Abstract
Background
The purpose of this review is to summarize our own experimental studies carried out over a 13-year period of time using the F98 rat glioma as model for high grade gliomas. We evaluated a binary chemo-radiotherapeutic modality that combines either cisplatin (CDDP) or carboplatin, administered intracerebrally (i.c.) by means of convection-enhanced delivery (CED) or osmotic pumps, in combination with either synchrotron or conventional X-irradiation.
Methods
F98 glioma cells were implanted stereotactically into the brains of syngeneic Fischer rats. Approximately 14 days later, either CDDP or carboplatin was administered i.c. by CED, followed 24 h later by radiotherapy using either a synchrotron or, subsequently, megavoltage linear accelerators (LINAC).
Results
CDDP was administered at a dose of 3 µg in 5 µL, followed 24 h later with an irradiation dose of 15 Gy or carboplatin at a dose of 20 µg in 10 µL, followed 24 h later with 3 fractions of 8 Gy each, at the source at the European Synchrotron Radiation Facility (ESRF). This resulted in a median survival time (MeST) > 180 days with 33% long term survivors (LTS) for CDDP and a MeST > 60 days with 8 to 22% LTS, for carboplatin. Subsequently it became apparent that comparable survival data could be obtained with megavoltage X-irradiation using a LINAC source. The best survival data were obtained with a dose of 72 µg of carboplatin administered by means of Alzet® osmotic pumps over 7 days. This resulted in a MeST of > 180 days, with 55% LTS. Histopathologic examination of all the brains of the surviving rats revealed no residual tumor cells or evidence of significant radiation related effects.
Conclusions
The results obtained using this combination therapy has, to the best of our knowledge, yielded the most promising survival data ever reported using the F98 glioma model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and background
The treatment of high grade glioma represents a therapeutic challenge that has defied the efforts of clinicians and researchers to achieve 5 year survival rates better than a few percent [1]. One of the major problems has been how to circumvent the blood–brain barrier (BBB) in order to improve drug delivery to the brain [2]. The development of convection-enhanced delivery (CED) as a means to improve brain tumor drug delivery was pioneered by Oldfield and Lonser and their co-workers at the National Institutes of Health in Bethesda, Maryland beginning approximately 25 years ago [3,4,5,6,7,8]. The principle upon which CED is based has been described in detail by Morrison [9] and initially was applied to the delivery of a variety of test agents to normal brain in primates and rats, and subsequently chemotherapeutic agents in brain tumor bearing rats. CED is based on the application of a continuous hydraulic force by means of a syringe pump to produce bulk flow thereby increasing the interstitial concentration of a drug beyond that which would occur by diffusion alone. Readers interested in more detailed information relating to CED are referred to a review by Lonser et al. [10].
There now is a voluminous literature on the use of CED to enhance drug delivery to the brain both in experimental animals and in humans. The clinical application of CED for the treatment of patients with high grade glioma has been reviewed by Jahangiri et al. [11] and more recently by Shi and Sanche [12]. Readers interested in this topic are referred to these two detailed, clinically oriented review articles. In the present review we will focus on our own experimental studies using CED or osmotic pumps to increase the concentration of either cisplatin (CDDP) or carboplatin followed by external beam photon irradiation to treat brain tumor bearing rats. The purpose of these studies was to lay the groundwork for future clinical studies in patients with high grade gliomas, as will be described later in this review.
Among all the cytoreductive chemotherapeutic agents available, platinum containing drugs have played an important role for the treatment of solid tumors [13]. However, their use for the treatment of brain tumors has been limited by their toxicity following systemic administration. Intracerebral administration of these drugs was first reported by Kroin and Penn et al. in the early 1980s [14, 15]. They demonstrated that chronic i.c. micro-infusion of Pt containing drugs into the brains of non-tumor bearing rats resulted in adequate and sustained therapeutic drug levels without producing any systemic toxicity [14]. They subsequently reported a small but statistically significant increases in the survival of 9 L glioma-bearing rats compared to untreated controls after a 7 day infusion of CDDP (0.5 mg/mL at a flow rate of 0.9 μL/h) [15]. Using the same tumor model, Kimler et al. [16] evaluated intratumoral (i.t.) administration of CDDP and several other antineoplasic agents, including 1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU), bleomycin, aziridinyl-benzoquinone (AZQ) and acivicin, administered in a single 5 μL injection. Their results suggested that i.c. administration of drugs possibly could represent a promising treatment for patients with brain tumors. Following up on this, Degen et al. [17] demonstrated that carboplatin or gemcitabine, delivered i.c. by CED, were effective in the treatment of 9L glioma-bearing rats. Among the rats that received carboplatin (40 μg/40 μL; 0.5 μL/min) on day 7 after tumor implantation, 3 of 4 animals survived to 120 days at which time the study was terminated. Two long term survivors also were observed in the group treated with 160 μg/40μL of gemcitabine (0.5 μL/min). The authors stated that it was unlikely that chemotherapeutic infusions could be uniformly curative since it only had a marginal effect in other tumor-bearing rats. They hypothesized that sporadic leakage of the infusate along the needle track could decrease the volume of distribution (Vd) in some animals, which underscored the importance of real-time imaging to monitor drug distribution. In the same study, the toxicity of carboplatin and gemcitabine, delivered by CED into the striatum or brainstem of non-tumor bearing rats, was evaluated. Toxicity occurred in rats that had received the highest doses of the drugs, but they were well tolerated if they were administered by CED at lower doses of carboplatin (40 µg/40 µL) or gemcitabine (160 µg/40 µL). It must be emphasized, however, that the 9L glioma is a highly immunogenic tumor and therapy studies employing this tumor model must be interpreted with caution since it can evoke a tumor specific immune response [18].
Basic principles and in vitro studies to evaluate synchrotron radiation in combination with CDDP
Our first studies were initiated by Jacques Balosso et al. [19] who carried out a series of experiments using the F98 glioma model. The F98 glioma (ATCC#CRL-2397) was produced by the intravenous (i.v.) administration of ethyl nitrosourea (ENU) to pregnant Fischer 344 rats on the 20th day of gestation [18]. Subsequently the progeny of these animals developed brain tumors that, after cloning, were designated the F98 glioma. The tumor is composed of a mixed population of spindle-shaped and polygonal cells. As shown in Fig. 1, when implanted intracerebrally, these cells invade contiguous normal brain with islands of tumor cells at varying distances from the main tumor mass, and many of them form perivascular clusters. This tumor has a number of features that resemble human high grade gliomas. These include an invasive pattern of growth within the brain, weak, if any, immunogenicity and the tumor is invariably fatal with an inoculum of as few as 100 cells [18]. F98 glioma cells overexpress RAS and PDGFB, EGFR, cyclin D1 and 2 relative to rat astrocytes [18]. The significant advantages of the F98 glioma now have made it a widely used brain tumor model for a wide variety of experimental studies [18]. Among the most important of these have been the studies by Barth and his co-workers to evaluate both low and high molecular weight boron containing delivery agents, administered by CED, for Boron Neutron Capture Therapy of brain tumors [20,21,22,23,24].
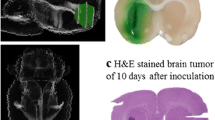
The F98 glioma. a Coronal section of the brain of a Fischer rat at the time of death (24–28 days) following implantation of 104 tumor cells into the right caudate nucleus. b Low power photomicrograph of the tumor showing a central cavity surrounded by proliferating tumor cells. c High power photomicrograph showing proliferating perivascular glioma cells. d High power photomicrograph showing tumor cell invasion into normal brain
Balosso et al.’s first study was aimed at evaluating the efficacy of CDDP in combination with irradiation using a monochromatic synchrotron X-ray beam tuned to energies of either 78.0 or 78.8 keV, which were slightly below or above the K edge (78.4 keV) of platinum [19]. The rationale for this approach was based on the predicted dose-enhancement triggered by the photoelectric effect on Pt atoms by X-rays tuned at the optimal energy above the K-edge of Pt following irradiation. Photo-electrons are extracted from the K-shell resulting in the creation of vacancies that subsequently are filled by radiative (96%) and non-radiative (4%) transitions from outer shells. This generates photo-electrons whose energies are directly linked to the difference between the photons’ energy and that of the Pt K-edge and characteristic low energy photons and Auger electrons. The latter have short path lengths in tissues and high linear energy transfer (LET). Consequently, they can produce lethal DNA double strand breaks (DSBs), providing that the high Z elements are in close proximity to DNA [25]. CDDP was chosen because of its DNA binding properties, the high Z number of Pt, and its frequent use as a chemotherapeutic agent for the treatment of various solid tumors in combination with conventional radiotherapy (XRT). The proximity of Pt atoms to DNA was seen as an advantage for generating lethal DNA DSBs.
Synchrotron radiation sources offer extremely high X-ray fluences, thereby allowing one to obtain a monochromatic X-ray beam. At the medical beamline of the European Synchrotron Radiation Facility (ESRF) in Grenoble, France, monochromatic X-ray beams (ΔE/E = 0.1%) are available within a broad energy range (25–150 keV). Initially, studies were carried out in vitro, following incubation of F98 glioma cells with 30 µM of CDDP for 6 h and irradiation with monochromatic beams tuned above or below the Pt K-edge at an X-ray dose of 30 Gy. A significantly higher number of radiation-induced DSBs (factor 1.3) was observed above the Pt K-edge (78.8 keV), compared to those seen below the Pt K-edge (78.0 keV) [19]. It must be emphasized however, that the in vitro conditions used for these molecular studies resulted in a much higher degree of DNA incorporation of Pt atoms than could have been achieved with in vitro clonogenic assays or in vivo studies. This enhancement, however, was not observed, even at the molecular level, if cells were incubated at concentrations of CDDP that were compatible with their survival [26].
Animal studies to evaluate synchrotron radiation in combination with i.c. CED of CDDP
Based on our in vitro studies, therapy studies were initiated in F98 glioma bearing rats. In order to obtain a sufficient concentration of Pt in the brain tumor, an intra-tumoral bolus injection of CDDP was given to bypass the BBB. In the first study [19] the rats received an i.t. injection of 3 µg of CDDP in 5 µL of isotonic NaCl, administered over few seconds to the tumor site. This was followed 24 h later by stereotactic synchrotron radiotherapy (SSRT) using X-rays with energies tuned slightly below or above the Pt K-edge in a single 15 Gy dose, delivered to the site of the tumor. The irradiation volume was a cylinder of 10 mm in diameter and 12 mm in height. Animals that had received either SSRT or CDDP alone had a median survival time (MeST) of 48 days and 37 days, respectively, and non-irradiated controls had MeST of 26 days. The MeSTs obtained for the two groups treated with the combination of CDDP and SSRT, when the energy was tuned below or above the Pt K-edge, were 214 days and 194 days, respectively (Fig. 2 and Table 1). The MeSTs of the two groups were not significantly different (P > 0.6), although better survival data would have been predicted if they had been irradiated above the Pt K-edge [19]. For the combined treatment, 33% long term survivors (LTS) were observed at 1 year, which at the time were the best survival data ever reported using the F98 glioma model [18].This experiment was repeated a few months later and similar results were obtained [19]. Histopathologic examination of the brains of the long-term survivors, which were euthanized 1 year after treatment, revealed no evidence of residual tumor but acellular pseudocysts, surrounded by a fibrous margin, were seen at the tumor implantation site.
Kaplan–Meier survival plots of rats bearing F98 glioma and subjected to the indicated treatments. The survivals are plotted against time (days) after tumor inoculation. (filled triangle), untreated controls (N = 12, MeST = 26 days); (inverted empty triangle), 3 μg CDDP alone (N = 10, MeST = 37 days); (empty circle), 15 Gy alone (N = 10, MeST = 48 days); (inverted filled triangle), 3 μg CDDP combined with irradiation at 78.0 keV (N = 9, MeST = 214 days); (filled circle), 3 μg CDDP combined with irradiation at 78.8 keV (N = 9, MeST = 194 days). The MeSTs of the two groups that had received CDDP and were irradiated either above or below the Pt K-edge were not significantly different (P > 0.6). Reprinted from [19]
Animal studies to compare CDDP in combination with SSRT or with conventional X-radiation
These prolonged survival times, obtained using the F98 glioma model following i.c. delivery of CDDP and SSRT, encouraged Hélène Elleaume and her research team to further evaluate this therapeutic approach. Since no differences were observed when SSRT was performed either above or below the Pt K-edge, they questioned their initial hypothesis and designed an experiment to compare the combined treatment using either low energy synchrotron or conventional megavoltage X-rays produced by a linear accelerator (LINAC). LINACs produce X-rays having energies for which Compton interactions are dominant in tissues in the presence or absence of high Z number elements such as Pt [27]. Therefore, negligible dose-enhancement would have been expected in the presence of Pt atoms. The next study was designed to determine if i.t. administration of CDDP in combination with LINAC irradiation also would increase the survival of F98 glioma bearing rats [28]. One thousand F98 glioma cells were implanted stereotactically into the brains of Fischer rats and 13 days later CDDP (6 µg in 20 µL) was administered i.c. by CED at a flow rate of 0.5 µL/min. On the following day the animals were irradiated with a single 15 Gy dose of X-rays, administered either using a 6 MV LINAC or 78.8 keV synchrotron X-rays at the ESRF medical beamline. At that time the tumors had a volume of approximately 30 mm3, as determined by synchrotron computed tomography or MRI. It is noteworthy that 13% LTS were observed in the group of rats that had received CDDP alone and their mean survival times (MST) and MeST were 59 ± 13 days (censored survival) and 32 days compared to a MST of 24 ± 1 days and of MeST = 25 days for the untreated controls (Fig. 3 and Table 1). Rats, which had received CDDP in combination with either 6 MV LINAC or 78.8 keV (SSRT), had almost identical censored MSTs of 75 ± 18 days and 74 ± 19 days, respectively, with 17 and 18% long-term survivors (MeST of 48 and 56 days, respectively) (Fig. 3 and Table 1). The survivals were not significantly different from each other (P = 0.9), Microscopic examination of the brains of the long-term surviving rats revealed an absence of viable tumor cells and cystic areas at the presumptive site of the tumor. These data established that i.c. CED of CDDP in combination with external X-irradiation significantly enhanced the survival of F98 glioma bearing rats [28]. This was independent of the X-ray beam energy and probably was not due to the production of Auger electrons, as previously had been hypothesized. Since a conventional LINAC can be used as the radiation source, this would significantly broaden the clinical applicability of this approach compared to SSRT, which could only be carried out at a very small number of specialized facilities.
Kaplan–Meier survival plots for F98 glioma-bearing rats after intra-tumoral CDDP with radiotherapy (6 MV or 78.8 keV photons). Untreated controls (empty diamond) (N = 27, MeST = 25 days), CDDP combined with 6 MV radiotherapy (filled triangle) (N = 12, MeST = 48 days, 17% long-term survivors), Group 6: CDDP combined with 78.8 keV radiotherapy (filled square) (N = 11, MeST = 56 days, 18% long-term survivors). The combined treatments were not significantly different from each other (P = 0.9), Reprinted from [28]
Studies to further define the therapeutic efficacy of CED of CDDP and carboplatin in combination with megavoltage X-irradiation
These very promising results, obtained by Elleaume and her co-workers at the ESRF, stimulated Rolf Barth and his research team at The Ohio State University to join together with Elleaume’s team to further evaluate the efficacy of this therapeutic modality. In parallel with studies to evaluate CED in combination with XRT, other studies were carried out in both laboratories to evaluate the efficacy of carboplatin [29,30,31,32,33,34]. Carboplatin is a CDDP analogue that has a similar range of clinical effectiveness but fewer deleterious side effects [35]. Although it has been reported that longer exposure times and higher drug concentrations were necessary in vitro when using carboplatin, both compounds induced the same number of Pt–DNA adducts [35, 36]. The goal of the next study [29] was to evaluate the efficacy of i.c. CED of carboplatin in combination with fractionated, external beam photon irradiation in F98 glioma-bearing rats.
The toxicity and efficacy of chemotherapy alone first were assessed at varying carboplatin concentrations and dosing schedules. Animals that received carboplatin at a dose of 20 μg in 20 μL had a MeST of 45 days, compared to 30 days for untreated controls. There was one long term survivor (120 days) in the treated group and no early deaths were observed. However, the highest doses of carboplatin tested (40 μg in 40 μL and 100 μg in 20 μL) were found to be toxic. Based on these data, Elleaume et al. chose a dose of 20 μg of carboplatin in 20 μL NaCl, at a flow rate of 0.5 µL/min, administered by CED 13 days after stereotactic implantation of 1000 F98 cells [29]. One day later, irradiations were carried out using either a conventional 6 MV LINAC or a monochromatic synchrotron source, tuned to an energy of 80 keV. The total 24 Gy X-ray dose was administered in three daily fractions of 8 Gy each. The MeSTs were 79 and 60 days, respectively, and the corresponding percent increase in life spans (%ILS) were 182% and 114%, respectively, for the combination of carboplatin chemotherapy and irradiation with either 6 MV or 80 keV photons [29] (Fig. 4 and Table 1). A subset of LTS > 200 days were observed in both chemo-radiotherapy groups: 16.6% and 8.3% for 6 MV and 80 keV, respectively. In contrast, the MeST of the 6 MV or 80 keV irradiated controls, chemotherapy alone, and untreated controls were 42, 51, 45, and 28 days, respectively.
Kaplan–Meier survival curves for F98 glioma bearing rats after chemoradiotherapy. Carboplatin was administrated on day 13 and X-ray dose fractions were delivered on days 14, 15, and 16 after tumor implantation. Survival times in days after tumor implantation for untreated animals (+ and ×, N = 9 and 6, MeSTs = 28 days); CED of carboplatin 20 μg/20 μL alone (empty diamond, N = 10, MeST = 45 days); irradiation at 6MV alone three fractions of 8 Gy (empty triangle, N = 11, MeST = 42 days); or in combination with CED of carboplatin 20 μg/20 μL (filled triangle, N = 12, MeST = 79 days); irradiation at 80 keV alone three fractions of 8 Gy (filled diamond, N = 11, MeST = 51 days) or in combination with CED of carboplatin 20 μg/20 μL (empty square, N = 12, MeST = 60 days). A subset of LTS > 200 days were observed in both chemo-radiotherapy groups: 16.6% and 8.3% for 6 MV and 80 keV, respectively. Reprinted from [29]
Studies to further improve the efficacy of the combination of carboplatin and X-irradiation
In order to improve the Pt distribution in the tumors, the following studies were performed using Alzet® osmotic pumps to administer carboplatin over a prolonged period of time [31, 34]. Carson and his co-workers previously had reported that carboplatin, delivered by Alzet® osmotic pumps to the brainstem of rats bearing the F98 glioma, had significantly prolonged survival times [37,38,39]. Based on this, we administered carboplatin i.c. by means of Alzet® osmotic pumps. Seven days after stereotactic implantation of 1000 F98 glioma cells into the brains of Fischer rats, carboplatin (72 μg in 144 μL) was delivered at a flow rate of 1 μL/h over 6 days, after which the pumps were removed. Rats were treated with a single 15 Gy X-ray dose, either delivered alone or 24 h after administration of carboplatin. Irradiation was performed in the first study at the ESRF with monochromatic X-rays tuned above the Pt K-edge [34]. Untreated rats had a MeST of 24 days, compared with 45 days for X-irradiated animals and 30 days for rats that received carboplatin alone, with 3 of 13 of the latter surviving > 195 days at which time the study was terminated. Animals that had received carboplatin followed by SSRT had a censored MST of 142 ± 21 days and a MeST of > 195 days, with 6 of 11 rats (55%) still alive at the end of the study (Fig. 5, Table 1). This was significantly different (P < 0.05) from irradiated or chemotherapy alone control animals. The corresponding %ILS, based on MeSTs, were 25%, 85%, and 713%, respectively, for carboplatin alone, SSRT alone, or the combination. Histologic examination of the brains of the rats that had received chemo-radiotherapy revealed no microscopic evidence of residual tumor in 7 out of 11 animals. These studies suggested that prolonged administration of carboplatin was superior to bolus administration by means of CED.
Kaplan–Meier survival plots for F98 glioma bearing rats after chemoradiotherapy. Carboplatin infusion (72 µg) was delivered from day 7 to day 13, using Alzet® osmotic pumps. Radiotherapy was delivered in a single 15 Gy fraction on day 14 using either 6 MV or monochromatic 78.8 keV X-rays. The origin of the x-axis corresponds to tumor implantation. 6 MV X-irradiation alone (filled triangle, N = 7, MeST = 38 days); Carboplatin in combination with 6 MV X-irradiation (filled square, N = 11, MeST > 180 days, 55% LTS); 78.8 keV synchrotron irradiation alone (empty triangle, N = 8, MeST = 45 days); Carboplatin in combination with 78.8 keV synchrotron irradiation (empty square, N = 11, MeST > 195 days, 55% LTS). Reprinted from [31, 34]
The next study [31] was carried out to demonstrate that this effect was independent of the radiation energy used. The survival of F98 glioma bearing rats was compared to that of rats receiving a similar chemotherapy regimen in combination with irradiation delivered using a LINAC. Carboplatin was administered i.c. to F98 glioma bearing rats over 6 days using Alzet® pumps starting 7 days after implantation of 1000 F98 cells. Radiotherapy was delivered in a single 15 Gy fraction on day 14. Untreated control animals had a MeST of 33 days. Animals that had received either carboplatin alone or XRT alone had MeSTs of 52 and 38 days, respectively. In the carboplatin alone group, one animal out of 7 survived until the end of the study (180 days after tumor implantation). Animals that received carboplatin in combination with XRT had a MST of 126 ± 8 days (censored data) and a MeST of > 180 days with a 55% cure rate (Fig. 5 and Table 1). The survivals of the chemoradiotherapy groups were not significantly different (P = 0.88). This study convincingly established that prolonged i.c. administration of carboplatin by means of Alzet® pumps in combination with irradiation produced the best survival data ever obtained with for the F98 glioma model [18], independently of the radiation energy used [31].
In vitro studies to define the radiobiologic basis for enhancement of the combination of carboplatin and megavoltage X-irradiation
The first study in the Barth laboratory [33] was initiated in 2008 to further extend the findings of Elleaume and her research team. Initially, in vitro clonogenic assays were carried out with F98 cells to define the relationship between the carboplatin concentrations alone or in combination with a 7 Gy dose of X-rays using a LINAC source. As shown in Fig. 6a, the D10 value for cells treated with carboplatin alone was 2.5 µg/mL and pretreatment with 2.5 µg of carboplatin followed by XRT in a single 7 Gy fraction reduced the Surviving Fraction (SF) to 1.94 × 10−2. Following pretreatment with 5 µg/mL of carboplatin and then X-irradiation, the SF was reduced to < 6.0 × 10–4 (Fig. 6a). The effects of varying doses of X-rays either alone or following pretreatment with either 1.0 or 2.5 µg/mL of carboplatin resulted in a dose-dependent reduction in the SF, as shown in Fig. 6b, with a maximum effect at the highest X-ray dose tested, 17.5 Gy (SF = 1.1 × 10−3 and 2.6 × 10−4, respectively). The sensitization enhancement ratios [40] were markedly increased when the cells were pretreated with 2.5 µg/mL of carboplatin followed by X-irradiation compared to those seen following pretreatment of 1 µg/mL of carboplatin (Fig. 6b). Analysis of the clonogenic survival data using a combination index (CI) plot, based on the Chou-Talay equation [41] (Fig. 7b) and computerized quantification [40], clearly indicated synergism (Fig. 7a, c) and a dose reduction index (DRI) of 2.10 to 6.79-fold for X-irradiation and 4.31 to 180.40 fold for carboplatin, which demonstrated a favorable DRI > 1 for the combination (Fig. 7c). In vivo, this could reduce normal brain toxicity and permit a slight escalation of the X-ray dose. Furthermore, the toxicities of carboplatin and X-irradiation would not overlap due to different molecular mechanisms by which they kill cells.
Clonogenic survival of F98 glioma cells after treatment with carboplatin alone or in combination with X-irradiation. a Surviving fractions were determined for the F98 glioma cells either treated with carboplatin alone (open circle) or followed by X-irradiation (7 Gy) (filled diamond). b Surviving fractions of F98 cells wither untreated (open circle) or pretreated with carboplatin at concentrations of 1 µg/mL (filled diamond) or 2.5 µg/mL (filled up-pointing triangle), followed by varying doses of X-rays (1–17.5 Gy). Reprinted from [33]
Analysis of clonogenic survival data shown in Fig. 6. a Combination index (CI) plot based on the Chou-Talalay equation and the computerized quantitation. The CI was plotted as a function of fractional effect levels (fa) (e.g., for 50% inhibition, fa = 0.5) where CI < 1, = 1, and > 1 indicate synergism, additive effect, and antagonism, respectively. b Normalized isobologram for the non-constant ratio combinations. A combination falling in the lower left or upper right quadrants of the diagonal, indicates synergism or antagonism, respectively. c Fa-DRI plot where the dose reduction-index (DRI) was plotted as a function of the fractional effect levels (fa). DRI indicates how many folds dose-reduction would be allowed at a given effect for the synergistic combination. Reprinted from [33]
In vivo studies to further refine this binary therapeutic modality
Based on the in vitro studies, a biodistribution study was initiated in rats bearing i.c. implants of the F98 glioma [33]. Immediately following a 30 min infusion by means of CED of 20 µg of carboplatin in 10 µL to the site of the tumor, the rats were euthanized and brain, tumor and various organs were removed for Pt determinations by means of Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES). The tumor drug concentration was 10.4 µg/g, with virtually undetectable amounts in the blood, kidneys and liver and very low concentrations (0.8 to 1.2 µg/g) in the left and right cerebral hemispheres. Neuropathologic examination of the brains of rats that had received 20 µg of carboplatin by CED over 30 min or 84 µg by Alzet® pumps over 7 days showed only mild reactive inflammation and gliosis in the regions immediately surrounding the needle track and these doses subsequently were used in the therapy studies described below.
Two in vivo studies were carried out to assess therapeutic efficacy. In the first, rats were treated 13 days following stereotactic implantation of 1000 F98 glioma cells into the caudate nucleus. The total radiation dose was 15 Gy, delivered in three 5 Gy fractions of 6 MV LINAC X-rays on days 14, 15, and 16. Animals that received carboplatin (20 µg in 10 µL on day 13) by CED had a MST of 55.2 ± 7.8 days (MeST of 46 days), which was significantly longer (P < 0.001) than that of rats that received radiotherapy alone with a MST of 31.8 ± 1.2 days and a MeST of 32 days (Fig. 8a and Table 1). Rats that received carboplatin (20 µg in 10 µL on day 13) followed 24 h later by the first of three 5 Gy fractions of X-rays to the right, tumor bearing hemisphere, had a censored MST of 83.4 ± 13.1 days and MeST of 58 days, with 22% surviving longer than 180 days. F98 glioma bearing rats that received 84 µg of carboplatin over 7 days (from day 7 to day 13) by means of Alzet® pumps followed 24 h later by three 5 Gy fractions of X-rays had the longest survival (censored MST of 111.8 ± 31.5 days and MeST of 78 days), with 40% long-term survivors (> 180 days), as shown graphically in Kaplan–Meier survival plots (Fig. 8b and Table 1). The surviving rats subsequently were euthanized and their brains were subjected to histopathologic examination. No residual tumor cells were identified and there was a mild infiltrate of lymphocytes with scattered clumps of dystrophic calcific debris but no evidence of radiation-related changes. If treatment was carried out at 17 days following tumor implantation, at which time the tumor volumes were 60–80 mm3, as determined by magnetic resonance imaging (MRI), the MST was only 44.9 ± 3.5 days (MeST of 43 days), indicating that rats bearing larger tumors were more resistant to treatment compared to those bearing smaller ones (Fig. 8b) [33]. In contrast to these results, F98 glioma bearing rats that received either oral or i.c. CED of temozolomide in combination with radiotherapy had MSTs of 23.2 ± 1.9 days and 29.3 ± 5.0 days, respectively, which were not significantly different (P > 0.6) from the irradiated group (21.2 ± 0.8 days) [33].
A. Kaplan–Meier survival plots of F98 glioma-bearing rats after administration of carboplatin by CED or by Alzet® pumps alone or in combination with X-irradiation. Survival times in days after implantation have been plotted for untreated animals (filled circle, N = 5, MeST = 23 days), X-irradiation only (15 Gy, open circle, N = 5, MeST = 32 days), carboplatin administered by Alzet® pumps (84 µg/g over 7 days) (filled square, N = 6, MeST = 59 days), carboplatin administered by Alzet® pumps + X-irradiation (open square, N = 5, MeST = 78 days). B. Kaplan–Meier survival plots of rats bearing F98 gliomas: small tumors (~ 20–25 mm3) or large tumors (~ 60–80 mm3). Survival times in days after implantation have been plotted for untreated controls (filled circle, N = 5, MeST = 23 days), irradiated rats (open circle, N = 5, MeST = 32 days), CED of carboplatin in rats with small tumors (filled down-pointing triangle, N = 18, MeST = 46 days), CED of carboplatin + X-irradiation in rats with small tumors (20 µg over 30 min) + X-irradiation (open triangle N = 18, MeST = 58 days), carboplatin administered by Alzet® pumps (84 mg/g over 7 days) (filled square N = 6, MeST = 59 days), CED of carboplatin + X-irradiation in rats with large tumors (open square N = 10, MeST = 43 days). Reprinted from [33]
Studies to further optimize this binary therapeutic modality
The next series of experiments in the Barth laboratory focused on attempts to further improve the therapeutic efficacy by attempting to optimize the dosing and radiation paradigms for the combination of carboplatin and radiotherapy [30]. Initially a biodistribution study was carried out in a group of 8 rats to determine the variability in tumor and normal brain carboplatin concentrations following CED of 20 µg of carboplatin in 10 µL to either untreated rats or those that received a combination of i.v. dexamethasone (D) followed by mannitol (M) and furosemide (F). The purpose of DMF treatment was to reduce the interstitial pressure within the tumor and thereby enhance the micro-distribution and uptake of carboplatin [42]. The tumor Pt concentrations in both groups showed significant variability from animal to animal and ranged from 3.02 to 17.10 µg/g tumor in the untreated rats and 8.55 to 18.51 µg/g tumor in the DMF-treated animals. In contrast, the normal brain concentrations in both the untreated and DMF-treated animals were in a very narrow range of 0.95 to 3.10 µg/g in the tumor bearing cerebral hemisphere. This variability is probably the most important factor in determining therapeutic efficacy and is the most likely explanation for the poor clinical results that have been seen clinically using CED to treat patients with high grade glioma [11].
As shown in the Kaplan Meier survival plots shown in Fig. 9, the best survival data (MST of 107.7 ± 21 days and MeST of 62 days, 37.5% LTS) were obtained from rats that received 84 µg of carboplatin, delivered by Alzet® pumps from days 7 to 13, in combination with 20 Gy of X-irradiation delivered in four 5 Gy fractions [30]. In contrast, the MSTs of untreated controls and irradiated controls were 24.6 ± 1.1 days and 35.3 ± 1.8 days, respectively. However, the iso-effective radiation dose to late responding tissues was 35 Gy compared to only 20.25 Gy for rats that received 15 Gy in three 5 Gy fractions. These were calculated using the linear quadratic model with an α/β ratio of 10 for acute (tumor) effects and 2 for late (normal brain) effects in conventional 2 Gy fractions. In a third experiment, a dose of 15 Gy (7.5 Gy × 2) in combination with carboplatin (10 µg/day × 2 days) was carried out to determine if the survival data could be improved if the carboplatin dose was divided so that a higher drug concentrations would be available at the time that radiotherapy was initiated. The MST of these rats was 82.1 ± 15.5 days and the MeST was 54.5 days with a 25% cure rate (i.e., survival > 180 days) [30]. As previously indicated, the purpose of these studies was to determine if modification of the XRT regimen and the dosing paradigm of carboplatin would result in improved survival compared to our earlier results [33]. The clear answer to this question was that sustained delivery of 84 µg carboplatin over 7 days by means of Alzet® pumps in combination with either three or four 5 Gy fractions at 24 h intervals resulted in the best survival MeSTs of 78 days and 62 days, respectively (Table 1 and Fig. 9) [30, 33]. Furthermore, there were no treatment-related deaths and less severe neuropathologic changes with the former regimen indicating its superiority. In contrast, rats that received an X-ray dose of 22.5 Gy in three 7.5 Gy fractions without the administration of carboplatin had a MST of 53.2 ± 4.6 days with significant radiation-related morbidity.
Kaplan–Meier survival plots of F98 glioma-bearing rats after administration of carboplatin by CED or Alzet pump in combination with X-irradiation. Survival times in days after implantation have been plotted for untreated animals (filled circle, N = 5, MeST = 25 days), X-irradiation only (5 Gy × 4) (open circle, N = 5, MeST = 35.5 days), CED of carboplatin (20 µg) + X-irradiation 6 h later (filled down-pointing triangle, N = 8, MeST = 48 days), CED of carboplatin at day 7 + X-irradiation (open up-pointing triangle, N = 8, MeST = 49 days), CED of carboplatin (20 µg) + X-irradiation 24 h later (filled square, N = 8, MeST = 55.5 days), carboplatin (84 µg) administered by Alzet® pump (between day 7 and 13) + X-irradiation delivered on day 14 (open square, N = 8, MeST = 62 days), and CED of carboplatin (10 µg × 2) on days 13 and 15 + X-irradiation (7.5 Gy × 2) on days 14 and 16 (filled diamond, N = 12, MeST = 54.5 days). Reprinted from [30]
Two possible ways that this combination therapy could be improved would be to increase the tumor uptake and micro-distribution of carboplatin. Although DMF treatment marginally increased the tumor drug concentrations, it is unlikely that this would have resulted in a significant increase in MST compared to animals that did not receive DMF treatment. The major problem, which has limited the strong synergy of XRT in combination with i.c administration of carboplatin, was the great variability in tumor drug concentrations following CED [30]. This led us to hypothesize that those animals that were cured of their tumors had higher tumor drug concentrations and better cellular micro-distribution of the carboplatin.
Another series of experiments were carried out in the Barth laboratory to determine if a liposomal formulation of CDDP could improve its tumor uptake [43]. Two formulations were used, a commercially available one, Lipoplatin™ (Regulon, Inc., Mountain View, CA, USA) [44] and another one, CHEMS liposomal formulation synthetized by Robert Lee and his co-workers at The Ohio State University. Unfortunately, Lipoplatin™ was highly neurotoxic when administered i.c. by CED to non-tumor bearing Fischer rats. CHEMS liposomes on the other hand, initially were well tolerated but a variety of dose dependent neuropathologic changes from none to severe were seen at either 10 or 14 days following their administration [43]. Based on these results, no further studies were carried out on CDDP containing liposomes.
The major problem associated with any therapeutic study carried out using rodent brain tumor models is their relevancy to patients with brain tumors. However, it can be stated with a reasonable degree of confidence, if it doesn’t “work” in rodents, it probably will not work in humans. The rat brain weighs approximately 1.2 g and a human brain weighs 1.3 to 1.4 kg, a thousand-fold difference. Although CED has been effective in improving the distribution of therapeutic agents in rats with brain tumors, its effectiveness in humans is much more problematic, as summarized by Jahangiri et al. [11] and in Shi and Sanche’s recent review [12].
Specific molecular targeting of the epidermal growth factor receptor using platinum containing bioconjugates
The last series of experiments in the Barth laboratory focused on specific molecular targeting of the Pt-containing drug by linking CDDP either to a specific human EGFR-targeting monoclonal antibody, C225 (Cetuximab) or to an EGF peptide [45]. The conjugation scheme for the former has been described in detail elsewhere [46]. Briefly summarized, CDDP molecules were linked to a fifth generation polyamidoamine (PAMAM) dendrimer containing 128 reactive terminal amino groups. This in turn was linked to C225 by means of two heterobifunctional reagents, as previously described [46]. In order to have a lower molecular weight targeting moiety, two EGF peptides were selected, a 13 mer B-cell epitope, designated PEP382, and the other, a 16 mer HER-1 epitope designated PEP455. The PEP-Pt conjugates were prepared by adding CDDP to the peptides and allowing them to react for 48 h, following which the reaction mixture was purified by passage through PD MiniTrap G-10 columns. The chemical reaction itself involved the replacement of one of the chlorides of CDDP with electron donor groups of the peptide [46]. Initially, the cellular uptake and cytocidal activity of the Pt bioconjugates were evaluated in vitro. The C225-Pt bioconjugates were devoid of in vitro cytotoxic activity compared to CDDP against F98 glioma cells that had been transfected with the human gene encoding EGFR (F98EGFR) [47]. C225-Pt was less cytotoxic in vitro than free CDDP up to a concentration of 1000 µM, suggesting that there was reduced release of the drug, even at higher concentrations of the bioconjugate. In contrast, PEP455-Pt and PEP382-Pt showed equivalent toxicity against F98EGFR cells and reduced cell viability to ~ 20% compared to non-platinated PEP455, which was devoid of intrinsic cytotoxicity.
Based on the in vitro data, therapy studies were initiated in F98EGFR glioma bearing rats. The C225-Pt bioconjugates and PEP455-Pt bioconjugates were administered i.c. by means of CED to in F98EGFR glioma bearing rats. The C225-Pt bioconjugate at a Pt dose of 46.16 µg had no effect on the MST of tumor bearing rats compared to that of untreated controls [46]. This was attributed to lack of release of CDDP, which also had been observed in vitro. The therapeutic efficacy of PEP455-Pt bioconjugate was evaluated in a different way from that we previously had employed, which was based on the increase in MST treated versus untreated control animals [33]. In this study the tumor bearing rats were euthanized at 4 weeks following i.c. CED of PEP455-Pt and 6 weeks following tumor implantation. Untreated control rats had a MST of 26.3 ± 2.5 days and all of the treated rats were euthanized at 42 days following tumor implantation. Their brains were removed, fixed in formalin, and processed for histopathologic examination in order to determine tumor status. All of the untreated control animals had macroscopic tumor, while in contrast 57% of the brains of the treated rats were tumor free, 14% had microscopic tumors, and 29% had macroscopic tumors. This difference in tumor status of treated versus untreated animals was statistically significant at the level P ≤ 0.01. On the basis of in vitro and in vivo data it was concluded that the EGFR-targeting PEP-Pt bioconjugates were superior to those employing C225 as the targeting moiety. The problem of release of the therapeutic payload is one that has been a key factor in limiting the effectiveness of drug-containing bioconjugates, and for this reason only a small number of drug antibody bioconjugates currently are in clinical use compared to “naked” monoclonal antibodies that now are in widespread clinical use. Based on our studies we concluded that further studies are warranted to assess the therapeutic potential of PEP-Pt bioconjugates as part of the expanding role of antibody and peptide drug conjugates for the treatment of cancer [48, 49].
Other experimental studies carried out to further define the efficacy of this binary therapeutic modality
Before concluding this review of our own studies relating to CED of CDDP or carboplatin either alone or in combination with external beam photon radiation, we briefly would like to summarize the studies carried out by David Fortin and his research team at the University of Sherbrooke in Quebec, Canada using the same therapeutic approach and the F98 glioma model [50, 51]. They followed a path similar to our own in evaluating the therapeutic efficacy of a variety of platinum containing drugs, including CDDP, carboplatin, lipoplatin™, and oxaliplatin [52,53,54]. These were administered i.v. or intra-arterially (i.a.) with or without blood–brain barrier disruption (BBB-D) or i.c. by CED alone or combination with external beam photon irradiation using a Gamma Knife source. The longest MeST was 54 days in animals that received 25 µg of carboplatin by CED delivered at a rate of 0.5 μL/min for 20 min in combination with a single 15 Gy dose of radiation. However, there were no long-term survivors [54].
In comparison, Barth and his research team obtained a MST of 83.4 ± 13.1 and a MeST of 58 days with 22% long term survivors using 20 µg of carboplatin in 10 μL, administered by CED to the site of the tumor in combination with XRT of 15 Gy in three 5 Gy fractions (Table 1) [33]. Similarly, Elleaume and her co-workers reported a MeST of 79 days and 60 days and 16 and 8% long term survivors, respectively, for the combination of carboplatin (20 µg/20 µL) and irradiation (Table 1) [29]. This was obtained with 24 Gy of either 6 MV or 78.8 keV X-rays, delivered in three fractions of 8 Gy each. The best survival data obtained with carboplatin were those reported by Elleaume et al. [31, 34] and Barth et al. [33], when carboplatin was administered by means of Alzet® pumps over 7 days and irradiation was given in a single fraction using either a LINAC or SSRT. The MeSTs of rats receiving the combined treatments were > 180 days with 55% LTS, irrespective of the radiation source. However, with prolonged infusions of carboplatin using Alzet® pumps, the treatment was started earlier, when the tumors were smaller, and therefore more responsive. In contrast to the results obtained with carboplatin in combination with XRT [29, 33], the results obtained by Fortin et al. following i.v. administration or i.a. administration of oxaliplatin or Lipoxal™ (a liposomal formulation of oxaliplatin) with or without BBB-D and XRT, the survival data were not as good as those obtained with liposomal carboplatin at a dose of 50 µg (MeST of 49.5 days) [55].
Conclusions
The survival data that we have obtained using i.c. CED of CDDP or carboplatin in combination with external beam photon irradiation are, to the best of our knowledge, the best that ever have been obtained with the F98 glioma model. However, the fact that we have not succeeded in obtaining a 100% cure rate using this rat brain tumor model, which resembles human high grade gliomas in a number of important ways, indicates that the human cerebral hemisphere, which weighs approximately 650 g, versus 0.60 g in the rat remains a challenge that CED has yet to meet. As stated earlier in this review, “If it doesn’t work in a rat brain”, it is highly unlikely that it will “work” in a human brain. The encouraging experimental results that we have obtained using CED of carboplatin in combination with external beam XRT is a start. The challenge is to translate our success, partial though it may be, suggests that CED of therapeutic agents for the treatment of brain tumors still has a long way to go before it can be successfully applied to patients with high grade gliomas. The singular advantage of CED is that it circumvents the BBB and results in tumor drug concentrations that could be orders of magnitude greater than would be achievable following intravenous injection (i.v.). This was clearly demonstrated in our own study using the F98 glioma where the tumor carboplatin concentration after administration of 20 µg in 10 µL of saline resulted in a tumor drug concentration of 10.4 µg/g tumor. In contrast, to obtain an equivalent concentration following i.v. injection required 25 mg/kg body weight. However, one of the most significant problems associated with the use of CED to improve drug delivery to brain tumors, both clinically and in experimental animal studies, has been the significant variability in the uptake and microdistribution of the therapeutic agent within the tumor itself. This was clearly demonstrated in one of our own studies [30] in which carboplatin uptake following CED of 20 µg in 10 µl was quantified in a group of eight F98 glioma-bearing rats. The carboplatin concentration, as determined by inductively coupled plasma-optical emission spectroscopy, ranged from 3.02 to 17.10 µg/g tumor with a mean concentration of 8.50 µg/g. In contrast, the normal brain concentrations were in a very narrow range (0.95–3.10 µg/g). If this high variability in tumor uptake was seen in a rat brain cerebral hemisphere weighing approximately 0.60 g, it could be orders of magnitude greater in a human high grade glioma in a cerebral hemisphere weighing approximately 650 g.
The failure of a number of clinical trials such as the phase III trial PRECISE, which compared an interleukin-13-Pseudomonas exotoxin conjugate to BCNU-containing Gliadel wafers, did not result in any significant increase in MeST of the patients with recurrent gliomas [56], as did several other trials [57, 58]. A Phase 1 clinical trial to evaluate the toxicity of i.c. CED of Carboplatin in patients with recurrent high grade gliomas was carried out by Bradley Elder and his colleagues in the Department of Neurosurgery at The Ohio State University [59]. Unfortunately, the U.S. Food and Drug Administration mandated initial doses of 1 µg and then 2 µg and 4 µg of carboplatin in a volume of 54 mL for administration by CED. This starting dose was one-tenth of the dose that which we have administered to a rat cerebral hemisphere weighing 600 mg. Clearly there was no chance whatsoever of demonstrating anything but the potential toxicity of the procedure itself. Sadly, this is but one example of the problems associated with clinical trials to evaluate the potential efficacy of any therapeutic agent administered by CED.
On the other hand, Souweidane et al. [60] have obtained encouraging clinical results treating children with diffuse intrinsic pontine gliomas (DIPG). The 124I radio-labelled monoclonal antibody 849, which recognizes the B7-H3 surface antigen that is overexpressed in high grade gliomas including DIPG, was administered by CED to the pons, weighing 16–20 g. The challenge of using CED to treat patients with recurrent, diffusely infiltrative, high grade gliomas in a 650 g cerebral hemisphere is orders of magnitude greater. The development of more effective catheters by Steven Gill and his research team is a step in the right direction [61]. Further efforts to improve the distribution of therapeutic agents in the adult brain, as recently has been reported by Gill et al. [62, 63], are promising; but it remains to be determined if CED can ever be used effectively to treat patients with recurrent, infiltrative high grade gliomas. Only time will tell.
Abbreviations
- AZQ:
-
Aziridinyl-benzoquinone
- BBB:
-
Blood–brain barrier
- BBB-D:
-
Blood–brain barrier disruption
- BCNU:
-
1,3-Bis (2-chloroethyl)-1-nitrosourea
- C225:
-
Cetuximab
- CED:
-
Convection-enhanced delivery
- CDDP:
-
Cisplatin
- CI:
-
Combination index
- D:
-
Dexamethasone
- DMF:
-
Dexamethasone, mannitol, furosemide
- DRI:
-
Dose reduction index
- DSBs:
-
DNA double strand breaks
- ESRF:
-
European synchrotron radiation facility
- F98EGFR :
-
F98 glioma cells transfected with the human gene encoding EGFR
- F:
-
Furosemide
- i.a.:
-
Intra-arterially
- i.c.:
-
Intra-cerebrally
- i.t.:
-
Intra-tumoral
- i.v.:
-
Intra-venous
- ICP-OES:
-
Inductively coupled plasma-optical emission spectroscopy
- %ILS:
-
Percent increase in life span
- LET:
-
Linear energy transfer
- LINAC:
-
Linear accelerator
- LTS:
-
Long term survivors
- M:
-
Mannitol
- MeST:
-
Median survival time
- MRI:
-
Magnetic resonance imaging
- PAMAM:
-
Polyamidoamine
- PEP382:
-
13 Mer B-cell epitope
- PEP455:
-
16 Mer HER-1 epitope
- SF:
-
Surviving fraction
- SSRT:
-
Stereotactic synchrotron radiotherapy
- Vd :
-
Volume of distribution
- XRT:
-
Conventional radiotherapy
References
Lapointe S, Perry A, Butowski NA (2018) Primary brain tumours in adults. Lancet 392(10145):432–446. https://doi.org/10.1016/S0140-6736(18)30990-5
Arvanitis CD, Ferraro GB, Jain RK (2020) The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer 20(1):26–41. https://doi.org/10.1038/s41568-019-0205-x
Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH (1994) Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 91(6):2076–2080. https://doi.org/10.1073/pnas.91.6.2076
Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH (1995) Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg 82(6):1021–1029. https://doi.org/10.3171/jns.1995.82.6.1021
Laske DW, Youle RJ, Oldfield EH (1997) Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med 3(12):1362–1368. https://doi.org/10.1038/nm1297-1362
Laske DW, Morrison PF, Lieberman DM, Corthesy ME, Reynolds JC, Stewart-Henney PA, Koong SS, Cummins A, Paik CH, Oldfield EH (1997) Chronic interstitial infusion of protein to primate brain: determination of drug distribution and clearance with single-photon emission computerized tomography imaging. J Neurosurg 87(4):586–594. https://doi.org/10.3171/jns.1997.87.4.0586
Lonser RR, Corthesy ME, Morrison PF, Gogate N, Oldfield EH (1999) Convection-enhanced selective excitotoxic ablation of the neurons of the globus pallidus internus for treatment of parkinsonism in nonhuman primates. J Neurosurg 91(2):294–302. https://doi.org/10.3171/jns.1999.91.2.0294
Lonser RR, Walbridge S, Garmestani K, Butman JA, Walters HA, Vortmeyer AO, Morrison PF, Brechbiel MW, Oldfield EH (2002) Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. J Neurosurg 97(4):905–913. https://doi.org/10.3171/jns.2002.97.4.0905
Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL (1994) High-flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol 266(1 Pt 2):R292–305. https://doi.org/10.1152/ajpregu.1994.266.1.R292
Lonser RR, Sarntinoranont M, Morrison PF, Oldfield EH (2015) Convection-enhanced delivery to the central nervous system. J Neurosurg 122(3):697–706. https://doi.org/10.3171/2014.10.JNS14229
Jahangiri A, Chin AT, Flanigan PM, Chen R, Bankiewicz K, Aghi MK (2017) Convection-enhanced delivery in glioblastoma: a review of preclinical and clinical studies. J Neurosurg 126(1):191–200. https://doi.org/10.3171/2016.1.JNS151591
Shi M, Sanche L (2019) Convection-enhanced delivery in malignant gliomas: a review of toxicity and efficacy. J Oncol 2019:9342796. https://doi.org/10.1155/2019/9342796
Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7(8):573–584
Kroin JS, Penn RD (1982) Intracerebral chemotherapy: chronic microinfusion of cisplatin. Neurosurgery 10(3):349–354
Penn RD, Kroin JS, Harris JE, Chiu KM, Braun DP (1983) Chronic intratumoral chemotherapy of a rat tumor with cisplatin and fluorouracil. Appl Neurophysiol 46(1–4):240–244
Kimler BF, Liu C, Evans RG, Morantz RA (1992) Intracerebral chemotherapy in the 9L rat brain tumor model. J Neurooncol 14(3):191–200
Degen JW, Walbridge S, Vortmeyer AO, Oldfield EH, Lonser RR (2003) Safety and efficacy of convection-enhanced delivery of gemcitabine or carboplatin in a malignant glioma model in rats. J Neurosurg 99(5):893–898
Barth RF, Kaur B (2009) Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol 94(3):299–312. https://doi.org/10.1007/s11060-009-9875-7
Biston MC, Joubert A, Adam JF, Elleaume H, Bohic S, Charvet AM, Esteve F, Foray N, Balosso J (2004) Cure of fisher rats bearing radioresistant F98 glioma treated with cis-platinum and irradiated with monochromatic synchrotron X-rays. Cancer Res 64(7):2317–2323
Barth RF, Yang W, Al-Madhoun AS, Johnsamuel J, Byun Y, Chandra S, Smith DR, Tjarks W, Eriksson S (2004) Boron-containing nucleosides as potential delivery agents for neutron capture therapy of brain tumors. Cancer Res 64(17):6287–6295. https://doi.org/10.1158/0008-5472.CAN-04-0437
Yang W, Barth RF, Wu G, Kawabata S, Sferra TJ, Bandyopadhyaya AK, Tjarks W, Ferketich AK, Moeschberger ML, Binns PJ, Riley KJ, Coderre JA, Ciesielski MJ, Fenstermaker RA, Wikstrand CJ (2006) Molecular targeting and treatment of EGFRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin Cancer Res 12(12):3792–3802. https://doi.org/10.1158/1078-0432.CCR-06-0141
Yang W, Wu G, Barth RF, Swindall MR, Bandyopadhyaya AK, Tjarks W, Tordoff K, Moeschberger M, Sferra TJ, Binns PJ, Riley KJ, Ciesielski MJ, Fenstermaker RA, Wikstrand CJ (2008) Molecular targeting and treatment of composite EGFR and EGFRvIII-positive gliomas using boronated monoclonal antibodies. Clin Cancer Res 14(3):883–891. https://doi.org/10.1158/1078-0432.CCR-07-1968
Kawabata S, Yang W, Barth RF, Wu G, Huo T, Binns PJ, Riley KJ, Ongayi O, Gottumukkala V, Vicente MG (2011) Convection enhanced delivery of carboranylporphyrins for neutron capture therapy of brain tumors. J Neurooncol 103(2):175–185. https://doi.org/10.1007/s11060-010-0376-5
Barth RF, Yang WL, Wu G, Swindall M, Byun YJ, Narayanasamy S, Tjarks W, Tordoff K, Moeschberger ML, Eriksson S, Binne PJ, Riley KJ (2008) Thymidine kinase 1 as a molecular target for boron neutron capture therapy of brain tumors. P Natl Acad Sci USA 105(45):17493–17497
Karnas SJ, Yu E, McGarry RC, Battista JJ (1999) Optimal photon energies for IUdR K-edge radiosensitization with filtered x-ray and radioisotope sources. Phys Med Biol 44(10):2537–2549. https://doi.org/10.1088/0031-9155/44/10/312
Corde S, Balosso J, Elleaume H, Renier M, Joubert A, Biston MC, Adam JF, Charvet AM, Brochard T, Le Bas JF, Esteve F, Foray N (2003) Synchrotron photoactivation of cisplatin elicits an extra number of DNA breaks that stimulate RAD51-mediated repair pathways. Cancer Res 63(12):3221–3227
Robar JL, Riccio SA, Martin MA (2002) Tumour dose enhancement using modified megavoltage photon beams and contrast media. Phys Med Biol 47(14):2433–2449. https://doi.org/10.1088/0031-9155/47/14/305
Rousseau J, Barth RF, Fernandez M, Adam JF, Balosso J, Esteve F, Elleaume H (2010) Efficacy of intracerebral delivery of cisplatin in combination with photon irradiation for treatment of brain tumors. J Neurooncol 98(3):287–295. https://doi.org/10.1007/s11060-009-0074-3
Rousseau J, Boudou C, Barth RF, Balosso J, Esteve F, Elleaume H (2007) Enhanced survival and cure of F98 glioma-bearing rats following intracerebral delivery of carboplatin in combination with photon irradiation. Clin Cancer Res 13(17):5195–5201
Yang W, Barth RF, Huo T, Nakkula RJ, Weldon M, Gupta N, Agius L, Grecula JC (2014) Radiation therapy combined with intracerebral administration of carboplatin for the treatment of brain tumors. Radiat Oncol 9:25. https://doi.org/10.1186/1748-717X-9-25
Bobyk L, Edouard M, Deman P, Rousseau J, Adam JF, Ravanat JL, Esteve F, Balosso J, Barth RF, Elleaume H (2012) Intracerebral delivery of carboplatin in combination with either 6 MV photons or monoenergetic synchrotron X-rays are equally efficacious for treatment of the F98 rat glioma. J Exp Clin Cancer Res 31:78. https://doi.org/10.1186/1756-9966-31-78
Barth RF, Yang W, Huo T, Riley KJ, Binns PJ, Grecula JC, Gupta N, Rousseau J, Elleaume H (2011) Comparison of intracerebral delivery of carboplatin and photon irradiation with an optimized regimen for boron neutron capture therapy of the F98 rat glioma. Appl Radiat Isot 69(12):1813–1816. https://doi.org/10.1016/j.apradiso.2011.03.019
Yang W, Huo T, Barth RF, Gupta N, Weldon M, Grecula JC, Ross BD, Hoff BA, Chou TC, Rousseau J, Elleaume H (2011) Convection enhanced delivery of carboplatin in combination with radiotherapy for the treatment of brain tumors. J Neurooncol 101(3):379–390. https://doi.org/10.1007/s11060-010-0272-z
Rousseau J, Barth RF, Moeschberger ML, Elleaume H (2009) Efficacy of intracerebral delivery of carboplatin in combination with photon irradiation for treatment of F98 glioma-bearing rats. Int J Radiat Oncol Biol Phys 73(2):530–536. https://doi.org/10.1016/j.ijrobp.2008.09.018
Knox RJ, Friedlos F, Lydall DA, Roberts JJ (1986) Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res 46(4 Pt 2):1972–1979
Hongo A, Seki S, Akiyama K, Kudo T (1994) A comparison of in vitro platinum-DNA adduct formation between carboplatin and cisplatin. Int J Biochem 26(8):1009–1016. https://doi.org/10.1016/0020-711x(94)90072-8
Wu Q, Guarnieri M, Tyler B, Clatterbuck RE, Liu Y, Carson BS (2004) Section on tumors: Young investigator award: local release of carboplatin via an Alzet mini-osmotic pump prolongs survival in a rat brainstem tumor model. Clin Neurosurg 51:332–339
Guarnieri M, Carson BS (2004) Chronic local therapy for brainstem tumors. Neurosurgery 54(4):1025–1026. https://doi.org/10.1227/01.neu.0000117119.32806.af
Carson BS Sr, Wu Q, Tyler B, Sukay L, Raychaudhuri R, DiMeco F, Clatterbuck RE, Olivi A, Guarnieri M (2002) New approach to tumor therapy for inoperable areas of the brain: chronic intraparenchymal drug delivery. J Neurooncol 60(2):151–158. https://doi.org/10.1023/a:1020626419269
Chou TC, Martin N (2005) Software and user's guide: a computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50, ED50 and LD50 values. ComboSyn, Paramus
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70(2):440–446. https://doi.org/10.1158/0008-5472.can-09-1947
Boucher Y, Salehi H, Witwer B, Harsh GR, Jain RK (1997) Interstitial fluid pressure in intracranial tumours in patients and in rodents. Br J Cancer 75(6):829–836
Huo T, Barth RF, Yang W, Nakkula RJ, Koynova R, Tenchov B, Chaudhury AR, Agius L, Boulikas T, Elleaume H, Lee RJ (2012) Preparation, biodistribution and neurotoxicity of liposomal cisplatin following convection enhanced delivery in normal and F98 glioma bearing rats. PLoS ONE 7(11):e48752. https://doi.org/10.1371/journal.pone.0048752
Boulikas T (2004) Low toxicity and anticancer activity of a novel liposomal cisplatin (Lipoplatin) in mouse xenografts. Oncol Rep 12(1):3–12
Barth RF, Wu G, Meisen WH, Nakkula RJ, Yang W, Huo T, Kellough DA, Kaumaya P, Turro C, Agius LM, Kaur B (2016) Design, synthesis, and evaluation of cisplatin-containing EGFR targeting bioconjugates as potential therapeutic agents for brain tumors. OncoTargets Ther 9:2769–2781. https://doi.org/10.2147/ott.s99242
Wu G, Barth RF, Yang W, Chatterjee M, Tjarks W, Ciesielski MJ, Fenstermaker RA (2004) Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy. Bioconjugate Chem 15(1):185–194. https://doi.org/10.1021/bc0341674
Yang W, Barth RF, Wu G, Ciesielski MJ, Fenstermaker RA, Moffat BA, Ross BD, Wikstrand CJ (2005) Development of a syngeneic rat brain tumor model expressing EGFRvIII and its use for molecular targeting studies with monoclonal antibody L8A4. Clin Cancer Res 11(1):341–350
Pallares R, Albergel R (2020) Nanoparticles for targeted cancer therapy. Nano Research. https://doi.org/10.1007/s12274-020-2957-8
Hoppenz P, Els-Heindl S, Beck-Sickinger A (2020) Peptide-drug conjugates and their targets in advanced cancer. Front Chem. https://doi.org/10.3389/fchem.2020.00571
Drapeau A, Fortin D (2015) Chemotherapy delivery strategies to the central nervous system: neither optional nor superfluous. Curr Cancer Drug Targets 15(9):752–768
Fortin D (2019) Drug delivery technology to the CNS in the treatment of brain tumors: the sherbrooke experience. Pharmaceutics. https://doi.org/10.3390/pharmaceutics11050248
Charest G, Sanche L, Fortin D, Mathieu D, Paquette B (2013) Optimization of the route of platinum drugs administration to optimize the concomitant treatment with radiotherapy for glioblastoma implanted in the Fischer rat brain. J Neurooncol 115(3):365–373. https://doi.org/10.1007/s11060-013-1238-8
Shi M, Fortin D, Paquette B, Sanche L (2016) Convection-enhancement delivery of liposomal formulation of oxaliplatin shows less toxicity than oxaliplatin yet maintains a similar median survival time in F98 glioma-bearing rat model. Invest New Drugs 34(3):269–276. https://doi.org/10.1007/s10637-016-0340-0
Shi M, Fortin D, Sanche L, Paquette B (2015) Convection-enhancement delivery of platinum-based drugs and Lipoplatin(TM) to optimize the concomitant effect with radiotherapy in F98 glioma rat model. Invest New Drugs 33(3):555–563. https://doi.org/10.1007/s10637-015-0228-4
Charest G, Sanche L, Fortin D, Mathieu D, Paquette B (2012) Glioblastoma treatment: bypassing the toxicity of platinum compounds by using liposomal formulation and increasing treatment efficiency with concomitant radiotherapy. Int J Radiat Oncol Biol Phys 84(1):244–249. https://doi.org/10.1016/j.ijrobp.2011.10.054
Vogelbaum MA, Sampson JH, Kunwar S, Chang SM, Shaffrey M, Asher AL, Lang FF, Croteau D, Parker K, Grahn AY, Sherman JW, Husain SR, Puri RK (2007) Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery 61(5):1031–1037. https://doi.org/10.1227/01.neu.0000303199.77370.9e(discussion 1037–1038)
Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, Balasubramaniam A, Nair S, Oliushine V, Parfenov V, Poverennova I, Zaaroor M, Jachimczak P, Ludwig S, Schmaus S, Heinrichs H, Schlingensiepen KH (2011) Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol 13(1):132–142. https://doi.org/10.1093/neuonc/noq142
Desjardins A, Gromeier M, Herndon JE 2nd, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, Bigner DD (2018) Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med 379(2):150–161. https://doi.org/10.1056/NEJMoa1716435
Wang J, Barth RF, Cavaliere R, Puduvalli V, Giglio P, Lonser RR, Elder JB (2020) Phase 1 trial of intracerebral convection-enhanced delivery of carboplatin for treatment of recurrent high-grade gliomas. Plos One
Souweidane MM, Kramer K, Pandit-Taskar N, Zhou Z, Haque S, Zanzonico P, Carrasquillo JA, Lyashchenko SK, Thakur SB, Donzelli M, Turner RS, Lewis JS, Cheung NV, Larson SM, Dunkel IJ (2018) Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol 19(8):1040–1050. https://doi.org/10.1016/S1470-2045(18)30322-X
Lewis O, Woolley M, Johnson DE, Fletcher J, Fenech J, Pietrzyk MW, Baruab NU, Bienemann AS, Singleton W, Evans SL, Gill SS (2018) Maximising coverage of brain structures using controlled reflux, convection-enhanced delivery and the recessed step catheter. J Neurosci Meth 308:337–345
Arshad A, Yang B, Bienemann AS, Barua NU, Wyatt MJ, Woolley M, Johnson DE, Edler KJ, Gill SS (2015) Convection-enhanced delivery of carboplatin PLGA nanoparticles for the treatment of glioblastoma. PLoS ONE 10(7):e0132266
Singleton WGB, Bieneman AS, Woolley M, Johnson D, Lewis O, Wyatt MJ, Damment SJP, Boulter LJ, Killick-Cole CL, Asby DJ, Gill SS (2018) The distribution, clearance, and brainstem toxicity of panobinostat administered by convection-enhanced delivery. J Neurosurg-Pediatr 22(3):288–296
Acknowledgements
We thank Michael Weldon, Nilendu Gupta, and John C. Grecula for their help in carrying out radiation studies for the Barth Laboratory, W. Hans Meisen, Balveen Kaur, Pravin Kaumaya and Robert Lee for their assistance in carrying out studies relating to molecular targeting, Gong Wu for preparation of bioconjugates and Delisa Watkins and David Carpenter for their assistance in the preparation of this manuscript. We thank the ESRF for their technical support and for providing beam time and special thanks to Thierry Brochard, Christian Nemoz and Dominique Dallery. Finally, we thank Marie-Claude Biston, Jean-François Adam, Anne-Marie Charvet, Caroline Boudou and François Estève, for their help in carrying out radiation studies in Grenoble.
Funding
Support for studies, carried out by Barth and his co-workers, has been provided by the Musella Foundation, Voices against Brain Cancer, The Ohio State University Department of Pathology and the Kevin J. Mullin Memorial Fund for Brain Tumor Research. Those carried out by Elleaume and her research team were supported by the University Grenoble Alpes, INSERM, the Auvergne Rhône Alpes region and the Labex Primes (ANR-11-LABX-0063).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All animal studies performed by Hélène Elleaume’s team were performed in compliance with the European Directive 2010/63/EU. The protocols were submitted to the ESRF ethical committee reference number ETHAX N°113. Animal studies carried out by Rolf Barth and his research team were in accordance with the Guide for the Care and the Use of Laboratory Animals (National Academy press, Washington DC, 1996) and the protocols were approved by the Institutional Laboratory Care and Use Committee of The Ohio State University.
Research involving human and animal participants
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elleaume, H., Barth, R.F., Rousseau, J. et al. Radiation therapy combined with intracerebral convection-enhanced delivery of cisplatin or carboplatin for treatment of the F98 rat glioma. J Neurooncol 149, 193–208 (2020). https://doi.org/10.1007/s11060-020-03600-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03600-x