Abstract
Question
What is the role of temozolomide in the management of adult patients (aged 65 and under) with newly diagnosed glioblastoma?
Target population
These recommendations apply to adult patients diagnosed with newly diagnosed glioblastoma.
Recommendation
Level I: Concurrent and post-irradiation Temozolomide (TMZ) in combination with radiotherapy and post-radiotherapy as described by Stupp et al. is recommended to improve both PFS and OS in adult patients with newly diagnosed GBM. There is no evidence that alterations in the dosing regimen have additional beneficial effect.
Question
Is there benefit to adjuvant temozolomide treatment in elderly patients (> 65 years old?).
Target population
These recommendations apply to adult patients diagnosed with newly diagnosed glioblastoma.
Recommendation
Level III: Adjuvant TMZ treatment is suggested as a treatment option to improve PFS and OS in adult patients (over 70 years of age) with newly diagnosed GBM.
Question
What is the role of local regional chemotherapy with BCNU biodegradable polymeric wafers in adult patients with newly diagnosed glioblastoma?
Target population
These recommendations apply to adult patients diagnosed with newly diagnosed glioblastoma.
Recommendation
Level III: There is insufficient evidence for the use of BCNU wafers following resection in patients with newly diagnosed glioblastoma who undergo the Stupp protocol after surgery. Further studies of higher quality are suggested to understand the role of BCNU wafer and other locoregional therapy in the setting of Stupp Protocol.
Question
What is the role of bevacizumab in the adult patient with newly diagnosed glioblastoma?
Target population
These recommendations apply to adult patients diagnosed with newly diagnosed glioblastoma.
Recommendation
Level I: Bevacizumab in general is not recommended in the initial treatment of adult patients with newly diagnosed GBM. It continues to be strongly recommended that patients with newly diagnosed GBM be enrolled in properly designed clinical trials to assess the benefit of novel chemotherapeutic agents compared to standard therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Rationale
Surgery is recommended for newly diagnosed brain tumors to provide a pathological diagnosis, and when safe, to maximally resect the tumor. Whether patients undergo a gross total resection, subtotal resection, or biopsy, chemotherapy is usually initiated afterwards. While the previous guideline reviewed the data through 2008 [1], additional studies continue to define the role of chemotherapy and periodic review is required to review the role of chemotherapeutic options in the management of adult patients with newly diagnosed glioblastoma in order to provide updated recommendations.
Objectives
The purpose of this update is to assess the literature since the last set of clinical guidelines for chemotherapy in the management of newly diagnosed glioblastoma in adult patients. We seek to review new evidence and update the recommendations in regards to chemotherapy.
Methods
Writing group and question establishment
The evidence-based clinical practice guideline taskforce members and the Joint Tumor Section of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS) have prioritized an update of the guidelines for management of newly diagnosed glioblastoma. The writers represent a multi-disciplinary panel of clinical experts encompassing neurosurgery, neurooncology, and radiation oncology. Together, they were recruited to develop this update on the evidence-based practice guidelines for newly diagnosed glioblastoma (GBM) in adults. The methodology and findings of the previous guidelines were reviewed, and additional questions were developed to incorporate recent literature addressing practice patterns in management of GBM patients.
Literature review and eligibility criteria
The following electronic databases were searched from January 1, 2008 to December 31, 2018: PubMed, Embase, and Cochrane Database of Systematic Reviews using relevant MeSH and non-MeSH terms, including “GBM”, “GBM multiforme”, “GBM”, “Newly-diagnosed”, “newly diagnosed”, and “clinical trial.”
To be included in the guideline, a publication had to meet the following criteria:
Inclusion criteria
-
Peer-reviewed publications
-
Clinical studies in patients with newly diagnosed glioblastoma/high grade glioma
-
Each study reporting on at least five or more subjects
-
Adult patients (> 18 years of age). Studies with mixed adult and child populations were included if the adult cohorts could be isolated and analyzed separately
-
Publications written in English
The search criteria were developed and performed by two independent reviewers. Citations were independently reviewed and included if they met the a priori criteria for relevance. No discrepancies in study eligibility were noted. Corresponding full-text PDFs were obtained for all citations meeting the criteria, and reviewed. Data was extracted by the first reviewer and verified by another, all of which were compiled into evidence tables. The tables and data were reviewed by all of the authors. Articles not meeting the selection criteria were removed.
Data collection process
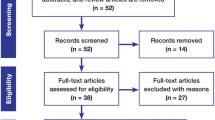
Our search criteria yielded a total of 271 publications, which were reviewed by two authors independently. Among these, 148 studies met the eligibility criteria and were further screened. 89/148 studies met all outlined selection criteria and specifically focused on chemotherapy for GBM.
Those abstracts that met with the selection criteria mentioned above were retrieved in full text form. The adherence to the selection criteria were confirmed. Corresponding full-text PDFs were obtained for all citations meeting the criteria, and reviewed. Data was extracted by the first reviewer and verified by another, all of which were compiled into evidence tables. The tables and data were reviewed by all of the authors. Articles not meeting the selection criteria were removed.
Scientific foundation
Classification of evidence and recommendation levels
Each reviewer independently determined the strength of the evidence, classified the level of evidence according to the criteria described in the Introduction section. Differences in classification of evidence and level of recommendations between the two reviewers were discussed between the reviewers to reach an agreement. If an agreement could not be reached the other three authors were asked to review the evidence and recommendations to allow the group to reach a consensus. Difference in level of recommendations were discussed amongst the reviewers and if a consensus could not be made discussed with the authors. For each article, a level of recommendation was achieved. Level I was reserved for well-designed, prospective, randomized and controlled studies with clear mechanisms to limit bias. Level II recommendations described studies that were randomized and controlled studies, but with design flaws leading to potential bias and limiting the paper’s conclusions, non-randomized cohort studies, and case–control studies. Level III recommendations were reserved for single surgeon, single institutional case series, comparative studies with historical control, and randomized studies with significant flaws related to studies with limited power and compromised statistical analysis. Additional information on study classification and recommendation development can be found at https://www.cns.org/guidelines/guideline-procedures-policies/guideline-development-methodology.
Summary of prior recommendations
In the previously published guidelines on the chemotherapeutic management of newly diagnosed GBM [1], the role of concurrent and post-irradiation temozolomide reached level I recommendations based upon a single class I study [2]. BCNU-impregnated biodegradable polymers were recommended as level II based upon two studies. The addition of temozolomide to radiation therapy for patients older than 70 with a Karnofsky performance status above 50 received a level III recommendation [1].
Assessment for risk of bias
Our search generated a list of abstracts, which were screened, and those articles that addressed our identified questions underwent full independent review by the authors. Reviewers were critical in their assessment, specifically in regard to trial design, such as randomization of treatment, blindedness, prospective character, etc., size of study population, baseline characteristics between study groups which could account for survivorship bias, selection bias, and appropriate statistical analyses of reported data.
Results of literature review
Temozolomide in patients with newly diagnosed GBM
Twelve studies examining the use of temozolomide for the treatment of newly diagnosed GBM (GBM) were eligible and were included in this analysis (Table 1).
Should patients with newly diagnosed GBM undergo temozolomide as adjuvant therapy?
In a phase III randomized controlled trial [2], Stupp et al. established level 1 evidence for the use of concurrent and post-irradiation temozolomide (TMZ) for the treatment of GBM. Since the previous guidelines publication in 2008 [1], no further large randomized controlled clinical studies have been done to address the role of chemotherapy in GBM. A small class II study by Karacetin et al. [3] showed statistically significant improvement in PFS and OS in GBM patients with concurrent/adjuvant TMZ with RT compared to RT only.
Does MGMT promoter methylation status predict benefit to adjuvant TMZ treatment?
We identified a total of six studies [4,5,6,7,8,9] that examined the role of MGMT methylation status with respect to response to TMZ treatment. All studies found that patients with newly diagnosed GBM with methylated MGMT promoter status had better outcomes with TMZ treatment compared to patients with MGMT promoter unmethylated tumors. Park et al. [4] found that only MGMT promoter-methylated patients benefited (improved PFS and OS) from adjuvant TMZ treatment compared to MGMT promoter-unmethylated patients. Further, patients with MGMT methylated tumors who underwent concurrent TMZ and radiation had significant improvement in PFS and overall OS compared to patients with MGMT methylated tumors receiving post-irradiation TMZ (OS 41 months vs. 17 months and PFS 24 months vs 3 months) [4]. This finding suggests that most treatment benefit from TMZ in MGMT promoter methylated tumors occurs during concomitant TMZ/RT treatment. In another study, Barbagallo et al. [5] found a direct correlation of median survival with MGMT promoter methylation status. Gilbert et al. [6], in a randomized controlled clinical trial, examined the benefit of dose dense adjuvant TMZ and again noted an association of MGMT promoter methylation with improved OS and PFS with the use of standard TMZ treatment. Weiler et al. [9] provided further evidence that MGMT promoter methylation status is an important predictive factor in response to TMZ treatment in a study investigating different dosing regimens for TMZ. Examining the role of TMZ therapy in elderly patients with GBM, Perez-Larraya et al. [7] found that MGMT promoter methylation was associated with improved response to TMZ therapy, consistent with the findings from other studies. Finally, in a more recent study examining standard TMZ treatment in combination with bevacizumab therapy, Gilbert et al. [8] confirmed the value of MGMT promoter methylation status as a positive prognostic factor, which was associated with significant improvement in PFS and OS. Although these studies were not specifically designed to compare treatment based on MGMT promoter methylation status, they all strongly suggest that methylation of the MGMT promoter in GBM is associated with improved outcome after TMZ treatment as compared to tumors with unmethylated MGMT promoter.
Is there benefit of adjuvant TMZ treatment in elderly patients (> 70 years of age)?
Two Class III studies [7, 10] examined the role of adjuvant TMZ for treatment of elderly patients with newly diagnosed GBM (Table 2). In a multicenter, prospective non-randomized phase II study of patients age 70 years or older with newly diagnosed GBM and postoperative KPS < 70, Perez-Larraya et al. [7] showed that these patients can tolerate TMZ treatment with improvement in PFS, OS, and functional status compared to reported supportive care data. In this study, MGMT promoter methylation was shown to indicate better response to TMZ therapy. In a retrospective study, Behm et al. [10] found statistically significant improvement in OS in elderly patients > 70 years of age with combined radiation and concomitant/adjuvant TMZ. Similar to previous findings in studies prior to 2008 [11,12,13,14], these studies indicate that treatment of elderly patients with adjuvant TMZ results in significant improvement in outcomes.
Synthesis
There is class I evidence that concurrent and post-irradiation temozolomide in combination with radiotherapy and post-radiotherapy as described by Stupp et al. is recommended to improve both PFS and OS in adult patients with newly diagnosed GBM.
Although the above studies were not specifically designed to compare treatment based on MGMT promoter methylation status, they all strongly suggest that methylation of the MGMT promoter in GBM is associated with improved outcome after TMZ treatment as compared to tumors with unmethylated MGMT promoter.
There is class III evidence that adjuvant TMZ treatment is recommended as a treatment option to improve PFS and OS in adult patients (over 70 years of age) with newly diagnosed GBM.
Timing of temozolomide in patients with newly diagnosed GBM?
When should temozolomide treatment be initiated after initial diagnosis of GBM?
Two Class III studies [15, 16] examined the effect of timing of initiation of concomitant TMZ/RT after diagnosis. In a single institution, retrospective assessment, Han et al. [15] found neither early (< 30 days) nor delayed (> 34 days) chemoradiation as beneficial. In their analysis, they showed that a short delay (30–34 days) is predictive of prolonged PFS and OS as compared to earlier or delayed chemoradiation. Sun et al. [16], in another retrospective study, found no significant effect on PFS or OS with a delay in initiation of treatment unless treatment was delayed by more than 6 weeks. Although these retrospective studies have significant limitations, they are consistent with previous studies [17,18,19] which have not shown any significant benefit of starting chemoradiation sooner than 4 weeks.
Does prolonged or non-standard temozolomide dosing regimens provide benefit compared to standard temozolomide dosing?
One class II study [20] and five class III studies [5, 21,22,23,24] examined the benefit of extended adjuvant TMZ treatment (> 6 cycles) compared to standard adjuvant therapy (6 cycles). In a prospective non-blinded randomized study, Bhandari et al. [20], examined the impact of six versus 12 cycles of adjuvant TMZ on OS in newly diagnosed postoperative patients. They found that that extended TMZ was well tolerated and lead to an increase in PFS (12.8 months vs. 16.8 months, p = 0.069) as well as OS (15.4 months vs. 23.8 months, p = 0.044) in newly diagnosed patients of GBM. They noted that the study was limited in the small number of patients (n = 40, 20 in each group) and lack of listing MGMT methylation status which is known prognosticator for response to TMZ therapy. In a retrospective matched cohort analysis of GBM patients treated with TMZ, Barbagallo et al. [5] examined two groups of patients: Group A, in which patients had greater than 6 cycles of adjuvant TMZ treatment (up to 101 cycles), and Group B, where patients did not receive more than 6 cycles of adjuvant TMZ treatment. The authors report that the median survival correlated with the number of TMZ cycles administered, however, this conclusion can be challenged based on significant limitations in the study design given important differences in the two groups. First, there were significantly more patients with MGMT promoter methylation in group A and the age of patients was significantly younger in group A compared to group B. Furthermore, a positive selection bias for patients to continue on monthly TMZ beyond 6 cycles was likely to play a role. Another retrospective study by Roldan et al. [21], also suggested improved median survival in patients receiving more than 6 cycles of TMZ compared to standard therapy of 6 cycles; however, the study had similar limitations in deriving this conclusion given that both groups had significant differences including that the extended adjuvant TMZ group had more patients with a methylated MGMT promoter than the group receiving standard therapy. In a larger study (n = 624) by Blumenthal et. al [22], which was a retrospective analysis of four multicenter randomized trials for newly diagnosed GBM patients, continuing TMZ beyond 6 cycles had some improvement in PFS, but did not show to increase overall survival after adjusting for prognostic factors. Similarly, in a retrospective single-center cohort study, Skardelly et al. [23] again did not find any evidence of improvement in overall survival with some improvement in PFS. Furthermore, Gramatzki et al. [24] after adjusting for age, extent of resection, KPS, presence of residual tumor, MGMT promoter methylation status, or IDH mutation status, not only did not find any benefit in OS, but, also they did not see any improvement in PFS. Ultimately, these studies do not provide sufficient evidence for extended adjuvant TMZ treatment. Future prospective trials will be required to better understand the effects of extended adjuvant TMZ treatment.
Three class II studies [6, 9, 25] investigated non-standard TMZ dosing regimens including dose dense and metronomic TMZ dosing strategies. In a prospective randomized phase II trial, Clarke et al. [25] compared post-irradiation adjuvant dose-dense TMZ (150 mg/m2 daily from days 1–7 and 15–21 of each cycle) and a metronomic TMZ regimen (50 mg/m2 daily from days 1–28 of each cycle) and found that either treatment was relatively well tolerated with the dose dense regimen trending towards better outcomes. They noted that the purpose of the study was not designed to compare the two treatment strategies but rather compare their treatment regimens to the historical control from the EORTC/NCIC phase III trial. Interestingly, the dose-dense TMZ group was shown to have a median survival of 15.4 months in unmethylated MGMT promoter GBM patients (compared to historical controls of 12.7 months), indicating a possible benefit of this treatment strategy. In another well designed randomized controlled trial, Gilbert et al. [6] did not demonstrate any significant benefit in PFS or OS from the use of dose-dense adjuvant TMZ therapy compared to standard therapy. Also of note, the dose-dense strategy was found to have statistically increased percentage of patient with Grade 3 or 4 toxicities. Weiler et al. [9] in a different dose-dense regimen, where TMZ was administered orally before and after RT in a weekly alternating schedule (50 mg/m2 during RT and 150 mg/m2 after RT with no maximal number of cycles defined) showed that this dose dense regimen showed some improvement in PFS in methylated MGMT promoter GBM patients compared to historical data from the EORTC/NCIC trial. Significant limitations in comparing the findings of this study with the historical data of the EORTC/NCIC trial include that this study’s patients were also all treated with indomethacin, the median KPS was 90, and the median number of cycles treated with adjuvant therapy was 6.5 (compared to median of 3 cycles in the EORTC/NCIC trial). The above studies did not show any significant evidence that alternative TMZ dosing strategies can lead to better outcomes compared to the standard Stupp protocol.
Synthesis
The above studies in regards to timing of TMZ treatment do not provide any significant evidence that alternative TMZ dosing strategies can lead to better outcomes compared to the standard Stupp protocol. In regards to the initiation of TMZ treatment, the retrospective studies above did not show any significant benefit of starting chemoradiation sooner than 4 weeks. Furthermore, the above studies do not provide sufficient evidence for extended adjuvant TMZ treatment or that alternative TMZ dosing strategies can lead to better outcomes compared to the standard Stupp protocol.
Adjuvant therapy in patients with newly diagnosed GBM?
Should temozolomide be given concomitantly with radiation therapy?
Two class III studies [4, 26] met the eligibility criteria for examining the role of concomitant TMZ therapy during radiation. In a retrospective analysis focusing on two prospective patient groups (RT followed by adjuvant TMZ and concomitant RT with TMZ followed by adjuvant TMZ) from a larger phase III clinical trial, Park et al. [4] found evidence that treatment with concomitant TMZ during radiation showed significant improvement in PFS and OS in patients with methylated MGMT promoter GBMs compared to treatment with post-irradiation TMZ only (PFS 24 months to 3 months and OS 41 months to 17 months, respectively). Interestingly, there was no significant difference in PFS or OS in patients with unmethylated MGMT promoter GBMs, indicating that the potential benefit of concomitant TMZ during radiation is only applicable to methylated MGMT tumors. In another retrospective study, Sher et al. [26] showed statistically significant improvement in OS with RT + concomitant TMZ + adjuvant TMZ compared to RT with adjuvant TMZ-only (OS 25.5 months compared 15.6 months, respectively). Unfortunately, this study did not test for MGMT methylation status, possibly accounting for the lack of significant differences in PFS between both groups. Although these studies have clear limitations, they highlight the important role of concomitant TMZ during RT, especially for methylated MGMT tumors in the overall benefit of adjuvant TMZ.
What is the role for local regional chemotherapy with BCNU biodegradable wafers in patients with newly diagnosed GBM?
Seven Class III studies [27,28,29,30,31,32,33] met our inclusion criteria which examined the use of BCNU biodegradable wafers (Gliadel wafers) as local therapy for patients with GBMs (Table 3). Affronti et al. [27] in single institution retrospective study comparing outcomes of newly diagnosed GBM patients receiving surgical resection with and without carmustine (BCNU) wafers followed by RT and concurrent TMZ plus rotational chemotherapy (temozolomide, carmustine, irinotecan) showed no statistically significant benefit in the use of BCNU wafers in addition to finding that this group had increased grade 3/4 toxicity (31% BCNU wafer group and 16 in the non-BCNU wafer group). They noted, however, though that the BCNU wafer group had better outcomes than the historical EORTC/NCIC data from the Stupp et al. paper [2], however this finding may be confounded by differences in the extent of resection in both studies. Specifically, patients in the BCNU wafter study had either gross total or subtotal resections, whereas 17% of patients in the EORTC/NCIC study underwent biopsy only. De Bonis et al. [28] found no substantial improvement in survival when adding loco-regional chemotherapy with BCNU wafers to standard therapy and found increased risk for adverse events. Furthermore, in a prospective non-randomized single arm study, Salmaggi et al. [29] found no significant improvement in survival with possibly slight improvement in PFS in newly diagnosed GBM patients treated with BCNU wafers in combination with 6-month metronomic temozolomide and radiation therapy. In another study examining newly diagnosed GBM patients aged > 65 years who underwent surgical resection with and without carmustine (BCNU) wafers, Chaichana et al. [30] showed statistically significant improvement in survival in patients older than 65 years old who underwent surgical resection with BCNU wafer placement compared to those that did not undergo BCNU wafer placement at surgical resection. However, the findings of this study is limited in terms of understanding efficacy with only 6/45 patients receiving temozolomide in either group. Similarly in a study by Noel et al. [31], the differences in the BCNU wafer treated group and the non-BCNU wafer treated group limits any significant conclusions made by the authors of this study. In this single institution, retrospective assessment of treatment of WHO Grade III or IV glioma patients who received surgical resection with and without BCNU wafers, the authors observed a trend, although not statistically significant, in improvement in outcome with treatment of patients with local regional therapy with BCNU, temozolomide, and radiotherapy. This trend in improvement in overall survival in GBM patients is difficult to claim given that 5 patients in the no-BCNU wafer group had biopsy only while 1 patient in the BCNU wafer group underwent biopsy only. This difference makes it difficult to conclude a positive trend in outcome with the use of BCNU wafers especially given their own univariate analysis of the quality of surgical removal (p = 0.03) which was a prognostic factor in overall survival. In another single institution, retrospective study by Akiyama et al. [32], therapy for newly diagnosed GBM patients who received surgical resection with BCNU wafers and bevacizumab or without BCNU wafer and without bevacizumab was examined. The study showed that patients who underwent BCNU wafer + bevacizumab group compared to no BCNU wafer and no bevacizumab group had significant benefit in PFS and OS (16.8 months vs. 7.3 months, p = 0.009; and 24.2 months vs. 15.3 months, p = 0.027). The study and its conclusions have significant limitations. The patients that are being compared are from two separate treatment eras 2010–2012 (no BCNU wafer) and 2013–2016 (BCNU wafer) which limits direct comparisons of results. The combination of BCNU wafers with bevacizumab is compared to treatment with neither BCNU wafers and bevacizumab, which limits direct comparison of either treatment. Roux et. al [33] also found benefit and relative safety of BCNU wafer compared to standard therapy without wafer, however, again this study was single institution retrospective study with potential selection bias.
In the prior guidelines publication in 2008 [1], the recommendation for the use of BCNU biodegradable wafers referenced two prospective studies [34, 35] which were randomized placebo controlled trials examining the efficacy of BCNU wafers in an era prior to the establishment of systemic TMZ as standard therapy. Both studies suggested a benefit from using BCNU wafers as locoregional chemotherapy.
The previous guideline detailed level II recommendations for the use BCNU wafers. Since then, concomitant temozolomide and radiation therapy (Stupp Protocol) has become the standard of care supported by level I evidence. The seven level III studies have not provided sufficient evidence that demonstrates significant improvement in overall survival or progression free survival to support the use of BCNU wafers. Additional studies of higher quality are required to understand the role of BCNU wafer and other locoregional therapy in the setting of Stupp Protocol are necessary.
What is the role of adjuvant bevacizumab (Avastin) in patients with newly diagnosed GBM?
Two Class I studies [8, 36] and 9 Class III studies [37,38,39,40,41,42,43,44,45] met our inclusion criteria to examine the benefit of adjuvant bevacizumab use in patients with GBM (Table 4). Both Class I studies (16,35) found no overall survival benefit in patients treated with adjuvant bevacizumab. In a multi-center, prospective randomized double-blind phase III trial examining the efficacy of bevacizumab added to standard therapy with RT/TMZ for the treatment of newly diagnosed GBM, Chinot et al. [36] found that the addition of adjuvant bevacizumab did not result in any overall survival benefit (OS 16.8 months with bevacizumab and 16.7 with placebo). They also noted an increased rate of adverse events, but they did see a benefit in terms of increased PFS and maintenance of performance status before deterioration with use of bevacizumab across different subgroups. Similarly, in another multicenter prospective randomized double-blinded phase III trial, Gilbert et al. [8] found that adding bevacizumab to standard therapy of concurrent TMZ + radiotherapy + adjuvant monthly TMZ did not result in any overall survival benefit (OS 15.7 months with bevacizumab and 16.1 months with placebo). Although this study did show improved PFS with bevacizumab similar to the study by Chinot et al. [35], it differed significantly in assessment of functional outcome since patients in this study were found to have worsened quality of life in the bevacizumab group. Furthermore, in another study examining the results of two consecutive phase II trials of hypofractionated-intensity modulated radiotherapy (hypo-IMRT) and TMZ with or without bevacizumab, Carlson et al. [37] did not find any significant benefit in adding bevacizumab to hypo-IMRT/TMZ, in addition to having a significant increase in Grade III toxicities with bevacizumab. In another Class III study, Van Linde et al. [40], in a single institution prospective non-randomized phase II study, evaluated the safety and efficacy of bevacizumab in combination with TMZ and RT in newly diagnosed GBM patients and found no benefit of bevacizumab treatment in terms of OS as compared to historical data with standard therapy (of note, in this study patients only received bevacizumab during concomitant RT + TMZ and did not receive bevacizumab during adjuvant TMZ therapy). In another prospective non-randomized single-arm phase II study, Omuro et al. [42] evaluated the use of hypofractionated stereotactic radiotherapy (HFSRT) combined with concomitant/ adjuvant TMZ and bevacizumab in the treatment of patients with newly diagnosed GBM. This study did not reveal any significant benefit in OS with bevacizumab treatment as compared to historical data. The study also provided interesting results regarding to molecular data demonstrating that the expression level of pro-angiogenic genes had no prognostic value in determining response to bevacizumab therapy.
Other class III studies that met inclusion criteria indicated a possible improvement in outcome with the use of adjuvant bevacizumab when compared to historical controls, however, these studies had specific characteristics in the study population that would limit their broader applicability. Lai et al. [39] in a multicenter, prospective non-randomized single-arm phase II study evaluating the efficacy of bevacizumab in combination with TMZ and RT in the treatment of patients with newly diagnosed GBM, commented on improved PFS and OS compared to historical control of the EORTC-NCIC data, however, their study population was significantly different from the historical control groups since in their study group only 3% of patients underwent biopsy while in the EORTC-NCIC group 17% underwent biopsy as compared to subtotal or gross total resection. Also, comparison of overall survival in the study group and the UCLA/KPLA control group is difficult to interpret given that over 50% of the UCLA/KPLA control group received bevacizumab as a salvage therapy at recurrence. Also of note, the EORTC/NCIC trial reported survival from the date of enrollment while this study reported survival from the date of diagnosis which could add to perceived OS. The study did provide important data regarding toxicity profile with use of bevacizumab therapy showing specifically increase in thromboembolic events and wound healing complications. Similarly, Narayana et al. [41] in a prospective nonrandomized single-arm phase II study concluded that there was apparent improvement of PFS and OS with the addition of bevacizumab to standard therapy as compared to the EORTC-NCIC trial, however, again the patient population presented here has substantial differences in the extent of resection (current study with reported 70% of patients with GTR compared to EORTC-NCIC study with only 39%). Likewise, in another prospective non-randomized single-arm phase II study evaluating the efficacy and safety of the addition of bevacizumab to RT + TMZ, followed by bevacizumab, TMZ, and irinotecan for newly diagnosed GBM patients. Vredenburgh et al. [43] asserted that there was an apparent improvement of PFS and OS with the addition of bevacizumab as compared to the EORTC trial, but, again the patient population presented here had substantial differences in the extent of resection (current study has no patients enrolled with biopsy compared with EORTC study which had 17% patients undergoing biopsy). Additionally, the study found significant toxicities of adjuvant TMZ + bevacizumab + irinotecan. Taking into consideration all the above studies, upfront use of bevacizumab is likely to improve PFS but has so far not been shown to extent OS in GBM patients. Moreover, conflicting data exists regarding the exact benefit of adjuvant bevacizumab in terms of quality of life. Another prospective nonrandomized single-arm phase II study by Reyes-Botero et. al [44] evaluated the efficacy and safety of upfront temozolomide (TMZ) and bevacizumab in patients aged ≥ 70 years and a KPS < 70 and found the combination to be well tolerated with potential to improve quality of life. Similarly, Hata et. al [45] found possible benefit in quality of life when adding bevacizumab to partially resected tumors in combination with combined chemoradiation. Again, these studies provide further support to performing a randomized controlled trial to assess benefit of bevacizumab on quality of life for GBM patients.
Is there a role for chemotherapy agents other than TMZ for the treatment of GBM?
A number of studies have examined the use of several chemotherapy agents other than TMZ for the treatment of GBM (Table 5). Studies that met our inclusion criteria examined the role of irinotecan, nitrosurea based chemotherapy agents (carmustine, nimustine), cisplatin, procarbazine, and gemcitabine. One Class I study [46] and two Class III studies [47, 48] examined the role of irinotecan in the treatment of GBM. In multi-center, prospective randomized phase II study examining the efficacy of bevacizumab combined with irinotecan (Bev-Iri) versus bevacizumab combined with temozolomide (Bev-Tem) before, during and after radiotherapy in newly diagnosed GBM patients, Hofland et al. [46] found that Bev-Iri did not provide any significant benefit when compared to Bev-Tem. This study did not show any benefit of Bev-Iri to Bev-Tem in terms of response rate and PFS. By interpreting the results of this study, one needs to consider that there were no study patients who underwent GTR as per protocol and concurrent chemoradiation was delayed compared to the standard Stupp et al. protocol. Furthermore, in a prospective study of RT and irinotecan followed by BCNU plus irinotecan in newly diagnosed GBM patients, Jaeckle et al. [47] did not find any benefit of this treatment regimen compared to standard Stupp protocol, in addition to finding that this combination was less well tolerated. Similarly, in a prospective trial evaluating the efficacy and safety of TMZ in combination with irinotecan before radiotherapy in patients with newly diagnosed GBM, Quinn et al. [29] did not show any benefit of combining TMZ with irinotecan compared to TMZ alone, their data suggest that this combination was found to be more toxic and poorly tolerated. They explained that the results were difficult to interpret and to compare with other studies as the majority of patients (81%) only underwent a biopsy. Moreover, 52% of patients (22/42) discontinued therapy after 1 or 2 cycles of treatment (due to disease progression or adverse events) and went on to immediate radiotherapy + TMZ, further limiting the interpretation of the study results. These studies did not show any benefit of irinotecan therapy compared to standard therapy with TMZ, in addition to some increased toxicity in many cases.
Two Class II studies [49, 50] and two Class III studies [51, 52] examined the role of nitrosurea based chemotherapies (nimustine (ACNU), carmustine (BCNU)) in the treatment of GBM. In a multicenter prospective randomized phase III study examining the efficacy of chemotherapy with nimustine (ACNU)- cisplatin (CDDP) when used in conjunction with radiotherapy plus adjuvant temozolomide in patients with newly diagnosed GBM, Kim et al. [49] found significant toxicity with neoadjuvant ACNU-CDDP treatment. Although, there appeared to be some survival benefit when comparing treatment to RT followed by adjuvant TMZ, the study lacked comparison to the standard Stupp protocol where patients were also treated with concomitant TMZ during radiation. Regardless, the study found serious toxicity with the ACNU-CDDP regimen, which further challenges its possible benefit. In another multicenter prospective randomized trial, Shibui et al. [50] found no benefit in PFS or OS when examining the efficacy of nimustine (ACNU) + procarbazine (PCZ) compared to ACNU alone for GBM and anaplastic astrocytoma. The authors noted that their study “was terminated early because temozolomide was newly approved in Japan.” Additionally, in a retrospective comparison of outcome of GBM patients treated with initial radiation and chemotherapy of TMZ or BCNU, Vinjamure et al. [51] found that TMZ treated GBM patients had better overall outcomes compared to BCNU treated patients, but they commented that this was due to newer salvage therapies in the era of TMZ treatment. This study had significant limitations such as non-randomized patients, tumor sizes were significantly larger in BCNU group, patients were treated in earlier years in BCNU group, and that not all patients in BCNU group received concomitant chemotherapy with radiation. Similarly, the results from another retrospective study comparing outcomes of GBM patients with at least near-total resection treated with initial radiation and TMZ or ACNU-based (ACNU plus teniposide or cisplatin) chemotherapy [52] are difficult to interpret given significant differences in treatment regimes. For example, the patients in ACNU-based group started therapy after completion of RT and, therefore, did not have any concomitant chemotherapy with radiation as the TMZ group. Also, greater than 40% of patients could not complete ACNU based treatment given significant toxicity. The authors note that while in a subgroup analysis of patients who were able to complete at least 4 cycles of ACNU-based therapy “a modest improvement in survival occurred in this ACNU subgroup, the efficacy was still inferior to that in the TMZ cohort.” Overall, these studies do not support the use of nitrosurea based chemotherapies such as BCNU or ACNU over standard treatment with TMZ.
One class III study [53] examined the effect of gemcitabine in the treatment of GBM. In this single institution, prospective phase II study of gemcitabine with radiotherapy (RT) as first line treatment for newly diagnosed GBM patients followed by adjuvant TMZ, Metro et al. [53] noted that there is some response to concomitant RT with gemcitabine but these results are difficult to compare to standard Stupp protocol given that no patients in this group had undergone gross total surgical resection. Also, given all the patients do receive adjuvant TMZ it is difficult to identify treatment with gemcitabine as cause of specific outcomes. Given small number of patients and lack of control group, the study did not provide any significant evidence that concomitant gemcitabine during RT has any benefit over standard therapy with TMZ.
Synthesis
In terms of adjuvant therapy for newly diagnosed GBM, there is class III evidence that highlights the important benefit of concomitant radiation with TMZ, especially for methylated MGMT tumors.
In regards to local regional chemotherapy with BCNU biodegradable wafers, the previous guidelines detailed level II recommendations, however, this recommendation was based on prior class I evidence where no systemic chemotherapy was used and/or TMZ had not been established as standard of care. In the current era, a number of level III studies have demonstrated no significant improvement in overall survival or progression free survival to support the use of BCNU wafers. Additional studies of higher quality are required to understand the role of BCNU wafer and other locoregional therapy in the setting of Stupp Protocol is necessary.
With respect to upfront use of bevacizumab, a number of studies have shown improved PFS but no improvement in OS in GBM patients. Moreover, conflicting data exists regarding the exact benefit of adjuvant bevacizumab in terms of quality of life requiring further rigorously designed clinical studies.
Furthermore, the above studies have not shown any convincing evidence that alternative chemotherapy regimens had any benefit over standard treatment with TMZ.
Conclusions and key issues for future investigation
Chemotherapy is essential in the management of newly diagnosed GBM. The use of temozolomide is supported by level I recommendations for patients with newly diagnosed GBM as previously shown by Stupp et al. and reported in the previous guidelines [2]. Although, many studies have identified the methylation of the MGMT promoter as a positive predictor for TMZ treatment in patients with newly diagnosed GBM, prospective randomized controlled trials with and without concurrent RT in methylated and non-methylated MGMT promoter GBM groups would help better elucidate and emphasize the benefit of TMZ treatment for these particular subsets of patients. Currently, while recognizing their limitations, the existing publications can be used to consider treatment options, but more importantly, to frame the important questions for future clinical trials.
Prospective randomized controlled trials in specific subsets of patients (i.e. by age group, molecular profile, extent of resection, previous treatment) would help define optimal timing and treatment regimens. Furthermore, given the poor prognosis of patients with GBMs, future clinical trials need to emphasize and prospectively consider quality of life measures in addition to PFS and OS. As additional clinical trials bring forth new chemotherapeutic and specific targeted options, the role of TMZ + concurrent RT followed by adjuvant TMZ treatment will need to be examined as standard therapy compared to new treatment regimens.
References
Fadul CE, Wen PY, Kim L, Olson JJ (2008) Cytotoxic chemotherapeutic management of newly diagnosed glioblastoma multiforme. J Neurooncol 89:339–357. https://doi.org/10.1007/s11060-008-9615-4
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Karacetin D, Okten B, Yalcin B, Incekara O (2011) Concomitant temozolomide and radiotherapy versus radiotherapy alone for treatment of newly diagnosed glioblastoma multiforme. J BUON 16:133–137
Park CK, Lee SH, Kim TM et al (2013) The value of temozolomide in combination with radiotherapy during standard treatment for newly diagnosed glioblastoma. J Neurooncol 112:277–283. https://doi.org/10.1007/s11060-013-1060-3
Barbagallo GMV, Paratore S, Caltabiano R et al (2014) Long-term therapy with temozolomide is a feasible option for newly diagnosed glioblastoma: a single-institution experience with as many as 101 temozolomide cycles. Neurosurg Focus 37:E4. https://doi.org/10.3171/2014.9.FOCUS14502
Gilbert MR, Wang M, Aldape KD et al (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31:4085–4091. https://doi.org/10.1200/JCO.2013.49.6968
Pérez-Larraya JG, Ducray F, Chinot O et al (2011) Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol 29:3050–3055. https://doi.org/10.1200/JCO.2011.34.8086
Gilbert MR, Dignam JJ, Armstrong TSTS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708. https://doi.org/10.1056/NEJMoa1308573
Weiler M, Hartmann C, Wiewrodt D et al (2010) Chemoradiotherapy of newly diagnosed glioblastoma with intensified temozolomide. Int J Radiat Oncol Biol Phys 77:670–676. https://doi.org/10.1016/j.ijrobp.2009.05.031
Behm T, Horowski A, Schneider S et al (2013) Concomitant and adjuvant temozolomide of newly diagnosed glioblastoma in elderly patients. Clin Neurol Neurosurg 115:2142–2146. https://doi.org/10.1016/j.clineuro.2013.08.002
Brandes AA, Vastola F, Basso U et al (2003) A prospective study on glioblastoma in the elderly. Cancer 97:657–662. https://doi.org/10.1002/cncr.11097
Glantz M, Chamberlain M, Liu Q et al (2003) Temozolomide as an alternative to irradiation for elderly patients with newly diagnosed malignant gliomas. Cancer 97:2262–2266. https://doi.org/10.1002/cncr.11323
Chinot OL, Barrle M, Frauger E et al (2004) Phase II study of temozolomide without radiotherapy in newly diagnosed glioblastoma multiforme in an elderly populations. Cancer 100:2208–2214. https://doi.org/10.1002/cncr.20224
Keime-Guibert F, Chinot O, Taillandier L et al (2007) Radiotherapy for glioblastoma in the elderly. N Engl J Med 356:1527–1535. https://doi.org/10.1056/NEJMoa065901
Han SJ, Rutledge WC, Molinaro AM et al (2015) The effect of timing of concurrent chemoradiation in patients with newly diagnosed glioblastoma. Neurosurgery 77:248–253. https://doi.org/10.1227/NEU.0000000000000766
Sun MZ, Oh T, Ivan ME et al (2015) Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J Neurosurg 122:1144–1150. https://doi.org/10.3171/2014.9.JNS14193
Blumenthal DT, Won M, Mehta MP et al (2009) Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol 27:733–739. https://doi.org/10.1200/jco.2008.18.9035
Seidlitz A, Siepmann T, Löck S et al (2015) Impact of waiting time after surgery and overall time of postoperative radiochemotherapy on treatment outcome in glioblastoma multiforme. Radiat Oncol 10:172. https://doi.org/10.1186/s13014-015-0478-5
Noel G, Huchet A, Feuvret L et al (2012) Waiting times before initiation of radiotherapy might not affect outcomes for patients with glioblastoma: a French retrospective analysis of patients treated in the era of concomitant temozolomide and radiotherapy. J Neurooncol 109:167–175. https://doi.org/10.1007/s11060-012-0883-7
Bhandari M, Gandhi AK, Devnani B, et al (2017) Comparative study of adjuvant temozolomide six cycles versus extended 12 cycles in newly diagnosed glioblastoma multiforme. J Clin Diagn Res 11:XC04–XC08. https://doi.org/10.7860/JCDR/2017/27611.9945
Roldán Urgoiti GB, Singh AD, Easaw JC (2012) Extended adjuvant temozolomide for treatment of newly diagnosed glioblastoma multiforme. J Neurooncol 108:173–177. https://doi.org/10.1007/s11060-012-0826-3
Blumenthal DT, Gorlia T, Gilbert MR et al (2017) Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: a secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol 19:1119–1126. https://doi.org/10.1093/neuonc/nox025
Skardelly M, Dangel E, Gohde J et al (2017) Prolonged temozolomide maintenance therapy in newly diagnosed glioblastoma. Oncologist 22:570–575. https://doi.org/10.1634/theoncologist.2016-0347
Gramatzki D, Kickingereder P, Hentschel B et al (2017) Limited role for extended maintenance temozolomide for newly diagnosed glioblastoma. Neurology 88:1422–1430. https://doi.org/10.1212/WNL.0000000000003809
Clarke JL, Iwamoto FM, Sul J et al (2009) Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol 27:3861–3867. https://doi.org/10.1200/JCO.2008.20.7944
Sher DJ, Henson JW, Avutu B et al (2008) The added value of concurrently administered temozolomide versus adjuvant temozolomide alone in newly diagnosed glioblastoma. J Neurooncol 88:43–50. https://doi.org/10.1007/s11060-008-9530-8
Lou AM, Heery CR, Herndon JE et al (2009) Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer 115:3501–3511. https://doi.org/10.1002/cncr.24398
De Bonis P, Anile C, Pompucci A et al (2012) Safety and efficacy of gliadel wafers for newly diagnosed and recurrent glioblastoma. Acta Neurochir (Wien) 154:1371–1378. https://doi.org/10.1007/s00701-012-1413-2
Salmaggi A, Milanesi I, Silvani A et al (2013) Prospective study of carmustine wafers in combination with 6-month metronomic temozolomide and radiation therapy in newly diagnosed glioblastoma: preliminary results. J Neurosurg 118:821–829. https://doi.org/10.3171/2012.12.JNS111893
Chaichana KL, Zaidi H, Pendleton C et al (2011) The efficacy of carmustine wafers for older patients with glioblastoma multiforme: prolonging survival. Neurol Res 33:759–764. https://doi.org/10.1179/1743132811Y.0000000006
Noël G, Schott R, Froelich S et al (2012) Retrospective comparison of chemoradiotherapy followed by adjuvant chemotherapy, with or without prior Gliadel implantation (Carmustine) after initial surgery in patients with newly diagnosed high-grade gliomas. Int J Radiat Oncol Biol Phys 82:749–755. https://doi.org/10.1016/j.ijrobp.2010.11.073
Akiyama Y, Kimura Y, Enatsu R et al (2018) Advantages and disadvantages of combined chemotherapy with carmustine wafer and bevacizumab in patients with newly diagnosed glioblastoma: a single-institutional experience. World Neurosurg 113:e508–e514. https://doi.org/10.1016/j.wneu.2018.02.070
Roux A, Peeters S, Zanello M et al (2017) Extent of resection and Carmustine wafer implantation safely improve survival in patients with a newly diagnosed glioblastoma: a single center experience of the current practice. J Neurooncol 135:83–92. https://doi.org/10.1007/s11060-017-2551-4
Westphal M, Hilt DC, Bortey E et al (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 5:79–88. https://doi.org/10.1215/S1522-8517-02-00023-6
Valtonen S, Timonen U, Toivanen P, et al (1997) Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery 41:44–8; discussion 48–9
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709–722. https://doi.org/10.1056/NEJMoa1308345
Carlson JA, Reddy K, Gaspar LE et al (2015) Hypofractionated-intensity modulated radiotherapy (hypo-IMRT) and temozolomide (TMZ) with or without bevacizumab (BEV) for newly diagnosed glioblastoma multiforme (GBM): a comparison of two prospective phase II trials. J Neurooncol 123:251–257. https://doi.org/10.1007/s11060-015-1791-4
Lai A, Filka E, McGibbon B et al (2008) Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys 71:1372–1380. https://doi.org/10.1016/j.ijrobp.2007.11.068
Lai A, Tran A, Nghiemphu PL et al (2011) Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 29:142–148. https://doi.org/10.1200/JCO.2010.30.2729
Van Linde ME, Verhoeff JJ, Richel DJ et al (2015) Bevacizumab in combination with radiotherapy and temozolomide for patientswith newly diagnosed glioblastomamultiforme. Oncologist 20:107–108. https://doi.org/10.1634/theoncologist.2014-0418
Narayana A, Gruber D, Kunnakkat S et al (2012) A clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastoma. J Neurosurg 116:341–345. https://doi.org/10.3171/2011.9.JNS11656
Omuro A, Beal K, Gutin P et al (2014) Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res 20:5023–5031. https://doi.org/10.1158/1078-0432.CCR-14-0822
Vredenburgh JJ, Desjardins A, Reardon DA et al (2011) The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res 17:4119–4124. https://doi.org/10.1158/1078-0432.CCR-11-0120
Reyes-Botero G, Cartalat-Carel S, Chinot OL et al (2018) Temozolomide plus bevacizumab in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial (ATAG). Oncologist 23:524–e44. https://doi.org/10.1634/theoncologist.2017-0689
Hata N, Yoshimoto K, Hatae R et al (2017) Add-on bevacizumab can prevent early clinical deterioration and prolong survival in newly diagnosed partially resected glioblastoma patients with a poor performance status. Onco Targets Ther 10:429–437. https://doi.org/10.2147/OTT.S125587
Hofland KF, Hansen S, Sorensen M et al (2014) Neoadjuvant bevacizumab and irinotecan versus bevacizumab and temozolomide followed by concomitant chemoradiotherapy in newly diagnosed glioblastoma multiforme: a randomized phase II study. Acta Oncol (Madr). https://doi.org/10.3109/0284186X.2013.879607
Jaeckle KA, Ballman KV, Giannini C et al (2010) Phase II NCCTG trial of RT + irinotecan and adjuvant BCNU plus irinotecan for newly diagnosed GBM. J Neurooncol 99:73–80. https://doi.org/10.1007/s11060-009-0103-2
Quinn JA, Jiang SX, Reardon DA et al (2009) Phase II trial of temozolomide (TMZ) plus irinotecan (CPT-11) in adults with newly diagnosed glioblastoma multiforme before radiotherapy. J Neurooncol 95:393–400. https://doi.org/10.1007/s11060-009-9937-x
Kim IH, Park CK, Heo DS et al (2011) Radiotherapy followed by adjuvant temozolomide with or without neoadjuvant ACNU-CDDP chemotherapy in newly diagnosed glioblastomas: a prospective randomized controlled multicenter phase III trial. J Neurooncol 103:595–602. https://doi.org/10.1007/s11060-010-0427-y
Shibui S, Narita Y, Mizusawa J et al (2013) Randomized trial of chemoradiotherapy and adjuvant chemotherapy with nimustine (ACNU) versus nimustine plus procarbazine for newly diagnosed anaplastic astrocytoma and glioblastoma (JCOG0305). Cancer Chemother Pharmacol 71:511–521. https://doi.org/10.1007/s00280-012-2041-5
Vinjamuri M, Adumala RR, Altaha R et al (2009) Comparative analysis of temozolomide (TMZ) versus 1,3-bis (2-chloroethyl)-1 nitrosourea (BCNU) in newly diagnosed glioblastoma multiforme (GBM) patients. J Neurooncol 91:221–225. https://doi.org/10.1007/s11060-008-9702-6
Wang Y, Chen X, Zhang Z et al (2014) Comparison of the clinical efficacy of temozolomide (TMZ) versus nimustine (ACNU)-based chemotherapy in newly diagnosed glioblastoma. Neurosurg Rev 37:73–78. https://doi.org/10.1007/s10143-013-0490-x
Metro G, Fabi A, Mirri MA et al (2010) Phase II study of fixed dose rate gemcitabine as radiosensitizer for newly diagnosed glioblastoma multiforme. Cancer Chemother Pharmacol 65:391–397. https://doi.org/10.1007/s00280-009-1155-x
Acknowledgements
We would like to acknowledge the significant contributions of Mary Bodach, Trish Rehring and the AANS/CNS Joint Guidelines Committee (JGC) for their review, comments and suggestions, as well as Martha Stone and Lisa Philpotts, for their valuable input as Medical Research Librarians. We also acknowledge the following individual peer reviewers their contributions to the development process: John O’Toole, MD, David Bauer, MD, Kimon Bekelis, MD, Andrew Carlson, MD, Isabelle Germano, MD, Catherine McClung Smith, MD, Jonathan Sherman, MD.
Disclaimer of liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Update on Newly Diagnosed Glioblastoma Task Force members were required to report all possible COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force Members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs.
Author | Conflicts |
|---|---|
Navid Redjal | None |
Brian Nahed | Research Funding: NIH, American Cancer Society Co-founder and consultant React Neuro |
Jorg Dietrich | Consulting: Blue Earth Diagnostics, Unum Therapeutics Royalties: Wolters Kluwer |
Jeffrey J. Olson | American Cancer Society, Editorial Consultant |
Disclosures
These guidelines were funded exclusively by the CNS Guidelines Committee and the AANS/CNS Joint Tumor Section Executive Committee with no funding from any outside commercial sources to support the development of this document.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sponsored by the American Association of Neurological Surgeons and Congress of Neurological Surgeons Joint Section on Tumors.
Reviewed for evidence-based integrity and endorsed by the American Association of Neurological Surgeons and Congress of Neurological Surgeons.
Rights and permissions
About this article
Cite this article
Redjal, N., Nahed, B.V., Dietrich, J. et al. Congress of neurological surgeons systematic review and evidence-based guidelines update on the role of chemotherapeutic management and antiangiogenic treatment of newly diagnosed glioblastoma in adults. J Neurooncol 150, 165–213 (2020). https://doi.org/10.1007/s11060-020-03601-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03601-w