Abstract
Emerging concepts in nanotechnology have gained particular attention for their clinical translation of immunotherapies of cancer, autoimmune and infectious diseases. Several nanoconstructs have been engineered with unique structural, physicochemical, and functional features as robust alternatives for conventional chemotherapies. Traditional cancer therapies like chemotherapy, radiotherapy, and ultimately surgery are the most widely practiced in biomedical settings. Biomaterials and nanotechnology have introduced vehicles for drug delivery and have revolutionized the concept of the modern immunotherapeutic paradigm. Various types of nanomaterials, such as nanoparticles and, more specifically, drug-loaded nanoparticles are becoming famous for drug delivery applications because of safety, patient compliance, and smart action. Such therapeutic modalities have acknowledged regulatory endorsement and are being used in twenty-first-century clinical settings. Considering the emerging concepts and landscaping potentialities, herein, we spotlight and discuss nanoparticle-based immunotherapies as a smart and sophisticated drug delivery approach to combat cancer metastasis. The introductory part of this manuscript discusses a broad overview of cancer immunotherapy to understand better the tumor microenvironment and nanotechnology-oriented immunomodulatory strategies to cope with advanced-stage cancers. Following that, most addressable problems allied with conventional immunotherapies are given in comparison to nanoparticle-based immunotherapies. The later half of this work comprehensively highlights the requisite delivery of various bioactive entities with particular cases and examples. Finally, this review also encompasses a comprehensive concluding overview and future standpoints to strengthen a successful clinical translation of nanoparticle-based immunotherapies as a smart and sophisticated drug delivery approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Noncommunicable diseases (NCDs) have a massive contribution to global deaths, and cancer is regarded as the most common cause of deaths. NCDs share 63% of global deaths, while cancers contribute 9% of global deaths [1, 2]. The incidence of cancer is growing sharply, and cancer-associated deaths are believed to be the main barrier to elevating life expectancy during the initial decades of the twenty-first century [2,3,4]. The tumor undergoes several molecular-level evolutionary processes during growth that consequently yield specific changes, allowing the immune response of the tumor cells. Similarly, tumor cells escape from the tumor niche, invade the neighboring tissues, enter through the circulatory pathways, and are implanted into distant tissues and organs by forming premetastatic niches that subsequently form metastatic lesions. It is driven by genetic and epigenetic factors of tumor cells and certain biomolecules produced in the tumor microenvironment (TME) [5,6,7]. Tumor metastasis is one of the main factors driving cancer-related fatalities [5, 6]. Metastasis is a complex nonlinear series of events and is considered to be the most crucial factor responsible for drug resistance and a potential barrier in the successful therapy of cancers. Besides, it can cause organ damage under critical conditions. Several authors organize detailed mechanisms of metastasis, readers are referred to go through these reviews to understand further metastasis and its impact on therapeutic outcome [5,6,7,8,9]. An overview of the complex and concurrent routes of metastasis is shown in Fig. 1 [6].
Overview of the complex and concurrent routes of metastasis. Reprinted from Suhail et al. [6] with permission under a Creative Commons license
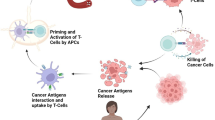
For a long time, oncologists have relied only on three types of therapies: surgery, chemotherapy, and radiotherapy for cancers [10]. Later, cancer treatments then evolved, and new therapeutic modalities were developed [11]. Nowadays, various types of therapies and strategies are being used to treat cancers, overcome the barrier of metastasis and eradicate cancer cells from the human body [12]. Most of the therapies lack discrimination between normal and cancer cells ideal immunotherapies should be able to tolerate normal cells and target cancer cells and are associated with off-target toxicities. Evidence indicates that the immune system can manage tumors effectively. For instance, revocation of the tumor without treatment, presence of various types of tumor-specific immune cells like cytotoxic T cells (CTCs), monocytes, lymphocytes and plasmacytic infiltrates, higher incidence of cancer in immunocompromised patients and cancer remission with immunomodulators can augment the role of the immune system in cancers [13]. Immunotherapies have shown the fastest growth during recent years and have proven to be the most promising in oncology and have a good impact on human health [14]. Immune system studies suggest that the efficacy of immunomodulatory approaches depends on activation of baseline immune response and untethering the pre-existing immunity [15]. However, effector T cells-induced immune response plays a pivotal role [14]. Recent progress in technology has allowed researchers to explore the mechanisms and role of tumor immune environment and immune systems in tumor growth and metastasis (Fig. 2) [13, 14]. Immunomodulatory approaches have offered opportunities and extended arms towards clinical translation of immunotherapies for cancer treatment. Optimization of emerging “cancer immunotherapy” is regarded as one of the major pillars in treating malignancies along with surgery, chemotherapy and radiotherapy. This review discusses the recent progress in understanding the tumor microenvironment and nanotechnology-oriented immunomodulatory strategies to treat initial and advanced-stage cancers. Besides, it also gives a comprehensive overview of the limitation of immunotherapies and current challenges associated with this nanotechnology.
The tumor–immune classification cycle as a tool to direct anticancer therapy. Reprinted from Galon and Bruni [14], with permission from Springer Nature. License Number: 5121760526516
Immunotherapies―potential therapeutic modalities
Immunotherapies are the immunomodulatory therapeutic regimens that boost up the immune system and bears specificity, potency, and memory as the potential as most important features. Inspired by these features immunotherapies are being considered for development in a wide range of different types of immune disease, infectious diseases and cancers at preclinical and clinical [16, 17]. The concept of cancer immunotherapies was probably hypothesized around the 1890s by William Coley, when he used bacterial toxins to treat cancer patients [11, 18]. This evidence was further endorsed by Thomas and Burnet theory of tumor immune surveillance. In the field of oncology, immunotherapies have seen alternate intervals of success and failure [18]. Previously, immunotherapies were restricted by severe toxicities and limited efficacy, while the recent breakthrough advances in understanding fundamental immunology and translational immunotherapy in cancer research have opened a new era of immunotherapies with a positive, encouraging impact in advanced stage cancers [19,20,21].
The immune system can eliminate cancers via two possible ways; (1) Natural or innate immune response or (1) acquired or adaptive immunity. But the tumor immune escape mechanism involves three phases of reaction; in the first phase immune system recognize the cancer cells and remove them from the body, the second phase immune system maintain a balance between cancer cells and immune cells and finally cancer cells immunoediting give rise the synthesis of immunocompatible cancer cells which have immune escape ability. Subsequently, these types of cells survive and develop tumors [22]. To mount an effective immunotherapeutic response, (1) released tumor cell antigens must be taken up by dendritic cells (DCs) must take up the tumor cell antigens with proper maturation signals to prompt differentiation and antigen presentation. (2) DCs should also be able to activate antitumor T cell responses driving their differentiation into tumor-specific activation of CTCs activating Natural Killer cells (NKCs) or Natural Killer T cells (NKTCs) response and heightening T helper cells 1 response. Subsequently, antitumor T cells must penetrate the tumor and elicit an antitumor response [23].
Immunotherapies are aimed to reprogram the tumor immune microenvironment, increase the cytotoxicity of cellular immunity by the CTCs and NKCs via administration of stimulators and strengthen the immune system to recognize the threat of cancers to eliminate it (Fig. 3) [24]. Immunotherapies mediate the activation, proliferation, differentiation and survival of antitumor lymphocytes and are elicit antitumor responses to eliminate tumor cells [25]. For instance, cytokines play an important role in regulation of anti-tumor CTCs or NKCs functions and significant evidence augments that interleukin-15 (IL-15) signaling must be optimal to maintain the full potential of NKCs antitumor activity [25]. Triggered by signals from surface receptors like NKG2D, DNAM1, NKp30, NKp44 and NKp46, NKCs release cytotoxic granules on target. NKCs bearing Fc-receptor CD-16 does not need activation signal by other NKCs, it mediates antibody-dependent cellular toxicity upon it interaction with antibody-coated cells. NKCs can also kill the target cell by tumor necrosis factor (TNF) related apoptosis-inducing ligand receptor (TRAILR) pathway and FAS–FAS ligand (also known as CD-95-CD-95 ligand) pathway [24]. While other specific monoclonal antibodies-based immunotherapies block the programmed death-1 (PD-1 or its receptors PD-L1/PD-L2) pathway [26]. For detailed mechanisms of action of immunotherapies, readers are referred to read the relevant literature.
Schematic overview of drugs that bolster NK cell antitumour immunity and their interaction points. Reprinted from Childs and Carlsten [24], with permission from Springer Nature. License Number: 5121760703231
Immunotherapies includes various modalities to deal with cancers including cytoimmunotherapies [23], growth factors or growth factor inhibitors [27, 28], vaccination [11], immunomodulatory strategies [14], cytokines [25], antigens and neoantigens [29], antibodies [30], etc. Among them, autologous tumor-directed T-cell therapy also known as adoptive cell therapy has shown high proportion of complete response in metastatic melanoma and other hematologic malignancies [19,20,21]. Immune checkpoint blockade therapy; administration of an antibody (ipilimumab) that hamper the inhibitory receptor of human cytotoxic T cell antigen-4 (CTCA-4) or PD-1/PD-L1 has expressed complete response in 20% of patients with advanced melanoma [31, 32]. Published literature shows that antibodies therapeutics possibly constitute a substantial part of biological drugs. A total of 79 antibodies have been approved by the United States FDA [33]. While upon combining both of the therapies (CTCA-4 and PD1) has shown quick and complete tumor regression in 10 weeks, thus indicating their synergistic therapeutic effects [30, 34]. Larkin et al. reported more than 5 years’ overall survival was 52% in combination of ipilimumab and nivolumab, nivolumab group showed 44% as compared with 26% overall survival of ipilimumab group [35]. Similarly, Hodi et al. report that median overall survival did not reach after 4 years follow up report of clinical trials [36] and Wolchok et al. observed that after 3 years overall survival rates were 58% in combination, 52% in nivolumab and 34% in ipilimumab administered patients [37].
Problems allied with conventional immunotherapies
After the complete treatment, follow-up care is critical to monitor for treatment relapse leading to secondary malignancies and post-therapeutic long-term complications [38]. The most important questions that were previously associated with immunotherapies are effectiveness and toxicity. However, the recent breakthroughs have given a landscaping direction for their success. At the same time, it has opened new challenges for immunotherapy of the modern era. Safe and accurate delivery of therapeutic agents at the target site is nanotechnology-based therapies' main aim, which possibly can maintain the efficacy of therapy and optimize the output while minimizing toxicities [39,40,41]. After injection, nanomedicine is subjected to different obstacles inside the body that can restrict drug from reaching the target. Various organs like liver, spleen, lymph nodes, lungs, and skin are considered the most important barriers among them [42]. Besides organs, significant data augments that various physiological and pathological anatomical structures like pleural fluid (surfactant) [43], blood–brain barrier [44,45,46], ocular structure [47,48,49], abnormal tumoral vasculature [50, 51], high intratumoral pressure [51], etc. are important barriers. Poor lymphatic drainage of the tumor area results in the accumulation of various substances at the regional or local level which are responsible for vascular permeability (such as angiotensin, bradykinins, angiotensin-converting enzyme inhibitors, nitric oxide, etc.) is also a hurdle in the successful delivery of nanoparticles [52,53,54]. Each entity regarded as a barrier in delivery has its mechanism, which unfavored the successful transportation of the carrier at the target site. Interested readers can go through the relevant literature, some of the manuscripts we have cited in this section at the appropriate place as well.
Since 1986 the concept of enhanced permeability and retention (EPR) effect has been a central paradigm in cancer nanomedicne. It is believed that increased vascular permeability optimize transport of high molecular weight drugs and prodrugs [55,56,57]. While, recent investigations by Sindhwani et al. report that although there are gaps in endothelium of tumor tissue size 2 µm. Still, the endothelium is mostly intact and active transport mechanism is followed for the remittance of nanoparticles through endothelial cells. He further shows that these interendothelial gaps are responsible for the extravasation of a limited (only 3%) amount nanoparticles into tumor tissue [58]. These challenges need to pay special attention to develop systematically developed nanotechnology-based targeted immunotherapies.
Besides anatomical and physiological barriers of nanotechnology-based systems of drug delivery, immunotherapy-related toxicities, resistance to immunotherapies and limited clinical benefits are major challenges associated explicitly with immunotherapies. Toxicities of immunotherapies can be explicated may range from general symptoms like fever or fatigue to organ damage like colitis, rash, adrenal and thyroid insufficiency [59]. Immunotherapies are believed to have fewer long-term toxicities than radiotherapy and chemotherapies, especially in pediatrics. Immunomodulatory agents such as cytokines and monoclonal antibodies (mAbs) are well tolerated. Fever myalgia’s, chills, headache, fatigues are common symptoms associated with cytokines while acute infusion-related reactions are associated with mAbs. Infusion-related acute reactions are well managed by administering antihistamines, antipyretics, and corticosteroids [60, 61]. Body of mAbs target bearing cells are indiscriminately diminished by the mAbs. For instance, rituximab depletes B cells and induce humoral immunosuppression, which can lead to higher risk of infection [60].
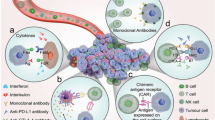
The main concern of the adoptive T-cell transfer is their lack of discrimination between cancerous and normal cells, so they also diminish the target bearing cells (even with low expression) in normal tissue. Cytokine release syndrome is another severe and fetal entity associated with adoptive T-cell therapy and may lead to organ failure and death [62, 63]. Checkpoint inhibitors show mild to moderate reactions such as hepatitis, pneumonitis, colitis and pancreatitis, thyroiditis, in young age patients treated with ipilimumab [64], while the PD-1/PD-L1 blockade showed hepatitis, pneumonitis, colitis, thyroiditis, dermatitis and hypophysitis and was comparably less toxic than ipilimumab [65,66,67]. However, these side effects were effectively managed by short course of steroids or interruption of therapy [68]. Michot et al. observed hemophagocytic syndrome in a patient after 8 weeks of immune checkpoint inhibitors administration, which was treated with corticosteroids. Unluckily, the patient developed a brain hemorrhage and died off later on [69]. In spite of significant advances and durable response of Immune checkpoint inhibitors, a significant proportion of patients do not benefit due to metastasis or acquired resistance due to intrinsic and extrinsic environmental factors [59]. Figure 4 shows these molecules and their targets [59].
Immune interactions involving antigen presenting cells or tumor cells, T cells, and tumor microenvironment. Reprinted from Marin-Acevedo et al. [59] with permission under a Creative Commons license
Resistance to immunotherapy
Complete response in patients is the forefront aim of therapies. Resistance to various therapies is a big challenge in clinical practice that leads to remission of disease, extended hospital stays, increased morbidity, mortality, and significantly affects the health budget [70]. Abundant literature justifies the rising tide of drug resistance in various types of cancers and against various therapeutic modalities derived by intrinsic and extrinsic factors [71]. Evidence of heterogeneous responses in different lesions in same patients exists. Tumor antigen disappearance, refashioned pathway of antigen presenting machinery like mutation in beta-2 microglobulin, downregulation or deletion of major histocompatibility complex (MHC), inhibition of immune cell infiltration or functions like mutation in PI3K, mitogen-activated protein kinase pathway and various mutations interferon gamma pathway are tumor intrinsic pathways responsible for resistance to therapies [72, 73]. Other extrinsic factors such as regulatory T cells (Tregs), myeloid derived suppressor cells, M2 macrophages, alongside other inhibitory immune checkpoints can also be important contributors in the way of antitumor immune response [74]. Disclosure of accurate molecular immune microenvironment of tumor such as PD-L1 expression, mutational loads, genes involved in chromatin remodeling, loss of various genetic information’s, etc. could be helpful in planning the efficient dosage administration and schedule of multiple immunotherapies while lowering the risk of disease remission or failure of therapy [75, 76].
Activation of dendritic cells through nanoparticles
Dendritic cells, B cells, and macrophages are the major components of the immune system and are prime representatives of antigen-presenting cells (APCs). These cells stimulate the adaptive immune response and through this stimulation exert antitumor efficacy [77]. These APCs possess the capabilities to phagocytize the antigens as well as damage-associated molecular patterns. When these APCs are stimulated, they present antigens on the major histocompatibility complexes to other immune cells like cytotoxic T-lymphocytes (CTLs) and downstream cytokines excretion [78]. Due to the key role of APCs in stimulating such responses, substantial efforts have focused on targeting the APCs with several kinds of nanoparticles having immunomodulating agents.
DCs are professional antigen-presenting cells that are skilled in propagating an antigen-specific immune response against pathogens. Hence, immunotherapeutic approaches exploit DCs to present antigens as a source of cell-mediated therapeutic vaccination in patients having advanced levels of malignancies. In this connection, ex vivo DCs are trained with antigens and then these are transferred back into patients for vaccination [79]. Although, a higher antigen-specific CTLs response is produced by this strategy at the metastatic tumor site, however, this approach lacks therapeutic efficacy in clinical trials. Additionally, this approach is also technically challenging and expensive as well. Thus, in situ targeting of DCs with adjuvant and antigen-loaded nanoparticles can significantly upsurge the clinical applications of dendritic cell-mediated immunotherapy.
Delivery of antigens
As compared with soluble formulation, loading of antigens into the nanoparticles presents greater advantages. Firstly, nanoparticles protect the antigens from degradation and deliver them to the target in a more precise and sustained manner than free antigens. Secondly, nanoparticles prevent the entry of the loaded antigens into the blood circulation thus reduced the off-target toxicity on one hand and provides targeted delivery to the resident immune cells on the other hand. The most important advantage of loading antigens into the nanoparticles is that the DCs tend to cross-present the antigens more effectively when conjugated with nanoparticles. Thus, to increase the efficacy of dendritic cells-mediated immunotherapy, several immunostimulatory and antigen-based compounds have been fabricated in nanoparticles to provide site-specific delivery in vivo [80].
In one study, Gao et al. formulated chitosan and carboxymethyl chitosan-based nanoparticles laden with extracellular material of Vibrio anguillarum. In this study, it was found that as compared with free extracellular material, the material laden within the nanoparticles exhibited enhanced innate as well as adaptive immune responses [81]. Similarly, Maji et al. evaluated the impact of cationic liposomes on the antigen-presenting and maturation capacity of the dendritic cells. From this study, it was found that as compares with anionic and neutral liposomes, the cationic liposomes were being taken up more efficiently by DCs and delivered to the target site for major histocompatibility complexes-based processing [82]. Rietscher et al. investigated the potential of PEG-b-PAGE-PLGA as a nanocarrier for prophylactic vaccination. They have also loaded ovalbumin as a model antigen within this nanocarrier. In this study, it was found that as compared with free and soluble ovalbumin antigen, the antigen that was loaded into nanoparticles exhibited significantly higher T-cell activation through antigen-presenting cells [83]. Several nanoparticles-based approaches for cancer immunotherapy are shown in Fig. 5 [84].
Visual illustration of several nanoparticles-based approaches for cancer immunotherapy. Reprinted from Qiu et al. [84] with permission from John Wiley and Sons. License Number: 5121761002630
Delivery of adjuvant
Vaccines are important form of immunotherapy and activate immune system. Vaccines efficient for antigen processing and presentation. When co-delivered with chemotherapeutics, they generate a synergistic effect and heighten the efficacy of therapies [85]. In immunotherapy, the vaccine adjuvants stimulate the immune system against a specific antigen. These adjuvants imitate the particular set of pathogenically preserved molecules called pathogen-associated molecular patterns. One such immune-stimulating compound is lipopolysaccharide which is the component of the bacterial cell wall, and nucleic acid situated in the pathological environment. As the immune system is involved to detect these moieties, thus the conjugation of the adjuvant with antigens can significantly upsurge the activity of the lymphocytes, dendritic cells, and macrophages [86]. Nevertheless, these immunostimulators can also promote undesired side effects like toxic shocks when administered systemically. In this regard, the delivery of chemotherapies through nanocarriers has significantly reduced the toxicity of various drugs (i.e. amphotericin B, and doxorubicin) through site-specific delivery at lower doses [87].
In one study, Sokolova et al. examined the potential of cationic gelatin-based nanoparticles for delivery of CpG adjuvant (a short single-stranded DNA that effectively stimulates DCs via toll-like receptors inside the phagosomes). In this study, it was observed that as compared with free CpG, the CpG delivered through nanoparticles exhibited significantly higher antigen-specific T-cell response, greater antitumoral immunity, and targeted delivery in a murine melanoma model [88].
In addition to the delivery of a single adjuvant, the nanocarriers also possess the potential to deliver multiple adjuvants in a single nanocarrier. In this regard, Schlosser et al. formulated the PLGA-based microspheres loaded with OVA antigen and CpG adjuvant. This combination system exhibited much better CTL responses in mice than alone OVA or CpG [89]. Similarly, Hamdy et al. observed that combined delivery of tyrosine-related protein 2 (i.e. poor immunogenic melanoma antigen) and monophosphoryl lipid A (i.e. an adjuvant) in a PLGA-based nanocarrier exhibited antitumor activities [90]. In another study conducted by Li et al. mice immunization with autophagosomes extracted from cancer cells and α-Al2O3 as an adjuvant resulted in tumor regression. They also reported that the combined delivery of two adjuvants without conjugation was inefficient, hence conjugation is very important to get the desired results. Fen et al. demonstrated that coating of nanoparticles, having MPL as an adjuvant, with a layer of cancer cells membrane possess the potential to produce a Tumor antigen associated (TAA) specific immune response [91].
Dendritic cells targetting
Active targeting of nanoparticles and their cargos to the DCs offers a promising approach for targetted and efficient delivery of immunotherapeutics. The nanoparticles' surface tailored with certain ligands like fucose, mannose, anti-CD11c, N-acetyl glucosamine, and anti-DEC205 have shown preferential higher uptake of nanoparticles within the cell through receptor-mediated endocytosis [92]. Furthermore, binding of these nanoparticles to the specific receptors expressed on the DCs also increases the maturation and hence further improves the efficacy of the vaccine formulation [93]. In this regard, Kempf et al. fabricated ligand conjugated PLGA-based nanoparticles for targeted delivery to the dendritic cells. as compared with blank nanoparticles, ligand conjugated nanoparticles exhibited more efficient delivery of nanoparticles to the dendritic cells, higher expression of IL-2, and higher upregulation of DCs maturation markers like CD 86 and CD 83 [94].
In this connection, Qian et al. formulated a vaccine that was functionalized with tumor antigen peptides that reach the target site via scavenger receptor-mediated class B1 pathway. The self-assembly, smaller size, and targetability of the prepared nanovaccine illustrated significant loading of tumor antigen peptide, considerable accumulation in lymph nodes (LNs), and increased antigen presentation. They also established that nanovaccine could be used alone as well as in combination with CpG as a therapeutic or prophylactic nanovaccine. Moreover, they also concluded that the size of the nanoparticles also plays an important role in site-specific delivery [95]. Mostly, large size particles (500–2000 nm) uptake by LNs relies on the DCs uptake, while the small-sized nanoparticles (20–200 nm) are freely uptaken by the LNs and subsequently target the LN-resident DCs [96].
Recntly, supramolecular peptides, protein and their derivatives are gaining special attention of researchers for immunological applications like vaccines and certain other immunotherapies [97]. Biomaterials created from Liu et al. admnistered CpG-DNA/peptide vaccines comprising an antigen and adjuvent cargo in tumor bearing mice. Authors observed 30 folds higher T cell priming, increased LN accumulation, lesser systemic disruption and optimized antitumor effect while minimizing toxicities [98]. However, the current approaches used in cancer vaccine delivery neccessitate direct peptide alteration that affect the vaccine efficacy. Li et al. has recently reported more effective adsorption approach using polyethyleneimine in mesoporous slica microrods (MSR) vaccines to the immunogenocty of vaccine. This atrategy resulted better outcome than that of existing MSR and bolus vaccines to activate the host HCs and T cells antitumor response. It eradicated established tumors in 80% mices and interestingly developed iimunological memory [99].
Kokate et al. investigated that RNA-lipoplexes could be efficiently targeted in vivo. Authors found that the lipoplexes could be targeted to the site of action (i.e. dendritic cells, and spleen) by simply adjusting the net charge on the nanoparticles. These nanoparticles have shown promising results in various cancer models and currently, these are in phase 1 clinical trials. In the future, several targeting mechanisms can also lead to state-of-the-art ways of prompting dendritic cell manipulation through nanocarriers [100]. Denditic cell based syntheitc multiepitope DNA vaccine delivery via liposomes was predicted by Yang et al.. Authors proposed that DNA vaccines were efficiently uptaken by DCs leading to significant tumor supression and recruitemnt of CD 8+ T cells at tumor cells [101].
The maturation of of DC and infiltration of effector T cells in tumor tissue and tumor-draining lymph nodes (tdLN) are pivotal for immunotherapy. Transdermal delivery of nonvaccine pembrolizumab complex microneedles optimizing transdermal immunization in skin tumors has recently been reported by Zhou et al. [102]. The authors observed accumulation of regimens at tdLN, DCs maturation activation and optimization Th1 immune responses. Moreover, pembrolizumab signifiantly activated CTCs and recruitment while reducing the Tregs in tdLN. Overall, the results indicate the transformation of immunosupressive TME to immunoactive TME.
Alterations in the tumor microenvironment through nanoparticles
There are certain characteristics associated with the tumor microenvironment like hypoxia, high proteolytic activity, irregular vascularization, and reduced extracellular pH, which can complicate immunotherapy. Moreover, the tumor microenvironment also produces an immunosuppressive environment through the release of several cytokines, tumor-associated macrophages, myeloid-derived suppressive cells, and Tregs. The presence of such an environment leads to treatment resistance and clinically poor prognosis [103]. Hence, there is a need for novel immunotherapeutic options that can control the tumor microenvironment on one hand and reverse the immunosuppressive conditions on the other hand. The utilization of nanoparticles-based immunotherapies presents an auspicious approach to remove this tumor persuaded immunosuppression.
In a current preclinical study, researchers developed nanolipogels in which the drug was loaded into cyclodextrin and cytokine-laden biodegradable polymers. The formulated nanolipogel exhibited the potential to deliver interleukin-2 and transforming growth factor-beta inhibitors in a sustained manner to the tumor microenvironment. The mice group treated with nanolipogel displayed a delay in tumor growth, increased survival rate, enhanced activity of natural killer cells, and intratumoral activated CD8+ cells [104].
To target immunosuppressive cells, Sacchetti et al. evaluated the potential of Treg-specific receptor ligands to enhance tumor-specific internalization of PEG-modified carbon nanotubes. The nanotubes complexed with glucocorticoid-induced TNFR-based receptor (i.e. overexpressed in intratumoral Tregs) exhibited higher accumulation in the target site than the non-targeted sites [105]. Similarly, Zhu et al. formulated PEG and mannose modified nanoparticles to enhance targeted delivery. The prepared nanoparticles exhibited stealth properties and reached the targeted site where the PEG was cleaved and mannose groups were exposed. After administration through the intravenous route, it was also observed that as compared with non-functionalized nanoparticles, the mannose decorated nanoparticles exhibited improved accumulation in the tumor microenvironment [106].
The applications of chemotherapy can also modify the tumor microenvironment and in turn, upsurge the therapeutic efficacy of the subsequent immunotherapy [107]. In this regard, Lu et al. investigated that a combination of TRP2 antigen and curcumin-PEG-based nanomicelles generated synergistic antitumor activity in melanoma mice model than alone treatment. In the components of the immune system, the combined therapy also remarkably increased the CTL response as well as the production of interferon-gamma. However, in the tumor microenvironment, the combination therapy reduced the expression of immunosuppressive factors. This reduction in the immunosuppression is in line with increased levels of CD8+ T-cell population and pro-inflammatory cytokines levels [108]. Similalrly, curcumin and loaded nanoparticles with encapsulated nanovaccines triggered residual tumor cells death and DCs recruitment, respectively. Besides, this hybrid delivery system also induced a strong T cells-specific immune response [109].
Immunotherapy can also be improved through physical manipulation. In combination with anti-CTCA-4 antibody therapy, some researchers investigated the potential of photothermal ablation of tumors with intratumorally injected PEG-conjugated single-walled carbon nanotubes. They found that the applied strategy had successfully modulated the adaptive immune responses, particularly cellular immunity against metastatic cancers. The mechanism through which it was achieved was based on photothermal-based cell death, which mediated the secretion of damage-associated molecular patterns and TAA that primes the immune system [110].
Immune modulating compounds delivery through nanoparticles
The EPR effect found in various disease conditions, including metastatic cancers, can be exploited for the targeted delivery of immune-modulating agents to the tumor site. Even though the concept of EPR is controversial in human beings, it is hypothesized that leaky blood vessels in the tumor site and the extended circulation time of the nanocarrier upsurges the targeted uptake of the nanocarriers to the tumor site [111]. To achieve passive targeted delivery, the most important consideration is that the nanoparticles must resist the uptake by the reticuloendothelial system. To overcome this problem, nanoparticles should be designed in such a way that they should be anionic or neutral, and must have a size in the range of 8 and 200 nm, and must be protected by a stealthing agent like PEG, that prevents opsonization. Thus, nanoparticles having these properties can be efficiently used as a nanocarrier for passive targeted delivery of immune-modulating agents [54].
Delivery of antibodies
Therapies based on antibodies have now become a promising strategy to efficiently treat metastatic cancers. The major advantage of this approach involves the targeted delivery of the drug while preventing off-target delivery. The antibodies bind specifically and preferentially to the target proteins, which are particularly overexpressed in the tumor microenvironment [42]. However, there are certain drawbacks associated with these delivery approaches such as poor pharmacokinetics, inadequate tissue penetration, and compromised interaction with the components of the immune system [112]. Researchers have employed various strategies to overcome these limitations and to improve the delivery efficiency of various antibodies. In this connection, Kim et al. modified the assembly of polyion complex micelles to load charged antibody derivatives for increased stability, efficient delivery to the cytosol, and the recognition of the antigens within the cells (Fig. 6) [113]. They observed that an optimum ratio of block catiomers and homopolymers-based micelles loaded with antibodies significantly upsurged the endosomal evasion on one hand and also increased the intracellular recognition of the antigens [113]. Chen et al. designed a nanocarrier consisting of PLGA having an anti-OX40-monoclonal antibody. As compared with the free monoclonal antibody, the antibody loaded into a PLGA-based nanocarrier exhibited significantly higher clinical activity when evaluated in a phase-1 clinical trial. Moreover, the prepared nanocarrier also showed substantially higher CTL-induced cell proliferation, antigen-specific cytotoxicity, and cytokine production than free antibodies [114].
A Pathways for successful intracellular antibody delivery with PIC micelles. B Formation of PIC micelles incorporating charge-converted igg antibody derivatives and strategies to engineer the systems in this study. Reprinted from Kim et al. [113] with permission from American Chemical Society
In clinical trials, the most effective immunotherapeutic-based antibodies block the immunosuppressive pathways that lead towards the progression of cancers. Among these antibodies, programmed death-1 and CTL-linked protein-4 allows the CTLs to target and destroy the cancer cells and produce an efficient clinical response in various kinds of metastatic cancers [115]. However, some patients exhibit poor response to such kind of therapies as well. To upsurge the clinical response, Lei et al. formulated mesoporous silica nanoparticles having CTCA-4 via non-covalent linkages at a very high density to provide targeted and long-lasting antibody release. The prepared formulation exhibited higher therapeutic efficacy than the same amount administered systemically. Additionally, alterations in the functionalities of the nanoparticles could also modify the rate of antibody release [116].
The codelivery of antibodies along with cytokines via nanoparticles also exhibited promising results. In this regard, Kwong et al. engineered pegylated nanoparticles having IL-2Fc fusion protein and T-cell stimulatory anti-CD137. The administration of the prepared formulation through intratumoral injection in the murine B16F10 model cured the primary tumor significantly. The prepared nanoparticles also reduced the lethal inflammation-based toxicities and produced antitumor memory than the equivalent dose of soluble antibodies [117]. Likewise, Li et al. formulated an alginate-based hydrogel delivery system having PD-1 monoclonal antibody and celecoxib. The combined delivery system significantly enhanced antitumor effects than alone. These positive results appeared due to sustained and high concentrations of drugs and antibodies in the tumor microenvironment [118].
Surprisingly, the codelivery of PD-1 antibody and celecoxib increased the levels of CD8+IFN-ɣ+ T-cells and CD4+IFN-ɣ+ within the immune components and the tumor microenvironment. Additionally, the expression of CD4+FoxP3+ Tregs and MDSC in the tumor site showed an immunogenic response increment. Kosmides et al. formulated a nanoparticles-based delivery system having combined T-cell stimulatory signals, anti-PD-L1, and anti-4-1BB. This dually targeted delivery system redirected the responses of effector T-cells to identify target cells whereas simultaneous blockage of various inhibitory pathways. In vitro, this response generated a six-fold increment in IFN-ɣ production through CD8+ T cells in the presence of tumor cells. Furthermore, the expression of PD-1 and tumor growth declined up to 30% in the tumor-infiltrating lymphocytes [119].
Delivery of immune-modulatory compounds through nanoparticles
Delivery of genes via nanoparticles
The delivery of small interfering ribonucleic acid (siRNA) can provide better utility in nanoparticles mediated metastatic cancer immunotherapy. In a recent study, Li et al. formulated cationic lipid-based PEG-PLGA nanoparticles for the efficient delivery of CTCA-4 siRNA and exhibited that this carrier system can efficiently enter T cells both in vitro and in vivo. It was observed that the prepared nanoformulation was internalized by tumor-infiltrating lymphocytes and increased T cell proliferation [120]. Wang et al. synthsized cationic polymer-lipid based hybrid nanoparticles (nanovesicles) to deliver doxorubicin and siRNA. Adjuvent delivery of doxorubicin with siRNA via nanovesicles showed antigen presentation and induced immunogenic cell death of B16 cells in multiple myeloma [121].
Similalrly, Huang et al. also used cationic lipid based polymeic nanoparticles to deliver siRNA and certain chemotherapeutics, which induced tumor regression and restricted tumor metastasis in colorectal and pancreatic cancers [122]. Li et al. investigated the efficacy of lipid micelles for the codelivery of shikon and siRNA and observed efficienttumor accumulation and cytoplasmic delivery. Authors reported that this micelles-based nanocarrierss efficiently delivered cargo, which effectively elicited the recruitment of CTCs and induced immunogenic cell death [123]. Furthermore, Wang et al. reported the successful delivery of intratumoral CRISPR activation libraries eliciting sufficient antitumor response to clear the local and distant established tumors by targeting mutated genes precisely [124]. Furthermore, the systemic delivery of the prepared nanoparticles also increased the number of effector cells, reduced the ratio of expression of CD4+ FOXP3+ Tregs, decreased the tumor growth, and significantly enhanced the survival time of mice bearing melanoma [120].
Chemical modification of nanoparticles with certain biomolecules likes tyrosine has also been recently reported for the efficient delivery of siRNA, biocompatibility and favorable physical properties in various in vitro, ex vivo and in vivo models [125]. Teo et al. evaluated the sensitization of the epithelial lining of ovarian cancer cells to CTL killing through the delivery of PD-L1 siRNA by folic acid functionalized polyethyleneimine nanocarriers. The prepared nanocarriers enhanced the cellular uptake of the PD-L1 siRNA into SKOV-3-Luc cells and reduced the nonspecific uptake into the monocytes [126]. Roeven et al. investigated that transfecting the PD-L1 and PD-L2 siRNA with SAINT-RED consisting of cationic amphiphilic lipid SAINT-18, produced the long-term knockdown of the PD-1 ligands without disturbing the maturation and viability of the dendritic cells. Moreover, they also found that the prepared transfection system in combination with a peptide of histocompatibility antigens mRNA could produce clinical-grade dendritic cell vaccines to upsurge antitumor immunity [127].
To further enhance the efficiency of vaccines in advanced stages of metastatic cancers, Xu et al. designed liposome-protamine-hyaluronic acid nanoparticles for the delivery of TGF-β siRNA. The resulting TGF-β downregulation upsurged the vaccine efficiency and reduced the tumor growth up to 52% than control groups [128].
Delivery of cytokines through nanoparticles
The capability of cytokines to affect the components and responses of the immune system has motivated researchers to use them in immunotherapy. In this regard, several cytokines like IL-2, TNF-α, and interferon α/ɣ (IFN-α/ɣ) have been approved by the food and drug administration authority (FDAA) for cancer therapy. However, these are rapidly metabolized and excreted from the body when administered in safe doses systemically. Hence, there is a need to administer higher doses to achieve desired therapeutic effects, however, higher doses are linked with higher adverse effects. Thus, there is a necessity to develop nanoparticles mediated carrier systems that can deliver cytokines specifically and preferentially to the target sites [129].
Considering the advantage of stealth liposomes, the delivery of IL-2 via the inhalational route in mice having mice with metastatic lung cancer can significantly decrease the tumor growth than free IL-2 [130]. Hagen et al. investigated that pegylated liposomes could be effective for systemic delivery of TNF-α along with liposomal chemotherapy for advanced stages of solid tumors. In this regard, in phase-I clinical trial, patients with follicular lymphoma receiving liposome-based nanoformulation of TAA and IL-2 illustrated an increment in tumor lymphocytes infiltration and tumor growth reduction [131]. Lastly, work by Anderson et al. illustrated that liposomes could serve as an efficient nanocarrier for efficient delivery of IL-6, IL-2, IL-1a, and granulucyte and macrophage colony-stimulating factor (GM-CSF) against metastatic cancers [132]. Visual presentation of mechanism of immunosuppression is shown in Fig. 7 [133].
Visual presentation of mechanism of immunosuppression. Reprinted from Kapadia et al. [133] with permission from Elsevier. License Number: 5121761230857
Conclusions and future prospects
Nanotechnology and its promises in cancer immunotherapies have been discussed in this review. But, exploring the complexity of TME, immunogenicity, and off-target toxicities are still challenging concerns of successful cancer immunotherapies. As costimulatory and inhibitory signals regulate tumor-specific CTCs. Currently immunotherapeutic approaches are based on reinstating their function by targeting their inhibitory pathways. However, various tumor cells such as tumor-infiltrating immune cells, tumor-associated stromal cells and cytokines and/or chemokines are crucial mediators of CTC functions. The immune system is a complex interaction of various farsighted stimulatory and inhibitory responses originating at subcellular, cellular and tissue levels. Under current challenging circumstances and limited technical understanding in immunotherapies, nanotechnology, especially nanomaterials, has a new epoch in immunotherapies and can have great promise and opportunities. Genetically engineered cells to harvest cellular nanocarriers expressing PD-1 to deliver immunological molecules have also been successfully used. These approaches are expected to explore new directions for personalized immunotherapy in medicine.
Bionanomaterials are believed to be the important player in delivering immunotherapeutic agents, but their compatibility is the main limiting factor. So it also necessitates the development of standard and optimized characterization and measurement techniques. Until now, limited data on the medical impact of bionanomaterials and their clinical transformation is available. Based on the current evidence, the effectiveness and selectivity of bio-nanocarriers hinder the target (tumor) site delivery of the drug. Hence restricting their large scale precise clinical translation. Which indicates further consideration of bionanomaterials biosafety as an immunotherapeutic carrier. Last but not least, in vivo pharmacokinetics and host immune responses of bionanomaterials needs to be further explored in detail.
Besides, revision of awareness protocols, refreshers course on evaluation of adverse reaction, and continued evaluation of quality clinical practices can effectively improve the outcomes [134, 135]. Although, there are several limitations in the clinical inauguration of bionanomaterials and fabrication. An improved design in light of deep clinical understanding will greatly help to establish effective and safe immunotherapies. As discussed earlier, systems of targeted delivery of drugs via carriers would be of great interest. At the same time, optimization of nanoparticles synthesis by exploring their interaction with various drug-like biological drugs or vaccines could be helpful in their appropriated and optimum delivery designs. Besides, it could be helpful to avoid the unnecessary and harmful interactions with drugs, especially with biological drugs. Interdisciplinary cooperation can enhance the chance of more accurate design of drug carriers.
Data availability
All data associated with this paper can be found in the main text.
Code availability
Not applicable.
References
Mathur P, Sathishkumar K, Chaturvedi M et al (2020) Cancer statistics, 2020: report from national cancer registry programme, India. JCO Glob Oncol 6:1063–1075
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Jubeen F, Liaqat A, Amjad F et al (2020) Synthesis of 5-fluorouracil cocrystals with novel organic acids as coformers and anticancer evaluation against HCT-116 colorectal cell lines. Cryst Growth Des 20:2406–2414
Jubeen F, Liaqat A, Sultan M, Zafar Iqbal S, Sajid I, Sher F (2019) Green synthesis and biological evaluation of novel 5-fluorouracil derivatives as potent anticancer agents. Saudi Pharm J 27:1164–1173
Zeeshan R, Mutahir Z (2017) Cancer metastasis—tricks of the trade. Bosn J Basic Med Sci 17:172–182
Suhail Y, Cain MP, Vanaja K et al (2019) Systems biology of cancer metastasis. Cell Syst 9:109–127
Chiang AC, Massague J (2008) Molecular basis of metastasis. N Engl J Med 359:2814–2823
Massague J, Obenauf AC (2016) Metastatic colonization by circulating tumour cells. Nature 529:298–306
Boulter L, Bullock E, Mabruk Z, Brunton VG (2020) The fibrotic and immune microenvironments as targetable drivers of metastasis. Br J Cancer 124(1):27–36. https://doi.org/10.1038/s41416-020-01172-1
Huang X, Khan MI, Wang J et al (2021) Role of receptor tyrosine kinases mediated signal transduction pathways in tumor growth and angiogenesis-New insight and futuristic vision. Int J Biol Macromol 180:739–752
Trapani JA, Darcy PK (2017) Immunotherapy of cancer. Aust Fam Physician 46:194–199
Khan MI, Batool F, Kalsoom F et al (2020) New insights on unique therapeutic potentialities of prostacyclin and prostacyclin synthase. Mater Today Chem 16:100258
Bergman PJ (2019) Cancer immunotherapies. Vet Clin North Am Small Anim Pract 49:881–902
Galon J, Bruni D (2019) Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 18:197–218
Wang W, Li F, Li S et al (2021) M2 macrophage-targeted iron oxide nanoparticles for magnetic resonance image-guided magnetic hyperthermia therapy. J Mater Sci Technol 81:77–87
Milling L, Zhang Y, Irvine DJ (2017) Delivering safer immunotherapies for cancer. Adv Drug Deliv Rev 114:79–101
Khalil DN, Smith EL, Brentjens RJ, Wolchok JD (2016) The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 13:273–290
van den Bulk J, Verdegaal EM, de Miranda NF (2018) Cancer immunotherapy: broadening the scope of targetable tumours. Open Biol 8(6):180037
Rosenberg SA, Restifo NP (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348:62–68
Fesnak AD, June CH, Levine BL (2016) Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 16:566–581
Rosenberg SA, Yang JC, Sherry RM et al (2011) Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17:4550–4557
Perazella MA, Shirali AC (2018) Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol 29:2039–2052
Sagnella SM, Yang L, Stubbs GE et al (2020) Cyto-immuno-therapy for cancer: a pathway elicited by tumor-targeted, cytotoxic drug-packaged bacterially derived nanocells. Cancer Cell 37:354–370
Childs RW, Carlsten M (2015) Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov 14:487–498
Rautela J, Huntington ND (2017) IL-15 signaling in NK cell cancer immunotherapy. Curr Opin Immunol 44:1–6
Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD (2017) Combination immunotherapy: a road map. J Immunother Cancer 5:16
Odero-Marah V, Hawsawi O, Henderson V, Sweeney J (2018) Epithelial-mesenchymal transition (EMT) and prostate cancer. Adv Exp Med Biol 1095:101–110
Viel S, Marcais A, Guimaraes FS et al (2016) TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci Signal 9:ra19
Desrichard A, Snyder A, Chan TA (2016) Cancer neoantigens and applications for immunotherapy. Clin Cancer Res 22:807–812
Wolchok JD, Kluger H, Callahan MK et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133
Zou W, Wolchok JD, Chen L (2016) PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aad7118
Lebbe C, Weber JS, Maio M et al (2014) Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann Oncol 25:2277–2284
Napoleone A, Laurén I, Linkgreim T et al (2021) Fed-batch production assessment of a tetravalent bispecific antibody: a case study on piggyBac stably transfected HEK293 cells. New Biotechnol 65:9–19
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23–34
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2019) Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535–1546
Hodi FS, Chiarion-Sileni V, Gonzalez R et al (2018) Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 19:1480–1492
Wolchok JD, Chiarion-Sileni V, Gonzalez R et al (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345–1356
Barakat LP, Schwartz LA, Szabo MM, Hussey HM, Bunin GR (2012) Factors that contribute to post-treatment follow-up care for survivors of childhood cancer. J Cancer Surviv 6:155–162
Zhao Y, Bilal M, Raza A et al (2020) Tyrosine kinase inhibitors and their unique therapeutic potentialities to combat cancer. Int J Biol Macromol 168:22–37
Sengupta S (2017) Cancer nanomedicine: lessons for immuno-oncology. Trends Cancer 3:551–560
Jiang W, Yuan H, Chan CK et al (2017) Lessons from immuno-oncology: a new era for cancer nanomedicine? Nat Rev Drug Discov 16:369–370
Wilhelm S, Tavares AJ, Dai Q et al (2016) Analysis of nanoparticle delivery to tumours. Nat Rev Mater 1:1–12
Alp G, Aydogan N (2018) Enhancing the spreading behavior on pulmonary mucus mimicking subphase via catanionic surfactant solutions: toward effective drug delivery through the lungs. Mol Pharm 15:1361–1370
Erickson MA, Banks WA (2018) Neuroimmune axes of the blood-brain barriers and blood-brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol Rev 70:278–314
Hladky SB, Barrand MA (2016) Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 13:19
Coureuil M, Lecuyer H, Bourdoulous S, Nassif X (2017) A journey into the brain: insight into how bacterial pathogens cross blood-brain barriers. Nat Rev Microbiol 15:149–159
Ruponen M, Urtti A (2015) Undefined role of mucus as a barrier in ocular drug delivery. Eur J Pharm Biopharm 96:442–446
Dunnhaupt S, Kammona O, Waldner C, Kiparissides C, Bernkop-Schnurch A (2015) Nano-carrier systems: strategies to overcome the mucus gel barrier. Eur J Pharm Biopharm 96:447–453
Peynshaert K, Devoldere J, De Smedt SC, Remaut K (2018) In vitro and ex vivo models to study drug delivery barriers in the posterior segment of the eye. Adv Drug Deliv Rev 126:44–57
Khawar IA, Kim JH, Kuh HJ (2015) Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release 201:78–89
Sriraman SK, Aryasomayajula B, Torchilin VP (2014) Barriers to drug delivery in solid tumors. Tissue Barriers 2:e29528
Hobbs SK, Monsky WL, Yuan F et al (1998) Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA 95:4607–4612
Hicks KO, Pruijn FB, Secomb TW et al (2006) Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxia-targeted anticancer drugs. J Natl Cancer Inst 98:1118–1128
Fang J, Nakamura H, Maeda H (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–151
Hoffman AS, Lai JJ (2020) Three significant highlights of controlled drug delivery over the past 55 years: PEGylation, ADCs, and EPR. Adv Drug Deliv Rev 158:2–3
Maeda H (2017) Polymer therapeutics and the EPR effect. J Drug Target 25:781–785
Maeda H (2012) Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Control Release 164:138–144
Sindhwani S, Syed AM, Ngai J et al (2020) The entry of nanoparticles into solid tumours. Nat Mater 19:566–575
Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y (2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol 11:39
Wayne AS, Capitini CM, Mackall CL (2010) Immunotherapy of childhood cancer: from biologic understanding to clinical application. Curr Opin Pediatr 22:2–11
Wedekind MF, Denton NL, Chen CY, Cripe TP (2018) Pediatric cancer immunotherapy: opportunities and challenges. Paediatr Drugs 20:395–408
Zhang Q, Ping J, Huang Z et al (2020) CAR-T cell therapy in cancer: tribulations and road ahead. J Immunol Res 2020:1924379
Corrigan-Curay J, Kiem HP, Baltimore D et al (2014) T-cell immunotherapy: looking forward. Mol Ther 22:1564–1574
Park JA, Cheung NV (2017) Limitations and opportunities for immune checkpoint inhibitors in pediatric malignancies. Cancer Treat Rev 58:22–33
Merchant MS, Wright M, Baird K et al (2016) Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin Cancer Res 22:1364–1370
Abdel-Rahman O, Helbling D, Schmidt J et al (2017) Treatment-related death in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Clin Oncol (R Coll Radiol) 29:218–230
Abdel-Wahab N, Shah M, Suarez-Almazor ME (2016) Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS ONE 11:e0160221
Yang JC, Hughes M, Kammula U et al (2007) Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 30:825–830
Michot JM, Pruvost R, Mateus C et al (2018) Fever reaction and haemophagocytic syndrome induced by immune checkpoint inhibitors. Ann Oncol 29:518–520
Khan MI, Xu S, Ali MM et al (2020) Assessment of multidrug resistance in bacterial isolates from urinary tract-infected patients. J Radiat Res Appl Sci 13:267–275
Pitt JM, Vétizou M, Daillère R et al (2016) Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and-extrinsic factors. Immunity 44:1255–1269
Zaretsky JM, Garcia-Diaz A, Shin DS et al (2016) Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 375:819–829
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A (2017) Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168:707–723
Jenkins RW, Barbie DA, Flaherty KT (2018) Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 118:9–16
Miao D, Margolis CA, Gao W et al (2018) Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359:801–806
Taube JM, Anders RA, Young GD et al (2012) Colocalization of inflammatory response with B7–h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4:127ra137
Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10
Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P (2015) Nanoparticle-based immunotherapy for cancer. ACS Nano 9:16–30
Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN (2014) Clinical use of dendritic cells for cancer therapy. Lancet Oncol 15:e257–e267
Fang RH, Kroll AV, Zhang L (2015) Nanoparticle-based manipulation of antigen-presenting cells for cancer immunotherapy. Small 11:5483–5496
Gao P, Xia G, Bao Z et al (2016) Chitosan based nanoparticles as protein carriers for efficient oral antigen delivery. Int J Biol Macromol 91:716–723
Maji M, Mazumder S, Bhattacharya S et al (2016) A lipid based antigen delivery system efficiently facilitates MHC class-I antigen presentation in dendritic cells to stimulate CD8+ T cells. Sci Rep 6:1–12
Rietscher R, Schröder M, Janke J et al (2016) Antigen delivery via hydrophilic PEG-b-PAGE-b-PLGA nanoparticles boosts vaccination induced T cell immunity. Eur J Pharm Biopharm 102:20–31
Qiu H, Min Y, Rodgers Z, Zhang L, Wang AZ (2017) Nanomedicine approaches to improve cancer immunotherapy. Wiley Interdiscip Rev: Nanomed Nanobiotechnol 9:e1456
Yang W, Zhu G, Wang S et al (2019) In situ dendritic cell vaccine for effective cancer immunotherapy. ACS Nano 13:3083–3094
Shapira L, Soskolne WA, Houri Y, Barak V, Halabi A, Stabholz A (1996) Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Infect Immun 64:825–828
Gavin AL, Hoebe K, Duong B et al (2006) Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 314:1936–1938
Sokolova V, Knuschke T, Kovtun A, Buer J, Epple M, Westendorf AM (2010) The use of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activation. Biomaterials 31:5627–5633
Schlosser E, Mueller M, Fischer S et al (2008) TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine 26:1626–1637
Hamdy S, Molavi O, Ma Z et al (2008) Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine 26:5046–5057
Li H, Li Y, Jiao J, Hu H-M (2011) Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat Nanotechnol 6:645–650
Fang RH, Hu C-MJ, Luk BT et al (2014) Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett 14:2181–2188
Klippstein R, Pozo D (2010) Nanotechnology-based manipulation of dendritic cells for enhanced immunotherapy strategies. Nanomed: Nanotechnol Biol Med 6:523–529
Kempf M, Mandal B, Jilek S et al (2003) Improved stimulation of human dendritic cells by receptor engagement with surface-modified microparticles. J Drug Target 11:11–18
Qian Y, Jin H, Qiao S et al (2016) Targeting dendritic cells in lymph node with an antigen peptide-based nanovaccine for cancer immunotherapy. Biomaterials 98:171–183
Bachmann MF, Jennings GT (2010) Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 10:787–796
Wen Y, Waltman A, Han H, Collier JH (2016) Switching the immunogenicity of peptide assemblies using surface properties. ACS Nano 10:9274–9286
Liu H, Moynihan KD, Zheng Y et al (2014) Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507:519–522
Li AW, Sobral MC, Badrinath S et al (2018) A facile approach to enhance antigen response for personalized cancer vaccination. Nat Mater 17:528–534
Kokate RA, Chaudhary P, Sun X et al (2016) Rationalizing the use of functionalized poly-lactic-co-glycolic acid nanoparticles for dendritic cell-based targeted anticancer therapy. Nanomedicine 11:479–494
Yang X, Fan J, Wu Y et al (2021) Synthetic multiepitope neoantigen DNA vaccine for personalized cancer immunotherapy. Nanomed: Nanotechnol Biol Med. https://doi.org/10.1016/j.nano.2021.102443
Zhou Z, Pang J, Wu X, Wu W, Chen X, Kong M (2020) Reverse immune suppressive microenvironment in tumor draining lymph nodes to enhance anti-PD1 immunotherapy via nanovaccine complexed microneedle. Nano Res 13:1509–1518
Estrella V, Chen T, Lloyd M et al (2013) Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 73:1524–1535
Park J, Wrzesinski SH, Stern E et al (2012) Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater 11:895–905
Sacchetti C, Rapini N, Magrini A et al (2013) In vivo targeting of intratumor regulatory T cells using PEG-modified single-walled carbon nanotubes. Bioconjug Chem 24:852–858
Zhu S, Niu M, O’Mary H, Cui Z (2013) Targeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticles. Mol Pharm 10:3525–3530
Zhao Y, Huo M, Xu Z, Wang Y, Huang L (2015) Nanoparticle delivery of CDDO-Me remodels the tumor microenvironment and enhances vaccine therapy for melanoma. Biomaterials 68:54–66
Lu Y, Miao L, Wang Y et al (2016) Curcumin micelles remodel tumor microenvironment and enhance vaccine activity in an advanced melanoma model. Mol Ther 24:364–374
Liu X, Feng Z, Wang C et al (2020) Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials 230:119649
Wang C, Xu L, Liang C, Xiang J, Peng R, Liu Z (2014) Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv Mater 26:8154–8162
Nakamura Y, Mochida A, Choyke PL, Kobayashi H (2016) Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem 27:2225–2238
Elhissi AM, Ahmed W, Hassan IU, Dhanak VR, D’Emanuele A (2012) Carbon nanotubes in cancer therapy and drug delivery. J Drug Deliv. https://doi.org/10.1155/2012/837327
Kim A, Miura Y, Ishii T et al (2016) Intracellular delivery of charge-converted monoclonal antibodies by combinatorial design of block/homo polyion complex micelles. Biomacromol 17:446–453
Chen M, Ouyang H, Zhou S, Li J, Ye Y (2014) PLGA-nanoparticle mediated delivery of anti-OX40 monoclonal antibody enhances anti-tumor cytotoxic T cell responses. Cell Immunol 287:91–99
Twyman-Saint Victor C, Rech AJ, Maity A et al (2015) Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520:373–377
Lei C, Liu P, Chen B et al (2010) Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. J Am Chem Soc 132:6906–6907
Kwong B, Gai SA, Elkhader J, Wittrup KD, Irvine DJ (2013) Localized immunotherapy via liposome-anchored Anti-CD137+ IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer Res 73:1547–1558
Li Y, Fang M, Zhang J et al (2016) Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology 5:e1074374
Kosmides AK, Schneck J (2014) Dual-targeting nanoparticles for reprogrammed T cell responses in the tumor microenvironment. J Immunother Cancer 2:P108
Li S-Y, Liu Y, Xu C-F et al (2016) Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J Control Release 231:17–28
Wang C, Shi X, Song H et al (2021) Polymer-lipid hybrid nanovesicle-enabled combination of immunogenic chemotherapy and RNAi-mediated PD-L1 knockdown elicits antitumor immunity against melanoma. Biomaterials 268:120579
Huang H, Jiang C-T, Shen S et al (2019) Nanoenabled reversal of IDO1-mediated immunosuppression synergizes with immunogenic chemotherapy for improved cancer therapy. Nano Lett 19:5356–5365
Li J, Zhao M, Xu Y, Hu X, Dai Y, Wang D (2021) Hybrid micelles codelivering shikonin and IDO-1 siRNA enhance immunotherapy by remodeling immunosuppressive tumor microenvironment. Int J Pharm 597:120310
Wang G, Chow RD, Bai Z et al (2019) Multiplexed activation of endogenous genes by CRISPRa elicits potent antitumor immunity. Nat Immunol 20:1494–1505
Karimov M, Schulz M, Kahl T et al (2021) Tyrosine-modified linear PEIs for highly efficacious and biocompatible siRNA delivery in vitro and in vivo. Nanomed: Nanotechnol Biol Med 36:102403
Teo PY, Yang C, Whilding LM et al (2015) Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: strategies to enhance T cell killing. Adv Healthc Mater 4:1180–1189
Roeven MW, Hobo W, van der Voort R et al (2015) Efficient nontoxic delivery of PD-L1 and PD-L2 siRNA into dendritic cell vaccines using the cationic lipid SAINT-18. J Immunother 38:145–154
Xu Z, Wang Y, Zhang L, Huang L (2014) Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano 8:3636–3645
Christian DA, Hunter CA (2012) Particle-mediated delivery of cytokines for immunotherapy. Immunotherapy 4:425–441
Kedar E, Braun E, Rutkowski Y, Emanuel N, Barenholz Y (1994) Delivery of cytokines by liposomes. II. Interleukin-2 encapsulated in long-circulating sterically stabilized liposomes: immunomodulatory and anti-tumor activity in mice. J Immunother 16:115–124
ten Hagen TL, Seynhaeve AL, van Tiel ST, Ruiter DJ, Eggermont AM (2002) Pegylated liposomal tumor necrosis factor-α results in reduced toxicity and synergistic antitumor activity after systemic administration in combination with liposomal doxorubicin (Doxil®) in soft tissue sarcoma-bearing rats. Int J Cancer 97:115–120
Anderson PM, Hanson DC, Hasz DE, Halet MR, Blazar BR, Ochoa AC (1994) Cytokines in liposomes: preliminary studies with IL-1, IL-2, IL-6, GM-CSF and interferon-γ. Cytokine 6:92–101
Kapadia CH, Perry JL, Tian S, Luft JC, DeSimone JM (2015) Nanoparticulate immunotherapy for cancer. J Control Release 219:167–180
Nisa ZU, Zafar A, Zafar F, Pezaro S, Sher F (2020) Adverse drug reaction monitoring and reporting among physicians and pharmacists in Pakistan: a cross-sectional study. Curr Drug Saf 15:137–146
Nisa ZU, Zafar A, Sher F (2018) Assessment of knowledge, attitude and practice of adverse drug reaction reporting among healthcare professionals in secondary and tertiary hospitals in the capital of Pakistan. Saudi Pharm J 26:453–461
Acknowledgements
Consejo Nacional de Ciencia y Tecnología (CONACYT) Mexico, is thankfully acknowledged for partially supporting this work under Sistema Nacional de Investigadores (SNI) program awarded to Hafiz M.N. Iqbal (CVU: 735340). The listed author(s) thankfully acknowledge the literature access provided by their representative organizations.
Funding
This work receives no external/internal funding.
Author information
Authors and Affiliations
Contributions
All listed authors have equal contributions from conceptualization to the compilation of this work.
Corresponding authors
Ethics declarations
Conflict of interest
The listed author(s) declare no conflicting interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Bilal, M., Qindeel, M. et al. Nanotechnology-based immunotherapies to combat cancer metastasis. Mol Biol Rep 48, 6563–6580 (2021). https://doi.org/10.1007/s11033-021-06660-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06660-y