Abstract
Background
We analyzed WHO grade II meningioma cases to identify factors influencing survival.
Materials and methods
Between January 2000 and August 2015, 206 cases of World Health Organization (WHO) grade II meningioma were operated at our institution. This population underwent a total of 298 surgical resections and 55 patients received a radiotherapy. A Cox multivariate regression was conducted on clinical and histological criteria.
Results
Sixty-four patients were deceased (31.1 %), of which 38 died following the disease progression (18.4 %). Overall survival probability at 1, 5, and 10 years were 95.4 %, 95 % CI [92.5, 98.4]; 84 %, 95 % CI [78.3, 90.2], and 72.9 %, 95 % CI [64.5, 82.4], respectively (Fig. 1a). At the end of the study, only 87 patients (42.2 %) were alive with no tumor residual or recurrence on the last scan. Age at diagnosis (hazard ratio (HR) = 0.31, 95 % CI [0.15, 0.63], p < 0.001), extent of resection (HR = 0.25, 95 % CI [0.12, 0.49], p < 0.001), and tumoral brain invasion (HR = 0.49, 95 % CI [0.25, 0.98], p = 0.040) were independent factors associated with the overall survival. The patients who received radiotherapy did not demonstrate a longer overall survival (p = 0.540).
Conclusions
WHO grade II meningioma significantly impaired the survival of the patients. In the adjusted Cox regression, a macroscopic gross total resection (Simpson grades 1, 2, and 3), an age below 62 years at diagnosis and the absence of brain invasion were independent factors associated with a longer survival. Radiotherapy may not increase the overall survival after complete or incomplete resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas that are thought to arise from arachnoidal cap cells account for 13–26 % of intracranial tumors and are benign in about 90 % of cases [19]. The 2000 and revised 2007 WHO classification of tumors affecting the central nervous system recognizes three grades of meningiomas. The chordoid, the clear cell, and the most common atypical meningioma correspond to a WHO grade II classification. WHO grade III meningiomas are associated with aggressive growth patterns reflecting their clinical and histopathological features of malignancy and can spread by metastatic dissemination [5]. WHO grade I meningiomas occur most often in women and are associated with a relatively good outcome. The behavior and outcome of WHO grade II meningiomas are intermediate. Histopathologically, atypical meningiomas are tumors with increased mitotic activity with four mitoses or more per ten high power fields (HPF) and/or have at least three of the following characteristics: sheet-like growth, spontaneous necrosis, increased cellularity, prominent nucleoli, and small cells with a high nuclear-to-cytoplasmic ratio. The number of grade II meningiomas increased when using the WHO 2007 classification (30 %) compared to previous editions, mainly due to the definition of brain infiltrating meningiomas as atypical (grade II) [3]. Complete surgical excision is the treatment of choice in all types of meningiomas. Further optimal management in the case of grade II meningioma is difficult to establish.
Several studies addressed the usefulness of radiotherapy in the management of WHO grade II meningiomas but none were able to demonstrate a significant improvement in any of the clinical outcomes [14]. The aim of this study was to investigate the clinical and histological prognostic factors that may be associated with overall survival and outcome of patients operated on for a WHO grade II meningioma.
Materials and methods
Clinical material
A retrospective neuropathology database search for meningioma was carried out at the Southern General Hospital, Glasgow. All patients with a diagnosis of WHO grade II/ atypical/ clear cell/ chordoid meningioma were included in this population-based series.
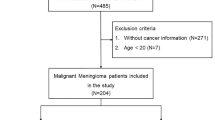
Inclusion criteria were: newly diagnosed meningioma between January 2000 and August 2015; a pathology diagnosis of grade II meningioma according to WHO 2000 or 2007 in use at time of surgery, including secondary WHO grade II meningioma that progressed from grade I. Exclusion criteria were inconclusive reports or ambiguous histology such as hemangiopericytoma.
Histology slides were not reviewed but all pathology reports were carefully examined. Cases diagnosed before 2007 were reclassified according to the last WHO grading system if necessary. Meningioma sub-type, mitoses count per ten high power fields (HPFs) also called mitotic index, Ki-67 index (MIB-1), presence of necrosis, brain invasion, architectural sheeting, and presence of psammoma bodies were separately extracted. In case of recurrence, histology reports were compared to those from previous resections.
Patient demographic and medical data were collected retrospectively. We used radiographic and surgical reports, and all available in- and out-patient records. Patients' CT and MRI images were studied pre and postoperatively. Tumor location was divided into ten categories.
Invasion of a venous sinus or its wall was separately noted for the four major sinuses. Volumes from the diameter method were calculated using the following formula to measure volumes of an ovoid object.
Methods
Age at diagnosis was defined according to the date of first surgery for a grade II meningioma. Surgical resection was evaluated according to the Simpson grading scale using the operative records and post-operative images [22]. We defined total resection (TR) as Simpson grades 1 and 2, gross total resection (MGTR) as Simpson grades 1, 2, and 3, and incomplete resection or subtotal resection (STR) as Simpson grades 4 and 5. If radiotherapy was given, data on the technique, overall dose, and time of completion after surgery were collected. We defined two types of recurrence. The first type was defined as a "surgical relapse", characterizing the patients who underwent a second surgical procedure for a WHO grade II meningioma recurrence (local control). The second type of recurrence was defined as a "radiological relapse" corresponding to radiological evidence of tumor regrowth in case of total resection, or to a residual tumor progressing or not, in case of incomplete resection (progression-free survival).
In case of death, the cause was searched and quoted differently if related or not to the surgery or the progressing meningioma disease.
A patient with no record for more than 2 years was considered as lost to follow-up. Patient outcome and clinical status were assessed through medical records, the patient database, and information obtained from general practitioners.
This retrospective study was conducted according to the ethical guidelines for epidemiological research in accordance with the ethical standards of the Helsinki Declaration.
Statistical analysis
Prior to modeling, the data were summarized with descriptive analysis including medians and inter-quartile ranges (IQR) for non-Gaussian distributed variables and frequencies for categorical variables. For exploratory purposes, an initial analysis of the data was performed using the Mann–Whitney–Wilcoxon test, χ2 and, Fisher’s exact tests to compare alive to dead patients, regardless of follow-up time. This allowed identification of potential prognostic factors. Survival statistics were based on time to death, which was measured from the age at diagnosis to the date of last follow-up or decease only if related to the meningioma surgery or progression. Survival function was assessed by the Kaplan–Meier method and the Mantel Cox log-rank test was used to compare different survival functions according to clinical and therapeutic factors. A univariate Cox regression was subsequently run. Independent prognostic factors with a p value < 0.20 were assessed with a multivariate stepwise Cox proportional regression model. We used Walds test, analysis of deviance, Akaike and Bayesian information criteria in the search of the best fitted model. Proportional hazard assumption was tested with Schoenfeld residuals. A p value < 0.05 was considered as statistically significant. In the survival analysis, some observations were automatically deleted due to missing of data, which were not imputed. For the analysis, we considered equally as radiotherapy any form of radiation therapy. Analyses were performed with the R programming language and software environment for statistical computing and graphics (R version 3.2.3 (2015-12-10)), the survival, the rms packages among others. The statistical program and workflow was written in R Markdown v2 with RStudio® for dynamic and reproducible research.
Results
Population description
Of the 206 cases collected, 95 patients were male (46.1 %). Median age at diagnosis was 57.2 years, IQR [45.5–67.9]. Seizures were the most frequent revealing symptom and the most common location was parafalcine meningioma, in 63 cases (34.1 %) (Table 1). There were four cases (1.9 %) of spinal grade II meningiomas. Seventeen patients (8.3 %) had initially a grade I meningioma that progressed to a grade II in a median time 5.7 years, IQR [2.7–8.5].
Concerning the past medical history, five patients had a type II neurofibromatosis and nine patients (6.5 %) had a presumed radiation-induced meningioma of whom four had had previous cranio-spinal radiotherapy for leukemia and five for radiation therapy for other types of previous brain tumors. Median follow-up was 4.1 years, IQR [1.6–7.3]. Only two patients were lost to follow-up.
Surgery
A total of 298 surgical resections was performed. Twenty-eight patients (13.6 %) had two surgical procedures and 24 (11.7 %) three or more; 156 patients (76.8 %) had a macroscopic gross total resection (MGTR). Forty-three patients (21.5 %) were re-operated for a relapse of their grade II meningioma. The median time between the first and second surgery was 4.2 years, IQR [1.4, 5.7]; 11.4 % of the patients experienced a postoperative infection, usually requiring a re-intervention for surgical site cleansing and infected bone flat removal; 7.1 % had a (titanium) cranioplasty inserted, mainly following an deep postoperative infection. Fourteen patients demonstrated a malignant transformation into a WHO grade III meningioma.
Radiotherapy
Fifty-one patients (26.6 %) received conventional radiotherapy. Twenty-two patients (11.2 %) had radiotherapy but were not re-operated on. The median delay between the WHO grade II meningioma surgery and the end of radiotherapy was 1 year, IQR [0.3–2.6]. Nine patients (4.7 %) received a stereotactic radiotherapy, mostly by Gamma Knife (median dose = 16 Gy), of which five had already had conventional radiotherapy. In the survival analysis, we considered as radiotherapy, whether conventional or stereotactic method (n = 55). Seventeen patients (36.2 %) had early adjuvant radiotherapy within the six postoperative months and 24 patients (31.9 %) received a late radiotherapy i.e., after the postoperative year; 21.8 % of the patients (n = 34) who had a MGTR underwent also radiotherapy compared to 42.6 % (n = 20) in the incomplete resection group. However, there is no statistical interaction between radiotherapy and completeness of resection (Wald test p value = 0.566). Therefore, radiotherapy is an independent predictor of overall survival.
Chemotherapy
Nine patients received conventional chemotherapy by hydroxycarbamide or somatostatin as palliative treatment without any remarkable efficiency. No one received newer targeted agents, such as MTOR inhibitors, bevacizumab, or sunitinib. Only the patient with tumor progression out of control received chemotherapy. Thus, chemotherapy is an independent predictor neither of the recurrence nor of the survival.
Outcome and survival analysis
Sixty-four patients were deceased (31.1 %), of which 38 died following the meningioma surgery or disease progression (18.4 %) (Fig. 1a). Overall survival probability at 1, 5, and 10 years were 95.4 %, 95 % CI [92.5, 98.4]; 91.09 %, 95 % CI [87, 95.39], 84 %, 95 % CI [78.3, 90.2] and 72.9 %, 95 % CI [64.5, 82.4], respectively (Figs. 1a and 2).
At the end of the study, only 87 patients (42.2 %) were alive with no tumor residual or recurrence on the last scan.
Univariate (unadjusted) Cox regression analysis performed on clinical and pathological criteria identified age at diagnosis, tumor volume, venous sinus invasion, completeness of resection, brain invasion and re-intervention for WHO grade II recurrence as predictive of the survival (Table 1). It suggested an association between previous surgery for a grade I meningioma, tumor location, and mitoses count that did not reach significance but did warrant inclusion in the subsequent multivariate analysis (Table 2). Age at diagnosis (HR = 0.31, 95 % CI [0.15, 0.63], p < 0.001), extent of resection (HR = 0.25, 95 % CI [0.12, 0.49], p < 0.001), and tumoral brain invasion (HR = 0.49, 95 % CI [0.25, 0.98], p = 0.040) were independent factors associated with the overall survival.
In the adjusted Cox regression, a gross total resection (Simpson grades 1, 2, and 3), an age below 62 years at WHO grade II meningioma diagnosis, and the absence of brain invasion were independent factors associated with a longer survival (Table 2 and Fig. 1b–d).
The patients who received radiotherapy did not demonstrate a longer overall survival (log-rank test p value = 0.540) (Table 2 and Fig. 1e, f).
Discussion
Despite its methodological limitations, this study on outcome and prognostic factors affecting the survival of WHO grade II meningioma is the largest series in the literature. We did not include any grade III meningiomas as it is well recognized that those two types of tumor behave very differently. Therefore, they should not be grouped together when assessing outcome and predictors [1, 7].
Predictors of survival
Age at diagnosis
The median age of 57.2 years at diagnosis is a prognostic factor of the survival (p value = 0.04). However, we found that the best statistical cut-off was 62 years (p < 0.001). Patients who are under 62 years old at WHO grade II surgery are less likely to die of their meningioma disease. This finding is consistent with previous reports where age at diagnosis has already been reported to be associated with the overall survival of grade II meningioma [7, 21, 27, 28]. Some authors have defined 65 years as the cut-off for a poor prognosis [7, 28]. Moreover, Aghi et al. found that older age was predictive of recurrence [2].
Surgery
Since the seminal publication of Simpson in 1957, there is general agreement about the importance of resection completeness, and it is clear that sub-totally removed meningiomas may continue to grow. The extent of resection (Simpson grading) is the most powerful prognostic factor for recurrence for all grades of meningiomas including grade II. GTR is associated with better local control than incomplete resection [8, 9, 12, 20, 28]. Surgical resection of grade II meningioma is not much more difficult compared to grade I. A Simpson grade I can still be achieved when the meningioma is located on the convexity. This becomes more difficult with para-sagittal meningiomas infiltrating a venous sinus wall. Invasive skull base meningiomas (e.g., petro clival) or those deeply infiltrating a venous sinus (tentorium cerebelli), cannot generally be removed completely without high risks of severe postoperative disabilities or stroke.
Being re-operated on for a grade II meningioma relapse impaired the survival in univariate analysis (HR = 2.04, Wald test p = 0.04) but not in multivariate (p = 0.27).
Histological brain invasion
The significance of brain invasion as a criterion of malignancy or determinant of poor survival has been controversial [25]. It has been traditionally considered to be indicative of malignancy but it was shown recently that brain-invasive meningiomas without anaplasia pursue a less aggressive course than histologically malignant meningiomas. In 2007, the WHO criteria were further modified to remove the automatic classification of tumors with brain invasion into the grade 3 category, thereby further increasing the proportion of grade 2 [15]. In some studies, brain invasion has been found to be associated with the recurrence risk or the progression-free survival [25, 27]. Its role as an independent predictor of overall survival has never been reported before. Yang et al. demonstrated a survival benefit with adjuvant radiotherapy for patients with atypical meningiomas only in the presence of brain invasion. This was not the case in our study (log-rank test p = 0.550).
Radiotherapy
Radiotherapy after surgical resection of WHO grade II meningiomas continues to be controversial. The studies addressing the usefulness of radiotherapy had a low level of evidence as no randomized clinical trials have been performed [9]. A particularly controversial management issue is the role of radiotherapy for WHO grade II meningioma treated with gross total resection. The treatment approach has largely been extrapolated from data on others meningioma grades, leading to non-uniform practices across institutions, adjuvant radiotherapy being used in many centers after subtotal resection of grade II meningioma [14, 17]. A total of 26.7 % of our patients received radiation therapy. This percentage is quite low compared to other series but still in previously reported ranges of 7.4–59.1 %. For grade II meningiomas, most neurosurgeons do not advocate adjuvant radiotherapy if the tumor is completely excised [16, 17]. However, the majority would recommend it in cases of incomplete resection [17, 28]. These practices are more or less in agreement with those in our department, which is to treat the patients with radiotherapy after the first recurrence, operated or not. Our data shows that the patients who received radiotherapy did not have a different overall survival. Our findings are consistent with many previously reported results [14, 23]. Moreover, this absence of effect on the survival was not influenced by the resection status of the meningioma (Fig. 1e, f).
According Kaur et al., the median 5-year overall survival of patients with atypical meningioma treated by radiotherapy was 67.5 %, and ranged from 51 to 100 % [14]. No study was able to demonstrate a statistically significant improvement in any of the clinical outcomes with adjuvant radiotherapy for WHO grade II meningioma. Systematic postoperative radiotherapy irrespectively of the resection extent failed to demonstrate its usefulness [24]. Therefore we think that it should be carefully considered regarding the side effects and, if possible, applied within research protocols. The relatively divergent results in the literature are most likely explained by bias of selection. Randomized clinical trials to adequately address this question are clearly necessary.
The “Radiotherapy versus Observation following surgical resection of Atypical Meningioma” (ROAM trial) may give us in 10 years time more clues about the usefulness of radiotherapy in cases of GTR [10, 11].
Comparison of outcome with existing literature
We reviewed published surgical series of grade II meningiomas in the English literature, since 2000 (Table 3). Among the several studies only dedicated to grade II meningiomas, there are noteworthy differences, especially in the number of irradiated patients, ranging from 12.7 to 59.1 %. Therefore it is difficult to compare the outcome results of median overall survival extending from 4.75 to 11.2 years and of 5-year overall survival expanding from 35 to 89.1 %.
Histological features of meningiomas are not fixed, and can evolve, like for gliomas. Fourteen progressed towards a WHO grade III meningioma. As only certain meningiomas undergo malignant transformation, there may be genetic predispositions or other factors influencing this outcome, mediated by numerous processes interacting via a complex matrix of signals [18, 26]. A greater understanding of tumor cells’ genetic mutations and molecular markers involved in critical signaling pathways may also aid in the identification of novel therapies targeted at distinct meningioma sub-types [4, 6, 13, 26]. So far, no chemotherapeutic options have demonstrated their usefulness in meningioma treatment. Further larger studies are needed to establish prognostic factors that may allow greater accuracy in predicting tumor behavior and aid in selecting optimal treatment regimens. The implementation of a national registry or a multicenter study could help us to achieve these goals.
Conclusions
WHO grade II meningiomas significantly impaired the survival of the patients. In the adjusted Cox regression, a macroscopic gross total resection (Simpson grades 1, 2, and 3), an age below 62 years at diagnosis, and the absence of brain invasion were independent factors associated with longer survival. Radiotherapy may not increase the overall survival after complete or incomplete resection.
References
Adeberg S, Hartmann C, Welzel T, Rieken S, Habermehl D, von Deimling A, Debus J, Combs SE (2012) Long-term outcome after radiotherapy in patients with atypical and malignant meningiomas–clinical results in 85 patients treated in a single institution leading to optimized guidelines for early radiation therapy. Int J Radiat Oncol Biol Phys 83(3):859–864
Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL, Curry WT, Barker FG (2009) Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64(1):56–60, discussion 60
Backer-Grøndahl T, Moen BH, Torp SH (2012) The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol 5(3):231–242
Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, Ligon KL, Palescandolo E, Van Hummelen P, Ducar MD, Raza A, Sunkavalli A, Macconaill LE, Stemmer-Rachamimov AO, Louis DN, Hahn WC, Dunn IF, Beroukhim R (2013) Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet 45(3):285–289
Champeaux C, Wilson E, Brandner S, Shieff C, Thorne L (2015) World Health Organization grade III meningiomas. A retrospective study for outcome and prognostic factors assessment. Br J Neurosurg 29(5):693–8
Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, Avşar T, Li J, Murray PB, Henegariu O, Yilmaz S, Günel JM, Carrión-Grant G, Yilmaz B, Grady C, Tanrikulu B, Bakircioğlu M, Kaymakçalan H, Caglayan AO, Sencar L, Ceyhun E, Atik AF, Bayri Y, Bai H, Kolb LE, Hebert RM, Omay SB, Mishra-Gorur K, Choi M, Overton JD, Holland EC, Mane S, State MW, Bilgüvar K, Baehring JM, Gutin PH, Piepmeier JM, Vortmeyer A, Brennan CW, Pamir MN, Kiliç T, Lifton RP, Noonan JP, Yasuno K, Günel M (2013) Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science (New York NY) 339(6123):1077–1080
Durand A, Labrousse F, Jouvet A, Bauchet L, Kalamaridès M, Menei P, Deruty R, Moreau JJ, Fèvre-Montange M, Guyotat J (2009) WHO grade II and III meningiomas: a study of prognostic factors. J Neurooncol 95(3):367–375
Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH (2000) Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys 46(1):57–61
Hammouche S, Clark S, Wong AHL, Eldridge P, Farah JO (2014) Long-term survival analysis of atypical meningiomas: survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir (Wien) 156(8):1475–1481
Jenkinson MD, Weber DC, Haylock BJ, Mallucci CL, Zakaria R, Javadpour M (2014) Radiotherapy versus observation following surgical resection of atypical meningioma (the ROAM trial). Neuro Oncol 16(11):1560–1561
Jenkinson MD, Weber DC, Haylock BJ, Mallucci CL, Zakaria R, Javadpour M (2015) Atypical meningioma: current management dilemmas and prospective clinical trials. J Neurooncol 121(1):1–7
Jo K, Park H-J, Nam D-H, Lee J-I, Kong D-S, Park K, Kim JH (2010) Treatment of atypical meningioma. J Clin Neurosci 17(11):1362–1366
Kalamarides M, Stemmer-Rachamimov AO, Niwa-Kawakita M, Chareyre F, Taranchon E, Han ZY, Martinelli C, Lusis EA, Hegedus B, Gutmann DH, Giovannini M (2011) Identification of a progenitor cell of origin capable of generating diverse meningioma histological subtypes. Oncogene 30(20):2333–2344
Kaur G, Sayegh ET, Larson A, Bloch O, Madden M, Sun MZ, Barani IJ, James CD, Parsa AT (2014) Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol 16(5):628–636
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
Mair R, Morris K, Scott I, Carroll TA (2011) Radiotherapy for atypical meningiomas. J Neurosurg 115(4):811–819
Marcus HJ, Price SJ, Wilby M, Santarius T, Kirollos RW (2008) Radiotherapy as an adjuvant in the management of intracranial meningiomas: are we practising evidence-based medicine? Br J Neurosurg 22(4):520–528
Moazzam AA, Wagle N, Zada G (2013) Recent developments in chemotherapy for meningiomas: a review. Neurosurg Focus 35(6):E18
Modha A, Gutin PH (2005) Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery 57(3):538–550, discussion 538–550
Moon H-S, Jung S, Jang W-Y, Jung T-Y, Moon K-S, Kim I-Y (2012) Intracranial meningiomas, WHO grade II: prognostic implications of clinicopathologic features. J Korean Neurosurg Soc 52(1):14–20
Sade B, Chahlavi A, Krishnaney A, Nagel S, Choi E, Lee JH (2007) World Health Organization grades II and III meningiomas are rare in the cranial base and spine. Neurosurgery 61(6):1194–1198, discussion 1198
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20(1):22–39
Stessin AM, Schwartz A, Judanin G, Pannullo SC, Boockvar JA, Schwartz TH, Stieg PE, Wernicke AG (2012) Does adjuvant external-beam radiotherapy improve outcomes for nonbenign meningiomas? A Surveillance, Epidemiology, and End Results (SEER)-based analysis. J Neurosurg 117(4):669–675
Sun SQ, Kim AH, Cai C, Murphy RK, DeWees T, Sylvester P, Dacey RG, Grubb RL, Rich KM, Zipfel GJ, Dowling JL, Leuthardt EC, Leonard JR, Evans J, Simpson JR, Robinson CG, Perrin RJ, Huang J, Chicoine MR (2014) Management of atypical cranial meningiomas, part 1: predictors of recurrence and the role of adjuvant radiation after gross total resection. Neurosurgery 75(4):347–354, discussion 354–355; quiz 355
Vranic A, Popovic M, Cör A, Prestor B, Pizem J (2010) Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: a study of 86 patients. Neurosurgery 67(4):1124–1132
Yew A, Trang A, Nagasawa DT, Spasic M, Choy W, Garcia HM, Yang I (2013) Chromosomal alterations, prognostic factors, and targeted molecular therapies for malignant meningiomas. J Clin Neurosci 20(1):17–22
Yoon H, Mehta MP, Perumal K, Helenowski IB, Chappell RJ, Akture E, Lin Y, Marymont MA, Sejpal S, Parsa A, Chandler JR, Bendok BR, Rosenow J, Salamat S, Kumthekar P, Raizer JK, Baskaya MK (2015) Atypical meningioma: randomized trials are required to resolve contradictory retrospective results regarding the role of adjuvant radiotherapy. J Cancer Res Ther 11(1):59–66
Zaher A, Abdelbari Mattar M, Zayed DH, Ellatif RA, Ashamallah SA (2013) Atypical meningioma: a study of prognostic factors. World Neurosurg 80(5):549–553
Zhao P, Hu M, Zhao M, Ren X, Jiang Z (2015) Prognostic factors for patients with atypical or malignant meningiomas treated at a single center. Neurosurg Rev 38:101–107
Acknowledgments
The authors thank the following for their assistance: Deborah Houston, Janice Lafferty, Department of Neurosurgery; Dr. Andres Kulla, Elizabeth Fraser, Jacqueline MacPherson Department of Neuropathology, Southern General Hospital, Glasgow; Melissa McEwan, Radiotherapy Department, The Beatson West of Scotland Cancer Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Comments
This is an important case series on atypical meningiomas. WHO grade II meningiomas have a somewhat unpredictable clinical behavior and studies with large cohorts do help in understanding disease characteristics. The authors have retrospectively reviewed 206 cases of WHO grade II meningiomas and have excluded anaplastic cases and other pathologies such as hemangiopericytomas. This is important, as most previous studies have analyzed grade II and III meningiomas as a single group. Some groups have also included hemangiopericytomas that have been established as a completely separate entity. Therefore, an analysis of this relatively more “purified” group is more desirable. Within the time frame of the reported cases from 2000 to 2015, the WHO pathological tumor classification has been revised twice and the authors indicate that they have reviewed the pathology records in detail to come to a definitive conclusion.
The low recurrence-free survival rate (42.2 %), despite exclusion of anaplastic cases, indicates that atypical meningiomas are high-risk pathologies and that they deserve the WHO grade II designation. The authors have found that an age above 62, extent of resection (Simpson 1 to 3), need for reoperation, and brain invasion as important variables influencing overall survival. Importantly, radiation therapy was not found to be a factor influencing patient survival. The conclusions of such a large cohort will certainly help clinicians to develop rational strategies in the management of atypical meningiomas.
Necmettin Pamir
Istanbul, Turkey
Rights and permissions
About this article
Cite this article
Champeaux, C., Dunn, L. World Health Organization grade II meningiomas. Acta Neurochir 158, 921–929 (2016). https://doi.org/10.1007/s00701-016-2771-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2771-y