Abstract
Previous systematic reviews and meta-analyses have identified cognitive deficits in adults with obstructive sleep apnoea (OSA). However, quantitative analysis of the association between OSA and neuropsychological performance has not been conducted specifically in older adults, for whom there is a greater risk of cognitive decline. We searched Medline, Embase and PsycINFO through August 2016 for studies describing associations between OSA and neuropsychological outcomes in people aged>50 years. Meta-analyses were performed on these studies for overall cognition and within cognitive domains. Subgroup analyses were performed taking into account risk of bias and moderating differences in study design. 13 studies met eligibility criteria for analysis. A small negative association was found between OSA and all neuropsychological outcomes combined, g=0.18(95% CI 0.04–0.32), and in memory and processing speed domains. Small case-control studies from sleep clinic populations observed the greatest associations, while larger cohort studies from community samples illustrated no association. Analysis accounting for publication bias resulted in a null overall association, g=0.02 (95%CI -0.12 to 0.16). Associations between OSA and cognition in later life are highly variable and the findings differ based on the type and setting of study. It appears some older adults may be at risk of cognitive impairments attributable to OSA; however, the risk of bias renders the evidence inconclusive. High quality research is warranted in clinically diagnosed OSA patients as well as those already experiencing neuropsychological impairment and who may be regarded at higher risk of further cognitive decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive decline in later life can have significant effects on daily functioning, quality of life and independence (Harada et al. 2013). With the increase in the ageing population and concomitant increase in the rates of dementia, efforts to understand the contributors to cognitive decline have become imperative. Between 30 and 50% of the risk for dementia could be due to modifiable risk factors such as depression, physical inactivity and cardiovascular disease (Norton et al. 2014). Over the last few years it has also become apparent that cognitive decline may also be linked to various forms of sleep disturbance (McKinnon et al. 2014; Naismith et al. 2014; Naismith et al. 2010; Naismith et al. 2011; Yaffe et al. 2011) to the extent that sleep disturbance is now also being considered as a potential modifiable risk factor (Mander et al. 2016).
There are various aetiological mechanisms by which sleep disturbance may be linked to cognitive decline, including disruption of critical neurotrophins and neurogenesis (Meerlo et al. 2009), disruption of sleep microarchitecture and the overnight consolidation of memories (Pace-Schott and Spencer 2015), or even excessive daytime sleepiness (Jaussent et al. 2012). There is also a well-established link between obstructive sleep apnoea (OSA) and cognitive dysfunction (Lal et al. 2012; Naismith et al. 2004). OSA is a common breathing disorder that involves repetitive cessation of breathing due to collapse of the pharyngeal airway during sleep. Immediate consequences of OSA are changes in blood oxygen and carbon dioxide levels, acute increases in blood pressure, as well as intermittent sleep fragmentation and arousals caused by the apnoeic events. In addition to being associated with multiple co-morbidities such as cardiovascular and metabolic dysfunction, neurobehavioral symptoms are often common, including excessive daytime sleepiness, increased risk of motor vehicle accidents, and neuropsychological dysfunction (for review, see(Jordan et al. 2014)).
There is substantial evidence surrounding the impact of OSA on neuropsychological function in adults. In younger and middle aged adults, meta-analytic studies suggest that OSA is associated with a decline in memory (Wallace and Bucks 2013), vigilance, executive functioning and motor coordination (Beebe et al. 2003; Bucks et al. 2013; Engleman et al. 2000). While the mechanisms have not been fully delineated, it appears that both hypoxemia and sleep fragmentation may contribute differentially to cognitive decline, with the former having potentially more deleterious effects. Of significance, effective treatment of OSA with continuous positive airway pressure (CPAP) appears to ameliorate some deficits associated with OSA but there is considerable heterogeneity in the outcomes. It appears that CPAP is associated with improvements in attention and vigilance, but deficits in other domains tend to persist despite adequate treatment (Kylstra et al. 2013; Pan et al. 2015).
However, the literature pertaining to the relationship between OSA and neuropsychological functioning in later life has not been specifically or quantitatively analysed. This is despite evidence that the risk and incidence of OSA increases with age (Launois et al. 2007; Heinzer et al. 2015). Affecting approximately 2–4% of middle aged adults (Young et al. 1993), OSA has been estimated to occur in anywhere from 15% to 60% of elderly adults (Ancoli-Israel et al. 1991; Young et al. 2002). The mechanisms behind an increased risk and prevalence of OSA in aged adults are not completely understood, but have been proposed to be due to a combination of anatomical and mechanical changes in the upper airway (Launois et al. 2007; Edwards et al. 2010). Importantly, older adults are at greater risk of medical burden. Not only does this reduce the reserve and resilience of older adults to OSA, these comorbidities are themselves associated with a greater risk for cognitive decline (Biessels et al. 2006; Justin et al. 2013). Of concern, there is longitudinal evidence of a link between OSA and increased risk of cognitive decline and dementia (Chang et al. 2013; Osorio et al. 2015; Yaffe et al. 2011). Therefore, to inform clinical screening and dementia prevention strategies (Naismith et al. 2009), it is important to clearly understand the relationship between OSA and cognitive function in older adults.
Unfortunately, the neuropsychology literature involving OSA can often be inconsistent and difficult to reliably quantify. Empirical studies differ significantly in their design and sampling of specific populations, increasing the risk of bias and reducing the generalisation of results to the general population. Studies also do not always use reliable or objective diagnosis measurements of both OSA such as polysomnography (PSG). Additionally, neuropsychological tests are not always well standardized, and since neuropsychological assessments are expensive and challenging to administer, especially in large samples, epidemiological or community studies may incorporate only gross and insensitive measures of cognition. These are important factors to be considered when reviewing and analysing the literature.
Therefore, the aim of this systematic review was to both find and examine the studies that have objectively investigated the effect of OSA on neuropsychological function with a specific focus on older adults. We also aimed to analyse the literature with a particular focus on investigating how differences in these studies may contribute as moderators of the effects, to better inform understanding in this area and potentially indicate which older adults may be more vulnerable to the cognitive consequences of OSA.
Methods
This work complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Liberati et al. 2009) (see Checklist S1). Methods of analysis and inclusion criteria were registered in advance with PROSPERO (2015:CRD42015020239) and are documented in Protocol S1.
Eligibility
This systematic review and meta-analysis aimed to answer the question: what is the association between OSA and cognitive function across various cognitive domains in older community-dwelling adults (aged >50 years)?
Participants
Eligible studies consisted of healthy participants with a mean participant age and lower bound of 1 standard-deviation above 50 years. Healthy participants were any adult without a diagnosis of mild cognitive impairment (MCI), dementia or other neurological disorders (e.g. stroke, epilepsy). Any studies that investigated the effects of medications that may have influenced cognition (e.g. anesthesia, benzodiazepines) were also excluded from the review.
Diagnosis Measures
Only objectively defined OSA as measured by a validated sleep apnoea diagnostic device or technique was eligible. Both in-lab and home-based PSG diagnostic sleep studies were acceptable as long as comparable measures of breathing and blood oxygen saturation had been derived in order to calculate the rate of apnoeas and hypopneas per hour of sleep (the apnoea-hypopnea index; AHI) and determine the severity of OSA. In concordance with the American Academy of Sleep Medicine guidelines (Epstein et al. 2009), OSA has been defined by a minimum of AHI ≥ 5, and severity rated as mild (5 ≤ AHI < 15), moderate (15 ≤ AHI < 30), or severe (AHI ≥ 30). Although there may have been differences between studies in their calculation of AHI indices (Ho et al. 2015; Ruehland et al. 2009), we extracted the AHI scores as reported by each of the individual studies.
This study only included cases of OSA. While central sleep apnoea is another sleep related breathing disorder of interest, the apnoeas and hypopnoeas are caused by a disturbance in neural output to the respiratory motor neurons rather than airway obstruction, such as in OSA (Young et al. 2002). Therefore the pathophysiology and characteristics of central sleep apnoea are distinct from OSA, justifying exclusion from this analysis.
Comparisons
Comparator groups must have consisted of older adults that shared the same age and gender distributions as OSA participants. The comparator participants must have also undergone a diagnostic PSG to rule out the presence of OSA. Absence of AHI was defined by an AHI < 5. Studies that compared cognitive outcomes with OSA severity measures were eligible if they included control participants in the sample (i.e. a complete range of OSA severity). Unlike previous meta-analyses in this area (Beebe et al. 2003; Wallace and Bucks 2013), we did not compare outcomes to published norms. This is because published neuropsychological normative data will have not taken into consideration the potential presence of OSA in reference samples. Additionally, the use of normative data will most likely differ across studies. We also did not include studies where OSA patients were their own controls before and after treatment (e.g. with CPAP). This is because changes in cognitive function associated with OSA may require treatment longer than can be seen in short-term intervention trials, or because practice effects on neuropsychological testing may bias observations. Therefore we reduced our analysis to simply look at cross-sectional data.
Outcome Measures
Outcomes included scores on standardised neuropsychological tests (Strauss et al. 2006) that measure performance in one or more of the following domains: global cognition, verbal memory, non-verbal memory, working memory, processing speed, attention and vigilance, language, visuospatial skills and executive functions. All outcomes that met this definition were included in the review, including secondary outcomes and multiple tests of the same cognitive domain.
Study Design
Published, peer-reviewed studies presenting cross-sectional or case-control data were selected for analysis. Studies reporting associations between cognitive outcomes and measure of OSA severity (i.e. AHI) were also included. Other study designs (e.g. cohort studies or trials) were eligible if they reported analyzable baseline outcome measures; only baseline data were included in the analyses.
Information Sources and Search Strategy
We searched Embase, PsychInfo and Medline from inception to August 2016. Search terms were: (“cognit*” OR “neurocognit*” OR “neuropsycholog*” OR “attention” OR “executive speed” OR “language” OR “visuospatial” OR “fine motor” OR “memory” OR “learning”) AND (“apnoea” OR “sleep apnea” OR “snoring” OR “sleep disordered breathing” OR “sleep AND apnoea” OR “sleep AND apnea” OR “sleep-disordered breathing” OR “sleep related breathing disorder” OR “sleep-related breathing disorder”). Syntax for the search in each database is provided in Protocol S1. There were no restrictions for date of publication.
Study Selection
Search results were screened for initial eligibility based on title and abstract by N.C. Full-text manuscripts of studies that could not be definitively screened out exclusively based on the information in their title or abstract were assessed by two reviewers (N.C. and J.P.) independently. Disagreements about study eligibility were resolved by A.L., who approved the final list of included studies. Language was limited to English, German, Portuguese, Polish or Hebrew, while conference abstracts with no corresponding full text manuscripts were excluded.
Risk of Bias
Within Studies
The risk of bias in studies was assessed by N.C. using criteria modified from the NIH National Heart, Lung and Blood Institute’s Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (National Institutes of Health 2014). Studies were regarded as being at high or unclear risk of bias if they did not meet core criteria (see table Table S1); all other studies we regarded as being at low risk of bias.
Across Studies
In order to evaluate risk of small-study effect, funnel plots of effect size against standard error for overall outcomes as well as for each cognitive domain were inspected for asymmetry (Sterne et al. 2011). When a meta-analysis included ten or more studies, further testing of funnel plot asymmetry was conducted using Egger’s test of intercepts (Egger et al. 1997). A positive intercept is indicative that studies with smaller samples were more likely to report more positive results than larger studies. When statistically significant asymmetry was found (defined as p < 0.1), a Trim and Fill analysis (Duval and Tweedie 2000) was used to quantify the impact of funnel plot asymmetry on the pooled effect size.
Data Extraction and Coding
Means and standard deviations of each neuropsychological test scores for OSA and comparator groups were extracted from studies and entered into a spreadsheet by N.C. Where available data regarding mean age of participants, years of education, the setting of the study (sleep clinic or community), and the percentage of females in the sample were also extracted from each study. Approximately 50% of the data were entered under the direct supervision of A.L. Primary outcome data from most studies were entered as means and standard deviations (SDs) for the OSA and comparator groups, however in some cases data was entered as a mean difference between groups with a p-value (Alchanatis et al. 2008; Hjelm et al. 2013), or as a correlation with OSA severity (Kim et al. 2011; Terpening et al. 2015; Yesavage et al. 1985). Outcome measures were coded into set cognitive domains by two reviewers (N.C. and A. L.) based on standard neuropsychological categorization (Strauss et al. 2006) or by consensus (for our classifications of neuropsychological tests into cognitive domains, see Table S2). Some studies reported findings between distinct groups based on OSA severity cutoffs (AHI > 5, AHI ≥ 15, AHI ≥ 30) (Ju et al. 2012; Lutsey et al. 2015; Blackwell et al. 2011). In these cases we combined means and SDs from the three groups into a single OSA arm using the formula provided in the Cochrane Handbook (Higgins and Green 2011).
Data Analysis
All analyses were performed using a random effects model in Comprehensive Meta-Analysis (CMA) version 3 (Biostat, Englewood, New Jersey). The primary outcome was standardised mean difference (SMD, calculated as Hedges’ g) in performance on neuropsychological tests between OSA and control groups, or the association between OSA severity measures. CMA flexibly allows for differently reported study outcomes to be entered into the model. Analyses were conducted for all cognitive results combined, as well as for each of the following cognitive domains: attention & vigilance, processing speed, memory, general cognitive or intellectual ability, executive function, language, visuospatial ability and motor learning. Precision of the SMD was calculated for each study by the 95% confidence interval (CI). A positive SMD suggests that controls performed better on cognitive tests than the OSA sample.
When the one study presented data from more than one neuropsychological test, these were combined in two ways. First, results for all tests were combined to produce a single SMD per study, following an established method (Lampit et al. 2014). Additionally, separate tests were classified by neuropsychological capability (see Table S1), such that each study could contribute to one or more cognitive-domain specific SMDs but no study could contribute more than once to each SMD calculated. When outcomes from a given study were combined, the effect estimate reflected the mean amongst the related tests, and the estimate’s variance was scaled up based on an assumed intercorrelation between the tests of 0.6 (Borenstein et al. 2009; Gleser and Olkin 2009). An assumed intercorrelation of 0.8 was used between tests when calculating the effect size for a specific cognitive domain within each study.
The main analysis consisted of combining all outcomes from each study and these were pooled to determine the association between OSA and overall cognition. We also performed domain-specific meta-analyses, in which only studies that reported outcomes on a specified cognitive domain were included, using one combined SMD per study. The overall and domain-specific meta-analyses were performed using a random-effects model. Heterogeneity among studies was quantified using I2 (Higgins et al. 2003).
Subgroup meta-analyses were conducted in order to examine between-study variability and identify how design elements may moderate these associations. These moderators were defined as: the setting of studies (sleep clinic or community-based), AHI cutoff used to define OSA and control participants, and risk of bias (high or low). The subgroup analyses were based on a mixed effects model, which uses a random-effects model to generate within-subgroup variance and a fixed-effects model to compare effects between subgroups. Between-subgroup heterogeneity was tested using the Cochrane’s Q statistic (Higgins and Green 2011) and was defined significant at the p < 0.05 level. Mean age of sample, years of education and percentage of females in study were examined as potential confounders through random effects meta-regression.
Results
Study Selection
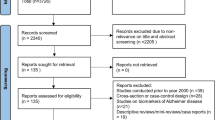
The search identified a total of 10,308 records. After duplicate search results were removed, 7757 studies were screened for eligibility based on abstract and title, of which 7560 were definitively excluded. One hundred and ninety-seven full-text articles were assessed for eligibility, of which 184 were excluded using our PICOS criteria (Fig. 1). In addition, 6 articles (Boland et al. 2002; Blackwell et al. 2015; Martin et al. 2015; Mathieu et al. 2007; Spira et al. 2008; Phillips et al. 1994) met our inclusion criteria however used the same or substantial proportions of participants from studies already included in the systematic review (Berry et al. 1990; Yaffe et al. 2011; Mathieu et al. 2008; Blackwell et al. 2011; Quan et al. 2011; Sforza et al. 2010). These were excluded in order to prevent replicate analysis of the same participants. Four studies were found that did not meet our criteria of a comparator group, as they compared samples of participants dichotomously split by a cutoff of (AHI < 15) (Hjelm et al. 2013; Ju et al. 2012; Sforza et al. 2010; Yaffe et al. 2011). However, we included these studies in the systematic review and meta-analysis for additional context. We found one study that met our inclusion criteria but did not report standard deviations or standard errors, and so could not be included in the meta-analysis (Foley et al. 2003). Thirteen articles were eventually deemed eligible and included in the meta-analysis (for a detailed description of each study, see Table 1).
Characteristics of Included Studies
The 13 studies included in the review reported a total of 5104 participants (OSA, n = 3128, median group size = 41, range 8–1840; controls n = 1879, median group size = 49, range 12–618; Table 1) and 38 neuropsychological test outcomes encompassing 8 cognitive domains (attention & vigilance; processing speed; memory; executive functioning; motor learning; language; visuospatial ability, and; general cognitive ability/intelligence). The most commonly reported cognitive domains were executive function (k = 10), processing speed (k = 8) and global cognition (k = 7). Mean participant age ranged from 55 to 82 years, and approximately 28% of participants were women where this was reported. The studies were largely from the US (Berry et al. 1990; Blackwell et al. 2011; Lutsey et al. 2015; Quan et al. 2006; Yaffe et al. 2011; Yesavage et al. 1985) or Europe (Alchanatis et al. 2008; Ferini-Strambi et al. 2003; Hjelm et al. 2013; Sforza et al. 2010), in addition to studies from Canada (Mathieu et al. 2008), Australia (Terpening et al. 2015) and Korea (Ju et al. 2012; Kim et al. 2011).
Ten of the studies (77%) reported differences in cognitive outcomes between an OSA group and a control group, while 3 studies reported correlations between OSA severity (AHI) and outcome measures (Kim et al. 2011; Terpening et al. 2015; Yesavage et al. 1985). Five of the case-control studies consisted of baseline data taken from a single time-point in cohort studies (Blackwell et al. 2011; Lutsey et al. 2015; Quan et al. 2006; Sforza et al. 2010; Yaffe et al. 2011).
Risk of Bias
The sampling of participants was the major source of bias, with studies either using convenience sampling or participants from a specific cohort study. Case-control studies arising from specialist sleep clinic populations were significantly smaller and were unclear when reporting whether cases and controls were recruited from the same populations. Three studies recruited their sample from different populations (Ju et al. 2012; Yaffe et al. 2011; Yesavage et al. 1985).
Three of the case control studies using sleep clinic populations examined specifically moderate (Mathieu et al. 2008) or severe (Ferini-Strambi et al. 2003; Alchanatis et al. 2008) cases of OSA only and excluded milder cases of OSA from their analyses. Four studies did not report whether the neuropsychological assessment was conducted in a standardized manner by a trained assessor (Alchanatis et al. 2008; Berry et al. 1990; Yaffe et al. 2011; Yesavage et al. 1985).
Overall Effect of OSA on Neuropsychological Performance
The overall effect size of OSA on neuropsychological performance in the combined sample of 13 studies was small and statistically significant (Hedge’s g = 0.18, 95% CI 0.04 to 0.32, p = 0.009; Fig. 2). Heterogeneity across studies was moderate (Tau2 = 0.029 I2 = 69.2%). The funnel plot showed significant asymmetry (Egger’s intercept = 1.52, p = 0.048; Fig. 3) suggesting smaller studies reported larger effects. A trim and fill analysis imputed 6 studies; the adjusted effect size was then negligible and statistically non-significant (g = 0.02, 95%CI -0.12 to 0.16).
Domain-Specific Effects
Executive functioning was the most reported cognitive domain (Fig. 4).
The effect size for this domain was small and statistically non-significant (k = 10, g = 0.12, 95% CI -0.03 to 0.27, p = 0.106, I2 = 69.9%, Tau2 = 0.03). The funnel plot did not reveal any asymmetry (Egger’s intercept = 1.14, p = 0.217; Fig. S1). In the processing speed domain, there was a small and statistically significant effect (k = 8, g = 0.20, 95% CI 0.02 to 0.38, p = 0.033, I2 = 57.6%, Tau2 = 0.03). The funnel plot did not reveal any asymmetry (Fig. S2).
The overall effect in declarative memory was small and statistically significant (k = 6, g = 0.17, 95% CI 0.04 to 0.30, p = 0.009, I2 = 22.5%, Tau2 = 0.01), and the funnel plot did not reveal any asymmetry (Fig. S3). The effect in working memory was negligible and statistically non-significant (k = 3, g = 0.04, 95% CI -0.08 to 0.15, p = 0.511, I2 = 0.0%, Tau2 = 0.0). The combined effect size was small and statistically non-significant for global cognition (k = 7, g = 0.14, 95% CI -0.09 to 0.36, p = 0.226, I2 = 78.3%, Tau2 = 0.05), and medium and statistically non-significant for motor learning (k = 3, g = 0.37, 95% CI -0.17 to 0.92, p = 0.18, I2 = 95.3%, Tau2 = 0.29). However, these effect sizes should be interpreted with caution as the small number of studies reporting on this cognitive domain may decrease the stability of these findings.
Moderators of the Effect of OSA on Neuropsychological Performance
Subgroup analysis based on the differences in study samples was also performed (Fig. 5). Firstly, the effect size on all combined cognitive domains in case-control studies of populations arising from sleep clinics (k = 6, g = 0.49, 95% CI 0.22 to 0.75, p < 0.001; I2 = 0.0%, Tau2 = 0.03) was significantly larger than that arising from baseline data from cohort studies (k = 5, g = 0.04, 95% CI -0.09 to 0.16, p = 0.320, I2 = 67.7%, Tau2 = 0.01; Q statistic for between-group heterogeneity = 8.08, df = 1, p = 0.004).
The analysis of studies was also stratified based around the differences in AHI cutoff used to define and separate groups. Negligible and statistically non-significant effects were observed for case-control studies split by AHI = 5 (k = 2, g = 0.14, 95% CI -0.12 to 0.40, p = 0.287, I2 = 89.7%, Tau2 = 0.03), or AHI = 15 (g = −0.02, 95% CI -0.16 to 0.13, p = 0.795, I2 = 33.1%, Tau2 = 0.01). Studies using a cutoff of AHI < 5 for controls but only including moderate to severe cases in the OSA group (i.E. minimum AHI of 10 to 40) exhibited a small trend toward better performance in controls (k = 5, g = 0.38, 95% CI -0.03 to 0.78, p = 0.077, I2 = 70.5%, Tau2 = 0.14). There were no significant differences in between-group heterogeneity (Q-between = 3.68, df = 2, p = 0.159). No asymmetry was observable on the funnel plot for these studies. There was a trend for studies with a higher risk of bias (k = 8, g = 0.40, 95% CI 0.07 to 0.73, p = 0.052, I2 = 73.0%, Tau2 = 0.15) to report larger effects than those considered at low risk (k = 5, g = 0.08, 95% CI -0.05 to 0.20, p = 0.250, I2 = 65.4%, Tau2 = 0.01; Q-between = 2.96, p = 0.067).
Meta-regression indicated that a greater mean age of study participants significantly predicted variance in the analysis and reduced the effect across all combined cognitive domains (beta = −0.02, df = 1,11, Q = 27.9, p = 0.027, R2 = 34.5%). Years of education, mean AHI or proportion of females in the study samples did not have any effect. Year of publication had no significant effect on the outcomes.
Discussion
This systematic review and meta-analysis is the first to specifically assess the evidence pertaining to the relationship between OSA and neuropsychological function in older adults. We identified thirteen articles that objectively measured the relationship between OSA and cognition in older adults. The meta-analysis of these studies showed a small negative association across a combined range of neuropsychology outcomes. However, this effect appears to be driven by publication bias, with larger studies reporting no link between the presence of OSA and cognition. Also analysis of those studies regarded as having a low risk of bias found no significant effect. Importantly the studies with low risk of bias were larger studies, and the actual pool of participants only declined by 14% within this analysis, indicating that the loss of effect was not due to a lack of remaining power.
Differences in study methodology appeared to influence the findings, and these were explored through the analysis of subgroups. Smaller case-control studies in samples of older adults from specialised sleep clinics showed a significant medium strength negative association between OSA and neuropsychological function, while no overall effect of OSA was observable in larger cohort studies sampled from the community. It is possible the larger effects seen in these sleep centre studies are due to clinical referral bias. Specifically, the types of individuals that present to these specialised clinics are generally more symptomatic, particularly with behavioural impairments such as severe hypersomnolence. Therefore, it is plausible that these patients would be at a higher risk of exhibiting neuropsychological deficits than individuals in the community who are not seeking medical care. The clinical significance of this message suggests that the link between OSA and cognition may be most pronounced in those seeking specialist assessments. In terms of dementia prevention, efforts to target OSA as a modifiable risk factor may be best focused towards older patients in sleep clinics, rather than screening in the general community. Unfortunately, however, once an individual attends a sleep clinic, irreversible cognitive impairment may have already occurred (Naismith et al. 2004).
Notwithstanding this possibility, it must be cautioned that the findings from sleep clinics were derived from studies with small samples with a higher risk of publication bias. Further, some studies were unclear with regards to the recruitment of patients and controls as to whether these arose from the same populations, while most of the clinical case-control studies investigated only severe cases of OSA, and excluded patients with mild or moderate OSA. Conversely, cohort studies included participants across a range of OSA severities. These factors could have potentially inflated the size of the association between OSA and neuropsychological function in the sleep clinic studies. However, while the effect size was larger in studies using more severe cases of OSA, between group heterogeneity was not influenced by the AHI cutoffs used to compare OSA cases with controls. Therefore these findings may suggest that neuropsychological deficits are only significantly observable between extremities of OSA severity and those without the disorder, and not within mild and moderate cases of OSA, however there is a lack of power in the current evidence to conclusively support this (Chowdhuri et al. 2016).
Therefore, while it is possible that older adults with OSA who present to sleep clinics may exhibit significant neuropsychological dysfunction, any conclusions are premature given the available evidence. Baseline data from larger cohort studies has failed to show any overall association between OSA and cognition in older adults. While these studies exhibit a lower risk of bias and consist of significantly larger samples from the community, the study designs are based on specific criteria, in effect reducing their representation of the general older population. It is also possible that OSA defined by the AHI metric is not a true reflection of the disease. A clinical diagnosis of OSA will incorporate the abnormal breathing events alongside neurobehavioral dysfunction (e.g. hypersomnolence). Therefore the presence of an elevated AHI alone may not result in neuropsychological impairment if this index is not an accurate measurement of the impact of OSA on the brain. Our choice to use the AHI as our exposure severity measure may have also been affected by the changes in the definition of that metric during the 30 year timespan of studies included in our review. Random misclassification errors in both our exposure and our outcome measures would have the effect of biasing towards a null finding– which is what we have observed. We also observed no association between year of publication and the strength of the association that might have indicated that the changes in AHI definition had caused the metric to become less predictive of dysfunction over successive revisions (Ho et al. 2015; Ruehland et al. 2009). Alternatively, there may be a particular phenotype of OSA patient with neuropsychological impairment. This review of the current evidence highlights the need for larger and higher quality studies using neuropsychological screening in clinical settings to definitively understand the association between clinical OSA and neuropsychological function in older adults. Following selective assessment and analysis of the evidence, it could be argued that OSA may have different associations with cognitive function depending on the type of older adults studied.
That certain individuals or their characteristics make some older people susceptible to the negative consequences of OSA is still unclear. In the neuropsychological context, it has been suggested that adults with increased cognitive reserve or brain resources may be more resilient to cognitive deficits associated with OSA (Adams et al. 2001; Alchanatis et al. 2005). Unfortunately the current evidence cannot answer this, as only around 60% of studies adequately reported years of education, even though this is regarded as one of the most important demographic factors in performance on neuropsychological tests (Strauss et al. 2006). Likewise, studies did not consider using more detailed measures of cognitive reserve and did not necessarily examine the potential for confounding effects of other key medical co-morbidities that are associated with cognitive decline and dementia. Determining the influence these factors may have on the association between OSA and cognition, as well as other possible covariates, should be a focus in future research in this area.
These current findings do not suggest that OSA should not be seriously considered in older adults. This review cannot rule out that neuropsychological deficits in certain older adults may be attributable to OSA, especially in light of the findings in studies from sleep clinics. Furthermore, there is longitudinal evidence linking long-term presence of OSA with an increased risk of cognitive decline, dementia and mortality (Chang et al. 2013; Pan and Kastin 2014; Marshall et al. 2014; Osorio et al. 2015; Yaffe et al. 2011). This may be because OSA is associated with a range of comorbidities that are also linked with dementia risk, such as elevated blood pressure and cholesterol, cardiovascular disease, and metabolic dysfunction (Norton et al. 2014; Calvin et al. 2009; Somers et al. 2008). There is also evidence from animal studies showing the negative effects of hypoxia and sleep fragmentation on the brain and neurobehavioral function (for review see (Pan and Kastin 2014)), while neuroimaging studies in humans have found associations with the presence of OSA and changes in grey (Torelli et al. 2011) and white matter (Kim et al. 2013), and even markers of oxidative stress (Duffy et al. 2016). Finally, OSA has been shown to significantly alter sleep microarchitecture and EEG brain activity (for review see (D’Rozario et al. 2016)), that have been linked to neurobehavioral dysfunction. Of particular interest from this review in older adults is the decline observed in declarative memory performance (e.g. word lists, stories), as these are the most affected domains in dementia. Sleep is strongly linked to memory consolidation; however research into how OSA may influence sleep-dependent memory consolidation is still in its infancy. Only a few small studies have been conducted in this area, suggesting that this process may be impaired in OSA (Kloepfer et al. 2009), possibly due to sleep fragmentation (Djonlagic et al. 2012) even in REM sleep (Varga et al. 2014).
These results are slightly different to those from previous meta-analyses of the younger and middle-aged literature. Unlike in younger adults (Beebe et al. 2003; Bucks et al. 2013; Saunamaki and Jehkonen 2007), the current analyses suggest that there is no association between OSA and executive dysfunction in older adults. A possible reason why the current results are inconsistent with previous analyses of the literature in younger cohorts could be our analysis method. The approach to average outcome data within each study to remove the dependence of effect sizes and calculate a composite score may have been a factor in the relatively small findings. However, given the level of information that was available, our approach is a robust and commonly employed method in the neuropsychological field. Furthermore, differences could be due to the particular grouping of cognitive domains. For instance, previously working memory has been included in measurements of executive function (Beebe et al. 2003; Bucks et al. 2013). However, we separated working memory as a distinct cognitive domain and coded our tests (e.g. digit span) by consulting a widely cited textbook (Strauss et al. 2006). Regardless, working memory was only reported in 3 studies with a negligible, very small pooled effect. Due to its role in managing the use of other cognitive domains, the integrity of executive functioning is considered a vital predictor for functional decline. However, it appears from these results that OSA has little effect on this cognitive domain in healthy older adults, and recent longitudinal evidence supports these findings in this domain (Martin et al. 2016).
This review only focused on studies of healthy adults (i.e. patients free of known or reported neurodegenerative or neurological diseases). Studying neuropsychological performance in cognitively intact participants can increase the risk of ceiling effects, which may reduce the chance of observing cognitive deficits. While it is difficult to ascertain whether this occurred in the reviewed studies and to what extent, this should have been accounted for by our systematic review only including studies that utilized standardized neuropsychological tests. Regardless, measuring OSA and cognitive function in adults who may be already experiencing cognitive impairments would be insightful, especially given the evidence suggesting an increased risk for further cognitive decline. Treatment with CPAP has shown positive effects in limited cognitive domains only (Kylstra et al. 2013; Olaithe and Bucks 2013; Pan et al. 2015), however some evidence suggests it may slow and reduce the progression of dementia (Osorio et al. 2015). Further research is needed to understand the effect of ongoing OSA treatment on neuropsychological performance in older adults. Ideally, this should be investigated through randomized controlled trials in individuals with increased risk of further cognitive decline such as those with MCI, in order to understand the reversibility of any association between OSA and cognitive decline.
Interestingly, while this systematic review focused on adult participants over the age of 50 years, meta-regression showed that as the mean age increased across the studies, the effect size slightly decreased. This suggests that greater age reduces the susceptibility to any cognitive impairment associated with OSA. This finding may illustrate a healthy survivor-effect which has been observed and discussed previously in a cardiovascular context (Gottlieb et al. 2010; Punjabi et al. 2009). As OSA is also associated with a range of negative health outcomes (e.g. cardiovascular disease), those individuals who show greater susceptibility to any negative consequences of OSA may have a higher mortality risk at an earlier age compared to those who are more resilient, making them less likely to be included in a research study. This possibility only serves to emphasise the importance of managing OSA in older adults for not only cognitive impairment, but overall health and quality of life.
Conclusions
The aim of this review was to refine the estimate of the association between OSA and cognitive functioning specifically in older adults. We found from our systematic search of studies that OSA appears to have no association with cognition overall, however the variance in the literature is significantly influenced by the setting. It is likely that there may be a relationship in some older adults, and evidence does suggest that OSA may increase the risk of cognitive decline. Further exploration of the factors that determine inter-individual differences in neuropsychological outcomes attributed to OSA will better aid in developing robust designs for early intervention trials aimed at slowing cognitive decline in older adults. These will better inform us whether management of OSA may significantly improve the quality of life in older adults, particularly those who are already experiencing cognitive impairment and at heightened risk for cognitive decline and dementia.
References
Adams, N., Strauss, M., Schluchter, M., & Redline, S. (2001). Relation of measures of sleep-disordered breathing to neuropsychological functioning. American Journal of Respiratory and Critical Care Medicine, 163(7), 1626–1631. doi:10.1164/ajrccm.163.7.2004014.
Alchanatis, M., Zias, N., Deligiorgis, N., Amfilochiou, A., Dionellis, G., & Orphanidou, D. (2005). Sleep apnea-related cognitive deficits and intelligence: An implication of cognitive reserve theory. Journal of Sleep Research, 14(1), 69–75.
Alchanatis, M., Zias, N., Deligiorgis, N., Liappas, I., Chroneou, A., Soldatos, C., et al. (2008). Comparison of cognitive performance among different age groups in patients with obstructive sleep apnea. Sleep & Breathing, 12(1), 17–24.
Ancoli-Israel, S., Kripke, D. F., Klauber, M. R., Mason, W. J., Fell, R., & Kaplan, O. (1991). Sleep-disordered breathing in community-dwelling elderly. Sleep, 14(6), 486–495.
Beebe, D., Groesz, L., Wells, C., Nichols, A., & McGee, K. (2003). The neuropsychological effects of obstructive sleep apnea: A meta-analysis of norm-referenced and case-controlled data. Sleep, 26(3), 298–307.
Berry, D. T., Phillips, B. A., Cook, Y. R., Schmitt, F. A., Honeycutt, N. A., Arita, A. A., et al. (1990). Geriatric sleep apnea syndrome: A preliminary description. Journal of Gerontology, 45(5), M169–M174.
Biessels, G. J., Staekenborg, S., Brunner, E., Brayne, C., & Scheltens, P. (2006). Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurology, 5(1), 64–74. doi:10.1016/s1474-4422(05)70284-2.
Blackwell, T., Yaffe, K., Ancoli-Israel, S., Redline, S., Ensrud, K. E., Stefanick, M. L., et al. (2011). Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: The osteoporotic fractures in men sleep study. Journal of the American Geriatrics Society, 59(12), 2217–2225. doi:10.1111/j.1532-5415.2011.03731.x.
Blackwell, T., Yaffe, K., Laffan, A., Redline, S., Ancoli-Israel, S., Ensrud, K. E., et al. (2015). Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: The osteoporotic fractures in men sleep study. Journal of the American Geriatrics Society, 63(3), 453–461. doi:10.1111/jgs.13321.
Boland, L. L., Shahar, E., Iber, C., Knopman, D. S., Kuo, T. F., & Nieto, F. J. (2002). Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: The sleep heart health study. Journal of Sleep Research, 11(3), 265–272.
Borenstein, M., Hedges, L., Higgins, J., & Rothstein, H. (2009). Introduction to meta-analysis. Chichester: Wiley.
Bucks, R. S., Olaithe, M., & Eastwood, P. (2013). Neurocognitive function in obstructive sleep apnoea: A meta-review. [meta-analysis review]. Respirology, 18(1), 61–70. doi:10.1111/j.1440-1843.2012.02255.x.
Calvin, A. D., Albuquerque, F. N., Lopez-Jimenez, F., & Somers, V. K. (2009). Obstructive sleep apnea, inflammation, and the metabolic syndrome. Metabolic Syndrome and Related Disorders, 7(4), 271–278. doi:10.1089/met.2008.0093.
Chang, W. P., Liu, M. E., Chang, W. C., Yang, A. C., Ku, Y. C., Pai, J. T., et al. (2013). Sleep apnea and the risk of dementia: A population-based 5-year follow-up study in Taiwan. PloS One, 8(10), e78655. doi:10.1371/journal.pone.0078655.
Chowdhuri, S., Quan, S. F., Almeida, F., Ayappa, I., Batool-Anwar, S., Budhiraja, R., et al. (2016). An official American Thoracic Society research statement: Impact of mild obstructive sleep apnea in Adults. American Journal of Respiratory and Critical Care Medicine, 193(9), e37–e54. doi:10.1164/rccm.201602-0361ST.
D’Rozario, A. L., Cross, N. E., Vakulin, A., Bartlett, D. J., Wong, K. K. H., Wang, D., et al. (2016). Quantitative electroencephalogram measures in adult obstructive sleep apnea-potential biomarkers of Neurobehavioural functioning. Sleep Medicine Reviews. doi:10.1016/j.smrv.2016.10.003.
Djonlagic, I., Saboisky, J., Carusona, A., Stickgold, R., & Malhotra, A. (2012). Increased sleep fragmentation leads to impaired off-line consolidation of motor memories in humans. PloS One, 7(3), e34106. doi:10.1371/journal.pone.0034106.
Duffy, S. L., Lagopoulos, J., Terpening, Z., Lewis, S. J., Grunstein, R., Mowszowski, L., et al. (2016). Association of Anterior Cingulate Glutathione with sleep apnea in older Adults at-risk for dementia. Sleep, 39(4), 899–906. doi:10.5665/sleep.5650.
Duval, S., & Tweedie, R. (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463.
Edwards, B. A., O'Driscoll, D. M., Ali, A., Jordan, A. S., Trinder, J., & Malhotra, A. (2010). Aging and sleep: Physiology and pathophysiology. Seminars in Respiratory and Critical Care Medicine, 31(5), 618–633. doi:10.1055/s-0030-1265902.
Egger, M., Davey Smith, G., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. [research support, U.S. Gov't, non-P.H.S.] BMJ, 315(7109), 629–634.
Engleman, H. M., Kingshott, R. N., Martin, S. E., & Douglas, N. J. (2000). Cognitive function in the sleep apnea/hypopnea syndrome (SAHS). Sleep, 23(Suppl 4), S102–S108.
Epstein, L. J., Kristo, D., Strollo Jr., P. J., Friedman, N., Malhotra, A., Patil, S. P., et al. (2009). Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. Journal of Clinical Sleep Medicine, 5(3), 263–276.
Ferini-Strambi, L., Baietto, C., Di Gioia, M., Castaldi, P., Castronovo, C., Zucconi, M., et al. (2003). Cognitive dysfunction in patients with obstructive sleep apnea (OSA): Partial reversibility after continuous positive airway pressure (CPAP). Brain Research Bulletin, 61(1), 87–92.
Foley, D. J., Masaki, K., White, L., Larkin, E. K., Monjan, A., & Redline, S. (2003). Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep, 26(5), 596–599.
Gleser, L., & Olkin, I. (2009). Stochastically dependent effect sizes. In Cooper H, Hedges L, & Valentine J (Eds.), The handbook of research synthesis and meta-analysis (2nd ed., pp. 357–376). New York: Russell Sage Foundation.
Gottlieb, D. J., Yenokyan, G., Newman, A. B., O'Connor, G. T., Punjabi, N. M., Quan, S. F., et al. (2010). Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation, 122(4), 352–360. doi:10.1161/circulationaha.109.901801.
Harada, C. N., Natelson Love, M. C., & Triebel, K. L. (2013). Normal cognitive aging. Clinics in Geriatric Medicine, 29(4), 737–752. doi:10.1016/j.cger.2013.07.002.
Heinzer, R., Vat, S., Marques-Vidal, P., Marti-Soler, H., Andries, D., Tobback, N., et al. (2015). Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. The Lancet Respiratory Medicine, 3(4), 310–318. doi:10.1016/s2213-2600(15)00043-0.
Higgins, J. P., & Green, S. (Eds.). (2011). Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0 ed.): The Cochrane Collaboration. Available from http://handbook.cochrane.org. Accessed 4/10/2017.
Higgins, J. P., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. doi:10.1136/bmj.327.7414.557.
Hjelm, C., Stromberg, A., Arestedt, K., & Brostrom, A. (2013). Association between sleep-disordered breathing, sleep-wake pattern, and cognitive impairment among patients with chronic heart failure. European Journal of Heart Failure, 15(5), 496–504.
Ho, V., Crainiceanu, C. M., Punjabi, N. M., Redline, S., & Gottlieb, D. J. (2015). Calibration model for apnea-hypopnea indices: Impact of alternative criteria for hypopneas. Sleep, 38(12), 1887–1892. doi:10.5665/sleep.5234.
Jaussent, I., Bouyer, J., Ancelin, M. L., Berr, C., Foubert-Samier, A., Ritchie, K., et al. (2012). Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep, 35(9), 1201–1207. doi:10.5665/sleep.2070.
Jordan, A. S., McSharry, D. G., & Malhotra, A. (2014). Adult obstructive sleep apnoea. Lancet, 383(9918), 736–747. doi:10.1016/s0140-6736(13)60734-5.
Ju, G., Yoon, I. Y., Lee, S. D., Kim, T. H., Choe, J. Y., & Kim, K. W. (2012). Effects of sleep apnea syndrome on delayed memory and executive function in elderly adults. Journal of the American Geriatrics Society, 60(6), 1099–1103.
Justin, B. N., Turek, M., & Hakim, A. M. (2013). Heart disease as a risk factor for dementia. Clin Epidemiol, 5, 135–145. doi:10.2147/clep.s30621.
Kim, H., Yun, C. H., Thomas, R. J., Lee, S. H., Seo, H. S., Cho, E. R., et al. (2013). Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep, 36(5), 709–715b. doi:10.5665/sleep.2632.
Kim, S. J., Lee, J. H., Lee, D. Y., Jhoo, J. H., & Woo, J. I. (2011). Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. The American Journal of Geriatric Psychiatry, 19(4), 374–381. doi:10.1097/JGP.0b013e3181e9b976.
Kloepfer, C., Riemann, D., Nofzinger, E. A., Feige, B., Unterrainer, J., O'Hara, R., et al. (2009). Memory before and after sleep in patients with moderate obstructive sleep apnea. Journal of Clinical Sleep Medicine, 5(6), 540–548.
Kylstra, W. A., Aaronson, J. A., Hofman, W. F., & Schmand, B. A. (2013). Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: A meta-analysis. Sleep Medicine Reviews, 17(5), 341–347. doi:10.1016/j.smrv.2012.09.002.
Lal, C., Strange, C., & Bachman, D. (2012). Neurocognitive impairment in obstructive sleep apnea. Chest, 141(6), 1601–1610. doi:10.1378/chest.11-2214.
Lampit, A., Hallock, H., & Valenzuela, M. (2014). Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PLoS Medicine, 11(11), e1001756. doi:10.1371/journal.pmed.1001756.
Launois, S. H., Pepin, J. L., & Levy, P. (2007). Sleep apnea in the elderly: A specific entity? Sleep Medicine Reviews, 11(2), 87–97. doi:10.1016/j.smrv.2006.08.005.
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Journal of Clinical Epidemiology, 62(10), e1–34. doi:10.1016/j.jclinepi.2009.06.006.
Lutsey, P. L., Bengtson, L. G., Punjabi, N. M., Shahar, E., Mosley, T. H., Gottesman, R. F., et al. (2015). Obstructive sleep apnea and 15-year cognitive decline: The atherosclerosis risk in communities (ARIC) study. Sleep, 39(2), 309–316.
Mander, B. A., Winer, J. R., Jagust, W. J., & Walker, M. P. (2016). Sleep: A novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends in Neurosciences, 39(8), 552–566. doi:10.1016/j.tins.2016.05.002.
Marshall, N. S., Wong, K. K., Cullen, S. R., Knuiman, M. W., & Grunstein, R. R. (2014). Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton health study cohort. Journal of Clinical Sleep Medicine, 10(4), 355–362. doi:10.5664/jcsm.3600.
Martin, M. S., Sforza, E., Crawford-Achour, E., Pascal, L., Lienard, G., & Frederic, R. (2016). Sleep breathing disorders and cognitive decline in healthy elderly followed for eight years: The PROOF cohort. Ann Phys Rehabil Med, 59s, e99. doi:10.1016/j.rehab.2016.07.221.
Martin, M. S., Sforza, E., Roche, F., Barthelemy, J. C., & Thomas-Anterion, C. (2015). Sleep breathing disorders and cognitive function in the elderly: An 8-year follow-up study. The proof-synapse cohort. Sleep, 38(2), 179–187A.
Mathieu, A., Mazza, S., Decary, A., Massicotte-Marquez, J., Petit, D., Gosselin, N., et al. (2008). Effects of obstructive sleep apnea on cognitive function: A comparison between younger and older OSAS patients. [research support, non-U.S. Gov't]. Sleep Medicine, 9(2), 112–120. doi:10.1016/j.sleep.2007.03.014.
Mathieu, A., Mazza, S., Petit, D., Decary, A., Massicotte-Marquez, J., Malo, J., et al. (2007). Does age worsen EEG slowing and attention deficits in obstructive sleep apnea syndrome? Clinical Neurophysiology, 118(7), 1538–1544.
McKinnon, A., Terpening, Z., Hickie, I. B., Batchelor, J., Grunstein, R., Lewis, S. J., et al. (2014). Prevalence and predictors of poor sleep quality in mild cognitive impairment. Journal of Geriatric Psychiatry and Neurology, 27(3), 204–211. doi:10.1177/0891988714527516.
Meerlo, P., Mistiberger, R. E., Jacobs, B. L., Heller, H. C., & McGinty, D. (2009). New neurons in the adult brain: The role of sleep and consequences of sleep loss. Sleep Medicine Reviews, 13(3), 187–194. doi:10.1016/j.smrv.2008.07.004.
Naismith, S., Hickie, I. B., Terpening, Z., Rajaratnam, S. M., Hodges, J. R., Bolitho, S., et al. (2014). Circadian misalignment and sleep disruption in mild cognitive impairment. Journal of Alzheimer's Disease, 38(4), 857–866. doi:10.3233/jad-131217.
Naismith, S., Winter, V., Gotsopoulos, H., Hickie, I., & Cistulli, P. (2004). Neurobehavioral functioning in obstructive sleep apnea: Differential effects of sleep quality, hypoxemia and subjective sleepiness. [comparative study]. Journal of Clinical and Experimental Neuropsychology, 26(1), 43–54. doi:10.1076/jcen.26.1.43.23929.
Naismith, S. L., Glozier, N., Burke, D., Carter, P. E., Scott, E., & Hickie, I. B. (2009). Early intervention for cognitive decline: Is there a role for multiple medical or behavioural interventions? Early Intervention in Psychiatry, 3(1), 19–27. doi:10.1111/j.1751-7893.2008.00102.x.
Naismith, S. L., Rogers, N. L., Hickie, I. B., Mackenzie, J., Norrie, L. M., & Lewis, S. J. (2010). Sleep well, think well: Sleep-wake disturbance in mild cognitive impairment. Journal of Geriatric Psychiatry and Neurology, 23(2), 123–130. doi:10.1177/0891988710363710.
Naismith, S. L., Rogers, N. L., Lewis, S. J., Diamond, K., Terpening, Z., Norrie, L., et al. (2011). Sleep disturbance in mild cognitive impairment: Differential effects of current and remitted depression. Acta Neuropsychiatr, 23(4), 167–172. doi:10.1111/j.1601-5215.2011.00555.x.
National Institutes of Health (2014). Quality assessment tool for observational cohort and cross-sectional studies. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort. National Heart, Lung, and Blood Institute.
Norton, S., Matthews, F. E., Barnes, D. E., Yaffe, K., & Brayne, C. (2014). Potential for primary prevention of Alzheimer's disease: An analysis of population-based data. Lancet Neurology, 13(8), 788–794. doi:10.1016/s1474-4422(14)70136-x.
Olaithe, M., & Bucks, R. S. (2013). Executive dysfunction in OSA before and after treatment: A meta-analysis. Sleep, 36(9), 1297–1305. doi:10.5665/sleep.2950.
Osorio, R. S., Gumb, T., Pirraglia, E., Varga, A. W., Lu, S. E., Lim, J., et al. (2015). Sleep-disordered breathing advances cognitive decline in the elderly. Neurology, 84(19), 1964–1971. doi:10.1212/wnl.0000000000001566.
Pace-Schott, E. F., & Spencer, R. M. (2015). Sleep-dependent memory consolidation in healthy aging and mild cognitive impairment. Current Topics in Behavioral Neurosciences, 25, 307–330. doi:10.1007/7854_2014_300.
Pan, W., & Kastin, A. J. (2014). Can sleep apnea cause Alzheimer's disease? [research support, N.I.H., extramural]. Neuroscience and Biobehavioral Reviews, 47, 656–669. doi:10.1016/j.neubiorev.2014.10.019.
Pan, Y. Y., Deng, Y., Xu, X., Liu, Y. P., & Liu, H. G. (2015). Effects of continuous positive airway pressure on cognitive deficits in middle-aged patients with obstructive sleep apnea syndrome: A meta-analysis of randomized controlled trials. Chinese Medical Journal, 128(17), 2365–2373. doi:10.4103/0366-6999.163385.
Phillips, B. A., Berry, D. T., Schmitt, F. A., Harbison, L., & Lipke-Molby, T. (1994). Sleep-disordered breathing in healthy aged persons: Two- and three-year follow-up. Sleep, 17(5), 411–415.
Punjabi, N. M., Caffo, B. S., Goodwin, J. L., Gottlieb, D. J., Newman, A. B., O'Connor, G. T., et al. (2009). Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Medicine, 6(8), e1000132. doi:10.1371/journal.pmed.1000132.
Quan, S. F., Chan, C. S., Dement, W. C., Gevins, A., Goodwin, J. L., Gottlieb, D. J., et al. (2011). The association between obstructive sleep apnea and neurocognitive performance--the apnea positive pressure long-term efficacy study (APPLES). Sleep, 34(3), 303–314B.
Quan, S. F., Wright, R., Baldwin, C. M., Kaemingk, K. L., Goodwin, J. L., Kuo, T. F., et al. (2006). Obstructive sleep apnea-hypopnea and neurocognitive functioning in the sleep heart health study. Sleep Medicine, 7(6), 498–507.
Ruehland, W. R., Rochford, P. D., O'Donoghue, F. J., Pierce, R. J., Singh, P., & Thornton, A. T. (2009). The new AASM criteria for scoring hypopneas: Impact on the apnea hypopnea index. Sleep, 32(2), 150–157.
Saunamaki, T., & Jehkonen, M. (2007). A review of executive functions in obstructive sleep apnea syndrome. Acta Neurologica Scandinavica, 115(1), 1–11. doi:10.1111/j.1600-0404.2006.00744.x.
Sforza, E., Roche, F., Thomas-Anterion, C., Kerleroux, J., Beauchet, O., Celle, S., et al. (2010). Cognitive function and sleep related breathing disorders in a healthy elderly population: The synapse study. Sleep, 33(4), 515–521.
Somers, V. K., White, D. P., Amin, R., Abraham, W. T., Costa, F., Culebras, A., et al. (2008). Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation, 118(10), 1080–1111. doi:10.1161/circulationaha.107.189375.
Spira, A. P., Blackwell, T., Stone, K. L., Redline, S., Cauley, J. A., Ancoli-Israel, S., et al. (2008). Sleep-disordered breathing and cognition in older women. Journal of the American Geriatrics Society, 56(1), 45–50.
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ, 343, d4002. doi:10.1136/bmj.d4002.
Strauss, E. H., Sherman, E. M. S., & Spreen, O. A. (2006). A compendium of neuropsychological tests: Administration, norms and commentary. Oxford: Oxford University Press.
Terpening, Z., Lewis, S. J., Yee, B., Grunstein, R., Hickie, I. B., & Naismith, S. L. (2015). Association between sleep-disordered breathing and neuropsychological performance in older Adults with mild cognitive impairment. Journal of Alzheimer's Disease. doi:10.3233/jad-141860.
Torelli, F., Moscufo, N., Garreffa, G., Placidi, F., Romigi, A., Zannino, S., et al. (2011). Cognitive profile and brain morphological changes in obstructive sleep apnea. NeuroImage, 54(2), 787–793. doi:10.1016/j.neuroimage.2010.09.065.
Varga, A. W., Kishi, A., Mantua, J., Lim, J., Koushyk, V., Leibert, D. P., et al. (2014). Apnea-induced rapid eye movement sleep disruption impairs human spatial navigational memory. The Journal of Neuroscience, 34(44), 14571–14577. doi:10.1523/jneurosci.3220-14.2014.
Wallace, A., & Bucks, R. S. (2013). Memory and obstructive sleep apnea: A meta-analysis. [meta-analysis]. Sleep, 36(2), 203–220. doi:10.5665/sleep.2374.
Yaffe, K., Laffan, A. M., Harrison, S. L., Redline, S., Spira, A. P., Ensrud, K. E., et al. (2011). Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA, 306(6), 613–619.
Yesavage, J., Bliwise, D., Guilleminault, C., Carskadon, M., & Dement, W. (1985). Preliminary communication: Intellectual deficit and sleep-related respiratory disturbance in the elderly. Sleep, 8(1), 30–33.
Young, T., Palta, M., Dempsey, J., Skatrud, J., Weber, S., & Badr, S. (1993). The occurrence of sleep-disordered breathing among middle-aged adults. The New England Journal of Medicine, 328(17), 1230–1235. doi:10.1056/nejm199304293281704.
Young, T., Peppard, P. E., & Gottlieb, D. J. (2002). Epidemiology of obstructive sleep apnea: A population health perspective. American Journal of Respiratory and Critical Care Medicine, 165(9), 1217–1239.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of Funding
This work was not supported by any project funding. N.C. is supported by an Australian Postgraduate Award and NHMRC ‘Neurosleep’ top-up funding. A.L. is supported by an NHMRC-ARC Dementia Fellowship. J.P. is partly supported by an NHMRC ‘Neurosleep’ fellowship. R.G. is supported by an NHMRC Senior Principal Research Fellowship.
Rights and permissions
About this article
Cite this article
Cross, N., Lampit, A., Pye, J. et al. Is Obstructive Sleep Apnoea Related to Neuropsychological Function in Healthy Older Adults? A Systematic Review and Meta-Analysis. Neuropsychol Rev 27, 389–402 (2017). https://doi.org/10.1007/s11065-017-9344-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-017-9344-6