Abstract
Cancer treatment through alternative strategies has a great contribution from immunotherapies nowadays with the latter inviting more intensive research for further advancement in this field. Current immunotherapies are being challenged by several issues like (1) less specificity among immune cells in reaching the target site and attacking cancer cells; (2) instability in vivo; (3) limited therapeutic effect; (4) chances of off-target cytotoxicity; (5) immunosuppressivity shown by tumour cells; and (6) heterogeneity of the disease among patient cohorts. To take a step ahead in overcoming these limitations, this review summarises the breakthroughs made by nano-sized biomaterials or nanocarriers for enhanced cancer therapy. With the simultaneous boom in the field of nanotechnology, a diverse range of artificial nanocarriers have come up which enhance specificity and reduce delivery time for encapsulated drugs at a stunning pace. These nanocarriers are mostly polymeric and inorganic-based nanoparticles, both of which have their own characteristic features. Another significant aim of biomaterials is safeguarding the drug from degrading enzymes and the action of adverse pH conditions. In addition, reducing the systemic toxicity of the nanodrug by decreasing its release time or after reaching the target tissue is also crucial. Moreover, biodegradable nanocarriers and several ways in which they can be engineered successfully in many ways to act as drug transporters for targeting the tumour microenvironment have also been discussed here.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to the most recent data compiled by the American Cancer Society (ACS), new cancer cases in 2022 are estimated to reach 2 million with approximately 0.6 million projected deaths in the USA. Interestingly, lung cancer has been proposed as the most lethal type by ACS, claiming almost 350 lives per day [1]. The data suggests that despite advancements made in traditional cancer treatment approaches like surgery, local radiotherapy and chemotherapy, the disease still prevails to be ubiquitous and fatal. This is because numerous insistent challenges continue to obstruct the aptness in (1) preventing cancer after diagnosis and (2) averting cancer relapse. They include drug intoxication and lack of selectivity [2], the immune system’s suppression by the tumour microenvironment [3] and polymorphism of the disease in the patient community [4]. Therefore after years of research for better alternatives, the paragon of cancer treatment has now relocated in a more specific, systemic and imperishable way is immunotherapy which mobilizes a patient’s own immunity. Though there is enough potential in immunotherapy-based cancer treatment especially in preventing cancer metastasis and inducing immunological memory, issues like therapeutics’ specific delivery and degradation inside the body are still lasting.
Biomaterials, initially developed for their use in medical devices and as prosthetics, have rapidly developed over the last few decades to become sophisticated devices which can illicit pre-defined biological responses. Available in a multitude of size scales and various forms like polymers, lipids, metal nanoparticles, quantum dots and various other self-assemble structures, the currently available range of biomaterials has found application in various aspects of diagnosis and treatment. They have been used to create 3D scaffolds for disease modeling, to construct biologically compatible implants [5, 6], as biosensors on microfluidic devices [7, 8] or as drug delivery vehicles in the form of micro- or nanoparticles [9]. When used for delivery, these nanoparticles can be loaded with therapeutics to facilitate a localized and sustained drug release with improved solubility and stability. In this review, we briefly focus on some modes of cancer immunotherapy, some artificial polymer-based nanocarriers and improved techniques to increase drug targeting and the future perspectives where biodegradable nanocarriers can be used.
2 Main Classifications of Cancer Immunotherapy
The history of treating cancer by triggering the patient’s immunity started more than a century ago. A blend of live and attenuated bacteria such as Streptococcus pyogenes and Serratia marcescens often referred to as ‘Coley’s toxin’ was the first documented immunotherapy approach and was administered to thousands of patients [9]. US Food and Drug Administration sanctioned recombinant versions of interferon-α as the primary drug against hairy cell leukaemia [10] in 1986. The next section gives a brief overview of the novel immunotherapies (Fig. 1) that have been explored for clinical use over the past several years.
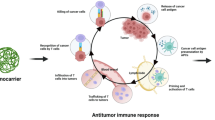
Several modes of immunotherapies to treat cancer. a Cytokines (e.g. interferons and interleukins) can stimulate and enhance the immune response to fight against cancer. b Therapeutic vaccines, which are employed to help kill cancer cells by enhancing active immune responses to tumours. c Adoptive cell transfer—a kind of immunotherapy in which T cells are genetically modified to express a chimeric antigen receptor (CAR) or T cell receptor (TCR) and reinfused into the body to combat cancer cells. d Checkpoint blockade targets immune checkpoints and key regulators of the immune system and leads to the activation of the immune system. e Binding between monoclonal antibodies and tumour antigens leads to better exposure of tumours to the immune system (adapted from Sang, W., et al. 2019, The Royal Society of Chemistry [11])
2.1 Cytokines
Passive immunotherapy refines the immune system to significantly superior levels by utilizing the power of modern technology. The first class of immunotherapeutics to be explored and assessed in clinics, cytokines like IFN-α act as immunomodulators and achieve their function by directly stimulating the activity of immune cells. IFNs are known to exert an anti-tumour effect and modulate tumour cell proliferation [12]. Interferon activation is also known to impede angiogenesis in the extracellular space [13]. However, a series of side effects including depression [14] and leukopenia [15] have been noted after the use. They have also shown limited success against advanced forms of cancers, often believed to be a consequence of massive tumour load and tumour immunosuppressive microenvironment [16]. It has been observed that besides IFNs, other cytokines including interleukins, TNFs and GM-CSF also remarkably enhance immune response [17]. It has been exhibited that interleukins have a role in vitalizing the growth and activity of both CD4 + helper T cells as well as CD8 + cytotoxic T cells [18,19,20,21]. Studies show that GM-CSF too assists T cell homeostasis and thus improves T cell survival. They are also known to aid in the differentiation of dendritic cells to exhibit tumour-specific antigens [22]. After chemotherapy, the retrieval of granulocyte was seen to have been elevated by G-CSF as well as GM-CSF. An agonist SD-208, an inhibitor of TGF-β receptor type 1 (TGFβR1) has been found to reinstate T cell activity and upgrade immune response [23]. It was also seen that, in comparison to IL-2, IL-21 and IL-15, had more significance in promoting the death of stimulated T cells while ensuring the survival of regulatory T cells [24, 25]. Currently, several more cytokines, e.g. IL-17 and IL-15 [26, 27], are in research and clinical trials. Cytokines are also being explored for their potential in combinational therapies. These include using two or more cytokines together, coupling cytokines and checkpoint inhibitors or using them together with other chemotherapies.
2.2 Vaccine
Cancer vaccines are mainly of two types, preventive vaccines (e.g. HPV in cervical cancer [28]) and treatment vaccines. The treatment vaccines are usually used to augment antigen presentation, enhance active immune responses and alter the immunosuppressive cancer microenvironment [29]. Dendritic cells (DC), tumour cell lysate and nucleic acids or neo-antigens are some of the treatment vaccines used based on the category of tumour antigen [30]. DC, which plays a pivotal role in antigen presentation and induction of T cell immunity to attack most cancer cells [31], are harvested from the patient’s blood, loaded ex-vivo with tumour-specific antigens, and subsequently activated and re-introduced into the affected person to make DC vaccines. Despite showing high safety profiles, dendritic cell-based vaccines have failed in clinical trials due to a shortfall of efficacy [32], sipuleucel-T being the exception which was approved for treating prostate cancer [33].
Ultraviolet B ray irradiation and freeze–thaw cycles are the two standard clinical methods to develop tumour cell lysates. These are reported to induce maturation of dendritic cells, contain tumour-associated antigens and are not restricted based on the patient’s HLA type [34]. The action of nucleic acid vaccines is based on transporting exogenous nucleic acids into target cells which are then translated into antigenic proteins by APCs [35]. RNA-based nucleic acid vaccines outshine DNA-based nucleic acid vaccines in several aspects, e.g. ease in intranuclear delivery; low preparation cost as it is naturally occurring does not get integrated into the genome hence no hereditary side effects and ability to extend its half-life through remoulding [36, 37]. To strengthen its intracellular delivery it demands special transfection reagents or delivery techniques [38]. Tumour somatic DNA is being used as antigens, only expressed in tumour cells, to boost the anti-tumour immune response in the case of neoantigen vaccines [39]. Advanced delivery technologies and materials can be proved substantially advantageous for NA vaccines.
2.3 Immunological Checkpoint Inhibitors
Immune checkpoints are immune cell surface receptors involved in inhibitory pathways. These are indulged in regulating the immune system primarily to maintain self-tolerance and firmly monitor cancer immunoediting [40]. Tumour cells are known to express PD-1 ligands which bind to the receptor PD-1, commonly expressed by activated T cells (Fig. 2). This interaction allows the tumour to inhibit the action of the T cells that become aware of tumour antigens [41]. This interaction is also believed to mediate the recruitment of tyrosine phosphatase SHP-2. The increase in concentration of the phosphatase in the vicinity of the intracellular domain of PD-1 lowers TCR phosphorylation and thus decreases TCR signaling and cytokine manufacturing. PD-1 has been seen to be overexpressed in several forms of cancer cells and is believed to play a major role in cancer immune evasion. CTLA-4, an inhibitory protein receptor, is another immune checkpoint that is known to act by binding to its ligands (CD80 and CD86, expressed by APCs). This binding results in the down-regulation of the initial stages of T cell activation and also stimulates tumour growth [42]. Therefore inhibitors (i.e. monoclonal antibodies) are being used to cease the interactions between the ligands and their receptor PD1 and CTLA-4 to raise T cell activity and have so far shown extraordinary anti-tumour responses. The use of anti-CTLA-4 antibodies has shown successful anti-tumour activities in various murine tumour models such as brain, bladder and ovarian cancer [43]. However, not all patients gain from checkpoint inhibitors. The systemic use of immunological checkpoint inhibitors may affect the functioning of normal organs, and its reactivity is still under research [44, 45]. Other novel molecules that are currently being explored in various stages of clinical trials include the T cell immunoglobulin and mucin domain-containing 3 (TIM3) proteins [46] and the lymphocyte activation gene 3 (LAG3) proteins [47].
T cell activation in the tumour milieu. a Active antigen-stimulated T cells exhibit an inhibitory receptor named PD1. Anti-tumour immune response gets blocked due to interactions between PD1 and its ligand, PD-L1, expressed in many tumours, and thus T cell activity is hindered. b Antibodies targeting PD1 or PD-L1 block the PD1 pathway and reactivate T cell activity. MCH, major histocompatibility complex; TCR, T cell receptor (adapted from Ribas A et al., 2015, The New England Journal Of Medicine [48])
2.4 Adoptive Cell Therapy (ACT)
ACT with tumour-infiltrating lymphocytes (TILs) is an approach where a combination of helper and cytotoxic T cells are harvested from resected metastatic tumour deposits and grown ex vivo beforehand[49, 50]. Because of the immune suppressive tumour microenvironment, the tumour-reactive T lymphocytes present inside the tumour get functionally impaired. Hence, to reactivate their potential, they are grown in a cytokine cocktail prior to the reinfusion [51]. Another strategy is based on the expression of the α and β chains of TCR to modulate the antigen specificity of the T cell. Here the T cells are isolated from the patient, and, instead of just stimulating the anti-tumour cells, these are supplied with a new TCR that specifically targets tumour antigens. One major limitation of the TIL and TCR treatment strategies is that they can only target and get rid of cancer cells where the antigens are bound by MHC. Another strategy that has shown significant promise is CAR T cell therapy. Here, T cells are isolated from the peripheral blood of a patient and are then modified in vitro to express chimeric antigen receptors or CARs, formed by fusing the variable domain of a tumour-specific immunoglobulin to the constant domain of TCR using a retroviral vector, which can then be reintroduced into the same patient [52]. So far two CAR T cell therapies, both targeting CD-19, were approved for the treatment of lymphoma by the US FDA. However, the overall process is tedious, expensive and technically demanding. Cytokine release syndrome and neurotoxicity [47] have also been seen in some cases which limits their universal use. Hence, there is a dire need for new biomaterials and techniques to improve their applicability to solid tumours.
2.5 Oncolytic Virus (OV) Therapy
Immunotherapy using oncolytic viruses is a novel approach that uses native or engineered viruses where disease-causing genes are removed and immune stimulating genes are inserted and which selectively infects and subsequently lyses cancer cells. This virus-mediated lysis is a highly immunogenic process and leads to the release of various tumour-specific antigens into the local environment. This then leads to the activation of adaptive immune response [49]. Hence, the introduction of these viruses triggers a multifaceted anti-tumour response. Furthermore, these viruses may also be used as vehicles carrying transgenes for various cytokines. Here, viral replication is coupled with cytokine production leading to enhanced immune cell recruitment and T cell activation [53], while numerous viruses (e.g. paramyxovirus, reovirus and picornavirus) have shown promising oncolytic potential; however, so far only one OV therapy, namely T-vec, has received FDA approval[54]. T-vec or Talimogene laherparepvec is a modified form of the type-I Herpes simplex virus where two ICP34.5 genes were replaced by genes for the cytokine GM-CSF [55]. In 2015, the virus was approved for use against advanced melanoma [56].
3 Artificial Nanocarriers in Cancer Therapy
While its high specificity has made cancer immunotherapy an effective treatment option, its success is still limited due to factors like high cost, lengthy processing and significant off-target effects. In recent years, bioactive carriers have been explored to mitigate these concerns and increase the efficacy and safety of these immunotherapy strategies by facilitating the targeting of therapeutic agents to specific immune reactions. Several biomaterials have been studied for this purpose. Of these, the use of bioactive nanoparticles has been of particular interest.
Nanomaterials having specific physical and biochemical properties can be used as carriers for therapeutic compounds to facilitate a controlled, localized and sustained drug delivery.
Several factors influence the design of nanoparticles for their use as successful carriers. For instance, the size and shape of the carrier can have significant effect on the uptake, transport and accumulation inside body [57, 58]. It has been found that particles within the range of 10 to 100 nm in diameter are usually most suitable for use in cancer therapy. Similarly, surface charge plays a determining role in internalization of these particles. It has been observed that particles with a positive charge accumulate more readily in tumour cells. Hence, particles are often modified to make them cationic and, thus, increase their toxicity and efficacy. Surface characteristics also play a role in determining bioavailability, with hydrophobic nanoparticles showing slower clearance and enhanced half-life [59]. These, and other such modifications, have helped nanocarriers to improve the pharmacokinetic properties of the associated payload drug.
3.1 Polymeric Nanoparticles
Of the various nanoparticle systems that have been explored for their potential use as carriers, the most common are polymeric nanocarriers.
3.1.1 PLGA
PLGA, or polylactic-co-glycolic acid (PLGA), is an FDA and EMA approved polymeric nanoparticle that has been extensively studied for its application in cancer immunotherapy. The polymer hydrolyses to give lactic acid and glycolic acid. Being endogenous molecules, both of these monomers are easily metabolized in the human body, making the polymer biodegradable as well as non-toxic. This, along with its enhanced permeability and retention capabilities, has made PLGA an attractive option for developing drug delivery systems.
Over the years, several different surface modifications have been explored to increase the efficacy of these molecules. Of these, the most commonly employed modification is the addition of a polyethylene glycol layer (PEGylation). This confers two added advantages to the nanocarriers: (1) it makes the particle hydrophilic. This helps it escape the reticulo-endothelial system (RES) which is responsible for eliminating hydrophobic particles from blood and thus increases its half-life by several folds [60]. (2) It confers a positive charge to the originally negatively charged PLGA molecules. This positive charge helps it interact with the negatively charged cell membranes and thus improves the internalization of particles [61].
PLGA nanocarriers have been used in several different strategies for cancer immunotherapy. For instance, they have been seen to successfully mediate the inhibition of STAT3. STAT3 plays a major role in promoting immunosuppression in the tumour environment. Thus, by inhibiting STAT3, either by acting as a carrier for STAT3 inhibitor JSI-124 [62] or anti-STAT3 siRNA [63], this approach was seen to restore the function of dendritic cells. PLGA nanoparticles have also been used in various combination therapy approaches. Particles carrying both paclitaxel, an anti-cancer drug, and SP-LPS, a non-toxic lipopolysaccharide derivative with an immunostimulatory effect, were seen to have much better anti-cancer activity than conventional Taxol treatment [64]. PLGA carriers loaded with anti-PD-1 peptide (APP) have also been used in combination with laser immunotherapy to treat metastatic tumour growths [65]. Furthermore, PLGA nanoparticles have been explored for their potential to mediate sustained drug release. Upon encapsulation of αCT1, a connexin43 mimetic peptide drug used for treatment of glioblastoma and chronic non-healing ulcers, into PLGA nanoparticles, a sustained in vitro release profile was observed. Here, 50% of the drug was released in the first 3 days, while the rest was released over the next two and a half weeks [66].
3.1.2 Liposomes
Liposomes, a type of lipid-based nanoparticles, are another class of nanocarriers that is commonly studied. First described in 1965, in 1995, the liposomal formulation Doxil became the first nanoscale drug approved for clinical use. Since then liposomes have undergone considerable advancements to make them highly effective as delivery systems. The liposome structure consists of an outer layer composed of phospholipids and an inner core where drugs can be loaded. Modifications in this basic structure allow liposomes to mimic the biophysical characteristics of a living cell, thus making it suitable as a therapeutic delivery agent.
Several strategies have been explored to improve the pharmaceutical applications of these nanoparticles. One of the major problems with the initial or ‘first-generation’ liposomes was their poor stability and rapid clearance. To overcome this, stealth liposomes were developed. Here, the nanoparticle was coated with hydrophilic PEG. This reduced its susceptibility to opsonisation and protein adsorption and hence increased blood circulation time [67]. Liposomal vesicles have also been used for trans-dermal drug delivery. This is achieved by developing deformable liposomes, called transferosomes, which have the ability to cross the stratum corneum of the epidermis and thus facilitate the transfer of drugs to the cells underneath. This serves as an alternative to intravenous drug administration and has several advantages like reduced side effects due to the absence of systemic absorption, better bioavailability due to avoidance of first-pass metabolism, better localized accumulation at the site of administration etc. [68, 69]. Other liposome modifications include pharmacosomes, which are liposomes designed for the delivery of poorly soluble or weakly lipophilic drugs, ethosomes, designed for enhanced skin permeation by increasing their ethanol content, etc.
At present, there are several clinically approved liposome-based drugs in the market, of which several have found applications in cancer therapy. Liposome nanoparticles have been successfully used as carriers of anticancer drugs like paclitaxel [70] and doxorubicin [71], as well as some nucleic acids [72]. Besides delivering traditional drugs, liposomes have also been used as a vehicle to deliver immunotherapeutic agents. The use of pH-sensitive liposomes containing a combination of ovalbumin and a pDNA that encodes IFN-γ and directed to the dendritic cells were seen to be successful in promoting the action of CTLs and thus mediated an effective anti-tumour response [73]. Similarly, pH-sensitive PEGylated dextran liposomes containing receptors for TGF-β 1 showed the ability to promote tumour infiltration by CD8 + cells [74]. Liposomes have also been used to promote immunotherapy through gene delivery. Liposomes carrying specific RNA molecules have been seen to activate INF-α-mediated immune response [75].
3.1.3 Dendrimers
Dendrimers are highly versatile three-dimensional globular polymers characterized by their hyper-branched and monodisperse structure (Fig. 3). The basic architecture consists of a central core made up by a single atom or a molecule, surrounding branched layers of repeating units (called ‘generations’) and a large number of terminal functional groups forming the dendritic surface [76]. The extensive branching around the central core leads to formation of several voids which act as flexible spaces where encapsulation of cargo molecules can occur. The presences of numerous terminal functional groups increase the molecules’ functionality and make them versatile and biocompatible. Additionally, dendrimers possess a uniform and well-defined size that is comparable to various biological structures [77], allowing them to easily cross biological barriers, circulate in the body and target specific areas. Another important aspect of dendrimer architecture is peripheral charge. Dendrimers have a polyvalent nature, owing to the various positive, negative or neutral charges on their terminal functional groups. Among the various types, cationic dendrimers or dendrimers with a positively charged surface have shown the most potential as drug carriers. This is because their positive charge allows them to easily bind to, and pass through, negatively charged biological membranes. However, these cationic molecules are known to cause cell-toxicity. To mediate this, the surface groups of such dendrimers are often modified with neutral molecules like carbohydrates, acetate and PEG [76]. Together, these characteristic features of dendrimers make them promising agents for therapeutic drug delivery.

Adapted from Jain et al. (2015); Nanomedicine: Nanotechnology, Biology and Medicine [78]
Schematic representation of dendrimer structure.
Over the years, various strategies have been developed to load drugs into the dendrimers. These include covalent conjugation of drug with the dendrimer surface, physical encapsulation in the inner core and complexing through electrostatic interaction. One approach that has shown significant promise is the functionalization of dendrimer surface with tumour-specific monoclonal antibodies. These dendrimers when loaded with drugs or toxic molecules through encapsulation serve as highly targeted drug delivery agents. For example, poly(propylene imine) dendrimers have been modified to carry monoclonal antibody K1 on their surface. This mAbK1 specifically recognizes mesothelin, a protein commonly overexpressed in ovarian adenocarcinoma. When the immunodendrimer was loaded with paclitaxel, the resulting conjugate showed enhanced drug uptake and tumour volume reduction, improved survival time and increased systemic circulation as compared to free paclitaxel [78]. Using a similar approach, dendrimers have also been designed to target dendritic cells (DCs) for improved antigen presentation. Dendrimers functionalized with Lewisb (Leb) glycopeptides, a natural ligand of DC receptor DC-SIGN, have been explored for this purpose and have shown encouraging results [79]. Another strategy is the incorporation of cytokine associated plasmid DNA (pDNA) into the dendrimers. A tumour-targeted lipid-dendrimer-calcium-phosphate (TT-LDCP) nanoparticle (NPs) with thymine-functionalized dendrimer was developed as a carrier for IL-2 pDNA. Study showed that this TT-LDCP facilitated enhanced endosomal escape and nuclear entry of cargo pDNA, leading to increased DC maturation as well as improved T cell activation [80]. Aside from these, dendrimers have also been explored as carriers for delivery of vaccines, immune checkpoint inhibitors etc. with varying levels of success [81].
3.1.4 Colloidal Nanocarriers
Colloidal nanocarriers have provided a wealth of new opportunities for the advancement of nanopharmaceutics, as they can be circulated throughout the human body as well as can penetrate tissue, or cell barriers, without the need for drug molecules to be integrated into the particles themselves. Anticancer agents linked with colloidal NPs are one of the techniques to boost the selectivity of medications towards cancer cells while minimizing their toxicity towards normal tissues and repressing resistance of other mechanisms. When an antigen is supplied through colloidal carrier systems rather than in soluble form, APCs are more likely to take it up and therefore develop more efficient immune responses [82, 83], which could be a result of the distinct ways that DCs conduct pinocytosis and phagocytosis. Gelatin is a natural biodegradable macromolecule, easy to crosslink and modifies chemically which offers great promise in the production of colloidal drug delivery systems like microspheres and nanoparticles. Since it has been used for decades as a plasma expander, is cheap, and can be sterilized, it stands a greater chance of being authorized as a biopolymer for nanoparticles [84].
Alum colloid encased in β-glucan particles has shown great promise as an adjuvant for tumour—vaccines based on the MUC1 antigen, according to new findings [85]. Since mucin 1 (MUC1) is improperly glycosylated and overexpressed in many types of epithelial cell carcinoma, it has emerged as a prospective target in designing cancer vaccines [86].
3.1.5 Polymeric Micelles
Polymeric micelles are a new class of nanocarriers that are being studied for the delivery of hydrophobic or poorly water-soluble compounds. Of the various types of micelles studied, the most common is the hydrophobically assembled amphiphilic micelles. These molecules undergo spontaneous self-assembly on being exposed to an aqueous environment forming a hydrophobic core into which the water-insoluble drug can be loaded. The hydrophilic segment of these micelles is usually made of PEG, though other polymers like poly(N-isopropyl acrylamide) (pNIPAM) and poly(N-vinyl pyrrolidone) (PVP) have also been used [87]. This part is responsible for providing steric stability and prolonging circulation time in the body. The hydrophobic core is usually composed of polyesters, polyamino acids or polyethers. The choice of polymer plays an important role in determining micelle stability, drug loading capacity and drug release profile. Amphiphilic micelles have several advantages including greater biocompatibility, better stability and lower CMC leading to prolonged circulation [88].
One of the major applications of polymeric micelles in cancer immunotherapy has been in delivering cytokines. Several immunostimulatory cytokines are known to play pivotal roles in mediating cancer immunity. However, their effectiveness is often restricted by their short half-life inside the body. This can be successfully overcome using polymeric micelles. A micelle formed by PEG-polyglutamate copolymer was used as a carrier for IL-2. This was seen to successfully increase the circulation time, improve the accumulation of CTLs as well as increase the efficiency of DC vaccines [89]. Another micelle composed of PEG-b-PGA polymer loaded with macrophage colony-stimulating factor was used to restrict tumour growth in the B16 cancer model by reversing TAM-mediated immunosuppression [90]. Similarly, a cationic diblock polymeric micelle carrying tyrosine-related protein 2 peptide antigen was seen to successfully increase the anti-cancer activity of T-lymphocytes in B16F0 melanoma models [91].
3.2 Inorganic NPs
3.2.1 Gold Nanoparticles
Gold nanoparticles have emerged as one of the most promising nanocarriers being explored at present. They have several advantages over other biomaterials including a high surface area to volume ratio, favourable optical, biochemical and electrical properties, ease of surface functionalization, etc. These, coupled with its non-toxicity and biocompatibility makes it an attractive tool for biomedical research. The action of gold nanoparticles mainly relies on its surface plasmon resonance (SPR) property. This is used to generate localized heating which either leads to cell destruction or mediates drug release from the carriers.
On being introduced into the cell, the nanoparticle interacts with proteins present in the microenvironment to form a nanoparticle-protein complex with the proteins forming a corona around the particle. This corona plays an important role in determining the bioavailability, internalization as well as other pharmacokinetic properties. Further, the particles may also be subjected to PEGylation to reduce their immune recognition and thus prolong circulation [92].
With respect to their use as drug carriers, these gold nanoparticles have been successfully used for delivering nucleotides, vaccines, recombinant proteins and small molecules. The use of gold nanoparticles conjugated with doxorubicin and targeted using anti-PD-L1 antibodies was shown to have improved drug targeting effect in colorectal cancer models. Here, the use of gold nanocarrier not only enabled efficient improved intracellular uptake of doxorubicin but also mediated NIR irradiation, which together led to a decrease in cell proliferation [93]. Another study aimed at using the targeted drug release capabilities of gold nanoparticles to mediate enhanced concentration of antibodies at the tumour site [94]. Gold nanoparticles have also been explored as potential carriers for PD-1/PD-L1 inhibitors. Since PD-1 and PD-L1 are commonly overexpressed in various forms of cancer, this approach has significant promise in developing successful anticancer strategies.
3.2.2 Silica Nanoparticles
Mesoporous silica nanoparticles (MSN) are another form of inorganic drug delivery system that has garnered considerable interest in recent years. Their unique properties like adjustable uniform pore size, easy large-scale production and large surface area and pore volume have made them an exciting new alternative for efficient drug encapsulation and delivery [95]. However, the feature that sets them apart from other nanoparticles is their ability to mediate a controlled drug release. By virtue of their design, MSNs make it possible to create a zero-release configuration where all the pore entrances are blocked by gatekeepers. Here, the drug is released only in response to particular stimuli. These stimuli may be internal, e.g. pH, redox potential or enzymatic activity, or they may be external like light, heat or ultrasound. These stimuli-responsive nanocarriers have garnered huge interest due to their potential role in minimizing side effects associated with early drug release.
The unique properties of MSNs have resulted in the development of several therapeutic drug delivery models. One popular approach has been the design of pH-sensitive MSNs which can respond to the acidic environment commonly seen in tumours. MSNs loaded with the cytotoxic drug 5-fluorouracil and coated with polyelectrolytes o-carboxymethyl chitosan and TEMPO-modified hyaluronic acid was seen to mediate the highly controlled release of the drug in the tumour while also maintaining good biocompatibility and stability [96]. Another widely explored stimulus has been light (visible, UV or NIR). Nanoparticles containing azobenzene derivatives are designed to have their pores closed in the dark. In presence of light, this azobenzene undergoes isomerization leading to the opening of pores and the release of the drug [97].
4 Improved Nanoformulations to Increase Drug Targeting and Its Consequent Release
Currently, around 150 registered clinical trials of cancer drugs are using chemotherapeutic nanomedicines formulated with advanced nano-biomaterials (https://clinicaltrials.gov). Interestingly, these nanomaterials/formulations have also been under evaluation for cancer immunotherapy [98]. Because the clinical potential of PD-1/L1 therapy is high, most nanomedicines are nowadays targeting immune checkpoint proteins like PD-1. Also, mRNA-based nanovaccines have now forayed into this field with 7 clinical trials already registered with https://clinicaltrials.gov for approval. In this review, Abraxane, mRNA-based nanovaccines, other specific peptide nanovaccines like IMF-001, DPX-survivac, JVRS-101 and some biomimetic carriers have been focused on.
4.1 Abraxane
Abraxane is a nanomedicine that traditionally uses albumin nanoparticles as the carrier to transport paclitaxel. Abraxane showed a response rate of 33% as compared to 19% in conventional Taxol, indicating much improvement in the response rates [99,100,101]. FDA has approved Abraxane for the clinical treatment of metastatic breast cancer, metastatic adenocarcinoma of the pancreas and locally advanced or metastatic non-small cell lung cancer. Apart from its usual mechanism of action, paclitaxel also has the potential for immunomodulation. It has been reported to activate the proliferation of dendritic cells (DCs) [102, 103], natural killer (NK) cells [104] and lymphocytes [105]. In addition to this, other reports suggest its role in reducing the regulatory T cell (Treg) count [106] and repolarization of macrophages toward the M-1 phenotype [105].
Abraxane has been combined with immunotherapeutics, especially PD-1/L1 blocking antibodies like atezolizumab, pembrolizumab and avelumab in approximately 70 clinical trials [107]. Among these antibodies, the combination of Abraxane and atezolizumab gave good results in locally advanced or metastatic triple-negative breast cancer. This chemo-immunotherapeutic combination improved both the median progression-free survival (mPSF) and median overall survival (mOS) in patient cohorts with mPSF increasing from 5.5 months to 7.2 months and mOS increasing from 17.6 to 21.3 months, respectively [108]. Also, this combination showed a higher therapeutic effect compared to PD-1/L1 monotherapy [109]. After promising results were seen in breast cancer, this immunotherapy was also tried on metastatic non-squamous non-small-cell lung cancer. Therefore, these reports suggest better clinical efficacy of chemo-immunotherapy of Abraxane and atezolizumab as compared to a single agent, making Abraxane a better alternative to Taxol in combination immunotherapies in terms of reduced toxicities and improved delivery of the drug [110].
4.2 mRNA-Based Nanovaccines
Epitopes expressed only on the surface of cancer cells, called neoantigens, are used to formulate vaccines. mRNA constructed from these identified neoantigens can be encoded inside APCs, which in turn present them to T cells [111]. One well-known example of mRNA nanovaccines is Lipo-MERIT, made by complexing negatively charged mRNA with cationic liposomes. These liposomes, in turn, are constituted of lipids like 1, 2-di-O-octadecenyl-3-trimethylammonium propane or 1,2-dioleoyl-3- trimethylammonium-propane. A very crucial parameter here is the lipid:mRNA ratio of the nanovaccines, which is within the range of 1.3:2, targets the spleen efficiently via intravenous injection [99]. This, as a result, leads to higher stimulation of NK, B, CD4 + , CD8 + T cells along with the production of interferon-alpha (IFN-α) [112].
Similarly, mRNA-4157 is another example where lipid nanoparticles are used to carry the mRNA cargo and used in adjuvant monotherapy in patients having resected solid tumours like bladder carcinoma and melanoma making this a personalized vaccine [reviewed in 114]. After following up with the patients for 8 months, 12 out of the 13 patients who were administered these vaccines were found to be cured. Despite some of these promising outcomes, mRNA nanovaccines have certain limitations like poor cell entry and low enzyme stability [113]. Therefore, only those nanovaccines which can be specifically targeted to APCs are considered for further research in cancer immunotherapy.
4.3 Cholesteryl Pullulan Nanoparticle-Based IMF-001
Cholesteryl Pullulan (CHP) nanoparticles are a novel tumour antigen-delivering system which is being exploited in making cancer vaccines. CHP helps in presenting multiple epitope peptides to both the MHC class I and class II, thus triggering their subsequent pathways [114, 115]. Kayegama and co-researchers have been trying to develop CHP-based protein vaccines against metastatic oesophageal cancer [116]. NY-ESO-1 is an antigen that is found to be expressed in the tumour tissue only, apart from its normal expression in the testis and placenta, therefore making this an ideal target for cancer immunotherapy [117, 118]. Thus, Kayegama et al., 2013 complexed the CHP nanoparticles with NY-ESO-1 antigen to get CHP-NY-ESO-1 (drug code: IMF-001) (see Fig. 4). The safety and immunogenicity of IMF-001 were tested and confirmed. Out of the two cohorts of patients, the 100 µg and 200 µg, the 200 µg dose induced more efficient immune response and overall survival time of 41 weeks as compared to 25 weeks in 100 µg dose group. Thus, IMF-001 showed a dose-dependent effect in the respective target patient groups. Moreover, clinical trials on this protein vaccine suggested that the toxicity here is mild.
4.4 DPX-Survivac
DPX-Survivac is a novel T cell immunotherapeutic, where the DPX platform is used to trigger a powerful T cell response against tumour cells [119]. The DPX is formulated in such a way that increases the duration and robustness of targeted immune responses which would need antigens to be taken up by APCs at the site of injection. It contains a synthetic peptide based on survivin in combination with a lipid NP-based adjuvant (https://immuno-oncologynews.com/dpx-survivac/). Similar to the antigen NY-ESO-1, survivin is also a tumour-associated protein which is upregulated in tumour tissue. Survivin has been detected in various cancers including ovarian, breast, colon and lung cancers, while found absent in healthy adult cells (https://immuno-oncologynews.com/dpx-survivac/). The safety and immunogenicity of DPX-Survivac were tested on patients detected with ovarian cancer in combination with and also without the drug. DPX-survivac showed favourable results in 80% of patients who were administered the drug. It successfully generated T cells specific for survivin and also led to their subsequent invasion into the tumour-microenvironment [119].
4.5 Cationic Lipid-DNA Complex: JVRS 100
JVRS 100 is a cationic lipid DNA complex (CLDC) comprising DNA both having CpG-rich motifs and non-CpG motifs to evoke immunomodulatory actions alongside a cholesterol-liposomal outer covering. Their route of administration to the system facilitates entry to dendritic cells (DCs) and macrophages. Moreover, the DNA present can activate the Toll-like receptors (TLRs) by binding to them and consequently generating NK cells and inducing T cell response (https://clinicaltrials.gov). The use of this delivery agent has been tested on animals suffering from acute leukaemia [120]. The results after this clinical trial suggested that the JVRS 100 elicited cytokines crucial for aiding the host system’s defence mechanism against cancer, especially IL-12, IFN-gamma and IFN-alpha. Furthermore, JVRS-100 was reported to have significantly delayed death from leukaemia in patients when delivered between 7 and 15 days following leukemic challenge, when injected intravenously [121].
5 Platelet-HSC Conjugation-Based S-P-aPD-1
Hu and co-workers developed a combination delivery system where cell membranes of haematopoietic stem cells (HSCs) were conjugated with platelets expressing antibodies against PD-1 (aPD-1) [122]. This delivery system named S-P-aPD-1 was carried to the bone marrow of leukaemia tumour-bearing mice. Thereafter, these platelets were activated by nearby macrophages, forming platelet-derived microparticles (PMPs) which release aPD-1 in situ (see Fig. 5). It was subsequently reported that this nanocarrier along with the drug activated a robust immunological response against the target tumour tissue by facilitating the release of several chemokines and cytokines along with the activation of T cells. Furthermore, it also extended the survival time of diseased mice. Thus, it was inferred that the drug delivery strategy successfully carried the cargo and also managed to occlude PD-1.
Schematic represents antibody aPD-1 expressing platelets which are conjugated with HSCs to get S-P-aPD-1. The drug delivery system gets transported to the bone marrow where the platelets get activated forming platelet-derived microparticles (PMPs). These PMPs release the antibodies in situ that in turn bind and block PD-1 from binding PD-L1 of leukaemia cells
6 Biodegradable Polymer-Based Nanocarriers
Although artificial nanoparticles are being extensively studied for disease diagnosis and therapy, their limitation lies in their accumulation and sequestration in the liver and spleen, thereby preventing proper delivery to the target tissue [123]. This brings the researchers to exploit biodegradable nanosystems for better drug delivery in vivo. Biodegradable nanopolymers can be constructed on the basis of chosen cargo and polymer into solid nanoparticles, core-shell structures, polymeric micelles and polyplexes. However, polymeric nanoparticles are usually constructed by either self-assembly or emulsion [124, 125]. Herein, we tried to discuss some recent examples of using biodegradable polymers in delivering drugs for cancer immunotherapy (also refer Table 1).
6.1 Exosome Nano-bioconjugate
Exosomes are membrane-bound vesicular particles in the nanoscale range, contained in almost all secretory cells. They have the capability to imitate the internal conditions and functions of those indigenous cells [126, 127]. In 2020, Nie et al. designed azide-modified exosome nano-bioconjugates (M1 Exo-Ab) which comprise of proinflammatory M1-macrophage exosomes and dibenzocyclooctyne-modified antibodies of CD47 and SIRPa (aCD47 and aSIRPa) through pH-sensitive benzoic-imine bonds [128]. After administering, M1 Exo-Ab was found to target the tumour actively by specifically recognizing CD47 antigen overexpressed on the tumour cell surface. Moreover, the nano-bioconjugate successfully disintegrated to ease the release of the encapsulated antibodies: aSIRPa and aCD47, which then respectively block SIRPa on macrophages and CD47. All this occurred in the acidic tumour microenvironment, finally leading to phagocytosis of macrophages and resistance towards the ‘don’t eat me’ signal [128].
6.2 Hyaluronic Acid (HA) Integrated with Dextran Nanoparticles
A naturally found biopolymer like hyaluronic acid was conjugated with dextran nanoparticles in order to form a microneedle. Usually, pH-sensitive dextran nanoparticles are chosen for this purpose. This biodegradable complex encapsulated anti-PD-1 antibodies were aimed at slow and regulated release of the immuno-drug in melanoma tissue. It was inferred from the observations recorded in the B16F10 melanoma model that the microneedle mediated delivery enabled the anti-PD-1 to trigger an elevated immune response compared to free anti-PD-1 antibody at the same dose [129].
6.3 Biodegradable Polymer-Coated Oncolytic Adenovirus (oAd)
In 2013, Kim and co-workers designed and developed a biodegradable methoxy poly (ethylene glycol)-b-poly {N- [N-(2-aminoethyl)-2-aminoethyl]-L-glutamate (PNLG) polymer which was made to coat oncolytic Ad (Ad-DB7- U6shIL8) (oAd/PNLG). This formulation resulted in higher tumour accumulation when systemically administered oAd/PNLG as compared with naked Ad and Ad conjugated with PEI. Interestingly, the oAd/PNLG also showed significantly lower innate and adaptive immune responses than only naked Ad (see Fig. 6A, B). Furthermore, the oAd/PNLG was reported to perform cancer cell killing more efficiently than both naked Ad and Ad/PEI [130].
Schematic representing oncolytic virus-mediated immunotherapy where A shows that when naked oAd is disabled by pre-existing Adenoviral-specific neutralizing antibodies and B shows revived oncolysis by coating with polymer, as anti-Ad antibodies can no longer neutralize the coated complex. This helps in higher targeting of tumour cells due to improvement in immunological response. oAd, oncolytic adenovirus; TAAs, tumour-associated antigens
6.4 Biodegradable Lignin-Based Nanoparticles
Lignin being one of the amplest aromatic polymers and renewable resources is a great applicant for the development of functional eco-friendly biomaterials due to its biodegradability, biocompatibility and low toxicity [143]. Figueiredo et al. constructed and characterized three lignin-based nano-systems that can be used—pure lignin NPs (pLNPs), iron(III)-complexed lignin NPs (Fe-LNPs) and Fe3O4-infused lignin NPs (Fe3O4-LNPs) [144] [145]. Flow cytometry was used to further quantitatively evaluate the cell-pLNP interaction. Poorly water-soluble drugs and different cytotoxic compounds [i.e. sorafenib (SFN), benzazulene (BZL)] have been used as model drugs for assessing the packaging ability of pLNPs, and their release profiles were also inspected thereafter. BZL-loaded pLNP’s antiproliferation effect was also analysed using various cancer cell lines. In addition, an assessment of water-soluble drug and their release profiles were done besides examining the antiproliferation effect of BZL-loaded pLNPs. Due to BZL’s promising anticancer properties, it was used in the experiments. Moreover, tricyclic benzazulenes and their derivatives are well known as specific Pim kinase inhibitors linked to cancer development in terms of advancing cell endurance and developing hindrance against chemotherapy and radiation therapy [146]. Due to the improved dissolution of BZL loaded in pLNPs, all cell lines which were used for this testing reported a significant cell growth inhibition. This growth inhibition impact was later compared with an identical molar ratio of unfastened BZL after 24-h incubation [147]. Pure BZL was not able to lessen the cellular viability to much less than 60%, whereas the BZL-pLNPs successfully did that from approximately 90 to 0% for entire cell traces, thereby proving its promise as an antiproliferative [148].
6.5 Polymeric Bio-nanoparticles
Methods like self-assembly of polymers, the macro-/micro-/mini-emulsion process and nanoprecipitation technique [149] [150] led to the evolution of polymeric bio-nanoparticles. These have characteristic features which make them very less toxic, thereby attracting their desirability in the field of immunotherapy. Zheng et al. reported a multifunctional NP self-assembly using widely used polylactic-co-glycolic acid (PLGA), a responsive amino-substituted HB derivative (DPAHB) and polyethylene glycol (PEG) [151]. PLGA efficiently blocked NP aggregation by creating a shell-like structure around DPAHB forming a sort of vesicle and PEG and, on the other hand, helped stabilize the same. Tumour regression has been observed beneficially by the nanovesicles which bring about high levels of singlet oxygen.
Kosmides and co-workers outlined and formulated a study where a PLGA-based artificial antigen-presenting cell was integrated with an antiPD1 monoclonal antibody. The MHC IgG dimer and anti-CD28 monoclonal antibody were added to the PLGA core to solutions to allow particles to react with the proteins for artificial antigen-presenting cell preparation [152]. While combined with checkpoint blockade, the NPs instigated IFN-g secretion and expanded the durability of tumour-bearing mice.
In experiments performed by Luo and co-workers, a polymer-based nano-vaccine (PC7A NP) was designed that could steer to a powerful cytotoxic T cell response [153]. Aggregation of PC7A NP with the checkpoint blockade confirmed synergistic anti-cancer efficacy in a TC-1 tumour model as per their illustration.
6.6 Biomimetic Nanoparticles
Li et al. designed some small-sized nanostructures called DNA origami molecules which are nanoscale folding of oligonucleotides that self-bring together into homogenous nanostructures as constructing blocks [154].
In cancer immunotherapy, purely DNA-based NPs have also emerged as a budding application. The nanostructures enhance immunostimulatory properties with synthetic CpG oligodeoxynucleotides which can bind to Toll-like receptor 9 (TLR9). They promote a signaling cascade besides being compact, stable, biocompatible and non-cytotoxic.
Another example is protein NP. It reportedly showed better anti-tumour activity as compared to non-capsulated phenanthriplatin when sheathed with tobacco mosaic virus (TMV) NPs according to Czapar et al. which scaled down the tumour size of the triple-negative breast cancer in mice [155].
7 Biomaterials for Non-specific Immune Stimulation
There have been some biomaterial-based delivery strategies to increase cytokine circulation, for instance, Park et al. developed a system referred to as nanolipogel, a nanoscale liposomal polymeric gel, evolved for the co-shipping of IL-2 and TGF-β, eased the entrapment of the drug-loaded β-cyclodextrins and IL-2 in a biodegradable polymer matrix with a PEGylated liposomal coating raised anti-cancer efficacy and survival in tumour-bearing mice [156]. PEG is coupled to a protein and thus is being modified for increasing circulation half-life and diminishing side effects of cytokines [157]. Poxylation (poly (2-oxazoline) polymers) [158] is an alternative polymer for the conjugation with cytokines for increased safety and efficiency beyond PEGylation. A pH-responsive NP stacked along with IL-12 was formulated by Wang and co-researchers. This delivery system was successfully released into the acidic tumour environment [159]. In another example, MPEG-PLA and 1, 2-dioleoyl-3-trimethylammonium-propane (DOTAP) were used to design a combination gene delivery system where plasmid interleukin-15 (IL-15) DNA was loaded as the cargo and subsequently given affirmative results, as shown in Fig. 7. MPEG-PLA and DOTAP were congregated into a new gene carrier, DOTAP/MPEG-PLA (DMA) micelles, which convey the pIL15 plasmid into cancer cells and after their transfection would express and secrete IL-15 (Fig. 7), which has been tested in preclinical studies to stimulate immune activation and regulate immune suppression [160]. The tumour volume, weight and normal body weight in a colon cancer mouse model are shown in Fig. 8A, B and C, respectively. The ex vivo images portray the tumour growth inhibition by DMA/pIL-15 (Fig. 8D), pointing towards higher chances of the combination formula in treating the tumour [160].
Transfection and targeting of pIL15 plasmid. DOTAP/MPEG-PLA (DMA) micelles composed of MPEG-PLA and DOTAP and help carry the pIL15 plasmid into cancer cells. They also aid the expression and secretion of the plasmid (adapted from Liu et al., 2018 [150])
Colon cancer model showcasing anti-tumour effect of DMA/pIL15. A Tumour growth curves from day 7 to 17. B Tumour weights after different treatment methods. C Body weight of mice. D Images of tumours, in different treatment groups (adapted from Liu et al., 2018 Theranostics [150])
8 Conclusion
Despite extensive research, cancer continues to be one of the most common fatal diseases affecting people worldwide. While treatment options like surgery, radiation and chemotherapy have been used extensively, the limitations associated with them, namely the higher probability of recurrence and severe side effects, have made it necessary to look for better alternatives. Over the last few decades, immunotherapy has developed as one of the leading strategies for cancer management. Its higher efficacy, paired with lower toxic effects, has made it an attractive option. Passive immunotherapy in the form of cytokines and active therapy using cancer vaccines has both shown promising results. Furthermore, advanced techniques like the use of oncolytic viruses, adoptive cell therapy and immune checkpoint inhibitors have also shown significant potential and are the basis of several clinical trials.
However, in spite of their various advantages, the use of these strategies has been limited so far. One of the major reasons for this is their propensity for off-target effects. To overcome this, targeted therapy using bioactive nanoparticles has emerged as a suitable solution. Here nanoparticles act as carriers for the antigen, adjuvant or other immunotherapeutics and help avoid non-specific uptake, increase tissue permeation and also enable specific intracellular localization.
Of the various biomaterials that have been explored clinically as well as preclinical, the most commonly used are polymeric nanoparticles. This includes PLGA, liposomes and micelle nanoparticles. Other than these, inorganic nanoparticles like gold, silica, iron oxide etc. have also been studied for their low toxicity and ease of manufacture. All of these particles have their unique properties and have established themselves as successful drug carriers in various studies.
At present, there are various nanoparticle-based therapies in different stages of clinical use. Nanoformulations like Abraxane have found application in cancer treatment, both by themselves and in conjugation with other immunotherapeutics. Nanovaccines carrying tumour-specific proteins or mRNA are another group that has shown promise in clinical trials.
While the field of nanocarriers as a whole is undergoing intense research, an avenue that has sparked special interest is the development of biodegradable nanoparticles. These molecules are designed to break down in physiological conditions and hence reduce any toxicity associated with the accumulation of macromolecules. Various materials have been explored for this purpose. These include natural materials like dextran and lignin as well as synthetic polymers like PLNG and functionalized PLGA. Aside from these, a range of biomimetics has also been explored as nanocarriers. DNA NP, protein NP and exosomes have all shown considerable promise as suitable carriers for desired drugs.
Data Availability
All the information has been gathered from research papers and review articles focusing on cancer immunotherapy and biomaterials used for better delivery of immunotherapeutics.
Reference
Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. (2022). Cancer statistics, 2022. A Cancer Journal for Clinicians, 72(1), 7–33.
Weber, J. S., Yang, J. C., Atkins, M. B., & Disis, M. L. (2015). Toxicities of immunotherapy for the practitioner. Journal of Clinical Oncology, 33, 2092-U2154.
Motz, G. T., & Coukos, G. (2013). Deciphering and reversing tumor immune suppression. Immunity, 39, 61–73.
Doroshow, J. H., & Kummar, S. (2014). Translational research in oncology-10 years of progress and future prospects. Nature Reviews Clinical Oncology, 11, 649–662.
Singh, A., & Peppas, N. A. (2014). Hydrogels and scaffolds for immunomodulation. AdvancedMaterials, 26, 6530–6541.
Kim, J., Li, W. A., Choi, Y., Lewin, S. A., Verbeke, C. S., Dranoff, G., & Mooney, D. J. (2015). Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nature biotechnology, 33(1), 64–72.
Reátegui, E., Aceto, N., Lim, E. J., Sullivan, J. P., Jensen, A. E., Zeinali, M., & Stott, S. L. (2015). Tunable nanostructured coating for the capture and selective release of viable circulating tumour cells. Advanced materials, 27(9), 1593–1599.
Domachuk, P., Tsioris, K., Omenetto, F. G., & Kaplan, D. L. (2010). Bio-microfluidics: Biomaterials and biomimetic designs. Advanced materials, 22(2), 249–260.
Shao, K., Singha, S., Clemente-Casares, X., Tsai, S., Yang, Y., & Santamaria, P. (2015). Nanoparticle-based immunotherapy for cancer. ACS nano, 9(1), 16–30.
Ahmed, S., & Rai, K. R. (2003). Interferon in the treatment of hairy-cell leukemia. Best Practice & Research Clinical Haematology, 16(1), 69–81.
Sang, W., Zhang, Z., Dai, Y., & Chen, X. (2019). Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chemical Society Reviews, 48(14), 3771–3810.
Maeda, S., Wada, H., Naito, Y., Nagano, H., Simmons, S., Kagawa, Y., Naito A, Kikuta J, Ishii, T., Tomimaru Y, Hama N, Kawamoto K, Kobayashi S, Eguchi H, Umeshita K, Ishii H, Doki Y, Mori M & Ishii, M. (2014). Interferon-α acts on the S/G2/M phases to induce apoptosis in the G1 phase of an IFNAR2-expressing hepatocellular carcinoma cell line. Journal of Biological Chemistry 289(34), 23786–23795.
Sun, T., Yang, Y., Luo, X., Cheng, Y., Zhang, M., Wang, K., & Ge, C. (2014). Inhibition of tumour angiogenesis by interferon-γ by suppression of tumour-associated macrophage differentiation. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics, 21(5), 227–235.
Elomaa, A. P., Niskanen, L., Herzig, K. H., Viinamäki, H., Hintikka, J., Koivumaa-Honkanen, H., & Lehto, S. M. (2012). Elevated levels of serum IL-5 are associated with an increased likelihood of major depressive disorder. BMC Psychiatry, 12(1), 1–8.
Müller, L., Aigner, P., & Stoiber, D. (2017). Type I interferons and natural killer cell regulation in cancer. Frontiers in immunology, 8, 304.
Lin, C. F., Lin, C. M., Lee, K. Y., Wu, S. Y., Feng, P. H., Chen, K. Y., & Tsai, T. T. (2017). Escape from IFN-γ-dependent immuno surveillance in tumourigenesis. Journal of Biomedical Science, 24(1), 1–9.
Lee, S., & Margolin, K. (2011). Cytokines in cancer immunotherapy. Cancers, 3(4), 3856–3893.
Cox, M. A., Harrington, L. E., & Zajac, A. J. (2011). Cytokines and the inception of CD8 T cell responses. Trends in immunology, 32(4), 180–186.
Ben-Sasson, S. Z., Hu-Li, J., Quiel, J., Cauchetaux, S., Ratner, M., Shapira, I., Dinarello, C.A. & Paul, W. E. (2009). IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proceedings of the National Academy of Sciences, 106(17), 7119–7124
Trinchieri, G. (2003). Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Reviews Immunology, 3(2), 133–146.
Itoh, K., & Hirohata, S. (1995). The role of IL-10 in human B cell activation, proliferation, and differentiation. The Journal of Immunology, 154(9), 4341–4350.
Yan, W. L., Shen, K. Y., Tien, C. Y., Chen, Y. A., & Liu, S. J. (2017). Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy, 9(4), 347–360.
Uhl, M., Aulwurm, S., Wischhusen, J., Weiler, M., Ma, J. Y., Almirez, R., Mangadu, R., Liu, Y.W., Platten, M., Herrlinger, U., & Murphy, A. (2004). SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Research, 64(21), 7954–7961.
Hasan, A. N., Selvakumar, A., Shabrova, E., Liu, X. R., Afridi, F., Heller, G., Riviere, I., Sadelain, M., Dupont, B., & O'Reilly, R. J. (2016). Soluble and membrane-bound interleukin (IL)-15 R α/IL-15 complexes mediate proliferation of high-avidity central memory CD8+ T cells for adoptive immunotherapy of cancer and infections. Clinical & Experimental Immunology, 186(2), 249–265.
Chapuis, A. G., Lee, S. M., Thompson, J. A., Roberts, I. M., Margolin, K. A., Bhatia, S., Sloan, H.L., Lai, I., Wagener, F., Shibuya, K & Cao, J.. (2016). Combined IL-21–primed polyclonal CTL plus CTLA4 blockade controls refractory metastatic melanoma in a patient. Journal of Experimental Medicine, 213(7), 1133–1139.
Perna, S. K., Pagliara, D., Mahendravada, A., Liu, H., Brenner, M. K., Savoldo, B., & Dotti, G. (2014). Interleukin-7 mediates selective expansion of tumor-redirected cytotoxic T lymphocytes (CTLs) without enhancement of regulatory T-cell inhibition IL-7 overcomes Treg inhibition of CAR-modified CTLs. Clinical Cancer Research, 20(1), 131–139.
Berger, C., Berger, M., Hackman, R. C., Gough, M., Elliott, C., Jensen, M. C., & Riddell, S. R. (2009). Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood, The Journal of the American Society of Hematology, 114(12), 2417–2426.
Barra, F., Leone Roberti Maggiore, U., Bogani, G., Ditto, A., Signorelli, M., Martinelli, F., ... & Ferrero, S. (2019). New prophylactics human papilloma virus (HPV) vaccines against cervical cancer. Journal of Obstetrics and Gynaecology, 39(1), 1–10
Conniot, J., Silva, J. M., Fernandes, J. G., Silva, L. C., Gaspar, R., Brocchini, S., ... & Barata, T. S. (2014). Cancer immunotherapy: Nanodelivery approaches for immune cell targeting and tracking. Frontiers in Chemistry, 2, 105.
Guo, C., Manjili, M. H., Subjeck, J. R., Sarkar, D., Fisher, P. B., & Wang, X. Y. (2013). Therapeutic cancer vaccines: Past, present, and future. Advances in cancer research, 119, 421–475.
Garg, A. D., Coulie, P. G., Van den Eynde, B. J., & Agostinis, P. (2017). Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends in immunology, 38(8), 577–593.
Rosenberg, S. A., Yang, J. C., & Restifo, N. P. (2004). Cancer immunotherapy: Moving beyond current vaccines. Nature medicine, 10(9), 909–915.
Kantoff, P. W., Higano, C. S., Shore, N. D., Berger, E. R., Small, E. J., Penson, D. F., Redfern, C.H., Ferrari, A.C., Dreicer, R., Sims, R.B. & Xu, Y. (2010). Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New England Journal of Medicine, 363(5), 411–422.
Chiang, C. L. L., Coukos, G., & Kandalaft, L. E. (2015). Whole tumour antigen vaccines: Where are we? Vaccines, 3(2), 344–372.
Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines—a new era in vaccinology. Nature reviews Drug discovery, 17(4), 261–279.
Liu, M. A. (2011). DNA vaccines: An historical perspective and view to the future. Immunological reviews, 239(1), 62–84.
Yang, B., Jeang, J., Yang, A., Wu, T. C., & Hung, C. F. (2014). DNA vaccine for cancer immunotherapy. Human vaccines & immunotherapeutics, 10(11), 3153–3164.
Kauffman, K. J., Webber, M. J., & Anderson, D. G. (2016). Materials for non-viral intracellular delivery of messenger RNA therapeutics. Journal of Controlled Release, 240, 227–234.
Li, L., Goedegebuure, S. P., & Gillanders, W. E. (2017). Preclinical and clinical development of neoantigen vaccines. Annals of Oncology, 28, xii11–xii17.
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer, 12(4), 252–264.
Alsaab, H. O., Sau, S., Alzhrani, R., Tatiparti, K., Bhise, K., Kashaw, S. K., & Iyer, A. K. (2017). PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Frontiers in pharmacology, 8, 561.
Yang, F., Shi, K., Jia, Y. P., Hao, Y., Peng, J. R., & Qian, Z. Y. (2020). Advanced biomaterials for cancer immunotherapy. Acta PharmacologicaSinica, 41(7), 911–927.
Grosso, J. F., & Jure-Kunkel, M. N. (2013). CTLA-4 blockade in tumour models: An overview of preclinical and translational research. Cancer Immunity, 13(1).
Friedman, C. F., Proverbs-Singh, T. A., & Postow, M. A. (2016). Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA oncology, 2(10), 1346–1353.
Byun, D. J., Wolchok, J. D., Rosenberg, L. M., & Girotra, M. (2017). Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nature Reviews Endocrinology, 13(4), 195–207.
Sakuishi, K., Apetoh, L., Sullivan, J. M., Blazar, B. R., Kuchroo, V. K., & Anderson, A. C. (2010). Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumour immunity. Journal of Experimental Medicine, 207(10), 2187–2194.
Fitzgerald, J. C., Weiss, S. L., Maude, S. L., Barrett, D. M., Lacey, S. F., Melenhorst, J. J., Teachey, D. T., Shaw, P., Berg, R.A., June, C.H., Porter, D.L. & Frey, N.V. (2017). Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Critical Care Medicine, 45(2), e124.
Robert, C., Schachter, J., Long, G. V., Arance, A., Grob, J. J., Mortier, L., & Ribas, A. (2015). Pembrolizumab versus ipilimumab in advanced melanoma. New England Journal of Medicine, 372(26), 2521–2532.
Hinrichs, C. S., & Rosenberg, S. A. (2014). Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunological reviews, 257(1), 56–71.
Kazemi, T., Younesi, V., Jadidi-Niaragh, F., & Yousefi, M. (2016). Immunotherapeutic approaches for cancer therapy: An updated review. Artificial cells, nanomedicine, and biotechnology, 44(3), 769–779.
Gilham, D. E., Anderson, J., Bridgeman, J. S., Hawkins, R. E., Exley, M. A., Stauss, H., Maher, J., Pule, M., Sewell, A.K., Bendle, G. and Lee, S., & Morris, E. (2015). Adoptive T-cell therapy for cancer in the United Kingdom: A review of activity for the British Society of Gene and Cell Therapy annual meeting 2015. Human Gene Therapy, 26(5), 276–285.
Scholler, J., Brady, T. L., Binder-Scholl, G., Hwang, W. T., Plesa, G., Hege, K. M., & June, C. H. (2012). Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Science translational medicine, 4(132), 132ra53-132ra53.
Gaston, D. C., Odom, C. I., Li, L., Markert, J. M., Roth, J. C., Cassady, K. A., Whitley, R.J., & Parker, J. N. (2013). Production of bioactive soluble interleukin-15 in complex with interleukin-15 receptor alpha from a conditionally-replicating oncolytic HSV-1. PloS One, 8(11), e81768.
Ledford, H. (2015). Cancer-fighting viruses near market. Nature, 526(7575), 622–623.
Liu, B. L., Robinson, M., Han, Z. Q., Branston, R. H., English, C., Reay, P., ... & Coffin, R. S. (2003). ICP34. 5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Therapy, 10(4), 292–303.
Lichty, B. D., Breitbach, C. J., Stojdl, D. F., & Bell, J. C. (2014). Going viral with cancer immunotherapy. Nature Reviews Cancer, 14(8), 559–567.
Danhier, Fabienne, et al. (2012). PLGA-based nanoparticles: An overview of biomedical applications. Journal of controlled release: official journal of the Controlled Release Society, 161(2), 505–22. https://doi.org/10.1016/j.jconrel.2012.01.043
Agarwal, Rachit, et al. (2013). Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proceedings of the National Academy of Sciences of the United States of America, 110(43), 17247–52. https://doi.org/10.1073/pnas.1305000110
Yang, Qi., et al. (2014). Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation. Molecular pharmaceutics, 11(4), 1250–8. https://doi.org/10.1021/mp400703d
Owens, Donald E., 3rd., & Peppas, Nicholas A. (2006). Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International journal of pharmaceutics, 307(1), 93–102. https://doi.org/10.1016/j.ijpharm.2005.10.010
Foged, Camilla, et al. (2005). Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. International journal of pharmaceutics, 298(2), 315–22. https://doi.org/10.1016/j.ijpharm.2005.03.035
Molavi, Ommoleila, et al. (2010). Development of a poly(d, l-lactic-co-glycolic acid) nanoparticle formulation of STAT3 inhibitor JSI-124: Implication for cancer immunotherapy. Molecular pharmaceutics, 7(2), 364–74. https://doi.org/10.1021/mp900145g
Alshamsan, Aws, et al. (2011). STAT3 Knockdown in B16 melanoma by siRNA lipopolyplexes induces bystander immune response in vitro and in vivo. Translational oncology, 4(3), 178–88. https://doi.org/10.1593/tlo.11100
Roy, A., Singh, M. S., Upadhyay, P., & Bhaskar, S. (2010). Combined chemo-immunotherapy as a prospective strategy to combat cancer: a nanoparticle based approach. Molecular pharmaceutics, 7(5), 1778–88. https://doi.org/10.1021/mp100153r
Luo, Lihua, et al. (2018). Laser immunotherapy in combination with perdurable PD-1 blocking for the treatment of metastatic tumors. ACS nano, 12(8), 7647–7662. https://doi.org/10.1021/acsnano.8b00204
Roberts, R., Smyth, J. W., Will, J., Roberts, P., Grek, C. L., Ghatnekar, G. S., Sheng, Z., Gourdie, R. G., Lamouille, S., & Foster, E. J. (2020). Development of PLGA nanoparticles for sustained release of a connexin43 mimetic peptide to target glioblastoma cells. Materials Science and Engineering: C, 108, 110191. https://doi.org/10.1016/j.msec.2019.110191
Van Slooten, M. L., et al. (2001). Liposomes as sustained release system for human interferon-gamma: Biopharmaceutical aspects. Biochimica et biophysica acta, 1530(2–3), 134–145. https://doi.org/10.1016/s1388-1981(00)00174-8
Gillet, A et al. (2011). Skin penetration behaviour of liposomes as a function of their composition. European Journal of Pharmaceutics and Biopharmaceutics: Official Journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnike, 79(1), 43–53. https://doi.org/10.1016/j.ejpb.2011.01.011
Elsayed, Mustafa M A., et al. (2006). Deformable liposomes and ethosomes: Mechanism of enhanced skin delivery. International journal of pharmaceutics, 322(1–2), 60–6. https://doi.org/10.1016/j.ijpharm.2006.05.027
Han, B., Yang, Y., Chen, J., Tang, H., Sun, Y., Zhang, Z., Wang, Z., Li, Y., Li, Y., Luan, X., & Li, Q. (2020). Preparation, characterization, and pharmacokinetic study of a novel long-acting targeted paclitaxel liposome with antitumour activity. International journal of nanomedicine, 15, 553–571. https://doi.org/10.2147/IJN.S228715
O’Brien, M. E. R., et al. (2004). Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Annals of oncology : Official journal of the European Society for Medical Oncology, 15(3), 440–449. https://doi.org/10.1093/annonc/mdh097
Chen, Yunching, et al. (2010). Nanoparticles modified with tumour-targeting scFv deliver siRNA and miRNA for cancer therapy. Molecular therapy : the journal of the American Society of Gene Therapy, 18(9), 1650–6. https://doi.org/10.1038/mt.2010.136
Yuba, Eiji, et al. (2015). pH-sensitive polymer-liposome-based antigen delivery systems potentiated with interferon-γ gene lipoplex for efficient cancer immunotherapy. Biomaterials, 67, 214–24. https://doi.org/10.1016/j.biomaterials.2015.07.031
Yuba, Eiji, Shinya Uesugi, Yuta Yoshizaki, Atsushi Harada, & Kenji Kono. (2017). Potentiation of cancer immunity-inducing effect by pH-sensitive polysaccharide-modified liposomes with combination of TGF-β type I receptor inhibitor-embedded liposomes. Medical Research Archives, 5.5: n. pag. Web. 29 May 2022
Kranz, Lena M., et al. (2016). Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature, 534(7607), 396–401. https://doi.org/10.1038/nature18300
Kesharwani, Prashant, et al. (2014). Dendrimer as nanocarrier for drug delivery. Progress in Polymer Science, 39(2), 268–307. https://doi.org/10.1016/j.progpolymsci.2013.07.005. Accessed 27 Dec. 2022.
Esfand, R., & Tomalia, D. A. (2001). Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discovery Today, 6(8), 427–436. https://doi.org/10.1016/S1359-6446(01)01757-3
Narendra, J., et al. (2015). The development, characterization and in vivo anti-ovarian cancer activity of poly(propylene imine) (PPI)-antibody conjugates containing encapsulated paclitaxel. Nanomedicine: Nanotechnology Biology and Medicine, 11(1), 207–218. https://doi.org/10.1016/j.nano.2014.09.006. Accessed 27 Dec. 2022.
García-Vallejo, Juan J., et al. (2013). Multivalent glycopeptide dendrimers for the targeted delivery of antigens to dendritic cells”. Molecular immunology, 53(4), 387–97. https://doi.org/10.1016/j.molimm.2012.09.012
Huang, Kuan-Wei., et al. (2020). Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Science advances, 6(3), eaax5032. https://doi.org/10.1126/sciadv.aax5032
Rawding, Piper A., et al. (2022). Dendrimers for cancer immunotherapy: Avidity-based drug delivery vehicles for effective anti-tumor immune response Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology, 14(2), e1752. https://doi.org/10.1002/wnan.1752
Diwan, M., Tafaghodi, M., & Samuel, J. (2002). Enhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. Journal of controlled release, 85(1–3), 247–262.
Elamanchili, P., Diwan, M., Cao, M., & Samuel, J. (2004). Characterization of poly (D, L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine, 22(19), 2406–2412.
Schwick, H. G., & Heide, K. (1969). Immunochemistry and immunology of collagen and gelatin. Modified gelatins as plasma substitutes, 33, 111–125.
Liu, Y., Li, M., Zhu, H., Jing, Z., Yin, X., & Wang, K.,… & Zhao, W. (2021). Alum colloid encapsulated inside β-glucan particles enhance humoral and CTL immune responses of MUC1 vaccine. Chinese Chemical Letters, 32(6), 1963–1966.
Wilson, R. M., & Danishefsky, S. J. (2013). A vision for vaccines built from fully synthetic tumor-associated antigens: From the laboratory to the clinic. Journal of the American Chemical Society, 135(39), 14462–14472.
Agrawal, R. D., Tatode, A. A., Rarokar, N. R., & Umekar, M. J. (2020). Polymeric micelle as a nanocarrier for delivery of therapeutic agents: A comprehensive review. Journal of Drug Delivery and Therapeutics, 10(1-s), 191–5. https://doi.org/10.22270/jddt.v10i1-s.3850
YousefpourMarzbali, M., & YariKhosroushahi, A. (2017). Polymeric micelles as mighty nanocarriers for cancer gene therapy: A review. Cancer chemotherapy and pharmacology, 79(4), 637–649. https://doi.org/10.1007/s00280-017-3273-1
Miki, Kenji, et al. (2014). Combination therapy with dendritic cell vaccine and IL-2 encapsulating polymeric micelles enhances intra-tumoural accumulation of antigen-specific CTLs”. International immunopharmacology, 23(2), 499–504. https://doi.org/10.1016/j.intimp.2014.09.025
Mao, Kuirong, et al. (2019). Intratumoural delivery of M-CSF by calcium crosslinked polymer micelles enhances cancer immunotherapy. Biomaterials science, 7(7), 2769–2776. https://doi.org/10.1039/c9bm00226j
Li, Hanmei, et al. (2017). Rational design of polymeric hybrid micelles to overcome lymphatic and intracellular delivery barriers in cancer immunotherapy. Theranostics, 7(18), 4383–4398. https://doi.org/10.7150/thno.20745
Kong, Fen-Ying., et al. (2017). Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules (Basel, Switzerland), 22(9), 1445. https://doi.org/10.3390/molecules22091445
Singh, Priyanka, et al. (2018). Gold nanoparticles in diagnostics and therapeutics for human cancer. International journal of molecular sciences, 19(7), 1979. https://doi.org/10.3390/ijms19071979
Emami, Fakhrossadat, et al. (2019). Doxorubicin and anti-PD-L1 antibody conjugated gold nanoparticles for colorectal cancer photochemotherapy. Molecular pharmaceutics, 16(3), 1184–1199. https://doi.org/10.1021/acs.molpharmaceut.8b01157
Meir, Rinat, et al. (2017). Fast image-guided stratification using anti-programmed death ligand 1 gold nanoparticles for cancer immunotherapy. ACS nano, 11(11), 11127–11134. https://doi.org/10.1021/acsnano.7b05299
Gao, Ying, et al. (2020). A review of mesoporous silica nanoparticle delivery Systems in chemo-based combination cancer therapies. Frontiers in chemistry, 8, 598722. https://doi.org/10.3389/fchem.2020.598722
Anirudhan, Thayyath Sreenivasan, et al. (2017). Layer-by-layer assembly of hyaluronic acid/carboxymethylchitosan polyelectrolytes on the surface of aminated mesoporous silica for the oral delivery of 5-fluorouracil. European Polymer Journal, 93, 572–589.
Tarn, Derrick, et al. (2014). A reversible light-operated nanovalve on mesoporous silica nanoparticles. Nanoscale, 6(6), 3335–43. https://doi.org/10.1039/c3nr06049g
Saeed, M., Gao, J., Shi, Y., Lammers, T., & Yu, H. (2019). Engineering nanoparticles to reprogram the tumor immune microenvironment for improved cancer immunotherapy. Theranostics, 9(26), 7981–8000.
Gradishar, W. J., Tjulandin, S., Davidson, N., Shaw, H., Desai, N., Bhar, P., Hawkins, M., & O’Shaughnessy, J. (2005). Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. Journal of Clinical Oncology., 23, 7794–7803.
Gradishar, W. J. (2006). Albumin-bound paclitaxel: A next-generation taxane. Expert Opinion on Pharmacotherapy, 7, 1041–1053.
Stinchcombe, T. E. (2007). Nanoparticle albumin-bound paclitaxel: A novel Cremophor-EL-free formulation of paclitaxel. Nanomedicine, 2(4), 415–423.
John, J., Ismail, M., Riley, C., Askham, J., Morgan, R., Melcher, A., & Pandha, H. (2010). Differential effects of paclitaxel on dendritic cell function. BMC Immunology, 11, 14.
Pfannenstiel, L. W., Lam, S. S. K., Emens, L. A., Jaffee, E. M., & Armstrong, T. D. (2010). Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunology, 263, 79–87.
Carson, W. E., Shapiro, C. L., Crespin, T. R., Thornton, L. M., & Andersen, B. M. (2004). Cellular immunity in breast cancer patients completing taxane treatment. Clinical Cancer Research, 10, 3401–3409.
Demaria, S., Volm, M. D., Shapiro, R. L., Yee, H. T., Oratz, R., Formenti, S. C., Muggia, F., & Symmans, W. F. (2001). Development of tumour-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. ClinicalCancer Research, 7, 3025.
Vicari, A. P., Luu, R., Zhang, N., Patel, S., Makinen, S. R., Hanson, D. C., Weeratna, R. D., & Krieg, A. M. (2009). Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumour effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunology, Immunotherapy, 58, 615–628.
Soliman, H. H. (2016). nab-Paclitaxel as a potential partner with checkpoint inhibitors in solid tumours. Onco Targets and Therapy, 10, 101–112.
Schmid, P., Adams, S., Rugo, S., Schneeweiss, A., Barrios, C. H., Iwata, H., Diéras, V., Hegg, R., Im, S. A., Shaw, W. G., Henschel, V., Molinero, L., Chui, S. Y., Funke, R., Husain, A., Winer, E. P., Loi, S., & Emens, L. A. (2018). Atezolizumab and Nab-paclitaxel in advanced triple-negative breast Cancer. New England Journal of Medicine, 379, 2108–2121.
Emens, L. A., Cruz, C., Eder, J. P., Braiteh, F., Chung, C., Tolaney, S. M., Kuter, I., Nanda, R., Cassier, P. A., Delord, J. P., Gordon, M. S., ElGabry, E., Chang, C. W., Sarkar, I., Grossman, W., O’Hear, C., Fassò, M., Molinero, L., & Schmid, P. (2019). Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer. JAMA Oncology, 5, 74.
Shi, Y. (2020). Clinical translation of nanomedicine and biomaterials for cancer immunotherapy: Progress and perspectives. Advanced Therapeutics, 3(1900215), 4.
Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines - A new era in vaccinology. Nature Reviews Drug Discovery, 17, 261.
Kranz, L. M., Diken, M., Haas, H., Kreiter, S., Loquai, C., Reuter, K. C., Meng, M., Fritz, D., Vascotto, F., Hefesha, H., Grunwitz, C., Vormehr, V., Hüsemann, Y., Selmi, A., Kuhn, A. N., Buck, J., Derhovanessian, E., Rae, R., Attig, S., … Sahin, U. (2016). Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature, 534(7607), 396–401.
Kim, J., Li, W. A., Choi, Y., Lewin, S. A., Verbeke, C. S., Dranoff, G., & Mooney, D. J. (2015). Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nature Biotechnology, 33, 64–72.
Ikuta, Y., Katayama, N., Wang, L., Okugawa, T., Takahashi, Y., Schmitt, M., Gu, X., Watanabe, M., Akiyoshi, K., Nakamura, H., Kuribayashi, K., Sunamoto, J., & Shiku, H. (2002). Presentation of a major histocompatibility complex class 1-binding peptide by monocyte-derived dendritic cells incorporating hydrophobized polysaccharide-truncated HER2 protein complex: Implications for a polyvalent immuno-cell therapy. Blood, 99, 3717–3724.
Hasegawa, K., Noguchi, Y., Koizumi, F., Uenaka, A., Tanaka, M., Shimono, M., Nakamura, H., Shiku, H., Gnjatic, S., Murphy, R., Hiramatsu, Y., Old, L. J., & Nakayama, E. (2006). In vitro stimulation of CD8 and CD4 T cells by dendritic cells loaded with a complex of cholesterol-bearing hydrophobized pullulan and NY-ESO-1 protein: Identification of a new HLA-DR15-binding CD4 T-cell epitope. Clinical Cancer Research, 12, 1921–1927.
Kageyama, S., Wada, H., Muro, K., Niwa, Y., Ueda, S., Miyata, H., et al. (2013). Dose-dependent effects of NY-ESO-1 protein vaccine complexed with cholesteryl pullulan (CHP-NY-ESO-1) on immune responses and survival benefits of esophageal cancer patients. Journal of Translational Medicine, 11, 246.
Chen, Y. T., Scanlan, M. J., Sahin, U., Türeci, O., Gure, A. O., Tsang, S., Williamson, B., Stockert, E., Pfreundschuh, M., & Old, L. J. (1997). A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proceedings of the National Academy of Sciences USA, 94, 1914–1918.
Jungbluth, A. A., Chen, Y. T., Stockert, E., Busam, K. J., Kolb, D., Iversen, K., Coplan, K., Williamson, B., Altorki, N., & Old, L. J. (2001). Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. International Journal of Cancer, 92, 856–860.
Dorigo, O., Fiset, S., MacDonald, L. D., Bramhecha, Y., Hrytsenko, O., Dirk, B., Rosu, G. N., & Stanford, M. (2020). DPX-Survivac, a novel T-cell immunotherapy, to induce robust T-cell responses in advanced ovarian cancer. Journal of Clinical Oncology., 38(5_suppl), 6–6.
Chang, S., Herse, Z., Claxton, D., Giffon, T., Lewis, D., & Fairman, J. (2008). Immunotherapy of acute leukemia with cationic lipid DNA complexes (JVRS-100). Experimental Biology, 22(1), 1077.17-1077.1.
National Library of Medicine. (2019). JVRS-100 for the treatment of patients with relapsed or refractory leukemia. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00860522.
Hu, Q., Sun, W., Wang, J., Ruan, H., Zhang, X., Ye, Y., Shen, S., Wang, C., Lu, W., Cheng, K., Dotti, G., Zeidner, J. F., Wang, J., & Gu, Z. (2018). Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nature Biomedical Engineering, 2, 831–840.
Ye Q.N., Wang Y., Shen S., Xu C.F. & Wang J. (2021). Biomaterials-based delivery of therapeutic antibodies for cancer therapy. Advanced Health Care Materials, 2002139.
Tsoi, K., MacParland, S., Ma, M., Spetzler, V., Echeverri, J., Ouyang, B., Fadel, S., Sykes, E., Goldaracena, N., Kaths, M., Conneely, J., Alman, B., Selzner, M., Ostrowski, M., Adeyi, O., Zilman, A., McGilvray, I., & Chan, W. (2016). Mechanism of hard-nanomaterial clearance by the liver. Nature Materials, 15, 1212–1221.
Karlsson, J., Vaughan, H. J., & Green, J. J. (2018). Biodegradable polymeric nanoparticles for therapeutic cancer treatments. Annual Review of Chemical and Biomolecular Engineering, 9, 105–127.
Raposo, G., & Stoorvogel, W. (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. Journal of Cell Biology, 200(4), 373–383.
Kourembanas, S. (2015). Exosomes: Vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annual Review of Physiology, 77, 13–27.
Nie, W., Wu, G., Zhang, J., Huang, L. L., Ding, J., Jiang, A., Zhang, Y., Liu, Y., Li, J., Pu, K., & Xie, H. Y. (2020). Responsive exosome nano-bioconjugates for synergistic cancer therapy. AngewandteChemie, 59, 2018–2020.
Wang, C., Ye, Y. Q., Hochu, G. M., Sadeghifar, H., & Gu, Z. (2016). Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Letters, 16(4), 2334–2340. https://doi.org/10.1021/acs.nanolett.5b05030
Kim, J., Li, Y., Kim, S. W., Lee, D. S., & Yun, C. O. (2013). Therapeutic efficacy of a systemically delivered oncolytic adenovirus - Biodegradable polymer complex. Biomaterials, 34(19), 4622–4631. https://doi.org/10.1016/j.biomaterials.2013.03.004
Teo, P. Y., Yang, C., Whilding, L. M., Parente-Pereira, A. C., Maher, J., George, A. J. T., et al. (2015). Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: Strategies to enhance T cell killing. Advances in Healthcare Materials, 4(8), 1180–1189. https://doi.org/10.1002/adhm.201500089
Rahimian, S., Fransen, M. F., Kleinovink, J. W., Amidi, M., Ossendorp, F., & Hennink, W. E. (2015). Polymeric microparticles for sustained and local delivery of Anticd40 and antiCTLA-4 in immunotherapy of cancer. Biomaterials, 61, 33–40. https://doi.org/10.1016/j.biomaterials.2015.04.043
Ye, Y., Wang, J., Hu, Q., Hochu, G. M., Xin, H., Wang, C., & Gu, Z. (2016). Synergistic transcutaneous immunotherapy enhances antitumour immune responses through delivery of checkpoint inhibitors. ACS Nano, 10(9), 8956–8963.
Mockel-Tenbrinck, N., Barth, C., Mauer, D., Meyer, M., Klumb, C., Schenk, T., et al. (2017). Safety assessment of the MACS GMP T cell TransAct, a robust and Potent polyclonal T cell activation and expansion tool for clinical scale manufacturing of gene-modified T cells. Molecular Therapy, 25(5), 227–7.
Wang, X., Stefanski, J., Borquez-Ojeda, O., Qu, J., Hack, A., He, Q., et al. (2015). Comparison of CTS (TM) Dynabeads (R) CD3/CD28, MiltenyiTransAct CD3/28 and ExpAct beads for large-scale CAR T cell manufacturing. Human Gene Therapy, 26(10), A31–A41.
Wang, X. Y., Qu, J. R., Stefanski, J., Borquez-Ojeda, O., Hack, A., He, Q., et al. (2016). Evaluation of MiltenyiExpAct and TransAct CD3/28 beads for CAR-T cell manufacturing. Molecular Therapy, 24, S182–S192. https://doi.org/10.1016/S1525-0016(16)33268-3
Smith, T. T., Stephan, S. B., Moffett, H. F., McKnight, L. E., Ji, W., Reiman, D., et al. (2017). In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nature Nanotechnology, 12(8), 813–820.
Le, T. M. D., Yoon, A. R., Thambi, T., & Yun, C. O. (2022). Polymeric systems for cancer immunotherapy: A review. Frontiers in Immunology., 13, 826876.
Lee, C. H., Kasala, D., Na, Y., Lee, M. S., Kim, S. W., Jeong, J. H., et al. (2014). Enhanced therapeutic efficacy of an adenovirus-PEI-bile-acid complex in tumors with low Coxsackie and adenovirus receptor expression. Biomaterials, 35(21), 5505–5516. https://doi.org/10.1016/j.biomaterials.2014.03.060
Park, Y., Kang, E., Kwon, O. J., Hwang, T., Park, H., Lee, J. M., et al. (2010). Ionically crosslinked Ad/chitosan nanocomplexes processed by electrospinning for targeted cancer gene therapy. Journal of Controlled Release, 148(1), 75–82. https://doi.org/10.1016/j.jconrel.2010.06.027
Kwon, O. J., Kang, E., Choi, J. W., Kim, S. W., & Yun, C. O. (2013). Therapeutic targeting of chitosan-PEG-folate-complexed oncolytic adenovirus for active and systemic cancer gene therapy. Journal of Controlled Release, 169(3), 257–265. https://doi.org/10.1016/j.jconrel.2013.03.030
Oh, E., Oh, J. E., Hong, J., Chung, Y., Lee, Y., Park, K. D., et al. (2017). Optimized biodegradable polymeric reservoir-mediated local and sustained codelivery of dendritic cells and oncolytic adenovirus co-expressing IL12 and GM-CSF for cancer immunotherapy. Journal of Controlled Release, 259, 115–127. https://doi.org/10.1016/j.jconrel.2017.03.028
Frangville, C., Rutkevicius, M., Richter, A. P., Velev, O. D., Stoyanov, S. D., & Paunov, V. N. (2012). Fabrication of environmentally biodegradable lignin nanoparticles. ChemPhysChem, 13, 4235e4243.
Lievonen, M., Valle-Delgado, J. J., Mattinen, M. L., Hult, E. L., Lintinen, K., Kostiainen, M. A., Paananen, A., Szilvay, G. R., Setala, H., & Osterberg, M. (2016). A simple process for lignin nanoparticle preparation. Green Chemistry, 18, 1416e1422.
Lintinen, K., Latikka, M., Sipponen, M. H., Ras, R. H., Österberg, M., & Kostiainen, M. A. (2016). Structural diversity in metal–organic nanoparticles based on iron isopropoxide treated lignin. RSC advances, 6(38), 31790–31796.
Kiriazis, A., Vahakoski, R. L., Santio, N. M., Arnaudova, R., Eerola, S. K., Rainio, E. M., & Koskinen, P. J. (2013). Tricyclic benzo [cd] azulenes selectively inhibit activities of Pim kinases and restrict growth of Epstein-Barr virus-transformed cells. PLoS One, 8(2), e55409.
Shah, N., Pang, B., Yeoh, K. G., Thorn, S., Chen, C. S., Lilly, M. B., & Salto-Tellez, M. (2008). Potential roles for the PIM1 kinase in human cancer–a molecular and therapeutic appraisal. European journal of cancer, 44(15), 2144–2151.
Brault, L., Gasser, C., Bracher, F., Huber, K., Knapp, S., & Schwaller, J. (2010). PIM serine/threonine kinases in the pathogenesis and therapy of hematologic malignancies and solid cancers. Haematologica, 95(6), 1004.
Nicolas, J., Mura, S., Brambilla, D., Mackiewicz, N., & Couvreur, P. (2013). Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chemical Society Reviews, 42(3), 1147–1235.
Li, K., & Liu, B. (2014). Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chemical Society Reviews, 43(18), 6570–6597.
Zheng, X., Ge, J., Wu, J., Liu, W., Guo, L., Jia, Q., Ding, Y., Zhang, H., & Wang, P. (2018). Biodegradable hypocrellin derivative nanovesicle as a near-infrared light-driven theranostic for dually photoactive cancer imaging and therapy. Biomaterials, 185, 133–141.
Kosmides, A. K., Meyer, R. A., Hickey, J. W., Aje, K., Cheung, K. N., Green, J. J., & Schneck, J. P. (2017). Biomimetic biodegradable artificial antigen presenting cells synergize with PD-1 blockade to treat melanoma. Biomaterials, 118, 16–26.
Luo, M., Wang, H., Wang, Z., Cai, H., Lu, Z., Li, Y., Du, M., Huang, G., Wang, C., Chen, X. & Porembka, M.R. (2017). A STING-activating nanovaccine for cancer immunotherapy. Nature Nanotechnology, 12(7), 648–654.
Li, J., Pei, H., Zhu, B., Liang, L., Wei, M., He, Y., Chen, N., Li, D., Huang, Q., & Fan, C. (2011). Self-assembled multivalent DNA nanostructures for noninvasive intracellular delivery of immunostimulatory CpG oligonucleotides. ACS Nano, 5(11), 8783–8789.
Czapar, A. E., Zheng, Y. R., Riddell, I. A., Shukla, S., Awuah, S. G., Lippard, S. J., & Steinmetz, N. F. (2016). Tobacco mosaic virus delivery of phenanthriplatin for cancer therapy. ACS Nano, 10(4), 4119–4126.
Park, J., Wrzesinski, S. H., Stern, E., Look, M., Criscione, J., Ragheb, R., ay, S.M., Demento, S.L., Agawu, A., Licona Limon, P. & Ferrandino, A.F. (2012). Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nature Materials, 11(10), 895–905.
Roberts, M. J., Bentley, M. D., & Harris, J. M. (2002). Chemistry for peptide and protein PEGylation. Advanced Drug Delivery Reviews, 54(4), 459–476.
Lühmann, T., Schmidt, M., Leiske, M. N., Spieler, V., Majdanski, T. C., Grube, M., Hartlieb, M., Nischang, I., Schubert, S., Schubert, U.S. & Meinel, L. (2017). Site-specific POxylation of interleukin-4. ACS Biomaterials Science & Engineering, 3(3), 304–312.
Wang, Y., Lin, Y. X., Qiao, S. L., An, H. W., Ma, Y., Qiao, Z. Y., Rajapaksha, R.Y.J., & Wang, H. (2017). Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumour microenvironment. Biomaterials, 112, 153–163.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AD, SM and SP have written the manuscript. DK has reviewed and edited the same.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Research Involving Humans and Animals
Not applicable as any animal study was done.
Informed Consent
Not applicable.
Consent for Publication
We, the undersigned, provide our consent to the BioNanoscience to publish.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhara, A., Majumder, S., Pahari, S. et al. Improved Targeting of Therapeutics by Nanocarrier-Based Delivery in Cancer Immunotherapy and Their Future Perspectives. BioNanoSci. 13, 278–299 (2023). https://doi.org/10.1007/s12668-023-01065-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01065-6