Abstract
Two-year (June 2003–May 2005) precipitation samples were collected from three monitoring sites with similar economy development level in the Yangtze River Delta Region of China to investigate the spatial–temporal variation of inorganic N wet deposition. The results showed that the Yangtze River Delta Region had higher inorganic N wet deposition than the northwestern, northern, or southern China. There was spatial variation of inorganic N wet deposition. The positive relationship between inorganic N deposition and precipitation suggested that rainfall amount might be an important factor influencing the wet deposition of inorganic N. Deposition of inorganic N occurred mainly in the spring and summer season (March–August; 70% of the annual total), which was in accord with seasonal distribution of precipitation. However, a negative logarithmic relation between rainfall and inorganic N concentration in rainwater indicated the dilution effect of rainwater on inorganic N concentration. Distinguished variation of NH4 +/NO3 − ratio in wet deposition existed in the different time scale. NH4 +/NO3 − ratio evidently decreased from 6 in 1980s to 1.2 in 2003/2005 and greatly varied between 0.3 and 9.9 within a year. NH4 +/NO3 − ratio peaked in accordance with nitrogen-fertilizing time during crop growing season. Annual alternate appearance of the 15N-enriched and 15N-depleted periods coincided with the temporal variation of NH4 +/NO3 − ratio, which was closely related to the timing of fertilization and seasonal climate changes, suggesting the effect of NH4 + sources in the wet deposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atmospheric nitrogen (N) deposition is an environmental consequence when biogeochemical N cycle undergoes intense perturbation by anthropogenic activities. Because of extensive use of fossil fuels in industry and transportation, use and sometimes overuse of chemical fertilizer in agriculture, and recent expansion in intensive animal husbandry, N deposition have been sharply increased in recent decades (Brimblecombe and Stedman 1982; Bartnichi and Alcamo 1989; Vitousek et al. 1997; Wright and Rasmussen 1998; Galloway et al. 2004). Currently, the global atmospheric N deposition reaches 70 Tg N year−1(Galloway et al. 2004). Even though N deposition does supplement N nutrition for plants (Russow et al. 2001), excessive N deposition induces a considerable burden on forest, grassland, and aquatic ecosystems, aggravates the eutrophication in waterbodies and soil acidification (Goulding et al. 1998; Vitousek et al. 1997; Bouwman et al. 2002), and causes undesirable changes in biodiversity (Stevens et al. 2004).
The increase of N deposition was reported by Chinese scientists as early as 1970s (Lu and Shi 1979). However, not until recently has limited information of N deposition on the regional scale been reported (Li and Li 1999; Larssen et al. 2006; Zhang et al. 2008). The Yangtze River Delta Region is one of the most important industrial–agricultural economic region in China. Rapid development of economy in this region results in the significant increase in reactive N creation. The total N input to the region was 2.94 Tg N in 2002 with an average of 291 kg N ha−1 (Deng et al. 2007), 4.5 times the national average (64 kg N ha−1) as estimated in 1995 (Xing and Zhu 2002). The total NO x (NO + NO2) emission in Jiangsu province, Zhejiang province, and Shanghai city averaged 1.71 Tg N year−1 from 1995 to 1998, accounting for 15% of the state total (Tian et al. 2001b). The average N application rate was up to 550 kg N ha−1year−1 under rice–upland crop annual rotation (Xing et al. 2002). Furthermore, excrements of human and animal contributed 24% (0.72 Tg N) of total N input to the study region in 2002 (Deng et al. 2007). It is believed that both the excessive use of chemical N fertilizer and increasing amount of human and livestock excrements may have enhanced NH3 emission. Atmospheric N deposition to the Yangtze River Delta Region, however, has not been well documented. To observe atmospheric N deposition in this region, four sites (Nanjing, Hangzhou, Changshu, and Wuxi) were established to continuously monitor wet N deposition from June 2003 to May 2005. Some of our results about N deposition in intensive agricultural district in this region were reported separately (Xie et al. 2008). Therefore, the emphasis of this paper will be on the patterns of spatial–temporal variation of inorganic N wet deposition at different motoring sites.
2 Materials and Methods
2.1 Study Sites
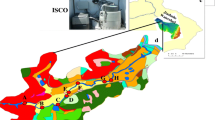
Nanjing site (118°47′ E, 32°4′ N) is located at Institute of Soil Science, Chinese Academy of Sciences (CAS), in Nanjing City, about 3 km away from city geographic center, with annual mean temperature of 16.5°C. Hangzhou site (120°10′ E, 30°15′ N) lies in the experimental farm of Zhejiang Academy of Agricultural Sciences. It belongs to typical rurbania and is about 12 km away from the center of Hangzhou city. It is surrounded by rice paddy, vegetable fields, and scattered industries, with annual mean temperature of 17.5°C. Changshu site (120°42′ E, 31°32′ N) is located at the Changshu Agro-Ecological Experimental Station, CAS, in Xinzhuang Town, Changshu City, with annual mean temperature of 17°C. It is typical intensive agricultural area under rice–wheat crop rotation (Fig. 1).
2.2 Sampling and Analysis
Each sampling site was equipped with a rain auto-sampler (APS-3, Wuhan Tianhong Instrument Company, China) to collect rainwater for the analysis of NH4 + and NO3 −. This rainfall-sensitive sampling system opens its cover automatically to collect rainwater sample when it rains and closes automatically when the rain stops, keeping the rainwater samples from contamination. After each rain event, rainwater samples were collected, thoroughly mixed, stored in 250-ml plastic bottles, and immediately frozen unfiltered at −20°C in a freezer until analysis.
Another home-made rain sampler was also installed at each site to collect a large amount of rainwater sample for the analysis of natural 15N abundance of NH4 + (δ 15NH4 +). This sampler is made of polyvinyl chloride plastic, with 0.5 m2 in area and same height from the ground as the auto-sampler. Its inner surface is in saucer shape so that rainwater can easily flow into the 10-L plastic cask via connecting pip. A top cover is attached during the dry period to keep the device from contamination until the rain event occurs. Because about 2 mg NH4 +-N is needed for precise mass spectrometry analysis, more than 2 L rainwater must be collected for every rain event. During the observation period, there were 57, 64, and 60 rainwater samples collected for δ 15NH4 + analysis in Changshu, Nanjing, and Hangzhou, respectively. All the rainwater samples passed through a column of cation exchange resin to adsorb NH4 + immediately, and the adsorbed NH4 + was eluted with 2 mol L−1 HCl into a 250-ml plastic bottle. The elute was concentrated under 80°C water bath until volume <10 ml, shifted into pyriform bottle, and then dried in oven (80°C) for N isotope analysis.
NH4 +-N and NO3 −-N in rainwater samples were measured colorimetrically by the indophenol blue method using ultraviolet spectrophotometer (Shimadzu UV mini-1240, Japan, with ±0.005 absorbance of photometric accuracy). δ 15N of NH4 + was determined in N2 derived from NH4 + using an isotope mass spectrometer (MAT-251, USA, with analytic error ±0.2 δ 15N).
NH4 + and NO3 − concentration and N deposition were calculated with the following equations:
where C refers to volume-weighted concentration of NH4 + or NO3 − (mg N L−1); C i is the concentration of NH4 + or NO3 − (mg N L−1) for an individual event with a precipitation amount of L i (L); n refers to numbers of times for rain event.
The δ 15N of NH4 + was calculated according to the following equation:
where “standard” refers to the atmospheric N2, which by definition has a 15N atom % value of 0.3663 and a δ 15N value of 0‰.
3 Results and Discussion
3.1 Spatial Variation of Inorganic Nitrogen in Wet Deposition
The average inorganic N wet deposition to the Yangtze River Delta Region was estimated to be 26.8 kg N ha−1 year−1 (Table 1). This value was 1.5 and 3.0 times that observed in the highest deposition areas by European Monitoring and Evaluation Programme (ranging from 1.04 to 18.4 kg N ha−1 year−1; EMEP 2003–2005) and National Atmospheric Deposition Program (ranging from 0.04 to 8.53 kg N ha−1 year−1; NADP 2003-2005) during the corresponding period. It is also far greater than that in the northwest China (averaged 16.3 kg N ha−1 year−1; Li and Li 1999), the North China Plain (averaged 20.4 kg N ha−1 year−1; Zhang et al. 2006), and the subtropical South China (averaged 14 kg N ha−1 year−1; Chen and Mulder 2007).
Remarkable spatial variations in N deposition were observed in a number of researches (Park and Lee 2002; Liu et al. 2006; Larssen et al. 2006; Chen and Mulder 2007; Zhang et al. 2008). In the present study, Hangzhou site had the highest N deposition, followed by Changshu and Nanjing. All these three sites have similar level of economic development, and therefore, the spatial variation of annual rainfall in three sites might be the primary cause for the variation of N deposition (Table 1). It is generally thought that the main anthropogenic source of NH4 + in rain is NH3 volatilizing from fertilizer and excrements of human and animal, and the major anthropogenic source of NO3 − are NO x emitted from fossil fuel combustion from power plants, automobiles, and biomass burning. Thus, NH4 +/NO3 − molar ratio might reflect the relative contribution of reactive N from industry and transportation, agriculture, and animal husbandry to N deposition on the local scale (Fahey et al. 1999; Liu et al. 2006; Anderson and Downing 2006; Larssen et al. 2006). In North America, with advanced industralization, NH4 +/NO3 − ratio for N deposition is usually much smaller than 1(Fahey et al. 1999). On the contrary, areas with intensive agriculture, such as the midwestern USA and parts of Europe, are characterized by N wet deposition with NH4 +/NO3 − ratio usually greater than 1 (Fahey et al. 1999). In China, NH4 +/NO3 − ratio for N deposition averaged 4.1 in the Guanzhong Plain of the northwest China (Li and Li 1999), 2 in the North China Plain (Zhang et al. 2008) and the South Acid Rain Area (Larssen et al. 2006), 1.2 in the current study area (Table 1). This indicates that NH4 + deposition from agriculture and excrements of human and animal is still the larger portion, as compared with NO3 − from fossil fuel combustion in industry and transportation. However, the contribution from NO3 − has been increasingly important in the total N deposition. Changshu site had the highest NH4 +/NO3 − ratio (1.5) because it located in an intensive agriculture region, and N fertilization had a great effect on NH4 + deposition. Nanjing site had the lowest NH4 +/NO3 − ratio (0.94) due to its location near the city center and relative larger effect of NO x emission from industry and transportation. NH4 +/NO3 − ratio in rain at Hangzhou site (1.3) was between the values of Changshu and Nanjing (Table 1). This may be because this site is located in a suburb area, about 12 km away from the urban center and surrounded by scattered rice paddy, villages, and factories. Therefore, this area may have a balanced emission of NH3 and NO x from agriculture, industry, or transportation.
3.2 Temporal Variation of Inorganic Nitrogen Wet Deposition
3.2.1 Comparison of Inorganic Nitrogen Wet Deposition in the Early 1980s with that Between 2003 and 2005
In the early 1980s, NH4 + was the dominant form of inorganic N deposition (Fig. 2a). Wet deposition of NH4 +and NO3 − to the Yangtze River Delta Region was estimated to be 12.1 and 2.07 kg N ha−1 year−1, respectively (Fig. 2b). In the years between 2003 and 2005, the concentration of NO3 − in deposition was dramatically increased to 0.97 mg N L−1, which was almost equal to the proportion of NH4 + (Fig. 2a). While wet deposition of NH4 + increased by 20% to the average of 14.9 kg N ha−1 year−1, the wet deposition of NO3 − was greatly elevated to 11.9 kg N ha−1 year−1, 5.7 times that in the early 1980s (Fig. 2b). As a result, the NH4 +/ NO3 − ratio in deposition sharply decreased from 5.8 to 1.2 (Fig. 2b), indicating a great enhancement of reactive N oxides emission in the past 20 years. It can be seen that the increase of NO3 − wet deposition is in correspondence with the rapid increase of fossil fuel consumption by the growth of industry and transportation in this area (Fig. 3a; Anon. 2006a, b). Although total consumption of chemical N fertilizer had also increased between 1980s and 2003/2005 period (Fig. 3b; Anon. 2006a, b), the increasing rate was insufficient to compensate the increase of fossil fuel combustions. This is partially attributed to the improved fertilizer application methods aiming at improving plant N utilization and reducing NH3 loss to the environment. It is expected that atmospheric dry and wet deposition of NH x (e.g., NH3 +, NH4 +) and NO y (e.g., NO x , NO3 −, HNO3 −, etc) to the Yangtze River Delta Region, especially NO y , will continuously exhibit an increasing trend with the rapid economy development in near future.
Changes of atmospheric N wet deposition of inorganic N to the Yangtze River Delta Region from early 1980s (Xing and Zhu 2001) to 2003/2005 (in the present study)
3.2.2 Seasonal Patterns of Inorganic Nitrogen Wet Deposition
Figure 4 shows the monthly variations in precipitation, inorganic N concentration in rainwater, and deposition of inorganic N at Nanjing site from June 2003 to May 2005. The precipitation at Nanjing site was more concentrated in the spring and summer seasons (March–August), accounting for 71% of the total annual precipitation (Fig. 4a). Monthly volume-weighted concentrations of inorganic N in rainwater showed large variation from 0.45 to 2.83 mg N L−1 for NH4 + and from 0.2 to 5.90 mg N L−1 for NO3 − (Fig. 4a). Because of the influence of N concentration and rainfall, the deposition of inorganic N varied from month to month. During the 2-year period, monthly deposition of inorganic N ranged from 0.03 to 3.11 kg NH4 +-N ha−1 and from 0.07 to 4.39 kg NO3 −-N ha−1 (Fig. 4b). Monthly volume-weighted concentrations of inorganic N in rainwater followed a trend opposite to that of precipitation (Fig. 4a). Meantime, a negative logarithmic relationship between inorganic N concentration and rainfall was observed (Fig. 5a, b). This indicates that there is dilution effect of rainwater on inorganic N concentration. However, the monthly deposition of NH4 + and NO3 − followed a trend similar to that of precipitation (Fig. 4b), and the positive relationship between monthly deposition of inorganic N and precipitation (Fig. 5c, d) suggests that rainfall amount is an important factor influencing the wet deposition of inorganic N. In fact, the total deposition of NH4 + and NO3 − in the rainy season (March–August) accounted for 70% of the annual total deposition during the 2-year period. Unlike the North China Plain (Liu et al. 2006), precipitation in the winter season (December–February) contributed 11% of the annual total precipitation due to the subtropical monsoon climate conditions (Fig. 4a). As a result, about 12% of total annual deposition of inorganic N was observed in the winter season at the monitoring site (Fig. 4b). These results are consistent with the results from the North China Plain (Liu et al. 2006; Zhang et al. 2006, 2008).
3.2.3 Effect of Nitrogen Fertilization During Crop Growing Season on Temporal Variation of NH4 +/ NO3 − Ratio in Precipitation
In Northern China, higher NO3 − concentration is in the wet deposition during the winter because large amount of coal is consumed for heating (Liu et al. 2006). On the contrary, there is almost no coal consumption for heating during winter in the southern region of the Yangtze River. Therefore, NO x emission and NO3 − deposition is relative steady throughout the year in the study region. Because NH3 has a relative shorter transportation distance than NO x (Asman et al. 1998; Krupa 2003; Akimoto 2003) and is readily converted to NH4 + (Krupa 2003), subjected to wet deposition near the source, localized NH3 emission from N fertilization may have great effect on NH4 + deposition and NH4 +/NO3 − ratio.
In the present study, NH4 +/NO3 − ratio in precipitation at the three sites showed large temporal variation from 0.3 to 9.9 (Fig. 6). However, it was frequently peaked at the periods of N fertilizer application, usually between late June and early August for rice and between early November and mid-March for wheat (Table 2 and Fig. 6). Changshu site was located in a typical intensive agricultural area under rice–wheat crop rotation, and the dynamics of NH4 +/NO3 − ratio at this site appeared to be closely related to the timing of N fertilization (Fig. 6c, Xie et al. 2008). Similarly, the NH4 +/NO3 − ratio at Nanjing and Hangzhou sites also had a similar temporal variation corresponding to the timing of N fertilization (Fig. 6a, b), implying that fertilization in surrounding fields had the great influence on NH4 + deposition to both urban and suburb areas.
Temporal variations of the NH4 +/NO3 − ratio in rainfall at three monitoring sites in Yangtze River Delta from June 2003 to May 2005. The arrow denotes the timing of N fertilizer application for rice and wheat. W wheat, R rice, B nitrogen basal dressing; T1 or T2 the first or second nitrogen top dressing. Date at Changshu site reported by Xie et al. (2008)
3.3 Effect of Nitrogen Fertilization During Crop Growing Season on Temporal Variation of δ 15NH4 + Value in Precipitation
After the first reported isotopic composition of ammonia in rain by Hoering (1957), results about δ 15NH4 + value in rainwater have been published extensively (Table 3). In the current study, considerable range of δ 15NH4 + value had been observed in the precipitation during the 2-year period in the Yangtze River Delta Region (Table 3). The regular fluctuation of δ 15NH4 + value in precipitation seemed to be related to the fertilization time and seasonal climate changes within a year (Fig. 7). Variation of δ 15NH4 + value had the similar trend across the three sites. The 15N-depleted period typically appeared from June to July and from December to March of the next year, whereas the 15N-enriched period appeared from April to May and from August or September to November (Fig. 7). These trends may be due to the changes of NH4 + sources for precipitation in different periods of crop growing season within a year.
Temporal variations of δ 15NH4 + value (per mill) in rainfall at three monitoring sites in Yangtze River Delta from June 2003 to May 2005. Dashed rectangles denotes the period of depleted δ 15NH4 + value. Date at Changshu site reported by Xie et al. (2008)
It is well known that the δ 15NH4 + value of ammonium-based N fertilizer is usually low or negative, while higher δ 15N value is found in excrements of human and animal (Table 4). Therefore, the 15N-depleted period in the precipitation in the current study may well be contributed by the application of chemical N fertilizers. The 15N-enriched period may be ascribed to NH3 emission from excrements of human and animal and other organic N sources. Although negative δ 15N values of NH3 were reported in cattle urine (Freyer 1978), sheep, and chicken shed (Heaton 1987), our results interestingly revealed positive δ 15N value in excrements of human and animal, which was also gradually increased with composting time (Table 5). According to other reported results (Table 4), NH3 emitted from those substrates with higher δ 15NH4 + value had relatively higher δ 15N value than that from synthetic nitrogen fertilizer.
In the present study, since rice base and the first topdressing fertilization (210 kg N ha−1, 70% of total N applied in rice season) happened in about 2 weeks during June and July (Table 2), NH4 + in precipitation in this period was probably dominated by NH3 volatilization of fertilizer, which was depleted in 15N. There is no fertilization from August to October, except for the second topdressing fertilization (90 kg N ha−1, surplus 30% of N applied) at the mid-August. Because of the high temperature and intense biological activities in this period, human and livestock excrements may predominate in NH3 emission. As a result, δ 15NH4 + value in the precipitation turned into positive. In early November, although it was the time for sowing of wheat and 100 kg N ha−1 of N base fertilizer was applied, NH3 volatilization during the wheat growing season was much smaller and almost negligible probably due to low temperature (Tian et al. 2001a). Therefore, relative higher δ 15NH4 + value was observed in November. From December to March of the next year are the winter and the early spring of a year. The temperature is the lowest, and biological activities are inhibited. The NH3 emission from human and livestock excrements decreases, whereas NH3 volatilization from fertilizer likely occurs due to the surface application of wheat first and second topdressing fertilization (Tian et al. 2001a). NH4 + in precipitation is gradually depleted in 15N. In April and May, wheat is at its mature growing stage without fertilization applied. The gradually rising temperature may facilitate NH3 volatilization from human and livestock excrements, again changing δ 15NH4 + value into positive (Fig. 7). The fact that the variation of δ 15NH4 + value is consistent with fertilization and seasonal climate changes can be informative to provide insights on the sources of NH4 + in precipitation (Russell et al. 1998).
4 Conclusion
The spatial and temporal variation of inorganic nitrogen wet deposition to some degree reflects the intense perturbation on atmospheric N cycle by anthropogenic activities. As one of the most developed and highly populated regions in China, NH4 +/NO3 − ratio in wet deposition in the Yangtze River Delta evidently decreased from 6 to 1.2 in two decades. This was probably due to the increasing emission of NO x from fossil fuel consumption derived by rapid growth of industry and transportation. However, the large quantity of nitrogen fertilization during crop growing season also had significant effects on temporal variation of NH4 +/NO3 − ratio. The amount of inorganic nitrogen wet deposition to the study region was greater than that in northern, northwestern, or southern China. Apparently, atmospheric N deposition was closely correlated to the growth rate and level of economy. Temporal variation of δ 15NH4 + value in precipitation coincided with variation of NH4 +/NO3 − ratio, which was closely related to the timing of fertilization and seasonal climate changes. The dynamics of δ 15NH4 + value mainly depended on variation of NH4 + source in precipitation at one or another time within a year. This not only corroborates the great effect of anthropogenic activities on atmospheric N deposition but also may shed light on the origins of NH4 +, which should be confirmed in the future.
References
Akimoto, H. (2003). Global air quality and pollution. Science, 302, 1716–1719. doi:10.1126/science.1092666.
Anderson, K. A., & Downing, J. A. (2006). Dry and wet atmospheric deposition of nitrogen, phosphorus and silicon in an agricultural region. Water, Air, and Soil Pollution, 176, 351–374. doi:10.1007/s11270-006-9172-4.
Anon. (2006a). China statistical yearbook. Beijing: China Statistical Press (in Chinese).
Anon. (2006b). Jiangsu statistical yearbook. Beijing: China Statistical Press (in Chinese).
Asman, W. A. H., Sutton, M. A., & SchjØrring, J. K. (1998). Ammonia: emission, atmospheric transport and deposition. The New Phytologist, 139, 27–48. doi:10.1046/j.1469-8137.1998.00180.x.
Bartnicki, J., & Alcamo, J. (1989). Calculating nitrogen deposition in Europe. Water, Air, and Soil Pollution, 47, 101–123. doi:10.1007/BF00469001.
Bouwman, A. F., Van Vuuren, D. P., Derwent, R. G., & Posch, M. (2002). A global analysis of acidification and eutrophication of terrestrial ecosystems. Water, Air, and Soil Pollution, 141, 349–382. doi:10.1023/A:1021398008726.
Brimblecombe, P., & Stedman, D. H. (1982). Historical evidence for a dramatic increase in the nitrate component of acid rain. Nature, 198, 460–462. doi:10.1038/298460a0.
Cao, Y. C., Sun, G. Q., Xing, G. X., & Xu, H. (1991). Natural abundance of 15N in main N-containing chemical fertilizers of China. Pedosphere, 1, 377–382.
Chen, X. Y., & Mulder, J. (2007). Atmospheric deposition of nitrogen at five subtropical forested sites in South China. The Science of the Total Environment, 378, 317–330. doi:10.1016/j.scitotenv.2007.02.028.
China Meteorological Data Sharing Service System (CMDSSS). (1971–2000). http://cdc.cma.gov.cn.
Deng, M. H., Xie, Y. X., Xiong, Z. Q., Xing, G. X., & Yan, X. Y. (2007). Nitrogen budgets of the Yangtze Delta Region and their effect on the environment. Acta Scientiae Circumstantiae, 27, 1709–1716 (in Chinese).
European Monitoring and Evaluation Programme (EMEP). (2003–2005). EMEP measurement data online. Acidifying and eutrophying compounds. http://www.nilu.no/projects/ccc/emepdata.html.
Fahey, T. J., Williams, C. J., Rooney-Varga, J. N., Cleveland, C. C., Postek, K. M., Smith, S. D., et al. (1999). Nitrogen deposition in and around an intensive agricultural district in central New York. Journal of Environmental Quality, 28, 1585–1600.
Fogg, G. E., Rolston, D. E., Decker, D. L., Louie, D. T., & Grismer, M. E. (1998). Spatial variation in nitrogen isotopic values beneath nitrate contamination sources. Ground Water, 36, 418–426. doi:10.1111/j.1745-6584.1998.tb02812.x.
Freyer, H. D. (1978). Seasonal trends of NH4 + and NO3 - nitrogen isotope composition in rain collected at Jülich, Germany. Tellus, 30, 83–92.
Freyer, H. D., & Aly, A. I. M. (1974). Nitrogen-15 variations in fertilizer nitrogen. Journal of Environmental Quality, 3, 405–406.
Galloway, J. N., Dentener, F. J., Capone, D. G., Boyer, E. W., Howarth, R. W., Seitzinger, S. P., et al. (2004). Nitrogen cycles: Past, present, and future. Biogeochemistry, 70, 153–226. doi:10.1007/s10533-004-0370-0.
Gao, Y. (2002). Atmospheric nitrogen deposition to Barnegat Bay. Atmospheric Environment, 36, 5783–5794. doi:10.1016/S1352-2310(02)00656-8.
Goulding, K. W. T., Bailey, N. J., Bradbury, N. J., Hargreaves, P., Howe, M., Murphy, D. V., et al. (1998). Nitrogen deposition and its contribution to nitrogen cycling and associated soil processes. The New Phytologist, 139, 49–58. doi:10.1046/j.1469-8137.1998.00182.x.
Heaton, T. H. E. (1987). 15N/14N ratios of nitrate and ammonium in rain at Pretoria, South Africa. Atmospheric Environment, 21, 843–852. doi:10.1016/0004-6981(87)90080-1.
Hoering, T. (1957). The isotopic composition of the ammonia and the nitrate ion in rain. Geochimica et Cosmochimica Acta, 12, 97–102. doi:10.1016/0016-7037(57)90021-2.
Krupa, S. V. (2003). Effects of atmospheric ammonia (NH3) on terrestrial vegetation: a review. Environmental Pollution, 124, 179–221. doi:10.1016/S0269-7491(02)00434-7.
Larssen, T., Lydersen, E., Tang, D., He, Y., Gao, J., Liu, H., et al. (2006). Acid rain in China. Environmental Science & Technology, 40, 418–425. doi:10.1021/es0626133.
Li, S. Q., & Li, S. X. (1999). Nitrogen added to ecosystems by wet deposition in Guanzhong Area in Shaanxi. Agricultural Environment Protection, 18, 97–101 (in Chinese).
Li, X. D., Masuda, H., Koba, K., & Zeng, H. A. (2007). Nitrogen isotope study on nitrate-contaminated groundwater in the Sichuan Basin, China. Water, Air, and Soil Pollution, 178, 145–156. doi:10.1007/s11270-006-9186-y.
Liu, X. J., Ju, X. T., Zhang, Y., He, C., Kopsch, J., & Zhang, F. S. (2006). Nitrogen deposition in agroecosystems in the Beijing area. Agriculture Ecosystems & Environment, 113, 370–377. doi:10.1016/j.agee.2005.11.002.
Lu, R. K., & Shi, T. J. (1979). The content of plant nutrients of precipitation in Jinhua district of Zhejiang Province. Acta Pedologica Sinica, 16, 81–84 (in Chinese).
Moore, H. (1977). The isotopic composition of ammonia, nitrogen dioxide, and nitrate in the atmosphere. Atmospheric Environment, 11, 1239–1243. doi:10.1016/0004-6981(77)90102-0.
National Atmospheric Deposition Program/National Trends Network (NADP/NTN).(2003–2005). Annual Data for Custom Site List. http://nadp.sws.uiuc.edu/nadpdata/annualReq.asp?site=Custom156.
Paerl, H. W., & Fogel, M. L. (1994). Isotopic characterization of atmospheric nitrogen inputs as sources of enhanced primary production in coastal Atlantic Ocean waters. Marine Biology (Berlin), 119, 635–645. doi:10.1007/BF00354328.
Park, S. U., & Lee, Y. H. (2002). Spatial distribution of wet deposition of nitrogen in South Korea. Atmospheric Environment, 36, 619–628. doi:10.1016/S1352-2310(01)00489-7.
Russell, K. M., Galloway, J. N., Macko, S. A., Moody, J. L., & Scudlark, J. R. (1998). Sources of nitrogen in wet deposition to the Chesapeake Bay region. Atmospheric Environment, 32, 2453–2465. doi:10.1016/S1352-2310(98)00044-2.
Russow, R., Böhme, F., & Neue, H. U. (2001). A new approach to determine the total airborne N input into the soil/plant system using 15N isotope dilution (ITNI): Results for agricultural areas in central Germany. TheScientificWorldJournal, 1(S2), 255–260.
Stevens, C. J., Dise, N. B., Mountford, J. O., & Gowing, D. J. (2004). Impact of nitrogen deposition on the species richness of grasslands. Science, 303, 1876–1879. doi:10.1126/science.1094678.
Tian, G. M., Cai, Z. C., Cao, J. L., & Li, X. P. (2001a). Factors affecting ammonia volatilisation from a rice–wheat rotation system. Chemosphere, 42, 123–129. doi:10.1016/S0045-6535(00)00117-X.
Tian, H. Z., Hao, J. M., Lu, Y. Q., & Zhu, T. L. (2001b). Inventories and distribution characteristics of NOx emission in China. China Environmental Science, 21, 493–497 (in Chinese).
Vitousek, P. M., Aber, J. D., Howarth, R. W., Likens, G. E., Matson, P. A., Schindler, D. W., et al. (1997). Human alterations of the global nitrogen cycle: Sources and consequences. Ecological Applications, 7, 737–750.
Wright, R. F., & Rasmussen, L. (1998). Introduction to the NITREX and EXMAN projects. Forest Ecology and Management, 101, 1–7. doi:10.1016/S0378-1127(97)00120-5.
Xiao, H. Y., & Liu, C. Q. (2002). Sources of nitrogen and sulfur in wet deposition at Guiyang, southwest China. Atmospheric Environment, 36, 5121–5130. doi:10.1016/S1352-2310(02)00649-0.
Xie, Y. X., Xiong, Z. Q., Xing, G. X., Yan, X. Y., Shi, S. L., Sun, G. Q., et al. (2008). Source of nitrogen in wet deposition to a rice agroecosystem at Tai lake region. Atmospheric Environment, 42, 5182–5192. doi:10.1016/j.atmosenv.2008.03.008.
Xing, G. X., & Zhu, Z. L. (2001). The environmental consequences of altered nitrogen cycling resulting from industrial activity, agricultural production and population growth in China. TheScientificWorldJournal, 1(S2), 70–80.
Xing, G. X., & Zhu, Z. L. (2002). Regional nitrogen budgets for China and its major watersheds. Biogeochemistry, 57, 405–427. doi:10.1023/A:1016508323200.
Xing, G. X., Cao, Y. C., Shi, S. L., Sun, G. Q., Du, L. J., & Zhu, J. G. (2001). N pollution sources and denitrification in waterbodies in Taihu Lake region. Science in China( Series B), 44, 304–314.
Xing, G. X., Cao, Y. C., Shi, S. L., Sun, G. Q., Du, L. J., & Zhu, J. G. (2002). Denitrification in underground saturated soil in a rice paddy region. Soil Biology & Biochemistry, 34, 1593–1598. doi:10.1016/S0038-0717(02)00143-8.
Yeatman, S. G., Spokes, L. J., Dennis, P. F., & Jickells, T. D. (2001). Comparisons of aerosol nitrogen isotopic composition at two polluted coastal sites. Atmospheric Environment, 35, 1307–1320. doi:10.1016/S1352-2310(00)00408-8.
Zhang, Y., Liu, X. J., Fangmeier, A., Goulding, K. T. W., & Zhang, F. S. (2008). Nitrogen inputs and isotopes in precipitation in the North China Plain. Atmospheric Environment, 42, 1436–1448. doi:10.1016/j.atmosenv.2007.11.002.
Zhang, Y., Liu, X. J., Zhang, F. S., Ju, X. T., Zou, G. Y., & Hu, K. L. (2006). Spatial and temporal variation of atmospheric nitrogen deposition in the North China Plain. Acta Ecologica Sinica, 26, 1633–1639. doi:10.1016/S1872-2032(06)60026-7.
Acknowledgments
The project was financially supported by the National Natural Science Foundation of China (no. 30390080). Deep appreciation goes to anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, X., Yan, X., Xiong, Z. et al. Spatial and Temporal Variation of Inorganic Nitrogen Wet Deposition to the Yangtze River Delta Region, China. Water Air Soil Pollut 203, 277–289 (2009). https://doi.org/10.1007/s11270-009-0011-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0011-2