concentrations were determined to indicate the available P concentration in the calcareous soil. DTPA-extractable (DTPA = diethylene triamine pentacetic acid) Zn, Fe, Cu, and Mn concentrations (denoted as DTPA-Zn, DTPA-Fe, DTPA-Cu, DTPA-Mn) were extracted with 5 mmol L−1 DTPA at pH 7.3 (180 rpm, 25 °C) and analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES, OPTIMA 3300 DV, Perkin-Elmer, USA) as proxies of available soil Zn, Fe, Cu and Mn (Lindsay and Norvell 1978).
Statistical analysis
Two-factor analyses of variance (ANOVAs) were used to evaluate the effects of soil type and P application on root and shoot dry weight and Zn accumulation. SAS software (SAS 8.0, USA) and SPSS software (SPSS 20.0, China) were used for the statistical analysis. When the effects were significant according to ANOVA, treatments were compared with the Duncan’s test at P < 0.05.
Results
Plant biomass
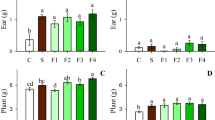
On the acid soil, shoot dry weight (DW) increased as the P application rate increased from 0 to about 2000 mg P2O5 kg−1 and then plateaued at higher application rates (Fig. 1a), and root DW increased as the P application rate increased from 0 to 100 mg kg−1 and then decreased at higher application rates (Fig. 1b). On the calcareous soil, shoot DW peaked at 2000 mg P2O5 kg−1 and then declined at higher P application rates (Fig. 1a), but root DW was not significantly affected by the P application rate (Fig. 1b).
Dry weight (a, b) and P concentration (c, d) of shoots and roots of wheat as affected by P application rate and soil, respectively. Values are means of four replications ± SE. Within each soil, means with different lowercase letters are significantly different at P < 0.05. Uppercase letters indicate significant differences between the soils at P < 0.05
Plant P and Zn contents
P application significantly increased the concentration of P in shoots (Fig. 1c) and roots (Fig. 1d) on both soils. On the acid soil, shoot P concentrations were not increased by P application rates <400 mg P2O5 kg−1. Shoot Zn concentrations decreased as the P application rate increased in both soils (Supplementary Fig. 1a). Root Zn concentrations were not significantly affected by P application on the acid soil, but they were decreased by P application on the calcareous soil (Supplementary Fig. 1b). On both soils, there was a negative correlation between shoot Zn and P concentrations (Fig. 2).
The P application effects on the P and Zn concentrations of roots and shoots and their biomasses translated into the following effects on the amounts of P and Zn accumulated by the plants. Shoot P and total P accumulation were significantly increased as the P application rate increased from 0 to 5000 mg P2O5 kg−1 on the acid soil and from 0 to 2000 mg P2O5 kg−1 on the calcareous soil (Supplementary Table 1). On the acid soil, specific P uptake (P uptake per unit of root DW) changed only weakly as the P application rate increased from 0 to 200 mg P2O5 kg−1, but increased strongly at higher P application rates. On the calcareous soil, specific P uptake increased as the P application increased from 0 to 2000 mg P2O5 kg−1 and then plateaued (Supplementary Table 1).
On the acid soil, shoot Zn and total Zn accumulation increased with increasing P application rate until reaching a plateau at rates between 400 and 2000 mg P2O5 kg−1 and then slightly declined again with further increase (Fig. 3a, c). Root Zn accumulation peaked at a P application rate of 100 mg P2O5 kg−1. On the calcareous soil, shoot and root Zn accumulation and total Zn accumulation remained unchanged when the P application rate was <100 mg P2O5 kg−1 but declined at higher rates (Fig. 3a, b and c). On the acid soil, specific root Zn uptake (total plant Zn uptake per unit of root DW) did not significantly differ among P application rates between 0 and 200 mg P2O5 kg−1 and between 400 and 5000 mg P2O5 kg−1, but increased from 200 to 400 mg P2O5 kg−1. On the calcareous soil, specific root Zn uptake decreased with increasing P application rate (Fig. 3d).
The Zn accumulation in shoots (a), roots (b), and total (c) Zn accumulation and Zn-specific uptake by roots (d) as affected by P application rates on the two soils. Total plant Zn uptake/root DW refers to the quantity of Zn taken by per unit dry weight of root. Values are means of four replications ± SE. Within each soil, means with different lowercase letters are significantly different at P < 0.05. Uppercase letters indicate significant differences between the soils at P < 0.05
Available soil P and Zn
Increasing P application significantly increased the Bray-P concentration in the acid soil (Fig. 4a) and the Olsen-P concentration in the calcareous soil (Fig. 4b). The increases were similar in the two soils. Increasing P application also increased the CaCl2-P concentration in both soils. In the acid soil, the CaCl2-P concentration increased slower at low (< 2550 mg P2O5 kg−1) than at high P application rates (Fig. 4c). In the calcareous soil, the CaCl2-P concentration increased rapidly as the P application rate increased from 0 to 1850 mg P2O5 kg−1 and then plateaued (Fig. 4c).
The DTPA-extractable soil Zn (DTPA-Zn) concentration of the acid soil increased as the P application rate increased from 0 to 400 mg P2O5 kg−1 and then decreased (Fig. 5a). The DTPA-Zn concentration of the calcareous soil remained nearly unaffected by P application in the range from 0 to 1000 mg P2O5 kg−1, but showed a significant decrease with increasing P application at rates exceeding 1000 mg P2O5 kg−1 (Fig. 5a). DTPA-Zn was first positively and then negatively related in the acid soil to the increasing Bray-P and CaCl2-P (Fig. 5c, d). It was also negatively related to Olsen-P and CaCl2-P in the calcareous soil, but only above a threshold of about 200 mg kg−1 and 30 mg kg−1 (Fig. 5e, f), respectively. The pH of the acid soil was not affected by the P application rate, while the pH of the calcareous soil showed a slightly increasing trend at P application rates from 0 to 200 mg P2O5 kg−1 and slight decrease at higher rates (Fig. 5b).
Effects of P application (rates given in mg P2O5 kg−1 soil) on (a) the concentration of DTPA-extractable soil Zn and (b) soil pH, and relationships of DTPA-extractable soil Zn with (c) Bray-P and (d) CaCl2-P in the acid soil and with (e) Olsen-P and (f) CaCl2-P in the calcareous soil. Log scale of x-axis was used in Fig. 5c-f. Values are means ± SE. Within each soil, means followed with different lowercase letters are significantly different at P < 0.05. *** indicates that the model is significant at P < 0.001
Relationship between plant Zn and available soil Zn and P
The relationships between root and shoot Zn concentrations and the concentrations of available soil P and Zn differed between the two soils. On the acid soil, root and shoot Zn concentrations were not related to the DTPA-extractable soil Zn concentration (Fig. 6a), while shoot Zn but not root Zn was negatively related to Bray-P and CaCl2-P (Fig. 6c, e). On the calcareous soil, root and shoot Zn concentrations were positively related to DTPA-Zn (Fig. 6b) and negatively related to Olsen-P and CaCl2-P (Fig. 6d, f).
Relationships of Zn concentrations in wheat shoots and roots with (a) DTPA-Zn, (c) Bray-P, and (e) CaCl2-P in the acid soil and with (b) DTPA-Zn, (d) Olsen-P, and (f) CaCl2-P in the calcareous soil. Log scale of x-axis was used in Fig. 6c-f. ** and *** indicate that the model is significant at P < 0.01 and P < 0.001, respectively
Discussion
Available soil P and Zn response to P application
Close positive relationships were found between P application rate and the indicators of available soil P (with the exception of CaCl2-P at the highest two application rates) on both soils, in line with many previous studies (Peck et al. 1971; Zhang et al. 2014, 2017b; ). However, in the latter studies, the available soil P concentrations did not extend to so high values as in our study. The negative correlations between available soil P and DTPA-Zn in our two soils were not consistent with the results of some other studies that found positive relationships between available soil P and DTPA-Zn (Rahman et al. 2007; Rezapour 2014). However, the available soil P concentrations in their experiments were not so high to represent the results from present study. The changes in available soil Zn were always associated with P condition in soil.
Increasing P application initially increased but then decreased available soil Zn in the acid soil, which agrees with analogous findings by Rupa et al. (2003). An increase in available soil Zn with P application at low rates was also observed by Drissi et al. (2015), who found that available soil Zn increased upon application of 72 mg P2O5 kg−1 in form of diammonium phosphate at a soil pH of 6.1. In our case, it may have been at least partly caused by competition between Zn and Ca cation for cation-exchanging sites, as we had used calcium superphosphate as P source in our experiment. Desorption of Zn due to competition with Ca has been reported for example by García-Miragaya and Dávalos (1986) and Escrig and Morell (1998). Besides, the complexes of ZnHPO4 and ZnH2PO4+ might, which are known to exist in soil solutions (Mattigod and Sposito 1977), might have increased the total Zn concentration in solution with increased P application rate in the acid soil, while at higher P application rates immobilizing effects became dominant. Negative influence of P application on available soil Zn may not appear at low application rate. A well-known major Zn immobilization effect of P on soil Zn is the formation of ternary Zn-phosphate-surface complexes (Sadiq 1991). In soil solutions, the main forms of P are HPO42− and H2PO4− (Havlin et al. 2005), and an increasing concentration of H2PO4− has been found to increase the adsorption of Zn to Fe and Al oxides (Jurinak and Inouye 1962). Thus, it can be expected that the increased concentration of the phosphate anion with increasing P application rate enhanced Zn sorption in the acid soil, counteracting the opposite effect of soluble Zn phosphate complex formation, and that above some threshold around 400 mg P2O5 kg−1 this immobilization effect became dominant over the mobilizing effect.
In contrast to the acid soil, DPTA-Zn was relatively constant in the calcareous soil at P application rates below 1000 mg P2O5 kg−1, but also here it decreased with P application at higher rates. This result differs from the finding of Saeed (1977) that P application increased available soil Zn concentration in a calcareous soil by decreasing Zn adsorption. The reason for the disagreement may be that the concentrations of available soil P (11–35 mg kg−1 NaHCO3-extractable P) and Zn were lower than in our study. Stukenholtz et al. (1966) found that P application rates between 0 and 1000 mg kg−1 did not affect the HCl-extractable Zn concentration of a calcareous soil, but increased it in an acid soil. In our study, DTPA-Zn decreased in the calcareous soil when the P application rate exceeded 1000 mg P2O5 kg−1 and Olsen-P reached about 200 mg kg−1 and CaCl2-P about 30 mg kg−1. At high rates of P application, precipitation of hopeite has been considered important for understanding the interaction between P and Zn in calcareous soils (Lindsay 1979).
Plant Zn concentration, accumulation, and uptake
Consistent with previous reports (Kizilgoz and Sakin 2010; Zhang et al. 2012, 2016), the shoot Zn concentration of our wheat plants was reduced by P application on both the acid and calcareous soil. The decrease in shoot Zn concentration was greater on the calcareous soil than on the acid soil though, as P application affected Zn accumulation more on the calcareous soil than on the acid soil at the same level of P application. The negative P effect on root Zn concentration on the calcareous soil agreed with the results of a recent field experiment in which it was attributed to increased soluble soil P (Olsen-P) and decreased root arbuscular mycorrhizal fungi (AMF) colonization (Zhang et al. 2016). The lack of a P treatment effect on root Zn concentration on the acid soil indicates that here the negative effect on shoot Zn was not due to reduced root Zn uptake.
While a similar negative relationship as found for both soils between available soil P and shoot Zn was found also between available soil P and root Zn in the case of the calcareous soil, there was no relationship between available soil P and root Zn in the acid soil. This finding is in line with the results of a pot experiment conducted by Zhu et al. (2001), who found that root Zn was not affected or even slightly increased with increasing P application on a slightly acid soil, and with similar results reported by Youngdahl et al. (1977). It is also consistent with the findings of Sharma et al. (1968), who reported that added P influenced Zn concentrations of shoots much more than those of roots in maize and tomato.
The responses of Zn accumulation to the P treatment were partially quite different from the responses of plant Zn concentrations, due to the fact that P application also had strong effects on biomass. On the calcareous soil, P application decreased shoot Zn accumulation and total Zn accumulation, as the decrease in shoot and root Zn concentration was not compensated by respective variations in biomass under our experiment condition. Also Kizilgoz and Sakin (2010) found that the Zn accumulation in wheat shoots decreased with increasing P application rate in a pot experiment with alkaline soil. In contrast, shoot Zn accumulation increased with P application on the acid soil at low P application rates, as the decrease in shoot Zn concentration was more than compensated by a substantial increase in shoot biomass. The change of Zn accumulation in the whole plant was similar to that in the shoot. Our results are in line with those of Stukenholtz et al. (1966), who found that low P application rates did not reduce Zn uptake by corn on an acid soil. As the P effect on shoot biomass levelled off at high P application rates, Zn accumulation reached a peak and then decreased also on the acid soil, following the respective trend in shoot Zn concentration. We found no other studies on shoot Zn accumulation in crops at similarly high rates of P application for comparison.
There are various reports in the literature indicating that high rates of P application can inhibit Zn uptake by roots (Rengel et al. 1999; Liu et al. 2000; Yang et al. 2011). In our study, P application significantly decreased specific Zn uptake by roots on the calcareous soil, which was consistent with recent field studies on wheat and maize (Zhang et al. 2016, 2017a). In a previous pot study using calcareous soil, P application did not greatly affect root growth, but as in our study also reduced Zn uptake (Gao et al. 2011). This effect was related to decreasing root colonization by AMF (Ova et al. 2015; Ryan et al. 2008). In contrast to the calcareous soil, total plant Zn uptake per unit mass of roots was not decreased by P application on the acid soil in our study, which may relate to the slightly increased available soil Zn on the acid soil. The result did not agree with the finding by Zhu et al. (2001) who found that an increase in P application caused a significant reduction in Zn uptake per unit of root weight on a slightly acid soil. The discrepancy may be due to the relatively low levels of P in the research of Zhu et al. (2001) compared with this study.
The relationships between soil P and Zn and plant Zn uptake
The decrease in total Zn uptake at the highest P application rates could have been at least partially due to the decrease in available soil Zn concentration observed in both soils. These high levels are not representative for agricultural practices in China, however. They were only chosen here to determine potential threshold levels for P effects on available soil Zn, wheat biomass and Zn uptake. Under normal field conditions less than 90 kg P ha−1 are applied per year in wheat-maize cropping systems in Northern China (Vitousek et al. 2009). In addition, from 1980 to 2007, the average concentration of Olsen-P increased from 7.4 to 24.7 mg kg−1 in Chinese agricultural soils (Li et al. 2011). Based on the results of our study, this increase in available soil P would not have decreased DTPA-Zn.
While our study did not give specific clues on the mechanisms that were responsible for the decrease in shoot Zn concentrations at lower P application, the results nonetheless suggest that the observed negative effects of P application on Zn uptake by wheat can be reduced by avoiding excessive P fertilization, especially on acid soils. The Zn nutrition of crops can also be improved by the application of Zn fertilizers, on calcareous soils in particular by foliar Zn application (Zhang et al. 2012; Cakmak 2008; Cakmak et al. 2010).
Conclusions
P application affected root and shoot Zn concentrations more on calcareous soil than on acid soil at the same level of P application. The critical P application level that reduced Zn accumulation in wheat was 2000 mg P2O5 kg−1 on the acid soil and 100 mg P2O5 kg−1 on the calcareous soil. DTPA-extractable soil Zn declined when Bray-P exceeded 34 mg kg−1 in the acid soil and Olsen-P exceeded 200 mg kg−1 in the calcareous soil. Such levels of available P are usually not reached in soils under current agricultural management practices though. On the acid soil, the decrease in shoot Zn density with increasing P application rate was associated with a substantial increase in shoot biomass. On the calcareous soil, in contrast, the decrease in shoot Zn density with increasing P application rate appeared to be mainly due to a decrease in Zn uptake by the roots.
References
Agbenin JO (1998) Phosphate-induced zinc retention in a tropical semi-arid soil. Eur J Soil Sci 49:693–700
Alloway BJ (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Hlth 31:537–548
Barber SA (1995) Soil nutrient bioavailability: a mechanistic approach. Q Rev Biol 161:140–141
Biswapati M, Mandal LN (1990) Effect of phosphorus application on transformation of zinc fraction in soil and on the zinc nutrition of lowland rice. Plant Soil 121:115–123
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–46
Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, L Nnerdal B, Ruel MT, Sandtr MB, Wasantwisut E, Hotz C (2004) International zinc nutrition consultative group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 25:S99–S203
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302:1–17
Cakmak I, Kalayci M, Kaya Y, Torun AA, Aydin N, Wang Y, Arisoy Z, Erdem H, Yazici A, Gokmen O, Ozturk L, Horst WJ (2010) Biofortification and localization of zinc in wheat grain. J Agric Food Chem 58:9092–9102
Drissi S, Houssa A, Bamouh A, Coquant J, Benbella M (2015) Effect of zinc-phosphorus interaction on corn silage grown on sandy soil. Agriculture 5:1047–1059
Escrig I, Morell I (1998) Effect of calcium on the soil adsorption of cadmium and zinc in some Spanish sandy soils. Water Air Soil Pollut 105:507–520
Gao X, Flaten DN, Tenuta M, Grimmett MG, Gawalko EJ, Grant CA (2011) Soil solution dynamics and plant uptake of cadmium and zinc by durum wheat following phosphate fertilization. Plant Soil 338:423–434
García-Miragaya J, Dávalos M (1986) Sorption and desorption of Zn on ca-kaolinite. Water Air Soil Pollut 27:217–224
George TS, Hinsinger P, Turner BL (2016) Phosphorus in soils and plants-facing phosphorus scarcity. Plant Soil 401:1–6
Havlin JL, Beaton JD, Tisdale SL, Nelson WL (2005) Soil fertility and fertilizers (7th ed.). ISBN: 0–13–027824-6 Pearson education limited USA
Henderson L, Gregory J, Swan G (2003) The national diet and nutrition survey: adults aged 19 to 64 years. Vitamin and mineral intake and urinary analytes, 3rd edn, London, pp 75
Imran M, Rehim A, Sarwar N, Hussain S (2016) Zinc bioavailability in maize grains in response of phosphorous-zinc interaction. J Plant Nutr Soil Sc 179:60–66
Jurinak JJ, Inouye TS (1962) Some aspects of zinc and copper phosphate formation in aqueous systems. Soil Sci Soc Am J 26:144–147
Kizilgoz I, Sakin E (2010) The effects of increased phosphorus application on shoot dry matter, shoot P and Zn concentrations in wheat (Triticum durum L.) and maize (Zea mays L.) grown in a calcareous soil. Afr J Biotechnol 9:5893
Kochian LV, Hoekenga OA, Pineros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459–493
Li H, Huang G, Meng Q, Ma L, Yuan L, Wang F, Zhang W, Cui Z, Shen J, Chen X, Jiang R, Zhang F (2011) Integrated soil and plant phosphorus management for crop and environment in China. A review. Plant Soil 349:157–167
Lindsay WL (1979) Chemical equilibria in soils. Clay Miner 28:319–319
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Liu A, Hamel C, Hamilton RI, Ma BL, Smith DL (2000) Acquisition of cu, Zn, Mn and Fe by mycorrhizal maize (Zea mays L.) grown in soil at different P and micronutrient levels. Mycorrhiza 9:331–336
Ma G, Jin Y, Li Y, Zhai F, Kok FJ, Jacobsen E, Yang X (2008) Iron and zinc deficiencies in China: what is a feasible and cost-effective strategy? Public Health Nutr 11:632–638
Mattigod SV, Sposito G (1977) Estimated association constants for some complexes of trace metals with inorganic ligands. Soil Sci Soc Am J 41:1092–1097
Nesme T, Colomb B, Hinsinger P, Watson CA (2014) Soil phosphorus management in organic cropping systems: from current practices to avenues for a more efficient use of P resources. In: Bellon S, Penvern S (eds) Organic farming, prototype for sustainable agricultures: prototype for sustainable agricultures. Springer, Netherlands Dordrecht, pp 23–45
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circ 939
Ova EA, Kutman UB, Ozturk L, Cakmak I (2015) High phosphorus supply reduced zinc concentration of wheat in native soil but not in autoclaved soil or nutrient solution. Plant Soil 393:147–162
Peck TR, Kurtz LT, Tandon HLS (1971) Changes in bray P-1 soil phosphorus test values resulting from applications of phosphorus fertilizer 1. Soil Sci Soc Am J 35:595–598
Pérez-Novo C, Bermúdez-Couso A, López-Periago E, Fernández-Calviño D, Arias-Estévez M (2011) Zinc adsorption in acid soils influence of phosphate. Geoderma 162:358–364
Rahman MA, Jahiruddin M, Islam MR (2007) Critical limit of zinc for rice in calcareous soils. J Agric Rural Dev 5:43–47
Rengel Z, Batten GD, Crowley DE (1999) Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crop Res 60:27–40
Rezapour S (2014) Effect of sulfur and composted manure on SO4-S, P and micronutrient availability in a calcareous saline-sodic soil. Chem Ecol 30:147–155
Rupa TR, Tomar KP (1999) Zinc desorption kinetics as influenced by pH and phosphorus in soils. Commun Soil Sci Plant Anal 30:1951–1962
Rupa TR, Srinivasa RC, Subba RA, Singh M (2003) Effects of farmyard manure and phosphorus on zinc transformations and phyto-availability in two alfisols of India. Bioresour Technol 87:279–288
Ryan MH, McInerney JK, Record IR, Angus JF (2008) Zinc bioavailability in wheat grain in relation to phosphorus fertilizer, crop sequence and mycorrhizal fungi. J Sci Food Agric 88:1208–1216
Sadiq M (1991) Solubility and speciation of zinc in calcareous soils. Water Air Soil Pollut 57-58:411–421
Saeed M (1977) Phosphate fertilization reduces zinc adsorption by calcareous soils. Plant Soil 48:641–649
Saeed M, Fox RL (1979) Influence of phosphate fertilization on zinc adsorption by tropical soils. Soil Sci Soc Am J 43:683–686
Schofield RK (1955) Can a precise meaning be given to ‘available’ soil phosphorus. Soils Fert 18:373–375
Sharma KC, Krantz BA, Brown AL, Quick J (1968) Interaction of Zn and P in top and root of corn and tomato. Agron J 60:453–456
Shewry PR (2009) Wheat. J Exp Bot 60:1537–1553
Stanton DA, Du R, Burger T (1970) Studies on zinc in selected orange free state soils: V. Mechanisms for the reaction of zinc with iron and aluminium oxides. Agrochemophysica 2:65–76
Stukenholtz DD, Olsen RJ, Gogan G, Olson RA (1966) On the mechanism of phosphorus-zinc interaction in corn nutrition. Soil Sci Soc Am J 30:759–763
Teng W, Deng Y, Chen XP, Xu XF, Chen RY, Lv Y, Zhao YY, Zhao XQ, He X, Li B, Tong YP, Zhang FS, Li ZS (2013) Characterization of root response to phosphorus supply from morphology to gene analysis in field-grown wheat. J Exp Bot 64:1403–1411
Vitousek PM, Naylor R, Crews T, David MB, Drinkwater LE, Holland E, Johnes PJ, Katzenberger J, Martinelli LA, Matson PA, Nziguheba G (2009) Nutrient imbalances in agricultural development. Science 324:1519–1520
Wang B, Xie Z, Chen J, Jiang J, Su Q (2008) Effects of field application of phosphate fertilizers on the availability and uptake of lead, zinc and cadmium by cabbage (Brassica chinensis L.) in a mining tailing contaminated soil. J Environ Sci 20:1109–1117
Xiang HF, Tang HA, Ying QH (1995) Transformation and distribution of forms of zinc in acid, neutral and calcareous soils of China. Geoderma 66:121–135
Yang XW, Tian XH, Lu XC, Cao YX, Chen ZH (2011) Impacts of phosphorus and zinc levels on phosphorus and zinc nutrition and phytic acid concentration in wheat (Triticum aestivum L.). J Sci Food Agric 91:2322–2328
Youngdahl LJ, Svec LV, Liebhardt WC, Teel MR (1977) Changes in Zn-65 distribution in corn root-tissue with a phosphorus variable. Crop Sci 17:66–69
Zhang YQ, Deng Y, Chen RY, Cui ZL, Chen XP, Yost R, Zhang FS, Zou CQ (2012) The reduction in zinc concentration of wheat grain upon increased phosphorus-fertilization and its mitigation by foliar zinc application. Plant Soil 361:143–152
Zhang YQ, Wen MX, Li XP, Shi XJ (2014) Long-term fertilization causes excess supply and loss of phosphorus in purple paddy soil. J Sci Food Agric 94:1175–1183
Zhang W, Liu DY, Li C, Cui ZL, Chen XP, Russell Y, Zou CQ (2015) Zinc accumulation and remobilization in winter wheat as affected by phosphorus application. Field Crop Res 184:155–161
Zhang W, Liu DY, Liu YM, Cui ZL, Chen XP, Zou CQ (2016) Zinc uptake and accumulation in winter wheat relative to changes in root morphology and mycorrhizal colonization following varying phosphorus application on calcareous soil. Field Crop Res 197:74–82
Zhang W, Chen XX, Liu YM, Liu DY, Chen XP, Zou CQ (2017a) Zinc uptake by roots and accumulation in maize plants as affected by phosphorus application and arbuscular mycorrhizal colonization. Plant Soil 413:59–79
Zhang W, Liu DY, Li C, Chen XP, Zou CQ (2017b) Accumulation, partitioning, and bioavailability of micronutrients in summer maize as affected by phosphorus supply. Eur J Agron 86:48–59
Zhu YG, Smith SE, Smith FA (2001) Zinc (Zn)-phosphorus (P) interactions in two cultivars of spring wheat (Triticum aestivum L.) differing in P uptake efficiency. Ann Bot-London 88:941–945
Acknowledgements
The work was supported by the National Natural Science Foundation of China (31672240, 31272252), the 973 project (2015CB150402), and the innovative group grant of NSFC (31421092). We thank reviewers great contributions to the improvement of the manuscript and Dr. Bruce Jaffee from USA for reviewing and improving the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philip John White.
Electronic supplementary material
ESM 1
(DOCX 442 kb)
Rights and permissions
About this article
Cite this article
Chen, XX., Zhang, W., Wang, Q. et al. Zinc nutrition of wheat in response to application of phosphorus to a calcareous soil and an acid soil. Plant Soil 434, 139–150 (2019). https://doi.org/10.1007/s11104-018-3820-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3820-5