Abstract
Deltamethrin is a type-II pyrethroid synthetic insecticide that is extensively used for controlling mosquitoes, flies, pests, and insects worldwide. This study was carried out to evaluate the likelihood protective effects of rutin, a natural antioxidant, against deltamethrin-induced liver and kidney toxicities in rats. Hepatotoxicity and nephrotoxicity were evaluated after the rats were treated orally with deltamethrin (1.28 mg/kg b.w.) alone or with rutin (25 and 50 mg/kg b.w.) for 30 days. Deltamethrin administration caused an increase in lipid peroxidation level and a decrease in activities of SOD, CAT, GPx, and GSH levels in the both tissues. Deltamethrin also increased serum ALT, AST, ALP, urea, and creatinine levels, while reduced nephrine levels in rats. In addition, deltamethrin increased the activation of inflammatory and apoptotic pathways by decreasing Bcl-2 and increasing TNF-α, NF-κB, IL-1β, p38α MAPK, COX-2, iNOS, beclin-1, Bax, and caspase-3 protein levels and/or activities. Furthermore, deltamethrin increased mRNA expression levels of PARP-1, VEGF, and immunohistochemical expressions of c-fos in the tissues. Rutin treatment significantly improved all examined parameters and restored the liver and kidney histopathological and immunohistochemical alterations. These findings demonstrate that rutin could be used to ameliorate hepatotoxicity and nephrotoxicity associated with oxidative stress, inflammation, and apoptosis in deltamethrin-induced rats.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exposure to environmental pollutants such as pesticides, xenobiotics, heavy metals, and radiation has deleterious effects on human health and causes serious pathophysiological disorders. In particular, the rate of pesticides usage (including insecticides) due to their broad-spectrum bioactivity and low prices is alarming in countries where the main source of the economy is agriculture (Arora et al. 2016). Humans are potentially exposed to pesticides either directly, as farmers and agricultural workers, or indirectly, through the ingestion of contaminated food (Abdel-Daim and El-Ghoneimy 2015). Therefore, the intense use of pesticides can cause severe ecological threats and likelihood health issues, such as acute, subacute, and chronic human and animal poisoning (Maalej et al. 2017).
Among pesticides, pyrethroid insecticides are one the most important classes of insecticides worldwide and account for more than 17% of the global agrochemical market (Li et al. 2017; Morgan et al. 2018). Deltamethrin is a broad-spectrum type-II synthetic pyrethroid insecticide, widely used to protect agricultural crops, fruits, and vegetables against pests including as beetles, mites, ants, and weevils (Abdel-Daim et al. 2013; Caglayan et al. 2020; Caglayan et al. 2019c). Accumulating evidence demonstrated that deltamethrin is readily absorbed through contaminated food and water (Ahmadvand et al. 2016; Barlow et al. 2001). Despite its rapid metabolism and low toxicity, recent studies have shown that chronic exposure to deltamethrin can cause adverse effects such as hepatotoxicity (Maalej et al. 2017), nephrotoxicity (Abdel-Daim and El-Ghoneimy 2015), neurotoxicity (Khalatbary et al. 2015), infertility (Abdallah et al. 2010), and metabolic disorders (Rjeibi et al. 2016). The accumulation of deltamethrin particularly in liver and kidney tissues increases the generation of the reactive oxygen species (ROS). Increased ROS damages proteins, nucleic acids, and lipids (Ahmadvand et al. 2016; Gulcin 2020; Gülçin 2012; Maalej et al. 2017).
In humans, deltamethrin acts as a pediculicide. The main toxic effects of deltamethrin are known as hyperexcitability, choreoathetosis, and salivation. These effects usually begin quickly and last for a short time. However, there is one reported case of human death involving a 30-year-old man who died 2 days after consuming approximately 30 ml of deltamethrin. Previous studies of deltamethrin metabolism in rats have been known to involve esterase-mediated cleavage of the ester linkage and ring hydroxylation by cytochrome P450s (Ding et al. 2004; Mirfazaelian et al. 2006). A prior study has reported that 27% of a 2.4 mg/kg intravenous dose of deltamethrin was excreted in the feces of female rats, possibly as metabolites (Ruzo et al. 1978).
Rutin is a flavonoid glycoside found in fruits and fruit peels, predominantly in citrus fruits such as grapefruit, oranges, and lemons. It is also present in spinach, onions, apples, buckwheat seeds, tea, and wine (Çelik et al. 2020; Nafees et al. 2015). It has several pharmacological properties such as anti-inflammatory, antioxidant, antihypertensive, antiulcer, antiallergic, anticarcinogenic, anti-mutagenic, immunomodulator, and potent scavenger of superoxide radicals (Caglayan et al. 2019b; Kandemir et al. 2020a; Nafees et al. 2015). Rutin has been documented to alleviate liver and/or kidney injuries induced by various toxic agents, including mercuric chloride (Caglayan et al. 2019a; Caglayan et al. 2019b), lead acetate (Ansar et al. 2016), carbon tetrachloride (Hafez et al. 2015), potassium bromate (Khan et al. 2012), acrylamide (Ahmed and Ibrahim Laila 2018), and hexachlorobutadiene (Sadeghnia et al. 2013).
Keeping in view the protective effects of rutin, the present study was designed to investigate the probable protective effects of rutin against deltamethrin-induced hepatotoxicity and nephrotoxicity in male rats.
Materials and methods
Chemicals
Deltamethrin (C22H19Br2NO3) is a synthetic pyrethroid insecticide (CAS number: 52918-63-5, 98% purity). The CAS chemical name (α-cyano-3-phenoxybenzyl (1R,3R)-3-(2,2-dibromovinyl)-2,2 dimethyl cyclopropane carboxylate), rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside, CAS number: 207671-50-9, 94% purity), and other chemicals used in the study were supplied by Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
Animals
Ten-week-old male Sprague Dawley rats (average body weight 250–300 g) were obtained from the Experimental Animal Center of Ataturk University, Erzurum, Turkey. The animal room was designed to maintain temperature at 24 ± 1 °C, relative humidity at approximately 45 ± 5%, and a 12 h dark/light photoperiod. The rats were acclimatized to the experimental condition for a period of 1 week and fed with standard laboratory feed and tap water ad libitum. The study was approved by the Animal Experiments Local Ethics Committee of the Atatürk University (approval no: 2020-4/65).
Experimental design
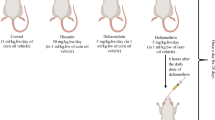
The animals were randomly subdivided into 5 different groups with 7 rats in each group and were treated as follows:
-
1)
Group I (control) received 0.5-ml corn oil per rat, which was given only once a day via oral gavage.
-
2)
Group II (rutin) received rutin (50 mg/kg, b.w.) (Manzoni et al. 2019).
-
3)
Group III (deltamethrin) received deltamethrin (1.28 mg/kg, b.w., dissolved in corn oil) (Yousef et al. 2006).
-
4)
Group IV (deltamethrin + rutin 25 mg/kg) was treated with rutin (25 mg/kg, b.w.) 30 min after deltamethrin administration.
-
5)
Group IV (deltamethrin + rutin 50 mg/kg) was treated with rutin (50 mg/kg, b.w.) 30 min after deltamethrin administration.
In the present study, deltamethrin and rutin were given orally through stomach gavage and continued for 30 consecutive days. The selected dose of deltamethrin was based on the previous study where 1/100 of the LD50 induced biochemical and histopathological alterations in rats without leading to morbidity (Yousef et al. 2006).
At the end of the experimental period, 24 h after receiving the last treatment, the rats were sacrificed under mild sevoflurane anesthesia. Blood samples were collected and centrifuged at 3000 rpm for 10 min to separate the serum. The liver and kidney tissues were used for biochemical, molecular, and histopathological analysis.
Determination of hepatic and renal function markers
Measurement of serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were done by enzymatic commercial kits (TML, Diagnostic Medical Products, Ankara, Turkey). Also, serum levels of urea, creatinine, and nephrin were measured by colorimetric kits (Diasis Diagnostic Systems, İstanbul, Turkey).
Determination of lipid peroxidation and antioxidant enzymes in the liver and kidney tissues
To obtain tissue homogenates, the liver and kidney tissues were ground using liquid nitrogen.
These tissues were homogenized in a homogenizer device with 1.15% potassium chloride to obtain a 1:10 (w/v) whole homogenate. The homogenates required for oxidative stress biomarkers and lipid peroxidation analyses were obtained as described in our previous study (Kandemir et al. 2020b). Superoxide dismutase (SOD) activity of the liver and kidney tissue homogenates was estimated according to the method of Sun et al. (1988), and it was expressed as units (U) per gram of protein. Catalase (CAT) activity was evaluated according to Aebi (1984), and it was expressed as katal per gram of protein. Glutathione peroxidase (GPx) activity was evaluated as U/g protein in the liver and kidney tissues according to Lawrence and Burk (1976) method. Glutathione (GSH) content was determined by the method of Sedlak and Lindsay (1968). As a marker of lipid peroxidation, the kidney and liver tissue malondialdehyde (MDA) concentrations were determined according to the method of Placer et al. (1966) and its levels were expressed as nanomole per gram of tissue. The GSH and MDA concentrations have been expressed as nanomole per gram tissue. The protein content of the liver and kidney tissue homogenates were determined using the Lowry et al. (1951).
Determination of inflammatory markers in the liver and kidney tissues
Frozen liver and kidney tissues were homogenized in ice-cold phosphate saline buffer (0.1 M; pH 7.4; 1:20 w/v) and then centrifuged at 3500 rpm for 15 min to obtain these tissue homogenates. The obtained supernatants were used in the ELISA assays. Interleukin-1 beta (IL-1β), nuclear factor kappa B (NF-κB), and tumor necrosis factor-α (TNF-α) levels and p38α mitogen-activated protein kinase (p38α MAPK), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) activities in liver and kidney tissues were determined using commercial rat ELISA kits according to the manufacturer’s procedure (YL Biont, Shanghai, China).
Determination of beclin-1 levels in the liver and kidney tissues
The levels of beclin-1 in the liver and kidney homogenates were determined using an ELISA kit according to the manufacturer’s instructions (YL Biont, Shanghai, China).
Real-time PCR
Total RNA was isolated from liver and kidney tissue of experimental and control groups with QIAzol Lysis Reagent (Qiagen, Cat: 79306, Germany) according to the manufacturer’s instructions. cDNA was synthesized with QuantiTect Reverse Transcription (Qiagen, Cat:330411, Germany) from total RNA according to the manufacturer’s instructions (Özdemir and Çomaklı 2018). Real-time PCR (RT-PCR) was conducted to measure the mRNA transcript level of Cas-3, Bax, Bcl-2, PARP-1, and VEGF in the liver and kidney tissues using Rotor-Gene Q 5plex HRM Platform (Qiagen, Germany). All primer sequences and reaction conditions are shown in Table 1. GAPDH was used as internal control gene. Relative folds of expressions were evaluated with the 2-ΔΔCT method (Livak and Schmittgen 2001).
Western blot analysis
Total protein was isolated from liver and kidney with radioimmunoprecipitation assay buffer (RIPA buffer) and the protein concentration was measured by Bradford assay. Total proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) on a 12% gel and transferred to a PDVF membrane. The membrane was incubated with primary antibodies, PARP (1:1000 dilution, Rabbit, ab227244, Abcam), VEGF (1:1000 dilution, Rabbit Polyclonal, ab46154, Abcam), and GAPDH (1:2000 dilution, Rabbit Polyclonal, ab9485, Abcam ) overnight at 4°C on a shaker. The membrane was incubated with the secondary antibodies (goat anti-rabbit IgG-HRP 1:5000 dilution, ab6721, Abcam) at room temperature (RT) for 1 h. The target protein bands were analyzed using the ChemiDoc™ MP Imaging System (Bio-Rad, CA, USA).
Histopathological processing
Liver and kidney tissues were first fixed in 10% neutral buffered formalin for 24 h and then were washed with tap water. Tissues were processed for dehydration with a series of graded ethanol (70, 80, 90, 96, 100%) and xylene series and embedded in paraffin. Five-micrometer thick were obtained with a microtome (Leica RM2255, Germany). Later, sections were deparaffinized, rehydrated, and stained with the hematoxylin-eosin solution for observation for pathological damage.
Immunohistochemistry
Immunohistochemical detection of c-fos was performed using the streptavidin-biotin peroxidase technique. Serial sections of 5-μm thickness were placed on poly-l-lysine-coated slides, deparaffinized, dehydrated, rinsed in tap water, and treated with 3 % H2O2 to quench the endogenous peroxidase activity for 10 min, then immersed in heated sodium citrate buffer (10 mM, pH 6.0) for 20 min, and then cooled for 20 min. The slides were incubated in a blocking solution to block nonspecific protein bindings for 10 min at room temperature. The slides were then incubated with the primary antibody (c-fos; Cat no: sc-166940, Santa Cruz Biotechnology) and diluted 1:100 for 1 h at room temperature. Thereafter, the sections were incubated with the appropriate biotin and then streptavidin peroxidase (UltraVision™ Large Volume Detection System, Thermo Scientific/Lab Vision, TP-125-HL) at room temperature, for 15 min each. The primary-secondary antibody complex was visualized using 3,3′diaminobenzidine (DAB) chromogen, and then tissues were counterstained with Mayer’s hematoxylin, dehydrated, cleared, and mounted with coverslips. The immunohistochemistry results were evaluated semiquantitatively as follows: none = −; mild = +; moderate = ++; intense = +++.
Statistical analysis
IBM SPSS 20 was used to perform statistical analyses. One-way analysis of variance (ANOVA) was used to detect statistically differences of Cas-3, Bax, Bcl-2, PARP-1, and VEGF expressions at mRNA level between control and treatment groups. RT-PCR results are expressed as mean ± SEM. Statistically differences were considered to be significant at p < 0.05, p < 0.01, and p < 0.001.
The immunohistochemistry data were analyzed using SPSS software and compared by one-way analysis of variance followed by Tukey’s test. Data are means ± standard errors of the mean (SEM). p < 0.05 was regarded as statistically significant.
Results
Rutin prevents deltamethrin-induced liver injury
Oral administration of deltamethrin caused a significant elevation (p<0.05) of serum ALT, AST, and ALP activities compared to the control group. Concurrent administration of rutin (25 and 50 mg/kg) significantly alleviated (p<0.05) serum AST, ALP, and ALT activities in deltamethrin-induced rats (Table 2).
Rutin protects deltamethrin-induced kidney injury
The effects of rutin treatment on the deltamethrin-induced kidney toxicity on serum urea, creatinine, and nephrin levels are shown in Table 3. Treatment with deltamethrin resulted in a highly significant increase in serum urea and creatinine levels (p<0.05) and a significant decrease in serum nephrin level (p<0.05) as compared to the control groups, while co-treatment of rutin caused a significant decline in the levels of serum urea and creatinine and a significant increase in the level of nephrin.
Effect of rutin and deltamethrin on liver and kidney oxidative stress markers
To research the roles of antioxidant enzymes in mediating the radical-scavenging activity of rutin, the lipid peroxidation and intracellular antioxidant enzyme activities were measured in the deltamethrin-induced hepatotoxicity and nephrotoxicity. It was observed that the MDA levels in liver and kidney tissues of rats treated with deltamethrin alone caused a significant increase (p<0.05) compared to the control group. This increase was attenuated by the treatment with rutin. Additionally, significantly reduced activities of the enzymatic (SOD, CAT, and GPx) and nonenzymatic (GSH) antioxidant molecules were seen in the liver and kidney tissues of deltamethrin-treated rats compared with the control group. Co-administration of rutin (25 and 50 mg/kg) with deltamethrin significantly increased the abovementioned parameters in a dose-dependent manner compared to the deltamethrin group alone. The results are listed in Tables 2 and 3.
Effect of rutin and deltamethrin on liver and kidney inflammatory markers
To explore the effects of rutin treatment on the inflammatory response with deltamethrin administration, we determined liver and kidney NF-κB, TNF-α, IL-1β, iNOS, and COX-2 levels. Deltamethrin-induced groups showed a significant increase (p<0.05) in liver and kidney NF-κB, TNF-α, and IL-1β levels when compared with the control groups as depicted in Fig. 1A–C. However, concurrent administration of rutin led to a significant decrease (p<0.05) in these levels in deltamethrin-induced groups.
A Effect of RUT on DLM-induced liver and kidney NF-κB levels. B Effect of RUT on DLM-induced liver and kidney TNF-α levels. C Effect of RUT on DLM-induced liver and kidney IL-1β levels. Values are expressed as mean ± SEM. Different letters (a–d) on the columns show a statistical difference (p < 0.05)
Activities of iNOS and COX-2 showed a significant (p<0.05) increase in the liver and kidney tissues of deltamethrin-induced rats when compared to the control group (Fig. 2A and 2B). The combination of deltamethrin and rutin downregulated (p<0.05) the high levels of these enzymes compared to the deltamethrin-induced group.
A Effect of RUT on DLM-induced liver and kidney iNOS activities. B Effect of RUT on DLM-induced liver and kidney COX-2 activities. C Effect of RUT on DLM-induced liver and kidney p38α MAPK activities. D Effect of RUT on DLM-induced liver and kidney beclin-1 levels. Values are expressed as mean ± SEM. Different letters (a–d) on the columns show a statistical difference (p < 0.05)
Effect of rutin and deltamethrin on liver and kidney p38α MAPK and beclin-1 levels
The levels of p38α MAPK and beclin-1 in liver and kidney tissues were studied by rat ELISA kits. The results showed that the levels of p38α MAPK and beclin-1 in these tissues increased significantly after deltamethrin treatment compared to the control group while both doses of rutin significantly (p<0.05) decreased p38α MAPK and beclin-1 levels compared to deltamethrin group (Fig. 2C and 2D).
Rutin decreased the apoptosis activity induced by deltamethrin
While Cas-3 and Bax mRNA transcript level was upregulated in the deltamethrin group compared to control (p < 0.01), these genes expression was downregulated in the deltamethrin+rutin-25 and deltamethrin+rutin-50 groups compared to the deltamethrin group (p < 0.05). Furthermore, Bcl-2 mRNA transcript level was downregulated in the deltamethrin group compared to control (p < 0.01). However, Bcl-2 gene expression was upregulated in the deltamethrin+rutin-25 and deltamethrin+rutin-50 groups compared to the deltamethrin group (p < 0.05) (Fig. 3A-3C). These results indicated that while deltamethrin treatment induced the apoptosis in the liver and kidney tissues, rutin decreased the apoptosis.
The mRNA transcript level of Cas-3, Bax, and Bcl-2 in the liver and kidney tissues of rat. Values represent the mean ± SEM of 3 indipendent samples; error bars indicate standard deviation. Statistical significance (*p ˂0.05, **p<0.01, ***p<0.001) was analyzed using one-way ANOVA. A Represent the relative mRNA expression levels of Cas-3. B Represent the relative mRNA expression levels of Bax. C Represent the relative mRNA expression levels of Bcl-2
Rutin reduced the expressions of PARP-1 and VEGF induced by deltamethrin
Poly[ADP-ribose]polymerase 1 and VEGF gene expression levels were only upregulated in the deltamethrin group compared to the control group (p < 0.01). In the rutin group, the mRNA transcript levels of PARP-1 and VEGF were similar as the control group for both liver and kidney tissue (p > 0.05). PARP-1 and VEGF gene expression levels were downregulated in the deltamethrin+rutin-25 and deltamethrin+rutin-50 groups compared to the deltamethrin group (p < 0.05) (Fig. 4A-4B). In addition, the Western blot results of PARP-1 and VEGF confirmed the RT-PCR results. While the protein levels of PARP-1 and VEGF were increased in the deltamethrin group, these protein levels were decreased in the deltamethrin+rutin-25 and deltamethrin+rutin-50 (p < 0.05) (Fig. 4C-4D). These results indicated that while deltamethrin treatment activated the PARP-1 and VEGF in the liver and kidney tissues of rats, rutin deactivated these gene expressions.
RT-PCR and Western blot results of PARP-1 and VEGF in liver and kidney tissues. A Represent the relative mRNA expression levels of PARP-1. B Represent the relative mRNA expression levels of VEGF. C Equal quantities of total PARP-1, VEGF, and GAPDH protein from different samples (from left to right: control, RUT, DLM, DLM+RUT25, and DLM+RUT50). Total protein was extracted from the liver tissues of rat. D Equal quantities of total PARP-1, VEGF, and GAPDH protein from different samples (from left to right: control, RUT, DLM, DLM+RUT25, and DLM+RUT50). Total protein was extracted from the kidney tissues of rat
Effect of rutin treatment on the histopathology of deltamethrin-treated rats
Normal hepatocytes with normal sinusoids and the central vein were seen both in control rats (Fig. 5A) and only rutin-treated rats (Fig. 5B). Deltamethrin caused extremely severe hemorrhage, necrosis, and dissociation in hepatocytes around the central vein in rats as shown in Fig. 5C. In the deltamethrin+rutin-25-treated group, it showed severe congestion, moderate necrosis (Fig. 5D). Rutin-50 treatment significantly alleviated deltamethrin-induced increase in congestion and necrosis (Fig. 5E).
Microscopic view of the liver section obtained from control-, RUT-, DLM-, DLM+RUT25-, and DLM+RUT50-treated rats. A, B Normal liver histology was shown in control and RUT-treated groups. C Severe hemorrhage (arrow), nuclear pyknosis (arrowhead), and dissociation in hepatocytes (asterisk) were shown in DLM-treated group. D In the DLM+RUT25 group, liver tissue seemingly severe congestion (arrow) and moderate necrosis (arrowhead) rather than to severe hemorrhage. E Mild congestion (arrow) and nuclear pyknosis (arrowhead) were shown in DLM+RUT50 group. (H&E stain, 400×)
There were not seen any pathological findings within the corticomedullary junction in the control (Fig. 6A) and only rutin-treated groups (Fig. 6B). Histopathological findings showed severe necrosis and hemorrhage in the corticomedullary junction in the deltamethrin-treated groups (Fig. 6C). In contrast, treatment with rutin-25 demonstrated a mild reduction of the hemorrhage in the corticomedullary junction (Fig. 6D). Treatment of deltamethrin+rutin-50 also provided a more significant reduction in hemorrhage (Fig. 6E).
Microscopic view of the kidney section obtained from control-, RUT-, DLM-, DLM+RUT25-, and DLM+RUT50-treated rats. A, B Normal kidney histology was shown in corticomedullary juntion of control and RUT-treated groups. C Severe hemorrhage (arrow) was shown in DLM-treated group. D A mild reduction of the hemorrhage (arrow) was shown in DLM+RUT25 group. E Mild hemorrhage (arrow) was shown in DLM+RUT50 group. (H&E stain, 400×)
Effect of rutin on the expression of c-fos in deltamethrin-treated rats
Immunohistochemical staining of c-fos in rat liver showed no c-fos expression in the control (Fig. 7A) and rutin-treated groups (Fig. 7B) with a significant increase in c-fos expression in the cytoplasm of hepatocytes in the deltamethrin-treated group (Fig. 7C) and a significant decrease in c-fos expression in the groups treated with deltamethrin+rutin-50 group (Fig. 7E), while the deltamethrin+rutin-25 group showed no a significant decrease in c-fos expression (Fig. 7D; Table 4, p<0.05). Immunohistochemical staining of c-fos in rats’ kidney showed no expression in the cytoplasm of tubular epithelium in the corticomedullary junction of the control (Fig. 8A) and rutin-treated groups (Fig. 8B). A significant increase in c-fos expression was recorded in the deltamethrin-treated group (Fig. 8C) as compared to the control group and rutin-treated group. A significant decrease was recorded in c-fos expression in the deltamethrin+rutin-25- (Fig. 8D) and deltamethrin+rutin-50-treated group (Fig. 8E) and showed a significant change as compared to the control group (Table 4, p<0.05).
Effect of RUT on immunohistochemical stain of c-fos in livers of DLM-treated rats. A Control group; B RUT-treated group; C DLM-treated group; D DLM+RUT25 group; and E DLM+RUT50 group. Immunostaining was performed using a specific antibody against c-fos. The positive staining of c-fos is presented as brown hepatocytes (arrows) (vc, central vein). (IHC stain, 400×)
Discussion
In this study, hepatotoxicity and nephrotoxicity caused by deltamethrin in rats were investigated. Experimental results suggest that treatment with rutin could decrease the deltamethrin-induced liver and kidney damages via modulating oxidative stress, inflammation, and apoptosis in experimental animals.
Free radicals and ROS may play crucial roles in the induction of pyrethroid-induced damage to proteins, lipids, and DNA in both invertebrates and vertebrates. Among the pyrethroid insecticides, deltamethrin has been reported to have some toxic effects (Abdou and Abdel-Daim 2014; El Golli-Bennour et al. 2019; Maalej et al. 2017). Liver microsomal enzymes which mainly metabolize deltamethrin and toxic metabolites of deltamethrin have been documented to accumulate in the liver (Abdel-Daim et al. 2016; Abdelkhalek et al. 2015; El Golli-Bennour et al. 2019). Toxic metabolites of deltamethrin mainly accumulates in liver as it is the main site of metabolism of xenobiotics and the kidney is the main excretory organ (Abdelkhalek et al. 2015; Sayeed et al. 2003). A group of scientists have shown that deltamethrin can easily penetrate the membrane cell due to its lipophilic nature and cause lipid peroxidation (Abdel-Daim et al. 2013). Based on the previous studies, it has been emphasized that one of the main causes of hepatotoxicity and nephrotoxicity caused by deltamethrin is oxidative stress (Abdel-Daim and El-Ghoneimy 2015; Rjeibi et al. 2016). Present study demonstrated that deltamethrin administration was associated with a significant increase in MDA level (as a marker of lipid peroxidation) as well as by a decrease in the activity of SOD, CAT, GPx, and GSH in both tissues (Tables 2 and 3). Rutin acts as a scavenger of ROS by donating hydrogen atoms to superoxide anions, hydroxyl radicals, and peroxyl radicals (Çelik et al. 2020). Therefore, rutin is thought to reduce the toxic effects of deltamethrin by scavenging ROS. In the present study, rutin treatment (25 and 50 mg/kg) reduced deltamethrin-induced liver and kidney damages by increasing antioxidant enzyme activities that had been reduced by deltamethrin. It has been determined that a dose of 50 mg/kg rutin used in combination with deltamethrin is more effective. In a similar study, it was found that rutin ameliorated reduced antioxidant enzyme activities in carbon tetrachloride-induced hepatotoxicity and nephrotoxicity in rats (Elsawy et al. 2019).
Liver biomarker enzymes such as ALP, ALT, and AST are used as markers of liver damage, while urea, creatinine, and nephrine are considered markers of kidney function, and in some recent studies, a notable elevation of levels of these biomarkers was observed after deltamethrin intoxication (Abdel-Daim et al. 2014; Abdel-Daim and El-Ghoneimy 2015; Maalej et al. 2017). The histopathologic observation of liver tissue in deltamethrin-treated rats showed severe hemorrhage, necrosis, and dissociation in hepatocytes around the central vein. Also, severe necrosis and hemorrhage in the corticomedullary junction have been observed in the deltamethrin-treated rat kidney tissues. In the present study, co-treatment with rutin significantly ameliorated deltamethrin-induced liver and kidney injuries and decreased elevations of abovementioned serum biomarker levels. These results revealed the antioxidant effect of rutin, which plays an important role in reducing toxicity and maintaining liver and kidney membrane integrity in deltamethrin-treated rats.
Reactive oxygen species can activate several transcription factors, which causes to the differential expression of some genes participated in inflammatory pathways (Hussain et al. 2016). Under different pathological conditions, the classical NF-κB inflammatory signaling pathway is activated by ROS. Following that, many pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, COX-2, and iNOS are released and this further exacerbates the inflammatory injury to the liver and kidney (Kandemir et al. 2019; Li et al. 2021; Temel et al. 2020). Arora et al. (2016) showed that DLM exposure increased the expression of inflammatory markers such as NFκB, TNFα, COX-2, and iNOS in primary hepatocytes of rats. However, the mechanism by which DLM induces inflammation remains unclear. In our study, we showed that rutin plays an important role in alleviating ROS-generated and NFκB-induced inflammation through reduction of TNF-α, IL-1β, p38α-MAPK, COX-2, and iNOS in the liver and kidney damages caused by deltamethrin. In our previous studies, we reported that different doses of rutin administered against mercury chloride toxicity decreased liver and kidney inflammation by reducing the levels of inflammation-related parameters (Caglayan et al. 2019a; Caglayan et al. 2019b).
Apoptosis is a normal cellular death process triggered by some factors that include toxins (Ahmadvand et al. 2016). Previously, in vivo and in vitro studies demonstrated that exposure to deltamethrin significantly affected cell survival and induced apoptosis in hepatocytes (Das et al. 2007), kidneys (Maalej et al. 2017), splenocytes (Kumar and Sharma 2015), neuronal cells (Wu et al. 2000), and PC12 cells (Park et al. 2017). Recent evidence has shown that deltamethrin exposure may induce caspase-independent/caspase-dependent death in a variety of cells/tissues (Arora et al. 2016; Kumar et al. 2016; Kumar and Sharma 2015; Park et al. 2017). It has also been reported that deltamethrin can induce cell damage through activation of multiple pathways, including but not restricted to caspase activation, ER stress signaling, eNOS/JNK/AR pathways, calpain-mediated cell death, altered intracellular calcium level, or autophagy modulation. (Hossain and Richardson 2011; Kumar et al. 2016; Magby and Richardson 2015; Park et al. 2017; Yu et al. 2014). Maalej et al. (2017) have manifested that deltamethrin induced apoptosis by increasing expression of p53 as well as decreasing bcl-2 in rat liver and kidney tissues. In the current study, we observed increased mRNA expression of apoptotic markers Bax and caspase-3, while Bcl-2 expression was decreased in deltamethrin-induced rat liver and kidney tissues. Conversely, co-treatment of rutin was significantly effective in reversing apoptosis in these tissues. In particular, it was determined that 50 mg/kg rutin dose was more effective in reducing apoptosis. Currently, the precise mechanisms by which rutin modulates genes associated with apoptosis are not clear. However, we believe that the anti-apoptotic effect of rutin is related to the upregulation of the Bcl-2/Bax ratio, because many antioxidants, including rutin, affect genes related to apoptosis in cells under oxidative stress, or activation of extracellular signal-related protein kinases (ERK1/2) or phosphoinositide-3-kinase (PI3K) may be responsible for the change of expression of genes related to the Bcl-2 family. Indeed, activation of PI3K and Akt has been shown to promote cell survival and suppress apoptosis (Jeong et al. 2009).
Vascular endothelial growth factor is an endothelial cell mitogen that is mainly synthesized due to tissue ischemia, hypoxia, and endothelial cell damage (Atakan et al. 2008). It plays a role in wound healing, vascular permeabilization, inflammation, embryogenesis, and tissue remodeling (Shihab et al. 2003; Yang et al. 2018). It has been documented that VEGF plays an important role in angiogenesis, nephrogenesis, and hepatic regeneration (Ferrara 1999; Papastefanou et al. 2007). PARP-1 is a nuclear protein involved in the routine repair of DNA damage by adding poly(ADP-ribose) polymers in response to various cellular stresses (Chaitanya et al. 2010). During oxidative stress, PARP-1 acts as a DNA break resulting in transient ribosylation using endogenous NAD+ as the ribose monomer substrate donor (Coyle et al. 2015). In addition, increased activation of PARP-1 can lead to cell death and organ damage as a result of depletion of cellular reducing equivalents (e.g., NADH) and cellular energy crisis (Hegedűs and Virág 2014). It has been reported that DNA damage associated with oxidative stress can induce PARP-1 activation (Hegedűs and Virág 2014; Virag 2005). RT-PCR and immunoblotting results in this study confirmed that deltamethrin toxicity caused an increase in PARP-1 and VEGF expression in liver and kidney tissues, while rutin treatment significantly decreased the expression of these parameters compared to only deltamethrin group.
C-fos is an important member of activator-protein 1 (AP-1) participated in cellular processes some of which include cell proliferation, differentiation, and apoptotic cell death (Kadry et al. 2018; Stanisavljević et al. 2019). C-fos also was shown to function as a suppressor protein in inflammatory responses. It directly interacts directly p65 subunit of NF-κB, consequently inhibiting the pathway downstream of NF-κB and triggering of pro-inflammatory cytokines such as TNF-α (Ray et al. 2006). In the present study, we found a remarkable increase in the expression of c-fos in the liver and kidney tissues of rats in the deltamethrin-induced group, and co-treatment with rutin reduced its overexpression.
There is no clinical antidote for deltamethrin poisoning and symptomatic treatment is the only option. Medicinal plants have traditionally been used for centuries to strengthen the immune system and prevent disease (Kumar et al. 2015). In this study, we report the protective role of rutin against deltamethrin-induced hepatorenal toxicity. Therefore, the role of plants in attenuating the different organ toxicities induced by deltamethrin should be investigated in the future.
Conclusions
The present study confirmed that the exposure to deltamethrin was potentially hepatorenal toxic and the obtained data showed that oxidative stress, inflammation, and apoptosis were involved in hepatotoxicity and nephrotoxicity in male rats intoxicated with deltamethrin for 30 days. However, treatment with rutin significantly ameliorated all biochemical, molecular, and histological changes, induced by deltamethrin. All data obtained from the study reveal the protective effects of rutin supplementation against deltamethrin-induced liver and kidney damage.
Abbreviations
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- Bax:
-
Bcl-2-associated x protein
- Bcl-2:
-
B-cell lymphoma-2
- Caspase-3:
-
Cysteine aspartate specific protease-3
- CAT:
-
Catalase
- COX-2:
-
Cyclooxygenase-2
- ELISA:
-
Enzyme-linked immunosorbent assay
- GPx:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- iNOS:
-
Inducible nitric oxide synthase
- MDA:
-
Malondialdehyde
- NF-κB:
-
Nuclear factor kappa B
- PARP-1:
-
Poly[ADP-ribose]polymerase 1
- p38α MAPK:
-
p38α mitogen-activated protein kinase
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor-α
- VEGF:
-
Vascular endothelial growth factor
References
Abdallah FB, Slima AB, Dammak I, Keskes-Ammar L, Mallek Z (2010) Comparative effects of dimethoate and deltamethrin on reproductive system in male mice. Andrologia 42:182–186
Abdel-Daim MM, El-Ghoneimy A (2015) Synergistic protective effects of ceftriaxone and ascorbic acid against subacute deltamethrin-induced nephrotoxicity in rats. Ren Fail 37:297–304
Abdel-Daim MM, Abuzead SM, Halawa SM (2013) Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One 8:e72991
Abdel-Daim MM, Abd Eldaim MA, Mahmoud MM (2014) Trigonella foenum-graecum protection against deltamethrin-induced toxic effects on haematological, biochemical, and oxidative stress parameters in rats. Can J Physiol Pharmacol 92:679–685
Abdel-Daim M, El-Bialy BE, Rahman HGA, Radi AM, Hefny HA, Hassan AM (2016) Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: Biochemical and histopathological studies. Biomed Pharmacother 77:79–85
Abdelkhalek NK, Ghazy EW, Abdel-Daim MM (2015) Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: impact on lipid peroxidation and oxidative stress. Environ Sci Pollut Res 22:3023–3031
Abdou RH, Abdel-Daim MM (2014) Alpha-lipoic acid improves acute deltamethrin-induced toxicity in rats. Can J Physiol Pharmacol 92:773–779
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Ahmadvand H, Ghabaee DNZ, Malekshah AK, Navazesh A (2016) Virgin olive oil ameliorates deltamethrin-induced nephrotoxicity in mice: a biochemical and immunohistochemical assessment. Toxicol Rep 3:584–590
Ahmed MM, Ibrahim Laila IM (2018) Rutin ameliorates acrylamide-induced hepatotoxicity and biochemical disturbance in male albino rats. SJO6U 4:8–13
Ansar S, Hamed S, AlGhosoon H, AlSaedan R, Iqbal M (2016) The protective effect of rutin against renal toxicity induced by lead acetate. Toxin Rev 35:58–62
Arora D, Siddiqui MH, Sharma PK, Shukla Y (2016) Deltamethrin induced RIPK3-mediated caspase-independent non-apoptotic cell death in rat primary hepatocytes. Biochem Biophys Res Commun 479:217–223
Atakan A, Arikan H, Macunluoglu B, Tuglular S, Ulfer G, Cakalagaoglu F, Ozener C, Akoglu E (2008) Renal protective effects of leukotrienereceptor blockers in an experimental model of cyclosporine nephrotoxicity. Transplant Proc 40:279–284
Barlow S, Sullivan F, Lines J (2001) Risk assessment of the use of deltamethrin on bednets for the prevention of malaria. Food Chem Toxicol 39:407–422
Caglayan C, Kandemir FM, Darendelioğlu E, Yıldırım S, Kucukler S, Dortbudak MB (2019a) Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J Trace Elem Med Biol 56:60–68
Caglayan C, Kandemir FM, Yildirim S, Kucukler S, Eser G (2019b) Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J Trace Elem Med Biol 54:69–78
Caglayan C, Taslimi P, Türk C, Kandemir FM, Demir Y, Gulcin İ (2019c) Purification and characterization of the carbonic anhydrase enzyme from horse mackerel (Trachurus trachurus) muscle and the impact of some metal ions and pesticides on enzyme activity. Comp Biochem Physiol Part - C: Toxicol Pharmacol 226:108605
Caglayan C, Taslimi P, Türk C, Gulcin İ, Kandemir FM, Demir Y, Beydemir Ş (2020) Inhibition effects of some pesticides and heavy metals on carbonic anhydrase enzyme activity purified from horse mackerel (Trachurus trachurus) gill tissues. Environ Sci Pollut Res Int 27(10):10607–10616
Çelik H, Kandemir FM, Caglayan C, Özdemir S, Çomaklı S, Kucukler S, Yardım A (2020) Neuroprotective effect of rutin against colistin-induced oxidative stress, inflammation and apoptosis in rat brain associated with the CREB/BDNF expressions. Mol Biol Rep 47:2023–2034
Chaitanya GV, Alexander JS, Babu PP (2010) PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 8:31
Coyle JP, Mayo-Perez A, Bourgeois M, Johnson G, Morris S, Harbison R (2015) The assessment of an in-vitro model for evaluating the role of PARP in ethanol-mediated hepatotoxicity. Int J Crit Illn Inj Sci 5:9
Das PC, Streit TM, Cao Y, Rose RL, Cherrington N, Ross MK, Wallace AD, Hodgson E (2007) Pyrethroids: cytotoxicity and induction of CYP isoforms human hepatocytes. Drug Met Drug Interact 23:211
Ding Y, White CA, Muralidhara S, Bruckner JV, Bartlett MG (2004) Determination of deltamethrin and its metabolite 3-phenoxybenzoic acid in male rat plasma by high-performance liquid chromatography. J Chromatogr B 810:221–227
El Golli-Bennour E, Timoumi R, Annaibi E, Mokni M, Omezzine A, Bacha H, Abid-Essefi S (2019) Protective effects of kefir against deltamethrin-induced hepatotoxicity in rats. Environ Sci Pollut Res 26:18856–18865
Elsawy H, Badr GM, Sedky A, Abdallah BM, Alzahrani AM, Abdel-Moneim AM (2019) Rutin ameliorates carbon tetrachloride (CCl4)-induced hepatorenal toxicity and hypogonadism in male rats. PeerJ 7:e7011
Ferrara N (1999) Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 56:794–814
Gülçin İ (2012) Antioxidant activity of food constituents: an overview. Arch Toxicol 86:345–391
Gulcin İ (2020) Antioxidants and antioxidant methods: an updated overview. Arch Toxicol 94:651–715
Hafez MM, Al-Harbi NO, Al-Hoshani AR, Al-Hosaini KA, Al Shrari SD, Al Rejaie SS, Sayed-Ahmed MM, Al-Shabanah OA (2015) Hepato-protective effect of rutin via IL-6/STAT3 pathway in CCl 4-induced hepatotoxicity in rats. Biol Res 48:30
Hegedűs C, Virág L (2014) Inputs and outputs of poly (ADP-ribosyl) ation: relevance to oxidative stress. Redox Biol 2:978–982
Hossain MM, Richardson JR (2011) Mechanism of pyrethroid pesticide–induced apoptosis: role of calpain and the ER stress pathway. Toxicol Sci 122:512–525
Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N (2016) Oxidative stress and inflammation: what polyphenols can do for us? Oxidative Med Cell Longev 2016:1–9
Jeong JJ, Ha YM, Jin YC, Lee EJ, Kim JS, Kim HJ, Seo HG, Lee JH, Kang SS, Kim YS, Chang KC (2009) Rutin from Lonicera japonica inhibits myocardial ischemia/reperfusion-induced apoptosis in vivo and protects H9c2 cells against hydrogen peroxide-mediated injury via ERK1/2 and PI3K/Akt signals in vitro. Food Chem Toxicol 47:1569–1576
Kadry MO, Abdel-Megeed RM, El-Meliegy E, Abdel-Hamid A-HZ (2018) Crosstalk between GSK-3, c-Fos, NFκB and TNF-α signaling pathways play an ambitious role in Chitosan Nanoparticles Cancer Therapy. Toxicol Rep 5:723–727
Kandemir FM, Yildirim S, Caglayan C, Kucukler S, Eser G (2019) Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats. Environ Sci Pollut Res 26:22562–22574
Kandemir FM, Caglayan C, Aksu EH, Yildirim S, Kucukler S, Gur C, Eser G (2020a) Protective effect of rutin on mercuric chloride-induced reproductive damage in male rats. Andrologia 52:e13524
Kandemir FM, Yıldırım S, Kucukler S, Caglayan C, Darendelioğlu E, Dortbudak MB (2020b) Protective effects of morin against acrylamide-induced hepatotoxicity and nephrotoxicity: A multi-biomarker approach. Food Chem Toxicol 138:111190
Khalatbary AR, Ghaffari E, Mohammadnegad B (2015) Protective role of oleuropein against acute deltamethrin-induced neurotoxicity in rat brain. Iran Biomed J 19:247
Khan RA, Khan MR, Sahreen S (2012) Protective effects of rutin against potassium bromate induced nephrotoxicity in rats. BMC Complement Altern Med 12:204
Kumar A, Sharma N (2015) Comparative efficacy of piperine and curcumin in deltamethrin induced splenic apoptosis and altered immune functions. Pestic Biochem Physiol 119:16–27
Kumar A, Bhaskar A, Chandra S, Sasmal D, Mukhopadhyay KM, Sharma N (2015) Mechanism of deltamethrin induced immunotoxicity: Current and future perspectives. Receptor Clin Invest 2:e578
Kumar A, Sasmal D, Bhaskar A, Mukhopadhyay K, Thakur A, Sharma N (2016) Deltamethrin-induced oxidative stress and mitochondrial caspase-dependent signaling pathways in murine splenocytes. EnTox 31:808–819
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. BBRC 71:952–958
Li H, Cheng F, Wei Y, Lydy MJ, You J (2017) Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: an overview. J Hazard Mater 324:258–271
Li S, Zheng X, Zhang X, Yu H, Han B, Lv Y, Liu Y, Wang X, Zhang Z (2021) Exploring the liver fibrosis induced by deltamethrin exposure in quails and elucidating the protective mechanism of resveratrol. Ecotoxicol Environ Saf 207:111501
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maalej A, Mahmoudi A, Bouallagui Z, Fki I, Marrekchi R, Sayadi S (2017) Olive phenolic compounds attenuate deltamethrin-induced liver and kidney toxicity through regulating oxidative stress, inflammation and apoptosis. Food Chem Toxicol 106:455–465
Magby JP, Richardson JR (2015) Role of calcium and calpain in the downregulation of voltage-gated sodium channel expression by the pyrethroid pesticide deltamethrin. J Biochem Mol Toxicol 29:129–134
Manzoni AG, Passos DF, da Silva JL, Bernardes VM, Bremm JM, Jantsch MH, de Oliveira JS, Mann TR, de Andrade CM, Leal DB (2019) Rutin and curcumin reduce inflammation, triglyceride levels and ADA activity in serum and immune cells in a model of hyperlipidemia. Blood Cells Mol Dis 76:13–21
Mirfazaelian A, Kim K-B, Anand SS, Kim HJ, Tornero-Velez R, Bruckner JV, Fisher JW (2006) Development of a physiologically based pharmacokinetic model for deltamethrin in the adult male Sprague-Dawley rat. Toxicol Sci 93:432–442
Morgan MK, MacMillan DK, Zehr D, Sobus JR (2018) Pyrethroid insecticides and their environmental degradates in repeated duplicate-diet solid food samples of 50 adults. J Expo Sci Environ Epidemiol 28:40–45
Nafees S, Rashid S, Ali N, Hasan SK, Sultana S (2015) Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: role of NFκB/MAPK pathway. Chem Biol Interact 231:98–107
Özdemir S, Çomaklı S (2018) Investigation of the interaction between bta-miR-222 and the estrogen receptor alpha gene in the bovine ovarium. Reprod Biol 18:259–266
Papastefanou VP, Bozas E, Mykoniatis MG, Grypioti A, Garyfallidis S, Bartsocas CS, Nicolopoulou-Stamati P (2007) VEGF isoforms and receptors expression throughout acute acetaminophen-induced liver injury and regeneration. Arch Toxicol 81:729–741
Park YS, Park JH, Ko J, Shin IC, Koh HC (2017) m TOR inhibition by rapamycin protects against deltamethrin-induced apoptosis in PC 12 Cells. EnTox 32:109–121
Placer ZA, Cushman LL, Johnson BC (1966) Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. AnBio 16:359–364
Ray N, Kuwahara M, Takada Y, Maruyama K, Kawaguchi T, Tsubone H, Ishikawa H, Matsuo K (2006) c-Fos suppresses systemic inflammatory response to endotoxin. Int Immunol 18:671–677
Rjeibi I, Saad AB, Hfaiedh N (2016) Oxidative damage and hepatotoxicity associated with deltamethrin in rats: the protective effects of Amaranthus spinosus seed extract. Biomed Pharmacother 84:853–860
Ruzo LO, Unai T, Casida JE (1978) Decamethrin metabolism in rats. J Agric Food Chem 26:918–925
Sadeghnia HR, Yousefsani BS, Rashidfar M, Boroushaki MT, Asadpour E, Ghorbani A (2013) Protective effect of rutin on hexachlorobutadiene-induced nephrotoxicity. Ren Fail 35:1151–1155
Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Raisuddin S (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf 56:295–301
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. AnBio 25:192–205
Shihab FS, Bennett WM, Isaac J, Yi H, Andoh TF (2003) Nitric oxide modulates vascular endothelial growth factor and receptors in chronic cyclosporine nephrotoxicity. Kidney Int 63:522–533
Stanisavljević A, Perić I, Gass P, Inta D, Lang UE, Borgwardt S, Filipović D (2019) Brain sub/region-specific effects of olanzapine on c-Fos expression of chronically socially isolated rats. Neuroscience 396:46–65
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Temel Y, Kucukler S, Yıldırım S, Caglayan C, Kandemir FM (2020) Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn Schmiedeberg's Arch Pharmacol 393:325–337
Virag L (2005) Structure and function of poly (ADP-ribose) polymerase-1: role in oxidative stress-related pathologies. Curr Vasc Pharmacol 3:209–214
Wu A, Ren T, Hu Q, Liu Y (2000) Deltamethrin induces altered expression of P53, Bax and Bcl-2 in rat brain. Neurosci Lett 284:29–32
Yang J, Yan J, Liu B (2018) Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol 9:978
Yousef MI, Awad TI, Mohamed EH (2006) Deltamethrin-induced oxidative damage and biochemical alterations in rat and its attenuation by vitamin E. Toxicology 227:240–247
Yu H-m, Wu Y, Ju P, Wang B-h, Yang X-d, Wang H-m, Xu L-c (2014) eNOS-JNK1-AR signaling pathway mediates deltamethrin-induced germ cells apoptosis in testes of adult rats. Environ Toxicol Pharmacol 38:733–741
Author information
Authors and Affiliations
Contributions
SK: investigation, methodology. FMK: investigation, data curation, review and editing, supervision, SÖ: methodology, software, formal analysis. SÇ: methodology, formal analysis. CC: investigation, writing (review and editing), supervision.
Corresponding authors
Ethics declarations
Ethics approval
The animal care and use procedures applied in the study were conducted under the protocols approved by the Animal Experiments Local Ethics Committee of the Atatürk University (approval no: 2020-4/65).
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Küçükler, S., Kandemir, F.M., Özdemir, S. et al. Protective effects of rutin against deltamethrin-induced hepatotoxicity and nephrotoxicity in rats via regulation of oxidative stress, inflammation, and apoptosis. Environ Sci Pollut Res 28, 62975–62990 (2021). https://doi.org/10.1007/s11356-021-15190-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15190-w