Abstract
The hepatic regulatory role in metabolism involves exposure to a diverse array of xenobiotic compounds leading to the potential accumulation of harmful toxins and subsequent manifestation of hepatic conditions such as steatosis, fibrosis, and cirrhosis. Herbs as part of culinary and traditional uses have demonstrated therapeutic effects against such conditions. Predominant among the dietary constituents are polyphenols and terpenoids, which are known for their liver-protective efficacies. Evident from their antioxidant and anti-inflammatory properties and modulation of antioxidant enzymes this class of phytochemicals regulates pivotal liver biomarkers. Ocimum species, notably Tulsi, are recognized for their abundant repertoire of terpenoids and polyphenols. From time immemorial, owing to its diverse biological properties, the Ocimum species has made its way into our culinary habits and traditions. Clinical findings have indicated the beneficial effects of Ocimum sanctum on the biochemical parameters of the liver in young overweight/obese subjects. However, despite several pre-clinical, and clinical studies elucidating the hepatoprotective potential of Ocimum species, a comprehensive understanding of the molecular mechanism underlying the actions of phenolic and terpenoid phytoconstituents is lacking till date. Consequently, targeted molecular therapies involving Ocimum species are yet to be developed. Thus, this mechanistic review was aimed at elucidating the intricate molecular pathways through which polyphenols and terpenoids derived from Ocimum species exert their hepatoprotective effects. By correlating these molecular mechanisms, insights into the hepatoprotective abilities of polyphenols and terpenoids found in Ocimum species were provided, which may pave the way for potential targeted therapeutic interventions.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver, being the largest glandular organ, assumes a pivotal role in biotransformation, as well as the synthesis and storage of carbohydrates, lipids, alcohol, and toxins, while concurrently executing the detoxification of xenobiotics and toxic by-products (Saha et al. 2019). Despite its protective mechanisms, the liver remains continuously exposed to harmful substances and bio-transformed toxic products, many of which accrue within its confines, thereby instigating pathological manifestations such as hepatic steatosis, fibrosis, and cirrhosis (Fig. 1). Hepatic pathology contributes to over two million fatalities annually, constituting 4% of global mortality, with a notable prevalence among females (Devarbhavi et al. 2023). Alcohol consumption escalates the hazard of hepatopathy-associated death by a staggering factor of 260. Excessive alcohol consumption leads to liver damage, including fatty liver, alcoholic hepatitis, and cirrhosis (Lieber 2020). The worldwide frequency of alcohol-induced hepatitis has surged in recent times, particularly among adolescents and females, concomitant with a heightened susceptibility to alcohol-induced hepatic cirrhosis. Hepatic cirrhosis precipitates a 5- to tenfold elevation in mortality risk, primarily attributable to complications such as ascites, variceal hemorrhage, hepatic encephalopathy, renal impairment, and infections, alongside instances of acute-on-chronic hepatic failure (Wu et al. 2024). Sametime, non-alcoholic fatty liver disease (NAFLD) afflicts 32.4% of the global populace, affecting one-fourth of adults worldwide, and ranks as the second principal etiology of end-stage hepatic afflictions and transplantation surgeries across Europe and the Americas (Le et al. 2024). Notably, 75% of hepatocellular carcinomas manifest in Asia, are primarily linked to hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. HBV constitutes a predominant catalyst for hepatic mortality across numerous nations, with China, India, and Nigeria bearing the brunt of HBV-associated hepatic morbidity (Balakrishnan and Rehm 2024).
Etiology of hepatotoxicity (Mężyńska et al. 2019). Persistent chronic exposure to hepatic insults caused by environmental factors (such as viral hepatitis, alcohol abuse, non-alcoholic steatohepatitis (NASH), exposure to toxins, and drug metabolites) repeatedly damages hepatocytes and induces inflammation, ultimately leading to liver cirrhosis. At this stage, the liver is prone to genomic instability. Consequently, hepatocytes are more likely to accumulate somatic changes, epigenetic modifications, and gene rearrangements, resulting in metabolic changes and alterations in molecular pathways. These dysregulations drive tumor progression and metastasis. ↓ Decrease, ↑ Increase
The liver plays a vital role in alcohol detoxification, especially in processing ethanol. Ethanol, the primary psychoactive component in alcoholic beverages, is metabolized in the liver by alcohol dehydrogenase (ADH) and other enzymes [cytochrome P450 2E1 (CYP2E1) and catalase] into acetaldehyde, which is further metabolized into acetate by aldehyde dehydrogenase (ALDH). Acetate is further broken down into water and carbon dioxide facilitating the elimination from the body. Alike alcohol liver also processes toxicants like acetaminophen and aflatoxins. Acetaminophen, a common over-the-counter antipyretic and pain reliever, causes liver damage primarily through the production of a toxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI). The liver normally detoxifies NAPQI by conjugating it with glutathione. However, in overdose situations, stored glutathione can become depleted, leading to liver injury (McGill and Jaeschke 2020; Ramachandran and Jaeschke 2021). Aflatoxins are toxic metabolites produced by certain fungi, such as Aspergillus flavus and Aspergillus parasiticus, which commonly contaminate food crops like peanuts, corn, and cereals. Continuous exposure to this toxin can cause liver damage and increase the risk of liver cancer. The liver metabolizes aflatoxins through a process involving cytochrome P450 enzymes, leading to the formation of reactive intermediates that can bind to DNA and proteins, causing cellular damage (Guengerich et al. 1996).
All these factors primarily function as prooxidants, instigating the generation of reactive oxygen species (ROS) or reactive nitrogen species (RNS), consequently inducing oxidative stress. In the context of chronic alcohol intoxication, the hydroxyethyl radical (CH3C·HOH), in conjunction with ROS, has the potential to initiate oxidative liver damage (Unsal et al. 2021). Cytochrome P450 isozymes significantly elevate endogenous production of CH3C·HOH in the endoplasmic reticulum. Free radicals can initiate cellular damage through diverse mechanisms, including lipid peroxidation (LPO), covalent binding, depletion of glutathione and protein thiols, disruption of intracellular free calcium homeostasis, and DNA fragmentation (Poli, 1993). LPO has emerged as a pivotal mechanism instigating irreversible hepatocyte damage and eliciting fibrotic responses induced by haloalkanes (Recknagel et al. 2020).
Dietary interventions have emerged as efficacious antioxidant strategies for mitigating and neutralizing the detrimental effects of ROS/RNS. A diet enriched in natural antioxidants (polyphenols, carotenoids, lignans, etc.) augments endogenous antioxidant enzyme defense including superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and catalase, or the fortification of non-enzymatic defenses, such as glutathione and vitamins. Antioxidants exhibit the capability to delay, inhibit, or prevent oxidation by scavenging free radicals and attenuating oxidative stress. However, in pathological conditions, the defense against ROS is compromised or impaired, leading to an increased oxidant load. Under such circumstances, an external supply of antioxidants becomes imperative to counteract the deleterious consequences of oxidative stress (Vladimir-Knežević et al. 2012).
While synthetic hepatoprotective drugs demonstrate adeptness in scavenging free radicals, their prolonged usage can induce toxicity, resulting in inflammation and carcinogenicity. Consequently, herbal therapeutic approaches are consistently regarded as alternatives, capable of targeting diseases with minimal side effects. The current utilization of plants to derive "lead molecules" in drug development underscores their potential as natural bioactive compounds or structural analogs, holding promise as drug candidates for hepatoprotection (Saha et al. 2019).
Natural phenolics and terpenoids represent prevalent components in plant-based diets globally. Polyphenols, renowned as leading antioxidants, exhibit mechanisms for scavenging free radicals, breaking free radical chain reactions, suppressing free radical formation by enzyme activity regulation, and chelating metal ions involved in free radical production (Vladimir-Knežević et al. 2012; Simón et al. 2020). Many of these activities are effective in mitigating oxidative liver damage. Similarly, terpenoids, naturally occurring hydrocarbon compounds, and their oxygenated derivatives like alcohols, aldehydes, and ketones (Gutiérrez-del-Río et al. 2021), showcase hydrogen-donating or radical-scavenging activities. Monoterpenes and diterpenes, along with their effectiveness in inhibiting LPO, protect the liver by binding with ions of toxic metals (Simón et al. 2020). In some instances, this can be attributed to their phenolic content, including carnosol, carnosic acid, carvacrol, or thymol (Graßmann, 2005). Thus, a diet rich in both polyphenols and terpenoids stands as an alternative to hepatoprotective drugs.
The genus Ocimum, or Tulsi, holds a sacred status in Hindu belief. It has secured its place in ancient Ayurvedic medical literature due to its myriad therapeutic values. Various species within the Ocimum genus possess medicinal properties, including hypoglycemic, antibacterial, antimicrobial, antifungal, cardiac, and hepatoprotective effects, pain management, and alleviation of depression and general stress. Traditionally, Tulsi is consumed in various forms such as herbal tea, dried powder, or fresh leaves for treating common colds, headaches, stomach disorders, inflammation, and various forms of poisoning (Zahran et al. 2020). Due to their diverse properties various marketed herbal formulations of Tulsi are available, such as Tulsi Hill capsules, Tulsi Ghanwati tablets, Tulasi Respiratory Wellness Tablets (Himalaya, Himalaya Wellness Company, Makali, Bangalore—562,162, Karnataka, India), Baidyanath Tulsi Tablets (Pandey et al. 2015).
Among the 150 species of the Ocimum genus, Krishna Tulsi or purple Basil (O. tenuiflorum), Green Tulsi or Rama Tulsi (O. sanctum), African Basil or clove Basil (O. gratissimum), Sweet Basil (O. basilicum), Damakese (O. lamiifolium), Rosary Tulsi (O. canum), and Hoary basil or (O. americanum) are the most prevalent. While these species are distributed widely across temperate zones globally, a majority is concentrated in Africa, cultivated throughout the Indian subcontinent, and Southeast Asia for their nutraceutical values and essential oil content (Pandey et al. 2014; Zahran et al. 2020; Gurav et al. 2022).
The Ocimum genus is recognized for its abundance of polyphenols (Apigenin, Quercetin, Rutin, Rosmarinic acid, Ferulic acid) and terpenoids (α-copaene, β-elemene, β-caryophyllene, α-humulene, and germacrene D). Various Ocimum species have undergone investigation for their hepatoprotective potential in diverse in vivo models of hepatotoxicity. Nevertheless, the precise molecular mechanisms underlying hepatoprotection attributed to the phenolic or terpenoid metabolites remain undefined thus far. Similarly, there exists a knowledge gap concerning the mechanistic insights into various other therapeutic compounds present in Ocimum. Consequently, this review aims to establish a correlation between the polyphenolic and terpenoid compounds within Ocimum species and their respective molecular mechanistic pathways involved in the prevention of hepatotoxicity.
Review strategy
To assess the real scenarios of polyphenolics and terpenoids from Ocimum for the treatment of different liver illnesses, a literature search was conducted for papers published till December 2023, without any restriction of time, using online databases including Science Direct, Springer, Wiley online library, Pubmed, Google Scholar, Web of Science and Scopus. Following search terms were used either alone or in combination: medicinal plants, herbal medicine, Ocimum species, hepatotoxicity, hepatoprotective, phytochemicals present in Ocimum species, polyphenols present in Ocimum species, terpenoids present in Ocimum species, polyphenols as antioxidant, polyphenols in hepatotoxicity, terpenoids in oxidative stress, terpenoids in hepatoprotection, Ocimum species hepatoprotection study. The accurate scientific designation of the plant was determined by consulting The Plant List database (theplantlist.org). Utilizing ChemDraw Professional v.17.1 software, the chemical structures of naturally transpiring metabolites previously recognized in Ocimum spp. were delineated.
The papers that were excluded were those written in any language other than English, conference proceedings, research involving non-phenolics and non-terpenoids from Ocimum, and articles without information on liver toxicity. Counting of research publications whose complete text or abstract is unavailable was avoided, well-illustrated, cited as well as recent full-text articles were chosen after careful consideration. The chosen studies were acquired, and pertinent papers were picked for full-text analysis. Following such a specific search, polyphenols and terpenoid compounds present in Ocimum have been reported from 40 reports, 40 pre-clinical and 2 clinical studies with Ocimum imparting hepatoprotective potential, over 86 recent studies of phenolic and terpenoid compounds present in Ocimum that can treat hepatic disorders were brought to light.
Natural molecules from Ocimum
Phenolic compounds and terpenoids are prominent classes of compounds abundantly found in Ocimum species (Pandey et al. 2016). Plant phenolics encompass simple phenols, phenolic acids, and flavonoids. Phenolic acids, deriving primarily from benzoic acid, cinnamic acid, and phenylacetic acid, exhibit a pharmacophore in the form of a methyl ester within the phenol ring, facilitating interaction with various protein targets in cell membranes. Bioactive flavonoids such as apigenin, luteolin, baicalin, quercetin, rutin, and kaempferol have been identified in Ocimum species (Table 1). Flavonoids, synthesized from cinnamic acid, exhibit a structural diversity that can undergo modifications such as skeleton dimerizations, oligomerizations, prenylations, glycosidations, and conjugation with other ring systems. O. tenuiflorum, or Krishna Tulsi, serves as a rich source of plant pigments, including seasonally varying quantities of anthocyanins and anthocyanidins, with peonidin, cyanidin, and delphinidin derivatives being common.
Terpenoids, distinguished by their diverse chemical structures, are categorized based on the number of isoprene units, encompassing monoterpenes, sesquiterpenes, diterpenes, sesterterpenes, triterpenes, tetraterpenes, and polyterpenes (Fox et al. 2011). Ocimum species contain various monoterpenes, such as camphor, pinene, thymol, eucalyptol, geraniol, and limonene. Additionally, hepatoprotective terpenoid compounds present in Ocimum species include linalool, borneol, ursolic acid, β-elemene, germacrene D, β-caryophyllene, epimaslinic acid, α-copaene, oleanolic acid, and humulene (Table 1; Fig. 2). The copious presence of phenolic compounds and terpenoids in Ocimum sp. imparts hepatoprotective effects (Table 2).
Hepatoprotective properties of Ocimum species
Hepatic steatosis, fibrosis, and cirrhosis constitute sequential manifestations of liver impairment induced by exposure to alcohol, pharmaceuticals, and environmental pollutants. These exposures lead to hepatocellular necrosis and heightened levels of various liver biomarkers and metabolites, indicative of pathological deviations (Leathers et al. 2019). Alcohol-induced impairment manifests in alterations to mitochondrial morphology and functionality, along with disruption of the antioxidant defense system. Concurrently, a conjugation process unfolds, resulting in escalated Caspase levels and diminished adenosine triphosphate (ATP) levels. These clinical manifestations precipitate hepatotoxicity and apoptosis accumulation (Madrigal-Santillán et al. 2015). When hepatocytes undergo injury, transaminases and glutathione enzymes emerge as primary indicators of bile metabolism. The quantification of alkaline phosphatase, alanine transaminase, and aspartate transaminase, pivotal hepatic enzymes in the serum, serves as a means to discern the clinical status of the hepatic milieu (Teofilović et al. 2021). Numerous studies involving Ocimum sp. have demonstrated the protection of the liver cells and their functions against hepatotoxic agents like drugs, chemicals, viral toxins, and apoptotic changes (Table 3).
In vitro and in vivo studies against drug-induced liver injury
Pre-treatment with Tulsi extracts proved instrumental in preserving hepatic antioxidant defenses, as evidenced by the downregulation of malondialdehyde levels and upregulation of catalase, glutathione S-transferase (GST), glutathione reductase (GR), glutathione peroxidase (GPx) levels (Table 3). Genfi et al. (2020) proposed that leaf and stem extracts of O. americanum 250 mg/kg BW administered orally in rats for 7 days, conferred substantial protection to the liver against acetaminophen-induced damage. An increased production of glutathione (GSH) and superoxide dismutase (SOD), inhibiting malondialdehyde (MDA) production, and downregulating the expression of nuclear factor-kappa B (NF-κB) and interleukin-1 (IL-1).
During hepatic injury, the formation of fibrous tissues attempts to repair the damage, impeding crucial functions of the liver such as drug detoxification, protein secretion, and albumin formation. Notably, the substantial bile acid production during ethanol oxidation induces cholestatic syndrome by activating Fas, an apoptotic element, and its expression on the plasma membrane. Consequently, total protein, bilirubin, albumin, urea, and creatinine levels serve as pivotal markers for assessing liver function (Cederbaum 2017). The majority of studies investigating the hepatoprotective effects of Tulsi extract against drug-induced liver injury have reported an elevation in total protein and albumin levels, coupled with a reduction in bilirubin, urea, and creatinine levels (Table 3). Teofilović et al. investigated the hepatoprotective potential of Ocimum basilicum (sweet basil) extract administered orally at a dose of 200 mg/kg for seven days in a model of acetaminophen-induced hepatotoxicity. The hepatoprotective effects were evidenced by an increase in the activity of antioxidant enzymes (catalase, GST, GR, and GPx), a reduction in lipid peroxidation, and a decrease in serum liver transferase enzyme activities. Additionally, the excretory liver function was preserved in animals pre-treated with the basil extract. Histopathological examination and morphometric analysis of the surface density of hepatic tissue damage corroborated the ameliorative effects of the aqueous basil extract in acetaminophen-induced liver injury as it showed a lower degree of parenchymal damage than the disease group (Teofilović et al. 2021).
ROS, potentiated by pro-inflammatory chemokine activity, are discharged through both neutrophil infiltration and Kupffer cell activation (Suraweera et al. 2015). This circumstance additionally facilitates the activation of hepatocellular stellate cells and triggers pro-fibrogenic pathways. The heightened activity of neutrophils, dormant under normal conditions but increasingly active with elevated cytokine levels, contributes to hepatic necrosis. Chronic inflammation and the concomitant ROS generation lead to hepatotoxicity, ultimately culminating in the development of the lethal condition known as hepatic cirrhosis. Nuclear involvement is evident in this condition, as numerous inflammation-associated transcription factors are upregulated. The combination of ROS and cell membrane adhesion molecules further propels liver fibrosis. Activated Kupffer cells contribute to elevated cytokine levels in circulation by inducing prostaglandin activation through COX-2, with arachidonic acid playing a pivotal role. The collective outcome of these actions results in inflammatory disorders and subsequent fibrogenesis (Zhang et al. 2019; Kanda et al. 2020; Yang et al. 2020). Histopathological examinations demonstrate that Tulsi extracts inhibit Kupffer cell stimulation leading to proinflammatory cytokine production and downregulating neutrophil infiltration (Suryani and Lubis 2019). Kumar et al. investigated the impact of methanol, ethanol, aqueous, and ethyl acetate extracts from the dried leaves and inflorescence of O. basilicum on the activity of cytochrome P450 enzymes (CYP2B6 and CYP3A4) and the esterase-mediated metabolism of rifampicin to 25-O-desacetyl rifampicin. Inhibition assays were conducted using human liver microsomes, while HepG2 cell assays were employed to measure the induction of CYP2B6/3A4 mRNA expression. These findings suggest that O. basilicum extracts have the potential to cause clinically significant herb-drug interactions (HDI) with CYP2B6 and the metabolism of rifampicin in vivo. The study identified phenolic and terpenoid compounds including rosmarinic acid (approximately 2298 mg/L in aqueous extract), caftaric acid, salvigenin (approximately 1855 mg/L in ethanolic extract), eupatorin (668.772 mg/L in ethanolic extract), rutin, and isoquercetin potentially responsible for the inhibitory effects. The predicted in vivo inhibition percentile was highest for the aqueous extract on CYP2B6 (96.7%) (Kumar et al.2020).
In vitro and in vivo studies against chemical-induced liver injury
Carbon tetrachloride is a toxic chemical compound that was historically used in cleaning agents and as a solvent. It is metabolized in the liver by cytochrome P450 enzymes, leading to the formation of highly reactive free radicals that can cause liver damage and fibrosis (Unsal et al. 2021). Nitric oxide (NO), one of those free radicals plays a regulatory role in organelle biogenesis and mitochondrial respiration. Simultaneously, nuclear factor-kappa B (NF-κB), a pro-inflammatory transcription factor, is activated and binds to the promoter of inducible nitric oxide synthase (iNOS), a crucial NO producer. iNOS, in turn, amplifies hepatic fibrosis and inflammatory cytokine expression (Cassini-Vieira et al. 2015). The aqueous extract of O. gratissimum leaves (OGE) at a dose of 0.2 mg/kg BW of male Wistar rats administered orally for 12 weeks, aids in managing acute liver injury by reducing hepatic heat shock protein 70 (HSP70) and iNOS proteins in the livers of CCl4-administered rats. Moreover, it diminishes the matrix metalloproteinases (MMP)-9/MMP-2 ratio, urokinase-type plasminogen activator (uPA) protein levels through ERK signaling, and NF-κB phosphorylation. MMP-9, a member of the MMP family, is crucial for fibrogenesis and malignancies in various liver diseases. The uPA further enhances MMP-9 expression, and ERK 1/2 and NF-κB signaling are pivotal for MMP-9 up-regulation (Chiu et al. 2012). A comparable study evaluated the in vivo and in vitro efficacy of OGE in a model of carbon tetrachloride (CCl4)-induced hepatocellular fibrosis in rats. Male Wistar rats were administered CCl4 via intraperitoneal injection and received varying oral doses of OGE (0–40 mg/kg body weight) for 8 weeks. The results demonstrated that OGE significantly attenuated liver damage, including steatosis and fibrosis, in a dose-dependent manner. Additionally, OGE inhibited the formation of lipid peroxidation products during CCl4 treatment. Furthermore, OGE reduced CCl4-induced hepatic collagen deposition and enhanced the expression of catalase, an antioxidant enzyme. Inhibition of fibrosis markers, specifically α-SMA expression, was also observed. In primary hepatic stellate cell (HSC) cultures, OGE significantly suppressed serum-induced activation and decreased both the protein and gene expression of α-SMA and type I collagen α (Chiu et al. 2012). Additionally, essential oil from O. basilicum (OBE), comprising primarily monoterpene hydrocarbons (70.3%), notably iso-menthone (38%), 1.8-cineole (13.9%), trans-sabiene (12%), and pulegone (6.4%), along with minor quantities of terpenoids (L-carvone) and sesquiterpenes (cis-α-bisabolene, trans-α-bergamotene, α-farnesene, α-humulene, trans-caryophyllene, δ-cadinene, and α-morphene) and methyl eugenol (7.5%), has been documented to stimulate hepatocyte growth factor (HGF) while concurrently downregulating CYP2E1 expression. This intervention (200 mg/kg BW IP for 8 weeks) results in reduced collagen deposition, and a decrease in α-smooth muscle actin (α-SMA) immuopositive cells, signifying mitigation of hepatic stellate cell activation by OBE in CCl4-induced liver fibrosis in rats (Ogaly et al. 2015). In another study, CCl4-induced liver fibrosis in male Wistar rats was treated with Ocimum gratissimum polyphenol extract (OGPE) at a dosage of 12 mg/kg body weight for 8 weeks. The results indicated that OGPE containing catechin, caffeic acid, and epicatechin preserved liver weight, significantly ameliorated CCl4-induced steatosis, and mitigated other histopathological alterations. Additionally, OGPE maintained serum levels of liver function markers like alanine aminotransferase (ALT) and aspartate aminotransferase (AST), as well as the levels of MDA, catalase, and α-SMA in hepatic tissues, counteracting the effects induced by CCl4. These findings suggest that the polyphenolic components present in OGPE were the primary factors for preventing fibrotic changes (Chen et al. 2015). Kamel et al. conducted a study on the hepatoprotective potential of Ocimum sanctum L. against galactosamine-induced hepatotoxicity and investigated the bioactive compounds present in its extract, alongside identifying serum metabolites. Hepatotoxicity was induced in adult albino rats through an intraperitoneal injection of galactosamine (400 mg/kg). The hydroalcoholic and alcoholic extracts of Ocimum sanctum L. (administered at 100 and 200 mg/kg body weight/day) containing bioactive compounds such as rutin, ellagic acid, kaempferol, caffeic acid, quercetin, and epicatechin were evaluated for their hepatoprotective potential. A significant reduction in serum enzymes and MDA was observed, indicating hepatoprotective effects. The study further fractionated the hydroalcoholic extract based on polarity into hexane, chloroform, and ethyl acetate fractions and assessed their hepatoprotective activity in vitro using Chang liver cells exposed to CCl4 toxicity (40 mM). Among these, the ethyl acetate fraction exhibited the highest hepatoprotective activity. This fraction contained substantial amounts of rutin (0.34% w/w), ellagic acid (2.32% w/w), kaempferol (0.017% w/w), caffeic acid (0.005% w/w), quercetin (0.038% w/w), and epicatechin (0.057% w/w), which were identified as key contributors to hepatoprotection. When compared to standard silymarin, the isolated bioactive molecules demonstrated significant hepatoprotective activity in Chang liver cells subjected to CCl4-induced toxicity (Kamel et al. 2023). To assess cytotoxicity in both malignant and non-malignant cells, specifically hepatocytes, a study was conducted utilizing HepG2 (human hepatocellular carcinoma) and freshly isolated porcine liver cells (PLP2). Among the aqueous and hydroethanolic extracts tested, the hydroethanolic extract of Ocimum citriodorum exhibited cytotoxic effects against the human tumor cell lines. Additionally, this extract demonstrated cytotoxicity towards non-malignant hepatocytes, although it presented a higher GI50 (concentration that inhibits 50% of cell growth) value compared to that observed for the cancer cell lines (Majdi et al. 2020). Selvarani et al. 2015 conducted a cytotoxicity study of silver nanoparticles synthesized using ethanol leaf extract from the stem of Ocimum kilimandscharicum against HepG2 cells. The study demonstrated an excellent IC50 value of 49 µg/mL and recommended the use of these silver nanoparticles for the effective control of liver cancer cell lines.
Clinical study against metabolic disorder-induced liver injury
A randomized, parallel-group, open-label pilot study was conducted to evaluate the effects of Ocimum sanctum on metabolic and biochemical parameters in thirty overweight/obese subjects, divided into two groups: Group A and Group B. Group A (n = 16) received one 250 mg capsule of O. sanctum extract twice daily on an empty stomach for 8 weeks, while Group B (n = 14) received no intervention. Statistically significant improvements were observed in Group A for serum triglycerides, low-density lipoprotein (LDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and Body Mass Index (BMI) after 8 weeks. The increase in HDL in the intervention group compared to the control group was also statistically significant. No significant changes were noted in liver enzymes, specifically serum glutamic-oxaloacetic transaminase (SGOT) and serum glutamic-pyruvic transaminase (SGPT), in either group. These findings indicate the beneficial effects of O. sanctum on various biochemical parameters in young overweight/obese subjects (Satapathy et al. 2017).
In vitro and in vivo studies against apoptotic changes in hepatocellular carcinoma
The in vitro and in vivo study by Huang et al. 2020, treatment with aqueous Ocimum gratissimum leaf extract (400, 600, 800 μg/mL for 24 h) in SK-Hep1 and HA22T cells demonstrated a dose-dependent reduction in apoptosis mediator caspase 3, poly (ADP-ribose) polymerase (PARP), cyclin-dependent kinase 4 (CDK4), and phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2) expressions, resulting in attenuated hepatocellular carcinoma (HCC) tumor growth. The in vivo study showed significant suppression of tumor weight in the treatment group (60 mg/kg daily oral administration for one month) compared to the control group.
In vitro and in vivo studies against viral hepatitis
Viral hepatitis is an infection that results in hepatic inflammation and damage. The various types of viral hepatitis include hepatitis A, B, C, D, and E. Hepatitis A and E are primarily transmitted via fecal–oral routes, typically through ingestion of food or water contaminated by an infected individual's feces. Hepatitis B, C, and D are transmitted through exposure to infected blood. Hepatitis B and D can also be transmitted through other bodily fluids. These transmissions can occur through activities such as sharing needles or engaging in unprotected sexual intercourse. Hepatitis A and E viruses generally cause acute infections, which are short-term and typically resolve as the immune system clears the virus. In contrast, hepatitis B, C, and D viruses can lead to both acute and chronic infections. Chronic hepatitis arises when the immune system fails to eliminate the virus, leading to persistent infection. Chronic hepatitis can result in severe complications, including cirrhosis, hepatic failure, and hepatocellular carcinoma. Studies have investigated the antiviral properties of O. basilicum extracts and its purified components against the hepatitis B virus (HBV). Results indicate that crude aqueous and ethanolic extracts, along with specific purified components such as apigenin, linalool, and ursolic acid, demonstrate significant antiviral activity. Apigenin exhibited the highest efficacy against the hepatitis B surface antigen, with a 50% effective concentration (EC50) of 7.1 mg/L, and against the hepatitis B e antigen with an EC50 of 12.8 mg/L (Chiang et al. 2005). Further research by Kubiça et al. (2014) explored the antiviral activity of O. basilicum essential oil and the monoterpenes camphor, thymol, and 1,8-cineole against the hepatitis C virus (HCV). The study assessed plaque inhibition percentages, expressed through metrics such as CC50 (50% cytotoxic concentration), IC50 (inhibitory concentration for 50% of plaques), and SI (selectivity index = CC50/IC50). Camphor (CC50 = 4420.12 µg/mL) and 1,8-cineole (CC50 = 2996.10 µg/mL) exhibited the lowest cytotoxicity and the most potent antiviral activities, with selectivity indices of 13.88 and 9.05, respectively, in the virucidal assay. These findings suggest that the monoterpenes exert their antiviral effects by acting directly on the viral particles. Apart from O. basilicum, other species, and their compounds need to be evaluated for their viral hepatitis protective efficacy.
Despite comprehensive investigations into the hepatoprotective efficacy of diverse Ocimum species against toxic agents, the majority of the research highlighted in Table 3 predominantly employed Ocimum extracts. A limited number of studies conducted phytochemical characterization to identify the specific compounds accountable for the observed protective effects. Furthermore, scant attention has been given to delineating the molecular mechanisms that underlie the hepatoprotective properties of these Ocimum species. The polyphenolic and terpenoid compounds identified in characterization studies of various Ocimum species were observed to demonstrate extensive associations with proteins and genes implicated in hepatoprotection in separate investigations.
Hepatoprotective polyphenols present in Ocimum species
Various polyphenols present in different Ocimum species have been studied for their signaling pathways, associated proteins, and genes involved in hepatoprotection. The outcomes of these studies correlate that phenolic compounds have protective potentials against hepatotoxicity (Table 4; Fig. 3). This section extensively explores and elucidates the broad molecular mechanisms of hepatoprotection by some polyphenols present in Ocimum species.
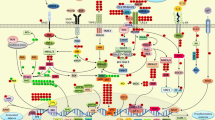
Intracellular signaling transduction mediated by polyphenols and terpenoids for the treatment of hepatocellular inflammation. Polyphenols may prevent injury in hepatocytes through several signaling pathways: (i) suppressing activation of MAPKs pathway to inhibit apoptosis; (ii) increasing β-fatty acid oxidation by upregulating PPARα; (iii) inhibiting lipogenesis via downregulation of SREBP-1c by AMPK and SIRT-1 activation; (iv) enhancing antioxidant defense through Nrf2-ARE pathway; (v) Bax, Caspase 3 suppression and Bcl 2 upregulation to promote antiapoptotic action CaA; Caffeic acid, p-CA; p- Coumaric acid, ChA; Chlorogenic acid, ChiA; Chicoric acid, RA; Rosmarinic acid, FA; Ferulic acid, GA; Gallic acid, Cya-3-g; Cyanidin-3-glucoside, OA; Oleanolic acid, UA; Ursolic acid, EA; Epimaslinic acid, AMPK; AMP-activated protein kinase, IL-6; Interleukin-6, MAPK; Mitogen-activated protein kinase, AP-1; activator protein 1, PARP; Poly (ADP-ribose) polymerases, ERK; Extracellular signal-regulated kinase, Nrf2; Nuclear erythroid related factor 2, ARE; Antioxidant response element, Keap1; Kelch like—ECH-associated protein 1, HO-1; Heme oxygenase 1, NQO1; NAD (P) H quinone oxidoreductase 1, SREBP-1c; Sterol regulatory element-binding protein 1c, ACC; Acetyl-CoA carboxylase, Bcl-2; B-cell lymphoma-2, Bax; Bcl-2-associated X protein, TNF; Tumor Necrosis Factor, NF-κB; Nuclear factor kappa B, JNK; c-Jun N-terminal kinase, IKK; Inhibitor of nuclear factor-κB kinase; RXR; Retinoid X receptor.  Inhibition;
Inhibition; Upregulation
Upregulation
Eugenol
Eugenol, belonging to the phenylpropanoid class of essential oils, is prominently present in various Ocimum species. Notably, substantial concentrations of eugenol are discerned in the leaves of O. gratissimum (79% or 0.0445 mg/g), O. sanctum (55% or 0.0943 mg/g), O. americanum (0.145 mg/g), O. basilicum (0.034 mg/g), and O. tenuiflorum (0.043 mg/g) (Pandey et al. 2016).
Recent investigations have explored the hepatoprotective potential of eugenol, unveiling its impact on molecular pathways. Fathy et al. (2019) demonstrated that eugenol activates peroxisome proliferator-activated receptor-gamma (PPAR-γ), mitigating CCl4-induced hepatotoxicity in vivo. PPAR-γ, a ligand-inducible nuclear hormone receptor, plays a pivotal role in regulating adipogenesis and metabolism. Activated PPAR-γ exhibits hepatocarcinogenesis and fibrosis-mitigating effects by downregulating NFκB, subsequently reducing the generation of profibrogenic factors, such as kupffer cells, leading to diminished NO formation and reactive species production.
In a parallel mechanism, eugenol was found to ameliorate cadmium toxicity in the liver, showcasing its versatility in hepatoprotection (Kumar et al. 2021). Additionally, eugenol contributes to a reduction in the activation of the microsomal enzyme CYP2E1, resulting in a decreased capacity for toxicant biotransformation within the liver (Yogalakshmi et al. 2010).
Rosmarinic acid
Rosmarinic acid (RA), classified as a phenolic acid within the hydroxy-cinnamic acid subclass, manifests hepatoprotective attributes, particularly evident in its ability to counter hepatic ischemia and reperfusion injury. This protective mechanism involves the inhibition of the NFκB signaling pathway, leading to a subsequent reduction in iNOS, endothelial nitric oxide synthase (eNOS), and NO levels (Ramalho et al. 2014).
Abundantly present in various Ocimum species, RA is notably found in O. tenuiflorum (8 mg/g of dried leaf extract), O. sanctum (1.653 mg/g of dried leaf extract or 0.27%), O. gratissimum (7.866 mg/g of dried leaf extract), O. basilicum (7.6 mg/g of dried leaf extract or 15.76%), O. americanum (10.966 mg/g of dried leaf extract), and O. lamiifolium (0.10 mg/g of dried leaf extract) (Pandey et al. 2016; Sundaram et al. 2012; Ibrahim et al. 2020; Rady and Nazif 2005).
RA exhibits remarkable efficacy in preventing oxidative stress-induced fibrogenesis by reducing the activity of MMPs and tissue inhibitors of metalloproteinases (TIMPs), both of which are NFκB-dependent and regulate extracellular matrix protein deposition (Lin et al. 2017). This antifibrotic effect is closely associated with the modulation of the hepatic transforming growth factor β1 (TGF-β1) pathway. RA reduces extracellular matrix-producing cells, such as portal myofibroblasts and hepatic stellate cells, linked with α-smooth muscle actin (α-SMA) immunoreactivity. Furthermore, RA downregulates TGF-β1 levels and its effectors, including hepatic protein phosphorylation of Smad 2/3, procollagen I, III, and connective tissue growth factor (CTGF) gene expression (Lin et al. 2017). In addition, RA diminishes oxidative stress by stimulating the antioxidant defense mechanism of the nuclear erythroid-related factor 2 (Nrf2)-Antioxidant response element (ARE) signaling pathway (Li et al. 2019; Lu et al. 2022). Under oxidative stress conditions, Nrf2 disassociates from the repressor protein Keap1, translocates into the nucleus, and activates ARE, leading to the upregulation of stress response-iron metabolism genes and antioxidant enzyme synthesis. RA also engages in hepatoprotection through the activation of AMP-activated protein kinase (AMPK) phosphorylation, which subsequently decreases the expression of the transcription factor sterol regulatory element-binding protein 1c (SREBP-1c), resulting in reduced fatty acid biosynthesis (Touiss et al. 2021). Moreover, studies by Khalaf et al. (2020) isolated RA from Rosmarinus officinalis L. plant leaves, demonstrating its activation of Nrf2 signaling, reduction in malondialdehyde (MDA) levels, and elevation of glutathione (GSH) concentration to attenuate hepatorenal oxidative damage induced by chromium.
The multifaceted mechanisms of hepatoprotection attributed to RA include its role in promoting antioxidant responses, modulating fibrogenic pathways, and regulating lipid metabolism, thereby positioning Ocimum species rich in RA as promising agents for liver protection (Guo et al. 2020).
Ferulic acid
Ferulic acid (FA), a prominent hydroxycinnamic acid within the phenolic compound group, is notably present in O. basilicum (1% or 0.546 mg/g of dried leaf extract), O. tenuiflorum (0.356 mg/g of dried leaf extract), O. sanctum (4.367 mg/g of dried leaf extract), O. gratissimum (0.446 mg/g of dried leaf extract), and O. americanum (0.336 mg/g of dried leaf extract) (Ibrahim et al. 2020).
FA exhibits hepatoprotective properties through multifaceted mechanisms. It upregulates AMP-activated protein kinase (AMPK) phosphorylation, thereby mitigating drug-induced acute liver injury via AMPK-mediated protected autophagy. Additionally, FA prevents both intrinsic and extrinsic apoptosis pathways by inhibiting TNFα-mediated Caspase-8 activation and suppressing the hepatic protein expression of pro-apoptotic Bcl-2 family members, including Bax, tBid, and Biml (Kim and Lee 2012). Furthermore, FA demonstrates antiapoptotic efficacy by preventing mitochondrial membrane potential reduction, increasing the Bax/Bcl2 ratio, and suppressing Caspase-3 expression (Wu et al. 2022). It enhances the antioxidant defense mechanism by upregulating the Nrf2-ARE signaling pathway and concurrently suppressing inducible nitric oxide synthase (iNOS), nuclear factor kappa B (NFκB), and other proinflammatory cytokine expressions (Mahmoud et al. 2020). In the context of hepatic ischemia/reperfusion-induced apoptosis, FA exerts protective effects by preventing the phosphorylation of Jun N-terminal Kinase-1 (JNK 1) and JNK 2 (Kim and Lee 2012). JNK, a member of the mitogen-activated protein kinase (MAPK) sub-family responsive to oxidative stress, regulates cellular processes such as migration, proliferation, and apoptosis. JNK 1 signaling induces activator protein-1, leading to Caspase-dependent hepatocellular apoptosis, while JNK 2 promotes TNF-induced apoptosis (Kim and Lee 2012).
Chicoric acid
Chicoric acid, another hydroxycinnamic acid abundantly present in O. basilicum (1.01% or 3.19–6.03 mg/g of dried aerial part extract) (Ibrahim et al. 2020), has been documented for its efficacy in preventing drug-induced hepatotoxicity. Its hepatoprotective mechanisms involve the prevention of oxidative stress and inflammation through the upregulation of hepatic Nrf2, HO-1, NQO-1, and PPARγ. Chicoric acid further inhibits apoptosis by upregulating Bcl-2 expression and suppressing Bax, cytochrome C (Cyt-C), and Caspase-3, highlighting its potential therapeutic role in safeguarding against liver damage (Hussein et al. 2020).
Luteolin
Luteolin, a bioactive flavone abundantly found in O. sanctum (1.116 mg/g of dried leaf extract), O. basilicum (5.94% or 0.683 mg/g), O. tenuiflorum (ranging from 0.031% to 0.046% or 1.530 mg/g), as well as 0.643 mg/g in O. gratissimum and O. americanum, among other Ocimum species (Pandey et al. 2016; Ibrahim et al. 2020), exhibits hepatoprotective properties. Luteolin effectively mitigates liver apoptosis by diminishing the expression of Bax, Cyt-C, Caspase-3, and Caspase-9, while concurrently elevating Bcl-2 expression.
In the context of mycotoxin-induced liver injury, Luteolin demonstrates protective effects by upregulating the Nrf2-ARE pathway and enhancing the activity of antioxidant enzymes such as catalase, GSH-Px, and SOD (Rajput et al. 2021). Its dietary intake proves beneficial in shielding against chronic liver injury induced by mercuric chloride, where it enhances the FoxO3a transcription factor, subsequently influencing the localization control of the tumor suppressor gene p53 by FoxO3a (Zhang et al. 2017). Moreover, Luteolin exhibits protective effects against acetaminophen-induced acute liver failure by combating endoplasmic stress. It achieves this by downregulating activating transcription factor 4 (ATF4) and C/EBP Homologous Protein (CHOP), as these proteins are notably elevated during endoplasmic stress (Tai et al. 2015).
Quercetin
Quercetin, the principal representative of flavonols, is discerned in varying concentrations across Ocimum species, including O. basilicum (0.047 mg/g of dried leaf extract), O. tenuiflorum (0.040 mg/g of dried leaf extract), O. sanctum (0.039 mg/g of dried leaf extract), O. gratissimum (0.067 mg/g of dried leaf extract), and O. americanum (0.039 mg/g of dried leaf extract) (Pandey et al. 2016). Differing from Luteolin by only one hydroxy group, quercetin emerges as a potent therapeutic agent in addressing liver fibrosis, liver steatosis, fatty hepatitis, and liver cancer. Its efficacy lies in its modulation of various targets and pathways integral to the pathogenesis and treatment of liver diseases. Quercetin exhibits preventive actions against liver steatosis, a disorder linked to lipid metabolism, often induced by excessive caloric intake or alcohol consumption. Mechanistically, it diminishes fat accumulation by downregulating both AMP-activated protein kinase (AMPK) and Sirtuin 1 (SIRT1), recognized targets in metabolic syndrome (Cao et al. 2023). Additionally, quercetin normalizes hepatic steatosis-related gene expressions by reducing the expression of peroxisome proliferator-activated receptor alpha (PPARα), which subsequently controls sterol regulatory element-binding protein 1c (SREBP1c) and fatty acid synthase (Zhao et al. 2021). Quercetin induces autophagy through the mammalian target of rapamycin (mTOR), influencing the expression of autophagy marker proteins such as microtubule-associated protein 1 and autophagic autophagosome bridging p62. Furthermore, it reduces oxidized low-density lipoprotein (Ox-LDL) accumulation in mice fed a high-fat diet. Quercetin also demonstrates anti-apoptotic effects by inhibiting factors such as p53 and Bax, while increasing the expression of Bcl-2 (Liu et al. 2017a, b; Lan et al. 2019).
In the context of hepatotoxicity induced by Triptolide, a potent hepatotoxic agent, Quercetin has been found to protect the liver by blocking Toll-like receptor 4 (TLR4). This protective effect is further emphasized by its modulation of T-cell immunoglobulin and mucin domain-containing protein 3, reduction of myeloid differentiation primary response gene 88, NFκB, and pro-inflammatory cytokines interleukin-17 (IL-17) and IL-6 related to T helper 17 (Th17) cells (Wei et al. 2017). Quercetin plays a pivotal role in regulating the balance between Th17 and T regulatory (T reg) cells, thereby maintaining T reg dominance and contributing to liver protection.
Quercetin exhibits notable efficacy in mitigating acute autoimmune hepatitis by suppressing the TNF receptor-associated factor 6 (TRAF6) / JNK pathway, thereby impeding autophagy and apoptosis processes (Wu et al. 2017). Rats treated with quercetin displayed reduced levels of 8-hydroxy guanosine, a marker indicative of oxidative damage to 2´-deoxy guanosine, suggesting the flavone's protective role against DNA damage (Ansar et al. 2016). In an in-vivo model of bile duct ligation-induced fibrosis in rats, quercetin demonstrated significant downregulation of TGF-β1 and miR-21 gene expression, while concurrently elevating miR-122 expression. This modulation supports the assertion that quercetin is effective in preventing liver fibrosis and cirrhosis (Nozari et al. 2020). A recent study revealed that quercetin down-regulates the Hedgehog pathway, an emerging target for hepatocellular damage repair. It attenuates the mRNA expressions of key Hedgehog pathway mediators and pro-inflammatory cytokines, including Serum sonic hedgehog (Shh), Patched-1 (Ptch-1), Gli-3, TNF-α, NFκB, and suppressor of cytokine signaling-3 (Socs-3) (Aslam et al. 2022).
Pérez-Ramírez et al. (2017) reported that O. sanctum flower extract, containing quercetin as a primary compound, exhibited anti-inflammatory and hypoglycemic potential by not up-regulating Glut4, IRS1, and PI3K genes while decreasing the levels of TNF-α and IL-6 compared to the negative control. A recent computational study focusing on quercetin from O. basilicum and O. tenuiflorum aimed at identifying anti-inflammatory responses through a non-steroidal mechanism. The study revealed that this flavonol modulates the carbonic anhydrase family and several key proteins from the arachidonic pathway, providing further insights into its anti-inflammatory properties (Beltrán-Noboa et al. 2022).
Rutin
Rutin, a significant flavonol, manifests in varying concentrations across different Ocimum species, including O. americanum (10.9 mg/g), O. basilicum (1.653 mg/g), O. gratissimum (0.920 mg/g), O. sanctum (0.074 mg/g), and O. tenuiflorum (0.173 mg/g) (Pandey et al. 2016). Its hepatoprotective properties extend to countering the adverse effects of toxic insecticides, exemplified by deltamethrin-induced hepatocellular inflammation, apoptosis, and necrosis. Küçükler et al. 2021, elucidated Rutin's role in attenuating lipid peroxidation (LPO) and downregulating proinflammatory cytokines, including TNF-α, NFκB, IL-1β, p38α MAPK, COX 2, iNOS, beclin 1, Bax, and Caspase 3. Rutin's impact also extends to reducing mRNA expression of PARP-1 and VEGF, indicating its potential in mitigating oxidative stress-associated DNA damage and promoting hepatic regeneration. Moreover, Rutin demonstrates efficacy in ameliorating cadmium-induced hepatotoxicity by inhibiting key members of MAPK and NFκB pathways such as JNK, ERK, and TNF-α. Liu et al. (2022) reported Rutin's ability to suppress stress response products, including HSP27, HSP40, HSP60, HSP 70, and HSP 90. Rutin's modulation of the iNOS-Nrf2 signaling pathway underscores its potential to maintain intracellular redox homeostasis (Singh et al. 2019). Choi et al. 2021, explored Rutin's protective effects on mitochondrial dynamics and alcohol-induced liver steatosis. The study revealed that Rutin inhibits lipid absorption in alcoholic fatty liver disease of zebrafish by suppressing c/ebpα and PPARγ mRNA expression. Additionally, Rutin restores mitochondrial morphology by inhibiting the drp1 expression-dependent mitochondrial fission mechanism.
p-Coumaric acid
p-Coumaric acid (p-CA) serves as a prominent hydroxycinnamic acid within O. basilicum, exhibiting a concentration of 1.653 mg/g. In O. sanctum and O. tenuiflorum, the quantities are 0.02 mg/g, 0.01 mg/g, and 0.06 mg/g respectively, in the entire plant extract (Pandey et al. 2016). Sabitha et al. (2020) elucidated the protective mechanisms of p-CA against alcohol-induced severe liver injury by mitigating reactive oxygen species (ROS) production, mitochondrial depolarization, and nuclear fragmentation in in-vitro cell lines L-02 and HepG2. Notably, p-CA treatment suppressed the expression of Bax, Caspases, and lipid biomarkers in rat liver tissue, thereby ameliorating hepatic injury. The inhibitory effect extended to mitogen-activated protein kinases (MAPKs) such as JNK, ERK, and p38 phosphorylation, while concurrently enhancing the antioxidant defense mechanism via upregulation of Nrf2 and HO-1. Additionally, p-CA treatment demonstrated efficacy in attenuating liver injury caused by the bioaccumulation of fipronil, a broad-spectrum insecticide. In the context of inflammation, p-CA exhibited anti-inflammatory properties by suppressing pro-inflammatory cytokines (TNF-α, IL-1β, IL-10) and myeloperoxidase (MPO) activity. Furthermore, it modulated antioxidant enzymes, contributing to the reduction of oxidative stress-mediated inflammation, as reported by Bal et al. (2022).
Apigenin
Apigenin (1,4´, 5,7-trihydroxy flavone), a compound ubiquitously present in various Ocimum species such as O. americanum (0.094 mg/g dried leaf extract), O. basilicum (0.134 mg/g of dried leaf extract), O. gratissimum (0.123 mg/g of dried leaf extract), O. sanctum (0.700 mg/g of dried leaf extract), and O. tenuiflorum (0.443 mg/g of dried leaf extract) as reported by Pandey et al. (2016). In the context of alcohol-induced liver injury, apigenin demonstrates efficacy in mitigating damage through the regulation of hepatic CYP2E1 enzyme, thereby mitigating oxidative stress. Additionally, it modulates lipogenic gene expression by upregulating hepatic PPARα (Wang et al. 2017). Furthermore, apigenin exhibits anti-inflammatory properties in hepatocytes, facilitating the translocation of Nrf2 from the cytoplasm to the nucleus. This results in the reduction of NFκB, TNFα, and intracellular nuclear factor-κB (IκB-α) protein expression (Zhou et al. 2020).
Caffeic acid and Chlorogenic acid
Similar to their counterparts, Caffeic acid (CA) and Chlorogenic acid (ChA) belong to the hydroxycinnamic acid class and are found in various Ocimum species. CA, a major phenolic acid in O. americanum, O. basilicum, O. gratissimum, O. sanctum, and O. tenuiflorum, occurs in quantities of 1.080, 0.920, 2.433, 0.390, and 1.006 mg/g of dried leaf extract, respectively (Pandey et al. 2016). Supplementation with CA has been shown to prevent hepatotoxicity induced by xenobiotics, such as fluoride, by modulating the expression of Bax and Caspase-3p20. CA exhibits protective effects on the liver, mitigating apoptosis and oxidative damage through the suppression of mitochondrial stress-associated factors like NADPH oxidase 4 (Nox4), p38αMAPK, Hsp 60, and the upregulation of Hsp 27 (Kanagaraj et al. 2015). Furthermore, CA plays a role in restoring energy metabolism, including hepatic fatty acid oxidation. This is achieved by upregulating transcriptional coactivators such as Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1α (PGC-1α), PPARα, farnesoid X receptor (FXR), and Liver X receptor (LXR) through the induction of SIRT 1 protein regulation (Xu et al. 2010; Zhu et al. 2018). Additionally, CA exhibits regulatory effects on bilirubin and bile acids, reducing their uptake and synthesis while accelerating their metabolism and efflux. This is accomplished by downregulating uptake transporters Ntcp, Oatp1a4, and Oatp1b2, and upregulating efflux transporters like Bsep and Mrp 2/3/4 (Buko et al. 2021).
Chlorogenic acid is found in varying quantities in O. americanum, O. basilicum, O. gratissimum, O. sanctum, and O. tenuiflorum, with concentrations of 0.460, 0.180, 0.803, 1.113, and 0.320 mg/g of dried leaf extract, respectively, as reported by Pandey et al. 2016. This compound exhibits anti-inflammatory and anti-apoptotic effects on hepatocytes and Kupffer cells by suppressing TLR4, TNFα, NF-κB p65, iNOS, COX-2, Bax mRNA expressions, as well as Caspase 3 and 9 activities. Additionally, ChA enhances mRNA expressions of AMPK-α, nuclear respiratory factor 1, and mitochondrial DNA transcription factor A. It plays a role in repairing mitochondrial dysfunction associated with acute or chronic hepatic injury by promoting ATP production and mitochondrial oxidative phosphorylation (Zhou et al. 2016).
Gallic acid
Ocimum species is a rich source of another small phenolic acid, 3,4,5- trihydroxy benzoic acid or gallic acid (GA). It is a major phenol present in leaves of O. americanum, O. basilicum, O. gratissimum, O. sanctum, and O. tenuiflorum at a quantity of 0.396, 0.255, 0.366, 0.282, 0.315 mg/g respectively (Pandey et al. 2016). GA serves as a key component in various Ayurvedic herbal formulations designed for diverse liver ailments. Its efficacy extends to mitigating hepatic damage induced by the anti-TB medications isoniazid and rifampicin, accomplished through the activation of Nrf2 and its downstream proteins or genes. When coadministered with these anti-TB drugs, GA effectively hinders the upregulation of the NFκB–TLR4 axis, as demonstrated in a study by Sanjay et al. 2021. In a separate investigation, gallic acid demonstrated its ability to alleviate hepatic lipid accumulation and impede the progression of non-alcoholic steatohepatitis by activating AMPK. This was achieved through the suppression of transcription factors ACCα, SREBP-1c, and LXRα, which play pivotal roles in fatty acid synthesis. The protective effects of GA were further evidenced by its prevention of apoptotic changes, as indicated by a decrease in Caspase 3/7 activity and ATF3 transcription factor levels, which regulate Bax/Bcl2 mRNA expression (Tanaka et al. 2020). Moreover, GA exhibited a notable impact on liver fibrosis markers, including the reduction of TIMP-1, TGFβ-1, platelet-derived growth factor B (PDGF-B), and hydroxyproline, while concurrently restoring expressions of α-SMA and Proliferating Cell Nuclear Antigen (PCNA) (El-Lakkany et al. 2019).
Apart from the mentioned polyphenols, many other similar compounds are present in Ocimum species in traces. However, not all have yet been evaluated for their oxidative stress-modulating potentials. In a recent in silico study by Rahayu et al. 2024 flavonoids (apigenin, rutin, and quercetin) and essential oils (α-bergamotene, α-cadinol, methyl cinnamate, and methyl eugenol) from O. basilicum were investigated for their involvement in the Keap1/SIRT1/NFκB pathway. The study demonstrated that apigenin, rutin, α-bergamotene, α-cadinol, and methyl cinnamate exhibit low toxicity. Pharmacokinetic analysis indicated that compounds from O. basilicum are primarily absorbed in the human intestine. Protein network analysis revealed the participation of NFκB and Nrf2 in the inflammatory response and regulation of the stress response. Rutin exhibited the highest binding affinity for Keap1, while α-bergamotene and α-cadinol showed the strongest binding affinities for NFκB and SIRT1, respectively. Further preclinical studies need to be conducted. The leaves of O. basilicum contain about 79% methyl chavicol, a phenylpropanoid group of compounds (Maurya and Sangwan 2020). This compound has been studied for less number of pharmacological activities. Santos et al. 2018 evaluated the In vitro antioxidant and anticipated activities of methyl chavicol, which may be promising molecular targets for the treatment of diseases associated with oxidative damage like hepatotoxicity.
Hepatoprotective terpenoids present in Ocimum species
A large number of terpenoids present in various Ocimum species (Table 2) have been studied for their mechanistic pathways in hepatoprotection. This can be corroborated by the fact that these terpenoids may be responsible for the protective role of Ocimum species against hepatotoxicity.
Ocimum species are rich in sesquiterpenoid compounds, including α-copaene, β-elemene, β-caryophyllene, α-humulene, and germacrene D, as reported by Maurya and Sangwan 2020. These compounds are recognized for their hepatoprotective potential. Paukku et al. (2009) have provided a comprehensive quantitative structure–activity relationship analysis of hepatoprotection by sesquiterpenoids, the largest class within the terpenoid category. The analysis outlines essential molecular variables associated with the hepatoprotective activity of sesquiterpenoid compounds. Notably, the hepatoprotective potency of these compounds is influenced by or can be inferred from specific variables, elucidating molecular electronic and geometrical components.
The variable dipole moment (µ) underscores the observation that the smallest charge separation or lowest µ corresponds to the highest hepatoprotective activity. Steric effects, attributed to the overall size of the molecule and functional groups with secondary sp3 hybridized carbons, contribute to a lowering effect on hepatoprotective activity. Additionally, compounds exhibiting the highest molecular walk count or a substantial number of substituted carbons demonstrate the most robust hepatoprotective activity (Paukku et al. 2009; Vinholes et al. 2014).
The following sections contain a detailed discussion of the hepatoprotective mechanisms of some terpenoids present in Ocimum species (Fig. 3).
β-elemene
Β-elemene, a sesquiterpenoid compound identified at a concentration of 21% in the stem of O. sanctum (Maurya and Sangwan 2020), demonstrates inhibitory effects against liver fibrosis, inflammation, and hepatocellular cancer in various in vivo and in vitro experimental models. The protective mechanism of β-elemene against CCl4-induced hepatic fibrosis in Wistar rats involves the down-regulation of the serum angiotensin II-hepatic angiotensin II type 1 (ANGII-AT1) receptor pathway, thereby suppressing hepatic collagen deposition. Additionally, β-elemene down-regulates the lipopolysaccharide signal transduction pathway (Zhu et al. 2009) and decreases TNF-α, plasma endotoxins, and hepatic CD14 expression (Liu et al. 2011). Furthermore, β-elemene exhibits inhibitory effects on the cell proliferation of the murine hepatocellular carcinoma cell line (H22) by elevating histone H1 protein levels (Bao et al. 2012). Supporting this finding, a study by Dai et al. 2013 delved into the anti-proliferative and apoptotic mechanisms of β-elemene on the human hepatoma (HepG2) cell line. The results revealed that β-elemene upregulates Fas/Fas L protein and gene expression, leading to the arrest of the cell cycle in the G2/M phase.
β-caryophyllene
Β-caryophyllene, a bicyclic sesquiterpene present in O. tenuiflorum, O. sanctum, O. basilicum, and O. americanum, serves as a natural antioxidant with the ability to inhibit hepatic stellate cell activation. This inhibition is achieved by reducing the activity of the fibrogenesis enzyme 5-lipoxygenase, subsequently suppressing the overproduction of extracellular matrix proteins and the expression of fibrotic marker genes such as COL1a1, TGFβ1, and TIMP1 (Calleja et al. 2013). In a study by Cho et al. 2015, the hepatoprotective mechanism of β-caryophyllene against D-galactosamine and lipopolysaccharide-induced liver injury was explored. It was revealed that β-caryophyllene down-regulates toll-like receptor (TLR4) and receptor for advanced glycation end products (RAGE) protein expression, as well as the phosphorylation of NFκB, ERK, p38, and c-JNK. Additionally, it inhibits the production of pro-inflammatory cytokines, reduces early growth response protein 1, and suppresses macrophage inflammatory protein 2 expression. Moreover, β-caryophyllene has been identified as a potential remedy for non-alcoholic steatohepatitis, as demonstrated by Arizuka et al. 2017. The compound achieves this by down-regulating the expression of the monocyte chemotactic and activating factor 1 gene. Chronic treatment with β-caryophyllene, as reported by Varga et al. 2018, also shows promise in improving alcoholic steatohepatitis. This improvement is attributed to the attenuation of kupffer cell-mediated pro-inflammatory cytokine response, up-regulation of PPAR-α, and suppression of neutrophil infiltration.
α-humulene/ α-caryophyllene
α-Humulene, alternatively recognized as α-caryophyllene, emerges as a prolific compound within O. tenuiflorum, O. sanctum, O. basilicum, and O. gratissimum. In a recent investigation, this 11-membered monocyclic terpene demonstrated the capability to impede Akt activation and facilitate Caspase 3 activation, thereby inducing mitochondrial apoptosis in hepatocellular carcinoma cells, both in vitro and in vivo contexts (Chen et al. 2019).
α-pinene
Α-pinene, a monoterpenoid present in O. tenuiflorum, O. sanctum, O. basilicum, O. gratissimum, O. americanum, O. lamiifolium and O. canum (Table 2) was found to have a similar apoptotic mechanism with β-elemene. α-pinene acts by suppressing human hepatoma tumor progression by down-regulating CDK1 and miR-221 levels (Xu et al. 2018).
Borneol
Borneol, a lipophilic monoterpenoid found in O. tenuiflorum, O. sanctum, O. basilicum, O. lamiifolium, and O. gratissimum, protects rat hepatocytes against exogenous oxidative DNA damage (Horváthová et al. 2012).
Oleanolic acid
Oleanolic acid (OA), a pentacyclic triterpenoid akin to the one reported in O. tenuiflorum, O. sanctum, O. basilicum, and O. canum, up-regulates multidrug resistance-associated proteins 2, 3, and 4. This action contributes to the reduction of cholestasis, facilitating normal bile flow from the liver (Sen 2020). Additionally, oleanolic acid demonstrates the ability to enhance the expression of various proteins and their associated genes. It elevates metallothionein, Nrf2, HO-1, and NQO1 expression, while also stimulating the potential of antioxidant enzymes such as SOD, GPx, and glutamate–cysteine ligases (Iranshahy et al. 2018). Notably, oleanolic acid increases the glutathione content in the liver, showcasing its antioxidant potential and promoting hepatic cell regeneration.
Epimaslinic acid
Epimaslinic acid, a member of the pentacyclic triterpenoid class found in O. basilicum, demonstrates a significant capacity to up-regulate Nrf2 in hepatocytes (Sen 2020).
Linalool
Linalool, a significant acyclic monoterpenoid compound identified in O. tenuiflorum, O. sanctum, O. basilicum, O. gratissimum, O. americanum, O. lamiifolium, and O. canum (Table 2), activates cytoprotective genes. This activation occurs through the inhibition of D-galactosamine/lipopolysaccharide-induced NF-κB up-regulation, as demonstrated in the study by Jadeja et al. 2016.
Eucalyptol
1,8-cineole, also known as eucalyptol and classified as a monoterpene oxide, is a significant compound present in O. tenuiflorum, O. americanum, and O. lamiifolium, exhibiting hepatoprotective activity. Identified as a potent drug candidate for treating non-alcoholic steatohepatitis, eucalyptol achieves this through the inhibition of the PI3K/Akt pathway. Notably, it hinders the progression of liver fibrosis by down-regulating collagen 1a1 expression (Murata et al. 2015). Additionally, eucalyptol has been found to effectively reduce myeloperoxidase activity, malondialdehyde (MDA) levels, and pro-inflammatory cytokines such as TNF-α, IL-8, IL-6, and IL-1β. Simultaneously, it increases the levels of the anti-inflammatory cytokine IL-10 and the antioxidant enzyme GSH (Lima et al. 2013). Furthermore, eucalyptol enhances PPAR-γ expression, leading to the down-regulation of NFκB (Linghu et al. 2019). These collective findings suggest that eucalyptol can mitigate oxidative stress within the liver through its anti-inflammatory mechanisms.
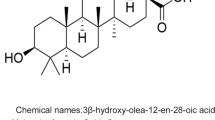
Ursolic acid
Ursolic acid (UA), a prominent pentacyclic triterpenoid, is found in significant quantities in O. basilicum, O. gratissimum, O. sanctum, O. tenuiflorum, and O. americanum, with concentrations of 8.033, 6.933, 1.473, 4.800, and 0.373 mg/g of dried leaf extract, respectively (Pandey et al. 2016). Research indicates its effectiveness in safeguarding against chronic alcoholic liver damage. This protection is achieved through the modulation of glutathione homeostasis and the downregulation of the NQO1 gene and protein expression, as NQO1 is a Phase II detoxifying enzyme belonging to the GST family and associated with Nrf2 activation (Yan et al. 2022). UA treatment has been observed to significantly impede the activation of CASP3 and cleavage of PARP, mitigating DNA degradation and apoptosis, thereby reducing the risk of alcoholic liver injury (Ma et al. 2021). Furthermore, UA treatment suppresses the phosphorylation of PI3K, Akt, and NFκB signaling processes, along with the associated cytokine oncostatin M production, contributing to the alleviation of hepatic inflammation (Han et al. 2022).
The molecular mechanistic pathways of numerous other terpenoids within Ocimum species have not been thoroughly explored. The 3D-QSAR model has been employed to analyze the molecular structure of α-copaene, a sesquiterpenoid found in O. sanctum and O. americanum. The analysis revealed that α-copaene possesses a compact structure with less molecular symmetry and electronegative substitution. Although the results of hepatoprotective activity analysis suggest its potential efficacy in liver protection (Vinholes et al. 2014), the specific molecular mechanisms underlying its hepatoprotective effects remain unknown.
Toxicity profile of the compounds preet al.,t in Ocimum species
To develop a safe and targeted phytomedicine, the toxicity profile of phenols and terpenoids in Ocimum species has been studied. Ocimum basilicum powder, identified as having the lowest toxicological risk, was extracted using various solvents, including n-hexane, dichloromethane, ethanol, and water. The ethanolic basil leaf extract was selected for further analysis due to its lower toxicological effects. Phytochemical analysis of the ethanolic extract identified rosmarinic acid, ellagic acid, catechin, liquiritigenin, and umbelliferone. Additionally, aqueous extracts of basil, at concentrations ranging from 10 to 1000 µg/mL, exhibited no toxicity (Nadeem et al. 2022). Ajayi et al. (2017) observed the acute toxicity effects of crude methanol extracts of Ocimum gratissimum leaves. Comparative acute toxicity tests revealed no mortality in rats administered doses of 2 and 5 g/kg. The extracts did not produce significant changes in the behavioral patterns, skin color, diarrhea, food intake, water consumption, or body weights of the treated animals immediately after administration or during the 14-day observation period. In another study, a 28-day subacute oral toxicity test of an aqueous extract of Ocimum basilicum leaves, containing phenolic acids (p-hydroxybenzoic, vanillic, caffeic, and rosmarinic acids) and flavonoids (naringenin, rutin, quercetin, and kaempferol), revealed no toxic effects in rats at doses of 50, 200, and 500 mg/kg (Housse et al. 2023). Ali et al. (2022) demonstrated the potential cytotoxicity of all crude extracts and fractions of Ocimum americanum L. on Clarkson’s scale, indicating low median lethal concentration (LC50) values. The chloroform and ethyl acetate fractions of the hydroethanolic crude extract exhibited the highest toxicological profiles against brine shrimp larvae. The chloroform fraction (LC50 0.59 µg/mL) had a lower median lethal concentration compared to the standard drug vincristine (LC50 11.83 µg/mL), suggesting a high potential for this fraction as a novel compound warranting further bioprospecting. Similarly, the ethyl acetate fraction (LC50 44.65 µg/mL) was highly toxic according to Clarkson’s criteria. The acetonic crude extract and fractions, as well as the aqueous fractions, exhibited moderate toxicity (LC50 303.39 µg/mL). The aqueous crude extract was slightly toxic (LC50 559.71 µg/mL), supporting the observation that acute use of herbal decoctions does not show toxicity in patients.
Conclusion
The effectiveness of phytomedicine is influenced by key groups of phytoconstituents present in plants, comprising alkaloids, terpenoids, tannins, flavonoids, saponins, quinones, and polyphenolics. Natural compounds, especially plant phenolics and terpenoids, have gained prominence due to their efficacy, minimal side effects, and protective properties. Polyphenols and terpenoids comprise the major share of all the phytoconstituents present in various Ocimum species. Pharmacological reports support the hepatoprotective properties of Ocimum species. Sametime, diverse polyphenols and terpenoids found in various Ocimum species have been investigated to understand their impact on signaling pathways, proteins, and genes associated with hepatoprotection. The findings of these investigations consistently demonstrated the effectiveness of terpenoids and phenolic compounds against hepatotoxicity. This mechanistic review provided a comprehensive exploration and clarification of the extensive molecular mechanisms involved in hepatoprotection by specific polyphenols present in Ocimum species. However, the limitations are that most of these hepatoprotective studies presented in this review have used different extracts of Ocimum, without identifying the individual compounds present in them. So, the specific mechanistic activities elicited by these individual phytochemicals remain unstudied and the antiviral properties of Ocimum in treating viral hepatitis remain largely unexplored. Consequently, targeted clinical trial investigations are necessary to unlock the full potential of Ocimum species for developing hepatoprotective therapies. As a result, various marketed herbal formulations of Tulsi are available as immunity boosters, and vitamin and nutrition supplements but no hepatoprotective herbal formulation from crude Tulsi or any of its isolated compounds has been marketed till now.
Future directions
Finally, to derive a potent hepatoprotective medication from this species, focused studies are required to elucidate the cellular molecular targets. Despite a rich reservoir of polyphenols and terpenoids, not all the species of Ocimum have been studied for hepatoprotective properties. Additionally, the natural variations of these compounds across different species are influenced by environmental factors such as weather and nutrients, posing a challenge in isolating lead molecules. Thus modern techniques, including tissue culture, biotransformation, and fermentation, need to be employed to overcome these challenges and extract hepatoprotective phytochemicals effectively. Information derived from human trials is also inconclusive regarding the use of Ocimum. In a parallel, randomized, single-blind trial to evaluate the effects of O. basilicum (dosage of 10 g/ day or a control group without a placebo for 12 weeks) in patients of NAFLD with hepatic steatosis did not produce significant anthropometric changes (Akbarian et al. 2016). Consequently, further research is required for the separation and isolation of phytoconstituents along with assessments of chronic toxicity, and clinical studies of the active biomolecules.
References
Adjou ES, Chougourou D, Soumanou MM (2019) Insecticidal and repellent effects of essential oils from leaves of Hyptis suaveolens and Ocimum canum against Tenebroides mauritanicus (L.) isolated from peanut in post-harvest. J Consum Protect Food Saf 14(1):25–30. https://doi.org/10.1007/s00003-018-1195-4
Ajayi AM, Naluwuge A, Buyinza P, Luswata I (2017) Comparative physicochemical, phytochemical and acute toxicity studies of two Ocimum species in Western Uganda. J Med Plants Res 11(1):1. https://doi.org/10.5897/JMPR2015.6025
Ajiboye TO, Ajala-Lawal RA, Adeyiga AB (2019) Caffeic acid abrogates 1, 3-dichloro-2-propanol-induced hepatotoxicity by upregulating nuclear erythroid-related factor 2 and downregulating nuclear factor-kappa B. Hum Exp Toxicol 38(9):1092–1101. https://doi.org/10.1177/0960327119851257
Akara EU, Emmanuel O, Ude VC, Uche-Ikonne C, Eke G, Ugbogu EA (2021) Ocimum gratissimum leaf extract ameliorates phenylhydrazine-induced anaemia and toxicity in Wistar rats. Drug Metab Personal Therapy 36(4):311–320. https://doi.org/10.1515/dmpt-2020-0185
Akbarian SA, Asgary S, Feizi A, Iraj B, Askari G (2016) Comparative study on the effect of Plantago psyllium and Ocimum basilicum seeds on anthropometric measures in non-alcoholic fatty liver patients. Int J Prev Med 7(1):114. https://doi.org/10.4103/2008-7802.191865
Ali H, Nguta J, Musila F, Ole-Mapenay I, Matara D, Mailu J (2022) Evaluation of antimicrobial activity, cytotoxicity, and phytochemical composition of Ocimum americanum L.(Lamiaceae). Evid-Based Complement Alternat Med. https://doi.org/10.1155/2022/6484578
Al-Rejaie SS, Aleisa AM, Sayed-Ahmed MM, Al-Shabanah OA, Abuohashish HM, Ahmed MM, Al-Hosaini KA, Hafez MM (2013) Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat. BMC Complement Altern Med 13(1):1–9. https://doi.org/10.1186/1472-6882-13-136
Alshehri AS, El-Kott AF, El-Gerbed MS, El-Kenawy AE, Albadrani GM, Khalifa HS (2022) Kaempferol prevents cadmium chloride-induced liver damage by upregulating Nrf2 and suppressing NF-κB and keap1. Environ Sci Pollut Res 29(10):13917–13929. https://doi.org/10.1007/s11356-021-16711-3
Anandjiwala S, Kalola J, Rajani M (2006) Quantification of eugenol, luteolin, ursolic acid, and oleanolic acid in black (Krishna Tulasi) and green (Sri Tulasi) varieties of Ocimum sanctum Linn. using high-performance thin-layer chromatography. J AOAC Int 89(6):1467–1474. https://doi.org/10.1093/jaoac/89.6.1467
Ansar S, Siddiqi NJ, Zargar S, Ganaie MA, Abudawood M (2016) Hepatoprotective effect of Quercetin supplementation against Acrylamide-induced DNA damage in wistar rats. BMC Complement Altern Med 16:1–5. https://doi.org/10.1186/s12906-016-1322-7
Arafah A, Rehman MU, Mir TM, Wali AF, Ali R, Qamar W, Khan R, Ahmad A, Aga SS, Alqahtani S, Almatroudi NM (2020) Multi-therapeutic potential of naringenin (4′, 5, 7-trihydroxyflavonone): experimental evidence and mechanisms. Plants 9(12):1784. https://doi.org/10.3390/plants9121784
Arizuka N, Murakami T, Suzuki K (2017) The effect of β-caryophyllene on non-alcoholic steatohepatitis. J Toxicol Pathol 30(4):263–273. https://doi.org/10.1293/tox.2017-0018
Aslam A, Sheikh N, Shahzad M, Saeed G, Fatima N, Akhtar T (2022) Quercetin ameliorates thioacetamide-induced hepatic fibrosis and oxidative stress by antagonizing the Hedgehog signaling pathway. J Cell Biochem. https://doi.org/10.1111/fcp.12896
Bal SS, Leishangthem GD, Sethi RS, Singh A (2022) P-coumaric acid ameliorates fipronil induced liver injury in mice through attenuation of structural changes, oxidative stress, and inflammation. Pestic Biochem Physiol 180:104997. https://doi.org/10.1016/j.pestbp.2021.104997
Balakrishnan M, Rehm J (2024) A public health perspective on mitigating the global burden of chronic liver disease. Hepatology 79(2):451–459. https://doi.org/10.1097/HEP.0000000000000679
Bao F, Qiu J, Zhang H (2012) Potential role of β-elemene on histone H1 in the H22 ascites hepatoma cell line. Mol Med Rep 6(1):185–190. https://doi.org/10.3892/mmr.2012.891
Beltrán-Noboa A, Proaño-Ojeda J, Guevara M, Gallo B, Berrueta LA, Giampieri F, Perez-Castillo Y, Battino M, Álvarez-Suarez JM, Tejera E (2022) Metabolomic profile and computational analysis for the identification of the potential anti-inflammatory mechanisms of action of the traditional medicinal plants Ocimum basilicum and Ocimum tenuiflorum. Food Chem Toxicol 164:113039. https://doi.org/10.1016/j.fct.2022.113039
Bhatt S, Tewari G, Pande C, Rana L (2018) Impact of drying methods on essential oil composition of Ocimum americanum L. from Kumaun Himalayas. J Essent Oil-Bearing Plants. 21(5):1385–1396. https://doi.org/10.1080/0972060X.2018.1543031
BinMowyna MN, AlFaris NA (2021) Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm Biol 59(1):146–156. https://doi.org/10.1080/13880209.2021.1877734
Borah R, Biswas SP (2018) Tulsi (Ocimum sanctum), excellent source of phytochemicals. Int J Environ, Agric Biotechnol. https://doi.org/10.22161/ijeab/3.5.21
Buko V, Zavodnik I, Budryn G, Zakłos-Szyda M, Belonovskaya E, Kirko S, Żyżelewicz D, Zakrzeska A, Bakunovich A, Rusin V, Moroz V (2021) Chlorogenic acid protects against advanced alcoholic steatohepatitis in rats via modulation of redox homeostasis, inflammation, and lipogenesis. Nutrients 13(11):4155. https://doi.org/10.3390/nu13114155
Calleja MA, Vieites JM, Montero-Meterdez T, Torres MI, Faus MJ, Gil A, Suárez A (2013) The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br J Nutr 109(3):394–401. https://doi.org/10.1017/S0007114512001298
Cao P, Wang Y, Zhang C, Sullivan MA, Chen W, Jing X, Yu H, Li F, Wang Q, Zhou Z, Wang Q (2023) Quercetin ameliorates nonalcoholic fatty liver disease (NAFLD) via the promotion of AMPK-mediated hepatic mitophagy. J Nutr Biochem 120:109414. https://doi.org/10.1016/j.jnutbio.2023.109414
Cassini-Vieira P, Araújo FA, da Costa Dias FL, Russo RC, Andrade SP, Teixeira MM, Barcelos LS (2015) iNOS activity modulates inflammation, angiogenesis, and tissue fibrosis in polyether-polyurethane synthetic implants. Mediators Inflamm. https://doi.org/10.1155/2015/138461
Cederbaum AI (2017) Cytochrome P450 and oxidative stress in the liver. Liver pathophysiology. Academic Press, London, pp 401–419
Chatterjee A, Kumar S, Sarkar SR, Halder R, Kumari R, Banerjee S, Sarkar B (2024) Dietary polyphenols represent a phytotherapeutic alternative for gut dysbiosis associated neurodegeneration: a systematic review. J Nutri Biochem. https://doi.org/10.1016/j.jnutbio.2024.109622
Chaudhary A, Sharma S, Mittal A, Gupta S, Dua A (2020) Phytochemical and antioxidant profiling of Ocimum sanctum. J Food Sci Technol. https://doi.org/10.1007/s13197-020-04417-2
Chen YH, Chiu YW, Shyu JC, Tsai CC, Lee HH, Hung CC, Hwang JM, Liu JY, Wang WH (2015) Protective effects of Ocimum gratissimum polyphenol extract on carbon tetrachloride-induced liver fibrosis in rats. Chin J Physiol 58(1):55–63. https://doi.org/10.4077/CJP.2015.BAD285
Chen H, Yuan J, Hao J, Wen Y, Lv Y, Chen L, Yang X (2019) α-Humulene inhibits hepatocellular carcinoma cell proliferation and induces apoptosis through the inhibition of Akt signaling. Food Chem Toxicol 134:110830. https://doi.org/10.1016/j.fct.2019.110830
Chiang LC, Ng LT, Cheng PW, Chiang W, Lin CC (2005) Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin Exp Pharmacol Physiol 32(10):811–816. https://doi.org/10.1111/j.1440-1681.2005.04270.x
Chigozie UU, Monanu MO (2016) Potential hepatoprotective effect of different solvent fractions of Ocimum gratissimum (OG) in a paracetamol-induced hepatotoxicity in Wistar albino rats. J Investig Biochem 5(1):10–16. https://doi.org/10.5455/jib.20160320033421
Chiu CC, Huang CY, Chen TY, Kao SH, Liu JY, Wang YW, Tzang BS, Hsu TC (2012) Beneficial effects of Ocimum gratissimum aqueous extract on rats with CCl4-induced acute liver injury. Evid-Based Complement Altern Med 2012:1–9. https://doi.org/10.1155/2012/736752
Cho HI, Hong JM, Choi JW, Choi HS, Kwak JH, Lee DU, Lee SK, Lee SM (2015) β-Caryophyllene alleviates d-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. Eur J Pharmacol 764:613–621. https://doi.org/10.1016/j.ejphar.2015.08.001
Choi Y, Seo H, Cho M, Kim J, Chung HS, Lee I, Kim MJ (2021) Rutin inhibits DRP1-mediated mitochondrial fission and prevents ethanol-induced hepatotoxicity in HepG2 cells and zebrafish. Animal Cells Syst 25(1):74–81. https://doi.org/10.1080/19768354.2021.1882565
Cummins CB, Wang X, Nunez Lopez O, Graham G, Tie HY, Zhou J, Radhakrishnan RS (2018) Luteolin-mediated inhibition of hepatic stellate cell activation via suppression of the STAT3 pathway. Int J Mol Sci 19(6):1567. https://doi.org/10.3390/ijms19061567
da Silva VD, Almeida-Souza F, Teles AM, Neto PA, Mondego-Oliveira R, Mendes Filho NE, Taniwaki NN, Abreu-Silva AL, da Silva Calabrese K, Mouchrek Filho VE (2018) Chemical composition of Ocimum canum Sims. essential oil and the antimicrobial, antiprotozoal, and ultrastructural alterations it induces in Leishmania amazonensis promastigotes. Indus Crops Prod 119:201–208. https://doi.org/10.1016/j.indcrop.2018.04.005
Dai ZJ, Tang W, Lu WF, Gao J, Kang HF, Ma XB, Min WL, Wang XJ, Wu WY (2013) Antiproliferative and apoptotic effects of β-elemene on human hepatoma HepG2 cells. Cancer Cell Int. https://doi.org/10.1186/1475-2867-13-27
Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS (2023) Global burden of liver disease: 2023 update. J Hepatol 79(2):516–537. https://doi.org/10.1016/j.jhep.2023.03.017
Devi R, Monika M, Dua A, Gupta SK, Mittal A, Sharma S (2024) Antioxidant potential and polyphenols analysis of medicinal herb Ocimum tenuiflorum (Shyama Tulsi). J Appl Nat Sci 16(1):289–298. https://doi.org/10.31018/jans.v16i1.5318
Domitrović R, Jakovac H, Tomac J, Šain I (2009) Liver fibrosis in mice induced by carbon tetrachloride and its reversion by luteolin. Toxicol Appl Pharmacol 241(3):311–321. https://doi.org/10.1016/j.taap.2009.09.001
Domitrović R, Jakovac H, Marchesi VV, Vladimir-Knežević S, Cvijanović O, Tadić Ž, Romić Ž, Rahelić D (2012) Differential hepatoprotective mechanisms of rutin and quercetin in CCl4-intoxicated BALB/cN mice. Acta Pharmacol Sin 33(10):1260–1270. https://doi.org/10.1038/aps.2012.62
Duan S, Du X, Chen S, Liang J, Huang S, Hou S, Gao J, Ding P (2020) Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed Pharmacother 121:109683. https://doi.org/10.1016/j.biopha.2019.109683
Ebhohon S, Akubuiro PC, Ogbu JC (2023) Protective effect of aqueous extract of Ocimum gratissimum leaf against cadmium-induced toxicity in male wistar rats. Trop J Nat Prod Res. https://doi.org/10.26538/tjnpr/v7i12.46
El-Lakkany NM, El-Maadawy WH, El-Din SH, Saleh S, Safar MM, Ezzat SM, Mohamed SH, Botros SS, Demerdash Z, Hammam OA (2019) Antifibrotic effects of gallic acid on hepatic stellate cells: In vitro and in vivo mechanistic study. J Tradit Complement Med 9(1):45–53. https://doi.org/10.1016/j.jtcme.2018.01.010
Enemali MO, Udedi SC (2018) Comparative evaluation of the protective effect of leaf extracts of Vernonia amygdalina (bitter leaf) and Ocimum canum (curry) on acetaminophen induced acute liver toxicity. J Pharmacogn Phytother 10(7):116–125. https://doi.org/10.5897/JPP2018.0497
Fathy M, Khalifa EM, Fawzy MA (2019) Modulation of inducible nitric oxide synthase pathway by eugenol and telmisartan in carbon tetrachloride-induced liver injury in rats. Life Sci 216:207–214. https://doi.org/10.1016/j.lfs.2018.11.031
Fox LT, Gerber M, Plessis JD, Hamman JH (2011) Transdermal drug delivery enhancement by compounds of natural origin. Molecules 16(12):10507–10540. https://doi.org/10.3390/molecules161210507
Genfi AK, Larbie C, Emikpe BO, Oyagbemi AA, Firempong CK, Adjei CO (2020) Modulation of oxidative stress and inflammatory cytokines as therapeutic mechanisms of Ocimum americanum L extract in carbon tetrachloride and acetaminophen-induced toxicity in rats. J Evid-Based Integr Med. 25:2515690X20938002. https://doi.org/10.1177/2515690X20938002
George S, Chaturvedi P (2008) Protective role of Ocimum canum plant extract in alcohol-induced oxidative stress in albino rats. Br J Biomed Sci 65(2):80–85. https://doi.org/10.1080/09674845.2008.11732802
Graßmann J (2005) Terpenoids as plant antioxidants. Vitam Horm 72:505–535
Green M, Pragada RR, Ethadi S, Rajanna B (2013) Comparative study on some selected species of Ocimum genus on free radical scavenging activity and hepatoprotective activity against CCl4 induced intoxication in rats. Am J Mol Biol 3:183–186. https://doi.org/10.4236/ajmb.2013.34024
Guengerich FP, Johnson WW, Ueng YF, Yamazaki H, Shimada T (1996) Involvement of cytochrome P450, glutathione S-transferase, and epoxide hydrolase in the metabolism of aflatoxin B1 and relevance to risk of human liver cancer. Environ Health Perspect 104(suppl 3):557–562. https://doi.org/10.1289/ehp.96104s3557
Guo C, Shangguan Y, Zhang M, Ruan Y, Xue G, Ma J, Yang J, Qiu L (2020) Rosmarinic acid alleviates ethanol-induced lipid accumulation by repressing fatty acid biosynthesis. Food Funct 11(3):2094–2106. https://doi.org/10.1039/C9FO02357G
Gupta P, Yadav DK, Siripurapu KB, Palit G, Maurya R (2007) Constituents of Ocimum sanctum with antistress activity. J Nat Prod 70(9):1410–1416. https://doi.org/10.1021/np0700164
Gurav TP, Dholakia BB, Giri AP (2022) A glance at the chemodiversity of Ocimum species: Trends, implications, and strategies for the quality and yield improvement of essential oil. Phytochem Rev 21(3):879–913. https://doi.org/10.1007/s11101-021-09767-z
Gutiérrez-del-Río I, López-Ibáñez S, Magadán-Corpas P, Fernández-Calleja L, Pérez-Valero Á, Tuñón-Granda M, Miguélez EM, Villar CJ, Lombó F (2021) Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 10(8):1264. https://doi.org/10.3390/antiox10081264
Hajdari A, Mustafa B, Hyseni L, Bajrami A, Mustafa G, Quave CL, Nebija D (2020) Phytochemical study of eight medicinal plants of the Lamiaceae family traditionally used as tea in the Sharri mountains region of the Balkans. Sci World J. https://doi.org/10.1155/2020/4182064
Hakkim FL, Arivazhagan G, Boopathy R (2008) Antioxidant property of selected Ocimum species and their secondary metabolite content. J Med Plants Res 2(9):250–257. https://doi.org/10.5897/JMPR.9000228
Han NR, Ko SG, Park HJ, Moon PD (2022) Ursolic acid suppresses oncostatin M expression through blockade of PI3K/Akt/NF-κB signaling processes in neutrophil-like differentiated HL-60 cells. Processes 10(2):220. https://doi.org/10.3390/pr10020220
He JD, Wang Z, Li SP, Xu YJ, Yu Y, Ding YJ, Yu WL, Zhang RX, Zhang HM, Du HY (2016) Vitexin suppresses autophagy to induce apoptosis in hepatocellular carcinoma via activation of the JNK signaling pathway. Oncotarget 7(51):84520
Hernández-Aquino E, Muriel P (2018) Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J Gastroenterol 24(16):1679
Hicks DF, Goossens N, Blas-García A, Tsuchida T, Wooden B, Wallace MC, Nieto N, Lade A, Redhead B, Cederbaum AI, Dudley JT (2017) Transcriptome-based repurposing of apigenin as a potential anti-fibrotic agent targeting hepatic stellate cells. Sci Rep 7(1):1–8. https://doi.org/10.1038/srep42563
Horváthová E, Kozics K, Srančíková A, Hunáková Ľ, Gálová E, Ševčovičová A, Slameňová D (2012) Borneol administration protects primary rat hepatocytes against exogenous oxidative DNA damage. Mutagenesis 27(5):581–588. https://doi.org/10.1093/mutage/ges023
Housse ME, Hadfi A, Karmal I, Ibrahimi BE, Jalal M, Ben-aazza S, Errami M, Belattar MB, Khrach S, Iberache N, Driouiche A (2023) Toxicity profile, phytochemical composition, and anti-scaling properties of the aqueous extract of Ocimum basilicum L. leaves as novel green and cost-effective inhibitor: experimental, MC/SAA and DFT approach. Waste Biomass Valoriz 14(11):3553–3573. https://doi.org/10.1007/s12649-023-02066-y
Huang CC, Hwang JM, Tsai JH, Chen JH, Lin H, Lin GJ, Yang HL, Liu JY, Yang CY, Ye JC (2020) Aqueous Ocimum gratissimum extract induces cell apoptosis in human hepatocellular carcinoma cells. Int J Med Sci 17(3):338
Hussain AI, Chatha SA, Kamal GM, Ali MA, Hanif MA, Lazhari MI (2017) Chemical composition and biological activities of essential oil and extracts from Ocimum sanctum. Int J Food Prop 20(7):1569–1581. https://doi.org/10.1080/10942912.2016.1214145
Hussein OE, Hozayen WG, Bin-Jumah MN, Germoush MO, El-Twab A, Sanaa M, Mahmoud AM (2020) Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARγ and Nrf2/HO-1 signaling. Environ Sci Pollut Res 27(17):20725–20735. https://doi.org/10.1007/s11356-020-08557-y
Hwang YJ, Lee EJ, Kim HR, Hwang KA (2013) Molecular mechanisms of luteolin-7-O-glucoside-induced growth inhibition on human liver cancer cells: G2/M cell cycle arrest and caspase-independent apoptotic signaling pathways. BMB Rep 46(12):611
Ibrahim RY, Mansour SM, Elkady WM (2020) Phytochemical profile and protective effect of Ocimum basilicum aqueous extract in doxorubicin/irradiation-induced testicular injury. J Pharm Pharmacol 72(1):101–110. https://doi.org/10.1111/jphp.13175
Iranshahy M, Iranshahi M, Abtahi SR, Karimi G (2018) The role of nuclear factor erythroid 2-related factor 2 in hepatoprotective activity of natural products: a review. Food Chem Toxicol 120:261–276. https://doi.org/10.1016/j.fct.2018.07.024
Jadeja RN, Upadhyay KK, Devkar RV, Khurana S (2016) Naturally occurring Nrf2 activators: potential in treatment of liver injury. Oxid Med Cell Longev. https://doi.org/10.1155/2016/3453926
Jiang X, Tang X, Zhang P, Liu G, Guo H (2014) Cyanidin-3-O-β-glucoside protects primary mouse hepatocytes against high glucose-induced apoptosis by modulating mitochondrial dysfunction and the PI3K/Akt pathway. Biochem Pharmacol 90(2):135–144. https://doi.org/10.1016/j.bcp.2014.04.018
Kamel FO, Karim S, Bafail DAO, Aldawsari HM, Kotta S, Ilyas UK (2023) Hepatoprotective effects of bioactive compounds from traditional herb Tulsi (Ocimum sanctum Linn) against galactosamine-induced hepatotoxicity in rats. Front Pharmacol 14:1213052
Kanagaraj VV, Panneerselvam L, Govindarajan V, Ameeramja J, Perumal E (2015) Caffeic acid, a phyto polyphenol mitigates fluoride induced hepatotoxicity in rats: a possible mechanism. BioFactors 41(2):90–100. https://doi.org/10.1002/biof.1203
Kanda T, Goto T, Hirotsu Y, Masuzaki R, Moriyama M, Omata M (2020) Molecular mechanisms: connections between nonalcoholic fatty liver disease, steatohepatitis and hepatocellular carcinoma. Int J Mol Sci 21(4):1525. https://doi.org/10.3390/ijms21041525
Khalaf AA, Hassanen EI, Ibrahim MA, Tohamy AF, Aboseada MA, Hassan HM, Zaki AR (2020) Rosmarinic acid attenuates chromium-induced hepatic and renal oxidative damage and DNA damage in rats. J Biochem Mol Toxicol 34(11):e22579. https://doi.org/10.1002/jbt.22579
Khlifi R, Dhaouefi Z, Toumia IB, Lahmar A, Sioud F, Bouhajeb R, Bellalah A, Chekir-Ghedira L (2020) Erica multiflora extract rich in quercetin-3-O-glucoside and kaempferol-3-O-glucoside alleviates high fat and fructose diet-induced fatty liver disease by modulating metabolic and inflammatory pathways in Wistar rats. J Nutr Biochem 86:108490. https://doi.org/10.1016/j.jnutbio.2020.108490
Kim HY, Lee SM (2012) Ferulic acid attenuates ischemia/reperfusion-induced hepatocyte apoptosis via inhibition of JNK activation. Eur J Pharm Sci 45(5):708–715. https://doi.org/10.1016/j.ejps.2012.01.010
Kim HM, Kim Y, Lee ES, Huh JH, Chung CH (2018) Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high-fat diet-induced obese mice by regulating autophagy. Nutrition 55:63–70. https://doi.org/10.1016/j.nut.2018.03.010
Kubiça TF, Alves SH, Weiblen R, Lovato LT (2014) In vitro inhibition of the bovine viral diarrhoea virus by the essential oil of Ocimum basilicum (basil) and monoterpenes. Braz J Microbiol 45:209–214. https://doi.org/10.1590/S1517-83822014005000030
Küçükler S, Kandemir FM, Özdemir S, Çomaklı S, Caglayan C (2021) Protective effects of rutin against deltamethrin-induced hepatotoxicity and nephrotoxicity in rats via regulation of oxidative stress, inflammation, and apoptosis. Environ Sci Pollut Res 28(44):62975–62990. https://doi.org/10.1007/s11356-021-15190-w
Kumar S, Bouic PJ, Rosenkranz B (2020) In vitro assessment of the interaction potential of Ocimum basilicum (L) extracts on CYP2B6, 3A4, and rifampicin metabolism. Front Pharmacol 11:500271. https://doi.org/10.3389/fphar.2020.00517
Kumar A, Siddiqi NJ, Alrashood ST, Khan HA, Dubey A, Sharma B (2021) Protective effect of eugenol on hepatic inflammation and oxidative stress induced by cadmium in male rats. Biomed Pharmacother 139:111588. https://doi.org/10.1016/j.biopha.2021.111588
Lan CY, Chen SY, Kuo CW, Lu CC, Yen GC (2019) Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J Food Drug Anal 27(4):887–896. https://doi.org/10.1016/j.jfda.2019.07.001
Latief U, Husain H, Mukherjee D, Ahmad R (2016) Hepatoprotective efficacy of gallic acid during Nitrosodiethylamine-induced liver inflammation in Wistar rats. J Basic Appl Zool 76:31–41. https://doi.org/10.1016/j.jobaz.2016.07.002
Le MH, Le DM, Baez TC, Dang H, Nguyen VH, Lee K, Stave CD, Ito T, Wu Y, Yeo YH, Ji F (2024) Global incidence of adverse clinical events in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Clin Mol Hepatol 30(2):235–246. https://doi.org/10.3350/cmh.2023.0485
Leathers JS, Balderramo D, Prieto J, Diehl F, Gonzalez-Ballerga E, Ferreiro MR, Carrera E, Barreyro F, Diaz-Ferrer J, Singh D, Mattos AZ (2019) Sorafenib for treatment of hepatocellular carcinoma. J Clin Gastroenterol 53(6):464–469. https://doi.org/10.1097/MCG.0000000000001085
Li Z, Feng H, Wang Y, Shen B, Tian Y, Wu L, Zhang Q, Jin M, Liu G (2019) Rosmarinic acid protects mice from lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting MAPKs/NF-κB and activating Nrf2/HO-1 signaling pathways. Int Immunopharmacol 67:465–472. https://doi.org/10.1016/j.intimp.2018.12.052
Lieber CS (2020) Metabolism of ethanol. Clin Liver Dis 24(1):1–21. https://doi.org/10.1016/j.cld.2019.08.002
Lima PR, de Melo TS, Carvalho KM, de Oliveira ÍB, Arruda BR, de Castro Brito GA, Rao VS, Santos FA (2013) 1, 8-cineole (eucalyptol) ameliorates cerulein-induced acute pancreatitis via modulation of cytokines, oxidative stress and NF-κB activity in mice. Life Sci 92(24–26):1195–1201. https://doi.org/10.1016/j.lfs.2013.05.009
Lima AS, Milhomem MN, Monteiro OS, Arruda AC, de Castro JA, Fernandes YM, Maia JG, Costa-Junior LM (2018) Seasonal analysis and acaricidal activity of the thymol-type essential oil of Ocimum gratissimum and its major constituents against Rhipicephalus microplus (Acari: Ixodidae). Parasitol Res 117(1):59–65. https://doi.org/10.1007/s00436-017-5662-0
Lin SY, Wang YY, Chen WY, Liao SL, Chou ST, Yang CP, Chen CJ (2017) Hepatoprotective activities of rosmarinic acid against extrahepatic cholestasis in rats. Food Chem Toxicol 108:214–223. https://doi.org/10.1016/j.fct.2017.08.005
Linghu KG, Wu GP, Fu LY, Yang H, Li HZ, Chen Y, Yu H, Tao L, Shen XC (2019) 1, 8-Cineole ameliorates LPS-induced vascular endothelium dysfunction in mice via PPAR-γ dependent regulation of NF-κB. Front Pharmacol 10:178. https://doi.org/10.3389/fphar.2019.00178
Lisboa CF, Melo EC, Demuner AJ, da Silva LC, Carneiro AP, Coelho AP (2020) Chemical composition of Lippia origanoides kunt. and Ocimum gratissimum L. essential oils stored at− 20° C. Indus Crops Prod 151:112485. https://doi.org/10.1016/j.indcrop.2020.112485
Liu J, Zhang Z, Gao J, Xie J, Yang L, Hu S (2011) Downregulation effects of beta-elemene on the levels of plasma endotoxin, serum TNF-alpha, and hepatic CD14 expression in rats with liver fibrosis. Front Med 5(1):101–105. https://doi.org/10.1007/s11684-011-0111-4
Liu Q, Pan R, Ding L, Zhang F, Hu L, Ding B, Zhu L, Xia Y, Dou X (2017a) Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int Immunopharmacol 49:132–141. https://doi.org/10.1016/j.intimp.2017.05.026
Liu Y, Gong W, Yang ZY, Zhou XS, Gong C, Zhang TR, Wei X, Ma D, Ye F, Gao QL (2017b) Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis 22:544–557. https://doi.org/10.1007/s10495-016-1334-2
Liu L, Zhao L, Liu Y, Yu X, Qiao X (2022) Rutin ameliorates cadmium-induced necroptosis in the chicken liver via inhibiting oxidative stress and MAPK/NF-κB pathway. Biol Trace Elem Res 200(4):1799–1810. https://doi.org/10.1007/s12011-021-02764-5
Lu YH, Hong Y, Zhang TY, Chen YX, Wei ZJ, Gao CY (2022) Rosmarinic acid exerts anti-inflammatory effect and relieves oxidative stress via Nrf2 activation in carbon tetrachloride-induced liver damage. Food Nutr Res. https://doi.org/10.29219/fnr.v66.8359
Luo C, Yang H, Tang C, Yao G, Kong L, He H, Zhou Y (2015) Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int Immunopharmacol 28(1):744–750. https://doi.org/10.1016/j.intimp.2015.07.018
Lv Y, Gao X, Luo Y, Fan W, Shen T, Ding C, Yao M, Song S, Yan L (2019) Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. J Nutr Biochem 71:110–121. https://doi.org/10.1016/j.jnutbio.2019.05.015
Ma XY, Zhang M, Fang G, Cheng CJ, Wang MK, Han YM, Hou XT, Hao EW, Hou YY, Bai G (2021) Ursolic acid reduces hepatocellular apoptosis and alleviates alcohol-induced liver injury via irreversible inhibition of CASP3 in vivo. Acta PharmacologicaSinica 42(7):1101–1110. https://doi.org/10.1038/s41401-020-00534-y
Madrigal-Santillán E, Bautista M, Gayosso-de-Lucio JA, Reyes-Rosales Y, Posadas-Mondragón A, Morales-González Á, Soriano-Ursúa MA, García-Machorro J, Madrigal-Bujaidar E, Álvarez-González I, Morales-González JA (2015) Hepatoprotective effect of Geranium schiedeanum against ethanol toxicity during liver regeneration. World J Gastroenterol: WJG 21(25):7718
Mahmoud AM, Hussein OE, Hozayen WG, Bin-Jumah M, El-Twab A, Sanaa M (2020) Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ Sci Pollut Res 27(8):7910–7921. https://doi.org/10.1007/s11356-019-07532-6
Majdi C, Pereira C, Dias MI, Calhelha RC, Alves MJ, Rhourri-Frih B, Charrouf Z, Barros L, Amaral JS, Ferreira IC (2020) Phytochemical characterization and bioactive properties of cinnamon basil (Ocimum basilicum cv.‘Cinnamon’) and lemon basil (Ocimum× citriodorum). Antioxidants 9(5):369. https://doi.org/10.3390/antiox9050369
Maurya S, Sangwan NS (2020) Profiling of essential oil constituents in Ocimum Species. Proc Nat Acad Sci, India Sect b: Biol Sci 90(3):577–583. https://doi.org/10.1007/s40011-019-01123-8
McCance KR, Flanigan PM, Quick MM, Niemeyer ED (2016) Influence of plant maturity on anthocyanin concentrations, phenolic composition, and antioxidant properties of 3 purple basil (Ocimum basilicum L.) cultivars. J Food Compos Anal 53:30–39. https://doi.org/10.1016/j.jfca.2016.08.009
McGill MR, Jaeschke H (2020) Metabolism and disposition of acetaminophen: recent advances to hepatotoxicity and diagnosis. Pharm Res 37(3):1–12. https://doi.org/10.1007/s11095-020-02898-3
Mężyńska M, Brzóska MM (2019) Review of polyphenol-rich products as potential protective and therapeutic factors against cadmium hepatotoxicity. J Appl Toxicol 39(1):117–145. https://doi.org/10.1002/jat.3709
Mith H, Yayi-Ladékan E, Sika Kpoviessi SD, Yaou Bokossa I, Moudachirou M, Daube G, Clinquart A (2016) Chemical composition and antimicrobial activity of essential oils of Ocimum basilicum, Ocimum canum and Ocimum gratissimum in function of harvesting time. J Essen Oil-Bearing Plants 19(6):1413–1425. https://doi.org/10.1080/0972060X.2014.890076
Mohamed WR, Kotb AS, Abd El-Raouf OM, Mohammad FE (2020) Apigenin alleviated acetaminophen-induced hepatotoxicity in low protein-fed rats: targeting oxidative stress, STAT3, and apoptosis signals. J Biochem Mol Toxicol 34(5):e22472. https://doi.org/10.1002/jbt.22472
Mohammadtaghvaei N, Afarin R, Mavalizadeh F, Shakerian E, Bavarsad SS, Mohammadzadeh G (2021) Effect of quercetin on the expression of NOXs and P-Smad3C in TGF-Β-activated hepatic stellate cell line LX-2. Hepat Mon. https://doi.org/10.5812/hepatmon.116875
Mohr FB, Lermen C, Gazim ZC, Gonçalves JE, Alberton O (2017) Antifungal activity, yield, and composition of Ocimum gratissimum essential oil. Genet Mol Res 16:1. https://doi.org/10.4238/gmr16019542
Mousavi L, Salleh RM, Murugaiyah V (2018) Phytochemical and bioactive compounds identification of Ocimum tenuiflorum leaves of methanol extract and its fraction with an anti-diabetic potential. Int J Food Prop 21(1):2390–2399. https://doi.org/10.1080/10942912.2018.1508161
Murata S, Ogawa K, Matsuzaka T, Chiba M, Nakayama K, Iwasaki K, Kurokawa T, Sano N, Tanoi T, Ohkohchi N (2015) 1, 8-Cineole ameliorates steatosis of Pten liver specific KO mice via Akt inactivation. Int J Mol Sci 16(6):12051–12063. https://doi.org/10.3390/ijms160612051
Nadeem HR, Akhtar S, Sestili P, Ismail T, Neugart S, Qamar M, Esatbeyoglu T (2022) Toxicity, antioxidant activity, and phytochemicals of basil (Ocimum basilicum L.) leaves cultivated in Southern Punjab, Pakistan. Foods 11(9):1239. https://doi.org/10.3390/foods11091239
Nozari E, Moradi A, Samadi M (2020) Effect of atorvastatin, curcumin, and quercetin on miR-21 and miR-122 and their correlation with TGFβ1 expression in experimental liver fibrosis. Life Sci 259:118293. https://doi.org/10.1016/j.lfs.2020.118293
Ogaly HA, Eltablawy NA, El-Behairy AM, El-Hindi H, Abd-Elsalam RM (2015) Hepatocyte growth factor mediates the antifibrogenic action of Ocimum bacilicum essential oil against CCl4-induced liver fibrosis in rats. Molecules 20(8):13518–13535. https://doi.org/10.3390/molecules200813518
Ogundipe OJ, Akinpelu OF, Oyerinde A, Oluwakemi OR (2021) Ocimum gratissimum (Linn) leaves extract attenuates oxidative stress and liver injury in gentamicin-induced hepatotoxicity in rats. Egypt J Basic Appl Sci. 8(1):146–155. https://doi.org/10.34119/bjhrv4n6-410
Owumi SE, Irozuru CE, Arunsi UO, Oyelere AK (2022) Caffeic acid protects against DNA damage, oxidative and inflammatory mediated toxicities, and upregulated caspases activation in the hepatorenal system of rats treated with aflatoxin B1. Toxicon 207:1–2. https://doi.org/10.1016/j.toxicon.2021.12.021
Pan PH, Lin SY, Wang YY, Chen WY, Chuang YH, Wu CC, Chen CJ (2014) Protective effects of rutin on liver injury induced by biliary obstruction in rats. Free Radical Biol Med 1(73):106–116. https://doi.org/10.1016/j.freeradbiomed.2014.05.001
Pandey R, Kumar B (2016) HPLC–QTOF–MS/MS-based rapid screening of phenolics and triterpenic acids in leaf extracts of Ocimum species and their interspecies variation. J Liq Chromatogr Relat Technol 39(4):225–238. https://doi.org/10.1080/10826076.2016.1148048
Pandey AK, Singh P, Tripathi NN (2014) Chemistry and bioactivities of essential oils of some Ocimum species: an overview. Asian Pacific J Trop Biomed. 4(9):682–694. https://doi.org/10.12980/APJTB.4.2014C77
Pandey R, Chandra P, Srivastava M, Mishra DK, Kumar B (2015) Simultaneous quantitative determination of multiple bioactive markers in Ocimum sanctum obtained from different locations and its marketed herbal formulations using UPLC-ESI-MS/MS combined with principal component analysis. Phytochem Anal 26(6):383–394. https://doi.org/10.1002/pca.2551
Pandey R, Chandra P, Kumar B, Dutt B, Sharma KR (2016) A rapid and highly sensitive method for simultaneous determination of bioactive constituents in leaf extracts of six Ocimum species using ultra high-performance liquid chromatography-hybrid linear ion trap triple quadrupole mass spectrometry. Anal Methods 8(2):333–341. https://doi.org/10.1039/C5AY01055A
Pang C, Zheng Z, Shi L, Sheng Y, Wei H, Wang Z, Ji L (2016) Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radical Biol Med 91:236–246. https://doi.org/10.1016/j.freeradbiomed.2015.12.024
Parasuraman S, Balamurugan S, Christapher PV, Petchi RR, Yeng WY, Sujithra J, Vijaya C (2015) Evaluation of antidiabetic and antihyperlipidemic effects of hydroalcoholic extract of leaves of Ocimum tenuiflorum (Lamiaceae) and prediction of biological activity of its phytoconstituents. Pharmacogn Res. 7(2):156
Park JY, Kang KS, Lee HJ (2017) Protection effect of cyanidin 3-O-glucoside against oxidative stress-induced HepG2 Cell death through activation of akt and extracellular signal-regulated kinase pathways. Bull Korean Chem Soc 38(11):1316–1320. https://doi.org/10.1002/bkcs.11290
Patidar S, Manigauha A, Dubey B (2019) Modulator efficacy of dietary inclusion of Ocimum sanctum leaves against gentamicin-induced hepatotoxicity in rats. Indian J Res Pharm Biotechnol (IJRPB). https://doi.org/10.31426/ijrpb
Paukku Y, Rasulev B, Syrov V, Khushbaktova Z, Leszczynski J (2009) Structure-hepatoprotective activity relationship study of sesquiterpene lactones: a QSAR analysis. Int J Quantum Chem 109(1):17–27. https://doi.org/10.1002/qua.21647
Pérez-Ramírez IF, González-Dávalos ML, Mora O, Gallegos-Corona MA, Reynoso-Camacho R (2017) Effect of Ocimum sanctum and Crataegus pubescens aqueous extracts on obesity, inflammation, and glucose metabolism. J Funct Foods 35:24–31. https://doi.org/10.1016/j.jff.2017.05.028
Poli G (1993) Liver damage due to free radicals. Br Med Bull 49(3):604–620. https://doi.org/10.1093/oxfordjournals.bmb.a072634
Rady MR, Nazif NM (2005) Rosmarinic acid content and RAPD analysis of in vitro regenerated basil (Ocimum americanum) plants. Fitoterapia 76(6):525–533. https://doi.org/10.1016/j.fitote.2005.04.001
Rahayu S, Widyarti S, Soewondo A, Prasetyaningrum DI, Umarudin U (2024) A computational insights of Ocimum basilicum flavonoid and essential oils interaction in the targeting keap1/SIRT1/NFKB signaling pathway. Trop J Nat Prod Res. https://doi.org/10.26538/tjnpr/v8i2.14
Rajendran P, Ammar RB, Al-Saeedi FJ, Mohamed ME, ElNaggar MA, Al-Ramadan SY, Bekhet GM, Soliman AM (2020) Kaempferol inhibits zearalenone-induced oxidative stress and apoptosis via the PI3K/Akt-mediated Nrf2 signaling pathway: in vitro and in vivo studies. Int J Mol Sci 22(1):217. https://doi.org/10.3390/ijms22010217
Rajput SA, Shaukat A, Wu K, Rajput IR, Baloch DM, Akhtar RW, Raza MA, Najda A, Rafał P, Albrakati A, El-Kott AF (2021) Luteolin alleviates aflatoxinB1-induced apoptosis and oxidative stress in the liver of mice through activation of Nrf2 signaling pathway. Antioxidants 10(8):1268. https://doi.org/10.3390/antiox10081268
Ramachandran A, Jaeschke H (2021) Oxidant stress and acetaminophen hepatotoxicity: mechanism-based drug development. Antioxid Redox Signal 35(9):718–733. https://doi.org/10.1089/ars.2021.0102
Ramalho LN, Pasta ÂA, Terra VA, Augusto MJ, Sanches SC, Souza-Neto FP, Cecchini R, Gulin F, Ramalho FS (2014) Rosmarinic acid attenuates hepatic ischemia and reperfusion injury in rats. Food Chem Toxicol 74:270–278. https://doi.org/10.1016/j.fct.2014.10.004
Ramesh B, Satakopan VN (2010) Antioxidant activities of hydroalcoholic extract of Ocimum sanctum against cadmium induced toxicity in rats. Indian J Clin Biochem 25:307–310. https://doi.org/10.1007/s12291-010-0039-5
Recknagel RO, Glende EA, Britton RS (2020) Free radical damage and lipid peroxidation inHepatotoxicology. CRC Press, Boca Raton, pp 401–436
Sabitha R, Nishi K, Gunasekaran VP, Agilan B, David E, Annamalai G, Vinothkumar R, Perumal M, Subbiah L, Ganeshan M (2020) p-Coumaric acid attenuates alcohol exposed hepatic injury through MAPKs, apoptosis and Nrf2 signaling in experimental models. Chem Biol Interact 321:109044. https://doi.org/10.1080/23312009.2018.1440894
Saha P, Talukdar AD, Nath R, Sarker SD, Nahar L, Sahu J, Choudhury MD (2019) Role of natural phenolics in hepatoprotection: a mechanistic review and analysis of regulatory network of associated genes. Front Pharmacol 10:509. https://doi.org/10.3389/fphar.2019.00509
Sanjay S, Girish C, Toi PC, Bobby Z (2021) Gallic acid attenuates isoniazid and rifampicin-induced liver injury by improving hepatic redox homeostasis through influence on Nrf2 and NF-κB signaling cascades in Wistar Rats. J Pharm Pharmacol 73(4):473–486. https://doi.org/10.1093/jpp/rgaa048
Santos BC, Pires AS, Yamamoto CH, Couri MR, Taranto AG, Alves MS, Araújo AL, de Sousa OV (2018) Methyl chavicol and its synthetic analogue as possible antioxidant and antilipase agents based on the in vitro and in silico assays. Oxid Med Cell Longev. https://doi.org/10.1155/2018/2189348
Sarkar B, Vyas P, Haque I, Mukhopadhyay K (2018) A rapid UPLC method for simultaneous separation and detection of anthocyanidins from Ocimum, Hibiscus and Syzygium species and estimation of their antioxidant activity. J Liq Chromatogr Relat Technol 41(10):658–667. https://doi.org/10.1080/10826076.2018.1506932
Satapathy S, Das N, Bandyopadhyay D, Mahapatra SC, Sahu DS, Meda M (2017) Effect of Tulsi (Ocimum sanctum Linn.) supplementation on metabolic parameters and liver enzymes in young overweight and obese subjects. Indian J Clin Biochem 32:357–363. https://doi.org/10.1007/s12291-016-0615-4
Selvarani S, Moorthi PV, Saranya P, Abirami M (2015) Anti-cancer activity of silver nanoparticle synthesized from stem extract of Ocimum Kilimandscharicum against Hep-G2, liver cancer cell line. J Nanotechnol Nanosci 1:100103
Sen A (2020) Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases. World J Clin Cases. 8(10):1767
Shi H, Shi A, Dong L, Lu X, Wang Y, Zhao J, Dai F, Guo X (2016) Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin Nutr 35(6):1366–1373. https://doi.org/10.1016/j.clnu.2016.03.002
Simón J, Casado-Andrés M, Goikoetxea-Usandizaga N, Serrano-Maciá M, Martínez-Chantar ML (2020) Nutraceutical properties of polyphenols against liver diseases. Nutrients 12(11):3517. https://doi.org/10.3390/nu12113517
Singh D, Chaudhuri PK (2018) A review on phytochemical and pharmacological properties of Holy basil (Ocimum sanctum L.). Indus Crops Prod 118:367–382. https://doi.org/10.1016/j.indcrop.2018.03.048
Singh S, Singh DK, Meena A, Dubey V, Masood N, Luqman S (2019) Rutin protects t-butyl hydroperoxide-induced oxidative impairment via modulating the Nrf2 and iNOS activity. Phytomedicine 55:92–104. https://doi.org/10.1016/j.phymed.2018.07.009
Stanojevic LP, Marjanovic-Balaban ZR, Kalaba VD, Stanojevic JS, Cvetkovic DJ, Cakic MD (2017) Chemical composition, antioxidant and antimicrobial activity of basil (Ocimum basilicum L.) essential oil. J Essent Oil-Bearing Plants. 20(6):1557–1569. https://doi.org/10.1080/0972060X.2017.1401963
Strazzer P, Guzzo F, Levi M (2011) Correlated accumulation of anthocyanins and rosmarinic acid in mechanically stressed red cell suspensions of basil (Ocimum basilicum). J Plant Physiol 168(3):288–293. https://doi.org/10.1016/j.jplph.2010.07.020
Sun G, Zhang S, Xie Y, Zhang Z, Zhao W (2016) Gallic acid as a selective anticancer agent that induces apoptosis in SMMC-7721 human hepatocellular carcinoma cells. Oncol Lett 11(1):150–158. https://doi.org/10.3892/ol.2015.3845
Sundaram RS, Ramanathan M, Rajesh R, Satheesh B, Saravanan D (2012) LC-MS quantification of rosmarinic acid and ursolic acid in the Ocimum sanctum Linn. leaf extract (Holy basil, Tulsi). J Liquid Chromatogr Related Technol 35(5):634–650. https://doi.org/10.1080/10826076.2011.606583
Suraweera DB, Weeratunga AN, Hu RW, Pandol SJ, Hu R (2015) Alcoholic hepatitis: the pivotal role of Kupffer cells. World J Gastrointest Pathophysiol 6(4):90. https://doi.org/10.4291/wjgp.v6.i4.90
Suryani D, Lubis HM (2019) Comparison between Ocimum Sanctum hepatoprotector extract and curcuma xanthorrhiza on the histological structure of aspartame-induced wistar rats. Budapest Int Res Exact Sci (BirEx) J. 1(4):45–52. https://doi.org/10.33258/birex.v1i4.476
Sutili FJ, Velasquez A, Pinheiro CG, Heinzmann BM, Gatlin DM III, Baldisserotto B (2016) Evaluation of Ocimum americanum essential oil as an additive in red drum (Sciaenops ocellatus) diets. Fish Shellfish Immunol 56:155–161. https://doi.org/10.1016/j.fsi.2016.07.008
Tai M, Zhang J, Song S, Miao R, Liu S, Pang Q, Wu Q, Liu C (2015) Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. Int Immunopharmacol 27(1):164–170. https://doi.org/10.1016/j.intimp.2015.05.009
Tanaka M, Sato A, Kishimoto Y, Mabashi-Asazuma H, Kondo K, Iida K (2020) Gallic acid inhibits lipid accumulation via AMPK pathway and suppresses apoptosis and macrophage-mediated inflammation in hepatocytes. Nutrients 12(5):1479. https://doi.org/10.3390/nu12051479
Tavallali V, Rowshan V, Gholami H, Hojati S (2020) Iron-urea nano-complex improves bioactive compounds in essential oils of Ocimum basilicum L. Sci Hortic 265:109222. https://doi.org/10.1016/j.scienta.2020.109222
Teofilović B, Tomas A, Martić N, Stilinović N, Popović M, Čapo I, Grujić N, Ilinčić B, Rašković A (2021) Antioxidant and hepatoprotective potential of sweet basil (Ocimum basilicum L.) extract in acetaminophen-induced hepatotoxicity in rats. J Funct Foods 87:104783. https://doi.org/10.1016/j.jff.2021.104783
Touiss I, Ouahhoud S, Harnafi M, Khatib S, Bekkouch O, Amrani S, Harnafi H (2021) Toxicological evaluation and hepatoprotective efficacy of rosmarinic acid-rich extract from Ocimum basilicum L. Evid-Based Complement Alternat Med. https://doi.org/10.1155/2021/6676998
Ugbogu OC, Emmanuel O, Agi GO, Ibe C, Ekweogu CN, Ude VC, Uche ME, Nnanna RO, Ugbogu EA (2021) A review on the traditional uses, phytochemistry, and pharmacological activities of clove basil (Ocimum gratissimum L). Heliyon. https://doi.org/10.1016/j.heliyon.2021.e08404
Unsal V, Cicek M, Sabancilar İ (2021) Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev Environ Health 36(2):279–295. https://doi.org/10.1515/reveh-2020-0048
Upadhyay R, Nachiappan G, Mishra HN (2015) Ultrasound-assisted extraction of flavonoids and phenolic compounds from Ocimum tenuiflorum leaves. Food Sci Biotechnol 24(6):1951–1958. https://doi.org/10.1007/s10068-015-0257-y
Usman LA, Yussuf AO, Saliu BK, Olanipekun BE, Elelu N (2017) Effect of collection time on Chemical composition and Antibacterial activity of Flower Essential oil of Ocimum canum (sims) grown in Nigeria. J Turkish Chem Soc Sect a: Chem. 4(1):149–164. https://doi.org/10.18596/jotcsa.287322
Varga ZV, Matyas C, Erdelyi K, Cinar R, Nieri D, Chicca A, Nemeth BT, Paloczi J, Lajtos T, Corey L, Hasko G (2018) β-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br J Pharmacol 175(2):320–334. https://doi.org/10.1111/bph.13722
Venuprasad MP, Kandikattu HK, Razack S, Khanum F (2014) Phytochemical analysis of Ocimum gratissimum by LC-ESI–MS/MS and its antioxidant and anxiolytic effects. S Afr J Bot 92:151–158. https://doi.org/10.1016/j.sajb.2014.02.010
Venuprasad MP, Kandikattu HK, Razack S, Amruta N, Khanum F (2017) Chemical composition of Ocimum sanctum by LC-ESI–MS/MS analysis and its protective effects against smoke induced lung and neuronal tissue damage in rats. Biomed Pharmacother 91:1–2. https://doi.org/10.1016/j.biopha.2017.04.011
Verma S (2016) Chemical constituents and pharmacological action of Ocimum sanctum (Indian holy basil-Tulsi). J Phytopharmacol. 5(5):205–207. https://doi.org/10.31254/phyto.2016.5507
Vinholes J, Rudnitskaya A, Gonçalves P, Martel F, Coimbra MA, Rocha SM (2014) Hepatoprotection of sesquiterpenoids: a quantitative structure–activity relationship (QSAR) approach. Food Chem 146:78–84. https://doi.org/10.1016/j.foodchem.2013.09.039
Vladimir-Knežević S, Blažeković B, Bival Štefan M, Babac M. Plant polyphenols as antioxidants influencing the human health. IntechOpen; 2012.
Wang F, Liu JC, Zhou RJ, Zhao X, Liu M, Ye H, Xie ML (2017) Apigenin protects against alcohol-induced liver injury in mice by regulating hepatic CYP2E1-mediated oxidative stress and PPARα-mediated lipogenic gene expression. Chem Biol Interact 275:171–177. https://doi.org/10.1016/j.cbi.2017.08.006
Wei CB, Tao K, Jiang R, Zhou LD, Zhang QH, Yuan CS (2017) Quercetin protects mouse liver against triptolide-induced hepatic injury by restoring Th17/Treg balance through Tim-3 and TLR4-MyD88-NF-κB pathway. Int Immunopharmacol 53:73–82. https://doi.org/10.1016/j.intimp.2017.09.026
Wei M, Zheng Z, Shi L, Jin Y, Ji L (2018) Natural polyphenol chlorogenic acid protects against acetaminophen-induced hepatotoxicity by activating ERK/Nrf2 antioxidative pathway. Toxicol Sci 162(1):99–112. https://doi.org/10.1093/toxsci/kfx230
Witkowska-Banaszczak E, Krajka-Kuźniak V, Papierska K (2020) The effect of luteolin 7-glucoside, apigenin 7-glucoside and Succisa pratensis extracts on NF-κB activation and α-amylase activity in HepG2 cells. Acta Biochim Pol 67(1):41–47. https://doi.org/10.18388/abp.2020_2894
Wu L, Zhang Q, Mo W, Feng J, Li S, Li J, Liu T, Xu S, Wang W, Lu X, Yu Q (2017) Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways. Sci Rep 7(1):1–3. https://doi.org/10.1038/s41598-017-09673-5
Wu J, Zhou F, Fan G, Liu J, Wang Y, Xue X, Lyu X, Lin S, Li X (2022) Ferulic acid ameliorates acetaminophen-induced acute liver injury by promoting AMPK-mediated protective autophagy. IUBMB Life. https://doi.org/10.1002/iub.2625
Wu XN, Xue F, Zhang N, Zhang W, Hou JJ, Lv Y, Xiang JX, Zhang XF (2024) Global burden of liver cirrhosis and other chronic liver diseases caused by specific etiologies from 1990 to 2019. BMC Public Health 24(1):363. https://doi.org/10.1186/s12889-024-17948-6
Xu Y, Chen J, Yu X, Tao W, Jiang F, Yin Z, Liu C (2010) Protective effects of chlorogenic acid on acute hepatotoxicity induced by lipopolysaccharide in mice. Inflamm Res 59(10):871–877. https://doi.org/10.1007/s00011-010-0199-z
Xu Q, Li M, Yang M, Yang J, Xie J, Lu X, Wang F, Chen W (2018) α-pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci Rep. https://doi.org/10.1042/BSR20180980
Yamani HA, Pang EC, Mantri N, Deighton MA (2016) Antimicrobial activity of Tulsi (Ocimum tenuiflorum) essential oil and their major constituents against three species of bacteria. Front Microbiol 7:681. https://doi.org/10.3389/fmicb.2016.00681
Yan X, Liu X, Wang Y, Ren X, Ma J, Song R, Wang X, Dong Y, Fan Q, Wei J, Yu A (2022) Multi-omics integration reveals the hepatoprotective mechanisms of ursolic acid intake against chronic alcohol consumption. Eur J Nutr 61(1):115–126. https://doi.org/10.1007/s00394-021-02632-x
Yang SY, Pyo MC, Nam MH, Lee KW (2019) ERK/Nrf2 pathway activation by caffeic acid in HepG2 cells alleviates its hepatocellular damage caused by t-butylhydroperoxide-induced oxidative stress. BMC Complement Altern Med 19(1):1–3. https://doi.org/10.1186/s12906-019-2551-3
Yang H, Xuefeng Y, Shandong W, Jianhua X (2020) COX-2 in liver fibrosis. Clin Chim Acta 506:196–203. https://doi.org/10.1016/j.cca.2020.03.024
Yogalakshmi B, Viswanathan P, Anuradha CV (2010) Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 268(3):204–212. https://doi.org/10.1016/j.tox.2009.12.018
Yu L, Zhang SD, Zhao XL, Ni HY, Song XR, Wang W, Yao LP, Zhao XH, Fu YJ (2020) Cyanidin-3-glucoside protects liver from oxidative damage through AMPK/Nrf2 mediated signaling pathway in vivo and in vitro. Journal of Functional Foods 73:104148. https://doi.org/10.1016/j.jff.2020.104148
Yuan L, Wang J, Xiao H, Wu W, Wang Y, Liu X (2013) MAPK signaling pathways regulate mitochondrial-mediated apoptosis induced by isoorientin in human hepatoblastoma cancer cells. Food Chem Toxicol 53:62–68. https://doi.org/10.1016/j.fct.2012.11.048
Yuan H, Duan S, Guan T, Yuan X, Lin J, Hou S, Lai X, Huang S, Du X, Chen S (2020) Vitexin protects against ethanol-induced liver injury through Sirt1/p53 signaling pathway. Eur J Pharmacol 873:173007. https://doi.org/10.1016/j.ejphar.2020.173007
Zahran EM, Abdelmohsen UR, Khalil HE, Desoukey SY, Fouad MA, Kamel MS (2020) Diversity, phytochemical and medicinal potential of the genus Ocimum L.(Lamiaceae). Phytochem Rev 19:907–953. https://doi.org/10.1007/s11101-020-09690-9
Zhang H, Tan X, Yang D, Lu J, Liu B, Baiyun R, Zhang Z (2017) Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/p53 signalling pathway in rats. Oncotarget 8(25):40982
Zhang LY, Zhan DL, Chen YY, Wang WH, He CY, Lin Y, Lin YC, Lin ZN (2019) Aflatoxin B1 enhances pyroptosis of hepatocytes and activation of Kupffer cells to promote liver inflammatory injury via dephosphorylation of cyclooxygenase-2: an in vitro, ex vivo and in vivo study. Arch Toxicol 93:3305–3320. https://doi.org/10.1007/s00204-019-02572-w
Zhao X, Wang J, Deng Y, Liao L, Zhou M, Peng C, Li Y (2021) Quercetin as a protective agent for liver diseases: A comprehensive descriptive review of the molecular mechanism. Phytother Res 35(9):4727–4747. https://doi.org/10.1002/ptr.7104
Zhou Y, Ruan Z, Zhou L, Shu X, Sun X, Mi S, Yang Y, Yin Y (2016) Chlorogenic acid ameliorates endotoxin-induced liver injury by promoting mitochondrial oxidative phosphorylation. Biochem Biophys Res Commun 469(4):1083–1089. https://doi.org/10.1016/j.bbrc.2015.12.094
Zhou RJ, Zhao Y, Fan K, Xie ML (2020) Protective effect of apigenin on d-galactosamine/LPS-induced hepatocellular injury by an increment of Nrf-2 nucleus translocation. Naunyn-Schmiedeberg’s Arch Pharmacol. 393(6):929–936. https://doi.org/10.1007/s00210-019-01760-w
Zhu R, Yang L, Shen L, Ye J, Liu J, Hu S (2009) ANG II-AT1 receptor pathway is involved in the anti-fibrotic effect of β-elemene. J Huazhong Univ Sci Technol 29(2):177–181. https://doi.org/10.1007/s11596-009-0208-z
Zhu L, Wang L, Cao F, Liu P, Bao H, Yan Y, Dong X, Wang D, Wang Z, Gong P (2018) Modulation of transport and metabolism of bile acids and bilirubin by chlorogenic acid against hepatotoxicity and cholestasis in bile duct ligation rats: involvement of SIRT1-mediated deacetylation of FXR and PGC-1α. J Hepatobiliary Pancreat Sci 25(3):195–205. https://doi.org/10.1002/jhbp.537
Acknowledgements
The authors acknowledge the DST-SERB, a Government of India-funded project [grant no. EMR/2016/005695] to Dr. Biswatrish Sarkar and Junior Research Fellowship to Amrita Chatterjee.
Author information
Authors and Affiliations
Contributions
Amrita Chatterjee: Literature Search, Analysis, Writing-original draft; Writing-review & editing; Biswatrish Sarkar: Conceptualization, Supervision, Editing and Revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chatterjee, A., Sarkar, B. Polyphenols and terpenoids derived from Ocimum species as prospective hepatoprotective drug leads: a comprehensive mechanistic review. Phytochem Rev (2024). https://doi.org/10.1007/s11101-024-09992-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11101-024-09992-2