Abstract
Aims
As an important contributor to carbon (C) flux in the global C cycle, fine root litter in forests has the potential to be affected by the elevated nitrogen (N) deposition observed globally. However, the direct effects (on current fine root decomposition) and indirect effects (on root quality and subsequent decomposition) of N deposition on fine root decomposition are poorly understood.
Methods
We conducted a 5-year field experiment in a Pleioblastus amarus bamboo forest in southwestern China. In the first 3 years of the experiment, N-treated sites (0, 50, 150, and 300 kg N ha−1 year−1, respectively) and fine roots under two N regimes (0 and 150 kg N ha−1 year−1; 0 N-Root/+N-Root, respectively) were prepared. Next, these two fine root treatments were applied to a 2-year decomposition experiment in the N-treated sites under continuous N treatment.
Results
Nitrogen additions increased fine root density, concentrations of N, P, and lignin in fine roots, and concentrations of TOC, TN, NH4 +-N and NO3 −-N in the soil, and decreased the soil pH. The decomposition constant k decreased under N addition treatments, and the decomposition rate of + N-Root was lower than that of 0 N-Root, suggesting that both the direct and indirect effects of N additions on fine root decomposition rates were negative. Both N addition and root substrate changes led to an increase in the residual lignin content during decomposition. Nitrogen additions significantly decreased the loss of C, N, P, K, Ca and Mg during decomposition.
Conclusions
The changes in the soil environment and increased root lignin concentration as a result of N additions may be the mechanism underlying the negative direct and indirect effects observed. Elevated fine root biomass input and slower degradation rates may be a potential mechanism explaining the increase in soil TOC and TN under N treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last two centuries, food and energy production, as well as other human activities, have substantially increased anthropogenic reactive nitrogen (N) production and deposition (Vitousek et al. 1997; Galloway et al. 2004; Denman et al. 2007), and are currently estimated to add more than 200 Tg yr−1 of N to terrestrial and aquatic ecosystems worldwide (Galloway et al. 2008). The amount of atmospheric N deposition is expected to increase over the next century, in view of continuing global development and industrialization (Dentener et al. 2006). The largest increases are forecast to occur in East and South Asia (Reay et al. 2008; Liu et al. 2013). In the 2000s, the average N deposition over China was 21.1 kg N ha−1 year−1 (Liu et al. 2013). Evidence indicates that increasing N deposition significantly impacts the properties of many terrestrial and aquatic ecosystems (NRC 1996; Vitousek et al. 1997; Aber et al. 1998; Matson et al. 2002; Rabalais 2002; Baron et al. 2013). In the context of global changes that involve global warming and increasing atmospheric carbon dioxide (CO2) concentrations, one of the major scientific issues regarding elevated atmospheric N deposition is to understand how it alters carbon (C) cycling and storage.
As an important component of the global C budget, forest litter decomposition regulates the transfer of C and nutrients to the soil, and is greatly affected by the elevated N deposition observed globally (Knorr et al. 2005). Nitrogen additions have shown variable effects on forest leaf litter decomposition, including decreases (Magill and Aber 1998; Fang et al. 2007; Hobbie, 2008), increases (Hobbie and Vitousek 2000; Ludovici and Kress 2006; Kaspari et al. 2008), or no effects on decomposition rates (Hobbie and Vitousek 2000). The response of litter decomposition to N additions is primarily controlled by the N addition rate, the background atmospheric deposition of N, and litter substrate quality (Knorr et al. 2005). It should be noted that most studies regarding the response of forest litter decomposition to N additions focused on aboveground litter (Berg and Matzner 1997; Knorr et al. 2005; Hobbie 2008; Song et al. 2013). To our knowledge, the effects of N additions on the decomposition of forest fine root litter have received less attention.
Fine roots (< 2 mm in diameter) are important structural and functional components of forest ecosystems (Fitter 2002). Fine roots play an important role in net primary production (NPP) in terrestrial ecosystems (Gill and Jackson 2000). Assuming conservatively that fine roots turn over once per year, they represent 33 % of terrestrial annual NPP (Jackson et al. 1997). The fluxes of C and N through fine root turnover may exceed the proportion of aboveground fluxes of these nutrients (Nadelhoffer and Raich 1992). Despite the challenges of estimating the rates of root decomposition, there exists an increasing corpus of literature on fine root decomposition in many forest ecosystems (Silver and Miya 2001; Lin et al. 2011; Sun et al. 2013). However, none of these studies were conducted under N addition treatments. Root turnover often plays a crucial role in underground C sequestration and nutrient cycling, and is closely related to many ecosystem processes and properties such as soil respiration (Mo et al. 2007; Cleveland and Townsend 2006; Jia et al. 2010; Tu et al. 2013a), above ground litter decomposition (Hobbie 2008; Song et al. 2013), soil enzyme activities (Carreiro et al. 2000; Keeler et al. 2009), soil chemistry (Ring et al. 2011), and soil detrital food webs (Gan et al. 2013), all of which are significantly affected by N deposition. Therefore, N deposition seems certain to have an impact on root turnover processes.
The effects of N deposition on litter decomposition can be divided into direct and indirect effects (Berg and Matzner 1997). Direct effects involve the decomposition of existing litter, while indirect effects involve the substrate quality of fresh litter and its subsequent decomposition. Numerous studies have conducted N addition experiments in different ecosystems to investigate the direct effects of N deposition on plant litter decomposition (Magill and Aber 1998; Fang et al. 2007; Hobbie, 2008; Tu et al. 2014a). Atmospheric N deposition is a long-term and continuous process, with substantial effects on substrate chemistry. For example, many studies have found that N deposition or N addition affects the concentrations of N, P, S, Ca, K, C, lignin and tannins in aboveground litter (Pregitzer and Burton 1992; Berg and Matzner 1997; Huttunen et al. 2009; Nikula et al. 2010); a similar phenomenon also occurs in fine roots (Helmisaari et al. 2009; Tu et al. 2011; Wang et al. 2012). Changes in substrate quality resulting from N additions are similar to those resulting from natural variation among litter types (Berg and Matzner 1997). Decomposition rates may increase if N additions increase low-molecular compounds (such as amino acids) in litter, while if N addition increase recalcitrant compounds such as lignin, decomposition rates of litters may be inhibited. However, Berg and Matzner (1997) hypothesized that increases in tissue N as a result of long-term N additions may increase decomposition rate in early stages, and retard lignin degradation and thus mass loss in later stages. At a global scale, root chemistry is the dominant factor controlling decomposition rates (Silver and Miya 2001; Zhang et al. 2008). Furthermore, substrate quality is one of the most important factors determining how litter decomposition responds to N additions (Knorr et al. 2005). Therefore, changes in fine root substrate quality caused by N deposition are bound to affect subsequent decomposition indirectly. It is probable that these indirect effects are more pronounced in regions with high N deposition rates relative to regions with low N deposition rates; however, this aspect has not been studied in detail.
In terms of spatial variability, the greatest increments in N deposition are predicted to occur in areas that currently experience high N deposition rates (Galloway et al. 2004). At present, southern China is experiencing rates of N deposition that are well above the global average (Jia et al. 2010; Hu et al. 2007; Zhao et al. 2009; Fang et al. 2011; Liu et al. 2013). In previous studies, we measured atmospheric N deposition in the region encompassing the western edge of the Sichuan Basin (southern China), over a 3 year period. The results of those studies indicated that wet deposition is the dominant N flux of N deposition in this region, ranged up to 95 kg N ha−1 year−1 (Xu et al. 2013 ; Tu et al. 2013b), which is considerably higher than the mean N deposition across China (15.75 kg N ha−1 year−1, Liu et al. 2013), Southeast China (30.49–37.37 kg N ha−1 year−1, Cui et al. 2014), and is also significantly higher than the mean reported across 50 forest ecosystems in China (16.6 kg N ha−1 year−1, Fang et al. 2011). To our knowledge, the N deposition rate in this region is likely on the highest level in the world, because this region borders one of the most important industrial-agricultural economic regions of China, with very highly annual precipitation (colloquially referred to as the “rainy zone of western China”). Furthermore, over the next few decades, the largest increases in N deposition in the world are projected to occur in southern China (Reay et al. 2008). This part of China is one of the major cultivation and distribution centers for bamboo (FAO 2010; SFAPRC 2010). Bamboo forests are one of the most important forest types in China (FAO 2010), contributing to about 10 % of the C stocks in the live biomass of Chinese forests (Chen et al. 2009). As such, bamboo forests play an important role in regional and global C cycles. In our previous studies, we conducted several N addition experiments in bamboo forests, with the finding that short-term exogenous N input continued to significantly affect the processes of soil respiration (Tu et al. 2013a), aboveground litter decomposition (Tu et al. 2014a), and C sequestration (Tu et al. 2011).
To evaluate the responses of fine root decomposition to elevated atmospheric N deposition, we conducted N addition treatments in a subtropical bamboo (Pleioblastus amarus) plantation over the course of 3 years. We tested the hypotheses that: (1) N additions inhibit fine root decomposition as a consequence of the potentially inhibitory effects of exogenous inorganic N on lignin decay (Berg and Laskowski 2006) (i.e. the direct effect of N deposition on root decomposition is negative); and (2) N additions may change the substrate quality such as increased N concentrations in fine roots, and N additions may stimulate consequent root decomposition in the early stages and inhibit the degradation in the later stages.
Materials and methods
Site description
The study was carried out in Liujiang, Sichuan, China (29°42′ N, 103°14′ E), at an elevation of about 600 m a.s.l.; this area experiences an elevation-modified humid subtropical climate. The annual mean relative humidity is 86 %, and the monthly mean temperature ranges from 6.6 °C in January to 25.7 °C in July. The mean annual precipitation (1980–2000 records) is 1490 mm. The annual atmospheric wet N deposition was 95 kg N ha−1 year−1 in the study site during the period from 2008 to 2010 (Tu et al. 2014a). Even under the background of such high level of N deposition rates, N addition still stimulated plant growth in this site, i.e. the plants’ growth in this site is N limited in the present state. The annual frost-free period ranges from 352 to 360 days. The study site (10 ha) was converted from cropland to a P. amarus plantation in 2000 as part of the National Project of Converting Farmland to Forests (NPCFF). This project has spearheaded the planting of P. amarus, a major bamboo species in the NPCFF, over a large area in this region. The mean density of the study stand was 52, 200 stems ha−1 in November 2007; and the mean diameter at breast height was 2.3 cm, with a canopy density of about 0.9. The soil at the site is classified as a Lithic Dystrudepts according to USDA Soil Taxonomy, derived from purple sandstone and shale, with a depth of about 100 cm and a pH around 4.6 (0–20 cm soil horizon, H2O extraction). In November 2007, the aboveground dry biomass was 25.4 kg m−2, and the surface organic layer was generally about 1 cm thick. There are very little shrubs or herbs in the understories of the stands studied. Additional details on soil properties in this area were presented in Tu et al. (2011).
In October 2007, a root decomposition plot (40 m × 40 m) and six root collection plots (10 m × 10 m) were established in the P. amarus plantation site. In the root decomposition plot, 12 sub-plots (3 m × 3 m) were established and randomly located throughout the plot, with a minimum of 5 m was between sub-plots. The sub-plots were randomly allocated to four treatments: control (CK, no N added), low-N (LN, 50 kg N ha−1 year−1), medium-N (MN, 150 kg N ha−1 year−1), and high-N (HN, 300 kg N ha−1 year−1), with three replicates for each treatment. The six root collection plots received two treatments (three plots each treatment): 0 N (no N added) and + N (150 kg N ha−1 year−1). Fertilizer additions ammonium nitrate (NH4NO3) occurred monthly in 12 equal applications beginning in November 2007. During each application, the fertilizer was weighed, dissolved in 1 L of water, and applied to each plot using a portable sprayer. The control plot received 1 L water without fertilizer. Addition of N to root collection plots lasted 3 years (November 2007 to October 2010); and N addition in root decomposition plot lasted 5 years (November 2007 to November 2012).
After 3 years of N additions (October 2010), P. amarus roots (which tend to be <2 mm in diameter in this species) were collected from the top 20 cm of the soil in all six root collection plots (three 0 N plots and three + N plots). All the roots were quickly washed in tap water and then in deionized water to remove adhered soil particles, after which they were air-dried for several days at room temperature to constant weight. Fine roots (<2 mm in diameter) were cut into pieces about 5 cm long. The fine roots derived from 0 N and + N plots are referred to as 0 N-Root and + N-Root, respectively, hereafter. For both treatments, fine root sub-samples were assessed to determine the initial water content and chemical properties (lignin, C, N, P, Ca, and Mg concentrations). Simultaneously, the fine root density was determined by collecting soil samples using a soil corer (8 cm in diameter and 20 cm deep). Ten cores were randomly collected from each root collection plot to yield one composite sample. Roots were weighed after separation by washing and sieving to a size of 2 mm, dried at 65 °C for 48 h, and then weighed (Powers et al. 2005).
Fine root decomposition
The decomposition experiment was conducted in the 12 aforementioned subplots of the root decomposition plot under four levels of N addition treatments. Fine root decomposition was measured using the standard litter-bag technique (Falconer et al. 1933). Briefly, a known amount of air-dried fine roots (5.00 g) was placed into a litter bag (10 × 10 cm), constructed from 100 μm nylon mesh. The bags were sewn closed, after which the bags were connected together, in groups of six, about 0.5 m away from each other, with a piece of nylon string. These six bags comprised three replicate bags for each of the two root treatments. Altogether, 288 bags were prepared for each root treatment. Litterbags were placed in the root decomposition plots in late November, 2010. This was accomplished by creating an incision in the soil at an angle of 45° to a depth of 10 cm where the bags were buried. Eight groups of litterbags were placed in each plot (96 groups in total).
One group of bags was collected from each plot at 3-month intervals for 2 years. Nine litterbags were collected for each N treatment and root type on each collection trip. The bags were air-dried and then cut open on all sides. Adherent soil and other extraneous materials were gently removed using a fine brush. The samples were then dried at 65 °C for 48 h, after which their dry weights were recorded. Root samples for initial and residual chemistry analysis were ground in a Wiley mill with a 1 mm mesh screen.
Chemical analyses
Lignin concentrations (mg g−1) in the litter were determined using the acid detergent fiber (ADF) method (Rowland and Roberts 1994). Carbon concentrations (mg g−1) in the litter were measured using the dry combustion method (Nelson and Sommers 1982). The total N concentration (mg g−1) in the litter was determined through acid digestion. Briefly, a 200-mg sample was digested in 5 ml of 1.84 g ml−1 (18.4 M) H2SO4 and then distilled using a UDK 142 automatic distillation unit (VELP, Milan, Italy). For the determination of P in the litter, samples were subjected to triple-acid digestion (nitric, perchloric, and sulfuric acid, 5:1:1, v/v/v) (Jackson 1958). Total P (mg g−1) was determined colorimetrically in digested samples using the ammonium molybdate/stannous chloride method (Olsen and Sommers 1982). The concentrations of K, Ca, and Mg (mg g−1) in the litter were analyzed using an atomic absorption spectrophotometer (TAS-986, PGENERAL, Beijing, China) following perchloric acid-nitric acid (HClO4-HNO3) digestion (Jones and Case 1990). Their concentrations were expressed per unit of oven dried sample (65 °C).
In June, 2012, five cores (2 cm in diameter and 20 cm deep) were collected from each N addition experimental plot. For each plot, composite samples were prepared through gentle mixing, and visible roots were removed using tweezers. Next, the soil samples were homogenized, passed through a 2 mm sieve, and stored at 4 °C until analysis (within 1 week). Soil pH was measured with a glass electrode in a 1:2 mixture (w/w) of soil and water. Soil TOC (mg g−1) was measured by the dichromate digestion method (Kalembasa and Jenkinson 1973). Total N concentration (mg g−1) in the soil was determined through acid digestion, using the Kjeldahl method (Grimshaw et al. 1989). A 5-g sample was digested in 5 ml of 1.84 g ml−1 H2SO4 and a 1.0-g mixture of potassium sulfate (K2SO4), copper sulfate (CuSO4), and selenium powder (100:10:1, w/w/w), and then distilled using a UDK 142 automatic distillation unit (VELP, Milano, Italy). Available ammonium-N and nitrate-N (mg g−1) was extracted with 2 M KCl solution and their concentrations were measured by colorimetry.
Data analyses
All statistical analyses were conducted using SPSS 15.0 for Windows (SPSS Inc. Chicago, USA). The percentage remaining (R) of mass, lignin and elements (C, N, P, K, Ca and Mg) for each period (X i ) was determined and compared to the initial values (X 0) using the formula: %R = (X i / X 0) × 100. The residual substrate mass, as a proportion of the initial mass, was plotted against time, using a single-exponent decomposition model, X = e –kt (Olson 1963), where X is the fraction of initial mass remaining at time t (year), and k (year−1) is the decomposition constant. The relationship between residual lignin and residual mass was measured using linear regression. Data from the two treatments of roots were pooled, yielding a sample size of 192 (two types roots × 4 N treatments × 8 sampling times × 3 replicate plots) for this aspect of the analysis.
To determine the effects of N additions on litter decomposition, repeated measures ANOVAs (RM-ANOVA) (N addition as main effect and time as within subject factor) were conducted to examine the percentages of residual mass, lignin, and elements, as well as trends in lignin concentrations, among the different N addition treatments. When Mauchly’s test of sphericity was not assumed, the data was adjusted by Greenhouse-Geisser method. To investigate N addition and root substrate type effects on root litter decomposition, the k value and final residual amounts of mass, lignin and elements were compared among root treatments and N treatments using two-way ANOVA.
Statistical differences in root density and root chemical properties (concentrations of C, N, P, K, Ca, Mg and lignin) between 0 N-Root and + N-Root were tested using a t-test. A one-way ANOVA was applied with Fisher’s LSD test for testing significant differences among treatments with respect to soil pH, TOC, TN, NH4 +- N and NO3 −- N.
Results
Effect of N additions on root chemistry
Following three consecutive years of N additions, significant changes in fine root chemistry were observed (Table 1). Nitrogen additions significantly decreased fine root C concentrations, but increased N and P concentrations. Although the mean lignin concentration was 7 % higher in HN than that in CK, this was not statistically significant.
Litter mass loss and k value of root decomposition
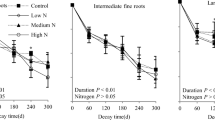
A single-exponent decomposition model generated a good fit for plotting the fraction of initial mass remaining in each root litter substrate and treatment as a function of time (P < 0.001) (Fig. 1a). Following 2 years of decomposition, the mass losses of 0 N-Root and + N-Root ranged from 54 to 69 %, and 53 to 64 %, respectively. Nitrogen additions significantly inhibited mass loss for both root treatments (P < 0.001). Both N additions and root substrate type effects significantly affected the k value and final residual mass content after 2 years of decomposition (Fig. 2a and b). The k value decreased with increases in N additions until the MN treatment; furthermore, the decomposition rate of + N-Root was significantly lower than that of 0 N-Root. The T 50% and T 95% values increased, respectively, by 0.2–0.5 years and 0.9–2.1 years for 0 N-Root, and by 0.2–0.4 year and 0.8–1.8 years for + N-Root (Table 2).
Percentages of mass (a) and lignin (b) remaining, and lignin concentrations of decomposing roots of Pleioblastus amarus (c), in different N treatments for each root type, in a 2-year (November 2010 to November 2012) root decomposition experiment. 0 N-Root and + N-Root are fine roots collected in plots received two N treatments for 3 years (November 2007 to October 2010): 0 N (no N added) and + N (150 kg N ha−1 year−1), respectively. CK, LN, MN and HN denote four treatments in the root decomposition plots: control (0 kg N ha−1 year−1), low nitrogen (50 kg N ha−1 year−1), medium nitrogen (150 kg N ha−1 year−1) and high nitrogen (300 kg N ha−1 year−1), respectively; and monthly N additions applied for 5 years from November 2007. Values are means ± SE. The results of repeated measures ANOVAs are shown for each parameter. * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001
Decomposition rate (k), and remaining mass of C, N, P, K, Ca and Mg in decomposing roots in each treatment after 2 years of decomposition. Results of two-way ANOVAs (with N additions and root types as main effects) are shown as “Nitrogen”, “Substrate” and “Nitrogen × Substrate” for each parameter. * P < 0.05; ** P < 0.01; *** P < 0.001. Different lowercase and uppercase letters indicate effect is significant among N addition treatments for 0 N-Root and + N-Root, respectively (P < 0.05, Fisher’s least significant difference test). Values are means + SE
Lignin dynamics during root decomposition
The dynamics of residual lignin was similar to those for residual mass i.e., N additions significantly decreased residual lignin content (Fig. 1b). The results of the two-way ANOVA indicated that N additions and root substrate type contributed significantly to increasing the residual lignin content after 2 years of decomposition (Fig. 2c). The lignin concentrations in root litter increased in the first 6 months, and decreased quickly in the 12 subsequent months (Fig. 1c). Lignin concentrations in both root treatments, across all the N treatments, generally stabilized at this point, decreasing very slowly in the remaining 6 months of the experiment. Further, there was a significant, positive, linear relationship between residual lignin and residual mass, based on the means of all root and N treatments (Fig. 3).
Elements dynamics during root decomposition
All the six elements assessed in this study exhibited significantly temporal patterns (Figs. 4 and 5). Three distinct patterns were observed during root decomposition. First, residual C in both root treatments decreased with time (Fig. 4a). Second, residual mass of N, P, Ca and Mg exhibited a loss pattern characterized by a rapid initial mass decrease followed by a more gradual decrease (Figs. 4b, c and 5b, c). More than 40 % of N and P, more than 60 % of Ca and more than 50 % of Mg was lost in the four initial months of decomposition. Subsequently, they were released very slowly into the environment through the rest of the study period. Finally, K exhibited a unique pattern in that its concentration decreased rapidly initially, and then decreased slowly in the intermediate stages of decomposition. Towards the end of the process, it began to accumulate in the litter (Fig. 5a). RM-ANOVA tests indicated that N additions significantly affected residual C and N in both 0 N-Root and + N-Root, and also affected residual Ca and Mg in 0 N-Root. A two-way ANOVA of the residual amounts of all six elements indicated that N additions significantly decreased the losses of all these elements, while, the proportions of these elements remaining, after decomposition for 2 years, were significantly different between 0 N-Root and + N-Root (Fig. 2d, e, f, g, h and i). The results of two-way ANOVA tests on absolute concentrations of elements after 2 years of decomposition showed that N addition affected P, K, Ca and Mg concentrations, and root type only affected final K and Mg concentrations (Fig. 6).
Root density and soil properties under N addition treatments
After 3 years of N additions at the rate of 150 kg N ha−1 year−1, fine root density was increased by 273 g m−2 (P < 0.001; Fig. 7a). The soil pH value decreased with the increase of the amount of N addition, with CK and LN being significant from HN (Fig. 7b). Soil TOC increased under N addition treatments, with TOC concentrations in LN and HN being significantly higher than in CK (Fig. 7c). For total N and NH4 +-N, N additions had a significant effect only in the HN treatment (Fig. 7d and e). The concentration of NO3 −-N increased by 23 to 90 % in the N treatments, relative to the control, with MN and HN having significantly higher values than the CK and LN treatments (Fig. 7f).
Root densities and soil properties in different treatments. Values are means + SE. The P value of a t-test is shown for root density. Results of a one-way ANOVA with Fisher’s LSD test are indicated: means of treatments with the same superscript are not significantly different (P < 0.05). All soil and root properties were determined in the 0–20 cm soil horizon. Root densities were measured root collect plots in October 2010 (i.e. after 3 years of N addition). Soil properties were measured based on soil samples collected in root decomposition plots in June 2012 (i.e. after 55 months of N additions)
Discussion
Confirming our first hypothesis, N additions significantly decreased the fine root decomposition rate. In general, the main factors affecting the decomposition rate vary according to the phase of decomposition (Berg and McClaugherty 2008). The degradation of soluble and low-molecular-weight compounds dominates the early stages of litter decomposition. Next, hemicelluloses and cellulose degradation is the dominant activity, and finally, lignin degradation becomes dominant (Berg and McClaugherty 2008). In our study, two evident stages of decomposition with different mass loss rates were observed. In the first 6 months, mass loss ranged from 30 to 50 %, while, very slow decomposition rates were observed in the following 18 months. A similar phenomenon was observed in fine root decomposition experiments in a subtropical Cunninghamia lanceolata and Tsoongiodendron odonum mixed forest (Yang et al. 2004). In our study, lignin was the most important components of fine roots (about 40 %); the inhibitory effect of exogenous inorganic N on lignin degradation may be the main reason for the observed decrease in fine root decomposition rates. After the first 6 months of decomposition, the relative concentration of lignin in fine roots attained approximately 50 %, in all treatments. The residual mass of fine roots was closely related to the amount of residual lignin; the latter explained 91 % of the variation in residual mass, which suggests that lignin may play a critical role in influencing fine root mass loss. Many previous studies on aboveground litter decomposition demonstrated that N additions inhibit lignin decomposition (Magill and Aber 1998; Fog 1988; Berg and Matzner 1997), and consequently, the loss in litter mass (Magill and Aber 1998; Carreiro et al. 2000; Hobbie, 2008). However, these studies were all based on leaf litter decomposition, which have much lower concentrations of lignin than do fine roots (Ostertag and Hobbie 1999; Gholz et al. 2000). This trait has often been considered to be one of the key reasons for the slower degradation rates in root litter compared to foliage litter (Fujimaki et al. 2008). Therefore, the mechanisms of the effects of N additions on root lignin may not be the same as those for leaves.
The following mechanisms may be involved in the inhibitory effects of exogenous inorganic N on lignin decay. First, N additions may reduce lignolytic enzyme production and decomposer efficiency. White-rot organisms (Basidiomycetes) and soft-rot organisms (Ascomycetes) are two types of aerobic fungi that are involved in breaking down lignin. The optimum pH ranges for the growth of Basidiomycetes and Ascomycetes are 4.0–5.0 and 6.0–7.5 respectively (Sinsabaugh 2010). After 4.5 years of N additions, the pH of the surface soil decreased from 4.46 (CK) to 4.12 (HN) in this study. Therefore, Basidiomycetes may be the main lignin-degrading fungal community in this ecosystem. In general, degradation carried out by Basidiomycetes tends to proceed to completion, whereas Ascomycetes are unable to degrade the lignin completely (Keyser et al. 1978). We may explain the decrease of soil pH value as the result of the loss of base cations under N additions (Tu et al. unpublished results). These changes in soil pH may have affected lignin-degrading decomposer efficiencies and reduced the production of lignolytic enzymes. In an adjacent bamboo (Bambusa pervariabilis × Dendrocalamopsis daii) stand (grown on an identical soil type, about 1 km apart from the stand in this study), we conducted a 2-year N addition experiment, revealing that N additions significantly depressed the activities of two important lignin-degrading enzymes, polyphenol oxidase and peroxidase, in the surface soil horizon (0–20 cm) (Tu et al. 2014b). Previous studies reported that Basidiomycetes do not synthesize lignin-degrading enzymes in the presence of ammonium and other low molecular weight N-rich compounds such as amino acids (Keyser et al. 1978). As a matter of fact, numerous studies have demonstrated that the inhibitory effects of high N environments on lignolytic enzyme production are widespread (Berg and Matzner 1997; Carreiro et al. 2000; Waldrop et al. 2004; Tu et al. 2014b). Second, Berg (1986) hypothesized that lignin decomposition intermediate products react with inorganic N to form additional compounds that are resistant to decomposition, during the stages controlled by lignin degradation. In our study, the higher N retention by fine roots, under both control and N addition treatments, observed after 2 years of decomposition, may be explained by the interaction of exogenous N with residual lignin. Lastly, cellulose in fine roots is generally shielded from degradation by lignin polymers (Berg and Laskowski 2006), and thus, the inhibition of lignin decomposition would also decrease cellulose degradation and mass loss. In our previous studies, we found that N additions suppressed the degradation of both lignin and cellulose in several aboveground litter types in forest ecosystems (Tu et al. 2014a).
Confirming our second hypothesis partly, N additions significantly changed the substrate quality of fine roots, and N additions inhibited the degradation in the later stages, while we did not observe increase decomposition rates in the early stages. We found that the decomposition rate calculated based on the 2 years period of + N-Root was significantly lower than that of 0 N-Root, indicating that substrate changes caused by N additions were responsible for the reduction in the decomposition rate. Roots are exposed to a different decomposition environment than aboveground litters, and root chemistry appears to be the primary controller of root decomposition on a global scale, in contrast to leaf litter decomposition (Silver and Miya 2001). A series of studies on fine roots suggested that lignin and nutrient concentrations are the main controllers of root decomposition (Silver and Miya 2001; Fujii and Takeda 2010; Sun et al. 2013). Similar to many fertilization experiments (Helmisaari et al. 2009; Wang et al. 2012), we observed higher concentrations of N and P in the fine roots after N additions, which may lead to greater decomposition rates as shown by other studies (Silver & Miya 2001; Zhang et al. 2008; Birouste et al. 2011). Our data contradict the hypothesis resulting from these two literature reviews on root litter and leaf litter, respectively. We may explain this phenomenon as the result of the following two reasons. First, higher tissue N may slow down decomposition rates. Berg and Matzner (1997) hypothesized that increases in tissue N in leaf litter as a result of long-term N deposition may have two consequences: an initially rapid decomposition rate, followed by a significantly slower rate of degradation in later stages. This hypothesis is based on the fact that lignin only controls the decomposition rate of leaf litter in later stages. However, many studies demonstrated a higher lignin concentration in root litter compared with leaf litter (Ostertag and Hobbie 1999; Fujii and Takeda 2010; Hobbie et al. 2010). Indeed, the lignin concentration was very high in fine roots of this bamboo species. We hypothesize that lignin control the decomposition rates not only in the later stages, but also in the early stages of decomposition. We observed that the residual mass and lignin concentrations were higher in + N-Root than those in 0 N-Root throughout the entire decomposition period. The higher tissue N concentration may strengthen the reaction of N and lignin degradation intermediate products. Second, higher tissue lignin may inhibit decomposition directly. The increase in lignin concentration in fine roots (although not statistically significant) as a result of N additions in the present study is similar to the results of a fertilization experiment conducted in Pinus sylvestris and Picea abies plantations (Berg and Staaf 1980). The inhibitory effect of exogenous N on lignin degradation has been suggested to be more prominent in forests characterized by higher-lignin species (Waldrop et al. 2004). Our study supports this observation, in that residual lignin masses after 2 years of decomposition were higher in + N-Root than in 0 N-Root, across all N treatments.
Following 3 years of N additions, fine root biomass in the P. amarus stand increased, similar to what has been observed in many forest ecosystems (Pregitzer and Burton 1992; Zogg et al. 1996; Burton et al. 2002; Lee and Jose 2003; Cleveland and Townsend 2006). Conversely, many other studies found that N additions decreased forest fine root biomass (Bowden et al. 2004; Mo et al. 2007; Jourdan et al. 2008). Tateno et al. (2004) found a significant relationship between soil N availability and fine root production in temperate forests. Root biomass generally exhibits a linear relationship with aboveground plant biomass (Cairns et al. 1997; Norby et al. 2004), so as NPP increases, plant root growth increases accordingly. In a previous study at the same site, we found that NPP (Tu et al. 2011) and aboveground litterfall (Xiao et al. 2013) increased under N additions. In general, in forested ecosystems, C distribution from plants to soil is predominantly accomplished through plant roots, particularly fine roots (Van Groenigen et al. 2006), with C inputs being primarily in the form of active rhizospheric deposits (Boddy et al. 2007). Thus, all the changes typically associated with fine root production, mortality and decomposition may impact the C pool in forest soils. Increases in fine root biomass may increase inputs of low-molecular-weight C compounds to soil, and thus increase the soil C concentration as we observed in this study. Furthermore, we found N concentration in fine roots increased under N treatments, it may imply shortened life-span and accelerated root turnover (Hendricks et al. 1993). In general, nutrient retrieval during fine root senescence tends to be very low (Nambiar and Fife 1991). Aboveground litterfall and dying fine roots are the primary plant residue inputs to forest soil (Van Groenigen et al. 2006). We hypothesize that C and nutrient inputs to soil increase in response to increases in the mass of aboveground litterfall and speed of turnover of fine roots. Corroborating this, we found that soil TOC increased significantly under N addition treatments, although this increase was small. Additionally, decreases in decomposition rates, in conjunction with increased lignin accumulation for aboveground litter (Tu et al. 2014a) and fine roots under N additions, suggest that the pool of stable soil organic C in this ecosystem may increase via these mechanisms.
The atmospheric wet N deposition rate is very high (95 kg N ha−1 year−1, Tu et al. 2014a) at the study site, relative to the global average. Ecosystem health may be significantly threatened when atmospheric N deposition exceeds 20 kg N ha−1 year−1 (Bobbink et al. 2010). Therefore, high N deposition may induce a cascade of negative effects on ecosystems in this region. Surprisingly, several studies revealed that such high N deposition rates have not yet resulted in any evident negative consequences for local ecosystems (Tu et al. 2011; Tu et al. 2013b). This could be attributed to the fact that these studies are relatively recent and negative effects may simply have not occurred yet. Open field N deposition through precipitation is main mechanism of reactive N input in this ecosystem (Tu et al. 2013b), but experimental N additions still stimulated plant growth during the first 2 years (Tu et al. 2011). It is possible that soil N availability was low at this site and was insufficient for the rapid growth rate of the studied bamboo species (Tu et al. 2013b). Since annual global atmospheric wet N deposition is forecast to increase further (Reay et al. 2008), local forest ecosystems similar to the study site can be expected to become N-saturated in the near future. There remains uncertainty regarding the long-term effects of chronic N deposition on C cycling in forest ecosystems in this region.
Overall, our study demonstrated that N additions decreased the decomposition rate of fine roots through direct inhibition of lignin degradation and indirect effects on fine root substrates. Elevated fine root biomass input and slower degradation rate may be the potential mechanisms explaining the increase in soil TOC and TN under N treatments. However, the long term status of C cycling and storage in this bamboo forest remains uncertain, given the continuous increases in annual N deposition. Further long-term studies regarding C cycling under different ambient N deposition rates are necessary to supplement our knowledge regarding how forest C processes respond to N deposition.
References
Aber JD, McDowell W, Nadelhoffer K, Magill A, Berntson G, Kamakea M, McNulty S, Currie W, Rustad L, Fernandez I (1998) Nitrogen saturation in temperate forest ecosystems. Biosci 48(11):921–934
Baron JS, Hall EK, Nolan BT, Finlay JC, Bernhardt ES, Harrison JA, Chan F, Boyer EW (2013) The interactive effects of excess reactive nitrogen and climate change on aquatic ecosystems and water resources of the United States. Biogeochemistry 114:71–92. doi:10.1007/s10533-012-9788-y
Berg B (1986) Nutrient release from litter and humus in coniferous forest soils – a mini review. Scand J Forest Res 1(1–4):359–369
Berg B, Laskowski R (2006) Litter decomposition: A guide to carbon and nutrient turnover. Elsevier Ltd., Burlington
Berg B, Matzner E (1997) Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ Rev 5:1–25
Berg B, McClaugherty C (2008) Plant litter: Decomposition, humus formation, carbon sequestration, 2nd edn. Springer, Heidelberg
Berg B, Staaf H (1980) Decomposition rate and chemical changes of scots pine needle litter II. Influence of chemical composition. Ecol Bull 32:373–390
Birouste M, Kazakou E, Blanchard A, Roumet C (2011) Plant traits and decomposition: are the relationships for roots comparable to those for leaves? Ann Bot. doi:10.1093/aob/mcr297
Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, Erisman J-M, Fenn M, Gilliam F, Nordin A, Pardo L, De Vries W (2010) Global assessment of nitrogen deposition on plant diversity : a synthesis. Ecol Appl 20:30–59. doi:10.1890/08-1140.1
Boddy E, Hill PW, Farrar J, Jones DL (2007) Fast turnover of low molecular weight components of the dissolved organic carbon pool of temperate grassland field soils. Soil Biol Biochem 39:827–835. doi:10.1016/j.soilbio.2006.09.030
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P (2004) Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. Forest Ecol Manag 196:43–56. doi:10.1016/j.foreco.2004.03.011
Burton AJ, Pregitzer KS, Reuss RW, Hendrick RL, Allen MF (2002) Root respiration in North American forests: effects of nitrogen concentration and temperature across biomes. Oecologia 131:559–568. doi:10.1007/s00442-002-0931-7
Cairns MA, Brown S, Helmer EH, Baumgardner GA (1997) Root biomass allocation in the world’s upland forests. Oecologia 111:1–11. doi:10.1007/s004420050201
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecol 81:2359–2365. doi:10.1890/0012-9658(2000)081[2359:MESELD]2.0.CO;2
Chen X, Zhang X, Zhang Y, Booth T, He X (2009) Changes of carbon stocks in bamboo stands in China during 100 years. Forest Ecol Manag 258:1489–1496. doi:10.1016/j.foreco.2009.06.051
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci U S A 103:10316–10321. doi:10.1073/pnas.0600989103
Cui J, Zhou J, Peng Y, He Y, Yang H, Mao J, Zhang M, Wang Y, Wang S (2014) Atmospheric wet deposition of nitrogen and sulfur in the agroecosystem in developing and developed areas of Southeastern China. Atmosph Envrion 89:102–108. doi:10.1016/j.atmosenv.2014.02.007
Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, Hauglustaine D, Heinze C, Holland E, Jacob D, Lohmann U, Ramachandran S, da Silva Dias PL, Wofsy SC, Zhang X (2007) Couplings between changes in the climate system and biogeochemistry. In: Solomon S, Qin D, Manning M, Marquis M, Averyt K, Tignor MMB, Miller HLR, Chen Z (eds) Climate change 2007, the physical science basis. Contribution of working group i to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 499–587
Dentener F, Drevet J, Lamarque JF, Bey I, Eickhout B, Fiore AM, Hauglustaine D, Horowitz LW, Krol M, Kulshrestha UC, Lawrence M, Galy-Lacaux C, Rast S, Shindell D, Stevenson D, Van Noije T, Atherton C, Bell N, Bergman D, Butler T, Cofala J, Collins B, Doherty R, Ellingsen K, Galloway J, Gauss M, Montanaro V, Müller JF, Pitari G, Rodriguez J, Sanderson M, Solmon F, Strahan S, Schultz M, Sudo K, Szopa S, Wild O (2006) Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Global Biogeochem Cy 20(4). doi:10.1029/2005GB002672
Falconer GJ, Wright JW, Beall HW (1933) The decomposition of certain types of fresh litter under field conditions. Am J Bot 20:196–203
Fang H, Mo J, Peng S, Li Z, Wang H (2007) Cumulative effects of nitrogen additions on litter decomposition in three tropical forests in southern China. Plant Soil 297:233–242. doi:10.1007/s11104-007-9339-9
Fang YT, Gundersen P, Vogt RD, Koba K, Chen F, Chen X, Yoh M (2011) Atmospheric deposition and leaching of nitrogen in Chinese forest ecosystems. J Forest Res 16:341–350. doi:10.1007/s10310-011-0267-4
FAO (2010) Global forest resources assessment 2010: Main report. Food and Agriculture Organization of the United Nations, Rome
Fitter A (2002) Characteristics and functions of root systems. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: The hidden half. Marcel Dekker, New York
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Fujii S, Takeda H (2010) Dominant effects of litter substrate quality on the difference between leaf and root decomposition process above- and belowground. Soil Biol Biochem 42:2224–2230. doi:10.1016/j.soilbio.2010.08.022
Fujimaki R, Takeda H, Wiwatiwitaya D (2008) Fine root decomposition in tropical dry evergreen and dry deciduous forests in Thailand. J Forest Res 13:338–346. doi:10.1007/s10310-008-0087-3
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vörösmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226. doi:10.1007/s10533-004-0370-0
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Sci 320:889–892. doi:10.1126/science.1136674
Gan H, Zak DR, Hunter MD (2013) Chronic nitrogen deposition alters the structure and function of detrital food webs in a northern hardwood ecosystem. Ecol Appl 23:1311–1321. doi:10.1890/12-1895.1
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Global Change Biol 6:751–765. doi:10.1046/j.1365-2486. 2000.00349.x
Gill RA, Jackson RB (2000) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147:13–31. doi:10.1046/j.1469-8137.2000.00681.x
Grimshaw HM, Allen SE, Parkinson JA (1989) Nutrient elements. In: Allen SE (ed) Chemical analysis of ecological material. Blackwell Scientific, Oxford, pp 81–159
Helmisaari HS, Saarsalmi A, Kukkola M (2009) Effects of wood ash and nitrogen fertilization on fine root biomass and soil and foliage nutrients in a Norway spruce stand in Finland. Plant Soil 314:121–132. doi:10.1007/s11104-008-9711-4
Hendricks JJ, Nadelhoffer KJ, Aber JD (1993) Assessing the role of fine roots in carbon and nutrient cycling. Trends Ecol Evol 8:174–178. doi:10.1016/0169-5347(93)90143-D
Hobbie SE (2008) Nitrogen effects on decomposition: a five-year experiment in eight temperate sites. Ecol 89:2633–2644. doi:10.1890/07-1119.1
Hobbie SH, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecol 81:1867–1877. doi:10.1890/0012-9658(2000)081[1867:NLODIH]2.0.CO;2
Hobbie SE, Oleksyn J, Eissenstat DM, Reich PB (2010) Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 162:505–513. doi:10.1007/s00442-009-1479-6
Hu ZY, Xu CK, Zhou LN, Sun BH, He YQ, Zhou J, Cao ZH (2007) Contribution of atmospheric nitrogen compounds to N deposition in a broadleaf forest of southern China. Pedosphere 17:360–365. doi:10.1016/S1002-0160(07)60043-5
Huttunen L, Aphalo PJ, Lehto T, Niemela P, Kuokkanen K, Kellomaki S (2009) Effects of elevated temperature, elevated CO2 and fertilization on quality and subsequent decomposition of silver birch leaf litter. Soil Biol Biochem 41:2414–2421. doi:10.1016/j.soilbio.2009.08.014
Jackson ML (1958) Soil chemical analysis. Prantice Hall Inc., Englewood Cliffs
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci U S A 94:7362–7366
Jia S, Wang Z, Li X, Sun Y, Zhang X, Liang A (2010) N fertilization affects on soil respiration, biomass and root respiration in Larix gmelinii and Fraxinus mandshurica plantations in China. Plant Soil 333:325–336. doi:10.1007/s11104-010-0348-8
Jones JB, Case VW (1990) Sampling, handling, and analyzing plant tissue samples. In: Westerman RL (ed) Soil testing and plant analysis. Soil Science Society of America, Inc., Madison, pp 390–428
Jourdan C, Silva EV, Goncalves JLM, Ranger J, Moreira RM, Laclau JP (2008) Fine root production and turnover in Brazilian Eucalyptus plantations under contrasting nitrogen fertilization regimes. Forest Ecol Manag 256:396–404. doi:10.1016/j.foreco.2008.04.034
Kalembasa SJ, Jenkinson DSA (1973) comparative study of titrimetric and gravimetric methods for determination of organic carbon in soil. J Sci Food Agr 24:1085–1090
Kaspari M, Garcia MN, Harms KE, Santana M, Wright SJ, Yavitt JB (2008) Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecol Lett 11:35–43. doi:10.1111/j.1461-0248.2007.01124.x
Keeler BL, Hobbie SE, Kellogg LE (2009) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosyst 12:1–15. doi:10.1007/s10021-008-9199-z
Keyser P, Kirk TK, Zeikus IG (1978) Ligninolytic enzyme of phanerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol 135:790–797
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecol 86:3252–3257. doi:10.1890/05-0150
Lee KH, Jose S (2003) Soil respiration, fine root production, and microbial biomass in cottonwood and loblolly pine plantations along a nitrogen fertilization gradient. Forest Ecol Manag 185:263–273. doi:10.1016/S0378-1127(03)00164-6
Lin CF, Yang YS, Guo JF, Chen G, Xie J (2011) Fine root decomposition of evergreen broadleaved and coniferous tree species in mid-subtropical China: dynamics of dry mass, nutrient and organic fractions. Plant Soil 338:311–327. doi:10.1007/s11104-010-0547-3
Liu XJ, Zhang Y, Han WX, Tang AH, Shen JL, Cui ZL, Vitousek P, Erisman JW, Goulding K, Christie P, Fangmeier A, Zhang FS (2013) Enhanced nitrogen deposition over China. Nat 494:459–462. doi:10.1038/nature11917
Ludovici KH, Kress LW (2006) Decomposition and nutrient release from fresh and dried pine roots under two fertilizer regimes. Can J Forest Res 36:105–111
Magill AH, Aber JD (1998) Long-term effects of experimental nitrogen additions on foliar litter decay and humus formation in forest ecosystems. Plant Soil 203:301–311. doi:10.1023/A:1004367000041
Matson P, Lohse K, Jall SJ (2002) The globalization of nitrogen: consequences for terrestrial ecosystems. Ambio 31:113–119. doi:10.1579/0044-7447-31.2.113
Mo J, Zhang W, Zhu W, Gundersen P, Fang Y, Li D, Wang H (2007) Nitrogen addition reduces soil respiration in a mature tropical forest in southern. Global Chang Biol 14:1–10. doi:10.1111/j.1365-2486.2007.01503.x
Nadelhoffer KJ, Raich JW (1992) Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecol 73:1139–1147
Nambiar EKS, Fife DF (1991) Nutrient retranslocation in temperate conifers. Tree Physiol 9:185–207. doi:10.1093/treephys/9.1-2.185
Nelson DW, Sommers LE (1982) Total carbon, OC, and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2. Agronomy Society of America and Soil Science Society of America, Madison, pp 539–577
Nikula S, Vapaavuori E, Manninen S (2010) Urbanization-related changes in European aspen (Populus tremula L.): Leaf traits and litter decomposition. Environ Poll 158:2132–2142. doi:10.1016/j.envpol.2010.02.025
Norby RJ, Ledford J, Reilly CD, Miller NE, O’Neill EG (2004) Fine-root production dominates the response of a deciduous forest to atmospheric CO2 enrichment. Proc Natl Acad Sci 101:9689–9693. doi:10.1073/pnas.0403491101
NRC (National Research Council (1996) Understanding marine biodiversity. National Academy Press, Washington
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2. Agronomy Society of America and Soil Science Society of America, Madison, pp 403–430
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecol 44:322–331
Ostertag R, Hobbie SE (1999) Early stages of root and leaf decomposition in Hawaiian forests: effects of nutrient availability. Oecologia 121:564–573. doi:10.1007/s0044 20050963
Powers JS, Treseder KK, Lerdau MT (2005) Fine roots, arbuscular mycorrhizal hyphae and soil nutrients in four neotropical rain forests: patterns across large geographic distances. New Phytol 165:913–921. doi:10.1111/j.1469-8137.2004.01279.x
Pregitzer KS, Burton A (1992) Foliar sulfur and nitrogen along an 800-km pollution gradient. Can J Forest Res 22:1761–1769
Rabalais NN (2002) Nitrogen in aquatic ecosystems. Ambio 31:102–112. doi:10.1579/ 0044-7447-31.2.102
Reay DS, Dentener F, Smith P, Grace J, Feely RA (2008) Global nitrogen deposition and carbon sinks. Nat Geosci 1:430–437. doi:10.1038/ngeo230
Ring E, Jacobson S, Högbom L (2011) Long-term effects of nitrogen fertilization on soil chemistry in three Scots pine stands in Sweden. Can J Forest Res 41:279–288. doi:10.1139/X10-208
Rowland AP, Roberts JD (1994) Lignin and cellulose fractionation in decomposition studies using acid-detergent fiber methods. Commun Soil Sci Plan 25:26–277
SFAPRC (2010) Statistics of forest resources in China (2004–2008). State Forestry Administration, People’s Republic of China, Available: http://cfdb.forestry.gov.cn:443/showpdf.action. Accessed 2014 Aug 16
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419. doi:10.1007/s004420100740
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404. doi:10.1016/j.soilbio.2009.10.014
Song X, Jiang H, Zhang Z, Zhou G, Zhang S, Peng C (2013) Interactive effects of elevated UV-B radiation and N deposition on decomposition of Moso bamboo litter. Soil Biol Biochem 69:11–16. doi:10.1016/j.soilbio.2013.10.036
Sun T, Mao Z, Han Y (2013) Slow decomposition of very fine roots and some factors controlling the process: a 4-year experiment in four temperate tree species. Plant Soil 372:445–458. doi:10.1007/s11104-013-1755-4
Tateno R, Hishi T, Takeda H (2004) Above- and belowground biomass and net primary production in a cool-temperate deciduous forest in relation to topographical changes in soil nitrogen. Forest Ecol Manag 193:297–306. doi:10.1016/j.foreco.2003.11.011
Tu LH, Hu TX, Zhang J, Li RH, Dai HZ, Luo SH (2011) Short-term simulated nitrogen deposition increases carbon sequestration in a Pleioblastus amarus plantation. Plant Soil 340:383–396. doi:10.1007/s11104-010-0610-0
Tu LH, Hu TX, Zhang J, Huang LH, Xiao YL, Chen G, Hu HL, Liu L, Zheng JK, Xu ZF, Chen LH (2013a) Nitrogen distribution and cycling through water flows in a subtropical bamboo forest under high level of atmospheric deposition. PLoS One 8:e75862. doi:10.1371/journal.pone.0075862
Tu LH, Hu TX, Zhang J, Li XW, Hu HL, Liu L, Xiao YL (2013b) Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol Biochem 58:255–264. doi:10.1016/j.soilbio.2012.12.005
Tu LH, Chen G, Peng Y, Hu HL, Hu TX, Zhang J, Li XW, Liu L, Tang Y (2014a) Soil biochemical responses to nitrogen addition in a bamboo forest. PLoS One 9:e102315. doi:10.1371/journal.pone.0102315
Tu LH, Hu HL, Chen G, Peng Y, Xiao YL, Hu TX, Zhang J, Li XW, Liu L, Tang Y (2014b) Nitrogen addition significantly affects forest litter decomposition under high levels of ambient nitrogen deposition. PLoS One 9:e88752. doi:10.1371/journal.pone.0088752
Van Groenigen KJ, Six J, Hungate BA, de Graaff MA, van Breemen N, van Kessel C (2006) Element interactions limit soil carbon storage. Proc Natl Acad Sci U S A 103:6571–6574. doi:10.1073/pnas.0509038103
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750. doi:10.1890/1051-0761(1997)007[0737:HAOTGN]2.0.CO;2
Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C (2004) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14:1172–1177. doi:10.1890/03-5120
Wang C, Han S, Zhou Y, Yan C, Cheng X, Zheng X, Li M (2012) Responses of fine roots and soil N availability to short-term nitrogen fertilization in a broad-leaved Korean pine mixed forest in northeastern China. PLoS One 7:e31042. doi:10.1371/journal.pone. 0031042
Xiao YL, Tu LH, Hu TX, Zhang J, Li XW, Hu HL (2013) Early effects of simulated nitrogen deposition on annual nutrient input from litterfall in a Pleioblastus amarus plantation in rainy area of West China. Acta Ecol Sin 33:7355–7363. doi:10.5846/stxb201208301224, in Chinese with English abstract
Xu ZF, Tu LH, Hu TX, Zhang J, Li XW, Hu HL (2013) Implications of greater than average increases in nitrogen deposition on the western edge of the Szechwan Basin, China. Environ Poll 177:201–202. doi:10.1016/j.envpol.2012.12.031
Yang YS, Chen GS, Guo JF, Lin P (2004) Decomposition dynamic of fine roots in a mixed forest of Cunninghamia lanceolata and Tsoongiodendron odonum in mid-subtropics. Ann Forest Sci 61:65–72. doi:10.1051/forest:2003085
Zhang DQ, Hui DF, Luo YQ, Zhou GY (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93. doi:10.1093/jpe/rtn002
Zhao X, Yan X, Xiong Z, Xie Y, Xing G, Shi S, Zhu Z (2009) Spatial and temporal variation of inorganic nitrogen wet deposition to the Yangtze River Delta region, China. Water Air Soil Poll 203:277–289. doi:10.1007/s11270-009-0011-2
Zogg GP, Zak DR, Burton AJ, Pregitzer KS (1996) Fine root respiration in northern hardwood forests in relation to temperature and nitrogen availability. Tree Physiol 16:719–725. doi:10.1093/treephys/16.8.719
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 31300522), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20125103120018) and the Scientific Research Fund of Sichuan Provincial Education Department of China (No. 12ZA118).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Simon Jeffery.
Rights and permissions
About this article
Cite this article
Tu, Lh., Peng, Y., Chen, G. et al. Direct and indirect effects of nitrogen additions on fine root decomposition in a subtropical bamboo forest. Plant Soil 389, 273–288 (2015). https://doi.org/10.1007/s11104-014-2353-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2353-9