Abstract
This study evaluated the protective effect of kaempferol, a natural flavonoid, against cadmium chloride (CdCl2)-induced liver damage and examined the possible anti-inflammatory and antioxidant mechanisms of protection. Adult male rats were divided into 4 groups (each of 8 rats) as control, kaempferol (50 mg/kg/day orally), CdCl2 (15 ppm/day), and CdCl2 (15 ppm/day) + kaempferol (50 mg/kg/day). All treatments were given for 30 days. With no effect on attenuating the reduced food intake, kaempferol significantly increased body weight and lowered serum levels of liver injury markers including bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyltransferase 1 (γ-GTT1) in the CdCl2-treated rats. It also restored normal liver architectures, prevented hepatocyte, loss, and swelling and reduced inflammatory cell infiltration. These effects were associated with a reduction in mitochondrial permeability transition pore, as well as in the expression of cytochrome-c and cleaved caspase-3, markers of mitochondrial damage, and intrinsic cell death. In both the control positive and CdCl2-treated rats, kaempferol significantly lowered the hepatic levels of reactive oxygen species, malondialdehyde (MDA), tumor necrosis factor-α (TNF-α), Interleukine-6 (IL-6), and the nuclear activity and localization of NF-κB p65. Besides, kaempferol significantly increased the hepatic total and nuclear levels of the nuclear factor erythroid 2–related factor 2 (Nrf2) and heme oxygenase-1, as well as levels of superoxide dismutase (SOD) and reduced glutathione (GSH) but reduced the cytoplasmic protein levels of keap1. In conclusion, the protective effect of kaempferol against CdCl2-induced hepatic damage is mediated by antioxidant and anti-inflammatory effects driven by upregulating Nrf2/HO-1 axis and suppressing the NF-κB p65 and keap1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is one of the most toxic non-essential heavy metals on the earth crust and is associated with renal, hepatic, reproductive, neural, and pulmonary toxicities in both humans and experimental animals (Rani et al. 2014; Go et al. 2015; Rana et al. 2020). Cd-induced hepatotoxicity is well established in both humans and animals and is a leading cause for the development of liver damage, steatosis, fibrosis, and failure (Ağır and Eraslan 2019; Arroyo et al. 2012; Hyder et al. 2013; Rana et al. 2020; Toppo et al. 2015). Currently, several studies have investigated the mechanism underlying Cd ions-induced liver damage where most of them pointed out the emerging role of oxidative stress (Abarikwu et al. 2017; Eybl et al. 2004; Rani et al. 2014; Sanjeev et al. 2019).
Indeed, overproduction of reactive oxygen species (ROS) and subsequent induction of oxidative stress, inflammation, and apoptosis are mechanisms that underlie the hepatotoxic effect of Cd. Within this view, Cd ions stimulate the production of ROS in the livers of mammals through several mechanisms including impairing metals hemostasis (i.e., Cu, Zn, Fe), binding and depleting the sulfhydryl groups containing proteins and thiols (i.e., glutathione/GSH and metallothionein/MTT), downregulating of antioxidant enzymes, and uncoupling the mitochondria oxidative phosphorylation (Arroyo et al. 2012; Rani et al. 2014; Rikans and Yamano 2000). Besides, Cd ions induce hepatic inflammation and increase the production of ROS by direct activation of Kuepfer cells (Yamano et al. 2000). Also, Cd ions activate the intrinsic (mitochondria-mediated) hepatocytes apoptosis by increasing intracellular Ca+2 levels, activating DNA damage, suppressing DNA repair, activating caspases, and promoting the opening of membrane permeability transition pore (MTP) (Arroyo et al. 2012; Liu et al. 2019; Rani et al. 2014).
Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is a natural flavonoid that is abundantly found in grapes, broccoli, tea, tomato, spinach, and beans (Devi et al. 2015). Studies have shown that dietary supplementation of kaempferol protects against several oxidants and inflammatory disorders including cancer and diabetes mellitus (DM), as well as other pulmonary, renal, hepatic, and cardiac disorders (Kim and Park 2020). The health beneficial effects of kaempferol were attributed to its potent antioxidant and anti-inflammatory properties mediated by scavenging ROS and suppressing NF-κB and apoptosis (Chen et al. 2013; Kim and Park 2020; Park et al. 2011; Tsai et al. 2018; Wang et al. 2020). Concerning its hepatoprotective effect, kaempferol also ameliorated liver injury in rodent’s models of carbon tetrachloride (CCL4), propacetamol; alcohol, D-galactosamine, and lipopolysaccharide (LPS)-induced liver damage by acting through several mechanisms including scavenging ROS, upregulating cellular antioxidants, inhibiting NF-κB and inflammatory cytokines production, and suppressing hepatic fibrosis, mitochondria damage, endoplasmic reticulum (ER) stress, and intrinsic cell apoptosis (Dong et al. 2017; Wang et al. 2019; Wang et al. 2015; Wu et al. 2020; Xu et al. 2019; Zang et al. 2017).
Whether kaempferol could prevent hepatic damage in Cd-treated rats is still not investigated. Of note, El-Kott et al. (El-kott et al. 2020a) have shown that kaempferol mitigates CdCl2-induced hippocampal damage and memory loss in CdCl2-treated rats, mainly by suppressing ROS generation, inhibiting NF-κB, and stimulating antioxidant levels. These data were very encouraging to us. Therefore, this study was designed to evaluate the protective effect of kaempferol against CdCl2-induced liver damage in rats and to investigate the possible underlying mechanisms.
Materials and methods
Animals
Healthy adult male rats (Wistar strain) (150 ± 10 g, 7 weeks old) were supplied from the animal house of the College of Science, King Khalid University, Abha. During the experimental period, all rats were housed under a controlled condition (temperature of 21°C, humidity of 50–60%, and 12 h/dark/light cycle) and had always free access to their water and diet. All procedures included in this study were approved by the animal ethical committee at the College of Science (Ethical number ECM#2020-1701) which followed the guidelines of the US National Institutes of Health (NIH publication No. 85-23, revised 1996).
Experimental design
All rats were randomly selected and divided into 4 groups (n=8 rats/each and 1 rat/cage) as (1) control rats: orally administered an equivalent volume of the vehicle (2% 2-methyl cellulose (Cat. No. M0512-100G, Sigma Aldrich, St. Louis, MO, USA) for 30 consecutive days; (2) kaempferol-treated rats: orally (intragastrically) administered kaempferol (Cat. No. 60010, Sigma Aldrich, St. Louis, MO, USA) dissolved in 2% 2-methyl cellulose to a final concentration of 50 mg/kg/day orally for 30 days (El-Kott et al. (2020a); (3) CdCl2-treated rats: administered CdCl2 (Cat. No. 202908 Sigma Aldrich, St. Louis, MO, USA) dissolved in drinking water (15 ppm) for 30 days (Bilgen et al. 2003; Koyu et al. 2006; Yazıhan et al. 2011); and (4) CdCl2 + kaempferol-treated rat: treated with CdCl2 in the drinking water and received a concomitant daily oral dose of kaempferol (50 mg/kg) for 30 days. Body weights and food intake were determined weekly. We have placed the rats in their cages individually to make sure to consume the whole water volume (20–25 ml) containing the daily dose of CdCl2.
Previously, we have shown that administration of either drinking water or 2% 2 methylcelluloses has no effect on body weight, food intake, and hippocampus structure, and function in rats (El-Kott et al. 2020a, 2020b). Also, in our preliminary experiments, we have found that administration of either drinking water or 2% 2 methylcelluloses to control rats did not affect the liver structure, liver enzymes, and hepatic markers of oxidative stress (data not shown). For this reason, we have omitted the control + drinking water from the experimental design for simplicity.
Serum and tissue collection
By the end of day 30, all rats of all groups were anesthetized by an intraperitoneal dose of sodium pentobarbital (55 mg/kg). Blood samples were directly collected from the heart into plain tubes and centrifuged (1200 × g/10 min/room temperature) to isolate the serum. All serum samples were stored at −20°C for further biochemical analysis. The animals were killed by cervical dislocation, and their livers were rapidly isolated on ice, weighed, and washed with ice-cold phosphate buffer saline (pH=7.4). The livers were then cut into smaller pieces. Parts of the livers were rapidly snap-frozen in liquid nitrogen and stored at −80°C until use. Other parts were rapidly fixed in 10% buffered formalin for 24 h and forwarded to the pathology laboratory at the College of Medicine, King Khalid University, and subjected to routine staining with hematoxylin and eosin.
Serum analysis
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyltransferase 1 (γ-GTT1) were assessed by rats’ ELISA kits (Cat. No. MBS2540581, Cat. No. MBS2540582, and Cat. No. MBS923419, respectively (MyBioSource CA, USA). All measurements were determined for n-8 rats/group and per the manufacturer’s instructions.
Preparation of tissue homogenates
Parts of the frozen liver samples (50 mg) were homogenized in 1 ml ice-cold 1X phosphate buffer saline containing a protease/phosphatase inhibitor cocktail (Cat. No. ab201119, Abcam, Cambridge, UK). The homogenates were centrifuged at 10,000 × g for 15 min at 4°C and the supernatants were isolated to new tubes. The tubes were kept at −20°C until further biochemical analysis. To prepare tissue homogenates for western blotting, frozen liver samples (40 mg) were humanized in 0.5 ml 1× radioimmunoprecipitation assay (RIPA) buffer (Cat. No. 91116, Abcam, Cambridge, UK) containing protease/phosphatase inhibitor, centrifuged (11,000 × g/4°C/10 min), and supernatants were isolated. Protein levels in all samples were determined using a commercially available kit (Cat. No. 704002, Caymen Chemicals, MI, USA).
Biochemical measurements in the liver homogenates
Hepatic levels of total free radicals (ROS&RNS) were measured using a fluorometric assay kit (Cat. No. E-BC-K138-F, Elabscience, USA). Levels of malondialdehyde (MDA), total glutathione (GSH), superoxide dismutase (SOD), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) were measured using special rats’ ELISA kits (Cat. No. MBS738685, Cat No. MBS265966, Cat. No: MBS036924, Cat. No. MBS2507393 Cat. No. MBS175908, respectively (MyBioSource, CA, USA). All procedures were done for n=8 samples/group and per each kit instruction.
Measurements of mitochondrial permeability transition pore (mtPTP)
The cytoplasmic/mitochondria fraction from frozen livers was prepared using a commercially available kit (Cat. No. Ab110168, Abcam, Cambridge, UK) as per the manufacturer’s instructions. To determine the mtPTP, we followed the procedures established by Adhihetty et al. (Adhihetty et al. 2005) and Kavazis et al. (Kavazis et al. 2017) who have shown that treating the mitochondria with CaCl2 or specific ROS-generating compound such as tert-butyl hydroperoxide (t-BuOOH) causes mitochondria swelling and parallel damage of the external membrane through the opening of the mtPTP. In the test, the increase in the decline of the absorbance (Vmax) with a concomitant decrease in the time required to reach Vmax indicates higher mtPTP values. Accordingly, freshly isolated mitochondria from each sample were suspended in 0.5 ml buffer containing 215 mM mannitol, 71 mM sucrose, 3 mM HEPES, and 5 mM succinate to a final concentration of 1 mg/ml. Then, each sample was incubated with CaCl2 (400 μM) and t-BuOOH (75 μM), and the decline in the absorbance was monitored over 10 min at an absorbance of 540 nm.
Determination of NF-κB activation in the nuclear extract
The cytoplasm/nuclear fractions of the frozen livers of all samples were prepared using a special kit (Cat. No. 78833). Protein levels in the nuclear extract were determined using the provided kit (Cat. No. 704002, Caymen Chemicals, MI, USA). The activity of NF-κB p65 was determined using a specific assay kit using 20 μg of the isolated nuclear proteins (Cat. No. 31102, Active Motif, Tokyo, Japan). A standard recombinant NF-κB p65 was used to generate the standard curve used to determine the nuclear levels of NF-κB p65. All procedures were done for n=8 sample/group as per the manufacturer’s instructions
Western blotting
Protein levels were measured using all supernatants of the liver RIPA-homogenates using the commercial kit. The proteins were prepared in the 2× Laemmli buffer at a final concentration of 3 μg/μl and then boiled for 5 min. Equal proteins from each sample (40 μg) were separated on different percentages of SDS polyacrylamide gel (100 v for 2h). Membranes were then transferred on nitrocellulose membranes (100 v for 2 h) and blocked 5% w/v non-fat dry milk. The membranes were then washed for 3 times with 1×TBST incubated with the primary antibody against cleaved caspase-3 (Asp175) (Cat. No. 9664, 17/19 kDa), cytochrome-c (Cat. No. 11940, 14 kDa, 1:1000), NF-κB p65 antibody (Cat. No. 3034, 65 kDa, 1:1000), Nrf2 (cat. No. 17212, 100 kDa, 1:1000), HO-1 (Cat. No. 70081, 28 kDa), β-actin (Cat. No. 4970, 45 kDa), keap 1 (Cat. No. 60 kDa) (cell signaling technology), and Lamin A (Cat. No. sc-293162, 69 kDa, 1:1000) (Santa Cruz Biotechnology). The incubation with the 1st and 2nd antibodies was done at room temperature for 2 h with continuous shaking. Washing between steps and preparation of the skimmed milk and antibodies were done in 1× tris-buffered saline tween 20 (TBST) buffer. Membranes were stripped up to 3 times in which the phosphorylated proteins were detected first. Bands were visualized using a price ECL substrate (Cat. No. 32109, ThrmoFisher Scientific). All bands were scanned and analyzed using the LI-COR C-DiGit scanner (USA). Normalization between the stripped gels was done using an internally known sample.
Histological evaluation
The protocol of the histological evaluation was done as previously shown by others (Fischer et al. 2008; Grosset et al. 2019). In brief, parts of the freshly collected liver samples were fixed in 10% buffered formalin for the next 24 h and then dehydrated with ascending alcohol (70–100%) and cleared in xylene. All tissues were then embedded in paraffin wax, cut at a thickness of 3 μM, and then stained with hematoxylin and eosin (H&E). The alterations in the liver architectures were done by a histologist who is unaware of the experimental groups. All photos were examined and photographed under a magnification of 200× using a light microscope (Nikon Eclipse, model ME600).
Statistical analysis
All data were analyzed using Graphpad Prism (V8, Australia). The differences in all measured parameters among all the study group parameters were analyzed using one-way ANOVA followed by Newman-Keuls Multiple Comparison Test. Data were presented as mean ± standard deviation (SD). Values were considered significantly different at P < 0.05.
Results
Kaempferol prevents CdCl2-induced liver damage
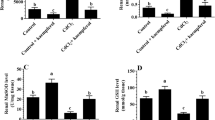
Final body weights and average daily food intake were significantly decreased in the CdCl2-treated rats as compared to control rats (P = 0.002 and P < 0.0001, respectively) (Figure 1A, B). However, no significant variations in body weights and food intake were observed when kaempferol or CdCl2+ kaempferol-treated rats were compared with the control- or CdCl2-treated rats, respectively (P >0.05) (Figure 1A, B). However, serum levels of ALT, AST, γ-GTT1, and bilirubin were not significantly different in kaempferol-treated rats (P > 0.05) but were significantly increased in the serum of CdCl2-treated rats, as compared to control rats (P < 0.0001, P =0.004, and P <0.0001, respectively) (Figure 1C–F). Besides, while normal liver architectures were observed in the control or kaempferol-treated rats (Figure 2A, B), livers from CdCl2-treated rats showed severe loss of hepatocytes, hepatocyte swelling, and damaged cell membranes with increased fatty cells, dilated central vein, and increased infiltration of inflammatory cells (Figures 2C and 3D). On the other hand, the livers obtained from CdCl2 + kaempferol-treated rats showed normal serum levels of all measured enzymes with a concomitant decrease in fatty cells and macrophage infiltration, as well as an obvious improvement in hepatocyte and liver structure (Figures 1A–D and 3E, F).
Final body weights (A), average daily food intake (B), and serum levels of hepatic markers (C–F) in all experimental groups of rats. Data are expressed as mean ± SD of 8 rats/group. Values are significantly different at P < 0.05.*,***Significantly different as compared to control rats at P< 0.05 and 0.001, respectively. #,###Significantly different as compared to kaempferol-treated rats at P < 0.05 and 0.001, respectively. $$$Significantly different as compared to CdCl2 (cd)-treated rats at P < 0.001
Photomicrographs of liver sections of some groups of rats after staining with hematoxylin and eosin (H&E). A,B Taken from control- and kaempferol-treated rats, respectively, and showed normal liver structure with normally sized central vein (CV) and hepatocytes (long arrow) and sinusoids (short arrow). C,D Taken from CdCl2-treated rats and showed dilated CV, severe loss of the hepatocyte parenchyma (short blue arrow), hepatocytes swelling (long black arrow), damage of hepatocytes membrane (short black arrow), dilated sinusoids (arrowhead), increased infiltration of inflammatory cells (thick long blue arrow). E,F Taken from CdCl2 + kaempferol-treated rats and showed improvement in hepatocytes structure and organization with a concomitant reduction in the diameter of the CV, amount of inflammatory cells, and hepatocytes loss. Most of the hepatocytes have intact membranes. However, some inflammatory cells (short blue arrow), hepatocytes damage (long black arrow), cell swelling (long blue arrow), damage in the CV (curved black arrow) were still observed
Levels of oxidative stress markers in the livers of all groups of rats (A–D). Data are expressed as mean ± SD of 8 rats/group. Values are significantly different at P < 0.05.*,***Significantly different as compared to control rats at P< 0.05 and 0.001, respectively. #,###Significantly different as compared to kaempferol-treated rats at P < 0.05 and 0.001, respectively. $$$Significantly different as compared to CdCl2 (cd)-treated rats at P < 0.001
Kaempferol suppresses oxidative stress and upregulates Nrf2 in the livers of both the control- and CdCl2-treated rats by suppressing keap1
Levels of ROS and MDA were significantly increased but the levels of SOD and GSH, as well as the total protein levels of HO-1 and Nrf2, were significantly decreased in the livers of CdCl2-treated rats as compared to control rats (P <0.0001 for all) (Figures 3A–D and 4A, B). Besides, nuclear protein levels of Nrf2 were significantly reduced (P < 0.0001) but the total cytoplasmic levels of keap1 were significantly increased (P < 0.0001) in the livers of CdCl2-treated rats as compared to control rats (Figure 4C, D). However, a significant reduction in the levels of ROS and MDA coincided with a significant increase in the levels of SOD and GSH, and protein levels of HO-1 and Nrf2 were seen in the livers of the kaempferol and CdCl2 + kaempferol-treated rats as compared to the control or CdCl2-treated rats (P <0.0001 and P = 0.0021 for HO-1 and P < 0.0001 and P = 0.0013 for Nrf2, respectively) (Figures 3A–D and 4A, B). Concomitantly, nuclear protein levels of Nrf2 were significantly increased but cytoplasmic levels of keap1 were significantly reduced in the livers of both kaempferol- and CdCl2 + kaempferol-treated rats as compared to their the control group or CdCl2-treated rats, respectively (P = 0.0012 and P <0.0001 for Nrf2, and P = 0.0214 and P < 0.0001 for keap1, respectively) for HO-1 and P < 0.0001 and P = 0.0013 for Nrf2, respectively (Figure 4C, D).
Total protein levels of heme oxygenase-1 (HO-1) (A) and nuclear factor erythroid 2-related factor 2 (Nrf2) (B), as well as the nuclear levels of Nrf-2 (C) and the cytoplasmic levels of keap1 (D) in the livers of all groups of rats. Data are expressed as mean ± SD of 8 rats/group. Values are significantly different at P < 0.05.**,***Significantly different as compared to control rats at P <0.01 and 0.001, respectively. ##,###Significantly different as compared to kaempferol-treated rats at P <0.01 and 0.001, respectively. $$$Significantly different as compared to CdCl2 (cd)-treated rats at P < 0.001
Kaempferol reduced mTPTP and inhibits apoptosis in the livers of CdCl2-treated rats
The value of Vmax and time to reach Vmax (markers of mTPTP), as well as total protein levels of cleaved caspase-3 were not significantly different between the control- and kaempferol-treated rats (P > 0.05) (Figure 5A–D). However, the value of Vmax was significantly increased (P < 0.0001), and the time needed to reach Vmax was significantly decreased (P = 0.0002) in the isolated mitochondria of CdCl2-treated rats, as compared to control rats, thus indicating an increase in mtPTP (Figure 5A, B). Besides, total protein levels of cleaved caspase-3 and cytoplasmic protein levels of cytochrome-c were significantly increased (P < 0.0001) in the livers of CdCl2-treated rats as compared to control rats (Figure 5C, D). CdCl2 + kaempferol-treated rats showed a significant reduction in the value of Vmax (P < 0.0001), as well as in the protein levels of cleaved caspase-3 (P < 0.0001) and cytochrome-c (P < 0.0001) with a concomitant increase in the time to reach Vmax (P < 0.0007) as compared to CdCl2-treated rats (Figure 5C, D).
Mitochondria membrane transition pores (mtMTP) function (A, B), protein levels of cleaved caspase-3 (B), and cytoplasmic protein cytochrome-c (C) in the livers of all groups of rats. Data are expressed as mean ± SD of 8 rats/group. Values are significantly different at P < 0.05.***Significantly different as compared to control rats at 0.001. ###Significantly different as compared to kaempferol-treated rats at P 0.001. $$$Significantly different as compared to CdCl2 (cd)-treated rats at P < 0.001. Vmax, the maximum decline in the absorbance of the isolated mitochondria after treatment with CaCl2 and tert-butyl hydroperoxide (t-BuOOH) (a reactive oxygen species generating molecule)
Kaempferol inhibits NF-κB p65 and inflammatory cytokines in the livers of both the control- and CdCl2-treated rats
Levels of TNF-α and IL-6, as well as the nuclear activity and protein levels of NF-κB p65, were significantly decreased (P = 0.0108, P = 0.027, P = 0.0189, and P = 0.0014, respectively) in the livers of kaempferol-treated rats as compared to control rats (Figure 6A–D). However, levels of TNF-α and IL-6, as well as the nuclear activity and protein levels of NF-κB p65 were significantly increased (P < 0.0001 for all) in the livers of CdCl2-treated rats as compared to control rats (Figure 6A–D). On the contrary, levels of all these markers were significantly reduced in the livers of CdCl2 + kaempferol-treated rats when compared to CdCl2-treated rats (P < 0.0001 for all) (Figure 6A–D).
Levels of inflammatory markers (A, B) and nuclear activity and levels of NF-κB p65 (C, D) in the livers of all groups of rats. Data are expressed as mean ± SD of 8 rats/group. Values are significantly different at P < 0.05.*,**,***Significantly different as compared to control rats at P < 0.05, 0.01, and 0.001, respectively. ##,###Significantly different as compared to kaempferol-treated rats at P < 0.01 and 0.001, respectively. $$$Significantly different as compared to CdCl2 (cd)-treated rats at P < 0.001
Discussion
The findings of this study confirm the previously reported data which have shown that administration of CdCl2 contributes significantly to liver damage in rodents (Ağır and Eraslan 2019; Arroyo et al. 2012; Hyder et al. 2013; Rana et al. 2020; Toppo et al. 2015). Besides, our data uniquely show that the concomitant administration of kaempferol is an excellent preventative strategy to alleviate CdCl2-induced hepatotoxicity. Accordingly, the administration of kaempferol along with CdCl2 preserved the rat’s liver structure and prevented hepatocyte damage, loss, and apoptosis. Mechanistically, our data suggest that such protective effect of kaempferol is attributed mainly to its ability to antioxidant and anti-inflammatory effects mediated by reducing the generation of ROS, Nrf2/HO-1-dependent increase in the levels of GSH and SOD, preventing mitochondria mtPTP, and suppressing the activation of NF-κB and the generation of some inflammatory cytokines. A graphical abstract demonstrating the basic suggested mechanism of protection of kaempferol is shown in Figure 7.
A graphical abstract showing the protective effect of kaempferol against CdCl2-induced hepatic damage. In the graph, CdCl2 stimulates hepatic oxidative stress and inflammation by suppressing Nrf2 and activating NF-κB in the liver of rats. The inhibition of Nrf2 leads to overproduction of reactive oxygen species (ROS) and oxidative stress by decreasing the expression of heme-oxygenase-1 (HO-1) and subsequent decrease in the levels of some antioxidant markers such as glutathione (GSH) and superoxide dismutase (SOD). On the other hand, CdCl2-induced activation of NF-κB stimulates hepatic inflammation by increasing the expression of interleukine-6(Il-6) and tumor necrosis factor (TNF-α). Kaempferol prevents these events by activating Nrf2 and inhibiting NF-κB
Cadmium ions accumulate at varying degrees in many tissues including the brain, liver, kidneys, and testes, leading to tissue damage and organ failure (Go et al. 2015; Rani et al. 2014). In vivo evidence suggests that Cd ions accumulate at the highest levels in the livers of rats at all measured intervals (4, 8, and 12 weeks after exposure) (Haouem and El Hani 2013), thus confirming that hepatotoxicity is the earliest damage seen after intoxication with Cd ions (Kawagoe et al. 2005; Pari and Murugavel 2005). Weight and appetite loss are two major symptoms associated with Cd toxicity in both humans and animals and are attributed to the Cd ions-induced systemic toxicity and the reduction in appetite due to abnormal neurotransmitters signaling in the hypothalamus (Amara et al. 2008; El-kott et al. 2020a; Hwang and Wang 2001; Nwokocha et al. 2012). This has been also confirmed in this study in CdCl2-treated rats where we have also found a significant reduction in rat’s final body weights of CdCl2-treated rats.
On the other hand, kaempferol prevented the loss of body weight without altering the rat’s food intake of the CdCl2-treated rats. These data support our previous data in the same animal model (El-kott et al. 2020a; El-Kott et al. 2020b). Based on these data, we concluded that the protective effect of kaempferol on rat’s body weights is not related to altering food intake but could be possibly due to its chelating properties or its protective effect on the liver and other peripheral organs. Unfortunately, we could not measure the levels of Cd ions in the livers and other tissues to confirm this.
Nevertheless, oxidative stress and inflammation are the best-known mechanisms mediating the hepatotoxic effect of Cd (Arroyo et al. 2012; Liu et al. 2019; Rani et al. 2014). Cadmium-induced liver damage is associated with an increase in the serum levels of ALT, AST, lactate dehydrogenase (LDH), alkaline phosphatase (ALP), γ-GTT, and bilirubin, all of which are considered conventional biomarkers of loss of cell integrity (Ağır and Eraslan 2019; Renugadevi and Prabu 2010). Also, Cd ions can induce severe pathological alterations, including hepatocytes damage, necrosis, apoptosis, and inflammatory cell infiltration (Ağır and Eraslan 2019; Rana et al. 2020). Besides, it is currently well-accepted that Cd ions are an independent cause for the development of NAFLD and non-alcoholic steatohepatitis (NASH) in both humans and animals by suppressing fatty acids (FAs) oxidation, stimulating FAs-synthesis, and induction of oxidative stress, inflammation, mitochondria damage, and endoplasmic reticulum (ER) stress (Go et al. 2015).
In the same line, these effects were also shown in this study where there was a significant increase in the serum levels of ALT, AST, γ-GTT, and bilirubin with increased hepatocyte damage, inflammatory cell infiltration, and accumulation of fat droplets in the CdCl2-treated rats. Therefore, we can strongly argue that CdCl2-intoxication is a leading cause of liver damage and idiopathic hepatic steatosis. However, the first evidence supporting the hepatic protective role of kaempferol in CdCl2-induced rats was observed by the obvious attenuation in the serum levels of all these markers and the improvement in the liver architectures of the treated rats. This is expected given the well-known previously reported hepatoprotective effects of kaempferol in several animal models of hepatic injury, including those induced by treatment with CCL4, paracetamol; alcohol, D-galactosamine, and lipopolysaccharide (Dong et al. 2017; Wang et al. 2019; Wang et al. 2015; Wu et al. 2020; Zang et al. 2017).
On the other hand, although Cd ions cannot generate ROS by themselves, they indirectly generate high levels of superoxide, hydrogen peroxide, and hydroxyl radical by several mechanisms (Arroyo et al. 2012). Within this view, it has been demonstrated that Cd ions bind to sulfhydryl groups-containing cellular components such as GSH and reduce their availability (Ağır and Eraslan 2019; Arroyo et al. 2012; Gebhardt 2009; Rani et al. 2014). Besides, Cd ions can suppress and downregulate several endogenous antioxidant enzymes such as GPx, SOD, and CAT by direct binding to their active sites or by increasing their consumption to detoxify higher levels of ROS (Koyu et al. 2006; Newairy et al. 2007). Also, Cd ions stimulate ROS by accumulating in the hepatocytes mitochondria, a critical event that leads to uncoupling oxidative phosphorylation, altering membrane fluidity, dissipation of transmembrane electrical potential, and induction of mitochondrial permeability transition (Belyaeva et al. 2011; Diep et al. 2005; Zhang et al. 2011). In this study, CdCl2-induced hepatotoxicity was also associated with higher hepatic levels of ROS and MDA and lower levels of GSH, SOD, and GPx. Besides, the administration of CdCl2 induced mtPTP as noticed by the increase in the value of Vmax and the parallel decrease in the time which is required to reach Vmax. These data support the above-mentioned studies and confirm that CdCl2-induced hepatic damage is an oxidative-stress-dependent mechanism that involves the depletion of antioxidant and mitochondria damage.
However, Nrf-2 and HO-1 are major transcriptional factors responsible for increasing GSH and antioxidant levels within the majority of cells including the hepatocytes (Luo et al. 2018). Nonetheless, the transactivation of Nrf2 is mainly regulated by the levels of keap1 which bind to it in the transcription factor thus impedes its nuclear translocation or stimulates its cytoplasmic degradation. Previous studies have shown that Cd ions could suppress the antioxidant potential by downregulating Nrf2 and /or stimulating its cytoplasmic degradation by upregulating keap1 (Liu et al. 2019). Similar to these data, our study also revealed a significant reduction in the total and nuclear levels of Nrf2 and its downstream target, HO-1, with a concomitant increase in the cytoplasmic levels of keap1 in the livers of CdCl2-treated rats. These data suggest that CdCl2 is able not only to downregulate Nrf2 but also to stimulate its cytoplasmic degradation. Therefore, we can strongly argue that CdCl2 shifts the redox environment of the hepatocytes toward an oxidative stress one by increasing the production of ROS, overwhelming endogenous antioxidants, impairing the mitochondria function, downregulating Nrf2, and suppressing the transactivation of Nrf2 by upregulating keap1. These data may suggest that Cd ions may suppress GSH and antioxidant enzymes in the livers of rats by downregulating Nrf2.
However, Cd-ions-derived ROS can also stimulate haptic inflammation by inducing NLRP3 inflammasome assembly and subsequently activate NF-κB p65 (Arroyo et al. 2012; Horiguchi et al. 2000; Liu et al. 2019; Rzepecka et al. 2015; Xu et al. 2019). Interestingly, a negative cross-talk between Nrf2 and components of NF-κB has been also established (Wardyn et al. 2015). In this context, pharmacological activation of Nrf2 protected the livers from Cd ions-induced injury by increasing antioxidants and inhibiting NF-κB P65 (Wu et al. 2012). On the contrary, the higher activity of NF-κB p65 reduced the expression and activation of Nrf2 by reducing its heterodimer formation and inhibiting its transcriptional activity, mainly by increasing the keap1 nuclear translocation and promoting the dissociation of Nrf2 from its transcriptional co-activator, the CREB-binding protein (Wardyn et al. 2015). Associated with CdCl2-induced liver damage, ROS, and suppression of Nrf2, we have also found an increase in the inflammatory cell infiltration with a concomitant increase in the nuclear level/activity of NF-κB p65 and levels of TNF-α and IL-6 in the livers of CdCl2-treated rats. These data suggest a vicious cycle between ROS, Nrf2, and NF-κB P65 in the process of CdCl2-induced hepatic damage. Besides, these data may indicate that CdCl2-induced upregulation of keap1 is mediated via the activation of NF-κB. However, further studies are required to precisely identify the major trigger responsible for all these connected events, which seems to be mainly induced by Cd-derived ROS.
Although the above discussion remains confirmatory to many previous studies, the novelist findings of this study are that the protective effect of kaempferol, as well its possible mechanism of protection against CdCl2-induced liver damage is the first to be shown. Herein, kaempferol significantly attenuated the increase in ROS and lipid peroxidation, increased levels of GSH and SOD, upregulated Nrf2 and HO-1, suppressed the nuclear translocation and activation of NF-κB p65, lowered the levels of TNF-α and IL-6, and downregulated keap1 not only in the livers of CdCl2-treated rats but also in the livers of the control-treated rats. Besides, kaempferol administration to CdCl2-treated rats significantly suppressed mtPTP, the release of cytochrome-c, and activation of caspase-3. Based on these data, it seems logical that the mechanism of protection of kaempferol is attributed to antioxidant and anti-inflammatory mechanisms mediated by scavenging ROS, suppression of NF-κB and keap1, and upregulation of Nrf-2 and HO-1, and upregulation of the cellular antioxidants.
Supporting our findings, the protective role of kaempferol against serval cardiovascular, renal, hepatic, and inflammatory disorders is well-reported in many well-designed studies, where these effects were attributed to its potent antioxidant, anti-inflammatory, and anti-apoptotic effects (Imran et al. 2019; Kim and Park 2020). Indeed, kaempferol attenuated doxorubicin (DOX)-induced mitochondria damage by reducing ROS generation, increasing GSH levels, caspase-3 inactivation, and preventing mtPTP (Wang et al. 2020). Also, kaempferol prevented CCL4-induced hepatic damage by increasing GSH, SOD, and CAT (Zang et al. 2017). Besides, kaempferol protected against CdCl2-induced memory loss and hippocampal damage by suppressing TNF-α and IL-6 release and upregulation of MnSOD and GSH, in a SIRT1-dependent mechanism (El-kott et al. 2020a). Similar effects were also observed in the livers of streptozotocin (STZ)-induced diabetic rats. In the same line, kaempferol prevented memory deficits and prevented brain cell apoptosis induced by chlorpyrifos, at least by, activating Nrf2 (Hussein et al. 2018). Also, Saw et al. (Saw et al. 2014) have confirmed that the potent antioxidant potential of kaempferol is related to its ability to activate/upregulate Nrf2.
Nonetheless, kaempferol prevented cardiac damage in isoproterenol-induced heart failure in diabetic rats by suppressing NF-κB and stimulating Nrf2/antioxidants axis (Zhang et al. 2019). In a mouse model of vascular injury, as well as oxidative stress-induced umbilical vein endothelial cells (HUVECs) injury, kaempferol protective effect was mediated by upregulation of the Nrf2/HO-1 axis, increasing the expression of the antioxidants, and inhibition of NF-κB, TNF-α, and IL-6 (Yao et al. 2020). The inhibitory effect of kaempferol on NF-κB and inflammatory cytokines production has been also demonstrated in lipopolysaccharides (LPS)-induced human aortic endothelial cells (HAECs) injury, low-density lipoprotein (ox-LDL)-induced apoptosis in human aortic endothelial cells chondrocytes, and H9N2 virus-induced inflammation and lung injury (Cui et al. 2019; Dong et al. 2017; Zang et al. 2017).
Despite these findings, this study still has some limitations. Although the protective effect of kaempferol in this study is mediated by acting through increasing the expression of Nrf2 and suppression of NF-κB, we still unable to determine the major target of kaempferol given the negative cross-talk between the two. Besides, the precise upstream signaling pathways regulating such effects were not studied yet. Also, kaempferol attenuated the fatty changes in the livers of the CdCl2-treated rats in this study. Similar to these findings, kaempferol also prevented dyslipidemia and hepatic lipid accumulation in high-fat diet-fed rats by increasing fatty acid oxidation through activating PPARα and suppressing fatty acid synthesis by inhibiting SREBP1 (Chang et al. 2011; Wang et al. 2020). Yet, we did not investigate the effect of kaempferol on lipid synthesis/oxidation pathways in the livers of CdCl2-treated rats which requires further investigation to illustrate if this effect is secondary to suppression of oxidative stress or a direct effect on these pathways.
In conclusion, the data in our hands support the hepatoprotective effect of kaempferol and add such protection in an animal model of CdCl2 intoxication. It also supports the potent antioxidant and anti-inflammatory effect of this flavonoid and demonstrates that the mechanism of protection is mediated by the upregulation of Nrf2 and the concomitant suppression of NF-κB p65. Our recommendation is to further study these effects in more in vivo and clinical trials in patients with liver disorders.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Abarikwu SO, Njoku RC, Lawrence CJ, Charles IA, Ikewuchi JC (2017) Rutin ameliorates oxidative stress and preserves hepatic and renal functions following exposure to cadmium and ethanol. Pharm Biol 55:2161–2169. https://doi.org/10.1080/13880209.2017.1387575
Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA (2005) Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol 289:C994–c1001. https://doi.org/10.1152/ajpcell.00031.2005
Ağır MS, Eraslan G (2019) The effect of diosmin against liver damage caused by cadmium in rats. J Food Biochem 43:e12966. https://doi.org/10.1111/jfbc.12966
Amara S, Abdelmelek H, Garrel C, Guiraud P, Douki T, Ravanat JL, Favier A, Sakly M, Ben Rhouma K (2008) Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. J Reprod Dev 54:129–134. https://doi.org/10.1262/jrd.18110
Arroyo V, Flores K, Ortiz L, Gómez-Quiroz L, Gutiérrez-Ruiz M (2012) Liver and cadmium toxicity. J Drug Metab Toxicol S 5
Belyaeva EA, Korotkov SM, Saris NE (2011) In vitro modulation of heavy metal-induced rat liver mitochondria dysfunction: a comparison of copper and mercury with cadmium. J Trace Elem Med Biol 25(Suppl 1):S63–S73. https://doi.org/10.1016/j.jtemb.2010.10.007
Bilgen I, Oner G, Edremitlioğlu M, Alkan Z, Cirrik S (2003) Involvement of cholinoceptors in cadmium-induced endothelial dysfunction. J Basic Clin Physiol Pharmacol 14:55–76. https://doi.org/10.1515/jbcpp.2003.14.1.55
Chang CJ, Tzeng TF, Liou SS, Chang YS, Liu IM (2011) Kaempferol regulates the lipid-profile in high-fat diet-fed rats through an increase in hepatic PPARα levels. Planta Med 77:1876–1882. https://doi.org/10.1055/s-0031-1279992
Chen HJ, Lin CM, Lee CY, Shih NC, Peng SF, Tsuzuki M, Amagaya S, Huang WW, Yang JS (2013) Kaempferol suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells. Oncol Rep 30:925–932. https://doi.org/10.3892/or.2013.2490
Cui S, Tang J, Wang S, Li L (2019) Kaempferol protects lipopolysaccharide-induced inflammatory injury in human aortic endothelial cells (HAECs) by regulation of miR-203. Biomed Pharmacother 115:108888. https://doi.org/10.1016/j.biopha.2019.108888
Devi KP, Malar DS, Nabavi SF, Sureda A, Xiao J, Nabavi SM, Daglia M (2015) Kaempferol and inflammation: from chemistry to medicine. Pharmacol Res 99:1–10. https://doi.org/10.1016/j.phrs.2015.05.002
Diep PT, Denizeau F, Jumarie C (2005) Kinetics of the early subcellular distribution of cadmium in rat hepatocytes. Biometals 18:255–267. https://doi.org/10.1007/s10534-005-1538-3
Dong L, Yin L, Quan H, Chu Y, Lu J (2017) Hepatoprotective effects of kaempferol-3-O-α-l-arabinopyranosyl-7-O-α-l-rhamnopyranoside on d-galactosamine and lipopolysaccharide caused hepatic failure in mice. Molecules 22doi:10.3390/molecules22101755
El-kott AF, Abd-El-Karim M, Khalifa HS, Morsy K, Ibrahim EH, Bin-Jumah M, Abdel-Daim MM, Aleya L (2020a) Kaempferol protects against cadmium chloride-induced hippocampal damage and memory deficits by activation of silent information regulator 1 and inhibition of poly (ADP-Ribose) polymerase-1. Science of The Total Environment, 138832.
El-Kott AF, Bin-Meferij MM, Eleawa SM, Alshehri MM (2020b) Kaempferol protects against cadmium chloride-induced memory loss and hippocampal apoptosis by increased intracellular glutathione stores and activation of PTEN/AMPK induced inhibition of Akt/mTOR signaling. Neurochem Res 45:295–309. https://doi.org/10.1007/s11064-019-02911-4
Eybl V, Kotyzová D, Bludovská M (2004) The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicol Lett 151:79–85. https://doi.org/10.1016/j.toxlet.2004.02.019
Fischer AH, Jacobson KA, Rose J, Zeller R (2008) Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc 2008, pdb.prot4986. doi:10.1101/pdb.prot4986
Gebhardt R (2009) Prevention of cadmium-induced toxicity in liver-derived cells by the combination preparation Hepeel(®). Environ Toxicol Pharmacol 27:402–409. https://doi.org/10.1016/j.etap.2009.01.006
Go YM, Sutliff RL, Chandler JD, Khalidur R, Kang BY, Anania FA, Orr M, Hao L, Fowler BA, Jones DP (2015) Low-dose cadmium causes metabolic and genetic dysregulation associated with fatty liver disease in mice. Toxicol Sci 147:524–534. https://doi.org/10.1093/toxsci/kfv149
Grosset AA, Loayza-Vega K, Adam-Granger É, Birlea M, Gilks B, Nguyen B, Soucy G, Tran-Thanh D, Albadine R, Trudel D (2019) Hematoxylin and eosin counterstaining protocol for immunohistochemistry interpretation and diagnosis. Appl Immunohistochem Mol Morphol 27:558–563. https://doi.org/10.1097/pai.0000000000000626
Haouem S, El Hani A (2013) Effect of cadmium on lipid peroxidation and on some antioxidants in the liver, kidneys and testes of rats given diet containing cadmium-polluted radish bulbs. J Toxicol Pathol 26:359–364. https://doi.org/10.1293/tox.2013-0025
Horiguchi H, Harada A, Oguma E, Sato M, Homma Y, Kayama F, Fukushima M, Matsushima K (2000) Cadmium-induced acute hepatic injury is exacerbated in human interleukin-8 transgenic mice. Toxicol Appl Pharmacol 163:231–239. https://doi.org/10.1006/taap.1999.8877
Hussein RM, Mohamed WR, Omar HA (2018) A neuroprotective role of kaempferol against chlorpyrifos-induced oxidative stress and memory deficits in rats via GSK3β-Nrf2 signaling pathway. Pestic Biochem Physiol 152:29–37. https://doi.org/10.1016/j.pestbp.2018.08.008
Hwang D, Wang L (2001) Effect of taurine on toxicity of cadmium in rats. Toxicology 167:173–180
Hyder O, Chung M, Cosgrove D, Herman JM, Li Z, Firoozmand A, Gurakar A, Koteish A, Pawlik TM (2013) Cadmium exposure and liver disease among US adults. J Gastrointest Surg 17:1265–1273. https://doi.org/10.1007/s11605-013-2210-9
Imran M, Rauf A, Shah ZA, Saeed F, Imran A, Arshad MU, Ahmad B, Bawazeer S, Atif M, Peters DG, Mubarak MS (2019) Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: a comprehensive review. Phytother Res 33:263–275. https://doi.org/10.1002/ptr.6227
Kavazis AN, Morton AB, Hall SE, Smuder AJ (2017) Effects of doxorubicin on cardiac muscle subsarcolemmal and intermyofibrillar mitochondria. Mitochondrion 34:9–19. https://doi.org/10.1016/j.mito.2016.10.008
Kawagoe M, Hirasawa F, Cun Wang S, Liu Y, Ueno Y, Sugiyama T (2005) Orally administrated rare earth element cerium induces metallothionein synthesis and increases glutathione in the mouse liver. Life Sci 77:922–937. https://doi.org/10.1016/j.lfs.2005.02.004
Kim JK, Park SU (2020) Recent studies on kaempferol and its biological and pharmacological activities. Excli j 19:627–634
Koyu A, Gokcimen A, Ozguner F, Bayram DS, Kocak A (2006) Evaluation of the effects of cadmium on rat liver. Mol Cell Biochem 284:81–85. https://doi.org/10.1007/s11010-005-9017-2
Liu C, Zhu Y, Lu Z, Guo W, Tumen B, He Y, Chen C, Hu S, Xu K, Wang Y, Li L, Li S (2019) Cadmium induces acute liver injury by inhibiting Nrf2 and the role of NF-κB, NLRP3, and MAPKs signaling pathway. Int J Environ Res Public Health 17doi:10.3390/ijerph17010138
Luo JF, Shen XY, Lio CK, Dai Y, Cheng CS, Liu JX, Yao YD, Yu Y, Xie Y, Luo P, Yao XS, Liu ZQ, Zhou H (2018) Activation of Nrf2/HO-1 pathway by nardochinoid C inhibits inflammation and oxidative stress in lipopolysaccharide-stimulated macrophages. Front Pharmacol 9:911. https://doi.org/10.3389/fphar.2018.00911
Newairy AA, El-Sharaky AS, Badreldeen MM, Eweda SM, Sheweita SA (2007) The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology 242:23–30. https://doi.org/10.1016/j.tox.2007.09.001
Nwokocha CR, Nwokocha MI, Owu DU, Obi J, Olatunde B, Ebe C, Nwangwu O, Iwuala MO (2012) Comparative analysis on the effect of palm oil (Elaeis guineensis) in reducing cadmium and lead accumulation in liver of Wistar rats. Pharmacognosy Res 4:214–218. https://doi.org/10.4103/0974-8490.102266
Pari L, Murugavel P (2005) Role of diallyl tetrasulfide in ameliorating the cadmium induced biochemical changes in rats. Environ Toxicol Pharmacol 20:493–500. https://doi.org/10.1016/j.etap.2005.05.009
Park SE, Sapkota K, Kim S, Kim H, Kim SJ (2011) Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br J Pharmacol 164:1008–1025. https://doi.org/10.1111/j.1476-5381.2011.01389.x
Rana K, Verma Y, Rana SVS (2020) Possible mechanisms of liver injury induced by cadmium sulfide nanoparticles in rat. Biol Trace Elem Resdoi:10.1007/s12011-020-02128-5
Rani A, Kumar A, Lal A, Pant M (2014) Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res 24:378–399. https://doi.org/10.1080/09603123.2013.835032
Renugadevi J, Prabu SM (2010) Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp Toxicol Pathol 62:171–181. https://doi.org/10.1016/j.etp.2009.03.010
Rikans LE, Yamano T (2000) Mechanisms of cadmium-mediated acute hepatotoxicity. J Biochem Mol Toxicol 14:110–117. https://doi.org/10.1002/(sici)1099-0461(2000)14:2<110::aid-jbt7>3.0.co;2-j
Rzepecka J, Pineda MA, Al-Riyami L, Rodgers DT, Huggan JK, Lumb FE, Khalaf AI, Meakin PJ, Corbet M, Ashford ML, Suckling CJ, Harnett MM, Harnett W (2015) Prophylactic and therapeutic treatment with a synthetic analogue of a parasitic worm product prevents experimental arthritis and inhibits IL-1β production via NRF2-mediated counter-regulation of the inflammasome. J Autoimmun 60:59–73. https://doi.org/10.1016/j.jaut.2015.04.005
Sanjeev S, Bidanchi RM, Murthy MK, Gurusubramanian G, Roy VK (2019) Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ Sci Pollut Res Int 26:20631–20653. https://doi.org/10.1007/s11356-019-05420-7
Saw CL, Guo Y, Yang AY, Paredes-Gonzalez X, Ramirez C, Pung D, Kong AN (2014) The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: involvement of the Nrf2-ARE signaling pathway. Food Chem Toxicol 72:303–311. https://doi.org/10.1016/j.fct.2014.07.038
Toppo R, Roy BK, Gora RH, Baxla SL, Kumar P (2015) Hepatoprotective activity of Moringa oleifera against cadmium toxicity in rats. Vet World 8, 537-40. doi:10.14202/vetworld.2015.537-540
Tsai MS, Wang YH, Lai YY, Tsou HK, Liou GG, Ko JL, Wang SH (2018) Kaempferol protects against propacetamol-induced acute liver injury through CYP2E1 inactivation, UGT1A1 activation, and attenuation of oxidative stress, inflammation and apoptosis in mice. Toxicol Lett 290:97–109. https://doi.org/10.1016/j.toxlet.2018.03.024
Wang H, Chen L, Zhang X, Xu L, Xie B, Shi H, Duan Z, Zhang H, Ren F (2019) Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed Pharmacother 111:468–475. https://doi.org/10.1016/j.biopha.2018.12.105
Wang M, Sun J, Jiang Z, Xie W, Zhang X (2015) Hepatoprotective effect of kaempferol against alcoholic liver injury in mice. Am J Chin Med 43:241–254. https://doi.org/10.1142/s0192415x15500160
Wang T, Wu Q, Zhao T (2020) Preventive effects of kaempferol on high-fat diet-induced obesity Complications in C57BL/6 Mice. Biomed Res Int 2020:4532482–4532489. https://doi.org/10.1155/2020/4532482
Wardyn JD, Ponsford AH, Sanderson CM (2015) Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans 43:621–626. https://doi.org/10.1042/bst20150014
Wu KC, Liu JJ, Klaassen CD (2012) Nrf2 activation prevents cadmium-induced acute liver injury. Toxicol Appl Pharmacol 263:14–20. https://doi.org/10.1016/j.taap.2012.05.017
Wu W, Yang B, Qiao Y, Zhou Q, He H, He M (2020) Kaempferol protects mitochondria and alleviates damages against endotheliotoxicity induced by doxorubicin. Biomed Pharmacother 126:110040. https://doi.org/10.1016/j.biopha.2020.110040
Xu T, Huang S, Huang Q, Ming Z, Wang M, Li R, Zhao Y (2019) Kaempferol attenuates liver fibrosis by inhibiting activin receptor-like kinase 5. J Cell Mol Med 23:6403–6410. https://doi.org/10.1111/jcmm.14528
Yamano T, DeCicco LA, Rikans LE (2000) Attenuation of cadmium-induced liver injury in senescent male fischer 344 rats: role of Kupffer cells and inflammatory cytokines. Toxicol Appl Pharmacol 162:68–75. https://doi.org/10.1006/taap.1999.8833
Yao H, Sun J, Wei J, Zhang X, Chen B, Lin Y (2020) Kaempferol protects blood vessels from damage induced by oxidative stress and inflammation in association with the Nrf2/HO-1 signaling pathway. Front Pharmacol 11:1118. https://doi.org/10.3389/fphar.2020.01118
Yazıhan N, Koçak MK, Akçıl E, Erdem O, Sayal A, Güven C, Akyürek N (2011) Involvement of galectin-3 in cadmium-induced cardiac toxicity. Anadolu Kardiyol Derg 11:479–484. https://doi.org/10.5152/akd.2011.130
Zang Y, Zhang D, Yu C, Jin C, Igarashi K (2017) Antioxidant and hepatoprotective activity of kaempferol 3-O-β-D-(2, 6-di-O-α-L-rhamnopyranosyl) galactopyronoside against carbon tetrachloride-induced liver injury in mice. Food science and biotechnology 26:1071–1076
Zhang L, Guo Z, Wang Y, Geng J, Han S (2019) The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug Dev Res 80:294–309. https://doi.org/10.1002/ddr.21495
Zhang Y, Li JH, Liu XR, Jiang FL, Tian FF, Liu Y (2011) Spectroscopic and microscopic studies on the mechanisms of mitochondrial toxicity induced by different concentrations of cadmium. J Membr Biol 241:39–49. https://doi.org/10.1007/s00232-011-9361-y
Funding
This study was supported by the deanship of Scientific Research at King Khalid University, Abha, KSA under grant number (R.G.P1 /227/41). Also, this research was funded by the Taif University Researchers Supporting under grant number (TURSP-2020/99), Taif University, Taif, Saudi Arabia. Also, this research work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Fast-track Research Funding Program.
Author information
Authors and Affiliations
Contributions
Ali S. Alshehri: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Supervision, Project administration, Funding acquisition, Writing—original draft. Mohamed S A. El-Gerbed and Heba S. Khalifa: Conceptualization, Validation, Formal analysis, Investigation, Writing—original draft. Ayman E. El-Kenawy and Attalla F. El-Kott: Conceptualization, Investigation, Methodology, Writing—review and editing. Ghadeer M. Albadrani: Investigation, Methodology, Writing—review and editing
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All applicable international, national, and/or institutional guidelines for the care and use of animals and cell lines were followed and approved by the ethics committee at the King Khalid University (Ethical number ECM#2020-1701). All authors equally participate in the study.
Consent for publication
All authors allow the publication of the paper.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alshehri, A.S., El-kott, A.F., El-Gerbed, M.S.A. et al. Kaempferol prevents cadmium chloride-induced liver damage by upregulating Nrf2 and suppressing NF-κB and keap1. Environ Sci Pollut Res 29, 13917–13929 (2022). https://doi.org/10.1007/s11356-021-16711-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16711-3