Abstract
Systemic administration of 3-nitropropionic acid (3-NPA) is commonly used to induce Huntington’s disease (HD)-like symptoms in experimental animals. Here, the potential neuroprotective efficiency of rutin and selenium (RSe) co-administration on 3-NPA-induced HD-like symptoms model in mice was investigated. 3-NPA injection evoked severe alterations in redox status, as indicated via increased striatal malondialdehyde and nitric oxide levels, accompanied by a decrease in levels of antioxidant molecules including glutathione, glutathione peroxidase, glutathione reductase, superoxide dismutase, and catalase. Moreover, 3-NPA potentiated inflammatory status by enhancing the production of interleukin-1β, tumor necrosis factor-α, and myeloperoxidase activity. Pro-apoptotic cascade was also recorded in the striatum as evidenced through upregulation of cleaved caspase-3 and Bax, and downregulation of Bcl-2. 3-NPA activated astrocytes as indicated by the upregulated glial fibrillary acidic protein and inhibited brain-derived neurotrophic factor. Furthermore, perturbations in cholinergic and monoaminergic systems were observed. RSe provided neuroprotective effects by preventing body weight loss, oxidative stress, neuroinflammation, and the apoptotic cascade. RSe inhibited the activation of astrocytes, increased brain-derived neurotrophic factor, and improved cholinergic and monoaminergic transmission following 3-NPA intoxication. Taken together, RSe co-administration may prevent or delay the progression of HD and its associated impairments through its antioxidant, anti-inflammatory, anti-apoptotic, and neuromodulatory effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Huntington’s disease (HD) is a progressive inherited neurological disorder, characterized by significant motor and cognitive impairments, associated with psychiatric and behavioral disturbances due to degeneration of striatal and cortical neurons (Dhadde et al. 2016). The precise cause of neuronal loss in HD is unknown. However, abnormal aggregation of mutant huntingtin protein has been suggested to alter neuronal function by attacking cellular proteins and thus enhancing several pathogenic aspects including oxidative reactions, neuroinflammation, apoptotic cascades, mitochondrial dysfunction, bioenergetic decline, excitotoxicity, and neurotransmission alterations (Dhadde et al. 2016; Jamwal and Kumar 2017).
Systemic administration of 3-nitropropionic acid (3-NPA) has been widely used to induce HD-like symptoms in experimental animals as it penetrates the blood-brain barrier, inhibits succinate dehydrogenase (SDH) activity, and subsequently causes mitochondrial malfunction and neuronal degeneration (Tunez et al. 2010). Therapeutic interventions for HD focus on alleviating symptoms associated with HD but fail to prevent or reverse its progression. However, several adverse events have been recorded with administration of current HD medications including fatigue, depression, anxiety, insomnia, dizziness, gastrointestinal problems, and even Parkinsonism (Frank 2014; Videnovic 2013).
Recently, there has been a growing interest in developing alternative effective natural multi-target treatments with minimum side effects. Numerous natural molecules have been used to provide neuroprotection against neurodegenerative disease symptoms owing to their availability, low cost, high safety margins, and wide range of pharmacological activities (Enogieru et al. 2018). Rutin (3,3′,4′,5,7 pentahydroxyflavone-3-rhamnoglucoside) is a flavonoid glycosidic compound, found mainly in lemon, orange, berries, limes, and grapes. It has also been extracted from many other natural sources. Rutin has a variety of biological and pharmacological activities, including antioxidant, antidiabetic, immunomodulator, cytoprotective, anti-inflammatory, and neuroprotective effects (Enogieru et al. 2018; Gautam et al. 2016; Patel et al. 2011).
Selenium is an essential micronutrient incorporated into selenoproteins that have selenocysteine at their active sites. Selenium plays a crucial role in several physiological processes such as in immune responses, fertility, and thyroid hormone function and metabolism (Kohrle 2015; Kudva et al. 2015). Its importance in the brain arises from its antioxidant capacity, maintaining redox status, regulation of Ca2+ homeostasis and mitochondrial function, and neurogenesis modulation. Se deficiency was found to be associated with disturbance in brain metabolism and the development of cognitive and motor impairments (Cardoso et al. 2015; Solovyev 2015). Hence, this investigation was undertaken to evaluate the potential neuroprotective efficiency of rutin and selenium co-administration against 3-nitropropionic acid-induced Huntington’s disease-like symptoms in a mouse model.
Materials and Methods
Chemicals
3-NPA was supplied by Sigma Chemical Co. (St. Louis, MO, USA). Selenium nitrate was purchased from Pharco Pharmaceuticals Co., Egypt. All solvents and other chemicals used for the experiments were of analytical grade.
Plant Material
Ruta graveneoles dried plant was purchased from a local market in Egypt in January 2017. The plant specimen was identified by the Botany Department, Faculty of Science, Helwan University.
Extraction and Isolation of Rutin
The dried and ground leaves of Ruta graveneoles (1000 g) were extracted five times with 70% ethanol at room temperature followed by filtration. After evaporation under reduced pressure, the aqueous layer was collected and left to stand for 3 days. A yellow precipitate separated out of the solution. The precipitate was filtrated and washed three times with dichloromethane: ethyl acetate (2:1) to give 500 mg of a yellow solid. Compound 1 was analyzed by HPLC-PDA (SYKM S500 series, Germany; C18 Column, 250 × 4.6 mm (Fig. 1). The mobile phase was gradients of CH3CN/H2O at flow rate of 1.0 ml min−1.
Compound 1: Yellow solid, 1H NMR (DMSO-d6, 100 MHz) δ 12.60 (s, 1H), 7.55 (dd, J = 2.1 Hz, 7.5 Hz, 2H), 6.83 (d, J = 8.7 Hz, 1H), 6.38 (d, J = 2.1 Hz, 1H), 6.19 (d, J = 2.1 Hz, 1H), 5.30 (t, J = 7.2 Hz, 9.9 Hz, 2H), 5.09 (t, J = 7.8 Hz, 5.4 Hz, 2H), 4.38 (brs, 4H), 3.70 (d, J = 9.9 Hz, 1H), 3.29–3.06 (m, 6H), 0.99 (d, J = 6.3 Hz, 3H); 13C NMR (DMSO-d6, 100 MHz) δ 157.1 (C-2),133.8 (C-3),177.8 (C-4), 157.1 (C-5), 99.1 (C-6), 164.6 (C-7), 94.1 (C-8), 161.7 (C-9), 104.4 (C-10), 122.1 (C-1′), 115.7(C-2′),145.2 (C-3′), 148.9 (C-4′), 116.7 (C-5′),121.6 (C-6′),101.2 (C-1″),74.5 (C-2″), 76.9 (C-3″), 72.3 (C-4″), 76.4 (C-5″), 67.5 (C-6″), 101.6 (C-1″′), 70.5 (C-2″′), 71.0 (C-3″′), 71.8 (C-4″′), 68.7 (C-5″′), 18.2 (C-6″′).
Animals and Experimental Design
Forty adult male albino mice weighing 22–25 g were attained from VACSERA, Egypt. Mice were given free access to water and commercial pelleted rodent feed ad libitum. The mice were kept in the animal facility of the Zoology Department at Helwan University, Cairo, Egypt, under standard laboratory conditions at a temperature of 22–25 °C and a 12-h artificial light/dark cycle.
To investigate the potential neuroprotective role of RSe in 3-NPA-induced HD-like symptoms model, the animals were divided randomly into four groups (n = 10 per group) after 10 days of acclimatization as follows:
-
1.
Control group (CNT): These animals received oral administration of normal saline (0.9% NaCl) daily for 30 days. After 1 h, the animals were intraperitoneally (i.p.) injected with saline from the 8th day to 21st day.
-
2.
Rutin and selenium nitrate co-treated group (RSe): These animals received oral administration of rutin (50 mg/kg) according to Domitrovic et al. (2012) and selenium nitrate (0.2 mg/kg) according to Mohammadi (2014) daily for 30 days. After 1 h, the animals were i.p. injected with saline from the 8th day to 21st day.
-
3.
Huntington’s disease model (3-NPA): These animals received oral administration of normal saline (0.9% NaCl) daily for 30 days. After 1 h, the animals were i.p. injected with 3-nitropropionic acid (10 mg/kg) from the 8th day to 21st day according to Suganya and Sumathi (2017).
-
4.
Rutin + selenium nitrate + 3-NPA-treated group (RSe + 3-NPA): These animals received oral administration of rutin (50 mg/kg) and selenium nitrate (0.2 mg/kg). After 1 h, the animals were i.p. injected with 3-NPA (10 mg/kg) from the 8th day to 21st day.
In the current experiment, 3-NPA, rutin, and selenium nitrate were dissolved in normal saline. The mice were rapidly decapitated 24 h after the last treatment. The striatum was rapidly dissected and washed with isotonic saline. To estimate biochemical parameters, striatal tissue of seven mice was homogenized in ice-cold 10 mM phosphate buffer (pH 7.4) to produce a 10% (w/v) homogenate. Striatal tissue was homogenized in 75% aqueous HPLC grade methanol (10% w/v). The homogenate was spun at 4,000 rpm for 10 min for the estimation of monoamines, monoaminergic metabolites, GABA, and glutamate. For histopathological investigation, striatal tissue of 3 mice was fixed in 10% neutral buffered formalin. Additionally, striatal protein content was assessed for all measurements using the protocols designed by Lowry et al. (1951).
Movement Score Analysis
3-NPA administration is strongly associated with motor impairments which prevent normal ambulatory pattern of the experimented mice. The Severity of motor disturbances in 3-NPA-treated group was estimated utilizing a quantitative movement score in comparison to control group as described by Ludolph et al. (1991) as follows: (score = 0, normal movement; 1, general slowness of displacement resulting from mild hind limb impairment; 2, in coordination and marked gait abnormalities; 3, hind limb paralysis; 4, incapacity to move resulting from forelimb and hind limb impairment; 5, recumbency). The test was performed between 09:00 and 12:00 h at the 29th day of the treatment.
Biochemical Analyses
LPO
Lipid peroxidation (LPO) was assessed by measuring the content of malondialdehyde (MDA) according to the procedures of Ohkawa et al. (1979). Accordingly, 100 mg of the striatal homogenate in phosphate buffer (pH 7.4) was mixed with 100 μl of sodium thioglycolate (1%), 100 μl 100% trichloroacetic acid (TCA), and 250 μl of 1 N HCl. The mixture was then incubated for 20 min at 100 °C and then centrifuged for 10 min (4000 rpm). The formed color was measured as thiobarbituric acid reactive substances (TBARS) at 532 nm.
NO Level
The concentration of nitric oxide (NO) in striatal homogenate was determined according to the protocol of Sastry et al. (2002) via the determination of the stable products represented in nitrite (NO2) nitrate (NO3) levels. In brief, 0.1 ml of the striatal homogenate was mixed with 0.4 ml carbonate buffer and traces of activated Cu-Cd alloy. The mixture was incubated at 25 °C with shaking. To stop the reaction, the alloy was removed after addition of 100 μl of 0.35 M NaOH and 120 Mm ZnSO4. The mixture was exposed to vigorous shaking and then left for 10 min. Afterward, the mixture was spun at 4000 rpm for 10 min at 25 °C. Fifty microliters of Griess reagent was mixed with 10 μl of the striatal supernatant and incubated for 10 min. Finally, the absorbance was estimated using a microplate enzyme-linked immunosorbent assay (ELISA) reader at 545 nm.
GSH
Glutathione level in the striatal tissue was estimated by the method of Sedlak and Lindsay (1968). 250 μl of 10% striatal homogenate was mixed with 50 μl of 50% TCA and 250 μl distilled water. The mixture was exposed to vigorous shaking for 15 min and the centrifuged at 3000 rpm for 10 min at room temperature. Ten microliters of striatal supernatant was added to 400 μl 0.4 M Tris buffer (pH 8.9) and 10 μl of 5,5-dithio-bis-2-nitrobenzoic acid (DTNB) with continuous shaking. The developed color was finally estimated at 512 nm by UV-VIS spectrophotometer (V-630; Jasco, Japan).
CAT Activity
The rate of decomposition of H2O2 per minute was used to determine the activity of catalase (CAT) enzyme. The activity unit of CAT has been expressed as U/mg protein (Aebi 1984). The enzymatic reaction mixture (total volume 1 ml) contained 50 mM potassium phosphate (pH 7.0), 19 mM H2O2, and 50 μl of homogenate supernatant. The molar attenuation coefficient of H2O2 was inspected by UV-VIS spectrophotometer at 240 nm. One unit of catalase activity was known as the amount of enzyme decomposing H2O2 (1 μmol) per min per milligram of tissue protein (U/mg protein).
SOD Activity
Estimation of striatal superoxide dismutase (SOD) activity was carried out according to the method of Misra and Fridovich (1972). The assay protocol depended on the ability of epinephrine to be oxidized at pH 10.2 and creating adrenochrome and superoxide radicals (O2·−). The inhibition of striatal SOD activity can be determined based on decreasing the absorbance at 480 nm.
GPx Activity
Glutathione peroxidase (GPx) activity in striatal tissue was studied according to Lawrence and Burk (2012). In brief, striatal supernatant (200 μl) was mixed with of 75 mM phosphate buffer (1 ml) at pH 7.0, 150 mM glutathione (10 ml), 340 U/mL glutathione reductase (10 ml), 25 mM EDTA (30 ml), 5 mM NADPH (30 ml), 20% Triton X-100 (10 ml), and 7.5 mM H2O2 (50 μl). The oxidation of NADPH (extinction coefficient = 6.22 3 × 103 M−1 cm−1) to NADP+ was monitored at 340 nm for 3 min. One unit of GPx activity was expressed as the quantity of reduced glutathione (GSH) (nanomoles) oxidized per minute per milligram of protein (U/mg protein).
GR Activity
The activity of glutathione reductase (GR) enzyme was determined by adding the striatal homogenate (20 μl) to 0.44 mM oxidized glutathione (GSSG), 0.30 M EDTA, in 0.1 M phosphate buffer at pH 7.0. The enzymatic reaction was started by pipetting 0.036 M NADPH. The oxidation rate of NADPH was followed by the decrease in absorbance at 340 nm with time. One unit of enzyme was known as the quantity of enzyme required to oxidize 1 μmol of NADPH per minute (Farias et al. 2010).
Determination of Striatal Levels of IL-1β and TNF-α Levels
Quantitative estimation of interleukin-1β (IL-1β; cat. no. EM2IL1B, ThermoFisher Scientific) and tumor necrosis factor-α (TNF-α; cat. no. EZMTNFA, Millipore) levels were achieved using ELISA kits specified for mice.
Determination of Striatal AChE Activity
Acetylcholinesterase assayed based on the method described by Ellman et al. (1961). The produced thiocholine by the action of acetylcholinesterase forms 5,5′-dithiobis (2-nitrobenzoic acid) which further reduced to thionitrobenzoic acid, a yellow color anion. The concentration of the developed thionitrobenzoic acid yellow color is determined at 412 nm and is proportional to the activity of acetylcholinesterase (AChE) in the striatal sample.
Quantification of Striatal BDNF
The level of brain-derived neurotrophic factor (BDNF) in the striatal homogenate was carried out using ELISA kits specified for mice (cat. no. EM2IL1B, ThermoFisher Scientific) based on the manufacturer procedures.
Determination of Monoamines and Free Amino Acids
The HPLC system consisted of quaternary pump; a column oven, Rheodine injector and 20 μl loop, UV variable wavelength detector. The report and chromatogram taken from data acquisition program purchased from ChemStation. The sample was immediately extracted from the trace elements and lipids by the use of solid-phase extraction CHROMABOND column NH2 phase cat. no. 730031. The sample was then injected directly into an AQUA column 150 mm 5 μ C18, purchased from Phenomenex, USA, under the following conditions: mobile phase 20 mM potassium phosphate, pH 2.5, flow rate 1.5 mL/min, UV 190 nm. Norepinephrine (NE), dopamine (DA), and serotonin (5-HT) were separated after 12 min. The resulting chromatogram identified each monoamine position and concentration from the sample as compared with that of the standard purchased from Sigma-Aldrich, and finally, the determination of the content of each monoamine as μg per gram brain tissue was calculated according to Pagel et al. (2000). Free amino acid neurotransmitters were detected by using the precolumn PITC derivatization technique employed by Heinrikson and Meredith (1984).
Real-Time PCR
TRIzol reagent was used in total RNA extraction from striatal tissue and then transformed to complementary DNA (cDNA) using cDNA Synthesis Kit (Bio-Rad, Belgium). For gene expression analysis by quantitative Real-Time PCR, cDNA of the oxidative stress enzyme markers (Bax, Bcl-2, glial fibrillary acidic protein (GFAP) and AChE) were utilized as a template. QuantiFast SYBR Green RT-PCR kit (Qiagen, Hilden, Germany) and the corresponding forward and reverse primers shown in Table 1 were used. Primers were obtained from (Jena Bioscience GmbH, Jena, Germany). All experiments were performed in triplicate using Applied Biosystems 7500 Instrument (Thermo Fisher Scientific, CA, USA). The following PCR cycling thermal conditions were used: initial denaturation at 95 °C for 12 min, then by 40 cycles of denaturation at 94 °C for 60 s and annealing at 55 °C for 60 s, extension at 72 °C for 90 s, and afterward held for a final extension at 72 °C for 10 min. The measured differences between delta-delta cycle threshold (Ct) gave the levels of expressed genes (Livak and Schmittgen 2001). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference housekeeping gene.
Histopathological Investigation
From the sacrificed animals, striatum was isolated and leaved for 24 h at ambient temperature in 10% neutral buffered formalin. Removal of water from the tissues were carried out using alcohol, followed by xylene, implanted in paraffin wax and then segmented to obtain 5 μm thickness. Hematoxylin and eosin stains were used to dye the paraffin sections (Drury and Wallington 1980). The slides were examined using optical microscope and images were captured at an original magnification of × 400 (Nikon Eclipse E200-LED, Tokyo, Japan).
Immunohistochemical Investigations
Immunohistochemistry was investigated on dewaxed glass slides. The antigen sites were revealed by washing sections with boiled water followed by 0.03% H2O2 in absolute methanol for 10 min. Sections were kept at 4 °C overnight with (1:50) polyclonal rabbit anti-Bcl-2 antibody, anti-Bax antibody, and anti-caspase-3 antibody (Santa Cruz, CA, USA). To remove the unbound primary antibodies, sections were washed with phosphate-buffered saline (PBS), then incubated for 30 min with goat-derived secondary anti-rabbit antibody conjugated to horseradish peroxidase at 37 °C. The interactions between antigen and antibody were recognized by incubating sections for 10 min at room temperature with the chromogen 3,3′-diaminobenzidine tetrachloride (DAB-H2O2) as substrate. With the Nikon Eclipse E200-LED, the striatal sections were envisioned using × 400 magnification lens. Afterward, the intensity of the color for each evaluated protein was semi-quantitatively analyzed. The intensity was presented as + (weak immunoreaction), ++ (moderate immunoreaction), +++ (strong immunoreaction), or ++++ (very strong immunoreaction).
Statistical Analysis
Our results were expressed as the mean ± standard error of the mean (SEM) values of seven mice for biochemical and molecular investigations and three mice to evaluate the histopathological changes. The data obtained in parametric tests from different assessments were investigated statistically by one-way analysis of variance (ANOVA) and Tukey’s post hoc test. The statistical significance of P values is less than 0.05.
Results
Isolation and Structure Elucidation of Rutin
Compound 1 was isolated from Ruta graveolens as a yellow precipitate. The UV spectrum of 1 (Fig. S1) exhibited absorption bands at 210, 255, and 362 nm. The 1H NMR spectrum (Fig. S2) of 1 showed the presence of one chelated hydroxyl group at δ 12.60 (s, 1H) and five aromatic protons at δ 7.55 (dd, J = 2.1 Hz, 7.5 Hz, 2H), 6.83 (d, J = 8.7 Hz, 1H), 6.38 (d, J = 2.1 Hz, 1H), and 6.19 (d, J = 2.1 Hz, 1H). Additionally, several signals for two sugar units at δ 3.70–3.21 (m, 6H of sugar moieties), 3.70 (d, 1H-Rham), 0.99 (3H, d, CH3-Rham), 5.09–4.38 (4H, H-1 Glu), and 5.30 (1H, d, H-6) were observed. The 13C NMR spectrum (Fig. S3) of 1 depicted 27 carbon signals; 15 of them may be due to the flavonol moiety. The spectrum also showed nine oxygenated carbon atoms (δc 76.9–67.5), two anomeric carbon signals (δc 101.6 and 101.2), and one methyl group (δC 18.2) of rhamnose. Based on the above data, compound 1 was identified as rutin and confirmed by comparing its spectroscopic data with the literature Rajendran et al. (2016).
Behavioral Observation Following 3-NPA Injection
According to the movement scale described by Ludolph et al. (1991), 3-NPA administration caused a disturbance in the movement of mice as compared with the control group. Pretreatment with RSe significantly improved the decrease in the movement when compared against 3-NPA-treated rats (Table 1).
Effect of RSe on Body Weight in 3-NPA-Injected Mice
The effect of RSe on body weight is illustrated in Fig. 2. Control mice showed no change in final body weight. The same results were obtained in RSe-administered mice. Compared with control mice, mice injected with 3-NPA intraperitoneally (10 mg/kg, 30 days) exhibited a significant decrease in final body weight (P < 0.001; F = 15.10). The combined treatment with RSe orally (50 and 0.2 mg/kg, respectively) for 30 days prevented significantly (P < 0.01) the decrease in final body weight produced by 3-NPA treatment.
The effect of oral rutin and selenium nitrate co-administration on body weight in mice after systemic administration of 3-nitropropionic acid. Data are presented as mean ± SD (n = 7); significant change was observed following analysis with Tukey’s post hoc test (P < 0.05). aSignificant change from the control group; bsignificant change from the 3-nitropropionic acid-injected group
Effect of RSe on Redox Balance in 3-NPA-Injected Mice
It has previously been documented that 3-NPA induces oxidative stress in neuronal cells. 3-NPA injection was associated with an imbalance between oxidants and antioxidants in striatal homogenate as represented by significant elevation in LPO (P < 0.001; F = 89.06) and NO (P < 0.001; F = 49.55). Conversely, RSe-treated mice exhibited restored LPO and NO levels approximating those of the control group (Fig. 3). Moreover, 3-NPA injection significantly inhibited cellular enzymatic and non-enzymatic antioxidant defense molecules including GSH (P < 0.001; F = 32.46), GPx (P < 0.001; F = 56.29), GR (P < 0.001; F = 38.11), SOD (P < 0.001; F = 15.35), and CAT (P < 0.001; F = 32.68) when compared with those of control mice. The RSe orally treated group exhibited a significant increase in the activity of GPx, SOD, and CAT when compared with control animals (P < 0.001). No significant changes were observed in GSH and GR levels. Mice concurrently treated with RSe showed a significant increase in GSH, GPx, GR, SOD, and CAT (P < 0.001, 0.001, 0.001, 0.01, 0.01, respectively) when compared with 3-NPA-administered mice (Fig. 4); this reflects the antioxidant activity of RSe against 3-NPA-induced striatal oxidative challenge.
Effects of rutin and selenium nitrate co-administration on a LPO, b NO, and c GSH in striatal tissue following 3-nitropropionic acid injection. Data are presented as mean ± SD (n = 7); significant change was detected following analysis with Tukey’s post hoc test (P < 0.05). aSignificant change from the control group; bsignificant change from the 3-nitropropionic acid-injected group
Effects of rutin and selenium nitrate co-administration on striatal a SOD, b CAT, c GPx, and d GR activity following 3-nitropropionic acid injection. Data are presented as mean ± SD (n = 7); significant change was observed following analysis with Tukey’s post hoc test (P < 0.05). aSignificant change from the control group; bsignificant change from the 3-nitropropionic acid-injected group
Effect of RSe on the Inflammatory Status in 3-NPA-Injected Mice
To study inflammatory responses following 3-NPA injection, levels of TNF-α, IL-1β, and MPO activity were measured in striatal homogenates in all experimental groups. Compared with control mice, 3-NPA mice demonstrated a state of neuroinflammation as indicated by a significant increase in pro-inflammatory cytokines including TNF-α and IL-1β, and increased striatal MPO activity (P < 0.001; F = 282.02, 95.62, and 76.32, respectively), which is used as a biomarker for oxidative stress and inflammation. RSe-gavaged mice demonstrated no significant changes in the levels of these inflammatory mediators. RSe co-administration significantly (P < 0.001) inhibited the increased levels of striatal TNF-α, IL-1β, and MPO activity in 3-NPA-treated mice (Fig. 5).
Effects of rutin and selenium nitrate co-administration on the concentration of a TNF-α, b IL-1β, and c MPO activity in mouse striatum following 3-nitropropionic acid injection. Data are represented as mean ± SD (n = 7); significant change at P < 0.05 was used after using Tukey’s post hoc test. aSignificant change from the control group; bsignificant change from the 3-nitropropionic acid-injected group
Effect of RSe on Striatal Apoptotic Proteins in 3-NPA-Injected Mice
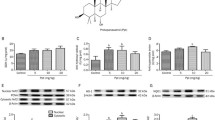
To investigate neuroapoptotic signaling following 3-NPA administration and the potential anti-apoptotic activity of RSe, Bcl-2, and Bax were evaluated using qRT-PCR, while caspase activity was evaluated using ELISA and immunohistochemical analysis. Compared with the control group, mice injected with 3-NPA showed a significant increase in the activity of cleaved caspase-3 and upregulation in Bax mRNA expression (P < 0.001; F = 122.82 and 64.57, respectively) accompanied with downregulation of Bcl-2 mRNA expression (P < 0.001; F = 60.93) No alterations in the tested apoptotic proteins were noted in RSe-treated mice compared with controls. The activity of cleaved caspase-3 and the expression of Bax, and Bcl-2 were partially modulated in RSe+3-NPA-treated mice (P < 0.001) when compared with those in 3-NPA-injected mice (Fig. 6). Additionally, immunohistochemical analysis revealed overactivation of caspase-3 in 3-NPA-treated mice relative to that in control mice (Fig. 7). However, this activation was decreased in RSe+3-NPA-administered mice (Supplementary Table 1S).
Effects of rutin and selenium nitrate co-administration on gene expression of aBcl-2, bBax, and c cleaved caspase-3 activity in mouse striatal neurons following 3-nitropropionic acid injection. Data for cleaved caspase-3 are represented as mean ± SD (n = 7); a significant change was observed following analysis with Tukey’s post hoc test (P < 0.05). aSignificant change from the control group; bsignificant change from the 3-nitropropionic acid-injected group. mRNA expression for Bax and Bcl-2 (mean ± SD of triplicate assays) were referenced to GAPDH and are depicted as fold change (log2 scale), with respect to mRNA levels in control and 3-nitropropionic acid-injected groups
Effect of RSe on Striatal BDNF Following 3-NPA Intoxication
BDNF plays a key role in the survival of nerve cells and has been implicated in several neurological disorders. BDNF levels were significantly decreased in the striatum in 3-NPA-injected mice compared with those in control mice (P < 0.001; F = 74.98). BDNF levels remained unchanged in the RSe-administered group. BDNF levels were significantly increased (P < 0.001) in the RSe+3-NPA group when compared with those in 3-NPA-intoxicated mice (Fig. 8).
Effects of rutin and selenium nitrate co-administration on level of brain-derived neurotrophic factor (BDNF) in mouse striatal neurons following 3-nitropropionic acid injection. Data are represented as mean ± SD (n = 7); significant change was observed after analysis using Tukey’s post hoc test (P < 0.05). aSignificant change from the control group; bsignificant change from the 3-nitropropionic acid-injected group
Effect of RSe on Glial Fibrillary Acidic Protein in Striatum Following 3-NPA Intoxication
GFAP is used as a marker for astroglial activity. qRT-PCR and immunohistochemical analysis revealed that mice intoxicated with 3-NPA showed a significant increase in GFAP mRNA expression compared with that in the control group (P < 0.001; F = 48.98). GFAP expression was unchanged in RSe-treated animals. GFAP was significantly inhibited (P < 0.001) in RSe-pretreated mice compared with 3-NPA-injected mice (Figs. 9 and 10).
Effects of rutin and selenium nitrate co-administration on glial fibrillary acidic protein (GFAP) expression in mouse striatal neurons following 3-nitropropionic acid injection. mRNA expression for GFAP (mean ± SD of triplicate assays) were referenced to GAPDH and expressed as fold change (log2 scale), with respect to mRNA levels in control and 3-nitropropionic acid-injected groups
Effect of RSe on Cholinergic Activity Following 3-NPA Intoxication
HD is associated with cognitive deficits. 3-NPA-injected mice showed a significant increase (P < 0.001; F = 61.90) in AChE activity in striatal homogenates. qRT-PCR findings revealed that mRNA expression levels of AChE were significantly increased (P < 0.05; F = 166.71) compared with control values. No alteration in AChE activity or mRNA expression was observed in RSe-treated mice. RSe-pretreated group exhibited partially restored activity of the cholinergic enzyme and its transcriptional expression (P < 0.01) relative to normal levels when compared with those in 3-NPA-injected animals (Fig. 11).
Effects of rutin and selenium nitrate co-administration on a acetylcholinesterase (AChE) activity and b mRNA expression in mouse striatal neurons following 3-nitropropionic acid injection. mRNA expression of AChE protein (mean ± SD of triplicate assays) were referenced to GAPDH and expressed as fold change (log2 scale), with respect to mRNA levels in control and 3-nitropropionic acid-injected groups
Effect of RSe on Monoamines and Metabolites Following 3-NPA Intoxication
3-NPA administration caused a significant depletion of striatal DA, NE, 5-HT, and GABA content; and increased levels of DOPAC, HVA, 5-HIAA, and glutamate compared with those of the control group (P < 0.001). Mice co-treated with RSe per se exhibited significantly increased concentration of striatal DA and 5-HT only (P < 0.001). No significant changes were noted in the other assessed neurochemical mediators and metabolites. Pretreatment with RSe successfully ameliorated the levels of the tested neurotransmitters compared with those in the 3-NPA intoxicated group (Table 2).
Effect of RSe on Striatal Histological Alterations Following 3-NPA Intoxication
Examination of striatal tissue in the control and RSe-treated groups showed normal nervous tissue structure. 3-NPA administered mice showed degenerated nerve cells coupled with condensed pyknotic cells and inflammatory cell infiltration with edema in striatal tissue. Pretreatment with RSe improved striatal tissue quality and ameliorated changes to approximate normalcy (Fig. 12).
Effect of RSe on striatal histological alterations following 3-NPA intoxication. Scale bar = 100 μm. a Control group. b Rutin and selenium nitrate co-administered group. c 3-Nitropropionic acid-injected group. d Co-treated group with RSe and 3-NPA. Black arrow: degenerated nerve cells coupled with condensed pyknotic cells. Red star: neuronal edema. Blue arrow: inflammatory cell infiltration
Discussion
3-NPA is a mitochondrial toxin that is widely used to induce HD-like symptoms and associated neurological impairments in experimental animals. 3-NPA crosses the blood-brain barrier, enhancing cortical and striatal neuronal loss, motor and cognitive dysfunctions, oxidative damage, pro-inflammatory cytokine release, neurotransmission alterations, ATP depletion, and neuronal apoptosis (Liu et al. 2018; Singh et al. 2015). In the current study and in agreement with previous studies, 3-NPA injection caused a marked decrease in body weight (Dhadde et al. 2016; Liu et al. 2018). The body weight loss may be due to energy metabolism alterations in peripheral tissues following mitochondrial dysfunction and degeneration of striatal and hypothalamic-pituitary axis (Gilbert 2009; Li et al. 2003). Pretreatment of mice with selenium and rutin prevented body weight reduction and 3-NPA-induced HD-like symptoms.
3-NPA causes mitochondrial dysfunction through different molecular pathways leading to reactive oxygen species (ROS) generation and subsequently oxidative damage. Our findings revealed imbalances between oxidants and enzymatic/non-enzymatic antioxidants as indicated by raised striatal LPO and NO levels accompanied with a depletion in GSH content and deactivation of SOD, CAT, GPx, and GR. Brain tissue is susceptible to oxidative stress progression more than other tissues due to its enriched lipid content, high oxygen consumption, and low antioxidant levels (Kassab et al. 2018). 3-NPA plays a fundamental role in the progression of oxidative stress by suppressing mitochondrial complex II and excessive influx of Ca2+ which further triggers ROS production in striatal neurons (Jamwal and Kumar 2017). 3-NPA systemic administration was associated with increased lipid peroxidation in brain tissue which is partially due to excessive production of superoxide anions (Sidhu et al. 2018). Overexpression of iNOS is responsible for NO increments following 3-NPA injection. NO further interacts with superoxide anions, forming peroxynitrite anions (NOO−), which cause severe cellular damage (Almeer et al. 2018). Previous studies recorded the suppression of antioxidant molecules in response to 3-NPA injection (Sidhu et al. 2018). The authors attributed the decrease in GSH, SOD, CAT, GPx, and GR to the elevation of lipid peroxidation.
Pretreatment with rutin and selenium decreased elevated lipid peroxidation and NO levels and restored the inhibited antioxidant defense system. In a previous reports, rutin and selenium compounds were found to protect striatal neurons against 3-NPA-induced HD-like symptoms by decreasing lipid peroxidation and NO, and increasing thiol molecules including GSH, SOD, CAT, GPx, and GR through increasing expression of antioxidant enzymes and deactivation of xanthine oxidase which has a role in ROS production (Bortolatto et al. 2013; Kosti 2015; Suganya and Sumathi 2017; Thome et al. 2018). Selenium acts as a selenocysteine residue in the active site of selenoproteins including GPx which has the ability to regulate oxidative balance by scavenging hydrogen peroxide radicals (Rayman 2000).
Neuroinflammation is associated with striatal degeneration and represents a characteristic feature of HD progression. In our model, excessive release of TNF-α, IL-1β, and MPO in the striatum was observed after 3-NPA treatment. The overproduction of pro-inflammatory cytokines enhances neuronal loss as a result of activating inflammatory and apoptotic cascades, and is involved in HD pathophysiology (Khan et al. 2015). TNF-α and IL-1β elevation has been attributed to the accumulation of mutant huntingtin protein which activates IκB kinase and subsequently triggers nuclear factor-kappa B (NF-κB) activity leading to release of these mediators (Hsiao et al. 2013). MPO is a protein produced by granulocytes in response to oxidative stress and inflammation. Excessive MPO release is linked with ROS generation, tissue damage, and the development of neurodegenerative diseases including HD (Pravalika et al. 2018). Agents which are able to modulate pro-inflammatory cytokine release may be used to delay neuroinflammation associated with HD. In the current study, pretreatment with rutin and selenium decreased the elevated inflammatory mediators following 3-NPA injection. The anti-inflammatory activity of rutin against neuronal inflammation has been investigated. Rutin downregulated hippocampal TNF-α and IL-1β mRNA expression by suppressing activated microglia following trimethyltin intoxication (Koda et al. 2009). In addition, rutin was able to decrease elevated MPO release in a cerebral ischemia-reperfusion model in rats (Annapurna et al. 2013). Moreover, selenium exhibited neuroprotective effects against cerebral ischemia-reperfusion by decreasing TNF-α and IL-1β in rats. This action may be due to the deactivation of NF-κB (Santamaria et al. 2005). Selenium was also found to decrease MPO release in lung tissue of septic rats (Zolali et al. 2014).
Neuroapoptosis represents an important molecular mechanism involved in neurodegeneration. In our model, 3-NPA injection potentiated striatal apoptotic cascade as indicated by increased expression of pro-apoptotic proteins (Bax and cleaved caspase-3) and decreased expression of Bcl-2, an anti-apoptotic protein. Our results are in agreement with previous reports (Ahmed et al. 2016; Duran-Vilaregut et al. 2011; Mahdy et al. 2014). Neuronal apoptosis is associated with oxidative stress progression, mitochondrial impairments, excessive Ca2+ influx, and ROS production (Kassab et al. 2018). On the other hand, pre-administration of rutin and selenium modulated the expression of apoptotic proteins in the striatum. In a previous study, rutin provided neuroprotective effects against cerebral ischemia-reperfusion in rats by inhibiting caspase-3 and Bax, and enhancing Bcl-2 expression (Zhang et al. 2013). The anti-apoptotic effect of rutin in different experimental models has been attributed to its ROS scavenging activity (Nkpaa and Onyeso 2018). The anti-apoptotic and cytoprotective effects of selenium have been recorded in a rhinosinusitis model in rats (Koc et al. 2016). The authors found that selenium blocked caspase-3 and Bax, and upregulated Bcl-2 expression in olfactory sensory neurons, an effect due to its antioxidant properties.
BDNF belongs to the neurotrophin family. BDNF is produced in the cortex and is transported into the striatum, where it is required for striatal neuronal activity and development (Zuccato and Cattaneo 2007). Our findings showed a decrease in striatal BDNF levels following 3-NPA systemic administration. It has been suggested that BDNF levels are decreased in HD animal models and postmortem studies (Shalaby et al. 2018). This decrease is due to ROS overproduction, BDNF mRNA downregulation, and dysregulation of huntingtin protein function (Zuccato and Cattaneo 2007). Therefore, antioxidant compounds that target neurotrophic factors are expected to have neuroprotective effects in neurodegenerative diseases (Ola et al. 2015). Co-administration of rutin and selenium increased BDNF levels, reflecting their potent neuroprotective role against 3-NPA-induced striatal degeneration and HD-like symptoms in mice.
GFAP represents the main filament in the astrocytic cytoskeleton and has been widely used as a marker for astrocytic activation (Suganya and Sumathi 2017). In the current study, 3-NPA injection decreased mRNA expression of Gfap in the striatum. Our results are consistent with previous studies (Gopinath and Sudhandiran 2016). Elevation of GFAP is correlated with neuroinflammation, oxidative stress, and neurodegeneration (Gopinath and Sudhandiran 2016), which were observed in our study. Pretreatment with rutin and selenium reversed the overexpression of GFAP following 3-NPA intoxication. Decreased GFAP expression following rutin and selenium reflects the deactivation of astrocytes and decrement of reactive gliosis, and may explain the decreased pro-inflammatory cytokine release in the present study.
Neurotransmission disturbances have been documented in HD (Suganya and Sumathi 2017). In the current experiment, 3-NPA intoxication caused a reduction in striatal DA, NE, 5-HT, and GABA content, and increased levels of DOPAC, HVA, 5-HIAA, glutamate, and AChE activity. Numerous studies showed alteration in neurotransmitters and their metabolites following 3-NPA injection (Jamwal and Kumar 2017; Kaur et al. 2015; Suganya and Sumathi 2017). Monoaminergic system impairment has been reported to be responsible for motor deficits in HD in human and animal studies. The decrease in monoamines in HD is due to several factors including the downregulation of tyrosine hydroxylase expression, monoamine oxidase upregulation, over activation of catechol-O-methyltransferase, monoaminergic neuron degeneration, and inhibition of monoaminergic receptors in striatum (Richards et al. 2011; Schwab et al. 2015). ROS overproduction and apoptosis have also been found to enhance neurodegeneration and subsequently decrease monoamines and their metabolites in the striatum (Singh et al. 2015). Cholinergic dysfunction plays a key role in HD pathophysiology and its associated cognitive deficits (D'Souza and Waldvogel 2016). According to our findings, the increase in AChE activity is due to the upregulation of AChE mRNA expression and disturbances in neurotrophic factors including BDNF which were found to be decreased in the current study. 3-NPA triggers degeneration of striatal GABAergic projection neurons through suppression of mitochondrial complex II which may explain the depletion of GABA content (Kaur et al. 2015). In addition, ROS produced in response to 3-NPA intoxication is associated with glutamate receptor activation and excessive glutamate production. Moreover, dopaminergic dysfunction in HD potentiates neuronal excitability via enhancing glutamatergic transmission and further neuronal death (Chen et al. 2013; Jamwal and Kumar 2017).
Thus, modulation of striatal neurotransmitter profiles may provide protective effects against 3-NPA-induced HD-like symptoms and associated motor impairments. Rutin and selenium co-administration significantly modulated neurochemical parameters in the striatum following 3-NPA intoxication, indicating their neuromodulatory activity. Rutin was found to enhance spatial memory in old rats by increasing the concentration of DA, NE, and 5-HT, and decreasing their metabolites in different brain regions (Pyrzanowska et al. 2012). Rutin has also been shown to act as an antidepressant drug by increasing synaptosomal 5-HT and NE via inhibition of monoamine oxidase activity and decreasing their turnover (Lin et al. 2013; Oboh et al. 2018). In addition, rutin was found to decrease extracellular glutamate content by enhancing its reuptake by astrocytes (Martini et al. 2007). Due to its antioxidant and inflammatory properties, selenium supplementation was found to increase the depleted DA levels in response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (Khan 2010). In the present study, rutin and selenium co-treatment downregulated striatal AChE mRNA expression that was elevated following 3-NPA intoxication; this may explain the decreased AChE activity. Flavonoids including rutin have been shown to exert sedative effects by enhancing GABA release and activating GABAA receptors (Fernandez et al. 2006). It has been suggested that flavonoids have the ability to inhibit the activity of AChE and may enhance cognitive functions in neurodegenerative diseases (Xie et al. 2014). Treatment with organoselenium compounds produced antidepressant activity during the tail suspension test in mice by enhancing dopaminergic, noradrenergic, and serotonergic systems, and inhibiting monoamine oxidase (Donato et al. 2013; Pinto Brod et al. 2016). The neuromodulatory role of selenium may be due to its influence on ion channels, protein phosphorylation, and calcium homeostasis alterations (Solovyev 2015). Disruption of selenoprotein expression in the rat brain decreased the activity of glutamic acid decarboxylase and GABAergic neuron density in the striatum (Seeher et al. 2014). Organoselenium compounds modulated the glutamatergic system and inhibited glutamate-induced ROS production in different brain areas (Dalla Corte et al. 2012). Selenothymidine intraperitoneally injected mice showed a decrease in AChE activity in an intracerebroventricular administration of streptozotocin-induced dementia model. The authors attributed this action to the antioxidant capacity of selenothymidine and its ability to antagonize AChE activity according to in silico analysis (Thome et al. 2018).
The neurochemical alterations in the current study were correlated with motor impairments upon 3-NPA administration. Our findings are in agreement with earlier studies (Kaur et al. 2015; Liu et al. 2018). The authors demonstrated that the changes in the neurotransmitters and their metabolites, oxidative stress indices and inflammatory mediators led to a decrease in the locomotor activity in 3-NPA-induced HD-like symptoms. On the other hand, antioxidants have been shown to improve the behavioral alterations in 3-NPA-induced HD-like symptoms (Kaur et al. 2015; Liu et al. 2018). Interestingly, the co-administration of RSe along with 3-NPA was found to minimize or even prevent the locomotor disturbances in the experimented rats.
Conclusion
Based on our findings, rutin and selenium co-administration exerted a neuroprotective effect against 3-NPA-induced HD-like symptoms and its associated impairments in the striatum, manifested via prevention of body weight loss, maintenance of normal oxidative status, attenuation of neuroinflammation, and anti-apoptotic activity. Moreover, rutin and selenium neuroprotection suppressed the activation of astrocytes and modulated brain-derived neurotrophic factor. In addition, rutin and selenium displayed a protective role in this HD-like symptoms model by modulating cholinergic, aminoacidergic, and the monoaminergic neurotransmission.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmed LA, Darwish HA, Abdelsalam RM, Amin HA (2016) Role of rho kinase inhibition in the protective effect of fasudil and simvastatin against 3-nitropropionic acid-induced striatal neurodegeneration and mitochondrial dysfunction in rats. Mol Neurobiol 53:3927–3938. https://doi.org/10.1007/s12035-015-9303-2
Almeer RS, Kassab RB, AlBasher GI, Alarifi S, Alkahtani S, Ali D, Abdel Moneim AE (2018) Royal jelly mitigates cadmium-induced neuronal damage in mouse cortex. Mol Biol Rep 46:119–131. https://doi.org/10.1007/s11033-018-4451-x
Annapurna A, Ansari MA, Manjunath PM (2013) Partial role of multiple pathways in infarct size limiting effect of quercetin and rutin against cerebral ischemia-reperfusion injury in rats. Eur Rev Med Pharmacol Sci 17:491–500
Bortolatto CF, Jesse CR, Wilhelm EA, Chagas PM, Nogueira CW (2013) Organoselenium bis selenide attenuates 3-nitropropionic acid-induced neurotoxicity in rats. Neurotox Res 23:214–224. https://doi.org/10.1007/s12640-012-9336-5
Cardoso BR, Roberts BR, Bush AI, Hare DJ (2015) Selenium, selenoproteins and neurodegenerative diseases. Metallomics 7:1213–1228. https://doi.org/10.1039/c5mt00075k
Chen JY, Wang EA, Cepeda C, Levine MS (2013) Dopamine imbalance in Huntington’s disease: a mechanism for the lack of behavioral flexibility. Front Neurosci 7:114. https://doi.org/10.3389/fnins.2013.00114
Dalla Corte CL, Bastos LL, Dobrachinski F, Rocha JB, Soares FA (2012) The combination of organoselenium compounds and guanosine prevents glutamate-induced oxidative stress in different regions of rat brains. Brain Res 1430:101–111. https://doi.org/10.1016/j.brainres.2011.10.049
Dhadde SB, Nagakannan P, Roopesh M, Anand Kumar SR, Thippeswamy BS, Veerapur VP, Badami S (2016) Effect of embelin against 3-nitropropionic acid-induced Huntington's disease in rats. Biomed Pharmacother 77:52–58. https://doi.org/10.1016/j.biopha.2015.11.009
Domitrovic R et al (2012) Differential hepatoprotective mechanisms of rutin and quercetin in CCl(4)-intoxicated BALB/cN mice. Acta Pharmacol Sin 33:1260–1270. https://doi.org/10.1038/aps.2012.62
Donato F, de Gomes MG, Goes AT, Seus N, Alves D, Jesse CR, Savegnago L (2013) Involvement of the dopaminergic and serotonergic systems in the antidepressant-like effect caused by 4-phenyl-1-(phenylselanylmethyl)-1,2,3-triazole. Life Sci 93:393–400. https://doi.org/10.1016/j.lfs.2013.07.024
Drury RAB, Wallington EA (1980) Preparation and fixation of tissues. In: Carleton’s Histological Technique, 5th edn. Oxford University Press, New York pp 41–54
D'Souza GX, Waldvogel HJ (2016) Targeting the cholinergic system to develop a novel therapy for Huntington’s disease. J Huntington's Dis 5:333–342. https://doi.org/10.3233/JHD-160200
Duran-Vilaregut J, Manich G, del Valle J, Pallas M, Camins A, Pelegri C, Vilaplana J (2011) Neuronal apoptosis in the striatum of rats treated with 3-nitropropionic acid is not triggered by cell-cycle re-entry. Neurotoxicology 32:734–741. https://doi.org/10.1016/j.neuro.2011.07.009
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Enogieru AB, Haylett W, Hiss DC, Bardien S, Ekpo OE (2018) Rutin as a potent antioxidant: implications for neurodegenerative disorders. Oxidative Med Cell Longev 2018:6241017. https://doi.org/10.1155/2018/6241017
Farias JG, Puebla M, Acevedo A, Tapia PJ, Gutierrez E, Zepeda A, Calaf G, Juantok C, Reyes JG (2010) Oxidative stress in rat testis and epididymis under intermittent hypobaric hypoxia: protective role of ascorbate supplementation. J Androl 31:314–321. https://doi.org/10.2164/jandrol.108.007054
Fernandez SP, Wasowski C, Loscalzo LM, Granger RE, Johnston GA, Paladini AC, Marder M (2006) Central nervous system depressant action of flavonoid glycosides. Eur J Pharmacol 539:168–176. https://doi.org/10.1016/j.ejphar.2006.04.004
Frank S (2014) Treatment of Huntington’s disease. Neurotherapeutics 11:153–160. https://doi.org/10.1007/s13311-013-0244-z
Gautam R, Singh M, Gautam S, Rawat JK, Saraf SA, Kaithwas G (2016) Rutin attenuates intestinal toxicity induced by methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement Altern Med 16:99. https://doi.org/10.1186/s12906-016-1069-1
Gilbert GJ (2009) Weight loss in Huntington disease increases with higher CAG repeat number. Neurology 73:572; author reply 572. https://doi.org/10.1212/WNL.0b013e3181af0cf4
Gopinath K, Sudhandiran G (2016) Protective effect of naringin on 3-nitropropionic acid-induced neurodegeneration through the modulation of matrix metalloproteinases and glial fibrillary acidic protein. Can J Physiol Pharmacol 94:65–71. https://doi.org/10.1139/cjpp-2015-0035
Heinrikson RL, Meredith SC (1984) Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem 136:65–74
Hsiao HY, Chen YC, Chen HM, Tu PH, Chern Y (2013) A critical role of astrocyte-mediated nuclear factor-kappaB-dependent inflammation in Huntington's disease. Hum Mol Genet 22:1826–1842. https://doi.org/10.1093/hmg/ddt036
Jamwal S, Kumar P (2017) L-theanine, a component of green tea prevents 3-nitropropionic acid (3-NP)-induced striatal toxicity by modulating nitric oxide pathway. Mol Neurobiol 54:2327–2337. https://doi.org/10.1007/s12035-016-9822-5
Kassab RB, Lokman MS, Essawy EA (2018) Neurochemical alterations following the exposure to di-n-butyl phthalate in rats. Metab Brain Dis 34:235–244. https://doi.org/10.1007/s11011-018-0341-0
Kaur N, Jamwal S, Deshmukh R, Gauttam V, Kumar P (2015) Beneficial effect of rice bran extract against 3-nitropropionic acid induced experimental Huntington’s disease in rats. Toxicol Rep 2:1222–1232. https://doi.org/10.1016/j.toxrep.2015.08.004
Khan HA (2010) Selenium partially reverses the depletion of striatal dopamine and its metabolites in MPTP-treated C57BL mice. Neurochem Int 57:489–491. https://doi.org/10.1016/j.neuint.2010.06.020
Khan A, Jamwal S, Bijjem KR, Prakash A, Kumar P (2015) Neuroprotective effect of hemeoxygenase-1/glycogen synthase kinase-3beta modulators in 3-nitropropionic acid-induced neurotoxicity in rats. Neuroscience 287:66–77. https://doi.org/10.1016/j.neuroscience.2014.12.018
Koc S, Cayli S, Aksakal C, Ocakli S, Soyalic H, Somuk BT, Yuce S (2016) Protective effects of melatonin and selenium against apoptosis of olfactory sensory neurons: a rat model study. Am J Rhinol Allergy 30:62–66. https://doi.org/10.2500/ajra.2016.30.4313
Koda T, Kuroda Y, Imai H (2009) Rutin supplementation in the diet has protective effects against toxicant-induced hippocampal injury by suppression of microglial activation and pro-inflammatory cytokines: protective effect of rutin against toxicant-induced hippocampal injury. Cell Mol Neurobiol 29:523–531. https://doi.org/10.1007/s10571-008-9344-4
Kohrle J (2015) Selenium and the thyroid. Curr Opin Endocrinol Diabetes Obes 22:392–401. https://doi.org/10.1097/MED.0000000000000190
Kosti et al (2015) Xanthine oxidase: isolation, assays of activity, and inhibition. J Chem 2015:8. https://doi.org/10.1155/2015/294858
Kudva AK, Shay AE, Prabhu KS (2015) Selenium and inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 309:G71–G77. https://doi.org/10.1152/ajpgi.00379.2014
Lawrence RA, Burk RF (2012) Glutathione peroxidase activity in selenium-deficient rat liver. 1976. Biochem Biophys Res Commun 425:503–509. https://doi.org/10.1016/j.bbrc.2012.08.016
Li SH, Yu ZX, Li CL, Nguyen HP, Zhou YX, Deng C, Li XJ (2003) Lack of huntingtin-associated protein-1 causes neuronal death resembling hypothalamic degeneration in Huntington's disease. J Neurosci 23:6956–6964
Lin SH, Chang HC, Chen PJ, Hsieh CL, Su KP, Sheen LY (2013) The antidepressant-like effect of ethanol extract of daylily flowers ( Jin Zhen Hua) in rats. J Tradit Complement Med 3:53–61. https://doi.org/10.4103/2225-4110.106548
Liu P, Li Y, Liu D, Ji X, Chi T, Li L, Zou L (2018) Tolfenamic acid attenuates 3-nitropropionic acid-induced biochemical alteration in mice. Neurochem Res 43:1938–1946. https://doi.org/10.1007/s11064-018-2615-7
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262S1046-2023(01)91262-9
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ludolph AC, He F, Spencer PS, Hammerstad J, Sabri M (1991) 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Can J Neurol Sci 18:492–498
Mahdy HM, Mohamed MR, Emam MA, Karim AM, Abdel-Naim AB, Khalifa AE (2014) The anti-apoptotic and anti-inflammatory properties of puerarin attenuate 3-nitropropionic-acid induced neurotoxicity in rats. Can J Physiol Pharmacol 92:252–258. https://doi.org/10.1139/cjpp-2013-0398
Martini LH, Jung F, Soares FA, Rotta LN, Vendite DA, Frizzo MES, Yunes RA, Calixto JB, Wofchuk S, Souza DO (2007) Naturally occurring compounds affect glutamatergic neurotransmission in rat brain. Neurochem Res 32:1950–1956. https://doi.org/10.1007/s11064-007-9393-y
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Mohammadi S (2014) Effect of selenium on neurotoxicity in adult male mice exposed to formaldehyde. Electron Physician 6:939–943. https://doi.org/10.14661/2014.939-943
Nkpaa KW, Onyeso GI (2018) Rutin attenuates neurobehavioral deficits, oxidative stress, neuro-inflammation and apoptosis in fluoride treated rats. Neurosci Lett 682:92–99. https://doi.org/10.1016/j.neulet.2018.06.023
Oboh G, Adewuni TM, Ademiluyi AO, Olasehinde TA, Ademosun AO (2018) Phenolic constituents and inhibitory effects of Hibiscus sabdariffa L. (Sorrel) calyx on cholinergic, monoaminergic, and purinergic enzyme activities. J Diet Suppl 15:910–922. https://doi.org/10.1080/19390211.2017.1406426
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ola MS, Ahmed MM, Ahmad R, Abuohashish HM, Al-Rejaie SS, Alhomida AS (2015) Neuroprotective effects of rutin in streptozotocin-induced diabetic rat retina. J Mol Neurosci 56:440–448. https://doi.org/10.1007/s12031-015-0561-2
Pagel P, Blome J, Wolf HU (2000) High-performance liquid chromatographic separation and measurement of various biogenic compounds possibly involved in the pathomechanism of Parkinson's disease. J Chromatogr B Biomed Sci Appl 746:297–304
Patel DK, Kumar R, Prasad SK, Sairam K, Hemalatha S (2011) Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 1:316–322. https://doi.org/10.1016/S2221-1691(11)60051-8
Pinto Brod LM, Fronza MG, Vargas JP, Ludtke DS, Luchese C, Wilhelm EA, Savegnago L (2016) Involvement of monoaminergic system in the antidepressant-like effect of (octylseleno)-xylofuranoside in the mouse tail suspension test. Prog Neuro-Psychopharmacol Biol Psychiatry 65:201–207. https://doi.org/10.1016/j.pnpbp.2015.10.008
Pravalika K, Sarmah D, Kaur H, Wanve M, Saraf J, Kalia K, Borah A, Yavagal DR, Dave KR, Bhattacharya P (2018) Myeloperoxidase and neurological disorder: a crosstalk. ACS Chem Neurosci 9:421–430. https://doi.org/10.1021/acschemneuro.7b00462
Pyrzanowska J, Piechal A, Blecharz-Klin K, Joniec-Maciejak I, Zobel A, Widy-Tyszkiewicz E (2012) Influence of long-term administration of rutin on spatial memory as well as the concentration of brain neurotransmitters in aged rats. Pharmacol Rep 64:808–816
Rajendran N, Subramaniam S, Lotha R, Pemaiah B, Sivasubramanian A (2016) Isolation and characterization of flavonoids and flavone glycosides from the ethnic traditional medicinal plant Cotoneaster bacillaris Wall. Ex Lindl Der Pharmacia Lettre 8:321–324
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241. https://doi.org/10.1016/S0140-6736(00)02490-9
Richards G, Messer J, Waldvogel HJ, Gibbons HM, Dragunow M, Faull RL, Saura J (2011) Up-regulation of the isoenzymes MAO-A and MAO-B in the human basal ganglia and pons in Huntington's disease revealed by quantitative enzyme radioautography. Brain Res 1370:204–214. https://doi.org/10.1016/j.brainres.2010.11.020
Santamaria A et al (2005) Selenium reduces the proapoptotic signaling associated to NF-kappaB pathway and stimulates glutathione peroxidase activity during excitotoxic damage produced by quinolinate in rat corpus striatum. Synapse 58:258–266. https://doi.org/10.1002/syn.20206
Sastry KV, Moudgal RP, Mohan J, Tyagi JS, Rao GS (2002) Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem 306:79–82. https://doi.org/10.1006/abio.2002.5676S0003269702956769
Schwab LC, Garas SN, Drouin-Ouellet J, Mason SL, Stott SR, Barker RA (2015) Dopamine and Huntington’s disease. Expert Rev Neurother 15:445–458. https://doi.org/10.1586/14737175.2015.1025383
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Seeher S, Carlson BA, Miniard AC, Wirth EK, Mahdi Y, Hatfield DL, Driscoll DM, Schweizer U (2014) Impaired selenoprotein expression in brain triggers striatal neuronal loss leading to co-ordination defects in mice. Biochem J 462:67–75. https://doi.org/10.1042/BJ20140423
Shalaby HN, El-Tanbouly DM, Zaki HF (2018) Topiramate mitigates 3-nitropropionic acid-induced striatal neurotoxicity via modulation of AMPA receptors. Food Chem Toxicol 118:227–234. https://doi.org/10.1016/j.fct.2018.05.022
Sidhu A, Diwan V, Kaur H, Bhateja D, Singh CK, Sharma S, Padi SSV (2018) Nicotinamide reverses behavioral impairments and provides neuroprotection in 3-nitropropionic acid induced animal model ofHuntington's disease: implication of oxidative stress- poly(ADP- ribose) polymerase pathway. Metab Brain Dis 33:1911–1921. https://doi.org/10.1007/s11011-018-0297-0
Singh S, Jamwal S, Kumar P (2015) Piperine enhances the protective effect of curcumin against 3-NP induced neurotoxicity: possible neurotransmitters modulation mechanism. Neurochem Res 40:1758–1766. https://doi.org/10.1007/s11064-015-1658-2
Solovyev ND (2015) Importance of selenium and selenoprotein for brain function: from antioxidant protection to neuronal signalling. J Inorg Biochem 153:1–12. https://doi.org/10.1016/j.jinorgbio.2015.09.003
Suganya SN, Sumathi T (2017) Effect of rutin against a mitochondrial toxin, 3-nitropropionicacid induced biochemical, behavioral and histological alterations-a pilot study on Huntington's disease model in rats. Metab Brain Dis 32:471–481. https://doi.org/10.1007/s11011-016-9929-4
Thome GR et al (2018) Selenothymidine protects against biochemical and behavioral alterations induced by ICV-STZ model of dementia in mice. Chem Biol Interact 294:135–143. https://doi.org/10.1016/j.cbi.2018.08.004
Tunez I, Tasset I, Perez-De La Cruz V, Santamaria A (2010) 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington’s disease: past, present and future. Molecules 15:878–916. https://doi.org/10.3390/molecules15020878
Videnovic A (2013) Treatment of Huntington disease. Curr Treat Options Neurol 15:424–438. https://doi.org/10.1007/s11940-013-0219-8
Xie Y, Yang W, Chen X, Xiao J (2014) Inhibition of flavonoids on acetylcholine esterase: binding and structure-activity relationship. Food Funct 5:2582–2589. https://doi.org/10.1039/c4fo00287c
Zhang S, Qi Y, Xu Y, Han X, Peng J, Liu K, Sun CK (2013) Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia-reperfusion injury through suppression of apoptosis and inflammation. Neurochem Int 63:522–532. https://doi.org/10.1016/j.neuint.2013.08.008
Zolali E, Hamishehkar H, Maleki-Dizaji N, Majidi Zolbanin N, Ghavimi H, Kouhsoltani M, Asgharian P (2014) Selenium effect on oxidative stress factors in septic rats. Adv Pharm Bull 4:289–293. https://doi.org/10.5681/apb.2014.042
Zuccato C, Cattaneo E (2007) Role of brain-derived neurotrophic factor in Huntington's disease. Prog Neurobiol 81:294–330. https://doi.org/10.1016/j.pneurobio.2007.01.003
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animals were treated according to the criteria of Investigations and Ethics for Laboratory Animal Care at the Zoology department, Faculty of Science, Helwan University (approval no. HU2017/Z/03).
Conflict of Interest
The authors declare that they have no conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelfattah, M.S., Badr, S.E.A., Lotfy, S.A. et al. Rutin and Selenium Co-administration Reverse 3-Nitropropionic Acid-Induced Neurochemical and Molecular Impairments in a Mouse Model of Huntington’s Disease. Neurotox Res 37, 77–92 (2020). https://doi.org/10.1007/s12640-019-00086-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-019-00086-y