Abstract
Background
Candida tropicalis is an important human pathogen that can undergo multiple forms of phenotypic switching.
Aim
We aimed to evaluate the effect of phenotypic switching on the adhesion ability of C. tropicalis.
Methods
C. tropicalis morphotypes included parental phenotypes (clinical isolates) and switch phenotypes (crepe, revertant of crepe—CR, rough, revertant of rough—RR, irregular center and revertant of irregular center—ICR). Adhesion to polystyrene and HeLa cells was determined by crystal violet assay. The percentage of HeLa cells with adhered yeasts and the number of adhered yeasts per HeLa cell were determined by light microscopy. Filamentation among adhered cells was assessed by direct counting.
Results
On polystyrene, 60% of the switch strains showed difference (p < 0.05) on adhesion ability compared to their parental counterpart strains, and altered thickness of adhered cells layers. Filamentation was increased among adhered cells of the switched strains compared to parental strains. A positive correlation was observed between adhesion on polystyrene and filamentation for morphotypes of the system 49.07. The majority of the switched strains showed higher adhesion capability to HeLa cells in comparison to the adherence of the clinical strains. All revertant strains showed a higher number of yeast cells per HeLa cell compared to their variant counterparts (p < 0.05), with exception of the ICR.
Conclusions
Our findings indicate that switching events in C. tropicalis affect adhesion and filamentation of adhered cells on polystyrene and HeLa cells. The rise of switch strains with increased adhesion ability may contribute to the success of infection associated with C. tropicalis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Candida tropicalis has become one of the most common clinical species of this genus, especially in tropical regions [1,2,3]. In some settings, bloodstream infections due to C. tropicalis have been associated with higher mortality than infections with other Candida species [4, 5]. Further, C. tropicalis is well known for its resistance to fluconazole [6, 7] which may implicate persistent infections.
A number of virulence factors contributing toward the pathogenicity of C. tropicalis have been proposed, including phenotypic switching [8]. For Candida species, the event of phenotypic switching allows adaptation to environmental changes and consequently contributes to their success as pathogens to humans [9]. C. tropicalis is capable of switching between a number of phenotypes, including strains that switch between nonhyphal to hyphal colonies morphologies [10, 11]. Soll et al. [10] described a high-frequency switch in C. tropicalis during the course of infection in an immunocompromised host. Studies in vitro have demonstrated the occurrence of distinct isolate-specific switching repertoires in clinical isolates of C. tropicalis and the impact of switching on several putative virulence attributes, including biofilm formation, hemolytic activity and citotoxicity [11, 12]. Moreover, studies have shown that distinct switch states of C. tropicalis exhibited increased virulence and altered host–pathogen interactions [13, 14].
Adhesion is essential for Candida species to develop their pathogenic potential since it triggers the process that leads to colonization and infection [15,16,17,18]. C. tropicalis is highly adherent to epithelial and endothelial cells [19, 20]. In addition, this species is considered one of the most adherent Candida species, surpassing C. albicans in some studies [8]. For instance, C. tropicalis displayed the highest ability of adherence to human buccal epithelial cells among Candida species [21, 22]. In the clinical environment, adherence to abiotic surfaces is also an important factor, since it is the first step for the formation of biofilm on medical devices, which presents important clinical repercussions, including increased tolerance to antifungal therapy [19, 23]. C. tropicalis has been considered a prominent biofilm producer, compared to other pathogenic Candida species [24, 25]. A recent study suggests that C. tropicalis biofilm production is related to higher mortality in candidemia [26].
The present study was conducted to evaluate the effect of phenotypic switching on the adhesion capability of C. tropicalis. Thus, we assessed adhesion on polystyrene and HeLa cells using C. tropicalis parental strains (clinical sources), and distinct switch states, including switch variants (strains that arose from phenotypic switching and exhibit altered colony morphology-structured dome) and switch revertants (strains that switched back from the variants to the original phenotype-smooth dome).
Materials and Methods
Candida Tropicalis Strains
Morphotypes of phenotypic switching systems of three C. tropicalis clinical isolates (49.07, 100.10 and 335.07) were used. Morphotypes comprised clinical isolates (parental smooth phenotype), switch variants (crepe, rough and irregular center phenotypes) and the switch revertants of the crepe, rough and irregular center types [11, 13]. The strains were obtained as a stock culture stored in 15% glycerol at -80 °C, from the Fungal Genetics Laboratory, The University of Londrina-Brazil.
Adhesion of C. Tropicalis Morphotypes to Polystyrene
C. tropicalis adhesion assay was performed as reported previously [27], with modifications. For each strain, 200 µl of standardized cell suspensions in RPMI 1640 medium (SIGMA-ALDRICH, St Louis, MO, USA), containing 1 × 107 cells/ml, were placed into wells of 96-well polystyrene microtiter plates and incubated at 37 °C without agitation for 1 h. After, the medium was aspirated, and non-adherent cells were removed by washing thrice with sterile PBS buffer.
Yeast adhesion was quantified using the crystal violet (CV) staining method. Adhered cells were fixed with 200 μl of methanol for 15 min and 200 μl of CV (1% v/v) was added to each well and incubated for 5 min. The wells were washed with PBS buffer twice, and 200 μl of acetic acid (33% v/v). The solution absorbance was read in triplicate in a microtiter plate reader (Bio-Tek EL 808) at 540 nm. The experiments were performed in triplicate and repeated three times.
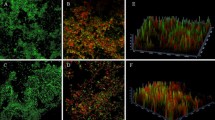
Confocal microscopy was performed as reported previously [12], with modifications. Cells of C. tropicalis morphotypes were adhered to polystyrene (SPL, Life Sciences Co., Ltd, Korea) coupons (1 cm) placed in 24-well tissue plates. One milliliter of standardized cell suspensions (1 × 107 cells/ml) was added to the wells, and the coupons were incubated at 37 °C without agitation for 1 h. Wells were gently washed with sterile PBS to remove non-adherent cells. Adhered cells were fixed with 10% paraformaldehyde (at room temperature in the dark for 10 min). After fixation, the samples were stained with calcofluor white M2R (10 μg/ml) (Sigma-Aldrich, USA) for 20 min. The representative images were obtained using a confocal microscope (SP8, Leica Microsystems, Mannheim, Germany), with the Las X software, at λex/λem = 405/411–491 nm.
For light microscopy, C. tropicalis strains (1 × 107 cells/ml) were adhered to polystyrene (SPL, Life Sciences Co., Ltd, Korea) coupons (1 cm) in 24-well plates for 1 h as described above. A total of 1000 cells per sample were direct counted. Each filamentous form (hyphae and pseudohyphae) was counted as 1 multicell unit. The representative images were obtained using a confocal microscope (SP8, Leica Microsystems, Mannheim, Germany) with software Las X using a black and white filter. The fields were randomly chosen.
Adhesion of C. Tropicalis Morphotypes to HeLa Cells
HeLa cells (ATCC, CCL2TM; Manassas, VA, USA), originating from human uterine cervix tumor, were acquired from the American Type Culture Collection. HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, USA) containing 10% fetal bovine serum and 1% penicillin/streptomycin (P/S; GIBCO, USA) (FBS; GIBCO, USA) at 37 °C in a 5% CO2 incubator. After, cell concentration was adjusted to 1 × 106 cells/ml with fresh DMEM without antimicrobial agents and added to each well of a 24-well plate, and incubated with 5% CO2 at 37 °C until achieving 80% confluence.
Adhesion to HeLa cells was conducted as reported previously by Negri et al. [16], with modifications. Yeast cells were suspended in DMEM medium, and a standardized cell suspension (1 × 107 cells/ml) was added to HeLa cells at a multiplicity of infection (MOI) of 10 and co-culture for 2 h at 37 °C, 5% CO2. After, wells were washed with PBS buffer to remove unattached yeast. Yeast cells were quantified using the crystal violet (CV) assay. Crystal violet (1 ml, 1%) was added to each well containing the epithelial cells with adherent yeasts for 5 min, and the wells were washed twice with PBS buffer. To remove color from the epithelial cells, 1 ml of ethanol:acetone (1:1) was added to the wells and removed immediately. Acetic acid (3 ml, 33%) was added to each well, and absorbance was read at 540 nm. The experiments were carried out in triplicate and repeated on three different occasions.
Imaging studies of C. tropicalis-infected (MOI 10) HeLa cells monolayers were performed by bright field microscopy, as previously described [28], with modifications. Briefly, HeLa cells (1 × 106 cells/ml) were adhered to 13-mm round coverslips (Perfecta, Brazil) with polarity previously adjusted with acetic acid, and incubated (24 h at 37 °C, 5% CO2). After achieving 80% confluence, the epithelial cells were co-cultivated with Candida cells (1 × 107 cells/ml) for 2 h at 37 °C and 5% CO2. After this period, the co-cultured cells were washed with PBS buffer and fixed with 4% of paraformaldehyde in PBS buffer for 30 min. After washing with PBS buffer, cells were stained with 1% methylene blue for 5 min, following washing with PBS buffer and analyzed by light microscopy (E100, Nikon-LED). The following parameters were analyzed: (1) Percentage of epithelial cells with adhered yeasts, (2) Mean of adhered yeasts per epithelial cell and (3) C. tropicalis adhered cell types (yeasts and filamentous forms). All tests were performed in triplicate and repeated thrice in independent assays. One hundred epithelial cells were accounted for three fields per coverslips.
Statistical analysis
For analysis of adhesion on polystyrene and HeLa cells, the t-paired test was used. Statistical significance was set at *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. The correlation of the data was verified through the calculation of the Pearson correlation coefficient. P < 0.05 was considered significant.
Results
Phenotypic Switching has Effect on C. Tropicalis Adhesion to Polystyrene.
Analysis of adhesion of distinct switch states of C. tropicalis on plastic surface (polystyrene) is shown in Fig. 1. Overall, six of the switched strains exhibited changed adhesion capability in comparison to their parental counterpart strains (clinical strains) (Fig. 1A).
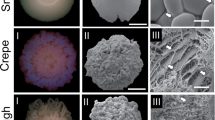
Adhesion of C. tropicalis morphotypes to polystyrene. A Adhesion of morphotypes of the switching systems 49.07 a, 100.10 b and 335.07 c determined by crystal violet assay. B Confocal microscopy analysis of adhered cells layers thickness. Representative side view of projection images of adhered cells on coverslips slides confocal microscopy (calcofluor-white-stained adhered cells). Switching systems 49.07-a parental strain, b crepe variant, c rough variant, d revertant of crepe—CR, e revertant of rough–RR; 100.10–f parental strain, g crepe variant, h rough variant, i revertant of crepe—CR, j revertant of rough –RR; 335.07 k parental strain, (l) variant irregular center and (m) revertant of irregular center. The data are means of three independent experiments performed in triplicate. Standard deviation is represented by bars. Asterisks represent a significant difference (Test-t paired, *P < 0.05; **P < 0.01; ***p < 0.001; ****p < 0.0001)
For the switching system 49.07, all switched strains (variants and revertants—strains that switched back from the variant phenotypes to the parental phenotype) exhibited higher adhesion than that observed for the parental strain (P < 0.001) (Fig. 1A). Revertant of crepe (CR) and revertant of rough (RR) showed lower adhesion than their variant counterparts. Thus, the order of adherence to polystyrene observed from highest to lowest was crepe > revertant of crepe > parental and rough > revertant of rough > parental.
Confocal microscopy of layers of adhered cells of C. tropicalis morphotypes revealed varied thickness (in micrometers) (Fig. 1B). Layers of adhered cells of switch variants and revertants of the switching system 49.07 were thicker compared to the thinner layer of adhered cells of the parental strain (Fig. 1B, lines a-e). Images obtained from the Z projection of different sections of adhered cells of the crepe and rough variants showed a thickness up to 6.25 times greater compared to the parental strain, and the presence of filamentous forms (Fig. 1B, lines b-c). Layers of adhered cells of revertant of crepe (CR) and revertant of rough (RR) were also thicker compared to the parental strain (Fig. 1B, lines d,e). In addition, the adherence layer topology also varied among morphotypes. The crepe and rough variants, and the revertant of rough exhibited a more heterogeneous topology (Fig. 1B, lines b,c,e).
We next assessed the morphology of adhered cells using light microscopy (Fig. 2). Adhered cells of the parental strain consist almost entirely of budding yeast cells (“low filamentation ability score”) (Fig. 2A,B). In contrast, morphologies of adhered cells of the switch variants (crepe and rough) consist of an increased number of filamentous forms (hyphae and pseudohyphae) (“high filamentation ability score”) than the parental strain (P < 0.001). Differently, adhered cells of revertant strains (CR and RR) exhibited a lower number of filamentous forms than their variant counterparts, and a higher number of filamentous forms than the parental strain (P < 0.001) (“intermediate filamentation ability score”) (Fig. 2A,B). The order of the percentage of filamentous forms among adhered cells on polystyrene, from highest to lowest, was crepe > revertant of crepe > parental and rough > revertant of rough > parental (Fig. 2A,B). A positive correlation (r2 0.874, p 0.005) was observed between adhesion ability and filamentation on polystyrene surface for strains of the switching system 49.07.
Filamentous growth among adhered cells of C. tropicalis morphotypes to polystyrene. A Percentage of filamentous forms (100% corresponds to 1 × 103 cells). Switching systems 49.07: parental strain, crepe variant, rough variant, revertant of crepe—CR, and revertant of rough–RR; 100.10: parental strain, crepe variant, rough variant, revertant of crepe—CR, and revertant of rough–RR; 335.07 parental strain, variant irregular center, revertant of irregular center. The data are means of three independent experiments performed in triplicate. Standard deviation is represented by bars. Asterisks represent a significant difference (Test-t paired, *P < 0.05; **P < 0.01; ***p < 0.001). B Light micrography of adhered cells of the switching system 49.07. White arrows indicate filamentous growth. Photomicrographs (400x). Scale bars = 10 µm. The warm tones indicate the filamentation profile of the morphotypes: red (high filamentation), orange (intermediate filamentation) and yellow (low filamentation). The cool tones indicate the adhesion profile: dark blue (high adhesion), blue (intermediate adhesion) and light blue (low adhesion)
For the switching system 100.10, the crepe variant presented higher adhesion than its parental counterpart (P < 0.01). The remaining switched strains showed the same extent of adhesion on polystyrene than the parental strain (Fig. 1A). Moreover, both revertant (CR and RR) strains exhibited lower adhesion than their variant counterparts (Fig. 1A). As expected, the crepe variant exhibited an increased thickness of the adherence layer compared to the adherence layer of the parental strain (Fig. 1B, lines f,g).
For the switching system 335.07, comprising a smooth parental strain, an irregular center variant and its revertant (ICR), adhesion capability was reduced for the revertant strain in comparison to the parental strain, while no difference in adhesion was observed between the irregular center variant and the parental strain (Fig. 1A), in accordance to images analysis of adherence on polystyrene (Fig. 1B, lines k-m). Additionally, morphotypes of the switching systems 100.10 and 335.07 showed a heterogeneous topology (Fig. 1B, lines f-m). For these two switching systems, all switched strains (variants and revertants) exhibited a higher percentage of filamentous forms among adhered cells than their parental counterparts (Fig. 2A).
Switch States of C. Tropicalis Show Varied Adhesion on HeLa Cells
All morphotypes of C. tropicalis were able to infect HeLa cells monolayers. Seven out of ten (70%) switch strains showed different adhesion capability to HeLa cells than that observed for the clinical strains (parental strains). Among them, five switch strains exhibited higher adhesion than their parental counterparts (P < 0.05), including the morphotypes rough and RR (revertant of rough) of both switching systems 49.07 and 100.10 (Fig. 3A). For the former switching system, all switched strains exhibited higher adhesion to HeLa cells than the parental strain, with the exception of the crepe variant as illustrated in Fig. 4. Further, microscopic analysis revealed distinct patterns of yeast distribution on HeLa cells. Revertants occurred as aggregates of cells, while the parental strain showed a diffuse pattern of cell adhesion (Fig. 4). Differently, switch strains of the system 335.07 showed reduced ability to infect HeLa cells compared to the parental strain.
Adhesion of C. tropicalis morphotypes to HeLa cells. A Adhesion of morphotypes of the switching systems 49.07, 100.10 and 335.07 determined by crystal violet assay. B Mean of C. tropicalis cells adhered per HeLa cells. C Percentage of filamentous forms among adhered cells to HeLa cells. D Percentage of HeLa cells with adhered C. tropicalis cells. The data are means of three independent experiments performed in triplicate. Standard deviation is represented by bars. Asterisks represent a significant difference (Test-t paired, *P < 0.05; **P < 0.01; ***p < 0.001; ****p < 0.0001)
Representative photomicrographs of C. tropicalis cells adhered to HeLa cells. Morphotypes: parental strain, crepe variant, rough variant, revertant of crepe—CR, and revertant of rough–RR. White arrows indicate filamentous growth. Black arrows indicate aggregate of yeast cells. Photomicrographs (400x). Scale bars = 10 µm. The warm tones indicate the filamentation profile of the morphotypes: red (high filamentation), orange (intermediate filamentation) and yellow (low filamentation). The cool tones indicate the adhesion profile: dark blue (high adhesion), blue (intermediate adhesion) and light blue (low adhesion)
We next assessed the number of adhered cells of morphotypes per HeLa cell. As shown in Fig. 3B, the revertants RR and CR of the system 49.07 exhibited a higher number of yeast cells per HeLa cell than that observed for the parental strain, while three switch strains showed the same extent of yeasts cells per epithelial cell compared to that observed for their parental counterparts. Moreover, revertant strains (RR and CR) of both switching systems 49.07 and 100.10 showed a higher number of yeast cells per HeLa cell than their variant counterparts (p < 0.05) (Fig. 3B, Fig. 4).
Using light microscopy, we examined filamentation ability among adhered cells of C. tropicalis morphotypes (Fig. 3C). A comparison of the percentage of filamentous forms between switched strains and their parental counterparts revealed that the rough variant of both switching systems (49.07 and 100.10) and the variant irregular center of the system 335.07 showed greater filamentous growth than their parental strains (Fig. 3C). The filamentation ability of the rough variant of the system 49.07 is shown in Fig. 4. For the systems 49.07 and 100.10, the revertants of rough showed the same extent of filamentation as the parental strains; thus, the reversion restored the morphogenesis of the original clinical strain. There was no correlation between the number of filamentous forms (filamentation ability) and the ability of the morphotypes to adhere to HeLa cells.
We also assessed the percentage of HeLa cells with adherent cells of C. tropicalis morphotypes. As shown in Fig. 3D, the percentage of infected HeLa cells was higher for four switch strains than their parental counterparts. There was no correlation between the percentage of HeLa cells with adhered yeasts and the average of yeasts per HeLa cell.
Discussion
C. tropicalis has been associated with systemic infections, specifically in patients admitted to ICUs, and requiring prolonged catheterization [8]. For this species, virulence attributes such as high adhesion capacity and ability to form biofilm on biotic and abiotic surfaces are tightly associated with its pathogenicity [8, 16].
In the present study, we examined the effect of phenotypic switching on the adhesion capability of C. tropicalis isolated from clinical samples. Our results demonstrated that the majority of switch states of C. tropicalis exhibited altered ability to adhere to polystyrene and human epithelial cells (HeLa cells) compared to the parental strains (clinical isolates) (Fig. 1A, Fig. 3A). On polystyrene surface, switch states of the system 49.07 showed an interesting feature, e.g., high adhesion of switch variants and intermediate adhesion of revertant strains, in comparison to the low adhesion of the parental strain (Fig. 1A).
The rise of switch strains with increased adhesion may have an impact on the ability of these strains to form biofilm. Indeed, our group recently described that strains of the system 49.07 produced more biofilm than the parental strain, and that sessile cells of switched strains adhered better to polystyrene surface compared to sessile cells of the parental strain [12]. Moreover, in the present study, the effect on adhesion ability to polystyrene was switching-dependent as no statistical differences were observed between most of the switch strains of the other two switching systems, 100.10 and 335.07, and their parental strains.
Adhesion to human epithelial cells was increased for switch strains of systems 49.97 and 100.10. Revertant strains (CR and RR) of the former switching system showed higher adhesion to HeLa cells and a higher number of yeast adhered to HeLa cells than the parental strain (Fig. 3A,B). In addition, these revertants infected a higher number of HeLa cells than the parental strain. These data highlight that strains that switched back from the variant phenotypes to the original parental phenotype, e.g., strains that exhibit colony morphology that resembles the original phenotype of the parental (clinical strain), do not restore the adhesion ability of the parental strain. This result corroborates our previous finding on other virulence attributes [13, 14] and suggests that switch states of C. tropicalis may be associated with distinct physiological status and virulence characteristics that are independent of their colony phenotype. Interestingly, the crepe phenotype of the system 49.07 exhibited increased infection to HeLa cells (Fig. 3D), corroborating its increased ability to infect and cause damage to FaDu cells as described previously [13]. Increased adherence of C. tropicalis to host cells may have clinical implications as adhesion comprises an essential initial step in the establishment of infection [29].
The changed ability of adhesion presented by switched strains suggests that switch states may exhibit altered cell wall properties. In our previous study, switch strains of the system 49.07 were differently recognized by the G. mellonella immune components, reinforcing the hypothesis of changes in C. tropicalis cell wall [14]. Adhesion of Candida cells is mediated through a large number of specific cell-wall proteins (adhesins), including the agglutinin‐like sequence (Als) proteins [30]. In C. tropicalis, the ALS‐like genes ALS1 and ALS2 were differently expressed on the surface of polystyrene compared to growth on epithelial cells.
Another important observation of the present study was that adhered cells of switch strains may exhibit higher levels of filamentous cells (hyphae and pseudohyphae) than adhered cells of the parental strain. When we evaluated the adhesion on polystyrene surface in relation to the filamentation capacity of the strains, we found that for the switching system 49.07, the filamentation ability of the strains was closely related to the adhesion capability. Crepe and rough variant strains with high filamentation ability showed high adhesion, compared to the parental strain with low filamentation ability and low adhesion, while revertant strains showed intermediate filamentation and adhesion ability (Fig. 1A, Fig. 2A,B). These results suggest that the differences in adhesion between parental strains and the four switch phenotypes (variants and revertants) reflect, at least in part, the amount of filamentous forms. According to Harun et al. [31], C. tropicalis expresses HWP1 (hyphal wall protein) gene that codifies for the adhesin-associated protein in the cell wall indicating the production of this adhesin in this species.
As shown in Fig. 3C, phenotypic switching also altered the filamentation ability of switch strains adhered to epithelial cells. Following adhesion on HeLa cells, four out of ten switch phenotypes showed an increased amount of filamentous forms than the parental strains. Some reports have suggested a correlation between C. tropicalis filamentation ability and the ability to invade and damage epithelial cells [19, 32, 33], though another recent study has not corroborated this trend [34]. Moreover, hyphal formation seems to play a vital role in the virulence of C. tropicalis [35]. A study of comparative analysis of the transcriptional profiles between the yeast and hyphal forms of C. tropicalis showed that 23 hyphae-related genes were up-regulated, including the ALS3 (agglutinin‐like)-encoding gene [36]. Thus, the changes in adhesion and filamentation observed in the present study may be due to the differences in transcriptional programs in distinct switch states. The pleiotropic effects of phenotypic switching on adhesion ability and on morphogenesis suggest that switching events in C. tropicalis may favor its virulence and as a consequence may contribute to the success of infection associated with this species.
In conclusion, this study shows that the adhesion of C. tropicalis was significantly altered depending on its phenotypic state. Besides, filamentation ability was positively correlated with adhesion on polystyrene. Phenotypic switching is associated with increased adhesion of C. tropicalis to HeLa cells, especially for revertant strains. Further studies on the molecular basis of these switching systems and their role in adhesion on biotic and abiotic surfaces will be important for the development of strategies for the control of C. tropicalis adhesion in the clinical environment.
References
Kothavade RJ, Kura MM, Valand AG, Panthaki MH. Candida tropicalis: its prevalence, pathogenicity and increasing resistance to fluconazole. J Med Microbiol. 2010;59:873–80.
Rodriguez L, Bustamamnte B, Huaroto L, Agurto C, Illescas R, et al. A multi-centric study of Candida bloodstream infection in Lima-Callao, Peru: species distribution, antifungal resistance and clinical outcomes. PLoS ONE. 2017;12:e0175172.
Medeiros MAP, Melo APV, Bento AO, de Souza LBFC, Bezerra Neto FA, et al. Epidemiology and prognostic factors of nosocomial candidemia in Northeast Brazil: A six-year retrospective study. PLoS ONE. 2019;14:e0221033.
Kontoyiannis DP, Vaziri I, Hanna HA, Boktour M, Thornby J, et al. Risk factors for Candida tropicalis fungemia in patients with cancer. Clin Infect Dis. 2001;33:1676–81.
Colombo AL, Guimarães T, Silva LR, Monfardini LPA, Cunha AKB, et al. Prospective observational study of candidemia in Sao Paulo, Brazil: incidence rate, epidemiology, and predictors of mortality. Infect Control Hosp Epidemiol. 2007;28:570–6.
Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty Years of the SENTRY antifungal surveillance program: results for Candida species from 1997–2016. Open Forum Infect Dis. 2019;6(Suppl. 1):S79–94.
Fan X, Xiao M, Liao K, Kudinha T, Wang H, et al. Notable increasing trend in azole non-susceptible Candida tropicalis causing invasive candidiasis in China (August 2009 to July 2014): molecular epidemiology and clinical azole consumption. Front Microbiol. 2017;8:464.
Zuza-Alves DL, Silva-Rocha WP, Chaves GM. An update on Candida tropicalis based on basic and clinical approaches. Front Microbiol. 2017;8:1927.
Soll DR. Why does Candida albicans switch? FEMS Yeast Res. 2009;9:973–89.
Soll DR, Staebell M, Langtimm C, Pfaller M, Hicks J, et al. Multiple Candida strains in the course of a single systemic infection. J Clin Microbiol. 1988;26:1448–59.
Moralez ATP, França EJG, Furlaneto-Maia L, Quesada RMB, Furlaneto MC. Phenotypic switching in Candida tropicalis: association with modification of putative virulence attributes and antifungal drug sensitivity. Med Mycol. 2014;52:106–14.
Moralez ATP, Perini HF, Paulo EA, Furlaneto-Maia L, Furlaneto MC. Effect of phenotypic switching on biofilm traits in Candida tropicalis. Microb Pathog. 2020;149:104346.
Moralez ATP, Perini HF, Furlaneto-Maia L, Almeida RS, Panagio LA, et al. Phenotypic switching of Candida tropicalis is associated with cell damage in epithelial cells and virulence in Galleria mellonella model. Virulence. 2016;7:379–86.
Perini HF, Moralez ATP, Almeida RSC, Panagio LA, Junior AOG, et al. Phenotypic switching in Candida tropicalis alters host-pathogen interactions in a Galleria mellonella infection model. Sci Rep. 2019;9:12555.
Sohn K, Senyürek I, Fertey J, Königsdorfer A, Joffroy C, et al. An in vitro assay to study the transcriptional response during adherence of Candida albicans to different human epithelia. FEMS Yeast Res. 2006;6:1085–93.
Negri M, Gonçalves V, Silva S, Henriques M, Azeredo J, et al. Crystal violet staining to quantify Candida adhesion to epithelial cells. Brit J Biomed Sci. 2010;67:120–5.
Silva-Dias A, Miranda IM, Branco J, Monteiro-Soares M, Pina-Vaz C, et al. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: relationship among Candida spp. Front Microbiol. 2015;6:205.
Yu SB, Li WG, Liu XS, Che J, Lu JX, et al. The Activities of adhesion and biofilm formation by Candida tropicalis clinical isolates display significant correlation with its Multilocus Sequence Typing. Mycopathologia. 2017;182:459–69.
Silva S, Negri M, Henriques M, et al. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2011;36:288–305.
Marcos-Zambrano LJ, Escribano P, Bouza E, Guinea J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: comparison of biomass production and metabolic activity and development of cut-off points. Int J Med Microbiol. 2014;304:1192–8.
Biasoli MS, Tosello ME, Luque AG, Magaro HM. Adherence, colonization and dissemination of Candida dubliniensis and other Candida species. Med Mycol. 2010;48:291–7.
Chaves GM, Diniz MG, da Silva-Rocha WP, Souza L, Gondim LAM, et al. Species distribution and virulence factors of Candida spp. isolated from the oral cavity of kidney transplant recipients in Brazil. Mycopathologia. 2013;175:255–63.
Brilhante RSN, Rodrigues TDJS, Souza D, Castelo-Branco DSCM, Teixeira CEC, et al. Antifungal susceptibility and virulence attributes of animal-derived isolates of Candida parapsilosis complex. J Med Microbiol. 2014;63:1568–72.
Kumari A, Mankotia S, Chaubey B, Luthra M, Singh R. Role of biofilm morphology, matrix content and surface hydrophobicity in the biofilm-forming capacity of various Candida species. J Med Microbiol. 2018;67:889–92.
Galán-Ladero MÁ, Blanco-Blanco MT, Fernández-Calderón MC, Lucio L, Gutiérrez-Martín Y. Candida tropicalis biofilm formation and expression levels of the CTRG ALS-like genes in sessile cells. Yeast. 2019;36:107–15.
Vitális E, Nagy F, Tóth Z, Forgács L, Bozó A, et al. Candida biofilm production is associated with higher mortality in patients with candidaemia. Mycoses. 2020;63:352–60.
Xu K, Wang JL, Chu MP, Jia C. Activity of coumarin against Candida albicans biofilms. J Mycol Medi. 2019;29:28–34.
Sanfelice RA, da Silva SS, Bosqui LR, Miranda-Sapla MM, Barbosa BF, et al. Pravastatin and simvastatin inhibit the adhesion, replication and proliferation of Toxoplasma gondii (RH strain) in HeLa cells. Acta Trop. 2017;167:208–15.
Munoz P, Giannella M, Fanciulli C, Guinea J, Valerio M, et al. Candida tropicalis fungaemia: incidence, risk factors and mortality in a general hospital. Clin Microbiol Infect. 2011;17:1538–45.
Zordan R, Cormack B. Adhesins in opportunistic fungal pathogens. In: Calderone RA, Clancy CJ, editors. Candida and Candidiasis. Washington, DC: ASM Press; 2012. p. 243–59.
Wan Harun WH, Jamil NA, Jamaludin NH, Nordin MA. Effect of Piper betle and Brucea javanica on the differential expression of Hyphal Wall Protein (HWP1) in Non-Candida albicans Candida (NCAC) Species. Evid Based Complement Alternat Med. 2013;2013:397268.
Jayatilake J, Samaranayake Y, Cheung L, Samaranayake L. Quantitative evaluation of tissue invasion by wild type, hyphal and SAP mutants of Candida albicans, and non-albicans Candida species in reconstituted human oral epithelium. J Oral Pathol Med. 2006;35:484–91.
Yu S, Li W, Liu X, Che J, Wu Y, Lu J. Distinct expression levels of ALS, LIP, and SAP genes in Candida tropicalis with diverse virulent activities. Front Microbiol. 2016;7:1175.
Banerjee M, Lazzell AL, Romo JA, Lopez-Ribot JL, Kadosh D. Filamentation is associated with reduced pathogenicity of multiple non-albicans Candida species. mSphere. 2019;4:e00656-e719.
Jiang C, Li Z, Zhang L, Tian Y, Dong D, Peng Y. Significance of hyphae formation in virulence of Candida tropicalis and transcriptomic analysis of hyphal cells. Microbiol Res. 2016;192:65–72.
Wu Y, Li Y-h, Yu S-b, Li W-g, Liu X-s, Zhao L, Lu J. A genome-wide transcriptional analysis of yeast-hyphal transition in Candida tropicalis by RNA-Seq. PLoS ONE. 2016;11:e0166645.
Acknowledgements
This work was supported by Fundação Araucária/SETI/Governo do Paraná–Brazil and PROPPG/UEL-Brazil. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. CMS was fellowship holder of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES). MCF is grateful to CNPq for the PQ fellowship.
Author information
Authors and Affiliations
Contributions
CMS, LF-M and MCF conceived and designed the experiments. CMS, HFP, WAVJr. and THZ performed the experiments. CMS, MCF and LF-M analyzed the data. CMS and MCF drafted the manuscript. CMS, LF-M and MCF reviewed and edited the manuscript. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
Ethical approval
This article does not contain any studies with human participants or animal experiments.
Consent for Publication
All authors agree to the submission of the manuscript in its actual format.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Marcia de Souza Carvalho Melhem.
Rights and permissions
About this article
Cite this article
de Souza, C.M., Perini, H.F., Verri, W.A. et al. Changes in Adhesion of Candida tropicalis Clinical Isolates Exhibiting Switch Phenotypes to Polystyrene and HeLa Cells. Mycopathologia 186, 81–91 (2021). https://doi.org/10.1007/s11046-020-00504-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-020-00504-2