Abstract
Purpose
Primary central nervous system diffuse large B-cell lymphoma (CNS-DLBCL) is a distinct clinicopathological entity with a poor prognosis. Concurrent MYC and BCL2 overexpression predicts inferior prognosis in systemic DLBCL, although their prognostic significance remains unclear in primary CNS-DLBCL.
Methods
Pretreatment diagnostic biopsy samples were retrospectively evaluated for 79 patients with primary CNS-DLBCL who were treated between January 2001 and December 2017. Histological and immunohistochemical testing were performed to evaluate the patients’ statuses for various markers, which were also evaluated for associations with survival outcomes.
Results
According to the Hans criteria, 26 patients (32.9%) had the germinal center B-cell subtype and 53 patients (67.1%) had the activated B-cell subtype. Forty-one cases (51.9%) were positive for MYC (expression of ≥ 40%), 33 cases (41.8%) were positive for BCL2 (expression of ≥ 70%), 22 patients (27.8%) were positive for both MYC and BCL2, and 27 patients (34.2%) were negative for both MYC and BCL2. There were no significant differences in survival between the germinal center and activated B-cell subtypes. Furthermore, MYC positivity was not associated with overall survival (p = 0.369) or progression-free survival (PFS) (p = 0.253). However, BCL2 positivity was significantly associated with poor overall survival (p = 0.039) and PFS (p = 0.036). Co-expression of MYC and BCL2 was not associated with survival.
Conclusion
Our data suggest that evaluating BCL2 expression may help predict the prognosis in cases of primary CNS-DLBCL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system diffuse large B-cell lymphoma (CNS-DLBCL) is an aggressive non-Hodgkin’s lymphoma that affects the brain or spinal cord without evidence of systemic involvement at the diagnosis. However, primary CNS-DLBCL is rare and only accounts for < 1% of all non-Hodgkin’s lymphomas and approximately 3% of all primary brain tumors [1]. Compared to systemic DLBCL, CNS-DLBCL has a poorer prognosis and it remains unclear whether this is related to the tumor’s location, the microenvironment, an inherently aggressive biology, or a combination of those factors. The cellular origin of CNS-DLBCL is thought to be a late germinal center B-cell (GCB), based on results from immunophenotypic analysis, gene expression profiling, and molecular characterization of the immunoglobulin genes [2, 3]. In addition, the GCB-like subtype of DLBCL is thought to have a better prognosis than the activated B-cell (ABC)-like subtype [4, 5]. However, most primary CNS-DLBCLs involve the ABC subtype, which may explain the poor prognosis of primary CNS-DLBCL [6].
The MYC gene has recently been shown to be translocated in 5–15% of systemic DLBCLs. This genetic alteration worsens the response to chemotherapy and prognosis, especially when it occurs with concomitant BCL2 and/or BCL6 translocations (double-hit and triple-hit lymphomas) [7,8,9,10]. Furthermore, recent studies have demonstrated that immunohistochemical detection of high MYC and BCL2 protein expressions (double expressing lymphomas) is associated with an aggressive behavior and inferior overall survival (OS), regardless of any MYC and BCL2 rearrangements [11,12,13,14,15]. However, these proteins’ relevance remains unclear in primary central nervous system lymphoma (PCNSL) without systemic involvement. Moreover, one large multicenter analysis revealed that the prognostic value of the cell of origin (COO) was lost after adjusting for double expressing lymphomas, which suggests that immunohistochemical detection of MYC and BCL2 co-expression was a more reliable prognostic factor among patients with DLBCL [12]. The present study used immunohistochemistry to evaluate the expressions of the MYC and BCL2 proteins, as well as their prognostic value relative to the COO concept, in CNS-DLBCL.
Materials and methods
Patients
Pretreatment diagnostic biopsy samples were retrospectively retrieved for 79 immunocompetent patients with newly diagnosed PCNSL who were treated between January 2001 and December 2017. The treatment involved induction therapy using a cycle of high-dose methotrexate (MTX) at 3.5 g/m2 on days 1, 22, and 43, which was followed by radiotherapy as previously described [16]. All patients had provided written informed consent for their treatment, and this study’s retrospective protocol was approved by our institutional ethics review board.
Histological and immunohistochemical testing
Tissue samples were fixed in formaldehyde and embedded in paraffin using standard methods. Sections were stained using hematoxylin and eosin before the immunohistochemical analysis. Routine deparaffinization, rehydration, and blocking of endogenous peroxidase activity were performed before the slide-mounted sections were immersed in 0.01 M sodium citrate buffer (pH 6.0) and heated at maximum power for 15 min in a 700-W microwave oven.
Immunohistochemical staining was performed for CD20 (L26; 1:200), CD10 (56C6; 1:25), and BCL6 (LN22; 1:100) using antibodies from Leica Biosystems (Wetzler, Germany). In addition, staining was performed for MUM-1 (MUM1p; 1:50), BCL2 (124; 1:200), and Ki-67 (MIB1; 1:50) using antibodies from DAKO (CA, USA). Staining for c-MYC (Y69; 1:200) was performed using antibodies from Abcam (Cambridge, UK). The staining pattern for MYC was distinctly nuclear and the staining pattern for BCL2 was clearly cytoplasmic. The cutoff values for positivity were defined as ≥ 40% for MYC and ≥ 70% for BCL2, as previously described [11]. The cutoff values for positivity were defined as ≥ 30% for CD10, BCL6, and MUM1. Cases were assigned to the GCB group based on positive results for CD10 or to the ABC group (non-GCB) based on negative results for CD10 and BCL6. If the results were negative for CD10 and positive for BCL6, the case was assigned to the GCB group if there was a negative result for MUM1 and to the ABC group if there was a positive result for MUM1 [17].
Statistical analysis
The survival intervals were calculated from the diagnosis to death or the last follow-up for OS and from the diagnosis to the first instance of disease progression, relapse, or death for progression-free survival (PFS). Survival curves were created using the Kaplan–Meier method and analyzed using the log-rank test and GraphPad Prism software (GraphPad Software Inc., San Diego, USA). The analyzed variables included patient age and sex, Karnofsky performance status (KPS) at admission, the number of lesions, and the expression statuses for MYC and/or BCL2. The Memorial Sloan Kettering Cancer Center prognostic score (MSKCC) was also calculated based on age and KPS [18]. All other statistical analyses were performed using StatView software (version 5; Abacus Concepts Inc., Berkeley, USA) and p-values of < 0.05 were considered statistically significant.
Results
The clinical features of the 79 patients with PCNSL are listed in Table 1. The median age was 68 years (range 41–86 years), the median KPS at admission was 40% (range 20–100%), and the median follow-up was 30.3 months (range 0.4–159.3 months). All patients were treated using high-dose MTX-based chemotherapy and achieved median OS and PFS values of 42.4 and 6.5 months, respectively. However, no significant differences in survival were observed according to the various clinical features, such as age, sex, KPS, number of lesions, and Memorial Sloan Kettering Cancer Center class (Table 2).
All cases had diffuse large cell morphology and were immunohistochemically positive for CD20, which indicated that all cases involved diffuse large B-cell lymphomas. The patients were subsequently grouped according to their immunohistochemical expressions of CD10, BCL6, and MUM1, with 26 patients (32.9%) having the GCB subtype and 53 patients (67.1%) having the ABC subtype (Table 1). No significant differences in OS and PFS were observed between the two subtypes (Table 2).
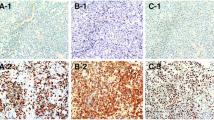
Immunohistochemical testing for the MYC and BCL2 proteins revealed that 41 patients (51.9%) were positive for MYC and 33 patients (41.8%) were positive for BCL2 (Table 2). Twenty-two patients (27.8%) were positive for both MYC and BCL2, while 27 patients (34.2%) were negative for both MYC and BCL2. Figure 1 shows the immunohistochemical findings from a representative case involving the GCB subtype. Figure 2 shows that univariate analyses of BCL2 expression revealed significant associations with poor OS (31.8 vs. 78.7 months; p = 0.039) and poor PFS (5.2 vs. 6.5 months; p = 0.036). However, MYC expression with or without BCL2 co-expression did not significantly affect OS (p = 0.369 and p = 0.136, respectively) or PFS (p = 0.253 and p = 0.056, respectively) (Table 2). The significant factors in the univariate analyses were included in the multivariate analyses of OS and PFS (Table 3), which revealed that BCL2 positivity independently affected OS (hazard ratio [HR]: 3.137, p = 0.016) but not PFS (HR: 2.134, p = 0.071).
A representative case of the GCB subtype. Homogenous enhancement of the lesion in the splenium of the corpus callosum during magnetic resonance imaging with gadolinium-DTPA (a). Diffusely infiltrating large neoplastic lymphocytes (b). Positive staining for CD20 (c). A high proliferative rate was detected via staining for MIB-1 (d). Negative staining for CD10 (e). Positive staining for BCL6 (f). Negative staining for MUM1 (g). Positive staining for MYC (h). Positive staining for BCL2 (i). Panels (b–i) are shown at ×200 magnification
Discussion
The present study evaluated the patients’ immunochemical statuses for MYC and BCL2, as well as the COO concept, to better predict the prognosis of primary CNS-DLBCL. Our results indicate that BCL2 expression had significant value for predicting both OS and PFS. However, MYC expression, co-expression of MYC and BCL2, the COO concept, and traditional prognostic factors (e.g., age and KPS) did not predict survival among our patients. Patient age and KPS have been suggested as prognostic factors and strongly influence the therapeutic decisions for PCNSL. For example, Abrey et al. [18] proposed the MSKCC model and indicated that patients who were < 50 years old had improved OS and PFS. However, we did not identify significant associations of these prognostic factors and the MSKCC score with our patients’ outcomes. Our previous study evaluated treatment results in patients with PCNSL and examined various prognostic factors including the MSKCC score [16]. That study revealed that completion of three cycles of high-dose MTX chemotherapy, rather than the traditional prognostic factors (e.g., age and KPS), independently predicted better OS relative to patients who did not complete three cycles of chemotherapy (56.4 vs. 24.0 months, p = 0.013). In this context, BCL2 is a known antiapoptotic molecule and its expression might be related to the response to MTX chemotherapy. The present study included 50 patients who completed three cycles of chemotherapy and experienced marginally better OS than patients who did not complete the chemotherapy (51.1 vs. 26.1 months, p = 0.071). Interestingly, the completion rates were similar among patients with and without BCL2 expression (63.6 and 63.1%, respectively). Thus, although we did not identify a significant relationship between BCL2 expression and response to high-dose MTX, we believe that further studies are needed to investigate this potential relationship.
The COO concept is based on gene expression profiles and is considered a strong prognostic factor for systemic DLBCL, with the COO subtypes having different responses to various therapeutic agents [19, 20]. In addition, several reports have indicated that the COO concept might predict prognosis among patients with DLBCL who underwent chemotherapy [17, 21, 22]. However, a few recent studies have indicated that COO defined using immunochemistry could not predict the prognosis of DLBCL [23, 24], which may be related to the immunohistochemical detection of COO producing inconsistent results among patients with primary CNS-DLBCL, similar to among patients with systemic DLBCL. Most primary CNS-DLBCLs are the non-GCB subtype, with Lin et al. reporting that 40 of 51 primary CNS-DLBCL cases (78%) involved the non-GCB subtype, while only 11 cases (22%) involved the GCB subtype [25]. Similarly, Hattab et al. found that 26 of 31 patients (84%) with primary CNS-DLBCL had the non-GCB subtype [26], while Camilleri-Broët et al. reported that 80 of 83 patients (94.3%) with primary CNS-DLBCL had the non-GCB subtype [6]. The present study revealed that 26 patients had the GCB subtype and that 53 patients (67.1%) had the ABC/non-GCB subtype, which is consistent with the findings of the previous reports. Interestingly, Camilleri-Broët et al. suggested that the poor prognosis of primary CNS-DLBCL might be partly related to its ABC subtype [6], which may indicate that the GCB subtype is associated with a better prognosis. However, several reports have described no significant difference in survival when the two subtypes were compared among patients with primary CNS-DLBCL [26,27,28]. Similarly, we failed to detect significant differences in OS and PFS when we compared the two subtype groups.
Double-hit lymphomas are rare (3–5% of DLBCL cases) and have chromosomal breaks involving MYC and BCL2 [12, 29]. In addition, DLBCL with co-rearrangement of MYC and BCL2 has poor OS and PFS outcomes [12, 29], while co-expression of MYC and BCL2 (double expressing lymphomas) is associated with a poor prognosis in systemic DLBCL, which is independent of the COO subtype [12, 13, 30]. Several studies have also examined co-expression and co-rearrangement of MYC and BCL2 in primary CNS-DLBCL. Although MYC expression in primary CNS-DLBCL significantly exceeds that in systemic DLBCL, MYC rearrangement is similar for primary CNS-DLBCL and systemic DLBCL [31]. Therefore, the rarity of MYC rearrangement and the high frequency of MYC overexpression is an interesting phenomenon that may be attributed to other genetic aberrations, such as MYC mutations or altered regulation of expression.
The reported rates of MYC expression are 70–90% for CNS-DLBCL [27, 31, 32], which are higher than the reported rates for systemic DLBCL [12, 13]. Although high MYC expression reportedly predicts poor OS and/or PFS for CNS-DLBCL [32,33,34], our results revealed no significant relationship between MYC expression and survival (OS: p = 0.369, PFS: p = 0.253) and similar results have also been reported [27]. Other reports have indicated that the rates of BCL2 expression are 59–73% for PCNSL [28, 32,33,34], with Shi et al. and Kim et al. reporting that high BCL2 expression was significantly associated with poor OS [32, 33], although other studies failed to detect an association with prognosis [28, 34]. The present study revealed a slightly lower BCL2 expression rate (41.8%), although we found that high BCL2 expression was significantly associated with poor OS and PFS.
Interestingly, co-expression of MYC and BCL2 in primary CNS-DLBCL did not have prognostic value, which conflicts with Guo et al.’s report that MYC expression and co-expression of MYC and BCL2 were significantly associated with outcomes among patients with primary CNS-DLBCL [35]. Moreover, Shi et al. investigated 77 patients with primary CNS-DLBCL and reported that co-expression of MYC and BCL2 strongly predicted the prognosis for primary CNS-DLBCL, independent of the COO concept [32]. Kim at al. have also reported that patients with primary CNS-DLBCL co-expressing MYC and BCL2 experienced poor PFS outcomes in the MTX-treated group [33]. In contrast, Gill et al. reported that expression of MYC and/or BCL2 was not associated with survival outcomes [27]. The present study revealed co-expression of MYC and BCL2 in 27.8% of cases, although this factor was not significantly associated with OS or PFS. These discrepancies may be related to the use of different antibodies and immunohistochemical cutoff values. For example, Kim et al. used antibodies against MYC (EP121, Cell Marque) and BCL2 (124, DAKO) with cutoff values of ≥ 40% for MYC and ≥ 30% for BCL2 [33], which generated positivity rates of 18.1% for MYC and 75% for BCL2 that predicted survival outcomes. In contrast, Gill et al. used different antibodies against MYC (9E10, Epitomics Inc.) and BCL2 (bcl-2/100/D5, Novocastera) with cutoff values of ≥ 40% for MYC and ≥ 70% for BCL2 [27], which generated positivity rates of 73% for MYC and 71% for BCL2 that did not predict survival outcomes. Moreover, Guo et al. and Shi et al. used different antibodies against MYC (Y69, Abcam) and BCL2 (124, DAKO) with cut-off values of 33.3 and 78% for MYC and 81.8 and 61% for BCL2 [32, 35]. Both studies used a ≥ 40% cutoff value for MYC, which agreed with previous studies, although the cutoff values for BCL2 that were 30 or 70% higher than in the other studies. Interestingly, Guo et al. only detected significant prognostic value for MYC expression, while Shi et al. detected significant prognostic value for both MYC and BCL2 expression. The present study used the same antibodies (Y69 for MYC and 124 for BCL2) with cutoff values of ≥ 40% for MYC and ≥ 70% for BCL2. The positivity rates were 51.9% for MYC and 41.8% for BCL2, but only BCL2 had significant prognostic value. Therefore, it is important to be aware of the specific antibodies and cutoff values that are being used to examine the prognostic values of these markers.
In conclusion, the present study revealed that BCL2 expression had good prognostic value, independent of the COO concept, among patients with primary CNS-DLBCL. The prognostic value of BCL2 is especially important in the era of targeted therapy, as this expression may predict a good response to anti-BCL2 targeted drugs.
References
Gerstner ER, Batchelor TT (2010) Primary central nervous system lymphoma. Arch Neurol 67:291–297
Montesinos-Rongen M, Brunn A, Bentink S et al (2008) Gene expression profiling suggests primary central nervous system lymphomas to be derived from a late germinal center B cell. Leukemia 22:400–405
Deckert M, Montesinos-Rongen M, Brunn A, Siebert R (2014) Systems biology of primary CNS lymphoma: from genetic aberrations to modeling in mice. Acta Neuropathol 127:175–188
Rosenwald A, Wright G, Chan WC et al (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346:1937–1947
Alizadeh AA, Eisen MB, Davis RE et al (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511
Camilleri-Broet S, Criniere E, Broet P et al (2006) A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood 107:190–196
Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, Roman E, Jack A (2010) Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 28:3360–3365
Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, Horsman DE, Gascoyne RD (2009) MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood 114:3533–3537
Johnson NA, Savage KJ, Ludkovski O et al (2009) Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood 114:2273–2279
Snuderl M, Kolman OK, Chen YB et al (2010) B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol 34:327–340
Green TM, Young KH, Visco C et al (2012) Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30:3460–3467
Hu S, Xu-Monette ZY, Tzankov A et al (2013) MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 121:4021–4031
Johnson NA, Slack GW, Savage KJ et al (2012) Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30:3452–3459
Horn H, Ziepert M, Becher C et al (2013) MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 121:2253–2263
Valera A, Lopez-Guillermo A, Cardesa-Salzmann T et al (2013) MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica 98:1554–1562
Makino K, Nakamura H, Hide T, Kuroda J, Yano S, Kuratsu J (2015) Prognostic impact of completion of initial high-dose methotrexate therapy on primary central nervous system lymphoma: a single institution experience. Int J Clin Oncol 20:29–34
Hans CP, Weisenburger DD, Greiner TC et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282
Abrey LE, Ben-Porat L, Panageas KS et al (2006) Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 24:5711–5715
Roschewski M, Staudt LM, Wilson WH (2014) Diffuse large B-cell lymphoma-treatment approaches in the molecular era. Nat Rev Clin Oncol 11:12–23
Lenz G, Wright G, Dave SS et al (2008) Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 359:2313–2323
Berglund M, Thunberg U, Amini RM et al (2005) Evaluation of immunophenotype in diffuse large B-cell lymphoma and its impact on prognosis. Mod Pathol 18:1113–1120
van Imhoff GW, Boerma EJ, van der Holt B, Schuuring E, Verdonck LF, Kluin-Nelemans HC, Kluin PM (2006) Prognostic impact of germinal center-associated proteins and chromosomal breakpoints in poor-risk diffuse large B-cell lymphoma. J Clin Oncol 24:4135–4142
Visco C, Li Y, Xu-Monette ZY et al (2012) Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Leukemia 26:2103–2113
Nyman H, Adde M, Karjalainen-Lindsberg ML, Taskinen M, Berglund M, Amini RM, Blomqvist C, Enblad G, Leppa S (2007) Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood 109:4930–4935
Lin CH, Kuo KT, Chuang SS, Kuo SH, Chang JH, Chang KC, Hsu HC, Tien HF, Cheng AL (2006) Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res 12:1152–1156
Hattab EM, Martin SE, Al-Khatib SM, Kupsky WJ, Vance GH, Stohler RA, Czader M, Al-Abbadi MA (2010) Most primary central nervous system diffuse large B-cell lymphomas occurring in immunocompetent individuals belong to the nongerminal center subtype: a retrospective analysis of 31 cases. Mod Pathol 23:235–243
Gill KZ, Iwamoto F, Allen A, Hoehn D, Murty VV, Alobeid B, Bhagat G (2014) MYC protein expression in primary diffuse large B-cell lymphoma of the central nervous system. PLoS ONE 9:e114398
Liu J, Wang Y, Liu Y, Liu Z, Cui Q, Ji N, Sun S, Wang B, Wang Y, Sun X, Liu Y (2017) Immunohistochemical profile and prognostic significance in primary central nervous system lymphoma: analysis of 89 cases. Oncol Lett 14:5505–5512
Niitsu N, Okamoto M, Miura I, Hirano M (2009) Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia 23:777–783
Perry AM, Alvarado-Bernal Y, Laurini JA et al (2014) MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. Br J Haematol 165:382–391
Brunn A, Nagel I, Montesinos-Rongen M et al (2013) Frequent triple-hit expression of MYC, BCL2, and BCL6 in primary lymphoma of the central nervous system and absence of a favorable MYC(low)BCL2 (low) subgroup may underlie the inferior prognosis as compared to systemic diffuse large B cell lymphomas. Acta Neuropathol 126:603–605
Shi QY, Feng X, Bao W, Ma J, Lv JH, Wang X, Rao Q, Shi QL (2017) MYC/BCL2 Co-expression is a stronger prognostic factor compared with the cell-of-origin classification in primary CNS DLBCL. J Neuropathol Exp Neurol 76:942–948
Kim S, Nam SJ, Kwon D, Kim H, Lee E, Kim TM, Heo DS, Park SH, Kim CW, Jeon YK (2016) MYC and BCL2 overexpression is associated with a higher class of Memorial Sloan-Kettering Cancer Center prognostic model and poor clinical outcome in primary diffuse large B-cell lymphoma of the central nervous system. BMC Cancer 16:363
Tapia G, Baptista MJ, Munoz-Marmol AM et al (2015) MYC protein expression is associated with poor prognosis in primary diffuse large B-cell lymphoma of the central nervous system. APMIS 123:596–603
Guo S, Bai Q, Rohr J, Wang Y, Liu Y, Zeng K, Yu K, Zhang X, Wang Z (2016) Clinicopathological features of primary diffuse large B-cell lymphoma of the central nervous system - strong EZH2 expression implying diagnostic and therapeutic implication. APMIS 124:1054–1062
Acknowledgements
We thank Ms. Masayo Obata for her histological assistance.
Funding
This work was supported by Grants-in-Aid for Scientific Research(C) to KM (17K10870).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Makino, K., Nakamura, H., Shinojima, N. et al. BCL2 expression is associated with a poor prognosis independent of cellular origin in primary central nervous system diffuse large B-cell lymphoma. J Neurooncol 140, 115–121 (2018). https://doi.org/10.1007/s11060-018-2940-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2940-3