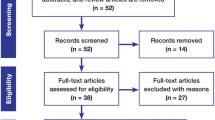
Abstract
Background
Combining Gliadel wafers and radiochemotherapy with TMZ may carry the risk of increased adverse events (AE). We analyzed the efficacy and safety in patients with glioblastoma who underwent multimodal treatment with implantation of Gliadel wafers.
Methods
One hundred sixty-five consecutive patients with newly diagnosed (77 patients) or recurrent (88 patients) glioblastoma were studied. Forty-seven patients underwent surgery + Gliadel. The impact of age (≥65 vs. <65), resection extent (gross total vs. partial), use of Gliadel and adjuvant treatment (TMZ vs. other schemes/no adjuvant therapy) on overall survival (OS, for patients with newly diagnosed glioblastoma) and on recurrence-survival (for patients with recurrent glioblastoma) was analyzed with Cox regression. The impact of age, history (newly diagnosed vs. recurrent glioblastoma), number of Gliadel wafers implanted (0 vs. <8 vs. 8), resection extent (gross-total vs. partial) and adjuvant treatment (TMZ vs. other schemes/no adjuvant therapy) on the occurrence of AE and on the occurrence of implantation site-related AE (ISAE) was analyzed with the logistic regression model. Significance was set at p < 0.05.
Results
Multivariate analysis showed the only factor associated with longer survival, both for newly diagnosed and for recurrent GBM, was resection extent. Both patients with a higher number of wafers implanted and patients with recurrent tumors were significantly at risk for AE and ISAE. Patients with eight Gliadel wafers implanted had a 3-fold increased risk of AE and a 5.6-fold increased risk of ISAE, and patients with recurrent tumor had a 2.8-fold increased risk of AE and a 9.3-fold increased risk of ISAE.
Conclusions
Adding Gliadel to standard treatment did not significantly improve the outcome. The toxicity after Gliadel use was significantly higher, both for patients with newly diagnosed and patients with recurrent glioblastoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, a Cochrane Database systematic review concluded that carmustine-impregnated wafers (Gliadel®) result in improved survival without an increased incidence of adverse events compared to placebo wafers when used for primary disease therapy for high-grade gliomas and that in recurrent disease Gliadel® does not appear to confer any additional benefit [4]. These reviewers analyzed the results of two randomized phase III trials published before 2005, with a total of 272 participants, showing significant improvement of survival 1, 2 and 3 years after implantation of Gliadel wafers for patients with newly diagnosed malignant glioma [12, 14, 15].
These studies and subsequent non-phase III studies have also shown risks associated with local chemotherapy within the central nervous system. Several complications have been associated with implantation of Gliadel wafers, including cerebral edema, healing abnormalities, cerebral spinal fluid (CSF) leaks, intracranial infections, seizures, hydrocephalus and cyst formation [1–3, 5, 6, 8, 10, 12, 14].
In 2005, Stupp et al. published the data of a phase III trial demonstrating the efficacy of radiation therapy and concomitant temozolomide (TMZ) in newly diagnosed glioblastomas [11]. The addition of TMZ to radiotherapy for newly diagnosed glioblastoma resulted in a clinically meaningful and statistically significant survival benefit (median survival 14.6 months vs. 12.1 months) with minimal additional toxicity. At present, after tumor resection, a 6-week protocol of concomitant radiochemotherapy with TMZ followed by six cycles of TMZ monochemotherapy can be regarded as the standard treatment for patients with newly diagnosed glioblastoma. Generally, this treatment is well tolerated. Grades 3 and 4 hematologic toxicity occur in 16 % of patients receiving chemoradiotherapy with TMZ [11].
Lately, this has resulted in clinical protocols combining local chemotherapy with BCNU wafers and concomitant radiochemotherapy with TMZ, although this may carry the risk of increased toxicity [2, 5, 6]. Recent retrospective studies have raised some concerns about the efficacy of Gliadel wafers in prolonging survival [2, 5, 6]. One of these studies showed no difference in survival between patients receiving standard adjuvant therapy with TMZ and patients receiving standard adjuvant therapy with TMZ and Gliadel [6].
The aim of this study was to analyze the efficacy and safety, comparing standard care (surgery followed by adjuvant radiochemotherapy with TMZ) and standard care plus Gliadel, in patients with newly diagnosed and recurrent glioblastoma treated at our institution.
Methods
Patients and treatment
From 2006 to 2011, 165 consecutive patients with newly diagnosed (77 patients) or recurrent (88 patients) glioblastoma were treated at the Departments of Neurosurgery and Radiation Therapy of Catholic University, Rome.
All patients received corticosteroids (betametasone, dose 4 mg/day to 16 mg/day) before (for 4-8 days before surgery) and after surgery. Antiepilectic drugs (both before and after surgery) were only administered if a history of two or more seizures was present: we administered levetiracetam (Keppra®) at a daily dose of 1,000/3,000 mg, or oxcarbazepine (Tolep®) at a daily dose of 600/1,200 mg or phenobarbital (Luminale®) at a daily dose of 100/200 mg. At induction of anesthesia, patients were given 2 g cephazolin intravenously. Postoperative doses of 2 g/day were administered every 12 h for 3 days after surgery. All patients received graduated compression (elastic) thigh-high stockings before surgery. Enoxaparin (Clexane®) was administered before surgery only for bedridden patients or for patients with paresis. Postoperatively, all bedridden patients received enoxaparin (daily dose: 4,000 UI).
All patients underwent craniotomy and tumor removal (with an intraoperative histological diagnosis of high-grade glioma). Contrast-enhanced MRI/CT studies were obtained shortly after surgery (within 72 h) in all patients and were used to determine the entity of tumor resection. Histological diagnosis was then confirmed for all cases (WHO grade IV glioblastoma).
Among these cases, 47 patients underwent surgery with intraoperative positioning of up to eight Gliadel wafers (19 patients with newly diagnosed glioblastoma and 28 patients with recurrent glioblastoma; see Table 1). All patients gave their written informed consent after being carefully informed concerning the potential risks and benefits of BCNU wafers prior to surgery.
Patients with newly diagnosed glioblastoma received adjuvant therapy 30 days after surgery (range, 3–9 weeks) and three-dimensional conformal radiation therapy (RT), delivered by 6-MV LINAC (total dose: 5,940 cGy–180 cGy/day). Patients received chemotherapy concomitant to RT with TMZ at a daily dose of 75 mg/m² per day. After a 1-month break, patients received up to 20 cycles of TMZ (mean 6 cycles) at a dose from 150 to 200 mg/m² per day on the standard schedule of 5 days per week every 28 days.
All patients with recurrent glioblastoma had undergone surgery followed by radio-chemotherapy at the first diagnosis. Most of these patients received TMZ (dose from 150 to 200 mg/m² per day on the standard schedule of 5 days per week every 28 days) after surgery.
Thirteen patients with newly diagnosed GBM and eight patients with recurrent GBM underwent surgery alone or received different chemotherapy schemes (because of TMZ-related toxicity) with cisplatin, fotemustine, BCNU and irinotecan. For patients who underwent surgery alone, adjuvant therapy was not administered because of poor clinical condition after surgery, subgaleal collection/hydrocephalus/wound dehiscence or patient/relative will.
Follow-up, adverse events and ethics
None of these patients was lost at follow-up. Patients were discharged between 8 and 46 days after surgery (median: 11 days). Radiological follow-up was performed in all cases: within 3 days (early postoperative contrast-enhanced CT/MRI), during hospitalization, at discharge (CT scan between 8 and 46 days after surgery), as outpatients 0–7 days before patients started radiation therapy (contrast-enhanced brain MRI between 18 and 51 days postoperatively), before adjuvant TMZ (contrast-enhanced MRI) and every three cycles during chemotherapy (contrast-enhanced MRI). Furthermore, we visit all patients as outpatients in the neurosurgery outpatient department 1 week/10 days after discharge in order to control the surgical wound, clinical status and medications. Patients were therefore monitored almost continually during the first 2 months after surgery. Contrast-enhanced MRI was then performed every 2 months for the first 2 years and every 3 months subsequently.
Follow-up data of all patients were obtained by the radiation therapy outpatient department, where patients were followed up initially once a week until adjuvant therapy completion and then every 2 months, even after tumor recurrence/disease progression. Weekly complete blood counts and platelets plus routine biochemistry analysis were performed. Toxicity and all adverse events were recorded. Minimum follow-up for this study was 12 months.
This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Statistical analysis
Statistical software used for analyses was SPSS (Statistical Package for the Social Sciences) 11.0 for Windows. Statistical analysis aimed to determine factors that could affect survival and potential predictors of adverse events.
Median recurrence-survival (RS, time between recurrence and death/last follow-up) and overall survival (OS) was determined. For patients with newly diagnosed glioblastoma, the impact on OS of age at diagnosis (≥65 vs. <65), resection extent (gross total vs. partial), use of Gliadel wafers and adjuvant treatment received (TMZ vs. other schemes/no adjuvant therapy) was analyzed. For patients with recurrent GBM, the impact on RS of age at recurrence (≥65 vs. <65), resection extent (gross total vs. partial), use of Gliadel wafers and adjuvant treatment received (TMZ vs. other schemes/no adjuvant therapy) was analyzed. Survival curves were obtained through the Kaplan–Meier method and were compared using the log-rank and Breslow tests. The Cox proportional hazards model was used for multivariate analyses.
Among the available clinical data, we analyzed the impact of age (≥65 vs. <65), history (newly diagnosed glioblastoma vs. recurrent glioblastoma), number of Gliadel wafers implanted (0 vs. <8 vs. 8), resection extent (gross total vs. partial) and adjuvant treatment received (TMZ vs. other schemes/no adjuvant therapy) on the occurrence of adverse events (AE), such as leucopenia/thrombopenia, deep venous thrombosis/pulmonary embolism, postoperative new-onset seizures, depression, severe brain edema, CSF leakage and intracranial abscess formation, and on the occurrence of implantation site-related AEs (ISAE), such as edema, CSF leak, abscess, seizures and depression/altered mental status. Dichotomous data were compared with Fisher’s exact test (two-sided). The logistic regression model was used to assess the independent contribution of predictive factors to the occurrence of AE or ISAE. Significance was set at p < 0.05.
Results
Survival: newly diagnosed GBM
The median OS for the 77 patients with newly diagnosed GBM was 13 months (95 % CI 9–16). OS was 14 months (CI 95 % 8–17) for 42 patients under 65 years of age and 11 months (95 % CI 7–15) for older patients. This difference was not significant (log-rank test, p = 0.48; Breslow test, p = 0.54). Patients who underwent gross total tumor removal (42 cases) had an OS of 16 months (95 % CI 13–20), and patients who underwent partial tumor removal had an OS of 10 months (95 % CI 9–11; log-rank test, p = 0.007; Breslow test, p = 0.02). Patients receiving Gliadel wafers (19 cases) had a longer OS (14 months, 95% CI 8–18 vs. 11 months, 95 % CI 8–14), but this difference was not statistically significant (log-rank test, p = 0.77; Breslow test, p = 0.72). OS was also longer for 10 patients receiving 8 Gliadel wafers (13 months, 95 % CI 11–16) compared to 9 patients receiving up to 7 wafers (11 months, 95 % CI 1–28) or 58 patients receiving no Gliadel (11 months, 95 % CI 8–14), but this difference was not statistically significant (log-rank test, p = 0.69; Breslow test, p = 0.50). OS was 15 months (95% CI 11–17) for patients receiving chemotherapy with TMZ and 10 months (95 % CI 8–12) for patients receiving other schemes/no adjuvant therapy (log-rank test, p = 0.01; Breslow test, p = 0.04). Multivariate analysis showed the only factor significantly associated with longer survival was gross total tumor removal (HR = 1.8, p = 0.048; see Table 2).
Survival: recurrent GBM
Median RS for the 88 patients with recurrent GBM was 8 months (95 % CI 6–10). RS was 8 months (CI 95 % 7–9) for 61 patients under 65 years of age and 7 months (95 % CI 5–9) for older patients. This difference was not significant (log-rank test, p = 0.7; Breslow test, p = 0.39). Patients who underwent gross total tumor removal (66 cases) had an RS of 9 months (95 % CI 6–12), and patients who underwent partial tumor removal had an RS of 6 months (95 % CI 4–8; log-rank test, p = 0.006; Breslow test, p = 0.03). Patients receiving Gliadel wafers (28 cases) had a shorter RS (6 months, 95 % CI 4–8 vs. 9 months, 95 % CI 7–11), but this difference was not statistically significant (log-rank test, p = 0.47; Breslow test, p = 0.38). RS was also similar for 17 patients receiving 8 Gliadel wafers (8 months, 95 % CI 5–11) compared with 11 patients receiving up to 7 wafers (6 months, 95 % CI 3–9) or 60 patients receiving no Gliadel (9 months, 95 % CI 7–11), but this difference was not statistically significant (log-rank test, p = 0.77; Breslow test, p = 0.68). RS was 8 months (95% CI 6–10) for patients receiving chemotherapy with TMZ and 7 months (95 % CI 1–13) for patients receiving other schemes/no adjuvant therapy (log-rank test, p = 0.79; Breslow test, p = 0.95).
Multivariate analysis showed the only factor significantly associated with longer survival was gross total tumor removal (HR = 2, p = 0.007; see Table 3).
Adverse events
Overall, complications of any type, as described below, occurred in 46/166 patients (27.7 %). The complication rate was higher for patients with recurrent GBM (33/88 patients, 37.5 %) than for patients with newly diagnosed GBM (13/78 patients, 16.6 %, Fisher’s exact test, two-sided, p = 0.003). Complication rates were higher for patients receiving 8 Gliadel wafers (14/27 patients, 51.8 %) compared to patients receiving up to 7 wafers (3/20 patients, 15 %) or no wafers (29/119, 24.3 %) (p = 0.006).
ISAE occurred in 32/166 patients (19.2 %). ISAEs were more frequent in patients with recurrent GBM (28/88 patients, 31.8 %) than in patients with newly diagnosed GBM (4/78 patients, 5 %; Fisher’s exact test, two-sided, p < 0.0001). ISAEs were more frequent in patients receiving 8 Gliadel wafers (13/27 patients, 48 %) than in patients receiving up to 7 wafers (2/20 patients, 10 %) or no wafers (17/119 patients, 14.2 %; p < 0.0001).
Multivariate logistic regression analysis revealed that both patients with a higher number of wafers implanted and patients with recurrent tumors were significantly at risk for adverse events of any type and for ISAE (Tables 4 and 5). In particular, patients with eight Gliadel wafers implanted had a 3-fold increased risk of AE and a 5.6-fold increased risk of ISAE, and patients with recurrent tumor had a 2.8-fold increased risk of AE and a 9.3-fold increased risk of ISAE complications (Tables 4 and 5).
Curiously, we also observed that wafers were still visible on the CT scan at 3 months postoperatively (Fig. 1) in seven patients. Six out of seven patients had a recurrent tumor.
Discussion
Studies performed before 2005 on patients with high-grade gliomas reported that the number of deaths, adverse events and laboratory abnormalities were high, as expected in this particular patient population [3, 12, 14]. The Gliadel arm and the placebo arm both experienced similar adverse events. In particular, according to Westphal et al., the most frequently reported AEs among the patients receiving Gliadel were hemiplegia, convulsions, confusion and brain edema. The most commonly reported adverse events among the patients in the placebo arm were convulsions, confusion, brain edema and aphasia. The only difference between the groups was that more patients in the Gliadel arm experienced intracranial hypertension (11 patients vs. 2 patients in the placebo arm, p = 0.019) [14, 15].
Similarly, another large randomized study by Brem et al. also found that both groups had similar occurrences of AE [3]. In particular, the overall incidence of serious intracranial infection was 2.2 %, but this complication was more common in the Gliadel arm than in the placebo arm (3.6 % and 0.89 %, respectively). This difference was statistically nonsignificant [3].
After the advent of TMZ, there have been no randomized studies on the efficacy and safety comparing standard care (surgery followed by adjuvant radiochemotherapy with TMZ) with standard care plus Gliadel [9]. In 2008, Attenello et al. retrospectively analyzed their large series of more than 1,000 patients (including 288 patients with Gliadel wafers implanted) and found no difference in the incidence of adverse events between groups [1]. In the last 2 years, three European studies have been published on this subject [2, 5, 6]. The multicenter retrospective study by Bock et al. reported an overall AE incidence of 52 % (23 patients), resulting in therapy delays for concomitant radiochemotherapy in eight patients (18 %). Seven patients (16 %) in that series required surgical treatment of AE. These authors concluded that observed adverse events appeared similar to complication rates published in the phase III trials for BCNU wafer implantation followed by radiation therapy alone and stated that Gliadel wafers “further add to the toxicity of concomitant radiochemotherapy with systemic TMZ” [2]. Menei et al. also concluded that the combination of Gliadel and radiochemotherapy with TMZ was well tolerated and appeared to increase survival (with respect to the Westphal study) without increasing AEs [5, 14]. Noel et al. compared a group treated with surgery, radiation therapy and TMZ, and a group in which Gliadel was added. They found no difference in survival between groups and observed a higher incidence of toxicity (grade 3 thrombopenia) in the Gliadel group [6].
In our study, we observed that the extent of surgery was the only factor associated with longer survival, both for newly diagnosed GBM and for recurrent GBM. No difference in survival was observed for patients receiving Gliadel wafers.
The incidence of AE reported in our study was comparable to that reported in the previously cited papers. During surgery, the number of wafers used was dependent on the cavity size. In our series, most patients with ISAE had received eight Gliadel wafers. Interestingly, multivariate analysis showed that the number of adverse events (both AE of any type and ISAE) was significantly higher for patients with recurrent GBM. The reason for this difference, which has not been previously observed, could be explained by the difficulty of the “injured” brain, meninges, bone and scalp to recover after repetitive mechanical and toxic traumas (surgical interventions, radiation therapy, local and systemic chemotherapy).
Multivariate analysis also showed that patients receiving eight Gliadel wafers had a significantly higher risk of AE and of ISAE compared with patients with fewer than eight wafers implanted or no wafers. This finding should be further confirmed in larger series.
In our study, we observed a high incidence of severe brain edema in patients with Gliadel wafers, especially for patients with recurrent GBM (Table 1). This has led us to modify our protocol for postoperative management of patients with recurrent tumors treated with Gliadel wafer implants. We have now increased the dose and length of administration of systemic therapy with steroids. Following the introduction and strict adherence to this risk management protocol, the incidence of severe brain edema dropped significantly. Two mechanisms of brain edema have been hypothesized: one occurring in the immediate postoperative period because of a greater distribution of carmustine and secondary to vasogenic edema induced by the surgery, with subsequent cytotoxicity, and the other occurring later, 2 weeks after the implantation, caused by a necrotic reaction [13]. We could hypothesize that in our cases brain edema was due to local damage of a more “fragile” brain parenchyma (which had already been irradiated prior to surgery for recurrence).
Finally, a curious finding in our study was that wafers were still visible on the CT scan at 3 months postoperatively in seven patients (in one case they were still visible at 5 months postoperatively). We do not have an explanation for this finding, as clinical characteristics of these patients were similar to those of other patients, with the exception that almost all of these patients (6/7) had a recurrent tumor. Possibly, brain scar/gliosis (because of the repeated surgery and previous radiation therapy) caused a sort of mechanical barrier to the penetration of the drug. The persistence of wafers after surgery has been investigated previously by Prager et al., who found that wafers were poorly seen after 2 months [7].
The main limitation of our study is its retrospective, nonrandomized nature (these studies may overestimate the effect of treatments because of attrition, detection or performance bias). Another limitation is the lack of information on the MGMT status (whose determination has been available in our hospital for 3 years).
In our study, many of these biases were avoided because no patients were lost to follow-up, the outcome and prognostic variables were standardized, the completeness and quality of the data were carefully checked and multivariate analyses were performed. We therefore acknowledge that our conclusions on both survival and complication rate should be tempered and should be confirmed by larger, randomized studies.
Conclusions
Adding Gliadel to standard treatment did not significantly improve the outcome for patients with newly diagnosed GBM or for patients with recurrent GBM. A trend towards longer OS was observed for patients with newly diagnosed glioblastoma in favor of the use of Gliadel. The toxicity after Gliadel use is significantly higher for both patients with newly diagnosed GBM and patients with recurrent tumor. Patients with recurrent GBM have a high risk of complications. Therefore, the results of our study suggest that a further selection of patients to follow this scheme should be considered, as the risk of toxicity after the use of Gliadel wafers could be high, especially for patients with recurrent tumor, without improving survival. According to this consideration, some stratification could be made in the future, taking into account survival and adverse events for the obvious purposes of model prediction before using the combination of surgery, Gliadel wafers, radiation and TMZ. Randomized trials comparing current standard therapy and standard therapy plus Gliadel wafers are greatly needed. Taking into account that all of these therapies are only performed in a palliative setting and so far the overall survival for glioblastoma is still only approximately 14 months, severe side effects leading to hospitalization and profound loss of quality of life are unacceptable for patients, their families and caregivers.
References
Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H (2008) Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol 15:2887–2893
Bock HC, Puchner MJ, Lohmann F, Schutze M, Koll S, Ketter R, Buchalla R, Rainov N, Kantelhardt SR, Rohde V, Giese A (2010) First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev 33:441–449
Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G et al (1995) Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The polymer-brain tumor treatment group. Lancet 345:1008–1012
Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K (2011) Chemotherapy wafers for high grade glioma. Cochrane Database Syst Rev 3:CD007294
Menei P, Metellus P, Parot-Schinkel E, Loiseau H, Capelle L, Jacquet G, Guyotat J (2010) Biodegradable carmustine wafers (Gliadel) alone or in combination with chemoradiotherapy: the French experience. Ann Surg Oncol 17:1740–1746
Noel G, Schott R, Froelich S, Gaub MP, Boyer P, Fischer-Lokou D, Dufour P, Kehrli P, Maitrot D (2011) Retrospective comparison of chemoradiotherapy followed by adjuvant chemotherapy, with or without prior gliadel implantation (carmustine) after initial surgery in patients With newly diagnosed high-grade gliomas. Int J Radiat Oncol Biol Phys
Prager JM, Grenier Y, Cozzens JW, Chiowanich P, Gorey MT, Meyer JR (2000) Serial CT and MR imaging of carmustine wafers. AJNR Am J Neuroradiol 21:119–123
Quinn JA, Jiang SX, Carter J, Reardon DA, Desjardins A, Vredenburgh JJ, Rich JN, Gururangan S, Friedman AH, Bigner DD, Sampson JH, McLendon RE, Herndon JE 2nd, Threatt S, Friedman HS (2009) Phase II trial of Gliadel plus O6-benzylguanine in adults with recurrent glioblastoma multiforme. Clin Cancer Res 15:1064–1068
Sabel M, Giese A (2008) Safety profile of carmustine wafers in malignant glioma: a review of controlled trials and a decade of clinical experience. Curr Med Res Opin 24:3239–3257
Smith KA, Ashby LS, Gonzalez LF, Brachman DG, Thomas T, Coons SW, Battaglia M, Scheck A (2008) Prospective trial of gross-total resection with Gliadel wafers followed by early postoperative gamma knife radiosurgery and conformal fractionated radiotherapy as the initial treatment for patients with radiographically suspected, newly diagnosed glioblastoma multiforme. J Neurosurg 109(Suppl):106–117
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T (1997) Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery 41:44–48, discussion 48-49
Weber EL, Goebel EA (2005) Cerebral edema associated with Gliadel wafers: two case studies. Neuro Oncol 7:84–89
Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 5:79–88
Westphal M, Ram Z, Riddle V, Hilt D, Bortey E (2006) Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 148:269–275, discussion 275
Funding
None.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Bonis, P., Anile, C., Pompucci, A. et al. Safety and efficacy of Gliadel wafers for newly diagnosed and recurrent glioblastoma. Acta Neurochir 154, 1371–1378 (2012). https://doi.org/10.1007/s00701-012-1413-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1413-2