Abstract
Dry evergreen forest (DEF) and dry deciduous dipterocarp forest (DDF) are major forest types extensively distributed in northeastern Thailand, exhibiting different nutrient cycling properties. This study aims to improve our understanding on the pattern of mass loss and nitrogen release from two categories of roots (fine, <2 mm and small, 2–5 mm) of Hopea ferrea at DEF and fine roots of mixed trees and dwarf bamboo (Arundinaria pusilla) at DDF sites. Decomposition experiment was performed for more than 12 months using buried litter bag technique. Initial chemistry was significantly different among the four root litters; fine root of H. ferrea exhibited a low ratios of C:N and acid-insoluble:N. The fine root of dwarf bamboo was characterized by high contents of total carbohydrate and ash. Decomposition rate constants (year−1) of ash-free weight remaining were 1.27 and 0.55 for fine and small roots of H. ferrea, and 0.73 and 0.66 for fine root of mixed trees and dwarf bamboo, respectively. At the end of the experiment, the N concentration in fine and small roots of H. ferrea increased to 1.5 times the initial concentration. Whereas, N mass of dwarf bamboo decreased during the experiment. This suggests a different pattern of root decomposition and N release in two forest ecosystems. Generally, the fine root decomposition was faster in the DEF than in the DDF. The role of initial litter chemistry was more pronounced than the climatic seasonality on the belowground decomposition pattern in our study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dry evergreen forest (DEF) and deciduous dry dipterocarp forest (DDF) are major types of dry tropical forests in northeastern Thailand (Kanzaki et al. 1995; Sahunalu and Dhanmaonda 1995). DEF is dominated by evergreen trees (mainly Hopea species), regarded as the climatic climax in dry tropical forests. The overstory of DDF is dominated by deciduous broad-leaved trees of Dipterocarpaceae, and the understory is dominated by dwarf bamboo (Arundinaria pusilla). Fire is common and occurs almost annually without active fire prevention efforts because of seasonally dry conditions and human activities (Sakurai et al. 1998). Besides tree species composition, these forests differ in terms of their nutrient cycling properties; e.g., their nutrient accumulations in soils, N dynamics, and leaf litter decompositions (Sakurai et al. 1998; Hirobe et al. 2003; Takeda and Tian 2003; Toda et al. 2007).
In forest ecosystems, litter decomposition is an inevitable process that regulates nutrient availability in forest soils through organic matter decomposition and nutrient release (Staaf and Berg 1982; Aber and Mellilo 2001). The rate of decomposition and nutrient release are regulated by several factors, including the soil environment, decomposers activity, and resource quality of detrital matter (Swift et al. 1979; Berg and McClaugherty 2003). Climate is known to be the dominant factor influencing litter decomposition on a large geographical scale, and litter decomposition in a tropical region is considered to be more rapid than in other regions (Tripathi and Singh 1992a; Aerts 1997; Takeda 1998). Study of aboveground litter has revealed that the rate of decomposition is closely related to initial litter chemistry; e.g., nutrient concentrations or carbon-to-nutrient ratios (Berg and Staaf 1980) and lignin content or lignin-to-nutrient ratio (Melillo et al. 1982; Aber et al. 1990). However, regulation by the seasonal pattern of rainfall at a local site has sometimes been observed in a temperate forest (Tripathi et al. 2006) and subtropical humid forest (Arunachalam et al. 1996). Thus, the importance of litter quality and local climate conditions is site-specific.
Plant litter quality is one of the most important factors controlling litter decomposition in a variety of litter from different forest ecocystems (Swift et al. 1979; Taylor et al. 1989; Tripathi and Singh 1992a; Couteaux et al. 1995; Hirobe et al. 2004). Most studies on decomposition have focused on aboveground litter (e.g., Berg and Ekbohm 1991), although some authors have examined the decomposition of roots in soil (e.g., Berg 1984; Fahey et al. 1988; Bloomfield et al. 1993; Ostertag and Hobbie 1999). These studies reported different patterns of decomposition, for example, roots decompose faster (Hobbie 1996; Ostertag and Hobbie 1999) or slower (Aber et al. 1990; Bloomfield et al. 1993) than foliage depending on their initial tissue chemistry, especially concentrations of lignin and N (Bloomfield et al. 1993; Hobbie 1996; Berg and McClaugherty 2003). The root chemistry has been also reported to differ for various tree species on increasing soil N availability gradients (Hendricks et al. 2000; Tripathi et al. 2005). The importance of fine roots in the biogeochemistry of forests has been recognized recently (Vogt et al. 1995; Jackson et al. 1997), but the studies on different aspects of fine roots including their decomposition and nutrient release patterns are still scanty.
The forest canopy in DEF closes over the entire year, whereas in DDF the canopy is open in the dry season because the vegetation consists of mainly deciduous trees (Sahunalu and Dhanmaonda 1995). Due to variations in litter quality and local climate conditions in two forests in northeastern Thailand, it is expected that the root decomposition may differ between DEF and DDF. Litter decomposition regulation by rainfall is expected under such a condition in DDF. In addition, different patterns of the decomposition of root litter for DEF and DDF may result in different contributions to nutrient cycling in the forest ecosystems. Here, we examined the root decomposition and associated nitrogen dynamics in two forest stands, a DEF and DDF, in northeastern Thailand. The objective of this study was to clarify the importance of root chemistry and local climate on the belowground decomposition pattern in two major types.
Materials and methods
Study site
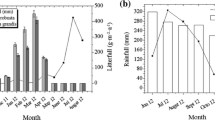
The study was conducted in a DEF and DDF at the Sakaerat Environmental Research Station [14°30′N, 101°55′E, 200–800 m in elevation], in the southeast fringe of the Korat Plateau in northern Thailand. The mean annual temperature and precipitation in this area are 26.2°C and 1,240 mm, respectively (Sakurai et al. 1998). However, precipitation during the present study (from January 2004 to February 2005) was 580 mm, a much drier condition than is usual (Fig. 1). The extremely dry season was between October and February with monthly precipitation of less than 30 mm, and this was a longer period than usually occurring. There was no rainfall from December 2004 to February 2005. General characteristics of the study sites are summarized in Table 1. The acidic forest soils are Ultisols derived from Mesozoic sandstone (Sahunalu and Dhanmanonda 1995; Sakurai et al. 1998; Soil Survey Staff 1992). The dominant tree in the DEF was Hopea ferrea, whereas the DDF site was dominated by mixed deciduous trees including Shorea obtusa (Dipterocarpaceae), Xylia xylocarpa, (Leguminosae–Mimosoideae), and Pterocarpus macrocarpus (Leguminosae–Papilionoideae) (Toda et al. 2007). No individuals of H. ferrea were observed at the DDF site. Dwarf bamboo (A. pusilla) dominated in understory vegetation in the DDF. Because of the seasonal dry conditions and human activities, the DDF area experiences frequent burning.
Litter bag preparation
In January 2004, roots were dug up from two forest sites using several soil monoliths approximately 15 cm in depth, washed with tap water and dried at room temperature for 2 days. Since the DEF site was a pure stand of H. ferrea, the roots recovered from this site were considered as H. ferrea roots. For this site, H. ferrea roots were categorized into fine root (<2 mm) and small root (2–5 mm) size classes. However, for the DDF site, the roots were separated into mixed tree fine roots and dwarf bamboo fine roots of less than 2 mm diameter. The mixed tree and dwarf bamboo roots were distinguished by texture and color. These roots were cut into small pieces (ca. 2 cm), mixed thoroughly and enclosed in 10 cm × 10 cm nylon net (mesh size 1 mm) bags. The litter bags contained 3 g of air-dried root material or 1 g in the case of bamboo roots. A total of 260 litter bags were prepared (65 for each root type).
Decomposition experiment
Sixty points for root bags in a 50 m × 50 m quadrat were established for each of the DEF and DDF sites. Adjacent points were at least 2 m apart. The decomposition experiments began on 24 and 25 January 2004. A fine root bag and small root bag of H. ferrea were buried in each point at the DEF site, and a mixed tree root bag and bamboo root bag in each point at the DDF site. The two root bags in each point were placed approximately 10 cm apart.
Split holes were carefully dug in the surface soil to a 5-cm depth at a 30–45° angle using a single-blade plow, with care being taken to avoid soil disturbance, and the litter bags were inserted. Five bags of each type were brought back to the laboratory for the analysis of initial litter chemistry. Ten litter bags of each root type were collected on each of six occasions at intervals of 2 or 3 months; litter bags were collected on 30 April, 29 June, 19 August, 21 October, 4 December 2004, and 19 February 2005. After recovery, litter bags were opened and roots were collected carefully avoiding adhering soil particles. The root materials were oven-dried at 70°C for 2 days and weighed.
Chemical analysis
The different root samples were ground with a vibrating sample mill (TI-100, CMT Co. Ltd) and chemically analyzed. The total C, N and ash contents were determined for all root litter samples, and the total carbohydrate (TCH) and acid-insoluble fraction (AIS) contents were determined for initial root litter samples. The AIS contents of the initial roots were determined by gravimetry according to a standardized method using hot sulfuric acid digestion (King and Heath 1967). The ethanol–benzene extractive-free residue was digested with 72% H2SO4 for 2 h, and secondary hydrolysis was performed in an autoclave at 120°C for 1 h after dilution of H2SO4 to a concentration of 2.5%. The autoclaved solution was filtered through a porous crucible (G4), and residue was dried at 105 °C and weighed as the AIS. The glucose equivalent TCH content of the filtrate was determined using the phenol–sulfuric acid method (Dubois et al. 1956).
Total C and N concentrations were determined by combustion (SUMIGRAPH NC-900, Sumika Chemical Analysis Service, Osaka, Japan). The residue after C and N combustion was weighed as an ash content measurement. As the litter bags settled in surface soils during the experiment, soil particles were likely to enter the bags, and this would lead to erroneous estimation of weight loss and chemical analysis. To avoid such an effect, the litter weight and AIS, TCH, C and N contents were expressed as ash-free weights, while ash concentrations in initial root litters were expressed as dry weights.
Statistical analysis
The decomposition rate of each type of root litter was calculated using an exponential decay model (Olson 1963):
where k is the decay rate per year, M 0 is the mean dry weight of the initial litter bag, and M t is the litter weight after t years. Linear regression was applied for the N concentration and accumulated mass loss of ash-free weight to explain the N dynamics of organic matter decomposition. The N concentration was treated as a dependent variable, and accumulated mass loss was treated as an independent variable.
An analysis of variance (ANOVA) test was used for comparison of initial chemistry of four root types. Concentrations of N, TCH and ash and ratios of C:N and AIS:N were log-transformed before the ANOVA test to improve the equalities of variance. Scheffe’s test was performed for a probability of 5% when significant difference was detected by the ANOVA test. The relationship between the mass loss of each root type and climate conditions comprising the mean temperature and sum of precipitation during each period of litter bag collection was tested by Pearson’s correlation analysis.
Results
Initial root chemistry
Significant differences in chemical characteristics were observed among root types (Table 2). The small root of H. ferrea had less N, AIS, ash and more TCH than the other roots. The fine root of dwarf bamboo in DDF was characterized by the high concentrations of TCH and ash. The initial chemistry of the mixed tree roots in DDF was similar to that of the fine root of H. ferrea, although it had higher C:N and AIS:N ratios. The order of C:N and AIS:N ratios was the fine root of H. ferrea < the fine root of dwarf bamboo in DDF < the fine roots of mixed trees in DDF < the small root of H. ferrea.
Decomposition pattern of roots
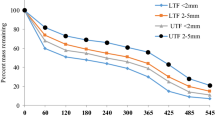
The decomposition rate of root litters varied among root types (Fig. 2), and the order was the fine root of H. ferrea (k = 1.27) > the fine roots of mixed trees in DDF (k = 0.73) > the fine root of dwarf bamboo (k = 0.66) > the small root of H. ferrea (k = 0.55). At the end of the experiment, the ash-free mass remainings of root litters were 36, 63, 54 and 53% of the fine root of H. ferrea, the small root of H. ferrea, the fine root of mixed trees in DDF and the fine root of dwarf bamboo, respectively. The exponential model fitted well for the fine roots of H. ferrea, mixed trees and dwarf bamboo in 1-year decomposition (R 2 = 0.53–0.61). The weight of root litter regularly decreased during the first 5 months for each root type of root (Fig. 2). After 5 months of the experiment, there was greater variation in the change in weight remaining. Mass loss during each period of litter bag collection did not significantly correlate to the mean temperature for each root type (r < 0. 49, P > 0.3 for all four roots). The mass loss of the dwarf bamboo root was relatively highly correlated to the precipitation during each period of litter bag collection (r = 0.65), although statistically insignificant (P = 0.18). For the other roots, mass loss during each period of litter bag collection did not significantly correlate to the precipitation (r < 0. 51, P > 0.3).
Mean weight remaining for litters of Hopea fine roots (a) and Hopea small roots (b) in the dry evergreen forest and mixed tree fine roots (c) and dwarf bamboo fine roots (d) in the dry dipterocarp forest. The weights remaining were calculated based on the ash-free weights of litters. Bars indicate standard deviations. Calculated decomposition rate constants (k, year−1) and their determinant coefficient (R 2) are also shown in the figure
N dynamics in root decomposition
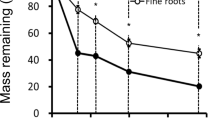
During the experiment, the N concentration in the ash-free faction of root litters increased in the fine and small roots of H. ferrea at the DEF site (Fig. 3). The C:N ratio decreased 65% by the end of the experiment owing to decomposition. In contrast, the N concentration and C:N ratio were constant in the root litters of mixed trees and dwarf bamboo at the DEF site (Fig. 3). Except for the small root of H. ferrea, the N content in the litter bags decreased with time (Fig. 4). Final N contents in the litter bags were 54, 91, 61 and 49% of initial N contents for the fine root of H. ferrea, the small root of H. ferrea in the DEF, and the fine roots of mixed trees and bamboo in the DDF, respectively.
The relationship between ash-free mass loss and N concentration differed for the two forests (Fig. 5). Fine and small roots of H. ferrea in the DEF site exhibited a significant increase in the N concentration with accumulated mass loss (P < 0.001), whereas linear regression of mass loss to N concentration was not significant for the fine roots of mixed trees and dwarf bamboo at the DDF site.
Relationship between accumulated mass loss of the ash-free fraction and nitrogen concentration during decomposition of Hopea fine roots (a), Hopea small roots (b), mixed tree fine roots (c) and bamboo fine roots (d). Results of linear regression are indicated in each graph. ***P < 0.001, ns not significant
Discussion
The rates of root decomposition in DEF and DDF were comparable to those previously reported in tropical or subtropical regions (Table 3). The ratios of C:N and AIS:N in the fine roots of H. ferrea, mixed trees and dwarf bamboo were also in the range reported by other studies. Generally, the initial litter chemistry affects the decomposition rates and nutrient dynamics. The litter decomposition rate inversely correlated to C:N and AIS:N (or lignin:N) ratios (Aber et al. 1990; Takeda and Abe 2001). In our study, the small root of H. ferrea had higher C:N and AIS:N ratios than the other studies, except that of Ostertag and Hobbie (1999). Such chemistry was likely to retard decomposition of the small root litters, resulting in a slower decomposition rate compared to the other studies.
The difference in decomposition rates among the woody root litters seems to be explained by the variation of C:N and AIS:N; the decomposition rates of the fine and small roots of H. ferrea and the fine roots of mixed trees were negatively related to C:N and AIS:N in our results (Fig. 2; Table 2) and in comparison to the other studies (Table 3). The only exception was the decomposition of bamboo roots. In spite of similar C:N and AIS:N ratios to those for the fine root of H. ferrea, the decomposition of the bamboo roots was slower than that for the fine roots of mixed trees at the DDF site. In addition, the decomposition of the bamboo root in our study was much slower than in a study in India (Tripathi and Singh 1992b). The reason for the slow decomposition of bamboo roots at our study site may be related to abiotic conditions prevailing at these sites and the intrinsic litter properties other than the litter quality determined by us and thus still remains to be clarified.
For our study sites, a leaf decomposition rate of 1.0–1.4 year−1 in H. ferrea at the DEF site and of 1.3–2.5 year−1 in trees at the DDF site have been reported (Takeda and Tian 2003). Except for the fine root of H. ferrea, the decompositions of the roots were slow compared to the leaf decomposition. The slow decomposition in roots could be related to the higher AIS concentrations or AIS:N of the four root litters than that of the lignin concentrations or lignin:N of leaves reported at the study site; lignin:N ratios in fresh leaf-litters ranged approximately from 15 to 30 (recalculated from Takeda and Tian 2003). Except for the fine root of H. ferrea, AIS:N in the root litters were higher than lignin:N of the leaves at the corresponding sites. Such differences could result in the slower decomposition of root litters, because a higher AIS:N ratio decreases the decomposition rate of fine litters (Aber et al. 1990).
We initially hypothesized that rainfall regulation of root decomposition would be observed in the DDF. However, the relationship between the decomposition rates of the roots and climatic conditions was weak in both the DEF and DDF, although the decomposition rate of the dwarf bamboo correlated with the seasonal change in precipitation. These results are in contrast to previous studies in India (Tripathi and Singh 1992b) and northern Japan (Tripathi et al. 2006). Ishikawa et al. (2007) reported that the moisture condition could not explain faster litter decomposition in a clear-cutting site in a temperate forest. They speculated the effect of clear-cutting on the mass loss of leaf litter varied among litter species in a temperate forest. Furthermore, the root decomposition in our study seems to be species specific and associated to the initial root chemistry rather than the local climate.
During the experiment, the N concentration increased in the fine and small roots of H. ferrea (Fig. 3). The increase in N concentration was explained by the accumulated mass loss at the DEF site (Fig. 5). Because of the rapid mass loss rate, N release was observed in the fine root of H. ferrea after 7 months decomposition, whereas N release was insignificant in the small root of H. ferrea during the 12-month decomposition (Fig. 4). The absence of N release in the small root of H. ferrea was partly due to the initial chemistry. Substrates with higher C:N and/or AIS:N ratios have low N availability for decomposers. In general, therefore, litters with high C:N and/or AIS:N ratios exhibit N immobilization associated with slow decomposition (Melillo et al. 1982; McClaugherty et al. 1984; Staaf and Berg 1982). In our study, the initial C:N and AIS:N ratios in the small root of H. ferrea were relatively high and thus N availability was likely to be low for decomposers. These chemical characteristics might prevent N release during the 12-month decomposition.
The N contents in the small root of H. ferrea and bamboo root increased over 11 months (until December), although differences between 9 and 11 months were not significant. At our study site, the dry season starts in December and soil moisture decreases. Although the xeric condition could retard the litter decay and N release, the absence of a significant seasonal pattern indicates that the effects of the xeric condition on root decomposition were weak in DEFs and DFFs in Thailand.
In spite of the similar C:N and AIS:N ratios to those of the fine root of H. ferrea, N release from the bamboo roots had a different pattern. The N concentration of the bamboo root litter did not correspond to the accumulated mass loss (Fig. 5), indicating the absence of N immobilization during decomposition. The highest N release was observed in the bamboo root decomposition among the roots we examined. By the end of the experiment, the N remaining in the bamboo roots had decreased to 42% of the initial N content, whereas the N remaining in woody roots was over 55% of the original content. In addition, a substantial decrease in N content in the bamboo roots was observed in the first 3 months, whereas the woody fine roots retained N over the first 5 months. This difference in N release indicates that decomposition and N dynamics in bamboo roots proceed under a mechanism different to that for woody species.
The rapid N release from bamboo roots suggests implications in N cycling in the DDF. Whereas N immobilization in root and leaf litters of woody plants (this study; Takeda and Tian 2003) acts as a N reservoir, bamboo root litter can be a N source in soil systems. The N release from root decomposition in bamboo may have a significant effect on N cycling at the DDF site, because a relatively large amount of N is accumulated in bamboo at this site (Toda et al. 2007). Our results suggest the litter production of dwarf bamboo would accelerate rapid N turnover in DDF.
In conclusion, the present study showed that the decomposition of H. ferrea fine roots at DEF sites decomposes faster than the roots at DDF sites. The difference in the decomposition rates of the roots was related to initial chemistry of the roots rather than the climate condition. The N concentration in the root litters increased with decomposition in the roots of H. ferrea at the DEF site. On the other hand, rapid N release was observed in the dwarf bamboo roots at the DDF site, suggesting a contribution to soil N availability.
References
Aber JD, Mellilo JM, McClaugherty CA (1990) Predicting long-term patterns of mass loss, nitrogen dynamics, and soil organic matter formation from initial fine litter chemistry in temperate forest ecosystems. Can J Bot 68:2201–2208
Aber JD, Mellilo JM (2001) Terrestrial ecosystems, 2nd edn. Academic, San Diego
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Arunachalam A, Pandey HN, Tripathi RS, Maithani K (1996) Fine root decomposition and nutrient mineralization patterns in a subtropical humid forest following tree cutting. For Ecol Manage 86:141–150
Berg B (1984) Decomposition of root litter and some factors regulating the process: long-term root litter decomposition in a Scots pine forest. Soil Biol Biochem 16:609–617
Berg B, Ekbohm G (1991) Litter mass-loss rates and decomposition patterns in some needle and leaf litter types: long-term decomposition in a Scots pine forest, VII. Can J Bot 69:1449–1456
Berg B, McClaugherty CA (2003) Plant litter, decomposition, humus formation, carbon sequestration. Springer, Berlin
Berg B, Staaf H (1980) Decomposition rate and chemical changes of Scots pine needle litter. Ecol Bull 32:373–390
Bloomfield J, Vogt KA, Vogt DJ (1993) Decay rate and substrate quality of fine roots and foliage of two tropical tree species in the Luquillo Experimental Forest, Puerto Rico. Plant Soil 150:233–245
Couteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fahey TJ, Hughes JW, Pu M, Arthur MA (1988) Root decomposition and nutrient flux following whole-tree harvest of northern hardwood forest. For Sci 34:744–768
Hendricks JJ, Aber JD, Nadelhoffer KJ, Hallet RD (2000) Nitrogen controls on fine root substrate quality in temperate forest ecosystems. Ecosystems 3:57–69
Hirobe M, Tokuchi N, Wachrinrat C, Takeda H (2003) Fire history influence on the spatial heterogeneity of soil nitrogen transformations in three adjacent stands in a dry tropical forest in Thailand. Plant Soil 249:309–318
Hirobe M, Sabang J, Bhatta BK, Takeda H (2004) Leaf-litter decomposition of 15 tree species in a lowland tropical rain forest in Sarawak: decomposition rates and initial litter chemistry. J For Res 9:341–346
Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66:503–522
Ishikawa H, Osono T, Takeda H (2007) Effects of clear-cutting on decomposition processes in leaf litter and the nitrogen and lignin dynamics in a temperate secondary forest. J For Res 12:247–254
Jackson RB, Mooney HA, Schulze E-D (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci USA 94:7362–7366
Kanzaki M, Yoda K, Dhanmanonda P (1995) Mosaic structure and tree growth pattern in a monodominant tropical seasonal evergreen forest in Thailand. In: Box EO, Peet RK, Masuzawa T, Yamada I, Fujiwara K, Maycock PF (eds) Vegetation science in forestry. Kluwer Academic Publishers, Dordrecht, pp 499–517
King HGC, Heath GW (1967) The chemical analysis of small samples of leaf material and the relationship between the disappearance and composition of leaves. Pedobiologia 7:192–197
McClaugherty CA, Aber JD, Melillo JM (1984) Decomposition dynamics of fine roots in forested ecosystems. Oikos 42:378–386
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Ostertag R, Hobbie SE (1999) Early stages of root and leaf decomposition in Hawaiian forests: effects of nutrient availability. Oecologia 121:564–573
Sahunalu P, Dhanmaonda P (1995) Structure and dynamics of dry dipterocarp forest, Sakaerat, northeastern Thailand. In: Box EO, Peet RK, Masuzawa T, Yamada I, Fujiwara K, Maycock PF (eds) Vegetation science in forestry. Kluwer Academic Publishers, Dordrecht, pp 465–494
Sakurai K, Tanaka S, Ishizuka S, Kanzaki M (1998) Differences in soil properties of dry evergreen and dry deciduous forests in the Sakaerat Environmental Research Station. Tropics 8:61–80
Soil Survey Staff (1992) Keys to soil taxonomy. SMSS Technical Monograph no 19, Pocahontas Press, Blacksburg
Staaf H, Berg B (1982) Release and accumulation of plant nutrients in needle litter during decomposition: long-term decomposition of Scots pine needle litter, II. Can J Bot 60:1561–1568
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. University of California Press, Berkeley
Takeda H (1998) Decomposition processes of litter along a latitudinal gradient. In: Sassa K (ed) Environmental forest science. Kluwer Academic Publishers, London, pp 197–206
Takeda H, Abe T (2001) Templates of food-habitat resources for the organization of soil animals in temperate and tropical forests. Ecol Res 16:961–973
Takeda H, Tian X (2003) Decomposition of leaf litter in DDF and DEF in the Sakaerat field station. In: Takeda H, Khamyong S, Wiwatiwitaya D (eds) Final report for NRCT project: decomposition and nutrient cycling processes in tropical seasonal forests in Thailand. Kyoto University, Kyoto, pp 52–68
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104
Toda T, Takeda H, Tokuchi N, Ohta S, Wacharinrat C, Kaitpraneet S (2007) Effects of forest fire on the nitrogen cycle in a dry dipterocarp forest, Thailand. Tropics 16:41–45
Tripathi SK, Singh KP (1992a) Abiotic and litter quality control during the decomposition of different plant parts in dry tropical bamboo savanna in India. Pedobiologia 36:241–256
Tripathi SK, Singh KP (1992b) Nutrient immobilization and release patterns during plant decomposition in a dry tropical bamboo savanna, India. Biol Fertil Soils 14:191–199
Tripathi SK, Sumida A, Shibata H, Uemura S, Ono K, Hara T (2005) Growth and substrate quality of fine root and soil nitrogen availability in a young Betula ermanii forest of northern Japan: effects of the removal of understory dwarf bamboo (Sasa kurilensis). For Ecol Manage 212:278–290
Tripathi SK, Sumida A, Shibata H, Ono K, Uemura S, Kodama Y, Hara T (2006) Leaf litterfall and decomposition of different above- and belowground parts of birch (Betula ermanii) trees and dwarf bamboo (Sasa kurilensis) shrubs in a young secondary forest in Northern Japan. Biol Fertil Soils 43:237–246
Vogt KA, Vogt DJ, Palmiotto PA, Boon P, O’Hara J, Asbjornsen H (1995) Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant Soil 187:159–219
Acknowledgments
The authors thank Dr. F. Hyodo, Dr. T. Osono, Mr. T. Toda and Mr. A. Shimizu at Kyoto University for their valuable comments on the experimental design. We also thank the staffs at the Royal Forest Department in Thailand and Sakaerat Environmental Research Station for their assistance in our experiment.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fujimaki, R., Takeda, H. & Wiwatiwitaya, D. Fine root decomposition in tropical dry evergreen and dry deciduous forests in Thailand. J For Res 13, 338–346 (2008). https://doi.org/10.1007/s10310-008-0087-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10310-008-0087-3