Abstract
Adhesion and biofilm formation, which can occur on abiotic and biotic surfaces, are key components in Candida pathogenicity. The aims of this study were to infer about the C. tropicalis clinical isolates ability to adhere and form biofilm on abiotic and biotic surfaces and to correlate that with the multilocus sequence typing and other virulence factors. Adhesion and biofilm formation were measured in 68 C. tropicalis isolates from 3 hospitals in China on abiotic (polystyrene) and biotic (human urinary bladder epithelial cell) surfaces by crystal violet assay and 2,3-bis (2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide reduction assay. In our study, almost all C. tropicalis isolates could adhere and produce biofilm on abiotic and biotic surfaces in a strain-dependent manner. The isolates from blood showed relatively lower adhesion and biofilm capacity on polystyrene surface, but had strong secreted aspartyl proteinase activity. Moreover, significant differences were found among MLST groups for adhesion and biofilm capacity. C. tropicalis in multilocus sequence typing group5 and group6 showed high adhesion and biofilm, while isolates in group1 exhibited low adhesion and biofilm formation. Overall, it is important to note that C. tropicalis isolates adhere to and produce biofilm on abiotic and biotic surfaces with strain specificity. These data will play an important role in subsequent research on the pathogenesis of C. tropicalis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the increasing number of immunocompromised patients, long-term hospitalized patients, and invasive medical inspection and therapy, the genus Candida has emerged as a major opportunistic pathogens that cause superficial and invasive infections in humans [1–3]. Although the majority of candidosis cases are attributed to C. albicans, a growing number of infections caused by non-Candida albicans Candida species, such as C. tropicalis, have been reported in recent years [4]. C. tropicalis is considered the leading pathogen in nosocomial fungemia and hepatosplenic fungal infections in patients with cancer, especially leukemia [5, 6]. In addition, C. tropicalis is the second most frequently isolated non-C. albicans Candida pathogen in the Asia–Pacific region and in Brazil [7, 8].
Several factors were reported to contribute to Candida pathogenicity, including adhesion to abiotic and biotic surfaces, biofilm formation (BF) and secretion of hydrolytic enzymes containing aspartyl proteases, phospholipases and hemolysins [9]. Adherence to host cells, which plays a vital role in its pathogenicity, is the first step in the invasive infections of Candida. Once the pathogens adhere to the host’s tissues or medical devices, cell division and proliferation will be activated, and biofilm will eventually develop [10]. Biofilm mainly consists of micro colonies of yeast, hyphae, pseudohyphae and extracellular matrix arranged in a complex structure [11, 12]. BF can confer significant tolerance to antifungal therapy primarily by limiting the penetration of substances through the biofilm matrix and protecting the embedded cells from host immune responses [9]. Another feature of Candida biofilms is their enhanced pathogenicity [13].
Although there is growing evidence of the importance of Candida adhesion and biofilm in their pathogenic mechanism, limited studies have been performed on C. tropicalis. The objective of this study was to determine the adhesion ability and biofilm production of C. tropicalis clinical isolates from China on abiotic (polystyrene) and biotic (human urinary bladder epithelial cell) surfaces, to analyze the correlation of adhesion/BF with the multilocus sequence typing (MLST) and other virulence factors, and to support further research on the role of virulent factors in pathogenesis of C. tropicalis.
Materials and Methods
Candida tropicalis Strains
A total of 68 C. tropicalis isolated from three general hospitals in Beijing, China, during a period of 3 years (2010.8–2013.9) were included in this study. These strains belong to the archive collection of the Chinese Centre for Disease Control and Prevention. The origins of these strains were as follows: 3 from blood, 3 from drainage, 7 from feces, 1 from prostatic secretion, 1 from sanies, 31 from sputum, 2 from throat swab, 10 from urea, 4 from vaginal secretion and 6 from unknown specimens. ATCC (American Type Culture Collection) 750 was used as a quality control. All strains were stored at −80 °C in brain–heart infusion (Oxoid) and maintained in Sabouraud dextrose agar media (SDA, Oxoid) at 25 °C during the study.
Determination of Adhesion and Biofilm Formation on Abiotic (Polystyrene) Surface
Adherence assay was performed in 96-well polystyrene flat-bottomed microtitre flat plates (PMP; Corning) according to Galan-Ladero et al. [14]. In short, C. tropicalis colonies from SDA plates were incubated in Sabouraud dextrose broth media (SDB, Oxoid) for 18 h at 120 rpm (revolutions per minute) at 37 °C. Then cells were harvested by centrifugation at 5000 g for 5 min and washed twice with phosphate buffer solution (PBS). The washed yeast cells were then suspended in PBS to 1 × 107 CFU ml−1. Next, the prepared suspension was distributed at 250 μl per well. After being incubated at 37 °C for 15 min and 24 h, unattached cells were removed by washing the wells three times with cold PBS. The immediate adhesion (IA) and the late adhesion (LA) were determined. All wells were detected at OD490. A well containing only PBS was used as a negative control.
BF assay was performed in 96-well PMP as according to Ramage et al. [15] with some modifications. Yeast suspension was prepared in RPMI-1640 culture medium (Gibco) with a concentration of 1 × 107 CFU ml−1. The plates were then incubated at 37 °C for 24 and 72 h. Non-adherent C. tropicalis cells were removed by washing three times with cold PBS. In accordance with previously described protocols [16, 17], BF was quantified by two methods: the crystal violet (CV) assay and the semi quantitative 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay. In addition, adhesion and BF on PMP surfaces were confirmed by inverted light microscopy (Nikon Eclipse Ti-S, 40× magnification).
Determination of Adhesion and Biofilm Formation on Biotic (Human Urinary Bladder Epithelial Cell) Surface
A human urinary bladder epithelial cell line (TCC-SUP; ATCC HTB-5™) was used in this study. Cells were cultured in minimum essential medium (Gibco) containing 10% fetal bovine serum (Gibco) supplemented with MEM non-essential amino acids solution (Gibco) and sodium pyruvate (Gibco) in cell culture flasks at 37 °C with 5% CO2. Cells were washed off using 0.25% trypsin–EDTA solution (Gibco). The cellular density was adjusted to 1 × 105 cells ml−1 in fresh minimum essential medium using a Neubauer chamber. Two milliliter of the suspension was added to a 12-well plate and incubated for 24 h. Before adhesion and BF assay on the TCC-SUP surface, the wells were washed twice with PBS.
For adhesion assay, mainly as according to Negri et al. [18], the washed yeast cells were resuspended in minimum essential medium, and the cellular density was adjusted to 1 × 107 cells ml−1. Two ml suspension of each C. tropicalis isolate was added to the corresponding well containing a confluent layer of epithelial cells. After being incubated at 37 °C with 5% CO2 for 2 h, the wells were washed twice with PBS to remove unattached cells.
For the BF study, the first step was the same as the adhesion assay. After the wells were washed twice with PBS, the washed wells were loaded with 2 ml minimum essential medium and incubated at 37 °C under 5% CO2 for 24 h. Later the wells were washed twice with PBS. Adhesion and BF on TCC-SUP surface were also confirmed by microscope observation (40× magnification) and determined by CV assay [19] and XTT assay [20]. There were a few differences with methods used for BF on PMP surface. Basically, for the CV assay, 1 ml 1% CV was added to the pre-washed wells and allowed to stain for 5 min. Then the CV was abandoned and the wells were washed three times with PBS. To remove the stain from the epithelial cells, 1 ml of ethanol: acetone (1:1) was added to the wells and discarded immediately. Acetic acid (33%, 2 ml) was added to the wells and incubated at 37 °C for 5 min. The absorbance was read at 570 nm. For the XTT assay, 200 μl XTT/menadione and 1.8 ml PBS were put into each well. Subsequently the plate was incubated in the dark at 37 °C for 2 h, and the absorbance was read at 420 nm. The well containing only TCC-SUP cells was used as a negative control. All experiments were performed at least three times for each strain.
Determination of Hydrolytic Enzymes Activity
The activity of hydrolytic enzymes, including the secretion of aspartyl proteinases, phospholipases and hemolysins, was determined on plates containing specific substrates by observing precipitation or translucent halo, mainly as according to Galan-Ladero et al. [21]. The activity of the secreted aspartyl proteinases was expressed according to PrZ_48 h (colony diameter/total diameter of the colony plus precipitation halo), the phospholipases activity was expressed according to PhZ (colony diameter/total diameter of the colony plus precipitation halo), and the hemolytic activity was expressed according to HZ_72 h (colony diameter/total diameter of the colony plus hemolysis halo).
MLST Analysis of Candida tropicalis Strains
The MLST type of 58 C. tropicalis isolates out of 68 isolates in this study was analyzed and published in our previous study [22]. In order to analyze the correlation between virulent activity and MLST types, the remaining 10 C. tropicalis isolates were also analyzed using MLST method following the same procedure as published [22]. The genetic relationship among the 68 strains and the MLST clonal clusters were determined using the minimum spanning tree of the BioNumerics software version 5.1 (Applied Maths, Kortrijk, Belgium). The method determines the putative relationships among isolates as related and records isolates as related when fewer than two of the six MLST gene loci are different. In addition, data from the variable loci from the six C. tropicalis alleles were conjoined into a single sequence, and then each base in the sequence was rewritten twice for a homozygous (A, C, G, or T) datum or as the two component bases for a heterozygous (K, M, R, S, W, Y) datum. These revised sequences could then be used to generate the genetic distance matrices.
Statistical Analysis
Analyses were performed using SPSS version 22.0. The results of the experiments were statistically described with frequency, medium and quartile range. Wilcoxon signed-rank test was used for comparison differences between two groups; the Kruskal–Wallis H test was used to determine statistically significant differences among groups; Spearman’s rank correlation was used for correlation analysis; Mantel test was used to compare two distance matrices; and “Corrplot” in R was applied in graphically illustrating the correlation coefficients in a circle graph. A Ρ value less than or equal to 0.05 was considered to be of statistical significance.
Results
Differentiated Adhesion Ability of Candida tropicalis Isolates on Abiotic and Biotic Surfaces
In this study, all C. tropicalis isolates displayed adhesion ability on PMP surface for both IA and LA (Figs. 1a, 2a, b). Several isolates (CYCT01, CYCT02, FXCT01, FXCT02, ZRCT16 and ZRCT32) showed the lowest IA ability with OD490 = 0.001. The FXCT01 isolate also exhibited the lowest LA with OD490 = 0.003 (Fig. 1a). Strain ZRCT47 displayed the highest IA and LA ability with OD490 = 0.021 and 0.056, respectively (Fig. 1a). The adhesion ability of LA was higher than IA in 97.1% of the strains, and only in two cases (ZRCT35 and ZRCT60) was it similar. This indicates that the adhesion ability strengthens over time, which is vital for subsequent BF and infection.
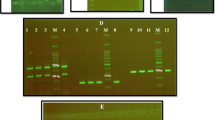
Adhesion and biofilm formation of typical C. tropicalis isolate on PMP surface by microscope. a Immediate and late adhesion of C. tropicalis. b Three adhesion levels of C. tropicalis. c Biofilm formation of C. tropicalis at 24 and 72 h. d Three biofilm formation levels of C. tropicalis. All panels were photographed at the same magnification (×40). The adhesion and biofilm formation were divided into three groups according to their distinct adhesion and biofilm ability on PMP surface using the following ranks: <25%, low; 25–75%, medium; and ≥75%, high
Adhesion of C. tropicalis on TCC-SUP surface was determined by CV and XTT assay. In the CV assay, a total of 61 (89.71%) isolates showed adhesion, and the remaining seven strains (CYCT02, ZRCT06, ZRCT48, ZRCT54, ZRCT61, ZRCT62 and ZRCT63) displayed no adhesion ability on the TCC-SUP surface (Figs. 1b, 3a). Strain ZRCT47 displayed the highest adhesion with OD570 = 0.027, while strains ZRCT02, ZRCT20, ZRCT42, ZRCT43 and ZRCT51 displayed the lowest adhesion (OD570 = 0.001) on the TCC-SUP surface (Fig. 1b). In the XTT assay, all 68 strains displayed adhesion on the TCC-SUP surface (Fig. 1b). Strain ZRCT45 displayed the highest adhesion (OD490 = 0.186), while ZRCT31 and ZRCT52 showed the lowest adhesion (OD490 = 0.006) (Fig. 1b). The tested value of all strains in the XTT assay was higher than that in the CV assay. This may indicate that the XTT assay was more sensitive than the CV assay in detecting the adhesion of C. tropicalis on the TCC-SUP surface.
Distinct levels of adhesion and biofilm formation by C. tropicalis on TCC-SUP surface in microscope. a Three adhesion levels of C. tropicalis. b Three biofilm formation levels of C. tropicalis. The red arrow points to TCC-SUP cells. All panels were photographed at the same magnification (×40). The adhesion and biofilm formation were divided into three groups according to their distinct adhesion and biofilm ability on TCC-SUP surface using the following ranks: <25%, low; 25–75%, medium; and ≥75%, high. (Color figure online)
Biofilm Formation Ability of Candida tropicalis Isolates on Abiotic and Biotic Surfaces
BF of C. tropicalis on PMP and TCC-SUP surfaces were also quantified by CV and XTT assay. The total biomass of biofilm was determined by CV assay, and the biofilm metabolic activity was determined by XTT assay. Regarding BF on PMP surface, all strains tested could form biofilm (Figs. 1a, 2d). The extent of biofilm formed at 24 and 72 h were similar (Fig. 2c). Thus, data of BF at 24 h were used in the following analysis. Strain CYCT01 produced the lowest biofilm in both CV and XTT assays (Fig. 1a). However, isolates producing the most massive mass of biofilm in these two methods were distinct: Strain ZRCT40 produced the strongest biofilm on PMP surface with OD570 = 3.804 in CV assay, while strain ZRCT51 produced the most biofilm with OD490 = 2.022 in XTT assay (Fig. 1a).
Regarding BF on TCC-SUP surface, all C. tropicalis isolates formed biofilm except for strains CYCT01, ZRCT16 and ZRCT51 by the CV method and strains CYCT02, ZRCT35 and ZRCT51 by the XTT method (Figs. 1b, 3b). In CV assay, ZRCT09 showed the largest biofilm biomass with OD570 = 0.140; while strain CYCT02, ZRCT52, ZRCT55 and ZRCT64 showed the smallest biofilm biomass with OD570 = 0.001. In XTT assay, ZRCT47 displayed the highest biofilm metabolic activity with OD490 = 0.570; while ZRCT50 showed the lowest biofilm metabolic activity with OD490 = 0.004 (Fig. 1b). Significant correlation was found between the two methods used for biofilm quantification on the abiotic surface (r s = 0.572) and the biotic surface (r s = 0.655) (Fig. 4).
Correlation coefficients of different virulence factors for C. tropicalis isolates. Correlation for each pair of virulence factors was determined using R statistical computing software (Spearman’s correlation and two-tailed probability of t for each correlation). Blue positive correlation, Red negative correlation; diameter of circles represents the absolute value of correlation for each pair of the virulence factor–virulence factor matrix. The time point of biofilm on polystyrene surface (BF_PMP_CV and BF_PMP_XTT) is 24 h. (Color figure online)
Relationship of Adhesion and BF Ability with MLST Types of Candida tropicalis Isolates
The minimum spanning tree dendrogram showed that most of the isolates were dispersed. Six groups were generated (Fig. 5 and Additional file 1). There were 7 diploid sequence types (DSTs) and 12 isolates in group1. Group2 had 5 DSTs and 6 isolates, group3 had 4 DSTs and 5 isolates, group4 had 3 DSTs and 3 isolates, group5 had 3 DSTs and 4 isolates, and group6 had 6 DSTs and 6 isolates, respectively (Fig. 5).
The analysis between MLST groups revealed differences in achieving statistical significance in both adhesion and BF (Table 1), thus indicating the existence of significant correlation between MLST types of C. tropicalis and adhesion or BF. C. tropicalis in group1 had the smallest mean ranks for adhesion on PMP surface, BF on PMP surface and BF on TCC-SUP surface (Table 1), which suggests that isolates of group1 had the lowest adhesion on abiotic surface, the lowest BF on abiotic surface and the lowest BF on biotic surface. C. tropicalis in group5 showed the highest adhesion (LA) on PMP surface and the highest BF on TCC-SUP surface. Isolates in group6 exhibited the highest adhesion (by XTT assay) on TCC-SUP surface and the highest BF (by XTT assay) on PMP surface. In general, C. tropicalis in MLST group5 and group6 showed high adhesion and BF, while isolates in group1 had low adhesion and BF.
In addition, significant correlation existed between the genetic distance matrices and the phenotypic distance matrices of BF_PMP_XTT (P = 0.013). But no significant correlations were found between the genetic distance matrices and the rest phenotypic distance matrices of IA_PMP, LA_PMP, Ad_cell_CV, Ad_cell_XTT, BF_PMP_CV, BF_cell_CV, BF_cell_XTT, PrZ_48 h, and HZ_72 h. The P values are 0.501, 0.479, 0.901, 0.119, 0.945, 0.219, 0.539, 0.991 and 0.535, respectively.
Correlation of Adhesion and Biofilm Formation with Other Virulence Factors
The correlation between adhesion and BF was firstly investigated. Significant associations were found between adhesion and BF on PMP surface (r s = 0.495 for IA and biofilm biomass; r s = 0.431 for IA and metabolic activity; r s = 0.315 for LA and biofilm biomass, and r s = 0.396 for LA and metabolic activity) (Fig. 4). Positive correlations were also found between adhesion and BF on TCC-SUP surface in CV analysis (r s = 0.244) and XTT analysis (r s = 0.602) (Fig. 4).
The hydrolytic enzymes (secreted aspartyl proteinases, phospholipases and hemolysins) for ZRCT01–ZRCT64 had previously been studied in our laboratory (Additional file 2) [23]. The determination of enzymatic activities by the remaining four strains (CYCT01, CYCT02, FXCT01 and FXCT02) was finished in this study (Additional file 2). Essentially, all C. tropicalis isolates produced proteinase and hemolytic activity, but no phospholipases were detected. Positive correlations were found between PrZ_48 h and IA, LA or biofilm biomass on PMP surface (r s = 0.390; r s = 0.228; and r s = 0.547, respectively) (Fig. 4). This indicates that a negative relationship exists between the activity of secreted aspartyl proteinases and IA, LA or biofilm biomass on PMP surface.
Activities of virulence factors by C. tropicalis were compared among anatomic sites with more than 3 samples, including those from blood, drainage, feces, sputum, urea and vaginal secretion. Isolates from blood had a mean rank of 5.67, 4.33 and 4.33 for IA, LA and secreted aspartyl proteinases (Table 2). Significant differences were observed for IA and secreted aspartyl proteinases among different anatomic sites (P is 0.018 and 0.050, respectively) (Table 2). This suggests that the adhesion on PMP of blood isolates was weak, while the activity of the secreted aspartyl proteinases was strong. These features of C. tropicalis isolated in blood may be associated with its invasive trait.
Discussion
Adhesion is considered to be the first event for colonization, BF and persistent infection of C. tropicalis [9]. In our study, most isolates exhibited adhesion on PMP and TCC-SUP surfaces. On PMP surface, the activity of adhesion increased with prolongation of culture, which is supported by a previous study [14]. This may be the reason why inpatients with longer stays in the hospital have much higher chances of getting infected. On TCC-SUP surface, the results of adhesion in CV and XTT assay were slightly deviated. Namely, all strains could adhere to TCC-SUP surface in XTT assay, while all isolates except for seven strains displayed adhesion in CV assay. These results suggest that the XTT method was more sensitive in detecting adhesion of C. tropicalis on TCC-SUP. It may indicate that XTT assay seems to be a good quantification method for adhesion of Candida on epithelial cells. C. tropicalis isolates from blood cultures demonstrated lower adhesion on PMP surface. It is worth noting that a similar phenomenon has been reported in a study on C. parapsilosis, where the systematic isolates demonstrated lower adherence to buccal epithelial cells than the superficial isolates [24]. At the same time, blood isolates exhibited strong secreted aspartyl proteinases activity. Secreted aspartyl proteinases are capable of degrading epithelial and mucosal barrier proteins, which facilitate invasion [25]. These results reveal that low adherence and strong activity of secreted aspartyl proteinases may be related to the invasive feature of C. tropicalis. This should be confirmed with an expanded sample size in a further study. At the same time, negative correlations existed between secreted aspartyl proteinases activity and adhesion on PMP, which was in accordance with the traits of blood strains.
We found that the tested C. tropicalis isolates produced large amounts of biofilm on the PMP surface as described previously [3, 26]. This has revealed that C. tropicalis show strain-dependent BF ability [2, 14]. According to the cutoffs (CV assay: low BF <0.44, medium BF = 0.44–1.17, high BF >1.17; XTT assay: low BF <0.097, medium BF = 0.097–0.2, high BF >0.2) proposed by Marcos-Zambrano et al. [27], 40 out of 68 C. tropicalis isolates were graded as low BF by CV method, while most (92.6%) strains were categorized as high BF by XTT assay in our study. It illustrates that although our strains displayed relatively small amounts of biomass, they showed higher rates of metabolic activity of BF [27]. Several studies indicate that biofilm metabolic activity is highly correlated with biomass (r > 0.7) [11, 28], but no correlation between those two methods was found in Silva’s study [26]. In our study, significant correlation existed between CV and XTT assay, but the coefficient of correlation was small (r s = 0.572). Interestingly, we also found that the majority of C. tropicalis with high biomass of biofilm showed relatively low metabolic activity. This phenomenon aligned very well with the paper reported by Marcos-Zambrano et al. [27] and Negri et al. [29]. Several reasons may be responsible for the differences between the two methods: First, CV can stain both active cells and the extracellular matrices, while XTT assay measures BF only through living cells’ metabolic activity [28]. Second, the compact structure and thick extracellular matrix of biofilm with high biomass can impair diffusion of nutrients and oxygen [30], thus leading to lower metabolic activity in cells. Although Candida could form biofilm on mucosal surfaces and trigger recurrent infections [31], there have not been many studies on BF of Candida on cell surface until now. BF of C. tropicalis on monolayer cell (TCC-SUP) surface was firstly analyzed in our study. Most (95.59%) C. tropicalis in CV and XTT assay could form biofilm on TCC-SUP surface. In our study, a positive correlation was found between adhesion and BF. Silva et al. also showed that positive association exists between adhesion and biofilm biomass for C. tropicalis [26]. However, both correlations were small. Therefore, we think that adhesion is likely to be related to BF for C. tropicalis, but adhesion is not the only factor for predicting BF of C. tropicalis. Besides, Galan-Ladero demonstrated that cellular surface hydrophobicity, growth and filamentation ability play a fundamental role in BF [14].
Until now, there have been few studies focusing on the association between virulent phenotypes and genotypes in Candida. In Li’s study, C. albicans within a clone or a clonal lineage determined by PCR–RFLP demonstrated phenotypic diversity. No significant differences were found among these clonal lineages. However, there were two groups containing strains with high BF [28]. Silva-Rocha et al. discovered that genotype C of C. albicans were more resistant to phagocytosis by polymorphonuclear neutrophils than genotypes A and B [32]. Our study firstly analyzed the relationship between virulence factors and MLST types. Significant differences were found among MLST groups for adhesion and BF. Most isolates in group5 and 6 displayed high adhesion or BF, while C. tropicalis of group1 exhibited lower adhesion and BF. This suggests that significant correlation exists between MLST types and virulence activity for C. tropicalis. In the future, with large population structure performed by whole genome sequencing, whether there are groups of C. tropicalis isolates with higher virulent or invasive ability or potential ability evolving to high virulent isolates will be determined.
In conclusion, the profile of adhesion and biofilm on abiotic and biotic surfaces by 68 C. tropicalis clinical isolates were analyzed. Almost all isolates could produce adhesion and BF with a strain-dependent feature, which is helpful for selecting representative strains in further studies. Blood isolates exhibited low adhesion on PMP surface and had strong activity of secreted aspartyl proteinases, which provides clues in studying the invasive mechanisms of C. tropicalis. In addition, significant correlations existed between adhesion/BF with MLST type. Most C. tropicalis in MLST group5 and group6 were associated with high adhesion and BF, while group1 contained strains with low adhesion and BF. Above all, the identification of virulence factors and their correlation with each other will aid in the understanding of C. tropicalis pathogenesis of infection.
References
Butler G, Rasmussen MD, Lin MF, Santos MA, Sakthikumar S, Munro CA, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–62.
Chaves GM, Diniz MG, da Silva-Rocha WP, de Souza LB, Gondim LA, Ferreira MA, et al. Species distribution and virulence factors of Candida spp. isolated from the oral cavity of kidney transplant recipients in Brazil. Mycopathologia. 2013;175:255–63.
Mohandas V, Ballal M. Distribution of Candida species in different clinical samples and their virulence: biofilm formation, proteinase and phospholipase production: a study on hospitalized patients in southern India. J Glob Infect Dis. 2011;3:4–8.
Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20:5–10.
Franca EJ, Furlaneto-Maia L, Quesada RM, Favero D, Oliveira MT, Furlaneto MC. Haemolytic and proteinase activities in clinical isolates of Candida parapsilosis and Candida tropicalis with reference to the isolation anatomic site. Mycoses. 2011;54:e44–51.
Lai HP, Chen YC, Chang LY, Lu CY, Lee CY, Lin KH, et al. Invasive fungal infection in children with persistent febrile neutropenia. J Formos Med Assoc. 2005;104:174–9.
Colombo AL, Nucci M, Park BJ, Nouer SA, Arthington-Skaggs B, da Matta DA, et al. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J Clin Microbiol. 2006;44:2816–23.
Pfaller MA, Segreti J. Overview of the epidemiological profile and laboratory detection of extended-spectrum beta-lactamases. Clin Infect Dis. 2006;42:S153–63.
Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 2011;19:241–7.
Ramage G, Martinez JP, Lopez-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 2006;6:979–86.
Hasan F, Xess I, Wang X, Jain N, Fries BC. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 2009;11:753–61.
Silva S, Henriques M, Martins A, Oliveira R, Williams D, Azeredo J. Biofilms of non-Candida albicans Candida species: quantification, structure and matrix composition. Med Mycol. 2009;47:681–9.
Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Kohler JR, et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010;6:e1000828.
Galan-Ladero MA, Blanco-Blanco MT, Hurtado C, Perez-Giraldo C, Blanco MT, Gomez-Garcia AC. Determination of biofilm production by Candida tropicalis isolated from hospitalized patients and its relation to cellular surface hydrophobicity, plastic adherence and filamentation ability. Yeast. 2013;30:331–9.
Ramage G, Vandewalle K, Wickes BL, Lopez-Ribot JL. Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol. 2001;18:163–70.
Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods. 2008;72:157–65.
Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, Ramage G, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3:1494–500.
Negri M, Botelho C, Silva S, Lopes LM, Henriques M, Azeredo J, et al. An in vitro evaluation of Candida tropicalis infectivity using human cell monolayers. J Med Microbiol. 2011;60:1270–5.
Negri M, Goncalves V, Silva S, Henriques M, Azeredo J, Oliveira R. Crystal violet staining to quantify Candida adhesion to epithelial cells. Br Biomed Sci. 2010;67:120–5.
Chandra J, Mukherjee PK, Ghannoum MA. In vitro growth and analysis of Candida biofilms. Nat Protoc. 2008;3:1909–24.
Galan-Ladero MA, Blanco MT, Sacristan B, Fernandez-Calderon MC, Perez-Giraldo C, Gomez-Garcia AC. Enzymatic activities of Candida tropicalis isolated from hospitalized patients. Med Mycol. 2010;48:207–10.
Wu Y, Zhou H, Wang J, Li L, Li W, Cui Z, et al. Analysis of the clonality of Candida tropicalis strains from a general hospital in Beijing using multilocus sequence typing. PLoS ONE. 2012;7:e47767.
Yu S, Li W, Che J, Bian F, Lu J, Wu Y. Study on virulence factors of Candida tropicalis isolated from clinical samples. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36:1162–6.
Panagoda GJ, Ellepola AN, Samaranayake LP. Adhesion of Candida parapsilosis to epithelial and acrylic surfaces correlates with cell surface hydrophobicity. Mycoses. 2001;44:29–35.
Borst A, Fluit AC. High levels of hydrolytic enzymes secreted by Candida albicans isolates involved in respiratory infections. J Med Microbiol. 2003;52:971–4.
Silva-Dias A, Miranda IM, Branco J, Monteiro-Soares M, Pina-Vaz C, Rodrigues AG. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: relationship among Candida spp. Front Microbiol. 2015;6:205.
Marcos-Zambrano LJ, Escribano P, Bouza E, Guinea J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: comparison of biomass production and metabolic activity and development of cut-off points. Int J Med Microbiol. 2014;304:1192–8.
Li X, Yan Z, Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology. 2003;149:353–62.
Negri M, Silva S, Capoci IR, Azeredo J, Henriques M. Candida tropicalis biofilms: biomass, metabolic activity and secreted aspartyl proteinase production. Mycopathologia. 2016;181:217–24.
Alnuaimi AD, O’Brien-Simpson NM, Reynolds EC, McCullough MJ. Clinical isolates and laboratory reference Candida species and strains have varying abilities to form biofilms. FEMS Yeast Res. 2013;13:689–99.
Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. Characterization of mucosal Candida albicans biofilms. PLoS ONE. 2009;4:e7967.
da Silva-Rocha WP, Lemos VL, Svidizisnki TI, Milan EP, Chaves GM. Candida species distribution, genotyping and virulence factors of Candida albicans isolated from the oral cavity of kidney transplant recipients of two geographic regions of Brazil. BMC Oral Health. 2014;14:20.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Youth Project No. 81301409), the National Sci-Tech Key Project (Grant No. 2013ZX10004203-002) and the National Key Technology Support Program (Grant No. 2012BAI11B05). We thank Yi Teing Cheung from Princeton University for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, S.B., Li, W.G., Liu, X.S. et al. The Activities of Adhesion and Biofilm Formation by Candida tropicalis Clinical Isolates Display Significant Correlation with Its Multilocus Sequence Typing. Mycopathologia 182, 459–469 (2017). https://doi.org/10.1007/s11046-017-0111-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-017-0111-2