Summary
Results of clinical trials with oxaliplatin in treating glioblastoma are dismal. Previous works showed that intravenous (i.v.) delivery of oxaliplatin did not increase the survival of F98 glioma-bearing Fisher rats. Low accumulation of the drug in tumor cells is presumed to be responsible for the lack of antitumor effect. In the present study, convection-enhanced delivery (CED) was used to directly inject oxaliplatin in brain tumor implanted in rats. Since CED can led to severe toxicity, the liposomal formulation of oxaliplatin (Lipoxal™) was also assessed. The maximum tolerated dose (MTD) of oxaliplatin was 10 μg, while that of Lipoxal™ was increased by 3-times reaching 30 μg. Median survival time (MeST) of F98 glioma-bearing rats injected with 10 μg oxaliplatin by CED was 31 days, 7.5 days longer than untreated control (p = 0.0002); while CED of 30 μg Lipoxal™ reached the same result. Compared to previous study on i.v. delivery of these drugs, their injection by CED significantly increased their tumoral accumulations as well as MeSTs in the F98 glioma bearing rat model. The addition of radiotherapy (15 Gy) to CED of oxaliplatin or Lipoxal™ increased the MeST by 4.0 and 3.0 days, respectively. The timing of radiotherapy (4 h or 24 h after CED) produced similar results. However, the treatment was better tolerated when radiotherapy was performed 24 h after CED. In conclusion, a better tumoral accumulation was achieved when oxaliplatin and Lipoxal™ were injected by CED. The liposomal encapsulation of oxaliplatin reduced its toxic, while maintaining its antitumor potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxaliplatin belongs to a third generation of platinum derivatives. It was developed to reduce the toxicity of parental platinum drugs, while maintaining or increasing the same antitumor efficiency. It has become one of the first line chemotherapy drugs for advance colorectal cancer and it is also being studied in the treatment of other types of cancers [1–3]. Several clinical trials have been carried out either using oxaliplatin as a single agent or combined with other antineoplastic drugs in the treatment of primary brain tumors [4–6]. However, either limited antitumor activities were observed or an insufficient number of patients were recruited to further support its use in clinic [4–6]. Studies were discontinued due to the dismal results of clinical trials. Our previous pre-clinical study also showed that intravenous injection (i.v.) of oxaliplatin does not increase the median survival time of F98 glioma-bearing rats [7]. This may be due to the presence of the blood-brain barrier (BBB) and impaired central nervous system (CNS) delivery, limiting drugs from accumulating in brain tumors [8]. In the same study, different platinum drugs were tested with different injection routes. Interestingly, higher tumoral accumulation of a drug correlated to the route of infusion, and was associated with the median survival time of glioma-bearing Fisher rats [7].
In that respect, convection-enhanced delivery (CED) is a particularly relevant strategy, since it allows delivery of high dose of antineoplastic agents directly in the tumor volume and maximize its residence for prolonged time [9]. As the platinum drugs are cell cycle specific agents, this approach allows for an increase of both the concentration and time of exposition. The teams of Elleaume and Barth have extensively studied the antitumor efficiency of cisplatin and carboplatin delivered by CED with and without radiotherapy [10–15]; a significant increase in median survival time was observed [10–15]. Therefore, it seems appropriate to evaluate the antitumor activity of oxaliplatin by this delivery method, despite its potential neurotoxicity [16].
Faced with the possibility that direct tumoral injection of oxaliplatin could lead to a worsen neurotoxicity profile, we intended to circumvent this risk by also testing the liposomal formulation of oxaliplatin (Lipoxal™). This formulation was developed by Regulon Inc. to reduce the toxicity of oxaliplatin. It was found safe within a range of dose of 100–250 mg/m2 by i.v. in human. Indeed, side effects such as mild myelotoxicity, nausea, peripheral neuropathy were only observed at doses of 300–350 mg/m2 [17].
Patients with glioblastomas have long benefited from post-operative radiotherapy [18]. It remains one of the main components of current glioblastoma treatment along with temozolomide chemotherapy [19]. We have recently studied the synergy between platinum-derivatives and radiation in glioblastoma cells, human colorectal cancer cells and in vivo colorectal tumor bearing nude mice [20–22]. The amount of platinum-DNA adducts has a positive correlation to the enhancement of the DNA damage by radiation [23]. At the time when the amount of platinum bound to the DNA of cancer cells is highest, optimal synergy is obtained between platinum-drugs and radiotherapy. Therefore, time-based radiotherapy studies of oxaliplatin and Lipoxal™ in F98 glioma-bearing rats were also performed with the goal of maximizing results of platinum-based chemo-radiation treatment.
Methods and materials
Chemicals
Oxaliplatin was purchased from Sanofi-Aventis (Laval, QC). Lipoxal™ (liposomal formulation of oxaliplatin) was generously provided by Dr. Teni Boulikas of Regulon Inc., (Athens, Greece).
Cell lines and animal model
The rat glioblastoma cell line F98 was purchased from American Type Culture Collection (ATCC). Male Fischer rats weighing 210 to 225 g were purchased from Charles River Laboratories International Inc. (Wilmington, MA). The experimental animal protocol was approved by the institutional ethical committee and complied with the regulations of the Canadian Council on Animal Care (protocol # 329-13B).
F98 glioma cell implantation in Fischer rats
The detailed implantation method has been described elsewhere [24]. Briefly, 5 μl of 10 000 F98 cells in Dulbecco’s Modified Eagle Medium (DMEM) without fetal bovine serum (FBS) were injected to a target site corresponding to 1 mm anterior and 3 mm right of the bregma, to a depth of 6 mm over 5 min.
CED procedure
Ten days after the implantation of F98 cells, CED procedure was performed with a 33 Ga Hamilton syringe (Hamilton Company, Reno, NV). Oxaliplatin and Lipoxal™ infusions were initiated at the same position as the F98 cell implantation, and done at a rate of 0.5 μl/min for 20 min [25]. Before and after infusion, the needle was maintained in a stationary position for 5 min and finally slowly withdrawn during 6 min. This step was performed to reduce the backflow and increase the convection volume by maintaining the interstitial pressure.
Maximum tolerated dose (MTD)
The MTD of oxaliplatin and Lipoxal™ were determined via a traditional 3 + 3 design [26]. After CED of platinum drugs, the rats were followed for 10 days; those who were unable to feed or groom, presented lethargy or weight loss by more than 30 % were considered to exhibit severe drug toxicity.
Drug distribution volume
Distributions of oxaliplatin and Lipoxal™ in the brain of implanted F98 Fischer rats were measured. To that end, the brains were extracted 30 min after CED and then sliced using a brain matrix. A 2 mm slice at the injection point was cut into several sections. Five sections representing proximal and distal to the target site were chosen and digested in a 1:1 mixture of 70 % HNO3 and 30 % H2O2 and their platinum content analyzed by inductively coupled plasma mass spectrometry (ICP-MS) (ELAN DRC-II, PerkinElmer, Woodbridge, ON). The distribution study was triplicated and average of the concentration in each section was mapped back into the slide.
Assessment of animal survival
Treatment groups were planned as follows: control (CED of 10 μl 5 % dextrose), radiotherapy alone (CED of 10 μl 5 % dextrose plus 15 Gy radiotherapy), chemotherapy alone (CED of 10 μl of 1 μg/μl oxaliplatin or 3 μg/μl Lipoxal™) and chemotherapy plus radiotherapy (CED of 1 μg/μl oxaliplatin plus 15 Gy radiotherapy or 3 μg/μl Lipoxal™ plus 15 Gy radiotherapy) (Fig. S1). Eight rats were included per group. The rats were monitored and assessed on a daily basis by the following criteria: weight, mobility, coordination, feeding and grooming. When an animal presented a loss of more than 30 % of its initial weight or one of the monitored indices reached a score of 1/10, it was euthanized.
Tissue platinum concentration and platinum-DNA adduct quantification
Ten days after F98 cell implantation, 10 μl of 1 μg/μl oxaliplatin or 3 μg/μl Lipoxal™ was infused by CED. Four h, 24 h, or 48 h later (3 rats/group), rats were anesthetized and 4 % paraformaldehyde was infused by intra-cardiac to evacuate the blood. Brains were extracted and a 2 mm thick slice at the injection point was cut using a rat brain matrix. The tumor area and the normal brain around tumor were collected and weighed. To determine the total platinum concentration, tissues were digested and platinum were quantified as described in the section “Drug distribution volume”. The tissue platinum concentration was expressed as μg platinum per g tissue. For platinum-DNA adduct quantification, DNA were extracted by the phenol/chloroform method and quantified by spectrophotometry, whereas platinum was quantified by ICP-MS [27]. The amount of platinum-DNA adducts were expressed as ng platinum per μg DNA.
Radiation treatment with a gamma knife
Either 4 or 24 h after CED, rats were treated with 15 Gy of radiation using a Gamma Knife PERFEXION (Elekta Instruments AB, Norcross, GA), as described previously [25].
Statistical analysis
Difference of the amount of platinum-DNA adducts at different times post-CED were assessed by two-way ANOVA. Survival data were plotted by Kaplan–Meier survival curves and the median survival time between groups were analyzed by log-rank test with GraphPad prism 6, (GraphPad Software Inc., San Diego, CA). A p value <0.05 was considered as statistically significant.
Results
Determination of MTD and neurotoxicology induced by platinum drugs
The toxicological profile of oxaliplatin can be significantly improved when it is shielded by liposome (Lipoxal™). As measured after delivery by CED, rats can tolerate a dose 3-times higher for Lipoxal™ than oxaliplatin. The MTD of Lipoxal™ was 30 μg, while the same signs of neurotoxity was observed after injecting 10 μg of oxaliplatin. When 30 μg of oxaliplatin was tested, unacceptable neurotoxicity was observed. One rat showed weakness in his left front paw and another had general touch hypersensitization for one day after the treatment. General signs such as reduced frequency of grooming, reduced feeding were also observed. On the third day, the rats had lost 12.4 % of initial weight. They were euthanized and their brains were extracted.
Histological analysis of rat’s brain treated at 30 μg of oxaliplatin showed a massive edema in the parenchyma, necrosis, vacuolization and focal hemorrhage (Fig. 1a); while a less severe and local edema, necrosis and hemorrhage were observed at the dose of 10 μg (Fig. 1b-d).
Neuropathologic changes upon injection of oxaliplatin or Lipoxal™ by CED. Three days after CED of 30 μg oxaliplatin, necrosis, massive edema, and heamorrage were observed (a). Three days after CED of 10 μg oxaliplatin, only local edema and heamorrage were detected (b). Using a higher magnification (c), focal hemorrhage, necrosis can be observed in the tumor site, while loss of glial cells was found in normal tissue near the tumor (d). Three days after CED of 30 μg Lipoxal™, focal heamorrage, necrosis were detected in the tumor site (e), while in normal tissue near the tumor, loss of glial cells was observed (f)
On the other hand, when animals were treated with Lipoxal™ at the same dose of 30 μg, only mild signs of toxicity such as reduced feeding were induced. A milder decrease of weight (4.0 %, p = 0.0045) was recorded at day 3. Histological analyses of brains treated with Lipoxal™ showed a necrosis and focal hemorrhage but only in the center of the tumor (Fig. 1e), and less edema was observed in the normal tissue surrounding the tumor than what was induced by the same dose of oxaliplatin (Fig. 1f). Interestingly, less glial cells were presented in normal tissue in both the oxaliplatin and Lipoxal™ animals treated at their respective MTD compared to untreated rats (Fig. 1d, f).
Volume of drug distribution
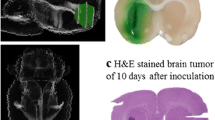
We determined whether the incorporation of oxaliplatin in a liposomal formulation altered its distribution when injected in glioma-bearing rats at their respective MTD. Concentrations of these drugs were determined by ICP-MS analysis and their distribution was mapped onto the brain slice (oxaliplatin, Fig. 2a; Lipoxal™, Fig. 2b). As expected, the drug concentrations were higher at the injection point. Most importantly, by comparing H&E brain tumor slices (Fig. 2c) with the drug distribution maps (Fig. 2a, b), we found that the distributions of both drugs were sufficient to cover the tumor area. On the other hand, drug diffusion in the contralateral hemispheres was limited since the concentration of oxaliplatin and Lipoxal™ were respectively 84 and 289 times lower (Table S1).
Distribution of oxaliplatin or Lipoxal™ in the rat brain bearing F98 tumor at 30 min after injection. Ten μl of 1 μg/μl oxaliplatin (a) or of 3 μg/μl Lipoxal™ (b) were infused by CED. H&E staining of brain tumor at 10 days after implantation (c). (Reprint permission obtained for Fig. 2 c)
In tumor samples corresponding to the injection site, only 1.9 times more Lipoxal™ that oxaliplatin was measured, although the amount of injected Lipoxal™ corresponding to its MTD was 3 times higher (Table 1). Conversely, in the farthest area to the brain tumor, the concentration of Lipoxal™ was 11.5 times more important than measured in brain injected with oxaliplatin (Lipoxal™ = 34. 0 μg/g tissue; oxaliplatin =3.0 μg/g tissue) (Fig. 2a, b; Table S1). These results support that the distribution volume of Lipoxal™ was much larger than the one of oxaliplatin.
Median survival time (MeST) of glioma-bearing rats
Oxaliplatin delivered by CED increased the MeST of F98 glioma-bearing rats by 7.5 days (p = 0.0002) to 31 days, when compared to rats treated with CED of dextrose (Fig. 3a and Table S1). The same MeST was obtained with Lipoxal™, supporting that the liposomal formulation did not hamper the overall antitumor effect (Fig. 3a and Table S1. When oxaliplatin and Lipoxal™ were combined to radiotherapy (15 Gy), the MeST increased by 4 and 3 days respectively (Fig. 3b, c).
Kaplan-Meier survival curve of F98 glioma bearing rats treated with oxaliplatin or Lipoxal™ with or without radiation and acute toxicity. Oxaliplatin and Lipoxal™ delivered by CED increased the MeST of F98 glioma bearing rats by 7.5 days (p = 0.0002) (a). After a radiation dose (15 Gy), the MeST was increased by 4 days with of oxaliplatin (b) and by 3 days with Lipoxal™ (c). MeST with radiotherapy performed at 4 or 24 h after oxaliplatin treatment (d). MeST and acute toxicity of F98 glioma bearing Fischer rats treated with radiotherapy at 4 or 24 h after CED of platinum-based drugs (e)
Kinetic of platinum-DNA adducts and optimized time for radiotherapy
After CED infusion, the quantity of oxaliplatin and Lipoxal™ in tumor and in surrounding normal tissue decreased from 4 to 48 h post-injection (Fig. 4a, b). Lipoxal™ shown higher tumoral and normal tissue uptake than oxaliplatin did when tested at their respective MTD. The quantity of DNA-bound oxaliplatin in tumor and surrounding normal tissue decreased gradually from 4 to 48 h (p < 0.05) (Fig. 4c). Conversely, the quantity of DNA-bound Lipoxal™, after an initial decrease between 4 and 24 h, was followed by a second maximal observed at 48 h post-CED, but only in tumor (p < 0.05) (Fig. 4d). At 4 and 48 h after CED, the quantity of Lipoxal™ bound to DNA was significantly more important than measured with oxaliplatin.
Quantification of oxaliplatin and Lipoxal™ in F98 tumor and normal tissue. Kinetics of oxaliplatin and Lipoxal™ accumulation in tumor (a) and normal tissue surrounding the tumor (b) after CED in F98 glioma bearing rats. Quantification of DNA-bound oxaliplatin (c) and Lipoxal™ (d) in F98 tumor and normal brain measured at different times after CED. All data points are the average of at least three measurements
Based on the significant decrease of DNA-bound oxaliplatin at 24 h relative to 4 h after CED, impact on the survival of rats irradiated at these time points was determined. Although MeST increased by 2 days when radiotherapy was administered 4 h after CED compared to 24 h, this difference was not found to be significant (p > 0.05) (Fig. 3d, e). Interestingly, animals irradiated 4 h after CED presented more signs of toxicities than those irradiated 24 h after CED of oxaliplatin (Fig. 3e). Indeed, in the early irradiated group (4 h), rats lost an average 14.7 % of their weight. In this group of 8 animals, 3 rats showed hypersensitivity and 1 rat had an eye dirt due to reduced grooming. In the late radiotherapy group (24 h post CED), no signs of toxicities were observed, except for a 4 % reduction in body weight.
Discussion
Distribution of drugs delivered by CED depends on the nature of drugs and their interactions with the brain extracellular environment [28]. Addition of the polymer polyethylene glycol (PEG) on the surface of liposomes is expected to reduce the binding of liposomes to cells, thus allowing for a greater distribution volume, slow release of the loaded drugs, all the while further reducing acute toxicity [29–31]. The expected benefits of pegylated liposomes were confirmed in our study since, at the same 30 μg of dose, less toxicity related symptoms were observed in rats treated with Lipoxal™ than those treated with oxaliplatin. Our results also support that higher concentration of Lipoxal™ was distributed outside the tumor volume. Indeed, the concentration of Lipoxal™ in the upper left part of brain tissue was more than 11 times higher than the one of oxaliplatin, although the infusion dose of Lipoxal™ was only 3 times that of oxaliplatin. Although it is distributed in a larger volume, the MTD of Lipoxal™ was better than that of oxaliplatin. These results corroborate with those obtained in other studies, where these drugs were delivered by i.v. [7, 17]. In human, the MTD of oxaliplatin was estimated at 200 mg/m2 or less, while that of Lipoxal™ at 300 mg/m2 [32, 33]. It was suggested that pegylated liposomes allow a stealthing from macrophages and immune cells in blood circulation increasing the circulation time, and reducing the systemic toxicity of the drug [32].
The MeST and tumor uptake of oxaliplatin and Lipoxal™ delivered by CED were compared with our previously published data on i.v. delivery of these platinium drugs using the same animal model in Table 1 [7]. CED of oxaliplatin significantly increased the MeST of F98 glioma-bearing rats by 7.5 days, while i.v. administration of this same agent did not. This was explained by the drug delivery issue across the BBB, resulting to a 47 times higher accumulation of oxaliplatin in the tumor area when injected by CED (Table 1). Similarly to oxaliplatin, Lipoxal™ delivered by i.v. did not improve the MeST, and tumoral uptake was low [7]; while Lipoxal™ CED allowed to increase by 200-fold its concentration in tumor and increased the MeST by 7 days.
Although encouraging, those results are not as good as those we reported with carboplatin or cisplatin CED, or those reported by the teams of Elleaume and Barth [10–15, 25]. However, due to the differential binding of DNA damage-recognition proteins on the DNA platinum adducts and differential replicative bypass [33], oxaliplatin do not express the cross-resistance to cisplatin in cisplatin resistant L1210 subline in vitro and in vivo [34]. Thus, even though it appears less effective in this specific glioma model, it remains another viable option for patients with glioma. On the other hand, the liposomal formulation of oxaliplatin (Lipoxal™) seems more promising than the one of cisplatin (Lipoplatin™). While Lipoxal™ was less toxic and shown a similar to antitumor potential than oxaliplatin, the liposomal formulation Lipoplatin™ has hampered the therapeutic effect of cisplatin without reducing its toxicity to normal brain tissue [25].
Several in vitro and in vivo studies have been carried out regarding radiosensitization by platinum drugs [20–23, 35]. In particular, Tippayamontri et al. found that tumor growth retardation was more significant when drug and radiation were combined at the time of maximum amount of platinum-DNA adducts formation in the cancer cells [22, 35]. For example, tumoral platinum-DNA adducts in colorectal tumor bearing mice was highest 48 h after i.v. delivery of oxaliplatin. Tumor irradiation at this time point after chemotherapy have resulted in the best anti-tumor response [22]. Time necessary to reach five-times the initial tumor volume (5Td) was 39 days, while that at 24 h post-chemotherapy, when the amount of tumoral platinum-DNA adducts was the lowest, was only 23 days [22]. In the present study, tumoral platinum-DNA adducts significantly decreased from 4 to 24 h after CED delivery of oxaliplatin. Radiotherapy performed at 4 h after CED delivery of oxaliplatin slightly increased the MeST compared to radiation performed at 24 h after, a difference that did not reach significance. Moreover, the 4 h radiotherapy group sustained more toxicity than the 24 h treatment group. This higher toxicity could be due to either the higher concomitant effect of CED chemotherapy and radiotherapy or simply because the combined radiation-CED induced edema was more prominent at 4 h after than 24 h after radiotherapy.
The tumor platinum-DNA adducts for Lipoxal™ decreased from 4 to 24 h after CED but interestingly bounced back at 48 h after CED. Lipoxal™ interacts with cells through two main pathways: fusion and endocytosis [36]. The liposome formulation of Lipoxal™ contains 1,2-dipalmitoyl-sn-glycero-3-phosphatidylglycerol (DPPG), a fusogenic lipid. It can fuse liposomes to the cell membrane, and directly release oxaliplatin to the cytoplasm. This can happen as early as 5 min after incubation, as shown in a fluorescent-labeled Lipoxal™ study in MCF-7 breast cancer cells [36]. This can explain why the 4 h Lipoxal™ group exhibited a high amount of platinum-DNA adducts. Subsequently, it is expected that the DNA repair system removes the platinum-DNA adducts reducing the level of oxaliplatin bound to DNA, as we observed in the 24 h group. On the other hand, Lipoxal™ can also enter the cell by endocytosis. The Lipoxal™ is engulfed by the cell membrane and processed to endosomes and lysosomes, then released oxalipaltin into the cytoplasm. This process takes 4–24 h, thus possibly explaining this biphasic uptake pattern [36]. Indeed, after its release in the cytoplasm, oxalipaltin then presumably enters the nucleus and causes a second, delayed increase in the amount of platinum-DNA adducts at 48 h. The combination of these 2 mechanisms could thereby explain the bi-phasic increase in the amount of platinum-DNA adducts.
In a previous study, we have reported a similar biphasic accumulation of platinum-DNA adducts in the colorectal tumor HCT116 after injecting the liposomal formulation of cisplatin (Lipoplatin™) [35]. Irradiation at the second increase of platinum-DNA adducts at 48 h post-injection has improved the control of tumor growth, compared to 24 h post-injection where platinum-DNA adducts was lower. In a subsequent study, it should be determined whether a similar finding could be found with the present brain tumor model. It is expected that brain tumor irradiation at 48 h after CED of Lipoxal™ would significantly increase the MeST, while leading to less toxicity since the level of platinum-DNA adducts in normal brain tissue is lower than at 24 h post-CED.
In conclusions, CED of oxaliplatin or its liposomal formulation Lipoxal™ led to higher tumoral accumulation of these drugs than obtained after i.v. delivery, and resulted in an increase of the median survival time of F98 glioma-bearing Fisher rats. The liposomal encapsulation of oxaliplatin reduced its toxic, while maintaining its antitumor potential.
References
Price TJ, Segelov E, Burge M, et al. (2013) Current opinion on optimal treatment for colorectal cancer. Expert Rev Anticancer Ther 13:597–611
Raez LE, Kobina S, Santos ES (2010) Oxaliplatin in first-line therapy for advanced non-small-cell lung cancer. Clin Lung Cancer 11:18–24
Okusaka T, Ikeda M, Fukutomi A, et al. (2014) Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci 105:1321–1326
Fouladi M, Blaney SM, Poussaint TY, et al. (2006) Phase II study of oxaliplatin in children with recurrent or refractory medulloblastoma, supratentorial primitive neuroectodermal tumors, and atypical teratoid rhabdoid tumors: a pediatric brain tumor consortium study. Cancer 107:2291–2297
Beaty O, Berg S, Blaney S, et al. (2010) A phase II trial and pharmacokinetic study of oxaliplatin in children with refractory solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer 55:440–445
Hartmann C, Weinel P, Schmid H, et al. (2011) Oxaliplatin, irinotecan, and gemcitabine: a novel combination in the therapy of progressed, relapsed, or refractory tumors in children. J Pediatr Hematol Oncol 33:344–349
Charest G, Sanche L, Fortin D, et al. (2013) Optimization of the route of platinum drugs administration to optimize the concomitant treatment with radiotherapy for glioblastoma implanted in the Fischer rat brain. J Neuro-Oncol 115:365–373
Fortin D, Desjardins A, Benko A, et al. (2005) Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in malignant brain tumors: the sherbrooke experience. Cancer 103:2606–2615
Bobo RH, Laske DW, Akbasak A, et al. (1994) Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A 91:2076–2080
Biston M-C, Joubert A, Adam J-F, et al. (2004) Cure of fisher rats bearing radioresistant F98 glioma treated with cis-Platinum and irradiated with monochromatic synchrotron X-rays. Cancer Res 64:2317–2323
Rousseau J, Boudou C, Barth RF, et al. (2007) Enhanced survival and cure of F98 glioma-bearing rats following intracerebral delivery of carboplatin in combination with photon irradiation. Clin Cancer Res 13:5195–5201
Rousseau J, Barth RF, Moeschberger ML, Elleaume H (2009) Efficacy of intracerebral delivery of carboplatin in combination with photon irradiation for treatment of F98 glioma-bearing rats. Int J Radiat Oncol Biol Phys 73:530–536
Rousseau J, Barth RF, Fernandez M, et al. (2010) Efficacy of intracerebral delivery of cisplatin in combination with photon irradiation for treatment of brain tumors. J Neuro-Oncol 98:287–295
Yang W, Huo T, Barth RF, et al. (2011) Convection enhanced delivery of carboplatin in combination with radiotherapy for the treatment of brain tumors. J Neuro-Oncol 101:379–390
Yang W, Barth RF, Huo T, et al. (2014) Radiation therapy combined with intracerebral administration of carboplatin for the treatment of brain tumors. Radiat Oncol 9:25
Pasetto LM, D’Andrea MR, Rossi E, Monfardini S (2006) Oxaliplatin-related neurotoxicity: how and why? Crit Rev Oncol Hematol 59:159–168
Boulikas T, Pantos A, Bellis E, Christofis P (2007) Designing platinum compounds in cancer : structures and mechanisms. Cancer Ther 5:537–583
Walker MD, Green SB, Byar DP, et al. (1980) Randomized comparisons of radiotherapy and nitrosoureas for the treatment of maligant glioma after surgery. N Engl J Med 303:1323–1329
Stupp R, Mason WP, van den Bent MJ, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Charest G, Paquette B, Fortin D, et al. (2010) Concomitant treatment of F98 glioma cells with new liposomal platinum compounds and ionizing radiation. J Neuro-Oncol 97:187–193
Tippayamontri T, Kotb R, Paquette B, Sanche L (2012) Synergism in concomitant chemoradiotherapy of cisplatin and oxaliplatin and their liposomal formulation in the human colorectal cancer HCT116 model. Anticancer Res 32:4395–4404
Tippayamontri T, Kotb R, Paquette B, Sanche L (2014) New therapeutic possibilities of combined treatment of radiotherapy with oxaliplatin and its liposomal formulations (Lipoxal™) in colorectal cancer using nude mouse xenograft. Anticancer Res 5312:5303–5312
Rezaee M, Hunting DJ, Sanche L (2013) New insights into the mechanism underlying the synergistic action of ionizing radiation with platinum chemotherapeutic drugs: the role of low-energy electrons. Int J Radiat Oncol Biol Phys 87:847–853
Mathieu D, Lecomte R, Tsanaclis AM, et al. (2007) Standardization and detailed characterization of the syngeneic Fischer/F98 glioma model. Can J Neurol Sci 34:296–306
Shi M, Fortin D, Sanche L, Paquette B (2015) Convection-enhancement delivery of platinum-based drugs and LipoplatinTM to optimize the concomitant effect with radiotherapy in F98 glioma rat model. Investig New Drugs 33:555–563
Korn EL, Midthune D, Chen TT, et al. (1994) A comparison of two phase I trial designs. Stat Med 13:1799–1806
Ausubel FM, Brent R, Kingston RE, et al. (1994) Current protocols in molecular biology. John Wiley and sons, New York
Allard E, Passirani C, Benoit J-P (2009) Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials 30:2302–2318
Saito R, Krauze MT, Noble CO, et al. (2006) Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neuro-Oncology 8:205–214
Perlstein B, Ram Z, Daniels D, et al. (2008) Convection-enhanced delivery of maghemite nanoparticles: increased efficacy and MRI monitoring. Neuro Oncol 10:153–6131
Raymond E, Chaney SG, Taamma A, Cvitkovic E (1998) Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol 9:1053–1071
Stathopoulos GP, Boulikas T, Kourvetaris A, Stathopoulos J (2006) Liposomal oxaliplatin in the treatment of advanced cancer: a phase I study. Anticancer Res 26:1489–1493
Wang D, Lippard SJ (2005) Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4:307–320
Tashiro T, Kawada Y, Sakurai Y, Kidani Y (1989) Antitumor activity of a new platinum complex, oxalato (trans-l-1,2-diaminocyclohexane)platinum (II): new experimental data. Biomed Pharmacother 43:251–260
Tippayamontri T, Kotb R, Paquette B, Sanche L (2013) Efficacy of cisplatin and Lipoplatin™ in combined treatment with radiation of a colorectal tumor in nude mouse. Anticancer Res 33:3005–3014
Stathopoulos GP, Boulikas T (2012) Lipoplatin formulation review article. J Drug Deliv 2012:581363
Acknowledgments
We would like to thank Dr. T. Boulikas for generously providing Lipoxal™, Dr. A-M Crous-Tsanaclis for her assistance in reviewing the histological samples and Dr. A.D. Bass for helpful suggestions and corrections.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
none.
Funding
This work was supported by Canadian Institutes of Health Research (grant # MOP 81356). David Fortin, Léon Sanche and Benoit Paquette are members of the Centre de Recherche du CHUS supported by the Fonds de la Recherche du Québec en Santé.
Rights and permissions
About this article
Cite this article
Shi, M., Fortin, D., Paquette, B. et al. Convection-enhancement delivery of liposomal formulation of oxaliplatin shows less toxicity than oxaliplatin yet maintains a similar median survival time in F98 glioma-bearing rat model. Invest New Drugs 34, 269–276 (2016). https://doi.org/10.1007/s10637-016-0340-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0340-0