Abstract
We have evaluated the efficacy of intracerebral (i.c.) convection-enhanced delivery (CED) of cisplatin in combination with photon irradiation for the treatment of F98 glioma-bearing rats. One thousand glioma cells were stereotactically implanted into the brains of Fischer rats and 13 days later cisplatin (6 μg/20 μl) was administered i.c. by CED at a flow rate of 0.5 μl/min. On the following day the animals were irradiated with a single 15 Gy dose of X-rays, administered by a linear accelerator (LINAC) or 78.8 keV synchrotron X-rays at the European Synchrotron Radiation Facility (ESRF). Untreated controls had a mean survival time (MST) ± standard error of 24 ± 1 days compared to >59 ± 13 days for rats that received cisplatin alone with 13% of the latter surviving >200 days. Rats that received cisplatin in combination with either 6 MV (LINAC) or 78.8 keV (synchrotron) X-rays had almost identical MSTs of >75 ± 18 and >74 ± 19 days, respectively with 17 and 18% long-term survivors. Microscopic examination of the brains of long-term surviving rats revealed an absence of viable tumor cells and cystic areas at the presumptive site of the tumor. Our data demonstrate that i.c. CED of cisplatin in combination with external X-irradiation significantly enhanced the survival of F98 glioma-bearing rats. This was independent of the X-ray beam energy and probably was not due to the production of Auger electrons as we previously had postulated. Our data provide strong support for the approach of concomitantly administering platinum-based chemotherapy in combination with radiotherapy for the treatment of brain tumors. Since a conventional LINAC can be used as the radiation source, this should significantly broaden the clinical applicability of this approach compared to synchrotron radiotherapy, which could only be carried out at a very small number of specialized facilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) is the most common primary malignant brain tumor, and with rare exception is invariably fatal [1]. The current standard of care for patients with GBMs consists of surgery and radiotherapy in combination with temozolomide, followed by repetitive cycles of temozolomide [2]. Although the survival advantage of this combined treatment regimen was still evident at 5 years, the increase in overall median survival was only 2.5 months (14.6 vs. 12.1 months) [2]. These dismal survival data provide a strong impetus to develop and evaluate new therapeutic approaches.
We previously have reported on the therapeutic efficacy of stereotactic synchrotron radiotherapy (SSR) [3–10]. In SSR, the tumor is first loaded with a high-Z number, dose-enhancing element, such as platinum or iodine, followed by irradiation with low energy monoenergetic beams of X-rays tuned to the energy of K-shell electrons of the sensitizer, or slightly above this energy [11]. Loading tumor cells with high-Z atoms increases the effective atomic number of the tumor and selectively enhances the absorbed dose following irradiation. This is dependent on the use of X-rays with energies that produce a dominant photoelectric effect and for which the interaction cross section exhibits a significant Z-dependence [12]. In vitro studies and in vivo preclinical studies of SSR have been carried out by our group at the ESRF in Grenoble, using either iodine dose-enhancers [3–7] or platinum compounds such as cisplatin [8, 13, 14] and carboplatin [9, 10]. Based on the same principles, others have investigated the possible use of gold [15, 16] and gadolinium [17] as radiation enhancers. Following photoelectric interactions with K-shell electrons, photoelectrons are generated and inner shell electrons are expelled. The resulting K-shell vacancies are filled almost instantaneously by radiative (fluorescence) and non-radiative transitions (Auger electrons) from outer shells. The latter have short path lengths in tissue, and produce high local energy deposition within a range of a few nanometers and can produce DNA double strand breaks, providing that the high-Z atoms are located in close proximity to DNA [11].
Platinated drugs, such as cisplatin and carboplatin, represent an important class of anti-tumor agents that are widely used to treat various malignancies [18]. Since DNA binding is the main biological event that triggers their tumoricidal effects [19], we hypothesized that they would be good candidates for the dose enhancement produced by Auger electrons. Despite producing significant toxicity, the oral and intravenous (i.v.) routes of administration of these drugs have not resulted in therapeutic concentrations for the treatment of patients with brain tumors. This is mainly due to their poor ability to penetrate an intact blood–brain barrier in areas where there are microinvasive deposits of tumor [20]. As originally proposed by Kroin and Penn, we have administered these drugs i.c. to overcome this limitation [21, 22]. The efficacy of i.c. administration of cisplatin in combination with SSR for the treatment of F98 glioma-bearing rats previously has been reported by us [8]. This treatment resulted in 35% long-term survivors at 1 year, and at the time were the best survival data ever reported using the F98 glioma model [23]. Our initial hypothesis was that therapeutic efficacy was based on the production of photoelectrons and Auger electrons, emitted by relaxation of excited Pt atoms following irradiation with low energy X-rays, tuned just above the Pt K-edge energy (78.4 keV). Unexpectedly, however, we observed the same therapeutic efficacy with X-rays below the Pt K-edge, although the production of photo- and Auger electrons was less than that produced by X-rays above the Pt K-edge.
In order to assess whether the therapeutic efficacy of this treatment was due to dose enhancement produced by the interaction of X-rays with Pt atoms or to the additive effects of platinum chemotherapeutic agents and radiotherapy, we designed an experiment to compare the combined treatment using either low energy synchrotron or megavoltage X-rays. For the latter, the vast majority of photons in the spectrum have energies for which Compton scatter is the predominant interaction, especially in tissue and tissue loaded with high-Z elements [12]. Therefore, a negligible dose enhancement effect would be expected from the Pt atoms. We recently have evaluated the combination of carboplatin, administered i.c. by CED, and photon irradiation consisting of 3 daily fractions of 8 Gy each. We used either low energy X-rays from a synchrotron source, tuned above the Pt K-edge, or high-energy 6 MV X-rays from a conventional LINAC. These treatments yielded similar survival data in F98 glioma-bearing rats [10]. The median survival times (MeSTs) were 79 and 60 days and the corresponding percent increase in life spans were 182 and 114%, respectively, for the combination treatment with either 6 MV or 80 keV photons. This strongly suggested that therapeutic efficacy was not related to the emission of Auger electrons from the Pt atoms. The formation of Pt adducts with DNA, which interfere with the repair of radiation-induced damage, could explain the synergistic interaction observed between carboplatin and ionizing radiation [24]. This led us to hypothesize that local administration of cisplatin in combination with 6 MV photon irradiation would also improve the survival of F98 glioma-bearing rats. In the present study, we have compared the therapeutic efficacy of i.c. convection-enhanced delivery (CED) of cisplatin, combined with either SSR or 6 MV X-irradiation, for the treatment of F98 glioma-bearing rats.
Materials and methods
Tumor model
F98 glioma cells (CRL-2397, American-Type-Culture-Collection, Manassus, VA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 10% fetal calf serum. For tumor cell implantation, male Fischer 344 rats (Charles River Laboratories), weighing 230–260 g, were anesthetized with isoflurane, followed by the intraperitoneal (i.p.) administration of ketamine (60 mg/kg body weight [b.w.]) and xylazine (7 mg/kg b.w.). The animals’ eyes were coated with an ocular lubricant to prevent keratitis. A 10 μl suspension of 1,000 F98 cells in serum-free DMEM containing 1% agarose (gelling temperature < 30°C), was injected stereotactically into the right caudate nucleus. The syringe pump (model KDS 310, GENEQ inc., Montréal, Canada) was directly mounted on the stereotactic frame (David Kopf Instruments, Tujunga, CA) and the syringe (model 702 N, Hamilton, Bonaduz, Switzerland) was attached to the pump. The cells were injected over 16 s via a 22-gauge needle, which was inserted 3.5 mm to the right of the bregma to a depth of 5.5 mm from the skull and then withdrawn to its target depth of 5.0 mm. Following implantation, the needle was left in place for 2 min and then withdrawn slowly. The burr hole in the calvarium was filled with bone wax and the operative field was cleaned with betadine before closure of the scalp incision. All operative procedures and animal care were carried out in conformity with the guidelines of the French Government (decree 87-848, 1987) and in accordance with the Laboratory Animal Care and Use Committee of the European Synchrotron Radiation Facility (ESRF).
Convection-enhanced delivery of cisplatin
Cisplatin (M.W. 300.04; Merck) was diluted in sodium chloride 0.9% solution (Laboratoire Aguettant, Lyon, France). Thirteen days after tumor implantation the animals were anesthetized, as described above, the scalp was re-opened and the bone wax was removed with a needle. A 32-gauge needle, attached to a 50 μl gastight syringe (model 1700, Hamilton, Reno, NV), was inserted into the tumor by using the same stereotactic coordinates as for tumor implantation. Cisplatin (6 μg in 20 μl) was administered over 40 min using the GENEQ syringe pump (rate: 0.5 μl/min). This dose was based on a study of Olivi et al. (25) that evaluated cisplatin neurotoxicity following delivery of the drug to the cerebrospinal fluid of rats. They showed that 6 μg of cisplatin in 20 μl was the highest non-lethal dose, although it produced some chronic toxicity.
Linear accelerator irradiation
Irradiations were performed using a 6 MV medical irradiator (Elekta Sli, Elekta Oncology Systems Ltd., Crawley, West Sussex, UK) [10]. Rats were irradiated, two at a time. The head of each animal was aligned in the middle of an 8 × 4 cm2 aperture, defined by the beam collimator. Only the right cerebral hemisphere was irradiated. A wax block was positioned between the rats’ heads and a 0.5 cm tissue equivalent bolus was placed on top to ensure electronic equilibrium. The dose of 15 Gy was prescribed at a 1.5-cm depth at a dose rate of 2 Gy/min (dosimetry treatment planning system: Dosigray®, DosiSoft, Cachan, France). After treatment the animals were transferred to the Animal Care Facility at the ESRF.
Stereotactic synchrotron irradiation
SSR was carried out at the ESRF, as previously described by us [4, 8, 10]. A single 15 Gy dose was delivered on day 14 after tumor implantation, using monochromatic 78.8 keV X-rays (80 eV energy-bandwidth) [25]. The right cerebral hemisphere was centred on the rotation axis of the irradiation system and the beam was shaped to 10 mm in width and 1 mm in height. The dose was delivered while the rat was being rotated, and translating upwards between each of the adjacent 360° arcs so that the irradiated target volume encompassed a cylinder 10 mm in diameter and 15 mm in height. The X-ray dose rate was 0.3 Gy/s at the tumor, determined by using an ion chamber (Unidos, PTW, Freiburg, Germany). In this group, tumor volumes were assessed the day after the irradiation by synchrotron radiation computed tomography (SRCT). CT images were acquired with 35 keV monochromatic X-rays after tail vein injection of 1 ml of prewarmed (37°C) iodinated contrast agent (Iomeron; iodine concentration: 350 mg/ml), followed by 1 ml of 0.9% NaCl solution. Three dimensional axial SRCT images (1 mm slice thickness, 1 mm spacing) were acquired, starting 5–10 min after completion of the i.v. iodine infusion [26].
Experimental groups
Cisplatin was administered by CED 13 days following stereotactic implantation of one thousand F98 glioma cells, at which time the tumors had a volume of about ~20–40 mm3, as determined by (SRCT) [26] or MRI [27]. Radiotherapy with 6 MV or monochromatic 78.8 keV X-rays was started 24 h later. The animals were stratified into six groups (Table 1), as follows: Group 1, untreated controls (n = 27); Group 2, chemotherapy alone, which consisted of 6 μg of cisplatin in 20 μl (n = 23); Groups 3 was irradiated with 6 MV X-rays administered by a LINAC (n = 13); Group 4 received cisplatin followed by 6 MV X-irradiation (n = 12); Groups 5 was irradiated at the ESRF with a monochromatic beam of 78.8 keV X-rays (n = 8); Group 6 received cisplatin followed by 78.8 keV X-irradiation (n = 11).
Monitoring of clinical status and neuropathologic evaluation
After treatment, the animals were weighed 3 times a week and their clinical status was monitored until the end of the study (200 days). Those animals showing a combination of sustained weight loss, ataxia and peri-orbital hemorrhage were euthanized by intracardiac injection of Dolethal (150 mg/kg; Vetoquinol, Lure, France). The brains were fixed in 10% buffered formalin, and cut coronally at the level of the optic chiasm and 2 mm anterior and posterior to it. Slices were embedded in paraffin, sectioned at 4 μm, stained with hematoxylin and eosin (H&E), and examined microscopically to assess histopathologic changes.
Statistical evaluation of survival data
Kaplan–Meier survival curves were plotted for each group. Differences between groups were assessed for statistical significance by means of the log-rank test (JMP®, SAS-Institute-Inc., Cary, NC). Minimal statistical significance was defined at P < 0.05. Those rats still alive at the end of the study (200 days) were euthanized. The mean survival times (MST), standard errors (SE), and median survival times (MeST) were calculated using the date of euthanization (200 days) for rats that were still alive at the end of the study (censored data). Percent increased lifespan (% ILS) was determined relative to MST or MeST of untreated controls, as previously described by us [10].
Results
Therapeutic response following cisplatin or X-irradiation alone
Survival data for the different treatments are shown in Table 1 and Kaplan–Meier plots for animals treated with either cisplatin or radiotherapy alone are shown in Fig. 1. The MST ± SE of untreated controls was 24 ± 1 days post tumor implantation compared to 59 ± 13 days for the rats that received cisplatin. This was significantly different from the untreated controls (P < 0.001). The corresponding % ILS relative to the mean was >145% (censored data) for cisplatin alone, with 3 of 23 animals cured (i.e. 13%) in this group. The survival plots of X-irradiated groups of animals were significantly different from that of the untreated controls (P < 0.001 for both). Rats that received a 15 Gy dose of 6 MV photons had a MST of 37 ± 2 days compared with a MST of 44 ± 3 days for those rats treated with 78.8 keV photons. All the animals treated only with radiation died within 57 days after tumor implantation.
Kaplan–Meier survival curves for F98 glioma-bearing rats after intratumoral radiotherapy alone or intracerebral cisplatin alone. The tumors were implanted on day 0. NB: The x-axis is in logarithmic scale. Group 1: untreated controls (open diamond), Group 2: cisplatin (*, dotted line), Group 3: 6 MV radiotherapy alone (open triangle), Group 5: 78.8 keV radiotherapy alone (open square)
Therapeutic response following combined therapy with cisplatin and X-irradiation
Survival data following chemo-radiotherapy are summarized in Table 1 and Kaplan–Meier survival plots are shown in Fig. 2. Animals treated with cisplatin, followed by 6 MV photon or 78.8 keV synchrotron X-irradiation had MSTs of >75 ± 18 and >74 ± 19 days, respectively, and MeSTs of 48 and 56 days, respectively, which were not significantly different from each other (P = 0.897), but they were different from that of the untreated controls (P < 0.001). The corresponding % ILSs, based on the MSTs, were 212 and 205% (both censored) for cisplatin plus 6 MV photons or cisplatin plus 78.8 keV X-rays, respectively. The log-rank test was not used for the other groups, since the survival plots crossed each other due to early deaths. The log-rank test is unlikely to detect differences between groups in such cases [28]. In both chemo-radiotherapy groups there were 2 long-term survivors (>200 days), i.e. 17 and 18% with 6 MV and 78.8 keV irradiation, respectively.
Kaplan–Meier survival curves for F98 glioma-bearing rats after intratumoral cisplatin with radiotherapy (6 MV or 78.8 keV photons). NB: The x-axis is in logarithmic scale. Group 1: untreated controls (open diamond), Group 4: cisplatin combined with 6 MV radiotherapy (closed triangle), Group 6: cisplatin combined with 78.8 keV radiotherapy (closed square)
Neuropathologic evaluation
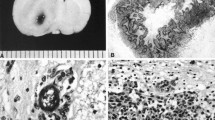
The tumors of untreated control animals microscopically were identical to those previously described by us [9, 10]. These were composed of a mixed population of spindle-shaped cells with fusiform nuclei, frequently forming a whorled pattern of growth, and a smaller subpopulation of polygonal cells with round to oval nuclei. There was extensive invasion of contiguous normal brain with islands of tumor cells at varying distances from the main tumor mass, which frequently formed perivascular clusters. Usually, there was a central area of necrosis filled with tumor cell ghosts. Rats that received X-irradiation alone (SSR) had very large tumors and one had a very small tumor at the time of death. Five of 11 of the animals that received CED of cisplatin in combination with X-irradiation (SSR) had evidence of residual tumor at the time of euthanization (Fig. 3A). Microscopically, there was a light infiltrate of mononuclear cells. However, leptomeningeal extension was seen in 4 of 11 rats and focal hemorrhage in 2 of 11 rats. Several of the brains of animals from the last two groups showed either extension into the lateral ventricle or ventricular dilatation. Neuropathologic evaluation of the brains of two longterm surviving rats that received cisplatin by CED in combination with synchrotron X-rays showed no gross or microscopic evidence of tumor (Fig. 3B). However, the microscopic findings indicate that these two animals both had tumors since these changes would not have been present if the tumors had failed to “take.” One of the animals’ brains showed dilatation of the right lateral ventricle and pseudocystic areas at the presumptive site of the original tumor. The surrounding white matter was rarified. The other brain showed an area of fibrosis and a small porencephalic cyst surrounded by a fibrous zone in which there were cells that were morphologically consistent with tumor cells. However, these appeared to be quiescent. In addition, there was basophilic debris consistent with focal dystrophic calcification. Two long-term surviving rats, which had received cisplatin in combination with 6 MV photons, showed no microscopic evidence of tumor (Fig. 3C, D). In one brain there was a small focus of lymphocytes and macrophages at the presumptive site of the original tumor. The brains of both of these animals showed marked dilatation of the right lateral ventricle. Similarly, no residual tumor was identified either grossly or microscopically in the brains of the animals that had received cisplatin alone. These findings again strongly indicate that the implanted tumors indeed had taken since otherwise these changes would not have been present. However, more detailed neuropathologic studies and neurofunctional studies are needed to further define the combined effects of platinated drugs and X-irradiation in normal brains and those of tumor bearing rats.
A Histology of the F98 glioma in a rat that received cisplatin and 15 Gy of synchrotron X-irradiation, failed treatment and died 47 days following tumor implantation The recurrent tumor shows a highly invasive pattern of growth with extensive invasion of white matter (×200). B Brain of a rat from the same experimental group that was alive at the time of euthanization on day 200. There was no evidence of viable residual or recurrent tumor. There was a cystic area with a focus of gliosis and fibrosis with mixed infiltrative of lymphocytes, macrophages and scattered pyknotic tumor cells (×200). There also was necrotic cellular debris with some dystrophic calcification (at the time of treatment, the CT images of this rat revealed a large tumor, visible over 6 slices (1 mm thickness), data not shown). C Low power photomicrograph (×40) of a porencephalic cyst measuring 4 × 5 mm from the right cerebral hemisphere of rat that had received 6 MV X-irradiation and cisplatin. This animal was a long-term survivor who has alive at the time of euthanization on day 200. No residual or recurrent tumor was seen. D Higher power view (×200) of the wall of the porencephalic cyst. There was mild gliosis and a light scattering of lymphocytes and macrophages immediately adjacent to the cavity
Discussion
In the present study, we have evaluated the efficacy of i.c. CED of cisplatin in combination with photon irradiation for treatment of F98 glioma-bearing rats. First, we showed that cisplatin alone, delivered i.c. by CED, was effective and that 13% of the animals in this group were cured. Second, we demonstrated that the therapeutic efficacy of i.c. cisplatin was further enhanced by the addition of X-irradiation. This combination was the most effective of the various treatments, irrespective of the X-ray beam energy since 6 MV and synchrotron X-rays resulted in almost identical numbers of long-term survivors (17 and 18%, respectively). These results confirm those previously reported by us with carboplatin [10], which showed that high-energy 6 MV X-rays were as effective as 78.8 keV synchrotron X-rays for treatment of the F98 glioma.
Among all chemotherapeutic agents available, platinum-based drugs have played an important role in the treatment of solid tumors [18], but their clinical use has been limited by their toxicity after systemic administration. Intracerebral administration of platinum compounds was first reported by Kroin and Penn and their collaborators in the early 1980s [21, 29]. Using the 9L gliosarcoma model, they demonstrated that a 7 days i.c. microinfusion of cisplatin (0.5 mg/ml at a flow rate of 0.9 μl/h), produced adequate and sustained therapeutic drug levels without producing systemic toxicity [21]. They further observed a small, but statistically significant increase in survival (27% ILS) compared to that of control rats after a 7 days infusion of cisplatin (0.5 mg/ml) at a flow rate of 0.9 μl/h in the rat 9L gliosarcoma model [29]. Using the same tumor model, Kimler et al. [30] evaluated the administration of cisplatin in combination with radiation therapy and reported that the combination was effective only when the drug was injected intra cerebrally. Intratumoral infusion of carboplatin also has been shown to be highly effective in treating brain tumors. Degen et al. [31] have demonstrated that carboplatin, delivered by CED, also was effective for the treatment of 9L gliosarcoma bearing rats. Three of 4 rats that received carboplatin (40 μg/40 μl; 0.5 μl/min) on day 7 after tumor implantation, survived to 120 days, at which time the study was terminated. However, since the 9L tumor is highly immunogenic, these results must be interpreted with caution [24].
To the best of our knowledge, there are only two clinical reports describing the direct i.c. administration of platinated drugs for glioma treatment. Bouvier et al. infused 8.2 mg of cisplatin over 10 days using 68 catheters, each connected to an Alzet pump, to treat a patient with a recurrent malignant glioma [32]. No adverse side effects associated with chemotherapy, catheter implantation or removal were observed. However, although chemotherapy halted progression of the tumor, it recurred and the patient died 6 months later. In another clinical study, Sheleg et al. administered cisplatin, incorporated into biodegradable polymers, after subtotal resection of the GBM. Twenty polymer discs loaded with cisplatin (1 mg/cm2) were placed in the tumor bed. Two to three weeks after surgery, the patients received radiotherapy (60 Gy). This combined treatment significantly increased the MST compared to that observed in patients who had received radiotherapy alone (427 vs. 211 days, respectively) [33].
Our own data have demonstrated that i.c. infusion of cisplatin, followed by either synchrotron or 6 MV LINAC photon irradiation, was highly effective for the treatment of F98 glioma-bearing rats. Studies to improve this chemo-radiotherapeutic approach are in progress and will focus on further optimizing the drug delivery by means of liposomal formulations, improving the radiation parameters, and on striking a balance between neurotoxicity and therapeutic efficacy. The present study confirm our previously reported data on carboplatin, establishing that the therapeutic gain obtained with i.t. injection of cisplatin or carboplatin, followed by X-irradiation, was not predominantly due to Auger electrons emitted from the Pt atoms, but rather was due to the cytotoxic effects of the drugs [34]. The intracerebral administration route of CDDP is probably a key element in the therapeutic efficacy of this combined treatment. It allows bypassing the BBB thereby achieving higher drug concentrations in the tumor than otherwise would be obtainable with systemic administration routes. In a previous study using the same tumor model, we measured 214 ± 17 μg/g of platinum in the tumor volume, 24 h after intracerebral injection of 5 μg of CDDP diluted in 5 μl of isotonic solution [35].
Other elements, such as gold nanoparticles, also would be of interest as radiation sensitizers [15, 16]. The dose enhancement effect produced by gold, as been shown by in vitro studies using microspheres [36], by preclinical rodent studies using gold nanoparticles [16] and by theoretical Monte Carlo simulations [37]. The main challenge for Pt-SSR is to obtain a sufficient subcellular concentration of Pt, preferably localized in the nucleus, and more specifically intercalated with DNA in order to produce enough photoelectric interactions in the tumor cells [34]. Other Pt-containing compounds, such as di-chloroterpyridine platinum (PtTC), which is known to be less cytotoxic than cisplatin or carboplatin, might accumulate in larger amounts in the tumor and this could help us to discriminate between Auger electrons effects and the chemotherapeutic efficacy of the drugs [38].
Studies to improve this chemo-radiotherapeutic approach are in progress and will focus on further optimizing the drug delivery by means of liposomal formulations [39, 40], improving the radiation parameters, and on striking a balance between neurotoxicity and therapeutic efficacy. The present study confirms our previously reported data on carboplatin, establishing that the therapeutic gain obtained with i.t. injection of cisplatin or carboplatin, followed by X-irradiation, was not predominantly due to Auger electrons emitted from the Pt atoms, but rather was due to the cytotoxic effects of the drugs reinforced by the intracerebral route of administration.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol
Adam JF, Elleaume H, Joubert A, Biston MC, Charvet AM, Balosso J, Le Bas JF, Esteve F (2003) Synchrotron radiation therapy of malignant brain glioma loaded with an iodinated contrast agent: first trial on rats bearing F98 gliomas. Int J Radiat Oncol Biol Phys 57:1413–1426
Adam JF, Joubert A, Biston MC, Charvet AM, Peoc’h M, Le Bas JF, Balosso J, Esteve F, Elleaume H (2006) Prolonged survival of Fischer rats bearing F98 glioma after iodine-enhanced synchrotron stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 64:603–611
Barth RF, Coderre JA, Vicente MG, Blue TE (2005) Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res 11:3987–4002
Boudou C, Balosso J, Esteve F, Elleaume H (2005) Monte Carlo dosimetry for synchrotron stereotactic radiotherapy of brain tumours. Phys Med Biol 50:4841–4851
Boudou C, Biston MC, Corde S, Adam JF, Ferrero C, Esteve F, Elleaume H (2004) Synchrotron stereotactic radiotherapy: dosimetry by Fricke gel and Monte Carlo simulations. Phys Med Biol 49:5135–5144
Biston MC, Joubert A, Adam JF, Elleaume H, Bohic S, Charvet AM, Esteve F, Foray N, Balosso J (2004) Cure of Fisher rats bearing radioresistant F98 glioma treated with cis-platinum and irradiated with monochromatic synchrotron X-rays. Cancer Res 64:2317–2323
Rousseau J, Barth RF, Moeschberger ML, Elleaume H (2009) Efficacy of intracerebral delivery of carboplatin in combination with photon irradiation for treatment of F98 glioma-bearing rats. Int J Radiat Oncol Biol Phys 73:530–536
Rousseau J, Boudou C, Barth RF, Balosso J, Esteve F, Elleaume H (2007) Enhanced survival and cure of F98 glioma-bearing rats following intracerebral delivery of carboplatin in combination with photon irradiation. Clin Cancer Res 13:5195–5201
Karnas SJ, Yu E, McGarry RC, Battista JJ (1999) Optimal photon energies for IUdR K edge radiosensitization with filtered x-ray and radioisotope sources. Phys Med Biol 44:2537–2549
Robar JL, Riccio SA, Martin MA (2002) Tumour dose enhancement using modified megavoltage photon beams and contrast media. Phys Med Biol 47:2433–2449
Corde S, Balosso J, Elleaume H, Renier M, Joubert A, Biston MC, Adam JF, Charvet AM, Brochard T, Le Bas JF, Esteve F, Foray N (2003) Synchrotron photoactivation of cisplatin elicits an extra number of DNA breaks that stimulate RAD51-mediated repair pathways. Cancer Res 63:3221–3227
Corde S, Biston MC, Elleaume H, Esteve F, Charvet AM, Joubert A, Ducros V, Bohic S, Simionovici A, Brochard T, Nemoz C, Renier M, Tropres I, Fiedler S, Bravin A, Thomlinson W, Le Bas JF, Balosso J (2002) Lack of cell death enhancement after irradiation with monochromatic synchrotron X rays at the K-shell edge of platinum incorporated in living SQ20B human cells as cis-diaminedichloroplatinum (II). Radiat Res 158:763–770
Hainfeld JF, Dilmanian FA, Slatkin DN, Smilowitz HM (2008) Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol 60:977–985
Hainfeld JF, Slatkin DN, Smilowitz HM (2004) The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 49:N309–N315
De Stasio G, Rajesh D, Ford JM, Daniels MJ, Erhardt RJ, Frazer BH, Tyliszczak T, Gilles MK, Conhaim RL, Howard SP, Fowler JF, Esteve F, Mehta MP (2006) Motexafin-gadolinium taken up in vitro by at least 90% of glioblastoma cell nuclei. Clin Cancer Res 12:206–213
Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7:573–584
Jung Y, Lippard SJ (2007) Direct cellular responses to platinum-induced DNA damage. Chem Rev 107:1387–1407
Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, Hall WA, Hynynen K, Senter PD, Peereboom DM, Neuwelt EA (2007) Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol 25:2295–2305
Kroin JS, Penn RD (1982) Intracerebral chemotherapy: chronic microinfusion of cisplatin. Neurosurgery 10:349–354
Boudou C, Tropres I, Rousseau J, Lamalle L, Adam JF, Esteve F, Elleaume H (2007) Polymer gel dosimetry for synchrotron stereotactic radiotherapy and iodine dose-enhancement measurements. Phys Med Biol 52:4881–4892
Barth RF, Kaur B (2009) Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol 94:299–312
Nias AH (1985) Radiation and platinum drug interaction. Int J Radiat Biol Relat Stud Phys, Chem Med 48:297–314
Elleaume H, Charvet AM, Berkvens P, Berruyer G, Brochard T, Dabin Y, Dominguez MC, Draperi A, Fiedler S, Goujon G, Le Duc G, Mattenet M, Nemoz C, Perez M, Renier M, Schulze C, Spanne P, Suortti P, Thomlinson W, Esteve F, Bertrand B, Le Bas JF (1999) Instrumentation of the ESRF medical imaging facility. Nucl Instrum Methods A 428:513–527
Rousseau J, Adam JF, Deman P, Wu TD, Guerquin-Kern JL, Gouget B, Barth RF, Esteve F, Elleaume H (2009) Intracerebral delivery of 5-iodo-2′-deoxyuridine in combination with synchrotron stereotactic radiation for the therapy of the F98 glioma. J Synchrotron Radiat 16:573–581
Yang WL, Barth RF, Wu G, Ciesielski LJ, Fenstermaker RA, Moffat BA, Ross BD, Wikstrand CJ (2005) Development of a syngeneic rat brain tumor model expressing EGFRvIII and its use for molecular targeting studies with monoclonal antibody L8A4. Clin Cancer Res 11:341–350
Moeschberger ML, Klein JP (1985) A comparison of several methods of estimating the survival function when there is extreme right censoring. Biometrics 41:253–259
Penn RD, Kroin JS, Harris JE, Chiu KM, Braun DP (1983) Chronic intratumoral chemotherapy of a rat tumor with cisplatin and fluorouracil. Appl Neurophysiol 46:240–244
Kimler BF, Liu C, Evans RG, Morantz RA (1993) Combination of aziridinylbenzoquinone and cis-platinum with radiation therapy in the 9L rat brain tumor model. Int J Radiat Oncol Biol Phys 26:445–450
Degen JW, Walbridge S, Vortmeyer AO, Oldfield EH, Lonser RR (2003) Safety and efficacy of convection-enhanced delivery of gemcitabine or carboplatin in a malignant glioma model in rats. J Neurosurg 99:893–898
Bouvier G, Penn RD, Kroin JS, Beique R, Guerard MJ (1987) Direct delivery of medication into a brain tumor through multiple chronically implanted catheters. Neurosurgery 20:286–291
Sheleg SV, Korotkevich EA, Zhavrid EA, Muravskaya GV, Smeyanovich AF, Shanko YG, Yurkshtovich TL, Bychkovsky PB, Belyaev SA (2002) Local chemotherapy with cisplatin-depot for glioblastoma multiforme. J Neurooncol 60:53–59
Bernhardt P, Friedland W, Paretzke HG (2004) The role of atomic inner shell relaxations for photon-induced DNA damage. Radiat Environ Biophys 43:77–84
Ortega R, Biston MC, Deves G, Bohic S, Carmona A (2005) Nuclear microprobe determination of platinum quantitative distribution in rat brain tumors after cisplatin or carboplatin injection for PAT treatment of glioma. Nucl Instrum Methods Phys Res Sect B-Beam Interact Mater At 231:321–325
Herold DM, Das IJ, Stobbe CC, Iyer RV, Chapman JD (2000) Gold microspheres: a selective technique for producing biologically effective dose enhancement. Int J Radiat Biol 76:1357–1364
Cho SH (2005) Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys Med Biol 50:N163–N173
Usami N, Furusawa Y, Kobayashi K, Lacombe S, Reynaud-Angelin A, Sage E, Wu TD, Croisy A, Guerquin-Kern JL, Le Sech C (2008) Mammalian cells loaded with platinum-containing molecules are sensitized to fast atomic ions. Int J Radiat Biol 84:603–611
Boulikas T, Vougiouka M (2003) Cisplatin and platinum drugs at the molecular level (review). Oncol Rep 10:1663–1682
Huynh GH, Deen DF, Szoka FC Jr (2006) Barriers to carrier mediated drug and gene delivery to brain tumors. J Control Release 110:236–259
Acknowledgments
We thank the European Synchrotron Radiation Facility for providing beam time; Mrs. C. Massart, Ms. E. Kerboul, Mr. T. Brochard, Dr. C. Nemoz for help during the experiments; Mr. D. Dallery and Mrs. A. Honkimaki for animal care; Dr. S. Corde for CHU dosimetry; Ms. M. Van Fossen for secretarial assistance. R.F.B. acknowledges the support of the Grey Ribbon Crusade, the Musella Foundation and The Ohio State University Department of Pathology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rousseau, J., Barth, R.F., Fernandez, M. et al. Efficacy of intracerebral delivery of cisplatin in combination with photon irradiation for treatment of brain tumors. J Neurooncol 98, 287–295 (2010). https://doi.org/10.1007/s11060-009-0074-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-0074-3