Abstract
Gliomas are common and aggressive brain tumors that carry a poor prognosis. The current multimodal therapeutic option for glioma includes surgery subsequently temozolomide chemotherapy and/or radiation; but gliomas are often associated with multidrug resistance, intensive adverse events, and tumor relapse. Thus, novel interventions that can enhance successful chemo-prevention and overcome therapeutic resistance are urgently needed. Phytochemicals have several biological properties with multi-target sites and relatively limited degrees of toxicity. Curcumin is a natural polyphenolic compound with several anti-tumor effects which potentially inhibit tumor growth, development, proliferation, invasion, dissemination, and angiogenesis in different human malignancies. Experimental model studies have demonstrated that curcumin attenuates glioma cell viability by G2/M cell cycle arrest, apoptosis, induction of autophagy, gene expression alteration, and disruption of multi-molecular pathways. Moreover, curcumin has been reported to re-sensitize cancer to chemotherapeutics as well as augment the effect of radiotherapy on glioma cells. In this review, we have provided an update on the in vitro and in vivo effects of curcumin-based therapy on gliomas. We have also discussed the use of curcumin in combination therapies, its effectiveness on drug-resistant cells, and new formulations of curcumin in the treatment of gliomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Gliomas are the most common and invasive malignant brain tumors, arising from the neuroglial stem or progenitor cells. The hallmarks of gliomas include local invasive growth and intense angiogenesis. Approximately 80% of malignant brain tumors, 30% of primary brain tumors, and the majority of fatalities from these tumors are attributed to glioma [1, 2]. The incidence of gliomas varies according to age, sex, ethnicity, and geographical location. Among them, glioblastoma multiforme (GBM) with an incidence of 3.21/100,000 people-year, is the most common and most aggressive glioma subtype [3]. Gliomas may be histologically defined by the World Health Organization (WHO) criteria into grades I–IV. Common gliomas in adults consist of infiltrative diffuse astrocytoma (grade II), anaplastic astrocytoma (grade III), GBM (grade IV), oligodendrogliomas, and mixed oligoastrocytomas. Pilocytic astrocytoma (grade I) and diffuse midline gliomas are the most prevalent glioma tumors in childhood. Thereafter, the WHO has modified the classification system of adult diffuse glioma by integrating the tumor morphology and its molecular changes [4, 5]. In this system, the best prognosis is usually seen in oligodendroglial tumors with mutation of isocitrate dehydrogenase (IDH) and simultaneous deletion of 1p/19q; intermediate outcome mostly present in astrocytic tumors with IDH mutation but not 1p/19q deletion; and poor prognosis is mostly linked in GBM with wild-type IDH. However, the majority of pediatric glioma tumors are characterized by limited growth rates, good prognosis, and frequentative fusions or mutations in the BRAF gene [6]. Despite recent advances in glioma treatment approaches such as surgery, chemotherapy, radiotherapy, and immunotherapy, patient mortality rates remain high and the median survival is about 9 months [7]. The fundamental issue in the field of glioma therapy is that candidate drugs cannot cross the blood–brain barrier (BBB) and desired specificity for malignant cells [8]. Hence, the development of tailored therapeutic platforms may ultimately improve outcomes.
It has been reported that more than 30% of glioma patients are prescribed supplementary and alternative therapies [9]. More recently, the therapeutic potential of herbal and traditional medicine on gliomas has been paid more attention by researchers. Different agents extracted from natural compounds or their derivatives have been reported to have inhibitory effects against glioma cells [10].
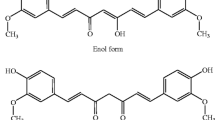
Curcumin (1,7- bis-(hydroxy-3-methoxyphenyl)-1,6-heptadiena-3,5-dione) is a hydrophobic and polyphenolic herbal supplement extracted from a spice known as turmeric (the rhizome Curcuma longa plant) [11]. There is growing evidence that this compound is safe and pleiotropic with a multitude of pharmacological impacts including anti-tumor [12,13,14], anti-inflammatory [15,16,17,18], antioxidant [19], antiangiogenic [20], neuroprotective [21, 22], hepatoprotective [23, 24], cardioprotective [25], pulmonoprotective [26], anti-ischemic [27, 28], lipid-modifying [29, 30], antidiabetic[31, 32], analgesic [33], vasculoprotective[34], anti-thrombotic[35], and immunomodulatory [36] effects. Curcumin has been shown to regulate multiple cellular pathways associated with cancer development, that include: nuclear factor-κB (NF-κB), Janus kinase (JAK)-Signal Transducer and Activator of Transcription (STAT3), Ras, PI3K/AKT, Notch1, forkhead box protein 1(FOXO1), Wnt/β-catenin, mitogen-activated protein kinases (MAPK), and p53, as well as oncogenic and tumor-suppressive miRNAs (Fig. 1) [37,38,39]. Because curcumin interacts differently with normal and cancer cells, in addition to its capacity to boost the effects of chemotherapy on tumor cells, and the absence of significant side effects have been reported [40, 41]. Hence, curcumin appears to be a candidate for use as an anticancer drug. Most importantly, the lipophilic characteristics of curcumin and therefore its potential permeability to the BBB make it a potential therapeutic agent against CNS-related disorders and malignancies [42]; as the protective effects of curcumin on Alzheimer disease[43], Parkinson[44], and GBM [45] have already been reported. The major limitations in curcumin’s clinical use are its low bioavailability, chemical mutability, fast metabolism, and short half-life [46]. To overcome these challenges, new formulations of curcumin have been designed to improve its efficacy [47]. In this review, we aim to provide an updated literature review on the in vitro and in vivo effects of curcumin on glioma (Fig. 2). We also scrutinized the use of curcumin in combination therapies against glioma, its effectiveness on resistant cells, and new formulations of curcumin in glioma.
Molecular target of curcumin in glioma. microRNA)miR(; cyclin-dependent kinase (CDK); vascular endothelial growth factor (VEGF); interleukin)IL (;Tumor necrosis factor alpha (TNF-α); matrix metalloproteinase-9 (MMP-9); Cyclooxygenase-2 (COX-2); poly (ADP-ribose) polymerase (PARP); signal transducers and activators of transcription (STAT); forkhead box protein O1 (FOXO1); inhibitor of growth (ING); extracellular signal-regulated kinases (ERK); prostate apoptosis response-4 (Par-4); mitogen-activated protein kinases (MAPK); angiopoietin-2 (Ang-2); thrombospondin 1 (TSP-1)
Molecular signaling pathways regulated by curcumin to induce glioma inhibition, apoptosis, and cell cycle arrest. Signal transducers and activators of transcription (STAT); mechanistic target of rapamycin complex 1 (mTORC1); cyclin-dependent kinase (CDK); Myeloid cell leukemia-1 (MCL-1); pyruvate dehydrogenase kinase 1 (PDK1)
In Vitro Effects of Curcumin on Glioma Cells
Inhibition of Growth, Migration, and Invasion
There is strong evidence that supports the potential benefits of curcumin in brain disorders including gliomas (Table 1) [48,49,50]. Curcumin has been shown to suppress the expression of neural precursor cell expressed developmentally downregulated protein 4 (NEDD4), an E3 ubiquitin ligase promoting PTEN degradation and subsequent activation of PI3K/AKT pathway in A1207 and SNB19 glioma cell lines [2]. PTEN/PI3K/AKT is considered to be an important pathway for regulating the signaling of various biological processes including metabolism, apoptosis, proliferation, and cell growth [51]. S-phase kinase-associated protein 2 (Skp2) is another major oncoprotein belonging to the ubiquitin–proteasome system which affected by curcumin in glioma cells [52]. It has been shown that curcumin mediates Skp2 downregulation and subsequent upregulation of its ubiquitination targets such as p57 which causes inhibited cell growth, migration, and invasion of U251 cells and SNB19 glioma cells [53]. Another mechanism for the anti-migration and anti-invasive effects of curcumin has been suggested that it inhibits fascin expression by inhibiting STAT3 phosphorylation in the U87 cell line [54]. Because fascin is an overexpressed actin-binding protein in the nervous system, its suppression in glioma cells alters the cell shape and reduces the formation of filopodia [55].
Downregulation of CD147, matrix metalloproteinase (MMP)-2/9, cyclin D1/CDK4/6, and BCL-2/BCL-XL due to inhibition of MAPK/extracellular signal-regulated kinases (ERK) pathway has been suggested as the mechanism of these antitumor effects of curcumin [56,57,58]. The MAPK axis is a critical pathway for human tumor cell survival, proliferation, differentiation, senescence, metastasis, and resistance to chemotherapy. The MAPK/ERK pathway is a convergent signaling node which has input from various internal and external stimuli. MAPK/ERK signaling has been specified to contribute in the motility and invasion of glioma cells [59].
Curcumin has been reported to downregulate JAK1,2/STAT3 signaling and downstream targets including c-Myc, MMP-9, Snail, Twist, and Ki67 proliferation marker, dose-dependently in glioma cell lines [60, 61]. Since JAK/STAT pathway is the cornerstone of cancer cell growth and invasion, curcumin therapy may be an impressive therapeutic approach for gliomas [62].
Induction of Cell Cycle Arrest and Apoptosis
Curcumin stimulates glioma cells to become more stem-like by inhibiting the transition from G1 to S phase and increasing the expression of transcription factors necessary to maintain the pluripotency of embryonic stem cells including SRY-related HMG box transcription factor 4 (SOX) 4, Sox2, and Oct4 [63]. In glioma-initiating cells, Oct4 is related to Sox4 and the Oct4-Sox4 complexes induce the enhancer effects of the SOX2 gene [64]. Curcumin has been shown to produce cell cycle arrest and enhance apoptosis by and mitigate exogenous norepinephrine-induced proliferation, migration, and G1 to S phase transition in LN229 and U87 malignant glioma cells, in a dose-dependent manner [56].
Curcumin-mediated pro-apoptotic mechanisms in glioma cells may be related to the induction of Bcl-2-associated X protein (BAX), caspase 3 gene, and poly ADP ribose polymerase (PARP) [65,66,67,68]. Curcumin stimulates caspase-dependent apoptosis in human glioma cells [69], and treatment with curcumin increases the expression pro-apoptotic proteins, including caspase-3, -7, -8, and -9, which initiate and develop the apoptotic process [69, 70]. Curcumin also exerts diverse pro-apoptotic effects. DNA fragmentation, cleavage of PARP-1 nuclear protein in glioma cells, as well as mitochondrial membrane potential loss and ROS generations are induced through curcumin treatment [71]. This indicates that curcumin induces the apoptotic pathways. Simultaneously, curcumin also exerts an anti-glioma effect via inhibiting anti-apoptotic signaling, as showed via an elevation in BAX:BCL2 ratio in various human glioma cells [69].
In curcumin-treated U138MG and C6 cells, downregulation of PI3K/AKT and NF-κB pathways, as well as NF-κB-regulated anti-apoptotic protein BCL-XL, have been reported as a prelude to apoptosis [72]. Another study identified overexpression of forkhead box protein O1 (FoxO1) and its potential target genes including cyclin G2, cleaved caspase-3, Fas ligand (FasL) as an underlying mechanism of curcumin-mediated G2/M cell cycle arrest and apoptosis in U87 cells [73]. Similarly, exposure of U251 cells with doses of curcumin greater than 10 µmol/L induced the same anti-tumor effects, which was related to the reduced p-Akt and increased PTEN protein expression [74].
Su et al.have reported that there are two main signaling pathways promoting apoptosis that are affected by curcumin in glioma cells: the p53 pathway by increasing p53 and p21 and repressing cdc2, and the retinoblastoma (RB) pathway via increasing CDKN2A/p16 and lowering phosphorylated RB [65].
Furthermore, curcumin stimulates p21 transcription independent of p53 by activating ERK and JNK MAPK pathways in U-87MG cells. As a result, phosphorylated Elk-1 increases the expression of early growth response-1 (Egr-1), a transcriptional activator that binds to the p21 promoter and further regulates cell cycle arrest, differentiation, and apoptosis [75]. In a p53-dependent manner, curcumin also could cause cell cycle arrest in the S and G2/M phase correlated with increased expression of a critical tumor suppressor belonging to the ING (inhibitor of growth) family, ING4 [76]. Previous studies have reported that ING4 triggers tumor apoptosis and cell cycle arrest while inhibiting cancer growth and angiogenesis in various malignant tumors, such as glioma [77, 78]. Moreover, curcumin was found to sensitize U251MG and U87MG glioma cells to tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-stimulated cell death via both receptor-mediated and chemical induction (corresponding to the extrinsic and intrinsic pathways of apoptosis, respectively), suggesting the potential application of curcumin in TRAIL-mediated immunotherapy of glioma cells [79, 80]. Zhou et al. have announced that curcumin administration downregulated the proliferating cell nuclear antigen (PCNA) in U251 cells and NADPH oxidase 4 (NOX4) in U87 cells, indicating a decrease in proliferation and reactive oxygen species (ROS) production as well as inducing the differential regulation of apoptosis-associated genes, respectively [66].
In contrast there is some evidence that higher doses of curcumin promoted ROS production and resulted in greater DNA damage and cell death in glioma U87 and rat glioma (C-6) cells [71, 81, 82]. In a ROS-dependent mechanism, low curcumin doses appreciably decreased proliferation, sphere-and colony-forming potential, and viability of glioma stem cells (GSCs). It is claimed that ROS induces anti-neoplastic impacts on GSCs by promoting the MAPK pathway and inhibiting the STAT3 activity and inhibitor of apoptosis (IAP) [83]. Moreover, loss of mitochondrial membrane potential following curcumin treatment has been reported as another factor in enhancing the apoptotic cascade activity of human glioma CHME cells. Curcumin’s pro-oxidant activity also led to a decrease in total antioxidant capacity and glutathione (GSH) content along with an increment in malondialdehyde content and superoxide dismutase activity in U87 cells [82]. Exposure of the glioma cell line A172 to curcumin leads to morphological alterations characteristic of paraptosis cell-death through affecting the integrity of the endoplasmic reticulum [84].
Autophagy-Induced Cell Death
Autophagic cell death is one of the main mechanism in the promotion of antineoplastic effects mediated by curcumin in U87MG and U118MG cells [85]. Curcumin’s pro-autophagic effect occurs through the production of ROS, resulting in upregulation of two distinct tumor suppressors, prostate apoptosis response-4 (Par-4) and ceramide [85]. As shown in A172 and U87 cells, curcumin-induced cell death was oppositely related to the baseline level of autophagic flux of these cells. In addition, autophagy induction due to serum starvation significantly alleviated the rate of cell death mediated by curcumin [86]. Curcumin-stimulated autophagic cell death may be correlated with suppression of the Akt/mammalian target of rapamycin (mTOR)/p70 ribosomal protein S6 kinase (p70S6K) pathway and activation of the ERK1/2 signaling [87]. These two major pathways contribute to the regulation of nutrient starvation-induced autophagy and oncogenesis in different cancer cell types [88]. Moreover, increased autophagy in glioma-initiating cells (GICs) following curcumin treatment initiates the differentiation cascade [89]. This evidence is consistent with the findings of a reduction in the side population (SP) of C6 cells, a rare population of stem cells, following curcumin treatment of these cells [90, 91]. Furthermore, curcumin could exert its antitumor effects on U251 cells by downregulating enolase 1 (ENO1) and hypoxia-inducible factor 1-alpha (HIF-1α) and subsequently inhibiting glycolytic processes [92]. It has been shown that the glycolytic enzyme ENO1 and its transcription activator, HIF-1α, are strongly expressed in various types of malignancies like glioma, and are directly related to tumor aggressiveness [93,94,95].
Anti-Inflammation and Anti-Angiogenesis
The anti-neuroglioma effects of curcumin on U87 cells are also mediated by suppressing the inflammatory HSP60/TLR4 signaling and its downstream proteins, such as myeloid differentiation primary response 88 (MYD88), NF-κB, inducible nitric oxide synthase (iNOS), and cytokines IL-6 and IL-1β [96]. While, the expression of apoptosis-dependent factors including TNF-α, caspase-3, and p53 was enhanced in U87 cells following the treatment [96]. Further, curcumin was shown to inhibit hepatoma-derived growth factor (HDGF), an angiogenesis-inducing growth factor overexpressed in U251 and LN229 cell lines. Since the HDGF constructs a complex with β-catenin from the Wnt pathway, its downregulation suppresses epithelial-mesenchymal transition (EMT) signals and thereby reduces the aggressiveness of human glioma cells [97].
Another possible mechanism underlying the curcumin-mediated attenuation of glioma cell invasion has been suggested to be the downregulation of atypical cadherin FAT1 and its transcriptional regulator, NF-κB [98]. Increased expression of the FAT1 gene has been specified in various cancers, including gliomas, which leads to upregulation of proinflammatory, stemness, and EMT markers [99]. Evaluation of curcumin’s deterrent effects on invasion and migration of glioma U87 cells by 3D spherical invasion assay has shown that this effect is gradual and begins at concentrations much lower than IC50 [100]. Furthermore, curcumin has been identified as a potential inhibitor of the sonic hedgehog (Shh) pathway by downregulating Shh, Smo, and glioma-associated oncogene homolog 1 (GLI1). Subsequently, the reduced expression of GLI1 target genes including CyclinD1, Bcl-2, and Foxm1 can suppress cell proliferation and migration while promoting apoptosis through the internal mitochondrial pathway in U87 and T98G cells [101]. The Shh is a well-known pathway for embryonic development, organogenesis, regeneration, and homeostasis, often associated with glioma tumorigenesis [102].
Modulation of microRNAs
Moreover curcumin reduces the carcinogenicity of glioma cells through modulating microRNAs (miRNAs) [103]. miRNAs are a group of small, non-coding, and single-stranded RNAs that post-transcriptionally modulate gene expression to maintain the exact balance of different biological processes. miRNAs may behave like oncogenes or tumor suppressors and play crucial roles in the formation and propagation of human malignancies (Fig. 3). miRNA-21 is a well-known oncogene correlated with the migratory and survival abilities of human glioma cells. It has been demonstrated that curcumin affects several distinct glioma tumor processes, including: proliferation, cell death, metastasis and chemoresistance through targeting miR-21. miR-21 mediates various effects of curcumin on multiple signaling pathways associate to such as PTEN, PI3K/AKT, PDCD4 and NF-κB [104]. Curcumin treatment of U251 cells reduced the miR-21 level and anti-apoptotic proteins expression while enhancing the pro-apoptotic proteins and microtubule-associated protein light chain 3-II expression [105]. miR-378 enhances the effects of curcumin on the U87 cell line by activating the P38 MAPK pathway [106].
miR-326 has been shown to be a tumor suppressor miR in several cancer types, and appears to mediate curcumin-induced cytotoxicity and apoptosis as well as inhibit proliferation and dissemination of glioma cells [107]. Curcumin induced properties of paraptosis in A172 cell line and causes over-expression of endoplasmic reticulum stress response genes IRE1 and ATF6 as well as altered levels of ER-associated miRs such as miR-27a, miR-222, and miR-449. These miRs regulated by curcumin interact with and involve the AKT-Insulin and p53-Bcl2 cascades [84]. This evidence suggest that miRNAs may be potential therapeutic target in glioma therapy (Fig. 3).
Therapeutic Effects of Curcumin on Glioma Cancer Cells in Animal Models
There is some in vivo evidence supporting the chemotherapeutic potency of curcumin against glioma (Table 2). Wang et al. have recently reported that the curcumin effectively alleviated growth and invasion of adverse psychological stress-induced glioma transplanted tumor in nude mice, which was associated with decreased serum levels of norepinephrine and epinephrine. The antitumor effects of curcumin were associated with inhibition of the MAPK/ERK signaling, which downregulated the MMP-2/9 and CD147 in tumor tissue [56]. Previously, increased growth and invasion of various tumors due to stimulation of the sympathetic nervous system and subsequently increased production of catecholamines have been reported [108, 109]. Administration of 0.01 ml/g of curcumin to a U87-derived xenograft glioma model repressed tumor growth and angiogenesis by 48% by downregulation of vascular endothelial growth factor (VEGF) and angiopoietin-2 (Ang-2) and upregulation of thrombospondin 1 (TSP-1) [110]. Also, the anti-angiogenic effects of curcumin in U87-derived xenografts have been demonstrated by reducing the gelatinolytic activity of MMP9, hemoglobin content of the tumor, and expression of endothelial cell markers assigned to newly formed vessels including CD105 and CD31 [45].
Intraperitoneal (IP) administration of curcumin into the nude mice model bearing a U87-implanted tumor, resulted in diminished tumor size and GLI1 expression and extended the survival time through inhibition of the Shh pathway [101]. In parallel with in vitro studies, there is in vivo evidence on curcumin-induced autophagy that resulted in decreased self-renewal and clonogenic potentials and yet augmented the transcription of neural differentiation markers in intracranial GICs implanted into mice [87, 89]. IP injection of 50 mg/kg/day curcumin to C6-implanted rats also caused a significant decrement in tumor volume of approximately 80% of the animals studied [72]. However, curcumin has been found to act as a histone acetyltransferase inhibitor in adult neuronal progenitor cells (NPCs) isolated from ICR mice. Given that histone hypoacetylation is crucial in determining stem cell fate, curcumin effectively suppressed glial differentiation while promoting neural differentiation [68]. Curcumin also suppressed the expression of phosphorylated JNK in lipopolysaccharide (LPS)-stimulated C6 orthotropic xenografts, thereby reducing CCL2 production [111]. Chemokine CCL2 has a decisive role in glioma progression by inducing mild leukocyte infiltration, BBB dysfunction, and upregulation of proinflammatory cytokines [112].
Potential of Novel Formulations of Curcumin in Glioma
Despite the reported anti-cancer properties, the clinical applications of curcumin have been limited because of its low water solubility and oral bioavailability. It is mainly due to low absorption besides rapid metabolism and excretion [113]. In addition, the capability to cross the BBB and achieve the desired concentration to inhibit or kill glioma tumor cells is another important challenge. The development of new formulations of curcumin, such as curcumin derivatives and nanoparticle delivery systems (NDDS), has somewhat alleviated these problems [114].
Curcumin Analogues
Curcumin and its analogs, bisdemethoxycurcumin (BDMC), demethoxycurcumin (DMC), and dimethoxycurcumin (DIMC) have all been shown to promote cell cycle arrest, apoptosis, and ROS generation in LN229 and GBM8401 glioma cells. However, these effects were observed more strongly and at a lower dose of DIMC than for the other analogs. DIMC was reported to decrease p-mTOR, p-CDC2, and BCL-2, dose-dependently, whereas increasing p-AKT, p-ERK, and autophagy markers LC3B-II and p62 in these glioma cells [115]. JNK, a major member of MAPKs, may be activated during apoptosis of glioma cells triggered via different agents. DMC-BH has been demonstrated to be superior to DMC in inhibiting the proliferation of U87 and SHG44 GSCs and inducing autophagy and apoptosis in them by promoting the JNK/ERK signaling. Also, DMC-BH reduced the growth of the GSCs derived-intracranial orthotopic tumor xenografts [116]. Through conjugation of triphenylphosphine (TPP) moiety with the phenolic hydroxyl group of DMC, Shi et al. designed a selective mitochondrial targeting compound with strong cytotoxicity in U251 cells and human glioma mouse model. DMC-TPP impressively repressed cellular thioredoxin reductase (TrxR) and yet induced mitochondrial-associated apoptosis by caspase activation, ROS generation, and mitochondrial membrane potential depletion [117]. It has been shown that miR-145 is a tumor-suppressor for gliomas by suppression of glioma cell proliferation, adhesion, and invasion as well as inducing apoptosis by targeting Notch and Sox9 signaling cascade. Notably, miR-145 promoted the chemosensitivity of glioma stem cells to DMC through targeting the SOX2-Wnt/β-catenin pathway [118]. Curcumin encapsulated in a nanocarrier, called dendrosomal curcumin, decreases the proliferation of U87 cells by the down-expression of OCT4 variants and SOX-2 in a miR-145-dependent way [119].
Hydrazinobenzoylcurcumin (HBC) has been found to be a Ca2+/Calmodulin (CaM) antagonist in U87MG and U373MG GSCs which downregulates CaM/CaM-dependent protein kinase II (CaMKII)/c-Met, thereby suppressing the stemness features of glioma cells [120]. Evidence suggests that CaMKII not only plays a vital role in the maintenance of cancer stem cells, but also in regulating the survival, proliferation, and migration of tumor cells [121, 122]. Curcumin inspired bis-chalcones robustly upregulated CCAAT-enhancer-binding protein homologous protein (CHOP), p-jun, and caspase 12 in the GSC line, which resulted in cell death via stimulation of endoplasmic reticulum stress as well as unfolded protein response (UPR) [123].
It has been claimed that the supercritical and hydroethanolic extract of turmeric rhizomes, called Turmeric Force™, is more cytotoxicity against tumor cell lines compared to turmeric [124]. Moreover, Curcuma amada supercritical extract suppresses AKT path, so inducing apoptosis via elevating BAX, BCL-X, BCL-2, BNIP3, mutant p53, caspase-3, and p21 proteins in human glioma cells. In addition, supercritical extract down-regulate the transcription levels of genes related with cell proliferation (i.e. Ki67) and angiogenesis (i.e. VEGF) as well as regulates HSP90 and AMPKα genes [125].
Curcumin Nanoparticles
Compared to free curcumin, curcumin-monomethoxy polyethylene glycol (MPEG)-polylactic acid (PLA) nanoparticles significantly inhibited the growth of GL261 cells and accelerated apoptosis, and folic acid (FA)-modified Cur/PEG-PLA micelles further augmented these inhibitory effects. In both subcutaneous and intracranial glioma models, Cur/Fa-PEG-PLA also represented the best therapeutic effect via inhibiting angiogenesis and promoting apoptosis [126]. One recent study has shown curcumin-loaded zein nanoparticles coated with polydopamine (pD) and functionalized with dodecamer peptide (G23), which specifically increases transcytosis in an in vitro BBB model as well as inhibited proliferation and migration, and increased ROS-induced apoptosis in C6 glioma cells. CUR-ZpD-G23 nanoparticles were also able to circulate after intravenous injection into zebrafish [127]. Moreover, encapsulation of curcumin nanopolymersomes in a thermo-sensitive and biodegradable composite hydrogel improved the sustained release of curcumin after intratumoral injection in ectopic C6 glioma tumor models [128]. GSH-sensitive biodegradable micelles constructed with polycaprolactone (PCL), polyethylenimine (PEI), and PEG, and conjugated with a cell-penetrating peptide (tLyp-1) could mediate simultaneous delivery of apoptosis-inducing ligand (pUNO1-hTRAILa) and curcumin to glioma cells. Hence, this nanocomplex exhibited much stronger anti-tumor effects on C6 cells and rats bearing in situ glioma [129].
Other Formulations
A novel drug delivery system has been developed in the form of a biodegradable soft scaffold using extrusion-based 3D printing technology for localized curcumin administration following resection surgery. This system sustainably released curcumin and exerted remarkable cytotoxic effects against the U87 human cell line [130]. Curcumin-based fluorescent probes are a promising approach for detecting glioblastoma cells during neurosurgical operations. These probes can only link to aldehyde dehydrogenase 1A3 (ALDH1A3), an enzyme upregulated in GSCs and related to their stemness and aggressiveness. Indeed, fluorescent signal was absent in cells without ALDH1A3 [131]. Compared to curcumin, solid lipid curcumin particles (SLCPs) significantly enhanced both apoptotic and autophagic cell death while inhibiting mitophagy markers and the PI3K/AKT/mTOR signaling [132, 133].
Curcumin Effects on the Resistant Glioma Cells
Curcumin can sensitize glioma cells to radiotherapy and various chemotherapeutic regimens such as cisplatin, etoposide, and doxorubicin through down-regulation of Bcl-2 and members of the IAP family as well as DNA repair enzymes such as MGMT, DNA-PK, and ERCC-1[134].
Temozolomide (TMZ), 3-methyl isolate of mitozolomide, is an orally active DNA alkylating prodrug, is the frontline standard treatment for glioma [135]. TMZ forms genomic O-6-methylguanine adducts and leads DNA single and double-strand breaks and cell cycle at G2/M phase, consequently cell apoptosis and autophagy. TMZ freely crosses the BBB; so, it is one of the most important cytotoxic agents for glioma treatment [136]. Although, at least 50% of treated patients develop TMZ-chemoresistance [137]. The emergence of chemotherapy recurrence and following disease relapse remains a major therapeutic challenge in these patients [138].
Recently, Huang et al. demonstrated the high expression of connexin 43 (Cx43) more than 2-times in TMZ-resistant glioblastoma cells than parental glioblastoma cells [139]. The gap junction channels and hemichannels protein, Cx43, have complicated roles in gliomagenesis, invasion, migration, and propagation. Connexins involve in the EMT of malignant glioma and Cx43 particularly is implicated in chemotherapeutic resistance to glioma cells [140]. Curcumin (10 μM) significantly decreased Cx43 protein amplification by nearly 40%. Moreover, curcumin promotes TMZ-activated apoptosis from 4 to 8%. Curcumin plus translation inhibitor cycloheximide synergically triggers Cx43 degradation. But, autophagy inhibitor 3-methyl adenine administration did not alter the effect of curcumin on Cx43 degradation. Notably, the combination of the proteasome inhibitor MG132 considerably neutralized the curcumin-mediated Cx43 degradation, which indicates that curcumin may enhance Cx43 degradation via the ubiquitin–proteasome proteolytic path [139].
Recently, androgen receptor (AR), has been reported to be an inducer of resistance to TMZ therapy. ALZ003, a novel curcumin analog, triggered ER stress, activated both extrinsic and intrinsic apoptotic pathways as well as ferroptosis in malignant glioma cells by down-expression of AR which regulates glutathione peroxidase 4 (GPX4)-mediated redox hemostasis. Interestingly, ALZ003 induces F-box and leucine-rich repeat protein 2 (Fbxl2)-mediated AR ubiquitination, mitigating AR amplification in glioma cells and negating the TMZ-resistance [141].
Combination Therapy with Curcumin
In anticancer drug development, combination therapy is one strategy to improve therapeutic success via the synergistic effects of each agent (Fig. 4).
Chemotherapy
Concerning the efficacy of curcumin in different experimental models, a series of investigations have been performed in order to assess the potency of combining curcumin with chemotherapeutic agents in models of human glioma. For instance, nanomicelle-curcumin (50 μM) plus erlotinib (50 μM) remarkably reduced the translational levels of angiogenesis and Wnt pathway-related genes than mono-treatments in U87 cells [142]. Combined diferuloylmethane (extracted from Curcuma longa Linn), and TMZ administration significantly inhibited U87 cell proliferation as well as stimulated apoptotic death versus each alone through over-expression of tumor suppressor miR-146a and blocking of NF-κB cascade [143]. Bagherian and colleagues reported that curcuminoids, nano-micelle curcumin, and TMZ all used as mono-treatment have anti-tumor activities on U87 cells. Concerning co-treatment, the effects of these agents were synergically enhanced through modulating various glioma-associated pathways, including the Wnt axis, apoptosis, and autophagy-associated genes and proteins. All treatment schedules down-regulate the levels of Wnt pathway-related genes, such as β-catenin, cyclin D1, Twist, and ZEB1 in glioma cell lines [144]. In another study, curcumin promoted the sensitizing to TMZ treatment in U87 cells by induction of apoptosis. In vitro and in vivo evidence indicated that higher generation of ROS and suppression of AKT/mTOR pathway may be involved in the increased apoptosis due to the curcumin/TMZ co-treatments [145]. Furthermore, by using a magnetic nanoparticles-based dual drug delivery system, it has been shown that curcumin plus TMZ has higher anti-tumor efficiency by apoptosis cell death induction [146]. However, Zanotto-Filho et al. reported that TMZ and curcumin synergism is doubtful to be successful. Whereas curcumin hinders the STAT3, NFκB, and PI3K/AKT axes to affect the survival of glioma cell lines, TMZ-induced autophagy is dependent on the DNA damage response and repair pathways. Although, both TMZ and curcumin needed ERK1/2 to activate autophagy. Inhibition of this ERK1/2-mediated TMZ and curcumin stimulated autophagy with resveratrol, a BBB permeable compound, enhanced TMZ/curcumin potency in brain-implanted tumors. Altogether, this finding indicates that autophagy disturbs the therapeutic potential of TMZ/curcumin, and blocking this phenomenon could be a novel therapeutic option to upgrade glioma therapy [147]. Co-delivery of the hydrophobic magnetic nanoparticles and a combination of paclitaxel and curcumin significantly inhibited cell proliferation triggered apoptosis, and mitochondrion damage as well as G2/M cell cycle arrest synergistically compared to each drug alone. Dual-targeting correspondence to more than 10-time enhancement in cellular uptake investigations, and over 5-times elevation in brain delivery than the non-targeting nanoparticles. Additionally, all mice having orthotopic glioma survived, versus 62.5% survival rate for the combination treatment with native drugs. It seems that the dual-targeting and drug co-loading approach provides novel opportunities for optimizing brain drug delivery and glioma management [148].
Transferrin is a hydrophilic transporter of iron ions in the blood, which can enter cells via its specific receptor-mediated endocytosis. Accumulating evidence demonstrated that the transferrin receptor is highly amplified in tumor tissues versus their healthy adjacent tissues because cancer cells are in an over-proliferative state. It has been shown that anti-Transferrin receptor monoclonal antibody and curcumin lonely could suppress proliferation of A172 and U87-MG cell lines via cell cycle arrest at the S and G2/M phases, respectively. Anti-Transferrin receptor monoclonal antibody induced apoptosis in tumor cells; though curcumin induce necrosis. Interestingly, combination therapy synergistically affects glioma cell growth inhibition and the activation of cells necrosis [149].
Methotrexate (MTX) is a folate analogue that is widely used as a prototype anticancer drug. In a recent report, curcumin-loaded poly[D,L-lactide-co-glycolide] (PLGA) nanoparticles plus MTX-loaded PLGA nanoparticles had an additive effect on cell apoptosis compared to single drug nanoparticles. Combination therapy alters the form of cell death from necrosis to apoptosis. Moreover, maximum levels of LDH activity are found when the U87MG cells are exposed to curcumin/MTX-co-loaded PLGA nanoparticles [150]. Nimustine hydrochloride (ACNU), is a nitrosourea compound, that inhibits cell replication, colony formation, dissemination, and invasion of glioma cells. Combination therapy with curcumin elevated ACNU-activated apoptosis via increasing cytochrome c release from mitochondria. Moreover, curcumin plus ACNU present synergistic anti-tumor activity through concurrent targeting N-cadherin/MMP2/9, cytochrome c/caspase, PI3K/AKT, as well as NF-κB/cyclooxygenase (COX)-2 pathways [151].
Radiotherapy
After the operation for surgical resection, radiotherapy is one of the frequently used adjuvant therapeutic strategies. Although, it has not been applied extensively under evaluation of combination therapy for glioma. The radios-ensitization potential of curcumin in vitro has been investigated in a few studies. Back et al. reported that combinatorial effects of curcumin (25 µM) and radiotherapy (5 Gy) were more effectively induce cell death through down-regulation of anti-apoptotic gene expression versus curcumin and radiation alone in U87 and T98 cells. The cell death effect of curcumin was p53- and caspase-independent, and therefore maybe involve a non-classical apoptotic way [134]. In another study, curcumin administration along with photodynamic therapy significantly decreased the viability of human glioma DKMG cell line dose-dependently [152]. Recently, it has been shown that treatment with curcumin and irradiation (2/4 Gy) significantly increased cell cytotoxicity by more than 94% in U87 and T98 cell lines in a dose-dependent manner. This combination provokes cell arrest at the G2/M phase and apoptosis synergistically [153]. The precise underlying mechanisms behind the photosensitizing activity of curcumin are unknown; although, it is well-established that compounds that induce G2/M arrest are powerful radiosensitizers.
In vivo and in vitro evidence also suggests that curcumin combined with radiation considerably promoted the anti-tumor effect compared to radiotherapy alone against both U87 cell line and U87 xenografted nude mice. Curcumin pretreatment inhibited radiation-induced ERK/JNK phosphorylation and triggered radiation-associated tumor cell apoptosis and G2/M arrest in U87 cells and subcutaneous xenografts. This effect is associated with the pro-apoptotic function of curcumin through induction of the dual-specificity phosphates (DUSP)-2 cascade [154, 155]. DUSPs as phosphatase enzymes are able to catalyze the dephosphorylation of both tyrosine and serine/threonine residues and have a crucial role in the inactivation of MAPK paths which are implicated in different human malignancies such as glioma [156]. Wang and co-workers developed a rat model with triple-reporter F98/FGT glioma to examine the effectiveness of curcumin addition on radiotherapy for glioma. In vitro experiments showed that curcumin induces G2/M cell cycle arrest and promotes radiation sensitivity of F98 glioblastoma cells. In vivo model also supported that curcumin as radiosensitizer synergistically boosts the anti-tumor effects of irradiation on transplanted glioma cells and in situ brain tumors, and considerably lengthen the overall survival compared to curcumin or radiation treatment solely [157]. However, in U251 glioma cells, curcumin pretreatment (5 μM for 72 h) for a single dose (1 to 6 Gy) or fractionated (5 × 2 Gy) radiation did not support the radiosensitizer effect of curcumin [158].
Conclusion and Future Prospective
There are several lines of evidence that support the potential of phytochemicals like curcumin in the treatment of different human cancers, that include brain tumors, and in particular glioma. Curcumin exerts its anticancer effects against glioma by several mechanisms: interfering with the cellular interactions necessary for the metastasis and recurrence of glioma cells, increasing proapoptotic proteins, or inducing or suppressing the production of different biomolecules including cytokines, transcription factors, enzymes, protein kinases, and growth factors such as MAPK, NF-κB, COX-2, IL-6, IL-8, FOXO1, STAT3, MMP-9, and TNF-α. MiRNAs are one of the potential therapeutic targets because of their involvement in the progression of gliomas. Curcumin could exert its therapeutic potentials through modulating miRNAs such as miR-21, miR-326 and miR-378 contributed in cellular and molecular signaling pathways of brain tumors. Furthermore, curcumin could affect miRs involved in the response to chemotherapy.
Curcumin has the potential to modulate different core signaling pathways which are aberrantly disrupted in glioma. Although, among these signaling axes, a more emphasis on PI3K/AKT, JAK1,2/STAT3, and MAPK/ERK pathways as well as over-expression of apoptotic pathways like p21, p53, and executor caspase 3 could be an area of interest in future pre-clinical studies, because the available evidence is still restricted.
In addition, the use of curcumin as adjuvants or as a chemotherapeutic agent to address major molecular and cellular glioma targets would be worthwhile since curcuminoids have the ability to resensitize chemo- and radio-resistant tumor cells through down-regulation of Bcl-2, members of the IAP family, DNA repair enzymes, Cx43 and AR. Moreover, greater attention should also be focused on designing a multimodal anticancer therapies approach by using the curcumin derivatives, analogues, and nano-formulations in future studies, in combination with the standard chemotherapy regimens; so this aspect of combinatorial treatment can synergically enhance the efficacy of current therapeutic agents and may provide a promising in glioma therapy.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Weller M, Wick W, Aldape K, Brada M, Berger M, Pfister SM, Nishikawa R, Rosenthal M, Wen PY, Stupp R (2015) Glioma. Nat Rev Dis Prim 1:1–18
Wang X, Deng J, Yuan J, Tang X, Wang Y, Chen H, Liu Y, Zhou L (2017) Curcumin exerts its tumor suppressive function via inhibition of NEDD4 oncoprotein in glioma cancer cells. Int J Oncol 51:467–477
Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR (2019) Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol 15:405–417
Chen R, Smith-Cohn M, Cohen AL, Colman H (2017) Glioma subclassifications and their clinical significance. Neurotherapeutics 14:284–297
Bush NAO, Chang SM, Berger MS (2017) Current and future strategies for treatment of glioma. Neurosurg Rev 40:1–14
Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Alifieris C, Trafalis DT (2015) Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther 152:63–82
Jovčevska I, Kočevar N, Komel R (2013) Glioma and glioblastoma: how much do we (not) know? Mol Clin Oncol 1:935–941
Pangal DJ, Baertsch H, Kellman EM, Cardinal T, Brunswick A, Rutkowski M, Strickland B, Chow F, Attenello F, Zada G (2021) Complementary and alternative medicine for the treatment of gliomas: scoping review of clinical studies, patient outcomes, and toxicity profiles. World Neurosurg. https://doi.org/10.1016/j.wneu.2021.04.096
Kundu M, Das S, Dhara D, Mandal M (2019) Prospect of natural products in glioma: a novel avenue in glioma management. Phytother Res 33:2571–2584
Slika L, Patra D (2020) Traditional uses, therapeutic effects and recent advances of curcumin: a mini-review. Mini Rev Med Chem 20:1072–1082
Hamzehzadeh L, Atkin SL, Majeed M, Butler AE, Sahebkar A (2018) The versatile role of curcumin in cancer prevention and treatment: a focus on PI3K/AKT pathway. J Cell Physiol 233:6530–6537
Mirzaei H, Naseri G, Rezaee R, Mohammadi M, Banikazemi Z, Mirzaei HR, Salehi H, Peyvandi M, Pawelek JM, Sahebkar A (2016) Curcumin: a new candidate for melanoma therapy? Int J Cancer 139:1683–1695
Bahrami A, A. Ferns G, (2021) Effect of curcumin and its derivates on gastric cancer: molecular mechanisms. Nutr Cancer 73:1553–1569
Ghandadi M, Sahebkar A (2017) Curcumin: an effective inhibitor of interleukin-6. Curr Pharm Des 23:921–931
Karimian MS, Pirro M, Majeed M, Sahebkar A (2017) Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev 33:55–63
Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M, Sahebkar A (2015) Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clin Nutr 34:1101–1108
Sahebkar A, Cicero AF, Simental-Mendía LE, Aggarwal BB, Gupta SC (2016) Curcumin downregulates human tumor necrosis factor-α levels: a systematic review and meta-analysis ofrandomized controlled trials. Pharmacol Res 107:234–242
Sahebkar A, Serban M-C, Ursoniu S, Banach M (2015) Effect of curcuminoids on oxidative stress: a systematic review and meta-analysis of randomized controlled trials. J Funct Foods 18:898–909
Shakeri A, Ward N, Panahi Y, Sahebkar A (2019) Anti-angiogenic activity of curcumin in cancer therapy: a narrative review. Curr Vasc Pharmacol 17:262–269
Ghosh S, Banerjee S, Sil PC (2015) The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol 83:111–124
Hu S, Maiti P, Ma Q, Zuo X, Jones MR, Cole GM, Frautschy SA (2015) Clinical development of curcumin in neurodegenerative disease. Expert Rev Neurother 15:629–637
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A (2017) Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res 67:244–251
Amel Zabihi N, Pirro M, JohnstonSahebkar PTA (2017) Is there a role for curcumin supplementation in the treatment of non-alcoholic fatty liver disease? the data suggest yes. Curr Pharm Des 23:969–982
Saeidinia A, Keihanian F, Butler AE, Bagheri RK, Atkin SL, Sahebkar A (2018) Curcumin in heart failure: a choice for complementary therapy? Pharmacol Res 131:112–119
Lelli D, Sahebkar A, Johnston TP, Pedone C (2017) Curcumin use in pulmonary diseases: state of the art and future perspectives. Pharmacol Res 115:133–148
Bavarsad K, Barreto GE, Hadjzadeh M-A-R, Sahebkar A (2019) Protective effects of curcumin against ischemia-reperfusion injury in the nervous system. Mol Neurobiol 56:1391–1404
Mokhtari-Zaer A, Marefati N, Atkin SL, Butler AE, Sahebkar A (2019) The protective role of curcumin in myocardial ischemia–reperfusion injury. J Cell Physiol 234:214–222
Ganjali S, Blesso CN, Banach M, Pirro M, Majeed M, Sahebkar A (2017) Effects of curcumin on HDL functionality. Pharmacol Res 119:208–218
Panahi Y, Khalili N, Hosseini MS, Abbasinazari M, Sahebkar A (2014) Lipid-modifying effects of adjunctive therapy with curcuminoids–piperine combination in patients with metabolic syndrome: results of a randomized controlled trial. Complement Ther Med 22:851–857
Panahi Y, Khalili N, Sahebi E, Namazi S, Simental-Mendía LE, Majeed M, Sahebkar A (2018) Effects of curcuminoids plus piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind placebo-controlled trial. Drug Res 68:403–409
Parsamanesh N, Moossavi M, Bahrami A, Butler AE, Sahebkar A (2018) Therapeutic potential of curcumin in diabetic complications. Pharmacol Res 136:181–193
Shakeri A, Sahebkar A (2016) Optimized curcumin formulations for the treatment of Alzheimer’s disease: a patent evaluation. J Neurosci Res 94:111–113
Bianconi V, Mannarino MR, Sahebkar A, Cosentino T, Pirro M (2018) Cholesterol-lowering nutraceuticals affecting vascular function and cardiovascular disease risk. Curr Cardiol Rep 20:1–20
Keihanian F, Saeidinia A, Bagheri RK, Johnston TP, Sahebkar A (2018) Curcumin, hemostasis, thrombosis, and coagulation. J Cell Physiol 233:4497–4511
Abdollahi E, Momtazi AA, Johnston TP, Sahebkar A (2018) Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: a nature-made jack-of-all-trades? J Cell Physiol 233:830–848
Kasi PD, Tamilselvam R, Skalicka-Woźniak K, Nabavi SF, Daglia M, Bishayee A, Pazoki-Toroudi H, Nabavi SM (2016) Molecular targets of curcumin for cancer therapy: an updated review. Tumor Biol 37:13017–13028
Wang M, Jiang S, Zhou L, Yu F, Ding H, Li P, Zhou M, Wang K (2019) Potential mechanisms of action of curcumin for cancer prevention: focus on cellular signaling pathways and miRNAs. Int J Biol Sci 15:1200–1214
Bahrami A, Majeed M, Sahebkar A (2019) Curcumin: a potent agent to reverse epithelial-to-mesenchymal transition. Cell Oncol 42:405–421
Allegra A, Innao V, Russo S, Gerace D, Alonci A, Musolino C (2017) Anticancer activity of curcumin and its analogues: preclinical and clinical studies. Cancer Invest 35:1–22
Marjaneh RM, Rahmani F, Hassanian SM, Rezaei N, Hashemzehi M, Bahrami A, Ariakia F, Fiuji H, Sahebkar A, Avan A (2018) Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. J Cell Physiol 233:6785–6798
Shabaninejad Z, Pourhanifeh MH, Movahedpour A, Mottaghi R, Nickdasti A, Mortezapour E, Shafiee A, Hajighadimi S, Moradizarmehri S, Sadeghian M, Mousavi SM, Mirzaei H (2020) Therapeutic potentials of curcumin in the treatment of glioblstoma. Eur J Med Chem 188:112040
Ahmed T, Gilani AH (2014) Therapeutic potential of turmeric in Alzheimer’s disease: curcumin or curcuminoids? Phytother Res 28:517–525
Pan J, Li H, Ma J-F, Tan Y-Y, Xiao Q, Ding J-Q, Chen S-D (2012) Curcumin inhibition of JNKs prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease through suppressing mitochondria dysfunction. Transl Neurodegener 1:1–9
Perry MC, Demeule M, Regina A, Moumdjian R, Beliveau R (2010) Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol Nutr Food Res 54:1192–1201
Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun D, Zhai G (2016) Oral bioavailability of curcumin: problems and advancements. J Drug Target 24:694–702
Ghasemi F, Bagheri H, Barreto GE, Read MI, Sahebkar A (2019) Effects of curcumin on microglial cells. Neurotox Res 36:12–26
Bhat A, Mahalakshmi AM, Ray B, Tuladhar S, Hediyal TA, Manthiannem E, Padamati J, Chandra R, Chidambaram SB, Sakharkar MK (2019) Benefits of curcumin in brain disorders. BioFactors 45:666–689
Shabaninejad Z, Pourhanifeh MH, Movahedpour A, Mottaghi R, Nickdasti A, Mortezapour E, Shafiee A, Hajighadimi S, Moradizarmehri S, Sadeghian M (2020) Therapeutic potentials of curcumin in the treatment of glioblstoma. Eur J Med Chem 188:112040
Eghbaliferiz S, Farhadi F, Barreto GE, Majeed M, Sahebkar A (2020) Effects of curcumin on neurological diseases: focus on astrocytes. Pharmacol Rep 72:769–782
Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF (2008) The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets 8:187–198
Wu J, Su H-k, Yu Z-h, Xi S-y, Guo C-c, Hu Z-y, Qu Y, Cai H-p, Zhao Y-y, Zhao H-f (2020) Skp2 modulates proliferation, senescence and tumorigenesis of glioma. Cancer Cell Int 20:1–11
Wang L, Ye X, Cai X, Su J, Ma R, Yin X, Zhou X, Li H, Wang Z (2015) Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget 6:18027
Park K-S, Yoon S-Y, Park S-H, Hwang J-H (2019) Anti-migration and anti-invasion effects of curcumin via suppression of fascin expression in glioblastoma cells. Brain Tumor Res Treat 7:16–24
Park K-S, Lee HW, Park S-H, Park TI, Hwang J-H (2016) The clinical significance of fascin expression in a newly diagnosed primary glioblastoma. J Neurooncol 129:495–503
Wang P, Hao X, Li X, Yan Y, Tian W, Xiao L, Wang Z, Dong J (2021) Curcumin inhibits adverse psychological stress-induced proliferation and invasion of glioma cells via down-regulating the ERK/MAPK pathway. J Cell Mol Med 25:7190–7203
Woo M-S, Jung S-H, Kim S-Y, Hyun J-W, Ko K-H, Kim W-K, Kim H-S (2005) Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun 335:1017–1025
Kim S-Y, Jung S-H, Kim H-S (2005) Curcumin is a potent broad spectrum inhibitor of matrix metalloproteinase gene expression in human astroglioma cells. Biochem Biophys Res Commun 337:510–516
Zohrabian VM, Forzani B, Chau Z, Murali R, Jhanwar-Uniyal M (2009) Rho/ROCK and MAPK signaling pathways are involved in glioblastoma cell migration and proliferation. Anticancer Res 29:119–123
Weissenberger J, Priester M, Bernreuther C, Rakel S, Glatzel M, Seifert V, Kögel D (2010) Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1, 2/STAT3 signaling pathway. Clin Cancer Res 16:5781–5795
Senft C, Polacin M, Priester M, Seifert V, Kögel D, Weissenberger J (2010) The nontoxic natural compound curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer 10:1–8
Brooks AJ, Putoczki T (2020) JAK-STAT signalling pathway in cancer. Cancers 12(7):1971
Shi L, Wang Z, Sun G (2015) Curcumin induces glioma stem-like cell formation. NeuroReport 26:167–172
Ikushima H, Todo T, Ino Y, Takahashi M, Saito N, Miyazawa K, Miyazono K (2011) Glioma-initiating cells retain their tumorigenicity through integration of the Sox axis and Oct4 protein. J Biol Chem 286:41434–41441
Su C-C, Wang M-J, Chiu T-L (2010) The anti-cancer efficacy of curcumin scrutinized through core signaling pathways in glioblastoma. Int J Mol Med 26:217–224
Zhou Y, Liu L (2021) Curcumin induces human glioma cell apoptosis by promoting reactive oxygen species production. Indian J Pharm Sci 83:714–722
Liu T, Huang W, D-m LAI, W-w CHENG, HUANG Q, LIU Z-x (2009) The regulatory effect of curcumin on the differential expression of Bcl-2 and Caspase 8 and its promotional effect of apoptosis mechanism in human glioma cells. Ch Oncol 4:252
Kang S-K, Cha S-H, Jeon H-G (2006) Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev 15:165–174
Huang T-Y, Tsai T-H, Hsu C-W, Hsu Y-C (2010) Curcuminoids suppress the growth and induce apoptosis through caspase-3-dependent pathways in glioblastoma multiforme (GBM) 8401 cells. J Agric Food Chem 58:10639–10645
Khaw AK, Hande MP, Kalthur G, Hande MP (2013) Curcumin inhibits telomerase and induces telomere shortening and apoptosis in brain tumour cells. J Cell Biochem 114:1257–1270
Zhang Y, Tu L, Zhou X, Li B (2018) Curcumin-mediated induction of apoptosis in human glioma CHME cells. Med Sci Monit Basic Res 24:216
Zanotto-Filho A, Braganhol E, Edelweiss MI, Behr GA, Zanin R, Schröder R, Simões-Pires A, Battastini AMO, Moreira JCF (2012) The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J Nutr Biochem 23:591–601
Cheng C, Jiao JT, Qian Y, Guo XY, Huang J, Dai MC, Zhang L, Ding XP, Zong D, Shao JF (2016) Curcumin induces G2/M arrest and triggers apoptosis via FoxO1 signaling in U87 human glioma cells. Mol Med Rep 13:3763–3770
ZeXia W, Ye F, Fei L, LiangZhu Y, MinCai L, MeiChun H (2019) Inhibitory effect and mechanism of curcumin on glioma cells. Chongqing Med 48:2903–2908
Choi BH, Kim CG, Bae Y-S, Lim Y, Lee YH, Shin SY (2008) p21Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: role of early growth response-1 expression. Can Res 68:1369–1377
Liu E, Wu J, Cao W, Zhang J, Liu W, Jiang X, Zhang X (2007) Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J Neurooncol 85:263–270
Du Y, Cheng Y, Su G (2019) The essential role of tumor suppressor gene ING4 in various human cancers and non-neoplastic disorders. Biosci Rep. https://doi.org/10.1042/BSR20180773
Shao B, Liu E (2017) Expression of ING4 is negatively correlated with cellular proliferation and microvessel density in human glioma. Oncol Lett 14:3663
Gao X, Deeb D, Jiang H, Liu YB, Dulchavsky SA, Gautam SC (2005) Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of procaspases and release of cytochrome c from mitochondria. J Exp Ther Oncol 5:39–48
X Gao D Deeb, Y Liu, RA Chapman, SC Gautam (2004) Curcumin (diferuloyl-methane) chemosensitizes human glioma cells (U87) to TRAIL-induced apoptosis. AACR
Seyithanoğlu MH, Abdallah A, Kitiş S, Güler EM, Koçyiğit A, Dündar TT, Papaker MG (2019) Investigation of cytotoxic, genotoxic, and apoptotic effects of curcumin on glioma cells. Cell Mol Biol 65:101–108
Guo-an L, Ya-dong J, Miao-miao R, Gui-chen L, Qi-li Y, Lan D (2020) Curcumin inducing apoptosis of U87 cells by promoting ROS production. Nat Prod Res Dev 32:541
Gersey ZC, Rodriguez GA, Barbarite E, Sanchez A, Walters WM, Ohaeto KC, Komotar RJ, Graham RM (2017) Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 17:1–11
Garrido-Armas M, Corona JC, Escobar ML, Torres L, Ordóñez-Romero F, Hernández-Hernández A, Arenas-Huertero F (2018) Paraptosis in human glioblastoma cell line induced by curcumin. Toxicol In Vitro 51:63–73
Thayyullathil F, Rahman A, Pallichankandy S, Patel M, Galadari S (2014) ROS-dependent prostate apoptosis response-4 (Par-4) up-regulation and ceramide generation are the prime signaling events associated with curcumin-induced autophagic cell death in human malignant glioma. FEBS Open Bio 4:763–776
Lee J-E, Yoon SS, Lee J-W, Moon E-Y (2020) Curcumin-induced cell death depends on the level of autophagic flux in A172 and U87MG human glioblastoma cells. Chin J Nat Med 18:114–122
Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y (2007) Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol 72:29–39
Shinojima N, Yokoyama T, Kondo Y, Kondo S (2007) Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy 3:635–637
Zhuang W, Long L, Zheng B, Ji W, Yang N, Zhang Q, Liang Z (2012) Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci 103:684–690
Fong D, Yeh A, Naftalovich R, Choi TH, Chan MM (2010) Curcumin inhibits the side population (SP) phenotype of the rat C6 glioma cell line: towards targeting of cancer stem cells with phytochemicals. Cancer Lett 293:65–72
Fong D, Chan MM (2012) Targeting cancer stem cells with phytochemicals: inhibition of the rat C6 glioma side population by curcumin. Stem Cells and Cancer Stem Cells. Springer, Netherlands, pp 61–68
Su X, Chen S, Lu H, Li H, Qin C (2021) Study on the inhibitory effect of curcumin on GBM and Its potential mechanism. Drug Des Dev Ther 15:2769
Jiang B-H, Agani F, Passaniti A, Semenza GL (1997) V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Can Res 57:5328–5335
Trojanowicz B, Winkler A, Hammje K, Chen Z, Sekulla C, Glanz D, Schmutzler C, Mentrup B, Hombach-Klonisch S, Klonisch T (2009) Retinoic acid-mediated down-regulation of ENO1/MBP-1 gene products caused decreased invasiveness of the follicular thyroid carcinoma cell lines. J Mol Endocrinol 42:249–260
Gao J, Zhao R, Xue Y, Niu Z, Cui K, Yu F, Zhang B, Li S (2013) Role of enolase-1 in response to hypoxia in breast cancer: exploring the mechanisms of action. Oncol Rep 29:1322–1332
Bi F, Wang J, Zheng X, Xiao J, Zhi C, Gu J, Zhang Y, Li J, Miao Z, Wang Y (2021) HSP60 participates in the anti-glioma effects of curcumin. Exp Ther Med 21:1–1
Luo Q, Luo H, Fu H, Huang H, Luo K, Li C, Hu R, Zheng C, Lan C, Tang Q (2019) Curcumin suppresses invasiveness and migration of human glioma cells in vitro by inhibiting HDGF/β-catenin complex. J South Med Univ 39:911–916
Srivastava C, Gupta Y, Irshad K, Chattopadhaya P, Sarkar C, Suri A, Sinha S, Chosdol K (2017) Curcumin downregulates FAT1 expression via NFkB in glioblastoma. Ann Oncol 28:x36
Srivastava C, Irshad K, Gupta Y, Sarkar C, Suri A, Chattopadhyay P, Sinha S, Chosdol K (2020) NFкB is a critical transcriptional regulator of atypical cadherin FAT1 in glioma. BMC Cancer 20:62
Zraikat M, Gharaibeh M, Alshelleh T (2020) The effect of curcumin on the invasion and migration of glioma cells. Eur J Med Plants. https://doi.org/10.9734/ejmp/2020/v31i730249
Du WZ, Feng Y, Wang XF, Piao XY, Cui YQ, Chen LC, Lei XH, Sun X, Liu X, Wang HB (2013) Curcumin suppresses malignant glioma cells growth and induces apoptosis by inhibition of SHH/GLI 1 signaling pathway in vitro and vivo. CNS Neurosci Ther 19:926–936
Carballo GB, Honorato JR, de Lopes GPF (2018) A highlight on sonic hedgehog pathway. Cell Commun Signal 16:1–15
Tan X, Kim G, Lee D, Oh J, Kim M, Piao C, Lee J, Lee MS, Jeong JH, Lee M (2018) A curcumin-loaded polymeric micelle as a carrier of a microRNA-21 antisense-oligonucleotide for enhanced anti-tumor effects in a glioblastoma animal model. Biomater sci 6:407–417
Chan JA, Krichevsky AM, Kosik KS (2005) MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Can Res 65:6029–6033
Yeh W-L, Lin H-Y, Huang C-Y, Huang B-R, Lin C, Lu D-Y, Wei K-C (2015) Migration-prone glioma cells show curcumin resistance associated with enhanced expression of miR-21 and invasion/anti-apoptosis-related proteins. Oncotarget 6:37770
Li W, Yang W, Liu Y, Chen S, Chin S, Qi X, Zhao Y, Liu H, Wang J, Mei X (2017) MicroRNA-378 enhances inhibitory effect of curcumin on glioblastoma. Oncotarget 8:73938
Yin S, Du W, Wang F, Han B, Cui Y, Yang D, Chen H, Liu D, Liu X, Zhai X (2018) MicroRNA-326 sensitizes human glioblastoma cells to curcumin via the SHH/GLI1 signaling pathway. Cancer Biol Ther 19:260–270
Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang L, Xu Z (2019) Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis 10:788
Guo K, Ma Q, Wang L, Hu H, Li J, Zhang D, Zhang M (2009) Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep 22:825–830
Zhang Z, Li C, Tan Q, Xie C, Yang Y, Zhan W, Han F, Shanker Sharma H, Sharma A (2017) Curcumin suppresses tumor growth and angiogenesis in human glioma cells through modulation of vascular endothelial growth factor/angiopoietin-2/thrombospondin-1 signaling. CNS Neurol Disord 16:346–350
Zhang Z-J, Zhao L-X, Cao D-L, Zhang X, Gao Y-J, Xia C (2012) Curcumin inhibits LPS-induced CCL2 expression via JNK pathway in C6 rat astrocytoma cells. Cell Mol Neurobiol 32:1003–1010
Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, Kanojia D, Pituch KC, Qiao J, Pytel P (2016) CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Can Res 76:5671–5682
Walker BC, Adhikari S, Mittal S (2021) Therapeutic potential of curcumin for the treatment of malignant gliomas. Exon Publ. https://doi.org/10.36255/exonpublications.gliomas.2021.chapter8
Li J, Zhao J, Tan T, Liu M, Zeng Z, Zeng Y, Zhang L, Fu C, Chen D, Xie T (2020) Nanoparticle drug delivery system for glioma and its efficacy improvement strategies: a comprehensive review. Int J Nanomed 15:2563
Luo S-M, Wu Y-P, Huang L-C, Huang S-M, Hueng D-Y (2021) The anti-cancer effect of four curcumin analogues on human glioma cells. Onco Targets Ther 14:4345
Shi L, Sun G, Zhu H (2020) Demethoxycurcumin analogue DMC-BH inhibits orthotopic growth of glioma stem cells by targeting JNK/ERK signaling. Aging 12:14718
Shi L, Gao L-l, Cai S-z, Xiong Q-w, Ma Z-r (2021) A novel selective mitochondrial-targeted curcumin analog with remarkable cytotoxicity in glioma cells. Eur J Med Chem 221:113528
Qian C, Wang B, Zou Y, Zhang Y, Hu X, Sun W, Xiao H, Liu H, Shi L (2019) MicroRNA 145 enhances chemosensitivity of glioblastoma stem cells to demethoxycurcumin. Cancer Manag Res 11:6829
Mirgani MT, Isacchi B, Sadeghizadeh M, Marra F, Bilia AR, Mowla SJ, Najafi F, Babaei E (2014) Dendrosomal curcumin nanoformulation downregulates pluripotency genes via miR-145 activation in U87MG glioblastoma cells. Int J Nanomed 9:403
Shin HJ, Lee S, Jung HJ (2019) A curcumin derivative hydrazinobenzoylcurcumin suppresses stem-like features of glioblastoma cells by targeting Ca2+/calmodulin-dependent protein kinase II. J Cell Biochem 120:6741–6752
Wang Y-y, Zhao R, Zhe H (2015) The emerging role of CaMKII in cancer. Oncotarget 6:11725
Chai S, Xu X, Wang Y, Zhou Y, Zhang C, Yang Y, Yang Y, Xu H, Xu R, Wang K (2015) Ca2+/calmodulin-dependent protein kinase IIγ enhances stem-like traits and tumorigenicity of lung cancer cells. Oncotarget 6:16069
Sansalone L, Veliz EA, Myrthil NG, Stathias V, Walters W, Torrens II, Schürer SC, Vanni S, Leblanc RM, Graham RM (2019) Novel curcumin inspired bis-chalcone promotes endoplasmic reticulum stress and glioblastoma neurosphere cell death. Cancers 11:357
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Ramachandran C, Portalatin G, Quirin K-W, Escalon E, Khatib Z, Melnick SJ (2015) Inhibition of AKT signaling by supercritical CO2 extract of mango ginger (Curcuma amada Roxb.) in human glioblastoma cells. J Complement Integr Med 12:307–315
He Y, Wu C, Duan J, Miao J, Ren H, Liu J (2020) Anti-glioma effect with targeting therapy using folate modified nano-micelles delivery curcumin. J Biomed Nanotechnol 16:1–13
Zhang H, van Os WL, Tian X, Zu G, Ribovski L, Bron R, Bussmann J, Kros A, Liu Y, Zuhorn IS (2021) Development of curcumin-loaded zein nanoparticles for transport across the blood–brain barrier and inhibition of glioblastoma cell growth. Biomater Sci. https://doi.org/10.1039/D0BM01536A
Babaei M, Davoodi J, Dehghan R, Zahiri M, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M (2020) Thermosensitive composite hydrogel incorporated with curcumin-loaded nanopolymersomes for prolonged and localized treatment of glioma. J Drug Deliv Sci Technol 59:101885
Xiang Y, Duan X, Feng L, Jiang S, Deng L, Shen J, Yang Y, Guo R (2019) tLyp-1-conjugated GSH-sensitive biodegradable micelles mediate enhanced pUNO1-hTRAILa/curcumin co-delivery to gliomas. Chem Eng J 374:392–404
Li R, Song Y, Fouladian P, Arafat M, Chung R, Kohlhagen J, Garg S (2021) Three-dimensional printing of curcumin-loaded biodegradable and flexible scaffold for intracranial therapy of glioblastoma multiforme. Pharmaceutics 13:471
Gelardi E, Caprioglio D, Colombo G, Mazzoletti D, Mattoteia D, Salamone S, Ferraris D, Aronica E, Nato G, Buffo A (2021) Curcumin-based-fluorescent probes targeting ALDH1A3 as a promising tool for glioblastoma precision surgery and early diagnosis
Maiti P, Al-Gharaibeh A, Kolli N, Dunbar GL (2017) Solid lipid curcumin particles induce more dna fragmentation and cell death in cultured human glioblastoma cells than does natural curcumin. Oxid Med Cell Longev. https://doi.org/10.1155/2017/9656719
Maiti P, Scott J, Sengupta D, Al-Gharaibeh A, Dunbar GL (2019) Curcumin and solid lipid curcumin particles induce autophagy, but inhibit mitophagy and the PI3K-Akt/mTOR pathway in cultured glioblastoma cells. Int J Mol Sci 20:399
Dhandapani KM, Mahesh VB, Brann DW (2007) Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFκB transcription factors. J Neurochem 102:522–538
Newlands E, Stevens M, Wedge S, Wheelhouse R, Brock C (1997) Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev 23:35–61
Alexiou GA, Vartholomatos E, Tsamis KI, Peponi E, Markopoulos G, Papathanasopoulou VA, Tasiou I, Ragos V, Tsekeris P, Kyritsis AP (2019) Combination treatment for glioblastoma with temozolomide, DFMO and radiation. J BUON 24:397–404
Lee SY (2016) Temozolomide resistance in glioblastoma multiforme. Genes Dis 3:198–210
Xia Q, Liu L, Li Y, Zhang P, Han D, Dong L (2021) Therapeutic perspective of temozolomide resistance in glioblastoma treatment. Cancer Invest 39:627–644
Huang B-R, Tsai C-H, Chen C-C, Way T-D, Kao J-Y, Liu Y-S, Lin H-Y, Lai S-W, Lu D-Y (2019) Curcumin promotes connexin 43 degradation and temozolomide-induced apoptosis in glioblastoma cells. Am J Chin Med 47:657–674
Grek CL, Sheng Z, Naus CC, Sin WC, Gourdie RG, Ghatnekar GG (2018) Novel approach to temozolomide resistance in malignant glioma: connexin43-directed therapeutics. Curr Opin Pharmacol 41:79–88
Chen T-C, Chuang J-Y, Ko C-Y, Kao T-J, Yang P-Y, Yu C-H, Liu M-S, Hu S-L, Tsai Y-T, Chan H (2020) AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-Mediated redox homeostasis. Redox Biol 30:101413
Bagherian A, Roudi B, Masoudian N, Mirzaei H (2021) Anti-glioblastoma effects of nanomicelle-curcumin plus erlotinib. Food Funct. https://doi.org/10.1039/D1FO01611C
Wu H, Liu Q, Cai T, Chen YD, Wang ZF (2015) Induction of microRNA-146a is involved in curcumin-mediated enhancement of temozolomide cytotoxicity against human glioblastoma. Mol Med Rep 12:5461–5466
Bagherian A, Mardani R, Roudi B, Taghizadeh M, Banfshe HR, Ghaderi A, Davoodvandi A, Shamollaghamsari S, Hamblin MR, Mirzaei H (2020) Combination therapy with nanomicellar-curcumin and temozolomide for in vitro therapy of glioblastoma multiforme via Wnt signaling pathways. J Mol Neurosci 70:1471–1483
Yin H, Zhou Y, Wen C, Zhou C, Zhang W, Hu X, Wang L, You C, Shao J (2014) Curcumin sensitizes glioblastoma to temozolomide by simultaneously generating ROS and disrupting AKT/mTOR signaling. Oncol Rep 32:1610–1616
Dilnawaz F, Sahoo SK (2013) Enhanced accumulation of curcumin and temozolomide loaded magnetic nanoparticles executes profound cytotoxic effect in glioblastoma spheroid model. Eur J Pharm Biopharm 85:452–462
Zanotto-Filho A, Braganhol E, Klafke K, Figueiró F, Terra SR, Paludo FJ, Morrone M, Bristot IJ, Battastini AM, Forcelini CM (2015) Autophagy inhibition improves the efficacy of curcumin/temozolomide combination therapy in glioblastomas. Cancer Lett 358:220–231
Cui Y, Zhang M, Zeng F, Jin H, Xu Q, Huang Y (2016) Dual-targeting magnetic PLGA nanoparticles for codelivery of paclitaxel and curcumin for brain tumor therapy. ACS Appl Mater Interfaces 8:32159–32169
Wen X, Cheng X, Hu D, Li W, Ha J, Kang Z, Zhang M, Huang Y, Wu S (2016) Combination of curcumin with an anti-transferrin receptor antibody suppressed the growth of malignant gliomas in vitro. Turk Neurosurg 26:209–214
Mujokoro B, Madani F, Esnaashari SS, Khosravani M, Adabi M (2020) Combination and co-delivery of methotrexate and curcumin: preparation and in vitro cytotoxic investigation on glioma cells. J Pharm Innov 15:617–626
Zhao J, Zhu J, Lv X, Xing J, Liu S, Chen C, Xu Y (2017) Curcumin potentiates the potent antitumor activity of ACNU against glioblastoma by suppressing the PI3K/AKT and NF-κB/COX-2 signaling pathways. Onco Targets Ther 10:5471
Jamali Z, Hejazi SM, Ebrahimi SM, Moradi-Sardareh H, Paknejad M (2018) Effects of LED-Based photodynamic therapy using red and blue lights, with natural hydrophobic photosensitizers on human glioma cell line. Photodiagn Photodyn Ther 21:50–54
Zoi V, Galani V, Vartholomatos E, Zacharopoulou N, Tsoumeleka E, Gkizas G, Bozios G, Tsekeris P, Chousidis I, Leonardos I (2021) Curcumin and radiotherapy exert synergistic anti-glioma effect in vitro. Biomedicines 9:1562
Zhang L, Ding X, Huang J, Jiang C, Cao B, Qian Y, Cheng C, Dai M, Guo X, Shao J (2015) In vivo Radiosensitization of human glioma U87 cells induced by upregulated expression of DUSP-2 after treatment with curcumin. Curr Signal Transduct Ther 10:119–125
Yu Q, Jianfen M, Xiaoyi G, Jun S, Yongtao Y, Boqiang C (2015) Curcumin enhances the radiosensitivity of U87 cells by inducing DUSP-2 up-regulation. Cell Physiol Biochem 35:1381–1393
Gao P-P, Qi X-W, Sun N, Sun Y-Y, Zhang Y, Tan X-N, Ding J, Han F (1876) Zhang Y (2021) The emerging roles of dual-specificity phosphatases and their specific characteristics in human cancer. Biochimica et Biophysica Acta 1:188562
Wang W-H, Shen C-Y, Chien Y-C, Chang W-S, Tsai C-W, Lin Y-H, Hwang J-J (2020) Validation of enhancing effects of curcumin on radiotherapy with F98/FGT glioblastoma-bearing rat model. Int J Mol Sci 21:4385
Sminia P, van den Berg J, van Kootwijk A, Hageman E, Slotman BJ, Verbakel WF (2021) Experimental and clinical studies on radiation and curcumin in human glioma. J Cancer Res Clin Oncol 147:403–409
Acknowledgements
We appreciate the assistance of the Clinical Research Development Unit of Akbar Hospital in conducting this review.
Funding
None.
Author information
Authors and Affiliations
Contributions
AB designed the article contents. MM wrote the original manuscript. GAF and SA made revisions to the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamadian, M., Ahmadi, S.S., Bahrami, A. et al. Review on the Therapeutic Potential of Curcumin and its Derivatives on Glioma Biology. Neurochem Res 47, 2936–2953 (2022). https://doi.org/10.1007/s11064-022-03666-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03666-1