Abstract
In the current report, we examined the potential beneficial role of soursop fruit extract (SSFE) on liver injury induced by a single paracetamol (APAP) overdose (2000 mg/kg). Thirty-five Wistar albino rats were randomly divided into five groups as follows: control, SSFE, APAP, SSFE+APAP, and silymarin (SIL)+APAP. APAP intoxication was found to elevate alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and total bilirubin levels. Moreover, it increased the levels of malondialdehyde, nitrites, and nitrates and depleted glutathione, superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase. APAP intoxication inactivated the nuclear factor erythroid 2-related factor 2 (Nrf2) defense pathway and upregulated the expression of heme oxygenase-1 (HO-1). APAP administration enhanced the activation of nuclear factor-kappa B (NF-κB), the elevation of tumor necrosis factor-alpha and interleukin 1-beta levels, and the upregulation of inducible nitric oxide synthase mRNA expression. In addition, APAP activated the overexpression of Bax protein, increased release of cytochrome c, and the downregulation of Bcl-2 protein. Finally, APAP-induced overexpression of transforming growth factor-beta (TGF-β) further suggested enhanced liver damage. On the other hand, SSFE pretreatment attenuated these biochemical, molecular, and histopathological alterations in the liver, which might be partially due to the regulation of hepatic Nrf2/HO-1 and downregulation of NF-κB and TGF-β.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The liver is the largest internal organ, regulating different physiological functions, such as metabolic processes, clotting factor production, bile secretion, protein synthesis, glycogen, and vitamin A storage, as well as detoxification and excretion of endogenous and exogenous substances, including chemotherapeutic agents. Drug-induced hepatotoxicity is a serious health concern, as it is the main cause of death among patients with acute liver failure worldwide (Chen et al. 2015). Acetaminophen (APAP), or paracetamol, is a frequently prescribed antipyretic and analgesic over-the-counter drug and has been categorized as the most commonly used drug that may cause acute and chronic liver injury when the recommended doses are exceeded (Yoon et al. 2016). However, therapeutic doses may also cause hepatotoxicity in individuals with malnutrition or in association with alcohol ingestion (Dokumacioglu et al. 2017). Excessive exposure to xenobiotics, toxins, and drugs may lead to liver damage as a result of reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation, resulting in perturbation of the hepatocyte membrane (Guan and He 2015).
It has been reported that 90% of the administered APAP is metabolized through glucuronidation or sulfation in the liver and then excreted in the urine; therefore, less than 10% is converted into N-acetyl-P-benzoquinone imine (NAPQI) by cytochrome p450 2E1 (CYP2E1). In normal physiological conditions, NAPQI is metabolized into a non-reactive metabolite via the glutathione (GSH) pool. When GSH is depleted, NAPQI attacks the intracellular organelles, resulting in oxidative stress, altered mitochondrial permeability, perturbed calcium homeostasis, and ATP depletion, leading to necrosis and, ultimately, to cell death (Chung et al. 2012; Hinson et al. 2010; Yoon et al. 2016).
For thousands of years, medicinal plants have been utilized to treat or prevent diseases by different human communities. About 80% of people in developing countries still use plants as a primary source of therapeutics because of their efficacy and low cost. Moreover, several effective medicinal plants or their active compounds have been widely used in the development of new therapeutic agents (Guan and He 2015). Soursop, or Graviola (Annona muricata), belongs to the Annonaceae family and is mainly distributed in the tropical regions. Phytochemical analysis showed that acetogenins, alkaloids, phenolic compounds, carotenoids, vitamins, cyclopeptides, and amides represent the major active constituents of the plant. All parts of the plant including seeds, fruits, leaves, barks, and roots showed several pharmacological and biological capacities (Moghadamtousi et al. 2015a). Florence et al. (2014) demonstrated the antidiabetic activity of Annona muricata aqueous extract in streptozotocin-treated rats. The authors attributed this effect to the antioxidant, its ability to lower the lipid profile and protecting pancreatic β-cells, which further modulate glucose metabolism. Moreover, Annona muricata leaf aqueous extract was found to provide protection against streptozotocin-induced hepatic injury through suppressing the lipid peroxidation, hypolipidemic effect, and enhancing the antioxidant defense molecules (Adewole and Ojewole 2008). This extract protected also the hepatocytes against jaundice in rats (Arthur et al. 2012). In addition, leaves of Annona muricata exhibited promising antioxidant, anti-inflammatory, and wound healing properties in a murine excisional wound model (Moghadamtousi et al. 2015b). Furthermore, Annona muricata leaf extract showed anti-ulcer effect in ethanol-treated rats through its rich antioxidant ingredients which could increase the mucosal nonprotein sulfhydryl group content (Hamid et al. 2012). It has been reported that Annona muricata leaf extract enhanced the immune response in RAW 264.7 macrophage cells through the activation of mitogen-activated protein kinase signaling pathway (Kim et al. 2016).
Hence, the aim of the current study was to evaluate the possible beneficial effect of the soursop fruit extract against paracetamol-induced hepatic intoxication in rats.
Materials and methods
Chemicals
Paracetamol was obtained from the Nile Pharmaceuticals Chemical Company. Tris–HCl and silymarin were procured from Sigma-Aldrich. Tumor necrosis factor-α, interleukin 1, and cytochrome c ELISA kits were obtained from R&D System. ALT, AST, ALP, and total bilirubin were provided by Biodiagnostic Co. The chemicals and reagents used were all of analytical grade.
Plant materials and extraction procedure
The plant was purchased from a local area in South-Cairo, Egypt, in the month of November 2017. The plant was identified and authenticated by a taxonomy specialist (Botany Department, Faculty of Science, Helwan University, Egypt). The pulps of soursop fruit (Annona muricata) were powdered using an electrical blinder. The powder crude was macerated three times for 24 h with methanol (70%) and the ratio between the plant powder and methanol was 1:10 (w/v). The solvent was concentrated under a vacuum evaporator and further lyophilized. The soursop fruit methanolic extract was stored at − 80 °C until the beginning of the current study.
HPLC analyses of soursop fruit extract (Annona muricata)
The fingerprint analysis of polyphenols and flavonoids constitutes of Annona muricata fruit pulp extract was performed using a Perkin Elmer Series 200 liquid chromatography (PerkinElmer, USA). The HPLC column was an AQUA column 150 mm 5 μ C18 (Phenomenex) and a detection wavelength of 280 nm. Elution was carried out using acetic acid (2%; A) and acetonitrile (B). The flow rate was set at 1 ml/min throughout the elution.
Experimental grouping
Thirty-five healthy male 8-week-old Wistar albino rats weighing 140–170 g were employed. Rats were supplied by the animal facility of the Holding Company for Biological Products and Vaccines (VACSERA). Animals were kept in plastic cages under continuous 12-h light/12-h dark cycles. Temperature ranged between 20 and 22 °C with 65% humidity. Rats were fed with standard rat pellet chow and had ad libitum access to water for 10 days before the experiment.
After acclimation, rats were randomly allocated into four groups of seven animals each as follows:
-
1.
Control group (CNT) was administered with distilled water for 7 days.
-
2.
APAP-intoxicated group was treated orally with a single APAP overdose (2000 mg/kg) and the animals were sacrificed after 24 h according to Rajasekaran and Periyasamy (2012).
-
3.
SSFE was orally administered at the dose of 300 mg/kg for 7 days.
An acute toxicity study performed using a maximum dose of 2000 mg/kg SSFE administered orally, which showed no signs of toxicity in rats and the oral dose of SSFE was selected based on a preliminary study using three doses of 100, 200, and 300 mg/kg showed that the oral administration of SSFE at a dose of 300 mg/kg was effectively prevented APAP-induced hepatotoxicity (data not shown).
-
4.
The SSFE+APAP rats were treated orally with SSFE (300 mg/kg) for 7 consecutive days and, after 3 h from the last dose, received a single APAP overdose (2000 mg/kg). Animals of this group were sacrificed 24 h after the last treatment.
-
5.
The SIL+APAP group was treated orally with SIL (50 mg/kg) for 7 consecutive days, and 3 h after the last dose, the rats received a single APAP overdose (2000 mg/kg). Animals of this group were sacrificed 24 h after the last treatment. Silymarin has been used as a standard hepatoprotective drug.
After the last treatment, rats were anesthetized and sacrificed. The liver was removed carefully and washed using ice-cold 50 mM Tris–HCl, pH 7.4. Each liver was weighed and homogenized in ice-cold medium containing 50 mM Tris–HCl, pH 7.4, to obtain a 10% (w/v) homogenate. The homogenates were spun for 10 min at 4 °C at 3000 rpm. The supernatants were then utilized for the determination of biochemical parameters. Total protein content was estimated using the Lowry protocol (Lowry et al. 1951).
Liver function parameters
Key parameters of liver functions including ALT, AST, ALP, and total bilirubin were calorimetrically assessed using standard kits according to the manufacturer’s specifications.
Hepatic oxidative damage
To estimate lipid peroxidation in liver, thiobarbituric acid-reactive substances (TBARS), a marker of lipid peroxidation, were determined in the homogenates as a function of malondialdehyde (MDA) levels (Janero 1990). Nitrite/nitrate levels and GSH content were assessed using the protocols described by Green et al. (1982) and Ellman (1959), respectively.
Antioxidant status
Liver homogenate supernatants were used for the determination of the activities of SOD, catalase, and GSH-Px and GSH-R according to the methods described by Fisher et al. (2003), Aebi (1984), and De Vega et al. (2002), respectively.
Quantification of hepatic TNF-α, IL-1β, and cytochrome c
The quantification of hepatic TNF-α (TNF-α; Cat. no. RTA00, R&D Systems), IL-1β (IL-1β; Cat. no. ERIL1B, ThermoFisher Scientific), and cytochrome c was performed in liver homogenate according to the suppliers’ instructions.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis
Total RNA was separated from liver tissues by using the RNeasy Plus Minikit from Qiagen. cDNA synthesis was performed using the Script™ cDNA synthesis kit (Bio-Rad, CA). Real-time PCR was performed using Power SYBR® Green from Life Technologies on an Applied Biosystems 7500 Instrument. The thermal profile for the PCR reaction was 95 °C for 4 min, followed by 40 cycles at 94 °C for 60 s, and 55 °C for 60 s. After PCR amplification, the ΔCt was determined. PCR primers for the Bax, Bcl-2, iNOS, Nrf2, and HO-1 genes were prepared by Jena Bioscience GmbH by using the Primer-Blast program from NCBI. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was employed as an internal control and appeared to be unchanged by the different therapies. The primer pairs used were previously described by Almeer and Abdel Moneim (2018).
Histopathological examination
Portions of rat liver tissue were fixed in 10% neutral buffered formaldehyde for 1 day, dehydrated in ethyl alcohol, cleared in xylene, and mounted in molten paraffin wax. Sections of 4–5 μm were obtained from paraffin-embedded livers and stained with hematoxylin and eosin. The sections were examined using a light microscope (Nikon; Eclipse E200-LED, Tokyo, Japan).
Immunohistochemistry analysis
To investigate the pro-inflammatory proteins, the prepared liver sections (4-μm thickness) were blocked with 0.1% hydrogen peroxide containing methanol for 15 min to inactivate the endogenous peroxidase. After blocking, the sections were incubated with rabbit polyclonal antibodies against iNOS, NF-κB, and TGF-β at 4 °C overnight. Thereafter, the sections were rinsed with phosphate-buffered saline and incubated with biotinylated goat anti-rabbit immunoglobulins, followed by incubation with streptavidin-peroxidase complexes at 30 °C for 30 min for the third time. The peroxidase activity was developed using diaminobenzidine (DAB)-hydrogen peroxide. Images were recorded at an original magnification of × 400 (Nikon Eclipse E200-LED, Tokyo, Japan).
Statistical analysis
All results were expressed as the mean ± standard error (SEM). Data for multiple variable comparisons were analyzed by one-way analysis of variance (ANOVA). For the between-group comparison, Duncan’s test was used as a post hoc test. The acceptable level of significance was established at P ˂ 0.05.
Results
HPLC analyses of soursop fruit extract
Polyphenols and flavonoids fingerprint of the soursop fruit pulp extract detected at 280 nm was illustrated in Fig. 1. The HPLC profile of soursop fruit pulp extract shows the presence of 12 peaks with retention times ranging from 2.853 to 22.728 min. Based on the UV-Visible spectral data and their retention time, the Annona muricata fruit pulp extract has UV band at 280 nm characteristic for polyphenols and flavonoids compounds, may be luteolin, quercetin and its derivatives, cinnamic acids, catechin, caffeic acid derivative, epicatechin gallate and its derivatives, and gallocatechin derivatives.
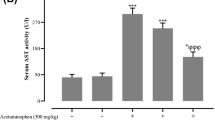
Effects of SSFE on liver function parameters following APAP intoxication
In the current study, liver functional parameters were assessed in rats following oral administration of paracetamol and/or soursop fruit extract. Rats treated with a single dose of APAP (2000 mg/kg) displayed a sharp and significant (P < 0.05) elevation in the levels of serum ALP, AST, ALT, and total bilirubin, as compared with the control animals. Notably, pretreatment with SSFE (300 mg/kg) for 7 days almost completely restored these parameters. In addition, the treatment with SIL prior to APAP alleviated significantly the levels of these physiological markers as compared against APAP groups, but their levels were still higher than the control values (Fig. 2).
Effect of 7-day SSFE supplementation (300 mg/kg) on the activities of hepatic alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total bilirubin (TB) levels in APAP overdose-treated rats. Data are expressed as the mean ± SEM (n = 7). #P < 0.05 indicates a statistically significant change with respect to the control group; $P < 0.05 indicates a statistically significant change with respect to the APAP-treated group using Tukey’s post hoc test
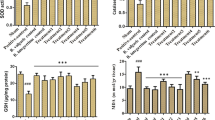
Effects of SSFE on hepatic oxidative status following APAP intoxication
To evaluate the oxidative status in the liver tissue following APAP administration, we estimated the level of oxidants, including MDA, nitrites/nitrates, and endogenous antioxidants GSH, SOD, CAT, GSH-R, and GSH-Px in hepatic homogenates. Figures 3 and 4 show that treatment with APAP significantly increased (P < 0.05) MDA and nitrite/nitrate levels, whereas it markedly reduced the GSH content, as well as the activities of GSH, SOD, CAT, GSH-R, and GSH-Px. Notably, SSFE significantly prevented the described alterations in the oxidative status caused by APAP intoxication, reflecting its antioxidant capacity against APAP-induced oxidative stress in liver tissue. Similarly, the pretreatment with SIL abrogated significantly the oxidative reactions recorded following APAP exposure.
Ameliorative effect of 7-day SSFE pretreatment (300 mg/kg) on the levels of hepatic malondialdehyde (MDA), nitrite/nitrate, and glutathione (GSH) content following APAP intoxication. Data are expressed as the mean ± SEM (n = 7). #P < 0.05 indicates statistically significant changes with respect to the control group; $P < 0.05 indicates a statistically significant change with respect to the APAP-treated group using Tukey’s post hoc test
Ameliorative effect of 7-day SSFE pretreatment (300 mg/kg) on the activities of hepatic superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GSH-R), and glutathione peroxidase (GSH-Px) after APAP intoxication. Data are expressed as the mean ± SEM (n = 7). #P < 0.05 indicates statistically significant changes with respect to the control group; $P < 0.05 indicates a statistically significant change with respect to the APAP-treated group using Tukey’s post hoc test
Effects of SSFE on Nrf2/HO-1 pathway following APAP intoxication
To explore the molecular mechanisms underlying the protective action of SSFE against APAP-induced-oxidative reactions, endogenous antioxidant systems were investigated. The expression of Nrf2 was evaluated in the liver tissue using quantitative real-time PCR. Whereas in APAP-treated animals, Nrf2 was downregulated, the pretreatment with SSFE and SIL was found to significantly restore Nrf2 mRNA expression. Moreover, the expression of HO-1 mRNA was significantly upregulated by APAP in the liver tissue. Notably, the pre-administration of SSFE and SIL also promoted HO-1 expression in response to APAP (Fig. 5).
The modulatory effect of 7-day oral administration of SSFE (300 mg/kg) on Nrf2 and HO-1 expression upon APAP overdose intake. Data are expressed as the mean ± SEM (n = 7). #P < 0.05 indicates a statistically significant change with respect to the control group; $P < 0.05 indicates a statistically significant change with respect to the APAP-treated group using Tukey’s post hoc test
Effects of SSFE on levels of TNF-α, IL-1β, and iNOS expression following APAP intoxication
To verify if the anti-inflammatory properties of SSFE could also possibly account for its protective effects against APAP intoxication, we evaluated TNF-α and IL-1β levels, as well as iNOS mRNA expression, in the hepatic tissue. Figure 6 shows that APAP increased TNF-α and IL-1β levels, as well as iNOS mRNA expression. However, a 7-day pretreatment with SSFE resulted in a significant attenuation of the APAP-induced TNF-α and IL-1β increase (P < 0.05). Similarly, drug-induced iNOS expression was significantly abolished by the SSFE pretreatment. Furthermore, SIL pre-administered rats showed a significant decrease in the levels of these pro-inflammatory cytokines and exhibited also downregulation of iNOS in hepatic tissue.
Effect of 7-day SSFE supplementation (300 mg/kg) on the hepatic levels of TNF-α, IL-1β, levels, and the expression of iNOS mRNA in APAP overdose-treated rats. Data are expressed as the mean ± SEM (n = 7). #P < 0.05 indicates statistically significant changes with respect to the control group; $P < 0.05 indicates a statistically significant change with respect to the APAP-treated group using Tukey’s post hoc test
Effects of SSFE on hepatic NF-κB and iNOS expression following APAP intoxication
To understand the molecular mechanism of the anti-inflammatory effect of SSFE using immunohistochemistry technique, the expression of nuclear factor-kappa B (NF-κB) and inducible nitric oxide synthase (iNOS), which are widely used as an indicator for oxidative stress and inflammation, was estimated in APAP-treated rats. NF-κB (Fig. 7a) and iNOS (Fig. 7b) were increased in the liver of APAP-intoxicated rats, compared with the control animals, confirming that oxidative stress and pro-inflammatory events are responsible for APAP toxicity. On the contrary, SSFE-pretreated rats demonstrated a decrease in the expression of these pro-inflammatory and oxidant markers, which further indicate the antioxidant and anti-inflammatory capacity of the extract. Moreover, SIL-pretreated group showed similar effects to SSFE-pretreated rats against APAP-induced inflammatory status in the hepatic tissue.
Effects of SSFE on hepatic TGF-β expression following APAP intoxication
To further characterize the potential hepatoprotective impact of SSFE on APAP-induced liver damage, the expression of transforming growth factor (TGF-β) was examined by immunohistochemistry. TGF-β has been identified as a key regulator in all stages of chronic liver disease. Liver tissues of APAP-treated rats showed stronger TGF-β immunoreactivity as compared with control animals. However, in rats treated with SSFE prior to APAP exposure, the expression of this cytokine was decreased, as compared with animals that had been solely exposed to the drug (Fig. 8). Treated rats with SIL+APAP exhibited a significant decline in TGF-β expression as compared with APAP-intoxicated rats.
Effects of SSFE on histological alterations following APAP intoxication
Results of the histopathological examination of the liver tissue following the administration of APAP are presented in Fig. 9. Control liver sections showed a normal histological structure of hexagonal hepatic lobules; hepatocytes radiated normally from central veins towards the lobule periphery, with clear cytoplasm, prominent nucleus, kupffer cells, and sinusoid appears normally. Meanwhile, APAP-treated rats showed dilated and congested central vein, degenerated granular liver cells, neutrophils infiltration accompanied with necrotic hepatocytes. SSFE pretreatment improved the hepatic injury through reversing most of histological alterations following APAP exposure; however, few apoptotic and vacuolated hepatocytes were recorded.
Effects of SSFE on apoptotic cascade following APAP intoxication
The anti-apoptotic effect of SSFE was also investigated in the present study following APAP administration. Rats treated with APAP showed significant upregulation in Bax mRNA expression, whereas Bcl-2 mRNA expression was downregulated in the hepatic tissue, as compared with the control group. Notably, both Bax upregulation and Bcl-2 downregulation were reverted upon the SSFE pretreatment in the APAP-treated group. In addition, whereas APAP significantly enhanced the cytoplasmic release of cytochrome c, this event was restrained by SSFE pretreatment (Fig. 10). The anti-apoptotic effect of SIL was also recorded, as indicated via the upregulation of Bcl-2 expression and decreasing Bax expression and cytochrome c release.
The anti-apoptotic effect of 7-day SSFE supplementation (300 mg/kg) on APAP overdose-induced apoptotic cascades in liver tissue. Data are expressed as the mean ± SEM (n = 7). #P < 0.05 indicates a statistically significant change with respect to the control group; $P < 0.05 indicates a statistically significant change with respect to the APAP-treated group using Tukey’s post hoc test
Discussion
Heedless consumption, or abuse, of specific medications, is strongly associated with mild-to-acute progression of liver dysfunction. Excessive dosage or chronic use of APAP provides a well-known example of this association (Dokumacioglu et al. 2017; Giannini et al. 2005; Lee and Seremba 2009). Therefore, agents capable of protecting the liver from APAP-induced damage, with little or negligible adverse effects are strongly desirable.
Aminotransferases and alkaline phosphatase are mainly found in the cytoplasm of hepatocytes and their presence in serum is routinely used as a key physiological marker for hepatic injury. The permeability of hepatocytes and their transport functions were perturbed following APAP intoxication, leading to the leakage of ALT, AST, ALP, and total bilirubin into the bloodstream (Das et al. 2010). The alteration of these functional parameters, described in the present study, upon exposure to a high dose of APAP, confirms the severity of the induced hepatocellular injury. Notably, a 7-day pretreatment with SSFE protected hepatocellular membrane structure and integrity and significantly ameliorated the liver damage induced by APAP, as indicated by the nearly complete restoration of these parameters to their basal values. Most likely, the hepatoprotective effects of SSFE are related to its high content of phytochemicals.
Oxidative stress has been reported to play a fundamental role in APAP-induced hepatic injury. Our findings showed that APAP altered the oxidative status in the hepatic tissue, as evidenced by the elevated MDA and nitrate/nitrite levels, GSH content depletion, and deactivation of enzymatic antioxidant defense system including SOD, CAT, GSH-R, and GSH-Px, whereas HO-1 mRNA expression was surprisingly upregulated. It is known that in response to intoxication by chemicals and subsequent ROS production, both enzymatic and non-enzymatic antioxidant defense systems are activated (Al-Olayan et al. 2014). In hepatocellular dysfunction, excessive ROS generation and depletion of antioxidants have been observed (Dokumacioglu et al. 2017). Excess APAP was reported to cause ROS generation and this, in turn, results in lipid peroxidation, with consequent structural and functional alterations of the hepatocyte membrane, formation of reactive aldehydes, and GSH depletion (Dokumacioglu et al. 2017). The APAP-induced alteration of the oxidative status that we observed in the hepatic tissue prompted us to investigate the possible impact of SSFE on these processes. We also observed iNOS upregulation and a consistent increase in the level of nitrates and nitrites upon APAP intoxication. These changes are indicative of elevated nitric oxide (NO) production. Excess NO may interact with superoxide (O2•−), thereby promoting the generation of peroxynitrite (ONOO−), a known powerful oxidant, causing deleterious cytotoxic effects and cell death (Waters et al. 2001). Of note, reactive compounds deriving from APAP metabolism were found to attack cellular macromolecules and inactivate endogenous antioxidants (Das et al. 2010; Horng et al. 2017).
Nrf2 is a transcription factor that binds to the antioxidant response element (ARE) to control the intracellular expression of various antioxidants and detoxifying molecules including HO-1. We found that Nrf2 mRNA expression was downregulated upon APAP treatment. A critical role of Nrf2 in hepatic metabolism has been established. The Nrf2 antioxidant defense system, consisting of GSH synthesis, phase II detoxifying enzymes, and ROS deactivating enzymes, is known to block the effects of NAPQI following treatment with acetaminophens (Reisman et al. 2009). A number of studies, employing different models, have shown that APAP-induced Nrf2 downregulation is associated with severe hepatic damage (Li et al. 2018; Lin et al. 2018; Ning et al. 2018). Thus, the ability of SSFE to restore Nrf2 expression may be a key element of its hepatoprotective properties. In addition, HO-1 catalyzing heme catabolism into biliverdin, free iron, and carbon monoxide was found to be upregulated by APAP in the hepatic tissue, possibly reflecting the activation of antioxidant defense mechanisms against APAP intoxication, suggesting that hepatocytes are able to suppress oxidative damage through HO-1 induction. Previous reports have shown that HO-1 plays a protective role in different experimental hepatic stress and inflammation models, including acetaminophen-induced hepatic dysfunction, through its antioxidant, anti-inflammatory, and anti-apoptotic activities (Chiu et al. 2002; Zuckerbraun and Billiar 2003). However, the overexpression of HO-1 was also reported to be associated with Fe2+ release and with the aggravation of iron-mediated ROS production (Araujo et al. 2012).
Based on previous findings, agents capable to increase GSH content, activate antioxidant enzymes, and regulate Nrf2 and HO-1 expression may exhibit the potential to abolish APAP hepatotoxicity. In the present study, SSFE pretreatment was found to prevent APAP-induced hepatic injury by decreasing the levels of oxidants (MDA and nitrate/nitrite), elevating GSH content, activating the antioxidant defense enzymes (SOD, CAT, GSH-R, and GSH-Px,), modulating the expression of HO-1, and activating Nrf2 in the liver tissue. These processes may play the key role in SSFE-mediated hepatoprotection versus APAP-induced liver toxicity.
These data reflect the potent hepatoprotective role of SSFE that is associated with its antioxidant capacity, which has been attributed to its ability to inhibit free radical production and deactivate NADPH oxidase that catalyzes the conversion of oxygen to superoxide anion (Zamudio-Cuevas et al. 2014). A. muricata leaf extracts were reported to inhibit lipid peroxidation, increase GSH content, and enhance the activity of SOD and CAT in an animal model of gastritis (Moghadamtousi et al. 2014b). In another study, watery extracts of A. muricata leaves suppressed lipid peroxidation and enhanced the cellular antioxidant enzyme activity in hepatic tissues of streptozotocin-treated diabetic rats (Adewole and Ojewole 2008). Moreover, ethyl acetate extracts of A. muricata leaves decreased lipid peroxidation and increased the activity of GPx, SOD, and CAT in a rat excisional wound model (Moghadamtousi et al. 2015b).
The role of inflammation was also explored in our model of APAP intoxication. Treatment with APAP increased the levels of the pro-inflammatory cytokines, namely, TNF-α and IL-1β and upregulated iNOS and NF-κB mRNA expression in the hepatic tissue. SSFE displayed anti-inflammatory activity as it decreased the levels of TNF-α and IL-1β, and downregulated the expression of iNOS and NF-κB. Inflammation was found to be involved in APAP-induced hepatocellular damage and the overproduction of pro-inflammatory cytokines and NO from Kupffer cells has been implicated in the development of cirrhosis (Bian et al. 2018; Guo et al. 2003; Kaya et al. 2018; Siddiqui et al. 2018). High doses of APAP may activate the NF-κB pathway by enhanced ROS production. NF-κB has been categorized as the major transcription factor promoting the expression of pro-inflammatory cytokines and of other mediators during inflammatory responses and oxidative reactions (Du et al. 2013; Jaeschke et al. 2003). APAP-induced overexpression of NF-κB may explain the elevated levels of TNF-α and IL-1β shown by our experiments. Agents capable of decreasing the level of pro-inflammatory cytokines have been proposed for the treatment of liver injury. A. muricata n-hexane leaf extract exerted an anti-inflammatory effect through the suppression of NF-κB translocation in a lung cancer-derived cell line (Moghadamtousi et al. 2014a). Another report showed that the same effect involves the inhibition of TNF-α, IL-1β, and NO production in lipopolysaccharide-stimulated macrophages (Laksmitawati et al. 2016). Furthermore, A. muricata aqueous leaf extracts were found to suppress the carrageenan-induced increase in both myeloperoxidase activity (MPO) and NO levels, in rodents (Quilez et al. 2015). The latter authors suggested that the anti-inflammatory activity of the extracts might be associated with decreased leukocyte migration, downregulation of TNF-α and IL-1β protein expression, and with suppression of the signaling intracellular pathways involving these inflammatory mediators.
Transforming growth factor β (TGF-β) is a cytokine mainly produced by hepatocytes, endothelial cells, and Kupffer cells and playing a crucial role in cell proliferation, differentiation, migration, and death. TGF-β is a central regulator in chronic liver disease and has been associated with the progression of liver fibrogenesis (Fabregat et al. 2016). The APAP-induced overexpression of TGF-β we observed in damaged liver tissue might be associated with the differentiation of stellate cells to myofibroblasts, which enhances cell death and promotes liver fibrosis and cirrhosis (Fabregat et al. 2016; Kaya et al. 2018). On the other hand, blunting of TGF-β signaling leads to decreased fibrosis. In the present study, SSFE pretreatment downregulated APAP-induced TGF-β expression, consistent with its potent hepatoprotective action against APAP intoxication.
A role of apoptotic cascades in the liver-damaging effects of excess APAP has been reported. In particular, a mitochondrial apoptotic cascade was shown to be a major mechanism underlying APAP-induced liver tissue damage, resulting in the overexpression of pro-apoptotic proteins and in depletion of the anti-apoptotic proteins, ultimately leading to the cell death (Hu and Colletti 2010; Dkhil et al. 2019; Wu et al. 2017). In addition, APAP was reported to enhance the release of mitochondrial cytochrome c, decrease Bcl2/Bax ratio, and trigger the activity of caspases-3, 6, 8, and 9 (Kumari and Kakkar 2012; Li et al. 2013). Consistent with these findings, our APAP-intoxicated rats exhibited a marked upregulation in pro-apoptotic proteins, including Bax, an enhanced release of cytochrome c and the downregulation of the anti-apoptotic protein, Bcl-2. These changes were substantially reversed by SSFE pretreatment, further confirming its potential hepatoprotective role.
Conclusion
In conclusion, our findings suggest that SSFE exerts ameliorative effects with respect to APAP-induced biochemical and histopathological alterations. SSFE actions appear to involve a wide spectrum of effector molecules and pathways, resulting in the activation of antioxidant mechanisms, inhibition of inflammatory and apoptotic cascades, and substantial prevention of histological damage. Collectively, our data demonstrated that SSFE deserves further consideration as a potential alternative agent for the prevention of APAP-induced liver injury. However, further studies on the molecular mechanisms of SSFE effect must be conducted to elucidate the main active ingredient responsible for its hepatoprotective effect.
Abbreviations
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- APAP:
-
Paracetamol
- AST:
-
Aspartate aminotransferase
- CAT:
-
Catalase
- GSH:
-
Glutathione
- GSH-Px:
-
Glutathione peroxidase
- GSH-R:
-
Glutathione reductase
- HO-1:
-
Heme oxygenase-1
- IL-1β:
-
Interleukin-1 beta
- iNOS:
-
Inducible nitric oxide synthase
- MDA:
-
Malondialdehyde
- NAPQI:
-
N-acetyl-P-benzoquinone imine
- NO:
-
Nitric oxide
- Nrf2:
-
Nuclear factor erythroid 2–related factor 2
- SIL:
-
Silymarin
- SOD:
-
Superoxide dismutase
- SSFE:
-
Soursop fruit extract
- TGF-β:
-
tumor growth factor-beta
- TNF-α:
-
tumor necrosis factor-alpha
References
Adewole SO, Ojewole JA (2008) Protective effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med 6:30–41
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Almeer RS, Abdel Moneim AE (2018) Evaluation of the protective effect of olive leaf extract on cisplatin-induced testicular damage in rats. Oxidative Med Cell Longev 2018:11
Al-Olayan EM, El-Khadragy MF, Aref AM, Othman MS, Kassab RB, Abdel Moneim AE (2014) The potential protective effect of Physalis peruviana L. against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of MMP-9 expression. Oxidative Med Cell Longev 2014:381413
Araujo JA, Zhang M, Yin F (2012) Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol 3:119
Arthur F, Woode E, Larbie C (2012) Bilirubin lowering potential of Annona muricata (Linn.) in temporary jaundiced rats. Am J Pharmacol Toxicol 7(2):33–40
Bian X, Wang S, Liu J, Zhao Y, Li H, Zhang L, Li P (2018) Hepatoprotective effect of chiisanoside against acetaminophen-induced acute liver injury in mice. Nat Prod Res:1–4. https://doi.org/10.1080/14786419.2018.1460841
Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI (2015) Drug-induced liver injury: interactions between drug properties and host factors. J Hepatol 63:503–514
Chiu H, Brittingham JA, Laskin DL (2002) Differential induction of heme oxygenase-1 in macrophages and hepatocytes during acetaminophen-induced hepatotoxicity in the rat: effects of hemin and biliverdin. Toxicol Appl Pharmacol 181:106–115
Chung RT, Stravitz RT, Fontana RJ, Schiodt FV, Mehal WZ, Reddy KR, Lee WM (2012) Pathogenesis of liver injury in acute liver failure. Gastroenterology 143:e1–e7
Das J, Ghosh J, Manna P, Sil PC (2010) Acetaminophen induced acute liver failure via oxidative stress and JNK activation: protective role of taurine by the suppression of cytochrome P450 2E1. Free Radic Res 44:340–355
De Vega L, Fernandez RP, Mateo MC, Bustamante JB, Herrero AM, Munguira EB (2002) Glutathione determination and a study of the activity of glutathione-peroxidase, glutathione-transferase, and glutathione-reductase in renal transplants. Ren Fail 24:421–432
Dkhil M, Abdel Moneim A, Hafez T, Mubaraki M, Mohamed W, Thagfan F, Al-Quraishy S (2019) Myristica fragrans kernels prevent paracetamol-induced hepatotoxicity by inducing anti-apoptotic genes and Nrf2/HO-1 pathway. Int J Mol Sci 20(4):993
Dokumacioglu E, Iskender H, Aktas MS, Hanedan B, Dokumacioglu A, Sen TM, Musmul A (2017) The effect of sulforaphane on oxidative stress and inflammation in rats with toxic hepatitis induced by acetaminophene. Bratisl Lek Listy 118:453–459
Du K, Williams CD, McGill MR, Xie Y, Farhood A, Vinken M, Jaeschke H (2013) The gap junction inhibitor 2-aminoethoxy-diphenyl-borate protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes and c-jun N-terminal kinase activation. Toxicol Appl Pharmacol 273:484–491
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Fabregat I, Moreno-Caceres J, Sanchez A, Dooley S, Dewidar B, Giannelli G, Ten Dijke P (2016) TGF-beta signalling and liver disease. FEBS J 283:2219–2232
Fisher AEO, Maxwell SC, Naughton DP (2003) Catalase and superoxide dismutase mimics for the treatment of inflammatory diseases. Inorg Chem Commun 6:1205–1208
Florence NT, Benoit MZ, Jonas K, Alexandra T, Desire DD, Pierre K, Theophile D (2014) Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J Ethnopharmacol 151:784–790
Giannini EG, Testa R, Savarino V (2005) Liver enzyme alteration: a guide for clinicians. CMAJ 172:367–379
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Guan YS, He Q (2015) Plants consumption and liver health. Evid Based Complement Alternat Med 2015:824185
Guo LM, Liu JY, Xu DZ, Li BS, Han H, Wang LH, Zhang WY, Lu LH, Guo X, Sun FX, Zhang HY, Liu XD, Zhang JP, Yao Y, He ZP, Wang MM (2003) Application of molecular adsorbents recirculating system to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int 23(Suppl 3):16–20
Hamid RA, Foong CP, Ahmad Z, Hussain MK (2012) Antinociceptive and anti-ulcerogenic activities of the ethanolic extract of Annona muricata leaf. Rev Bras 22:630–641
Hinson JA, Roberts DW, James LP (2010) Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 196:369–405
Horng CT, Liu ZH, Huang YT, Lee HJ, Wang CJ (2017) Extract from Mulberry (Morus australis) leaf decelerate acetaminophen induced hepatic inflammation involving downregulation of myeloid differentiation factor 88 (MyD88) signals. J Food Drug Anal 25:862–871
Hu B, Colletti LM (2010) CXC receptor-2 knockout genotype increases X-linked inhibitor of apoptosis protein and protects mice from acetaminophen hepatotoxicity. Hepatology 52:691–702
Jaeschke H, Knight TR, Bajt ML (2003) The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett 144:279–288
Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9:515–540
Kaya H, Polat B, Albayrak A, Mercantepe T, Buyuk B (2018) Protective effect of an L-type calcium channel blocker, amlodipine, on paracetamol-induced hepatotoxicity in rats. Hum Exp Toxicol 37(11):1169–1179
Kim GT, Tran NK, Choi EH, Song YJ, Song JH, Shim SM, Park TS (2016) Immunomodulatory efficacy of standardized Annona muricata (Graviola) leaf extract via activation of mitogen-activated protein kinase pathways in RAW 264.7 macrophages. Evid Based Complement Alternat Med 2016:2905127
Kumari A, Kakkar P (2012) Lupeol prevents acetaminophen-induced in vivo hepatotoxicity by altering the Bax/Bcl-2 and oxidative stress-mediated mitochondrial signaling cascade. Life Sci 90:561–570
Laksmitawati DR, Prasanti AP, Larasinta N, Syauta GA, Hilda R, Ramadaniati HU, Widyastuti A, Karami N, Afni M, Rihibiha DD, Kusuma HSW, Widowati W (2016) Anti-inflammatory potential of gandarusa (Gendarussa vulgaris Nees) and soursoup (Annona muricata L) extracts in LPS stimulated-macrophage cell (RAW264.7). J Nat Remed 16:73–81
Lee WM, Seremba E (2009) Drug-induced liver disease. In: Yamada T (ed) Textbook of gastroenterology. Blackwell Publishing, Oxford, pp 2167–2184
Li G, Chen JB, Wang C, Xu Z, Nie H, Qin XY, Chen XM, Gong Q (2013) Curcumin protects against acetaminophen-induced apoptosis in hepatic injury. World J Gastroenterol 19:7440–7446
Li L, Huang W, Wang S, Sun K, Zhang W, Ding Y, Zhang L, Tumen B, Ji L, Liu C (2018) Astragaloside IV attenuates acetaminophen-induced liver injuries in mice by activating the Nrf2 signaling pathway. Molecules 23. https://doi.org/10.3390/molecules23082032
Lin G, Luo D, Liu J, Wu X, Chen J, Huang Q, Su L, Zeng L, Wang H, Su Z (2018) Hepatoprotective effect of polysaccharides isolated from Dendrobium officinale against acetaminophen-induced liver injury in mice via regulation of the Nrf2-Keap1 signaling pathway. Oxidative Med Cell Longev 2018:6962439
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Moghadamtousi SZ, Kadir HA, Paydar M, Rouhollahi E, Karimian H (2014a) Annona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-kappaB. BMC Complement Altern Med 14:299
Moghadamtousi SZ, Rouhollahi E, Karimian H, Fadaeinasab M, Abdulla MA, Kadir HA (2014b) Gastroprotective activity of Annona muricata leaves against ethanol-induced gastric injury in rats via Hsp70/Bax involvement. Drug Des Devel Ther 8:2099–2110
Moghadamtousi SZ, Fadaeinasab M, Nikzad S, Mohan G, Ali HM, Kadir HA (2015a) Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci 16:15625–15658
Moghadamtousi SZ, Rouhollahi E, Hajrezaie M, Karimian H, Abdulla MA, Kadir HA (2015b) Annona muricata leaves accelerate wound healing in rats via involvement of Hsp70 and antioxidant defence. Int J Surg 18:110–117
Ning C, Gao X, Wang C, Kong Y, Liu Z, Sun H, Sun P, Huo X, Ma X, Meng Q, Liu K (2018) Ginsenoside Rg1 protects against acetaminophen-induced liver injury via activating Nrf2 signaling pathway in vivo and in vitro. Regul Toxicol Pharmacol 98:58–68
Quilez AM, Montserrat-de la Paz S, De la Puerta R, Fernández-Arche MA, García-Giménez MD (2015) Validation of ethnopharmacological use as anti-inflammatory of a decoction from Annona Muricata leaves. Afr J Tradit Complement Altern Med 12:14–20
Rajasekaran A, Periyasamy M (2012) Hepatoprotective effect of ethanolic extract of Trichosanthes lobata on paracetamol-induced liver toxicity in rats. Chin Med 7:12
Reisman SA, Csanaky IL, Aleksunes LM, Klaassen CD (2009) Altered disposition of acetaminophen in Nrf2-null and Keap1-knockdown mice. Toxicol Sci 109:31–40
Siddiqui RA, Simjee SU, Kabir N, Ateeq M, Shah MR, Hussain SS (2018) N-(2-hydroxyphenyl)acetamide and its gold nanoparticle conjugation prevent glycerol-induced acute kidney injury by attenuating inflammation and oxidative injury in mice. Mol Cell Biochem 450(1–2):43–52
Waters E, Wang JH, Redmond HP, Wu QD, Kay E, Bouchier-Hayes D (2001) Role of taurine in preventing acetaminophen-induced hepatic injury in the rat. Am J Physiol Gastrointest Liver Physiol 280:G1274–G1279
Wu H, Zhang G, Huang L, Pang H, Zhang N, Chen Y, Wang G (2017) Hepatoprotective effect of polyphenol-enriched fraction from Folium Microcos on oxidative stress and apoptosis in acetaminophen-induced liver injury in mice. Oxidative Med Cell Longev 2017:3631565
Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N (2016) Acetaminophen-induced hepatotoxicity: a comprehensive update. J Clin Transl Hepatol 4:131–142
Zamudio-Cuevas Y, Diaz-Sobac R, Vazquez-Luna A, Landa-Solis C, Cruz-Ramos M, Santamaria-Olmedo M, Martinez-Flores K, Fuentes-Gomez AJ, Lopez-Reyes A (2014) The antioxidant activity of soursop decreases the expression of a member of the NADPH oxidase family. Food Funct 5:303–309
Zuckerbraun BS, Billiar TR (2003) Heme oxygenase-1: a cellular Hercules. Hepatology 37:742–744
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were conducted according to the ethical standards approved by the Committee on Research Ethics for Laboratory Animal Care at the Department of Zoology, Faculty of Science, Helwan University (approval no, HU2017/Z/03).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Brakati, A.Y., Fouda, M.S., Tharwat, A.M. et al. The protective efficacy of soursop fruit extract against hepatic injury associated with acetaminophen exposure is mediated through antioxidant, anti-inflammatory, and anti-apoptotic activities. Environ Sci Pollut Res 26, 13539–13550 (2019). https://doi.org/10.1007/s11356-019-04935-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04935-3