Abstract
Astrocytes are the most abundant glial cells in the central nervous system, and are important players in both brain injury and neurodegenerative disease. Curcumin (1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione), the major active component of turmeric, belongs to the curcuminoid family that was originally isolated from the plant Curcuma longa. Several studies suggest that curcumin may have a beneficial impact on the brain pathology and aging. These effects are due to curcumin’s antioxidant, free-radical scavenging, and anti-inflammatory activity. In light of this, our current review aims to discuss the role of astrocytes as essential players in neurodegenerative diseases and suggest that curcumin is capable of direct inhibition of astrocyte activity with a particular focus on its effects in Alexander disease, Alzheimer's disease, ischemia stroke, spinal cord injury, Multiple sclerosis, and Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian brain consists of two main groups of glial cells: microglia and macroglia. Microglias are macrophages in the central nervous system (CNS) that are responsible for the removal of toxic cellular debris. Macroglia consist of ependymal cells, Schwann cells, oligodendroglia, and astroglia, the latter including astrocytes from the brain and spinal cord, Bergmann cells in the cerebella cortex, and tanycytes in the hypothalamus [1].
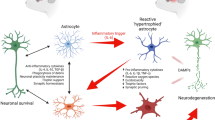
Astrocytes are the most abundant class of glial cell in the CNS and are important mediators of brain development, function, and plasticity [2]. Astrocytes play a vital role in homeostasis, being involved in glutamate uptake, buffering of K+, H+, Ca2+ ions, nutrient support, regulation of synaptic transmission and neuronal excitability [3]. Astrocytes also contribute to detoxification of ammonia, free-radical scavenging, metal sequestration, and control of the blood–brain barrier [4]. In addition, these cells produce several factors, including neurotrophic factors (GDNF, BDNF, NT-3, CNTF) and growth factors (TGF-β, FGF-2, NGF, EGF), that are necessary for the survival of neurons [5]. In several neurodegenerative diseases, the up regulation of glial fibrillary acidic protein (GFAP), vimentin, and nestin intermediate filament proteins lead to activation of astrocytes, or astrogliosis which is a pathological hallmark [6]. Activation of astrocytes leads to the production of neurotrophic factors and regulation of ion homeostasis necessary for neuronal survival but, once activated, astrocytes lose their regulatory function and initiate the inflammatory responses that cause the death of neurons amongst other detrimental effects [7]. Thus, activation of astrocytes or impairment of astrocyte function may contribute to the pathogenesis of many acute and chronic disorders, such as neurodegenerative disease, cancer, and oxidative stress [8].
Curcumin is a polyphenolic compound and a major coloring agent derived from the dried rhizomes of Curcuma longa (turmeric). Curcumin has received much attention for its neuroprotective [9], hepatoprotective [10], wound healing [11], pulmonoprotective [12], antitumor, and chemopreventive [13,14,15], anti-ischemic [16], and anti-inflammatory [17] activities. The curcumin molecule has two similar aromatic rings where O‐methoxy phenolic groups are located and are linked to α, β‐unsaturated β‐diketone moiety. In many redox reactions, curcumin serves as an electron donor owing to the presence of conjugated double bonds. Curcumin is capable to cross the cell membrane due to its lipophilic property. However, several factors, such as low aqueous solubility, insufficient tissue absorption, rapid systemic removal and degradation at alkaline pH, decrease the bioavailability of curcumin, thus limiting its pharmaceutical role [18]. The anti-inflammatory effects of curcumin involve various mechanisms of action including inhibition of NF-ҡB activation and its translocation to the nucleus, suppression of downstream pro-inflammatory cytokine-like, interleukin (IL)-23, IL-1β, IL-6, tumor necrosis factor (TNF)-α, and nitric oxide (NO) release, and suppression of MAPK signal pathway in a dose-dependent manner [19]. Curcumin is a powerful antioxidant and significantly decreases lipid peroxidation, regulates antioxidant enzymes and scavenges reactive-oxygen species (ROS). The anti-inflammatory and antioxidant activities of curcumin are linked to its therapeutic ability.
In the present review, we discuss the role of astrocytes as essential players in neurodegenerative diseases, and investigate the therapeutic effect of curcumin in brain pathologies (Fig. 1). An extensive search in electronic databases (PubMed, Scopus, and Science Direct) was used to identify the effects of curcumin on neurological diseases: Focus on astrocytes, without any time limitation, using “curcumin”, “Curcuma longa”, or “turmeric” and “astrocytes”, “neurological diseases”, “Brain”, or “neurodegenerative disease” as search words.
Neuroprotective actions of curcumin. Curcumin regulates neuroinflammation in a variety of neurological ailments. Most of its actions are related to regulating (1) NF-kB signaling, (2) the activation of NO, Wnt/β-catenin, CISD2, MEK1/ERK1, BDNF, and JAK2-STATE3 (3) the inhibition of MAPK, Iba-1, TNF-α, MMp-1, NF-κβ, iNOs, RANTES, and COX-2
Bioavailability of curcumin
Despite the efficacy of curcumin against various diseases, due to many problems, such as low aqueous solubility, low intestinal absorption, low oral bioavailability and rapid metabolism in the gastrointestinal tract, its clinical application is limited [20]. The water-soluble glucuronide and sulfate conjugates were the main metabolites of curcumin, whereas the minor components were hexahydrocurcuminol, hexahydrocurcumin, and hexahydrocurcumin glucuronide. Pharmacokinetic studies have demonstrated poor bioavailability of curcumin in both rodent and human models. For example, the orally administration of curcumin at a dose of 300 mg/kg has shown the maximum rat plasma level of 131.26 μg/L at 4.13 h after administration [21]. Also, the orally administered curcumin in human up to dose 8 g resulted in less than 1 μg/mL curcumin plasma concentration. Thus, in order to increase the metabolism, serum levels, tissue distribution and half-life of curcumin, the use of nanoparticles, polymeric micelles, adjuvants, liposomes, complexation with transition metal ions and phospholipid complexes were suggested. For example, in healthy human subjects the use of curcumin liquid droplet micromicellar (CLDM) formulation instead of curcumin cause improvement in absorption up to 522 times greater. Also, γ-cyclodextrin curcumin formulation (CW8) can increase oral bioavailability in human subjects up to 39 times higher than unformulated curcumin extract. These studies indicated promising results to overcome many problems of curcumin in clinical application [22].
Curcumin in acute and chronic neurodegenerative diseases
Several studies have implicated astrocytes in the pathogenesis of neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), tumor, stroke, and amyotrophic lateral sclerosis (ALS) [23].
Alzheimer’s disease
Alzheimer’s disease is characterized by neuronal cell death, dysfunction of synaptic integrity, perturbation of neuronal communication, and cognitive impairment. Aggregated amyloid-β peptide (Aβ) causes activation of microglia and astrocytes that leading to the production of inflammatory factors, such as TNF-α, NO, prostaglandins, and interleukins which may cause neuronal death [24].
Activation of astrocytes and microglia are key features of neuroinflammation and is implicated in the pathogenesis of many neurodegenerative disorders, such as AD, ischemic stroke, CNS injury, chronic pain, multiple sclerosis, and PD [25]. In the p25 transgenic mouse model of AD, curcumin ameliorated cognitive impairment via reduction of p25-induced tau/amyloid pathology. However, the major underlying mechanism responsible for curcumin’s beneficial effects in Alzheimer models is suppression of neuroinflammation, regulation of the cPLA2/LPC signaling pathways and a reduction of glial activation, especially of astrocytes [26]. Curcumin decreases the production of microglia, astrocytes, and cytokines, and increases PPARγ transcriptional activity, by inhibiting NF-ҡB signaling, thereby alleviating neuroinflammation in a rat model of AD [27]. In a rat model of AD, curcumin enhanced the RANTES expression in astrocytes, activated MAPK, and PI-3K signaling pathways, inhibited iNOS expression and exhibited neuroprotective activity [28]. Zhang et al. observed that curcumin effectively treated neuroinflammatory-associated disorders, especially AD. The anti-neuroinflammatory properties of curcumin could be attributed to regulation of CCL2 production and reduction of JNK phosphorylation in astrocytoma cells [25]. Curcuminoid submicron particles (CSP) significantly improved the memory impairment and pathological deficits in a mouse model of AD. Treatment with CSPs increased microglial Aβ phagocytosis in vitro and decreased astrogliosis and amyloid plaques in vivo, these being the major mechanisms responsible for the beneficial effects of CSPs in enhancing amyloid clearance and modulating neuroinflammation [29]. In the 5xFAD mouse model of AD, solid lipid curcumin particles (SLCPs) showed greater anti-inflammatory and neuroprotective effects than curcumin. This study showed that curcumin and SLCPs significantly reduced microglia (Iba-1-IR) and astrocyte activity, as well as decreased glial fibrillary acidic protein (GFAP)-IR and neuroinflammation in AD [30]. Lim et al. reported that curcumin at 160 ppm dose significantly reduced oxidative damage, decreased insoluble and soluble amyloid, and inhibited both the cytokine IL-1and GFAP (a marker of astrocytic inflammation) in an Alzheimer transgenic mice. Owing to its high efficacy and low toxicity, curcumin is; therefore, a potential therapeutic agent for the treatment of AD [31].
Kuo et al. reported that WGA (wheat germ agglutinin)-conjugated CL-incorporated liposomes (WGA-CL-liposomes) increased the anti-inflammatory activity of curcumin and produced nerve growth factor (NGF), useful in AD therapy. NGF regulated and improved the survival of sympathetic neurons and curcumin proved effective in the treatment of AD [32]. Curcumin ameliorated spatial memory disorders in AD model rats; this effect was attributed to the inhibition of astrocyte activity and suppression of GFAP expression [33]. The effect of curcumin-loaded lipid–core nanocapsules (Cur-LNC) in a rat model of AD was investigated. Curcumin was found to be effective in preventing, neuroinflammation, tau hyperphosphorylation, and behavioral impairments and the cell signaling disturbances triggered by Aβ. In a study conducted by Ganugapati et al., curcumin was found to inhibit amyloid-β protein and reduce the activity of astrocytes, and can therefore be considered as a possible drug candidate for the prevention of AD [34]. In transgenic mice, curcumin suppressed activated astroglia, decreased the expression of amyloid-β protein, and inhibited inflammation; thereby, treating the AD [35]. In another study, curcumin prevented and treated AD in Aβ-infused rats and transgenic APPS mouse model (Tg2576). Concentrations of curcumin between 15 μM and 30 μM were suitable for short studies (< 24 h), whilst concentrations between 5 μM and 15 μM were more effective for longer trials of 4 to 6 days to demonstrate the anti-inflammatory and antioxidant properties of curcumin [36].
Parkinson’s disease
Parkinson’s disease is one of the most common progressive neurodegenerative disorders among elderly people. The major pathological symptoms of PD are instability, rigidity, resting tremor, and difficulty in walking due to the progressive loss of dopaminergic neurons (DNs) in the nigra striatal and the striatum [37]. The activated astrocytes and microglia cause production of neurotoxic molecules, such as pro-inflammatory cytokines and prostaglandins, and reactive oxygen and nitrogen species. Therefore, the suppression of reactive glial cells causes reduction in neuroinflammation and alleviated neuronal damage [38].
In PD, both inflammation and oxidative stress affect the nigrostriatal dopaminergic (DA) pathway and are responsible for neuronal degeneration. Curcumin demonstrated neuroprotective effects in the 6-hydroxydopamine (6-OHDA) lesion in mouse model of PD via two mechanisms: (1) inhibition of glial response and a reduction in the activity of astrocytic and microglial activity (anti-inflammatory) and (2) promoting an increase in SOD1 expression (antioxidant activity) [39]. Curcumin showed anti-inflammatory and antioxidant activity in 1-methyl-4-phenylpyridinium ion (MPP+)-stimulated mesencephalic astrocytes by the suppression of both MyD88-dependent and TRIF-dependent pathways in toll-like receptor (TLR4) This mechanism could be a potential therapeutic target in treating PD [40]. In primary mouse mesencephalic astrocytes, curcumin exhibited a cytoprotective effect against MPP+ and LPS-induced toxicity due to reduction in ROS and inhibition of CYP2E1 activity [41]. Long-term consumption of curcumin decreased the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in a mouse model of PD through enhancement of GDNF and TGFβ1 expression [42].
Sharma et al. reported that curcumin exerted neuroprotective effect in the LPS-induced PD model due to inhibition of glial cell activation, generation of NO, inhibition of NF-ҡB activation and amelioration of microglial NADPH oxidase (PHOX) activity, as well as alleviating neuroinflammation [43]. Curcumin exerted its neuroprotective effects via inhibition of α-synuclein aggregation, production of GSH, suppression of the glial response and prevention of oxidative stress [44]. Moreover, in a 6-hydroxydopamine hemi-parkinsonian mouse model, curcumin exhibited a neuroprotective effect by reduction of Iba1-IR in microglia, GFAP-IR in astrocytes in the ipsilateral striatum, and protected nigrostriatal DA neurons [45]. In a rat model of PD, curcumin reduced oxidative stress mediated by the activation of the Wnt/β-catenin signaling pathway, and increased glutathione peroxidase and superoxide dismutase [37]. Abbaoui et al. demonstrated that the consumption of excessive Cu plays an important role in the pathogenesis of many neurodegenerative disorders, especially PD. Their study revealed that treatment with curcumin decreased Cu neurotoxicity and that this effect was related to the antioxidant property of curcumin [46].
Cerebral ischemia
Cerebral ischemia including stroke is the major cause of death and disability worldwide. It causes loss of neuronal function and irreversible brain damage. Cerebral ischemia is characterized by the lack of oxygen, cessation of blood flow, and an influx of calcium with enhancement of reactive-oxygen species (ROS), mitochondrial dysfunction, and neuronal apoptosis [47].
In a study conducted by Wang et al., curcumin demonstrated neuroprotective effect against ischemic damage. This effect was related to curcumin’s antioxidant ability in reducing oxidative stress, lipid peroxidation, and activated microglial cells and in ameliorating mitochondrial function [47]. Curcumin can protect against cerebral ischemia through inhibition of iNOS expression in astrocytes, scavenging of oxygen-free radicals and suppression of ONOO− that causes blood–brain barrier disruption [48]. The histological effects of chronic administration of first-generation, in comparison to second generation, antipsychotics, together with the ameliorating effect of curcumin, were evaluated in adult rat cerebral cortex. Treatment with haloperidol and risperidone decreased microglia cells and increased astrocyte number and altered neurodegenerative activity; curcumin ameliorated these side effects [49]. Curcumin at doses of 50 and 100 mg/kg treated the cerebral ischemia in rat. The results showed that curcumin in a dose-dependent manner, upregulated the expression of GFAP, and attenuated the cerebral ischemia [50]. The neuroprotective effect of curcumin in an okadaicacid-induced cerebral ischemic model was affected via activation of astrocytes, reduction in GFAP expression, and increased caspase-9 mRNA expression. The neuroprotective effect of curcumin may be related to its antioxidant, anti-inflammatory, and anti-apoptotic activities [51].
Nanomaterials, such as zinc oxide (ZnO) are becoming popular in many fields, including industry, nutrition, and medicine. Pollution of the environment by nanoparticles (NPs) under lays many neurodegenerative disorders, especially cerebral ischemia. Amer et al. reported that treatment of rat cerebellar cortex with curcumin ameliorated the harmful effects of ZnO NPs. The effects of curcumin attributed to scavenging of reactive-oxygen species (antioxidant activity), reduction of immune expression of caspase-3 and P53 (antiapoptotic), inhibition of COX-2 and proinflammatory cytokines (anti-inflammatory effects) [52]. The combination of embryonic stem cell exosomes and curcumin (MESC-exocur) effectively treated ischemia–reperfusion injury in mice; the data suggested that MESC-exocur attenuates the expression of astrocytic GFAP and NeuN positive neuron expression and decreases the tight, adherent junctions and vascular inflammation [53].
Cerebral edema
Cerebral edemais a leading cause of death and disability in approximately 10–20% of all strokes worldwide. The development of brain edema with an increase in intracranial pressure causes decreased cerebral blood flow and cerebral perfusion pressure, and leads to poor patient prognosis [54].
Wang et al., invistigated the relationship between aquaporins (AQPs) and curcumin in the treatment of brain edema in mice. AQP1, AQP4, and AQP9 play an important role in edema formation in different types of brain diseases. Curcumin attenuated brain edema and decreased both AQP4 and AQP9 expression in astrocytes and the degradation of hemoglobin through reduced activity of the NF-κB pathway [55].
Epilepsy
Epilepsy is a chronic neurological disease affecting 1% of the world’s population, the main symptom being spontaneous recurrent seizures (SRS). The most common type of refractory partial epilepsy in adults is temporal lobe epilepsy (TLE), and this condition demonstrates several pathological features, such as hippocampal sclerosis (neuronal loss and gliosis), dysfunction of the blood–brain barrier and an alteration of the neuronal network involved in hippocampal formation [56].
Drion et al. compared the anti-inflammatory and antioxidant effects of curcumin and rapamycinin the treatment of epileptogenesis. Curcumin, but not rapamycin, decreased the levels of the inflammatory markers, IL-6 and COX-2, in cultured astrocytes, inhibited the MAPK pathway and showed antioxidant effects via induction of heme oxygenase, there by improving the epilepsy [57]. Also, the effect of curcumin in model of TLE induced by kainic acid was invistigated. A beneficial effect of curcumin on modifying epileptogenesis in model of TLE induced by kainic acid was attributed to a reduction in proinflammatory cytokines, attenuation of abnormal mossy fiber sprouting (MFS) and astrocyte reactivation and a decrease in the severity of spontaneous recurrent seizures (SRS) [58]. Curcumin attenuated cognitive deficits in an experimental model of PTZ-induced chronic epilepsy through a reduction of GFAP and Iba-1 expression, inhibition of astrocyte and microglia activity and a reduction of proinflammatory cytokine expression [59].
Spinal cord injury
Spinal cord injury (SCI) is defined as damage to the spinal cord that causes neuronal death and irreversible tissue necrosis. Spinal cord injury (SCI) is a complex pathological process that includes an inflammatory response leading to glial scar formation by astrocytes, which severely hinders neural regeneration. The neuroprotective effect of curcumin was investigated in both rat model of SCI and in cell culture model of astrocyte reactivation. The neuroprotective effect was related to the attenuation of RANTES and iNOS mRNA expression and a reduction in the neurotoxicity of the LPS-conditioned astrocyte medium [60]. Lin et al. reported that curcumin can increase CISD2 expression in spinal cord injury and LPS-treated astrocytes. CDGSH iron sulfur domain 2 (CISD2) is regarded as a survival gene and contribute to the antagonism of neuroinflammation. Curcumin decreased the expression of GFAP and RANTES, and increased CISD2 expression, thus, reducing neuroinflammation and thereby improved the SCI [61]. Curcumin was observed to inhibit apoptosis and neuron loss, quench astrocyte activation, decrease macrophage and T-cell infiltration, reduce the expression ofthe chemokines MCP-1, RANTES and CXCL10 in astrocytes and thus reduce the inflammation in the glial scar and regulate both the NF-κB and SOX9 signaling pathways [62]. Curcumin was shown to significantly decrease the expression of IL-1β, as well as the number of Iba1+ inflammatory cells, and repaired the spinal cord through inhibiting glial scar formation and inflammation, thereby exhibiting a neuroprotective activity [63].
Ethidium bromide (EB) injections into the CNS are known to induce a loss of local oligodendroglial and astrocytic loss. Through increasing the immuno reactivity to GFAP EB injection also induces the development of peripheral astrogliosis around the injury site. In the rat brain stem, curcumin reduced the GFAP-stained area around the lesion at all time points after EB injection (compared with untreated animals) and attenuated glial scar formation [64].
Depression
The hypothalamic–pituitary–adrenal (HPA) axis is activated following stress and is involved in the pathogenesis of depression that causes increased concentrations of glucocorticoids in the circulating blood. Conversely, high levels of glucocorticoids induce a depressive-like behavior in rodents [65]. The antidepressant effect of curcumin and its possible mechanisms were studied in a corticosterone (CORT)-induced depression model in rats. BDNF is one of the most neurotrophic factors that produces antidepressant activity in the brain. Curcumin, enhanced the BDNF expression in the hippocampus and frontal cortex, and exerted an antidepressant activity [66]. Due to the low solubility and poor bioavailability of curcumin, the combination of Cur, Dexanabinol (HU-211), and SLNs was used by He et al. to develop Cur/SLNs-HU-211 nanoparticles as a potential tools for major depression. In vivo, Cur/SLNs-HU-211 increased the distribution of Cur in the brain and exhibited antidepressant effect in CORT-induced depression in mice. The main mechanism responsible for this effect was the reduction of the astrocytes and GFAP, an increase in CB1expression and activation of MEK1/ERK1/2 signaling [67].
Neuroinflammation
Neuroinflammation plays a pivotal role in the pathogenesis of chronic pain. Glial cells attenuate inflammatory or neuropathic pain and inhibit neuroinflammation. The antinociceptive and antineuroinflammatory effects of curcumin were studied on complete Freund’s adjuvant (CFA)-induced pain hypersensitivity. The intrathecal injection of curcumin decreased CFA-induced pain hypersensitivity, attenuated the activation of astrocytes and microglia and reduced the expression of the proinflammatory cytokine (IL-1β) and chemokines (MCP-1 and MIP-1α) [68].
The beneficial effect of curcumin and the role of fractalkine (FKN) and its receptor, CX3CR1 fructose-induced neuroinflammation were examined. FKN and CX3CR1 expression were increased and fructose stimulated neuroinflammation and activated the hippocampal microglia by activation of Toll-like receptor 4 (TLR4)/NF-ҡB signaling. In this study, curcumin attenuated neuroinflammation via inhibition of microglia and astrocyte activity and suppression of FKN/CX3CR1 up-regulation [69]. In the central nervous system, macrophage inflammatory protein-2 (MIP-2) plays an important role in the induction of inflammation that is produced by astrocytes. Curcumin inhibited the induction of MIP-2 gene expression and the production of the MIP-2 protein, and treated inflammatory diseasesof the CNS [70].
Multiple sclerosis (MS)
Multiple sclerosis (MS) is considered to be a chronic disorder of the central nervous system, the main symptoms of which are inflammation, neuronal injury, axonal degeneration and breaching of the blood–brain barrier [71]. In the disease process, curcumin offers neuroprotective and neurodegenerative activity through reduction of astrocyte activity. Curcumin reduced astrocyte activation by inhibition of MMP-9, MCP-1 expression and secretion of IL-6. The levels of IL-6 level are known to increase in neurodegenerative disorders, a [18]. In a study conducted by Naeimi et al., curcumin-loaded nanoparticles alleviated the multiple sclerosis disease process, indicating that curcumin-loaded NPs are an effective strategy for improving the solubility of curcumin. The protective effects of curcumin-loaded NPs might be partly mediated through anti-inflammatory activity, the inhibitory effects of glial and attenuation of oxidative stress in the context of demyelination [72].
Neuropathic pain
Neuropathic pain (NP) is defined as pain caused by disorders of the peripheral nervous system or the central nervous system, with damage or disease affecting the somato sensory nervous system.
Astrocytes are the major subset of glial cells and microglia in the CNS and play an important role in the development and maintenance of long-term chronic pain. Research on curcumin has suggested some mechanisms of action mediating the therapeutic effects of curcumin on NP. These mechanisms include inhibition of TNF-α-induced astrocyte migration, decrease in MCP-1 expression, up-regulation of SOD2 expression in TNF-α-induced astrocytes [73], increased expression of GFAP and phosphorylated ERK, inhibition of aggregation of the NALP1 inflammasome and activation of the JAK2-STAT3 cascade in astrocytes [74].
Oxidative stress in the brain and related pathology
Oxidative stress results from an imbalance between antioxidative defenses and the formation of ROS, is involved in a variety of neurodegenerative diseases. Astrocytes support neuronal survival by activitation of antioxidants and glutathione that inactivate ROS. A deficiency of antioxidative function in astrocytes leads to a variety of neurodegenerative disorders [75].
Curcumin protects primary spinal cord astrocytes against oxidative stress. Phase II enzymes by the detoxication of toxin and scavenge of free radicals play an important role in protecting cells against stress. Furthermore, antioxidant response element (ARE) increase the many phase II antioxidant enzymes genes including glutamate cysteine ligase (GCL), heme oxygenase 1 (HO1) and reduced quinine oxidoreductase 1, nicotinamide adenine dinucleotide phosphate (NADPH) [76]. In the nucleus, Nrf2 binds to ARE that reduce the ROS and restore redox balance. Thus, curcumin in astrocytes activated the Nrf2 cytoprotective signaling and activation of antioxidative function that led to reduce the level of reactive-oxygen species and attenuated oxidative damage [75]. Curcumin inhibited oxidative stress in astrocytes by attenuating the astrogliosis markers, GFAP and vimentin, inhibition of Prdx6 and suppression of apoptosis [8]. Daverey et al., showed the protective effect of curcumin and resveratrol against oxidative stress in astrocytes; both compounds protected astrocytes but curcumin was most effective in first few hours and showed better anti-inflammatory action against NF-ҡB [77]. The protective effect of the three different antioxidants (curcumin, Trolox, and N-acetylcysteine) against oxidative stress induced by acrylonitrile (AN) in rat astrocytes was investigated by Yu et.al. Both pre- and post-treatments with N-acetylcysteine and curcumin significantly reduced oxidative stress in AN-induced toxicity in vitro and post-treatment with Trolox showed high efficacy against AN-induced oxidative damage, this effect being related to activation of the Nrf2 signaling pathway [78]. In a study conducted by Lavoie et al., the ability of curcumin, quercetin, and tBHQ to increase GSH synthesis in cultured astrocytes and neurons were assessed. All three compounds increased GSH levels and GCL activity in astrocytes, but only curcumin increased GSH synthesis and inhibited oxidative stress in neurons [79]. The interaction between astroglia and curcumin led to an increase in the cellular content of GSH in parallel with an elevated basal and stimulated efflux, which could be a prerequisite for astroglial mediated neuroprotection [80]. Heme oxygenase-1 (HO-1) provides efficient cytoprotection against oxidative stress and is a redox-sensitive inducible stress protein. Caffeic acid phenethyl ester and curcumin were found to significantly increase HO-1 expression and heme oxygenase activity in astrocytes and protected endothelial cells against oxidativestress. In cultured astrocytes and neurons, curcumin-activated phase II enzymes, increased HO-1 expression and exhibited cytoprotective effects against oxidative damage by activation of the transcription factor Nrf2 [81].
The most important underlying reason for seizure-induced neuronal damage is increased oxidative stress. Kainic acid (KA), the analog of the excitatory amino acid l-glutamate, and KA-subtype receptors are particularly abundant in the astrocytic end feet and glial fibers that surround hippocampal capillaries. Connected to the non-N-methyl-d-aspartate (non-NMDA) glutamate receptors is an analogue of the excitotoxin glutamate that causes neurodegeneration, plasticity, memory loss, and neuronal cell death. Some antioxidants exist at high concentrations in astrocytes and protect the surrounding cells from oxidative stress-induced cell death. Some studies have demonstrated that curcumin, a potent antioxidant, may provide protection for KA-induced oxidative stress. Curcumin also showed an inhibitory effect against reactive astrocyte expression and microglial activation, and reduced the KA-induced immunoreactivity of caspase-3 [82].
Exposure to organophosphate pesticides (OPs) have been considered to be an important factor in the pathophysiology of various neurodegenerative diseases. Thus, Canales et al., investigated the protective effect of curcumin against OP-induced toxicity in rat hippocampus. The administration of curcumin at a dose of 200 mg/kg diminished the oxidative damage and reduced lipid peroxidation in rats exposed to Ops [83]. Finally, curcumin, via other mechanisms, such as enhancement of antioxidative function, reduction of oxidative stress and amelioration of mitochondrial function, exerted a protective effect against neurodegenerative disorders and diseases exacerbated by oxidative stress.
Curcumin and glial tumors
Several studies have indicated that curcumin is the active molecule responsible for various biological effects, including anticancer activity. In phase I clinical trials,curcumin was used for the treatment of some high-risk cancers. In vitro and in vivo, the inhibitory effect of curcumin was studied against abnormal expression of matrix metalloproteinases (MMPs). In vitro, curcumin had an effect on proliferation, apoptosis via the classical GBM survival pathways (PI3K/Akt and NF-ҡB), and selectivity to cancer. In the C6 implant mouse model of glioblastoma (GBM), curcumin activity was observed irrespective of the p53 and PTEN mutational status of the cells and reduced the size of the brain tumors in C6-implanted rats [84]. In addition, Kim et al. suggested that curcumin has broad-spectrum inhibitory activity against MMP gene expression in human astroglioma cells via suppression of PMA-induced MAP kinase activity, repression of DNA binding and the transcriptional activity of AP-1. These findings suggest that curcumin might be of therapeutic value for treating gliomas [85]. Curcumin also showed an inhibitory effect on MMP-9 expression via NF-ҡB and AP-1, their DNA binding activities being suppressed by curcumin. Furthermore, PMA-induced phosphorylation of ERK, JNK, and p38 MAP kinase were strongly inhibited by curcumin [86].
In malignant astrocytoma cells, nuclear factor (NF)-ҡB is activated aberrantly and promotes proliferation. The inhibitory effects of curcumin on NF-ҡB activity in a NP-2 human malignant astrocytoma cell line were investigated by DNA-fragmentation analysis, morphological observation and cellular proliferation; curcumin inhibited the cellular proliferation and induced apoptosis [87]. Romero-Hernández et al. showed that curcumin at a concentration < 100 µM increased morphological changes and induced apoptosis in the astrocytoma cell line without altering the DNA [88].
Developing novel and potent drugs is critical in the treatment of cancer. New derivatives of curcumin are far more active than natural curcumin against brain tumors. A new synthetic compound of curcumin with 2-ethylamino groups in a chalcone structure and variously substituted triazole groups as side chains (designed with a mimic library) were evaluated against TRAIL resistant human CRT-MG astroglioma cells. Among them, the derivatives of curcumin with the triazole groups were promising candidates as TRAIL-sensitizers with potential use in combination chemotherapy for brain tumor [89].
Cancer nanotherapeutics is becoming an area of intense global reasearch interest. In 2014, development of a stable nanostructured lipid carrier (NLC) system as a carrier for curcumin (CRM) on astrocytoma–glioblastoma cell line (U373MG) was investigated; particle size, polydispersity index, and entrapment efficiency (EE) were 146.8 nm, 0.18, and 90.86%, respectively. The cytotoxicity of CRM–NLC was higherthan CRM in the astrocytoma–glioblastoma cell line and suggested CRM–NLC to bea promising drug delivery system for brain cancer therapy [90].
The BBB limits the access of the majority of anticancer drugs to the brain tissue. Therefore, a variety of approaches, such as receptor-mediated endocytosishas garnered attention because of its compelling advantages. Gold–iron oxide nanocomposites (NCs) are one of the most utilized nanocarriers in the field of drug delivery. The synthesis of Fe3O4@Au NCs of curcumin with a mean size of less than 50 nm was introduced as a novel anticancer drug, enhancing cytotoxicity compared with free curcumin. In addition, these nanoparticles were less toxic in comparison to free curcumin, especially for normal astrocyte cells [91].
Glo1 (a type of glyoxalase) is ubiquitously expressed in all mammalian cells and is suggested as promoting cellular aging and cell death. It is known that inhibitors of Glo1, structurally related to glutathione, have anti-proliferative properties. Curcumin is known as a strong antioxidant and anti-inflammatory compound. Therefore, the inhibitory effect of curcumin was investigated in various tumor cell lines (PC-3, JIM-1, MDA-MD 231, and 1321N1) in terms of LPS-induced cytokine production (cytometric bead-based array), cell proliferation (WST-1 assay), and cytosolic Glo1 and Glo2 enzymatic activity. The results showed that curcumin exhibited a potent inhibitory effect on Glo1 (Ki = 5.161.4 mM) [92].
Malignant gliomas are brain tumors that are resistant to both radiation and chemotherapy. Overexpression of transcription factors, such as NF-ҡB and AP-1 are associated with enhanced glioma survival. There is evidence suggesting a role for curcumin as an adjunct to chemotherapy and radiation in the treatment of brain cancer. The effect of curcumin on glioma survival was therefore investigated in human (T98G, U87MG, and T67) and rat (C6) glioma cell lines. Curcumin reduced the cell survival via p53-mediated mechanism and inhibited the AP-1 and NF-ҡB signaling pathways via prevention of constitutive JNK and Akt activation [93]. Kang and collaborators observed that curcumin induced histone hypoacetylation in brain cancer cells, and induced apoptotic cell death viaa (PARP)-, and caspase 3-mediated mechanism. This study suggested that curcumin is a histone acetyltransferase inhibitor that simultaneously controls the expression of many genes [94].
Other neurodegenerative diseases
Alexander disease is a devastating, progressive and fatal disorder of the CNS that is characterized by intracytoplasmic Rosenthal fibers (RFs) in dystrophic astrocytes. Bachetti et al. investigated the effects of curcumin in a cellular model of Alexander disease. Curcumin is able to induce both HSP27 and alpha B-crystallin, and reduced levels of endogenous GFAP, both at RNA and protein levels, and induced autophagy [95].
Pelizaeus–Merzbacher disease (PMD) is a progressive and lethal leukodystrophy that is characterized by cognitive impairment, ataxia, seizures, and spasticity. This disease is caused by various mutations within the proteolipid protein gene (PLP1). Curcumin ameliorates symptoms of PMD in a Plp1 transgenic mouse model through reduction of astrocytosis, microgliosis, and lymphocytic infiltration with preservation of myelinated axons [96].
Diabetes mellitus is a widely prevalent metabolic disorder, placing a huge burden on public health systems. It is characterized by the presence of hyperglycemia resulting from defective insulin action, deficient levels of the hormone insulin, or both [97]. Diabetes mellitus is associated with an increased risk for dementia and lower cognitive performance. Curcumin reduced the deterious effect of diabetes in a STZ-induced diabetes model. Curcumin caused a reduction of GFAP in astrocytes, reduced expression of pancreatic and cardiac caspase-3 content, decreased serum glucose levels, and exerted a neuroprotective effect [98].
Chronic intermittent hypoxia (CIH) is a main characteristic of obstructive sleep apnea (OSA). Curcumin mitigated the brain edema in a dose-dependent manner and exhibited a neuroprotective effect in CIH-induced brain injury by the inhibition of AQP4 expression, and the p38MAPK pathway [99]. Multiple forms of cellular stress, such as nutrient deprivation, toxins, limitation of energy, radiation, hypoxia, DNA damage, and intracellular pathogens can stimulate autophagy. Recently, the neuroprotective effect of curcumin on cell cultures of normal rat astrocytes, subjected to 0.01 μM lipopolysaccharide (LPS), was investigated. Curcumin significantly increased the cell viability, and moderated the cytoskeleton rearrangements, and disordered NF-ҡB-dependent regulation in the LPS-activated astrocytes [100].
Gulf War Illness (GWI) is a chronic condition, the major symptoms of which are reduced ability to concentrate, sleep disturbance, and chronic pain. Kodali et al. reported that curcumin treatment is effective in an animal model of GWI. Curcumin exerted its effect through increased antioxidant activity, reduced astrocyte hypertrophy, and inhibition of inflammation [101].
Conclusions
In our study, we discussed the role of astrocytes as essential players in neurodegenerative diseases, and elaborated on the therapeutic effect of curcumin in brain pathologies. To our knowledge, this is the first review devoted on this subject matter. Monroy et al. (2013) discussed neuroprotective effect of curcumin especially on Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease [102]. Shuxin Hu (2015) reviewed the clinical development of curcumin in neurodegenerative disease and addresses the rationale for its use in neurodegenerative disease, particularly Alzheimer’s disease. We have been aware of these reviews and can ensure the subject matter and discussed contents are quite different [103].
There have been numerous experimental data supporting the potential efficacy of curcumin in neurodegenerative diseases by improving the learning and neuroprotection against disease-producing insults in animal models. However, due to interactions with specific efflux transporters produced in the BBB or its lipophilic nature, the in vivo use of curcumin as a neuroprotective agents is limited. Nevertheless, curcumin is a potent bioactive molecule and its beneficial therapeutic effects are well recognized.
Astrocytes are the most structurally complex cell type in the CNS, activation leading to a wide spectrum of CNS injury and disease. Astrocytes respond to CNS injuries, such as cerebral ischemia, cancer or neurodegenerative disease, by becoming activated, a process termed reactive gliosis and caused by astrocytic hypertrophy, proliferation, and altered gene expression, including regulation of glial fibrillary acidic protein (GFAP). Several reports suggest that activated astrocytes play an important role in neurodegenerative disorders, such as epilepsy, AD, PD, and even depression and schizophrenia. Extant evidence from in vitro (Table 1) and in vivo (Table 2) studies has shown that curcumin exerts its therapeutic effect through downregulation of the expression of the chemokines MCP-1, RANTES, and CXCL10 released by astrocytes, suppression of proinflammatory cytokines, and reduction of the expression of GFAP (Fig. 1).
In summary, it is evident that curcumin is a potent bioactive molecule that can modulate cell signaling, inhibit neurodegeneration, and modulate brain function. Further investigation is needed into neuron–astrocyte interactions during brain development as well as discovery of novel neuroprotective strategies for neurodegenerative disease treatment, especially involving astrocytic pathways.
Abbreviations
- TNF:
-
Tumor necrosis factor
- ROS:
-
Reactive-oxygen species
- NO:
-
Nitric oxide
- MAPK:
-
Mitogen-activated protein kinases
- Iba-1:
-
Ionized calcium binding adaptor molecule 1
- MCP-1:
-
Monocyte chemoattractant protein-1
- JAKs:
-
Janus kinases
- STATs:
-
Signal transducer and activator of transcription proteins
- MMP-9:
-
Matrix metallopeptidase 9
- IL-6:
-
Interleukin 6
- IL-1β:
-
Interleukin 1 beta
- MIP-1 α:
-
Macrophage inflammatory protein-1 alpha
- MIP-2:
-
Macrophage inflammatory protein 2
- NF-ҡB:
-
The inducible transcription factor nuclear factor-kappa B
- RANTES:
-
Regulated upon activation, normal T cell
- iNOS:
-
Inducible nitric oxide synthase
- BDNF:
-
Brain-derived neurotrophic factor
- CB1:
-
Cannabinoid receptor type 1
- GFAP:
-
Glial fibrillary acidic protein
- COX-2:
-
Cyclooxygenase-2
- MEK1:
-
Mitogen-activated protein kinase
- ERK1/2:
-
Extracellular signal-regulated kinase 2
- CISD2:
-
The causative gene in Wolfram syndrome 2
References
Kettenmann H, Ransom B. Neuroglia. 2nd ed. Oxford: Oxford University Press; 2005.
Sandhu JK, Sodja C, Byrd A, Cadieux C, Lanthier ZP, Roy Walker P, et al. The significance of astrocytic glutathione system in neuroprotection: a potential role for curcumin. Glutathione: biochemistry, mechanisms of action and biotechnological implications;2013. pp. 95–110.
Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7(4):338–53.
Nag S. Morphology and properties of astrocytes. The blood-brain and other neural barriers. New York: Springer; 2011. p. 69–100.
Aloisi F. The role of microglia and astrocytes in CNS immune surveillance and immunopathology. The functional roles of glial cells in health and disease. New York: Springer; 1999. p. 123–133.
Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harbor perspectives in biology. 2015;2015:a020628.
Ben Haim L, Carrillo-de Sauvage M-A, Ceyzériat K, Escartin C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci. 2015;9:278.
Daverey A, Agrawal SK. Curcumin alleviates oxidative stress and mitochondrial dysfunction in astrocytes. Neuroscience. 2016;333:92–103.
Chin D, Huebbe P, Pallauf K, Rimbach G. Neuroprotective properties of curcumin in Alzheimer's disease–merits and limitations. Curr Med Chem. 2013;20(32):3955–85.
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res (Stuttg). 2017;67(4):244–51.
Fereydouni N, Darroudi M, Movaffagh J, Shahroodi A, Butler AE, Ganjali S, et al. Curcumin nanofibers for the purpose of wound healing. J Cell Physiol. 2018;234(5):5537–54.
Panahi Y, Ghanei M, Bashiri S, Hajihashemi A, Sahebkar A. Short-term curcuminoid supplementation for chronic pulmonary complications due to sulfur mustard intoxication: positive results of a randomized double-blind placebo-controlled trial. Drug Res. 2014;65(11):567–73.
Hamzehzadeh L, Atkin SL, Majeed M, Butler AE, Sahebkar A. The versatile role of curcumin in cancer prevention and treatment: A focus on PI3K/AKT pathway. J Cell Physiol. 2018;233(10):6530–7.
Rezaee R, Momtazi AA, Monemi A, Sahebkar A. Curcumin: A potentially powerful tool to reverse cisplatin-induced toxicity. Pharmacol Res. 2017;117:218–27.
Iranshahi M, Sahebkar A, Takasaki M, Konoshima T, Tokuda H. Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo. Eur J Cancer Prev. 2009;18(5):412–5.
Sahebkar A. Molecular mechanisms for curcumin benefits against ischemic injury. Fertil Steril. 2010;94(5):e75–e7676.
Karimian MS, Pirro M, Majeed M, Sahebkar A. Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev. 2017;33:55–63.
Seyedzadeh MH, Safari Z, Zare A, Gholizadeh Navashenaq J, Razavi SA, Kardar GA, et al. Study of curcumin immunomodulatory effects on reactive astrocyte cell function. Int Immunopharmacol. 2014;22(1):230–5.
Abdollahi E, Momtazi AA, Johnston TP, Sahebkar A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: a nature-made jack-of-all-trades? J Cell Physiol. 2018;233(2):830–48.
Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal. Chem Curcumin J Med Chem. 2017;60(5):1620–37.
Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1411–7.
Shakeri A, Zirak MR, Hayes AW, Reiter R, Karimi G. Curcumin and its analogues protect from endoplasmic reticulum stress: mechanisms and pathways. Pharmacol Res. 2019;146:104335.
Nones J, Stipursky J, Costa SL, Gomes FCA. Flavonoids and astrocytes crosstalking: implications for brain development and pathology. Neurochem Res. 2010;35(7):955–66.
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421.
Zhang ZJ, Zhao LX, Cao DL, Zhang X, Gao YJ, Xia C. Curcumin inhibits LPS-induced CCL2 expression via JNK pathway in C6 rat astrocytoma cells. Cell Mol Neurobiol. 2012;32(6):1003–100.
Sundaram JR, Poore CP, Sulaimee NHB, Pareek T, Cheong WF, Wenk MR, et al. Curcumin ameliorates neuroinflammation, neurodegeneration, and memory deficits in p25 transgenic mouse model that bears hallmarks of Alzheimer’s disease. J Alzheimers Dis. 2017;60(4):1429–42.
Liu ZJ, Li ZH, Liu L, Tang WX, Wang Y, Dong MR, et al. Curcumin attenuates beta-amyloid-induced neuroinflammation via activation of peroxisome proliferator-activated receptor-gamma function in a rat model of Alzheimer's disease. Front Pharmacol. 2016;7:261.
Lin MS, Hung KS, Chiu WT, Sun YY, Tsai SH, Lin JW, et al. Curcumin enhances neuronal survival in N-methyl-d-aspartic acid toxicity by inducing RANTES expression in astrocytes via PI-3K and MAPK signaling pathways. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):931–8.
Tai YH, Lin YY, Wang KC, Chang CL, Chen RY, Wu CC, et al. Curcuminoid submicron particle ameliorates cognitive deficits and decreases amyloid pathology in Alzheimer's disease mouse model. Oncotarget. 2018;9(12):10681–97.
Maiti P, Paladugu L, Dunbar GL. Solid lipid curcumin particles provide greater anti-amyloid, anti-inflammatory and neuroprotective effects than curcumin in the 5xFAD mouse model of Alzheimer's disease. BMC Neurosci. 2018;19(1):1–18.
Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21(21):8370–7.
Kuo YC, Lin CC. Rescuing apoptotic neurons in Alzheimer’s disease using wheat germ agglutinin-conjugated and cardiolipin-conjugated liposomes with encapsulated nerve growth factor and curcumin. Int J Nanomed. 2015;10:2653–72.
Wang Y, Yin H, Wang L, Shuboy A, Lou J, Han B, et al. Curcumin as a potential treatment for Alzheimer's disease: a study of the effects of curcumin on hippocampal expression of glial fibrillary acidic protein. Am J Chin Med. 2013;41(1):59–70.
Ganugapati J, Babu R, Ahuja SJ, Mukundan M, Vutukuru SS. Screening and molecular docking studies of curcumin and its derivatives as inhibitors of amyloid-β protein: a key protein in Alzheimer’s disease. Asian J Pharm Clin Res. 2015;8(2):98–101.
Wang HM, Zhao YX, Zhang S, Liu GD, Kang WY, Tang HD, et al. PPARγ agonist curcumin reduces the amyloid-β-stimulated inflammatory responses in primary astrocytes. J Alzheimers Dis. 2010;20(4):1189–99.
Ambegaokar SS, Wu L, Alamshahi K, Lau J, Jazayeri L, Chan S, et al. Curcumin inhibits dose-dependently and time-dependently neuroglial cell proliferation and growth. Neuroendocrinol Lett. 2003;24(6):469–73.
Wang Y-L, Ju B, Zhang Y-Z, Yin H-L, Liu Y-J, Wang S-S, et al. Protective effect of curcumin against oxidative stress-induced injury in rats with parkinson’s disease through the Wnt/β-catenin signaling pathway. Cell Physiol Biochem. 2017;43(6):2226–41.
Norenberg MD. Reactive astrocytosis. In: Aschner A, Kimelberg HK, editors. The role of glia in neurotoxicity. Boca Raton, FL: CRC Press; 1996. p. 93–107.
Tripanichkul W, Jaroensuppaperch EO. Ameliorating effects of curcumin on 6-OHDA-induced dopaminergic denervation, glial response, and SOD1 reduction in the striatum of hemiparkinsonian mice. Eur Rev Med Pharmacol Sci. 2013;17(10):1360–8.
Yu S, Wang X, He X, Wang Y, Gao S, Ren L, et al. Curcumin exerts anti-inflammatory and antioxidative properties in 1-methyl-4-phenylpyridinium ion (MPP+)-stimulated mesencephalic astrocytes by interference with TLR4 and downstream signaling pathway. Cell Stress Chaperones. 2016;21(4):697–705.
Gui HY, Chen RN, Peng Y, Hu JH, Mao Z, Ning R, et al. Curcumin protects against 1-methyl-4-phenylpyridinium ion- and lipopolysaccharide-induced cytotoxicities in the mouse mesencephalic astrocyte via inhibiting the cytochrome P450 2E1. Evid Based Complement Alternat Med. 2013;2013:523484. https://doi.org/10.1155/2013/523484.
He XJ, Uchida K, Megumi C, Tsuge N, Nakayama H. Dietary curcumin supplementation attenuates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity in C57BL mice. Exp Toxicol Pathol. 2015;28(4):197–206.
Sharma N, Sharma S, Nehru B. Curcumin protects dopaminergic neurons against inflammation-mediated damage and improves motor dysfunction induced by single intranigral lipopolysaccharide injection. Inflammopharmacology. 2017;25(3):351–68.
Sharma N, Nehru B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology. 2018;26(2):349–60.
Tripanichkul W, Jaroensuppaperch EO. Curcumin protects nigrostriatal dopaminergic neurons and reduces glial activation in 6-hydroxydopamine hemiparkinsonian mice model. Int J Neurosci. 2012;122(5):263–70.
Abbaoui A, Gamrani H. Neuronal, astroglial and locomotor injuries in subchronic copper intoxicated rats are repaired by curcumin: a possible link with Parkinson's disease. Acta Histochem. 2018;120(6):542–50.
Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005;82(1):138–48.
Jiang J, Wang W, Sun YJ, Hu M, Li F, Zhu DY. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood–brain barrier damage. Eur J Pharmacol. 2007;561(1–3):54–62.
El-Haroun H. Microscopic study of a possible ameliorating role of curcumin against the effects of first-generation and second-generation antipsychotic drugs in adult rat cerebral cortex. Egypt J Chem. 2016;39(1):96–108.
Zhang P, Yu T, Zhang X, Li Y. Curcumin alters expression of glial fibrillary acidic protein and nestin following chronic cerebral ischemia. Neural Regen Res. 2011;6(9):651–5.
Rajasekar N, Dwivedi S, Tota SK, Kamat PK, Hanif K, Nath C, et al. Neuroprotective effect of curcumin on okadaic acid induced memory impairment in mice. Eur J Pharmacol. 2013;715(1–3):381–94.
Amer MG, Karam RA. Morphological and biochemical features of cerebellar cortex after exposure to zinc oxide nanoparticles: possible protective role of curcumin. Anat Rec. 2018;301(8):1454–66.
Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol. 2016;79:360–9.
Levin HS, Eisenberg HM, Gary HE, Marmarou A, Foulkes MA, Jane JA, et al. Intracranial hypertension in relation to memory functioning during the first year after severe head injury. Neurosurgery. 1991;28(2):196–200.
Wang BF, Cui ZW, Zhong ZH, Sun YH, Sun QF, Yang GY, et al. Curcumin attenuates brain edema in mice with intracerebral hemorrhage through inhibition of AQP4 and AQP9 expression. Acta Pharmacol Sin. 2015;36(8):939–48.
Banerjee PN, Filippi D, Hauser WA. The descriptive epidemiology of epilepsy—a review. Epilepsy Res. 2009;85(1):31–45.
Drion CM, van Scheppingen J, Arena A, Geijtenbeek KW, Kooijman L, van Vliet EA, et al. Effects of rapamycin and curcumin on inflammation and oxidative stress in vitro and in vivo—in search of potential anti-epileptogenic strategies for temporal lobe epilepsy. J Neuroinflammation. 2018;15(1):212.
Jiang Z, Guo M, Shi C, Wang H, Yao L, Liu L, et al. Protection against cognitive impairment and modification of epileptogenesis with curcumin in a post-status epilepticus model of temporal lobe epilepsy. Neuroscience. 2015;310:362–71.
Kaur H, Patro I, Tikoo K, Sandhir R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem Int. 2015;89:40–50.
Lin MS, Lee YH, Chiu WT, Hung KS. Curcumin provides neuroprotection after spinal cord injury. J Surg Res. 2011;166(2):280–9.
Lin CC, Chiang TH, Chen WJ, Sun YY, Lee YH, Lin MS. CISD2 serves a novel role as a suppressor of nitric oxide signalling and curcumin increases CISD2 expression in spinal cord injuries. Injury. 2015;46(12):2341–50.
Yuan J, Liu W, Zhu H, Chen Y, Zhang X, Li L, et al. Curcumin inhibits glial scar formation by suppressing astrocyte-induced inflammation and fibrosis in vitro and in vivo. Brain Res. 2017;1655:90–103.
Wang YF, Zu JN, Li J, Chen C, Xi CY, Yan JL. Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci Lett. 2014;560:51–6.
Bondan E, Cardoso C, Martins MF. Curcumin decreases astrocytic reaction after gliotoxic injury in the rat brainstem. Arq Neuropsiquiatr. 2017;75(8):546–52.
Johnson SA, Fournier NM, Kalynchuk LE. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res. 2006;168(2):280–8.
Huang Z, Zhong X-M, Li Z-Y, Feng C-R, Pan A-J, Mao Q-Q. Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neurosci Lett. 2011;493(3):145–8.
He X, Yang L, Wang M, Zhuang X, Huang R, Zhu R, et al. Targeting the endocannabinoid/CB1 receptor system for treating major depression through antidepressant activities of curcumin and dexanabinol-loaded solid lipid nanoparticles. Cell Physiol Biochem. 2017;42(6):2281–94.
Chen JJ, Dai L, Zhao LX, Zhu X, Cao S, Gao YJ. Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis. Sci Rep. 2015;5:10278.
Xu MX, Yu R, Shao LF, Zhang YX, Ge CX, Liu XM, et al. Up-regulated fractalkine (FKN) and its receptor CX3CR1 are involved in fructose-induced neuroinflammation: suppression by curcumin. Brain Behav Immun. 2016;58:69–81.
Tomita M, Holman BJ, Santoro CP, Santoro TJ. Astrocyte production of the chemokine macrophage inflammatory protein-2 is inhibited by the spice principle curcumin at the level of gene transcription. J Neuroinflamm. 2005;2:8.
Haider L, Zrzavy T, Hametner S, Höftberger R, Bagnato F, Grabner G, et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain. 2016;139(3):807–15.
Naeimi R, Safarpour F, Hashemian M, Tashakorian H, Ahmadian SR, Ashrafpour M, et al. Curcumin-loaded nanoparticles ameliorate glial activation and improve myelin repair in lyolecithin-induced focal demyelination model of rat corpus callosum. Neurosci Lett. 2018;674:1–10.
Qin X, Qiao H, Wu S, Cheng J, Wan Q, Liu R. curcumin inhibits monocyte chemoattractant protein-1 expression in TNF-α induced astrocytes through AMPK pathway. Neurochem Res. 2018;43(4):775–84.
Liu S, Li Q, Zhang MT, Mao-Ying QL, Hu LY, Wu GC, et al. Curcumin ameliorates neuropathic pain by down-regulating spinal IL-1β via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci Rep. 2016;6:1–4.
Jiang H, Tian X, Guo Y, Duan W, Bu H, Li C. Activation of nuclear factor erythroid 2-related factor 2 cytoprotective signaling by curcumin protect primary spinal cord astrocytes against oxidative toxicity. Biol Pharm Bull. 2011;34(8):1194–7.
Zhao L, Liu Z, Jia H, Feng Z, Liu J, Li X. Lipoamide acts as an indirect antioxidant by simultaneously stimulating mitochondrial biogenesis and phase II antioxidant enzyme systems in arpe-19 cells. PLoS ONE. 2015;10:e0128502.
Daverey A, Agrawal SK. Pre and post treatment with curcumin and resveratrol protects astrocytes after oxidative stress. Brain Res. 2018;1692:45–55.
Yu B, Changsheng Y, Wenjun Z, Ben L, Hai Q, Jing M, et al. Differential protection of pre- versus post-treatment with curcumin, Trolox, and N-acetylcysteine against acrylonitrile-induced cytotoxicity in primary rat astrocytes. NeuroToxicol. 2015;51:58–66.
Lavoie S, Chen Y, Dalton TP, Gysin R, Cuénod M, Steullet P, et al. Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: importance of the glutamate cysteine ligase modifier subunit. J Neurochem. 2009;108(6):1410–22.
Stridh MH, Correa F, Nodin C, Weber SG, Blomstrand F, Nilsson M, et al. Enhanced glutathione efflux from astrocytes in culture by low extracellular Ca2+ and curcumin. Neurochem Res. 2010;35(8):1231–8.
Scapagnini G, Colombrita C, Amadio M, D'Agata V, Arcelli E, Sapienza M, et al. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal. 2006;8(3–4):395–403.
Shin HJ, Lee JY, Son E, Lee DH, Kim HJ, Kang SS, et al. Curcumin attenuates the kainic acid-induced hippocampal cell death in the mice. Neurosci Lett. 2007;416(1):49–544.
Canales-Aguirre AA, Gomez-Pinedo UA, Luquin S, Ramírez-Herrera MA, Mendoza-Magaña ML, Feria-Velasco A. Curcumin protects against the oxidative damage induced by the pesticide parathion in the hippocampus of the rat brain. Nutr Neurosci. 2012;15(2):62–9.
Zanotto-Filho A, Braganhol E, Edelweiss MI, Behr GA, Zanin R, Schröder R, et al. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J Nutr Biochem. 2012;23(6):591–601.
Kim SY, Jung SH, Kim HS. Curcumin is a potent broad spectrum inhibitor of matrix metalloproteinase gene expression in human astroglioma cells. Biochem Biophys Res Commun. 2005;337(2):510–6.
Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH, Kim WK, et al. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun. 2005;335(4):1017–25.
Nagai S, Kurimoto M, Washiyama K, Hirashima Y, Kumanishi T, Endo S. Inhibition of cellular proliferation and induction of apoptosis by curcumin in human malignant astrocytoma cell lines. J Neurooncol. 2005;74(2):105–11.
Romero-Hernández MA, Eguía-Aguilar P, Perézpeña-Diazconti M, Rodríguez-Leviz A, Sadowinski-Pine S, Velasco-Rodríguez LA, et al. Toxic effects induced by curcumin in human astrocytoma cell lines. Toxicol Mech Methods. 2013;23(9):650–9.
Ahn Y, Oh S, Lee SJ, Park BG, Park YS, Shin WS, et al. Synthesis of diethylamino-curcumin mimics with substituted triazolyl groups and their sensitization effect of TRAIL against brain cancer cells. Bioorg Med Chem Lett. 2014;24(15):3346–50.
Madane RG, Mahajan HS. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: design, characterization, and in vivo study. Drug Deliv. 2016;23(4):1326–34.
Ghorbani M, Bigdeli B, Jalili-baleh L, Baharifar H, Akrami M, Dehghani S, et al. Curcumin-lipoic acid conjugate as a promising anticancer agent on the surface of gold-iron oxide nanocomposites: a pH-sensitive targeted drug delivery system for brain cancer theranostics. Eur J Pharm Biopharm. 2018;114:175–88.
Santel T, Pflug G, Hemdan NYA, Schäfer A, Hollenbach M, Buchold M, et al. Curcumin inhibits glyoxalase 1—a possible link to its anti-inflammatory and anti-tumor activity. PLoS One. 2008;3(10):e3508. https://doi.org/10.1371/journal.pone.0003508.
Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFκB transcription factors. J Neurochem. 2007;102(2):522–38.
Kang SK, Cha SH, Jeon HG. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006;15(2):165–74.
Bachetti T, Di Zanni E, Balbi P, Ravazzolo R, Sechi G, Ceccherini I. Beneficial effects of curcumin on GFAP filament organization and down-regulation of GFAP expression in an in vitro model of Alexander disease. Exp Cell Res. 2012;318(15):1844–54.
Epplen DB, Prukop T, Nientiedt T, Albrecht P, Arlt FA, Stassart RM, et al. Curcumin therapy in a Plp1 transgenic mouse model of Pelizaeus-Merzbacher disease. Ann Clin Transl Neurol. 2015;2(8):787–96.
Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement 1):S81–S90.
Faheem NM, Askary AE. Neuroprotective role of curcumin on the hippocampus against the structural and serological alterations of streptozotocin-induced diabetes in sprague dawely rats. Iran J Basic Med Sci. 2017;20(6):690–9.
Wang B, Li W, Jin H, Nie X, Shen H, Li E, et al. Curcumin attenuates chronic intermittent hypoxia-induced brain injuries by inhibiting AQP4 and p38 MAPK pathway. Respir Physiol Neurobiol. 2018;255:50–7.
Nedzvetsky VS, Agca CA, Kyrychenko SV. Neuroprotective effect of curcumin on LPS-activated astrocytes is related to the prevention of GFAP and NF-κB upregulation. Neurophysiology. 2017;49(4):305–7.
Kodali M, Hattiangady B, Shetty GA, Bates A, Shuai B, Shetty AK. Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav Immuny. 2018;69:499–514.
Monroy A, Lithgow GJ, Alavez S. Curcumin and neurodegenerative diseases. BioFactors. 2013;39(1):122–32.
Hu S, Maiti P, Ma Q, Zuo X, Jones M, Cole GM, et al. Clinical development of curcumin in neurodegenerative disease. Expert Rev Neurother. 2015;15(6):629–37.
Lin MS, Sun YY, Chiu WT, Hung CC, Chang CY, Shie FS, et al. Curcumin attenuates the expression and secretion of RANTES after spinal cord injury in vivo and lipopolysaccharide-induced astrocyte reactivation in vitro. J Neurotrauma. 2011;28(7):1259–69.
Hoppe JB, Coradini K, Frozza RL, Oliveira CM, Meneghetti AB, Bernardi A, et al. Free and nanoencapsulated curcumin suppress β-amyloid-induced cognitive impairments in rats: involvement of BDNF and Akt/GSK-3β signaling pathway. Neurobiol Learn Mem. 2013;106:134–44.
Author information
Authors and Affiliations
Contributions
Conceived the idea: SE, FF, MM, AS. Wrote the manuscript: SE, FF, GEB, MM, AS. Reviewed critically for content: GEB, AS. All authors approved the final manuscript and submission.
Corresponding author
Ethics declarations
Conflict of interest
Muhammed Majeed is the founder of Sabinsa Corporation and Sami Labs Ltd. Other authors have no direct competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eghbaliferiz, S., Farhadi, F., Barreto, G.E. et al. Effects of curcumin on neurological diseases: focus on astrocytes. Pharmacol. Rep 72, 769–782 (2020). https://doi.org/10.1007/s43440-020-00112-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00112-3