Abstract
To compare progression-free (PFS) and overall survival (OS) in patients treated in two consecutive phase II trials of hypofractionated-intensity modulated radiotherapy (hypo-IMRT) and temozolomide (TMZ) with or without bevacizumab (BEV). Patients with newly diagnosed glioblastoma multiforme (GBM) after biopsy or resection were enrolled on a clinical trial with hypo-IMRT and TMZ (hypo-IMRT/TMZ alone) from 2008 to 2010, or in the second protocol with the same hypo-IMRT and TMZ plus BEV (hypo-IMRT/TMZ/BEV) from 2010 to 2013. All patients received postoperative hypo-IMRT to the surgical cavity and residual tumor plus margin to a total dose of 60 Gy and to the T2 abnormality with margin to 30 Gy, both in ten fractions. Concurrent TMZ (75 mg/m2/day) was given to all patients for 28 consecutive days followed by adjuvant TMZ (150–200 mg/m2/day). Patients enrolled on the hypo-IMRT/TMZ/BEV trial received concurrent and adjuvant BEV (10 mg/kg) on days 1 and 15 of each 28-day cycle. Hazard ratios of PFS and OS were compared between trials in a Cox proportional hazards model. Twenty-six patients were enrolled on the hypo-IMRT/TMZ alone trial and 30 patients on the hypo-IMRT/TMZ/BEV trial. Median follow-up was 13.9 and 14.7 months, respectively. Median PFS was 3.4 months longer with hypo-IMRT/TMZ/BEV but the difference was not statistically significant (12.8 vs. 9.4 months, p = 0.58). Median (OS) was 16.3 months for both trials. The addition of BEV to TMZ and hypo-IMRT did not improve OS for patients with GBM in two phase II trials with small patient numbers; PFS was longer with BEV, but the difference was not statistically significant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) is the most common primary brain cancer in adults. Prognosis remains poor despite advances in imaging and treatment. Median overall survival (OS) with current standard of care therapy with conventional radiation and temozolomide (TMZ) is 14.6 months with 5 year OS of 10 % [1, 2]. Pattern of failure has been overwhelmingly local tumor recurrence/progression. Radiation dose escalation seems logical to increase local tumor control. However, radiation dose escalation studies with conventional fractionation (i.e., ≤2 Gy per fraction) have all failed to either increase local tumor control or to improve patient survival.
Hypofractionated radiation therapy represents an alternative method for biological effective dose (BED) escalation, in hope of increasing local tumor control and ultimately survival. We previously reported a phase I fractional dose escalation trial in which radiation fractional dose was safely escalated from 3 Gy per fraction to 6 Gy per fraction [3]. A total radiation dose of 60 Gy was given with concurrent and adjuvant TMZ. Hypofractionated-intensity modulated radiotherapy (hypo-IMRT) technique was used. To further investigate safety and tolerability of hypo-IMRT to 60 Gy in 6-Gy fractions over 2 weeks with concurrent and adjuvant TMZ, the highest dose per fraction cohort (6 Gy/fraction) of the phase I trial was subsequently expanded to a phase II trial. A total of 26 patients were enrolled and outcomes of the first 24 patients were previously reported [4]. The treatment regimen was well tolerated with no grade 3–4 non-hematologic toxicity. The median OS was 16.6 months, comparable to outcomes with standard therapy. Hypo-IMRT also seemed to have altered the patterns of failure in this group of GBM patients, with less local but more distant failures, which is likely due to the higher biologically effective dose delivered with hypo-IMRT, resulting in higher rates of local tumor control [5].
GBMs are characterized by extensive angiogenesis, with increased vascular endothelial growth factor (VEGF) expression correlated with poor prognosis [6, 7]. Mouse xenograft models showed that targeting of VEGF inhibited tumor growth [8, 9]. Bevacizumab (BEV), a humanized monoclonal antibody against the VEGF ligand, initially showed promising results in phase II clinical trials for recurrent GBMs with response on imaging and improved progression free survival (PFS) leading to FDA approval for recurrent GBMs in 2009 [10–12]. Subsequent trials with radiation in both the recurrent and upfront settings showed safety and promising outcomes [13, 14].
Since 2010 we have completed a second phase II trial of the same hypo-IMRT with concurrent and adjuvant TMZ as well as BEV [15]. Here, we compare the outcome results of these two consecutive phase II trials of hypo-IMRT and TMZ with or without BEV.
Materials and methods
The two phase II trials have been completed sequentially. Both trials were approved by our institutional review board and were registered with ClinicalTrials.gov, numbers NCT00792012 and NCT01209442.
Eligibility
Eligible patients were ≥18 years old with histopathologically confirmed GBM. Karnofsky performance status (KPS) had to be ≥60, with an estimated survival of ≥3 months. One difference between trials was tumor size requirements. In the hypo-IMRT/TMZ alone trial the surgical cavity plus the T1-weighted enhancing residual tumor on MRI had to be ≤6 cm; the hypo-IMRT/TMZ/BEV trial did not have a size constraint.
Hypo-IMRT
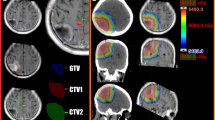
The hypo-IMRT technique was previously reported [3]. In brief, IMRT with a simultaneous integrated boost was used to deliver differential radiation doses to different targets. The gross tumor volume (GTV) was defined as any contrast-enhancing residual tumor on the post-operative T1-weighted post-contrast MRI plus the entire surgical cavity. The clinical tumor volume (CTV) was defined as the abnormality on the T2-weighted MRI. For the hypo-IMRT/TMZ alone trial, planning target volume 1 (PTV1) was defined as the GTV plus a 0.5 cm margin and planning target volume 2 (PTV2) the CTV plus a 0.5 cm margin. In the hypo-IMRT/TMZ/BEV trial, PTV1 was defined as the GTV plus a 1 cm margin and PTV2 the CTV plus a 1 cm margin. On both trials PTV1 received 60 Gy and PTV2 30 Gy simultaneously in ten fractions over 2 weeks.
Temozolomide chemotherapy
All patients were to receive TMZ concurrently with hypo-IMRT and adjuvant TMZ after hypo-IMRT. TMZ was administered orally, once daily at 75 mg/m2, starting on the first day of hypo-IMRT and continuing for 28 consecutive days. Adjuvant TMZ was administered at 150–200 mg/m2 orally once daily for 5 consecutive days every 28 days, for a total of six cycles. Adjuvant TMZ beyond 6 cycles was at the discretion of the treating neuro-oncologist.
Bevacizumab therapy
Patients enrolled on the hypo-IMRT/TMZ/BEV trial were to receive BEV concurrently with hypo-IMRT and TMZ and adjuvant BEV with TMZ after hypo-IMRT. BEV was administered intravenously at 10 mg/kg on day 1 and day 15 during hypo-IMRT. Adjuvant BEV was administered at 10 mg/kg on days 1 and 15 of every TMZ cycle for a total of six cycles.
Patient follow-up and toxicity evaluation
Patients in both trials were evaluated on a weekly basis during radiation, on a monthly basis during adjuvant therapy, and once every 3 months thereafter. Evaluation included history and physical examination, blood tests, KPS, and toxicity assessment. Common Terminology Criteria for Adverse Events version 3.0 was used for grading of toxicity. Contrast-enhanced MRI was performed within 28 days before study treatment and then at 1 and 3 months after hypo-IMRT, and once every 3 months thereafter. Acute toxicities were classified as occurring within 30 days of hypo-IMRT and late toxicities developed ≥30 days after hypo-IMRT.
The hypo-IMRT/TMZ/BEV trial was closed early after enrolling 30 of a planned 35 patients due to a higher than expected rate of presumed radiation necrosis (RN) with clinical decline.
Statistical analysis
All patients for whom treatment was initiated were included in the analysis of both trials, including the last two patients who were enrolled on the hypo-IMRT/TMZ trial but not included in the initial publication due to short follow-up. Demographic and clinical characteristics were summarized as mean, median and range for continuous measure, frequency and percentage for categorical measures and compared between the two trials using t tests for continuous measures and χ 2 tests for categorical measures.
OS was measured from the day of diagnosis to death from any cause. Follow-up time and PFS were measured from the day of hypo-IMRT completion. The Kaplan–Meier estimate of PFS and OS was compared between groups using a log-rank method for unadjusted comparison. To adjust for population factors by which the two groups differed, a Cox proportional hazards model was used for PFS and OS, and a logistic regression model was used for 6 month PFS and clinically significant presumed RN.
Results
Patients
Between 2008 and 2010 26 patients were enrolled on the hypo-IMRT/TMZ alone trial. Between 2010 and 2013, 30 patients were enrolled on the hypo-IMRT/TMZ/BEV trial. Mean age was 59.5 in the hypo-IMRT/TMZ alone trial and 55.9 in the hypo-IMRT/TMZ/BEV trial (p = 0.466). Mean KPS and PTV1 were higher in the hypo-IMRT/TMZ/BEV trial compared to the hypo-IMRT/TMZ alone trial, 83 and 77.7 % (p = 0.025), and 127.7 versus 100 ml (p = 0.029), respectively. The majority of patients in both trials were in RPA class 4. Patient demographics for both trials are summarized in Table 1.
Treatment compliance
All patients on both trials received hypo-IMRT and concurrent systemic therapy as prescribed. In the hypo-IMRT/TMZ alone trial, the mean number of adjuvant TMZ cycles was six and the hypo-IMRT/TMZ/BEV trial 7 (p = 0.532). 54 % of patients in the hypo-IMRT/TMZ alone trial completed 6 cycles of TMZ per protocol, compared to 70 % of patients in the hypo-IMRT/TMZ/BEV trial. All patients in the hypo-IMRT/TMZ/BEV trial received two doses of BEV on days 1 and 15 per the protocol; a mean of five cycles of adjuvant BEV were administered (Table 1).
Outcomes
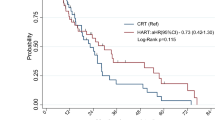
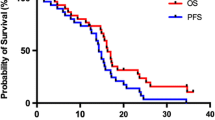
The median follow-up times for the hypo-IMRT/TMZ alone trial and the hypo-IMRT/TMZ/BEV trial were 13.9 and 14.7 months, respectively. Median PFS was increased by 3.4 months in the hypo-IMRT/TMZ/BEV trial, compared to the hypo-IMRT/TMZ alone trial (12.8 vs. 9.4 months, p = 0.58) (Fig. 1). PFS at 6 months was not significantly different between the two trials (84 and 83 %, respectively, p = 0.702). Median OS was the same in both trials at 16.3 months (Fig. 2).
At the time of data analysis for this report four patients treated on the hypo-IMRT/TMZ alone trial are still alive with a median OS of 65 months (range 42.1–66.8). Four patients treated on the hypo-IMRT/TMZ/BEV trial remain alive with a median OS of 32.7 months (range 10.5 – 34.7). Two year OS for the combined patient population was 25.5 %. Five year OS for the hypo-IMRT/TMZ alone trial was 12 %.
At the time of progression patients on both trials were treated with additional surgery, chemotherapy, radiation or phase I trial. On the hypo-IMRT/TMZ trial 6 patients underwent surgery, 2 underwent radiation [fractionated stereotactic radiosurgery (SRS)], and 11 patients received additional chemotherapy including bevacizumab, irinotecan, temozolomide, and BCNU. Two patients were also treated on a clinical trial with a study drug. On the hypo-IMRT/TMZ/BEV trial 6 patients underwent surgery, 2 underwent radiation (one fractionated IMRT; one single fraction SRS), and 8 patients received additional chemotherapy including bevacizumab, CCNU, and temozolomide. One patient was treated with Novocure TTF100A.
Toxicity and adverse events
In the hypo-IMRT/TMZ alone trial, no early or late grade 3 or higher non-hematologic toxicity was observed. Acute grade 1 and 2 toxicities included fatigue, headache, nausea, insomnia, confusion, partial seizure, and anorexia. Late grade 1 or 2 toxicities included fatigue, nausea, insomnia, headache, confusion, partial seizure, and anorexia.
In the hypo-IMRT/TMZ/BEV trial a higher rate of grade 3 toxicity was seen, with 30 % (9/30) of patients experiencing non-hematologic grade 3 toxicity including fatigue, nausea, anorexia, and wound dehiscence, as well as one instance of pulmonary embolism and one instance of stroke. Grade 1 and 2 toxicities were similar to those seen in the hypo-IMRT/TMZ alone trial with the addition of deep vein thrombosis and intracranial hemorrhage.
Clinically significant presumed radiation necrosis
There was a higher rate of clinically significant presumed RN with hypo-IMRT/TMZ/BEV compared to hypo-IMRT/TMZ alone, 50 versus 15 %, respectively (p = 0.012) with an odds ratio of 4.88 (CI 1.17–20.4). After adjusting for KPS and PTV1 size, however, the odds ratio was no longer statistically significant (OR 3.53; CI 0.90–13.8). Presumed radionecrosis was defined as progressive MRI changes not attributable to tumor progression resulting in neurological deficits. Differentiation from tumor progression was based on perfusion weighted MR imaging showing hypoperfusion in the areas of MRI change and consensus after presentation at a multidisciplinary brain tumor board. Clinically significant presumed RN was a late toxicity occurring at a median of 15.8 months (range 8.6–34.5), and an incidence at 6, 12, and 24 months of 0, 3 and 43 %.
Reoperation
In the hypo-IMRT/TMZ alone trial, six patients underwent a second craniotomy due to increased T1-weighted post-contrast enhancement seen on follow-up brain MRI, at a median of 10.3 months post hypo-IMRT (range 1.3–20.7 months). Four of the 6 patients were found to have >80 % necrosis with 2 patients having 100 % necrosis. The other 2 patients’ specimens contained 30–60 % recurrent tumor. In the hypo-IMRT/TMZ/BEV trial, 6 patients also underwent a second craniotomy for MRI changes alone (2 patients) and MRI changes with symptoms (4 patients), at a median of 13.6 months post hypo-IMRT (range 2.0–23.4 months). Two patients had extensive RN (>80 %) with residual glial tumor cells. Two patients had 30–40 % residual tumor in the setting of extensive necrosis. One patient had mixed tumor and necrosis, with the proportions of each not further quantified. The final patient was found to have high grade GBM with absence of therapy induced necrosis.
Discussion
Hypo-IMRT represents an alternative way to escalate BED for GBM patients without increasing treatment time in this poor prognosis population. Results from our first phase II trial demonstrated comparable OS outcomes to standard of care therapy [4]. Additionally, patterns of failure appeared to be altered with fewer local failures within the high dose region and more distant intracranial recurrences [5].
The addition of bevacizumab to hypofractionated radiation was thought to be a logical therapeutic strategy, both because of the intrinsic characteristics of GBM, as well as the demonstrated potential of bevacizumab to decrease rates of clinical/radiographic RN [16–18]. VEGF is overexpressed in malignant gliomas and has been shown to promote both angiogenesis and invasion of tumor cells [19, 20]. Initial results from phase 2 trials evaluating the addition of bevacizumab to standard of care therapy in both the upfront and recurrent setting were promising in terms of OS [11, 13]. RN is considered a delayed radiation toxicity, resulting in part from vascular endothelial injury [21]. Based on this mechanism, bevacizumab, which normalizes tumor vasculature, has been used to treat RN with documented success on imaging in a number of studies [16–18].
In the hypo-IMRT/TMZ/BEV trial we sought to evaluate the effect of bevacizumab in combination with hypo-IMRT and temozolomide on PFS survival as a primary endpoint, and OS and toxicity as secondary endpoints. This comparison study demonstrated a statistically non-significant increase in PFS with bevacizumab of 3.4 months (p = 0.39), and no difference in OS. Two recently published large randomized trials, the AVAGlio and RTOG 0825, have reported similar findings with standard fractionation radiation, temozolomide and BEV. The addition of bevacizumab improved progression free survival by 3.4–4.4 months, but had no effect on OS [22, 23].
Both the radiation dose and volume are predictors of RN [24, 25]. Larger PTV volumes may have contributed to the high rate of clinically significant presumed RN in the hypo-IMRT/TMZ/BEV trial. Recently, the Memorial Sloan Kettering group published results from a phase II trial of hypofractionated stereotactic radiotherapy with TMZ and BEV for newly diagnosed glioblastoma. They enrolled 40 patients with tumor volumes ≤60 cc and treated to 36 in 6 Gy fractions to the T1-contrast enhancing tumor and 24 in 4 Gy fractions to the FLAIR signal abnormality over 2 weeks. Treatment was well tolerated with no reports of RN. Median PFS was 10 months with a 1-year OS of 93 % and median OS of 19 months [26]. Reducing the high-dose treatment volume by selecting patients with smaller tumors as well as reducing the total radiation dose might help balance this increased risk of tissue necrosis and improve the tolerability to treatment and local tumor control. The reasons that a size limit was not set in the hypo-IMRT/TMZ/BEV trial were: (1) no grade 3–4 non-hematologic toxicity was observed in first trial; (2) to reduce patient selection bias in a single arm trial; (3) it was anticipated that BEV may have protective effects from RN. Further research is needed to define the suitable patient population and optimal total and fractional radiation dosage.
It was postulated that BEV would protect against the development of necrosis. A high rate of clinically significant presumed RN in our hypo-IMRT/TMZ/BEV trial did not support that. Clinically significant presumed RN was defined as MRI changes not attributable to tumor progression, with associated neurologic deficits. Specifically, differentiation from tumor progression was based on perfusion-weighted imaging showing hypoperfusion in the areas of MRI change, consensus after presentation at a multi-disciplinary brain tumor board, and ongoing follow-up of the patients revealing stabilization of imaging changes [15]. The “radioprotective” theory is primarily based on decreasing vascular permeability by bevacizumab, which renders radionecrosis invisible and less symptomatic; however, it is unknown whether BEV actually prevents tissue damage. Furthermore, it is possible that the addition of BEV may have contributed to necrosis in the hypo-IMRT/TMZ/BEV trial. Prolonged exposure to anti-angiogenic agents may result in “overpruning” of diseased blood vessels exacerbating hypoxia, necrosis and ischemia [27]. Exacerbation of RN has been described in one case report with a patient initially showing improvement in symptoms followed by deterioration during four cycles of therapy [28].
This study has certain limitations. Although the two trials compared were prospective trials, they were not randomized studies. Significantly larger PTVs were treated in the hypo-IMRT/TMZ/BEV trial. Small patient numbers may have limited statistical power.
Conclusion
Based on our two trial comparison, the addition of BEV does not improve OS in patients with GBM treated with hypo-IMRT to 60 Gy delivered in 6 Gy fractions over 2 weeks with concurrent and adjuvant TMZ. This finding is in line with recently published randomized trials which showed no survival benefit when BEV was added to conventional radiation with TMZ in GBM patients.
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Chen C, Damek D, Gaspar LE et al (2011) Phase I trial of hypofractionated intensity-modulated radiotherapy with temozolomide chemotherapy for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys 81:1066–1074
Reddy K, Damek D, Gaspar LE et al (2012) Phase II trial of hypofractionated IMRT with temozolomide for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys 84:655–660
Reddy K, Gaspar LE, Kavanagh BD et al (2014) Hypofractionated intensity-modulated radiotherapy with temozolomide chemotherapy may alter the patterns of failure in patients with glioblastoma multiforme. J Med Imaging Radiat Oncol 58:714–721
Birner P, Piribauer M, Fischer I et al (2003) Vascular patterns in glioblastoma influence clinical outcome and associate with variable expression of angiogenic proteins: evidence for distinct angiogenic subtypes. Brain Pathol 13:133–143
Godard S, Getz G, Delorenzi M et al (2003) Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res 63:6613–6625
Stefanik DF, Fellows WK, Rizkalla LR et al (2001) Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol 55:91–100
Kim KJ, Li B, Winer J et al (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841–844
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Vredenburgh JJ, Desjardins A, Herndon JE et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Lai A, Filka E, McGibbon B et al (2008) Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys 71:1372–1380
Gutin PH, Iwamoto FM, Beal K et al (2009) Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 75:156–163
Ney D, Carlson JA, Damek DM et al (2015) Phase II trial of hypofractionated intensity-modulated radiation therapy combined with temozolomide and bevacizumab for patients with newly diagnosed glioblastoma. J Neurooncol 122:135–143
Gonzalez J, Kumar AJ, Conrad CA et al (2007) Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 67:323–326
Torcuator R, Zuniga R, Mohan YS et al (2009) Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol 94:63–68
Wong ET, Huberman M, Lu XQ et al (2008) Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol 26:5649–5650
Salmaggi A, Eoli M, Frigerio S et al (2003) Intracavitary VEGF, bFGF, IL-8, IL-12 levels in primary and recurrent malignant glioma. J Neurooncol 62:297–303
Lamszus K, Ulbricht U, Matschke J et al (2003) Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin Cancer Res 9:1399–1405
Coderre JA, Morris GM, Micca PL et al (2006) Late effects of radiation on the central nervous system: role of vascular endothelial damage and glial stem cell survival. Radiat Res 166:495–503
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709–722
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708
Minniti G, Clarke E, Lanzetta G et al (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 6:48
Blonigen BJ, Steinmetz RD, Levin L et al (2010) Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 77:996–1001
Omuro A, Beal K, Gutin P et al (2014) Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res 20:5023–5031
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62
Jeyaretna DS, Curry WT Jr, Batchelor TT et al (2011) Exacerbation of cerebral radiation necrosis by bevacizumab. J Clin Oncol 29:e159–e162
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carlson, J.A., Reddy, K., Gaspar, L.E. et al. Hypofractionated-intensity modulated radiotherapy (hypo-IMRT) and temozolomide (TMZ) with or without bevacizumab (BEV) for newly diagnosed glioblastoma multiforme (GBM): a comparison of two prospective phase II trials. J Neurooncol 123, 251–257 (2015). https://doi.org/10.1007/s11060-015-1791-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1791-4