Abstract
In the present study, root cell suspension cultures of W. somnifera were elicited with mycelial extract (1% w/v) and culture filtrate (5% v/v) of their native endophytic fungus Aspergillus terreus 2aWF in shake flask. Culture filtrate of A. terreus 2aWF significantly elicits withanolide A at 6H (12.20 ± 0.52 µg/g FCB). However, with A. terreus 2aWF mycelial extract, withanolide A content was higher at 24H (10.29 µg/g FCB). Withanolide A content was maximum with salicylic acid (0.1 mM) treatment at 24H (8.3 ± 0.20 µg/g FCB). Further, expression analysis of withanolide pathway genes, hydrogen peroxide production, and lipid peroxidation was carried out after 48H of elicitation with 2aWF mycelial extract and culture filtrate. The expression levels of withanolides biosynthetic pathway genes, viz. HMGR, DXR, FPPS, SQS, SQE, CAS, SMT1, STE1 and CYP710A1 were quantified by real time PCR at 48H of elicitation. In all the treatments, the expression levels of key genes were significantly upregulated as compared to untreated suspension cells. Hydrogen peroxide was noticeably enhanced in SA, mycelia extract and culture filtrate, at 20% (115 ± 4.40 nM/g FCB), 42% (137.5 ± 3.62 nM/g FCB), and 27% (122.8 ± 1.25 nM/g FCB) respectively; however, lipid peroxidation was 0.288 ± 0.014, 0.305 ± 0.041 and 0.253 ± 0.007 (µM/gm FCB) respectively, higher than the control (0.201 ± 0.007 µM/gm FCB).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Withania somnifera bioactives are a part of traditional medicine in various health supplements that can enhance immunity and longevity. Root and leaf bioactive compounds from W. somnifera have been reported to be adaptogenic, cardio-protective, anti-oxidative, immunomodulatory, and possessing anti-cancer and anti-Alzheimer properties [1,2,3]. Pharmaceutically important bioactive compounds within leaf or root tissue of W. somnifera contain a group of 28 carbon steroidal lactone triterpenoids, collectively called ‘Withanolides’ that includes withaferin A, withanolide A, withanolide D, withanoside IV or VI, withanolide sulfoxide, etc. [4]. Withaferin A also disrupts cancerous cell by generating ROS, leakage of mitochondrial membrane potential [5], inhibiting telomerase [6], and changing the signaling pathways [7].
With increasing demand for withanolides, various attempts were made to increase the production of withanolides under in-vivo or in-vitro conditions. Under these circumstances, the application of biotechnological approaches to enhance withanolide production was made possible through cell/organ culture, which could provide new landscape to fulfill market demands. Ever since last decade, numerous avenues have successfully been explored to improve withanolides production through cell and organ culture [8]. Production of withanolide A, withaferin A, withanone, and others minor withanolides was successfully reported from shoot cell suspension, hairy root, and root suspension culture [9,10,11,12]. Increase in biomass and withanolide A accumulation in suspension culture was attempted by application of plant growth regulators, and nitrogen and carbon sources ratio in suspension media [13, 14]. Maximum biomass accumulation (147.81 g/L fresh weight) was recorded in medium containing 0.5× concentration of NH4NO3, however, maximum production of withanolide A content (4.36 mg/g DW) was recorded in the 2× concentration of KNO3 [13]. Ciddi et al. [9] inoculated salacin (750 μM) into cell suspension culture in production medium, leading to significantly enhanced production levels of withaferin A compared to unelicited controls. Sivanandhan and his group applied a variety of abiotic and biotic elicitors to hairy root culture and suspension culture to achieve maximum production of withanolides with enhanced biomass [15,16,17]. Amendment of aluminium chloride to hairy root culture results in substantial enhancement of withaferin A, withanolide A, withanolide B, withanoside V, and withanoside IV, as compared to control [16, 17]. However, treatment with biotic elicitor chitosan (100 mg/L) to root suspension culture leads to substantial enhancement of the total withanolides content both in shake-flask culture and bioreactor [17]. Squalene feeding to root suspension culture also plays a role in syntheses of key precursors withanolide A, withanolide B, withaferin A, withanone, 12 deoxywithanstramonolide, withanosides IV and withanosides V [18].
Among biotic elicitors, the application of fungal elicitors resulted in substantial augmentation in the production of a number of phytochemicals in plant tissue cultures [19, 20]. Cell wall-released heat-soluble elicitor prepared from Rhizoctonia solani was found to increase specific indirubin content in suspension culture of Polygonum tinctorium by 168% of the unelicited cell [19]. Catharanthus suspension cultures elicited with fungal extract (5% v/v) of T. viride for 48 H showed a threefold increase in ajmalicine content, whereas A. niger and F. moniliforme induced twofold increase in ajmalicine content in control sample [20]. Ahlawat and his group worked on the application of fungal cell homogenate and culture filtrate to bring about enhancement in withanolides content in suspension culture of W. somnifera [21, 22]. Culture filtrates and cell homogenate of A. alternata, V. dahliae, F. solani and P. indica at different concentration (1.0, 3.0, 5.0 and 7.0% v/v) have been applied to the suspension culture of W. somnifera in shake flask but P. indica cell homogenates (3.0% v/v) were resulted in maximum production of withanolide A (7.12 ± 0.07 mg/g DW), withaferin A (0.33 ± 0.04 mg/g DW) and withanone (5.97 ± 0.06 mg/g DW) [22]. Amendment of 10% v/v T. harzianum culture filtrate enhanced maximum biomass accumulation and total alkaloid content both in Vinca minor hairy roots culture as well as in cell suspension cultures [23]. However, only 3.3% v/v T. harzianum culture filtrate accelerated biomass growth and sanguinarine production (0.090 ± 0.008% DW) in Papaver somniferum cell suspension culture [24]. Many research groups have not explored the biotic elicitors of inherent endophytic fungi in cell suspension cultures, which could probably bring up an immense potential for mass production of secondary metabolites.
The complete biosynthetic pathways of withanolides and their derivatives are still elusive [25]. Since withanolides originated from plant sterols, any modulation in sterol biosynthetic pathway genes could directly affect withanolides content in plants [26]. Thus, sterols of endophytic fungus of native host plant could also elicit withanolides production.
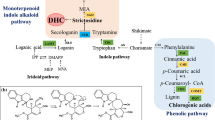
In plants, the formation of 3-isopentenyl pyrophosphate (IPP) follows the common chloroplastic MEP and cytoplasmic MVA pathway; from IPP to withanolide precursor 24-methylenecholesterol, various steps proceed through GPP, FPP, SQ, 2,3-oxidosqualene, cycloartenol, cycloartenol, 24-methylene cycloartenol, 24-methylene lupinol, episterol, and finally 5-dehydroepisterol (Fig. 1). Till date, no report is available for the production of transgenic W. somnifera at commercial level, which could be cultivated for substantially enhancing the withanolides content within plants. Due to limited knowledge of the complete pathway, some transgenic plants were developed which expressed an intermediate pathway gene; For example, the overexpression of WsCAS or GrDXS gene in transgenic W. somnifera have been shown to exhibit higher withanolides content in leaves as compared to control [27, 28]. Overexpression of squalene synthase (SQS) in W. somnifera leads to enhanced withanolide biosynthesis in roots stem and leaves [29, 30]. However, the transient suppression of squalene synthase gene in W. somnifera results into substantial decrease in phytosterol and withanolide content in leaves, and plants become susceptible for pathogens [31].
Schematic presentation of important steps of withanolides biosynthetic pathway in W. somnifera. Solid arrows indicate single-step reactions, dashed arrows indicate several steps, dotted arrows represent unidentified steps and the double arrow show isomers conversion or the exchange between cytosolic and plastid compartments. Bold with underlined genes are quantified in this study. CAS (cycloartenol synthase), CYP710A1 (C-22 sterol desaturase), DMAPP (dimethylallyl diphosphate), DOXP (1-deoxy-d-xylulose 5-phosphate), DXR (1-deoxy-d-xylulose 5-phosphate reductoisomerase), FPP (farnesyl diphosphate), FPPS (FPP synthase), GPP (geranylgeranyl diphosphate), GPPS (geranyl diphosphate synthase), HMBPP (4-hydroxy-3-methylbut-2-enyl diphosphate reductase), HMG-CoA (3-hydroxy-3-methylglutaryl-CoA), HMGR (HMG-CoA reductase), IPP (isopentenyl diphosphate), MEP (2-C-methyl-d-erythritol 4-phosphate), MTs (methyl transferase), MVA (Mevalonate), 3-PGAL (glyceraldehyde 3-phosphate), SGT (sterol glycosyl transferase), SMT1 (sterol methyltransferase 1), SQE (squalene monooxygenase/epoxidase), SQS (squalene synthase), SQ (squalene), STE1 (C-5 sterol desaturase). WFA withaferin A, WLA withanolide A
The present study was designed to evaluate the elicitation potentiality of native endophytic fungus, Aspergillus terreus 2aWF, in root cell suspension cultures of W. somnifera. Here we report, for the first time, the effect of culture filtrate and mycelial extract of native endophytic fungus, A. terreus 2aWF in root cell suspension cultures of W. somnifera, in eliciting withanolide A and modulation of key withanolide biosynthetic pathways genes in comparison to salicylic acid (SA). Additionally, lipid peroxidation and hydrogen peroxide content was estimated in the presence of SA, culture filtrate, and mycelial extract of A. terreus.
Materials and methods
Aseptic plantlet development and establishment of callus culture
W. somnifera Dunal var. Poshita seeds were washed 5 times with autoclaved distilled water, then soaked in 0.01% mercuric chloride for 30 s and washed 5 times with autoclaved distilled water. Thereafter, seeds were treated with 70% ethanol for 90 s, and washed 5 times with autoclaved distilled water. Surface sterilized seeds were inoculated in tissue culture bottle containing half strength MS media [32] and maintained at 25 ± 2 °C at 16H light and 8H dark cycle for 5 weeks. Five weeks grown roots were used as explants for tissue culture. Aseptically treated roots were chopped into small pieces (0.5 cm), transferred onto callus induction media (CIM) plate (MS 4.4 g/L, sucrose 3%, 2,4-dichlorophenoxyacetic acid (2,4-D) 2 mg/L, kinetin 0.2 mg/L, agar 0.8%, pH 5.8) [33], and kept at 25 ± 2 °C in dark for 4 weeks. The pH of the medium was maintained using 0.1 N NaOH/HCl prior to the addition of agar. The medium and accessory materials were autoclaved at 121 °C at 15 lbs for 20 min.
Cell suspension culture establishment
After 4 weeks, creamish color callus was formed around the roots; the new growth was subcultured onto fresh callus induction media. After 4 weeks of subculture, around 500 mg of fragile creamish color callus was inoculated in 250 mL Erlenmeyer flask containing 50 mL of cell suspension medium supplemented with the same hormone concentration without agar. The cultures were maintained on gyratory shaker at 100 rpm under total darkness at 25 ± 2 °C. Cell suspension cultures were maintained in 250 mL Erlenmeyer flask through sub-culturing every 4 weeks at 1:4 dilutions in 50 mL of cell suspension medium supplemented with the same hormone concentrations without agar.
Preparation of elicitor from native leaf endophytic fungi
Aspergillus terreus strain 2aWF as native endophytic fungus was previously isolated from the leaf of W. somnifera (L) Dunal. Complete procedure for elicitor’s preparation from native endophytic fungi is depicted in Fig. 3. Agar plugs, 6 mm each, were punched out from actively growing edges of the PDA plates with fungus growth across the plate, cultured with a sterilized cork-borer, and inoculated in 500 mL Erlenmeyer flask containing 100 mL modified CzapekDox (Sucrose 15 g/L, NaNO3 1 g/L, KH2PO4 1 g/L, MgSO4·7H2O 0.5 g/L, KCl 0.5 g/L, FeSO4·7H2O 0.1 g/L) and kept at 28 °C for 10 days in static conditions. Thereafter, mycelia were separated from the culture filtrate with the help of Whatman filter paper No. 1 and washed twice with autoclaved distilled water to remove traces of modified Czapek Dox culture filtrate. Filtered culture filtrate was considered as an elicitor, as previously reported in [23]. Filtered culture filtrate (100% v/v) was sterilized with syringe filter membrane (0.22 µM, Merck Millipore) and directly used as elicitor called ‘culture filtrate’. Washed mycelia were semidried with blotting paper followed by grinding in liquid nitrogen with the help of mortal pestle and finally dissolved in double distilled water (10% w/v). Water dissolved mycelial extract was sterilized by autoclaving, followed by filtration of the supernatant with syringe filter membrane (0.22 µM, Merck Millipore), and used as elicitor called ‘mycelia extract’.
Elicitor treatment in cell suspension culture and biomass estimation
Cell suspension culture was maintained in the same hormone composition of medium without agar, subcultured every 4 weeks in 1:4 dilutions, and kept on gyratory shaker at 100 rpm under total darkness at 25 ± 2 °C. Exponential growing cell suspension cultures (28th day of subculture) were accounted for culture filtrate and mycelia elicitor treatment. 2.5 mL of culture filtrate (100%) or 5 mL of water extract mycelia elicitor (10% w/v) were added individually to 50 mL of growing cell suspension to reach final concentrations 5% (v/v) and 1% (w/v) respectively. Here, we used reported salicylic acid concentration (SA 0.1 mM) as positive elicitor, which was induced the withanolides content in leaves of W. somnifera [34]. After treatment, samples were collected at different time points, such as 6H, 12H, 24H and 48H, for biomass and withanolide estimation. For RNA extraction, samples were stored at − 80 °C. At each time point, suspension cells culture were collected from suspension media and filtered with Whatman paper for fresh biomass measurement. Each treatment was preceded with three biological replicates.
Withanolides isolation from fresh cell biomass and HPLC analysis
Fresh cell biomass (2.5 g) was dissolved in 5 mL ethyl acetate for withanolide extraction and kept overnight on shaker at 100 rpm at room temperature. Ethyl acetate extract was collected and the procedure repeated thrice for complete extraction. Pooled ethyl acetate extract was dried at room temperature and dissolved in HPLC grade methanol. Dissolved samples were subjected to HPLC analyses (Shimadzu 10AVP; Shimadzu, Kyoto, Japan) Zodiac C-18 (250 mm × 4.6 mm, 5 µ, S.N.220814A516). Two solvents were used for the analysis that consisted of 0.1M of ammonium acetate (CH3COONH4) dissolved in 18 MΩ MQ water (solvent A) and acetonitrile (solvent B). Gradient program was set for 40 min run, having solvent A at 95%, comprising 55% A at 18 min and then to 20% A at 25 min. Constantly, 20% A was maintained from 25 to 28 min, and elevated to 55% of A at 38 min and finally run stabilized to 95% A at 40 min. The flow rate was set at 0.5 mL/min, and the chromatograms were monitored at a wavelength of 227 nm with UV detector. Peaks of withanolide A were identified by comparing with their retention time similar to standards.
RNA extraction and cDNA synthesis
Total RNA was isolated from 100 mg of treated and untreated cell suspension cultures using the RNA isolation kit (Sigma-Aldrich) according to the manufacturer’s instructions. Genomic DNA contamination in extracted RNA was removed by treatment with RNase-free DNase I enzyme (Ambion). Quantity and quality of RNA were checked at OD 260/280 by using the spectrophotometer (Eppendorf biospectrometer). 2 µg of RNA was preceded for first-strand cDNA synthesized using the ReadyScript® cDNA Synthesis Kit (Sigma-Aldrich) according to the manufacturer’s instructions.
Real-time-quantitative PCR (RT-qPCR) analysis
Primers for the real-time quantitative PCR (RT-qPCR) were followed as previously designed by Singh and his group [31] (Suppl. Table 1). RT-qPCR was performed using SYBR® Green JumpStartTMTaqReadyMix™ (Sigma-Aldrich) detection on technical replicates of triplicate biological samples from each of the three plants. PCR conditions followed were: initial denaturation for 10 min at 95 °C, followed by 40 cycles as of denaturation at 95 °C for 15 s, and annealing or extension at 60 °C for 1 min. Fluorescent signal was captured and analyzed by StepOnePlus™ Real-Time PCR System (Applied Biosystems). For specificity, evaluation amplicons were subjected to melting curve analysis using the dissociation method (Applied Biosystems). Threshold cycle (Ct) value of each gene was recorded and normalized against the Ct value of 18S gene (as the constitutive reference transcript). For relative quantification, 2− ΔΔCt method was followed, which was carried out with average Ct values of three technical repeats of each transcript in treated and untreated cell suspension culture [35].
Hydrogen peroxide determination
Hydrogen peroxide production was estimated at 48H of elicitor treatment according to Junglee and co-worker [36]. 200 mg of cell suspension culture was grounded in 1 mL of 0.1% (w/v) TCA in chilled mortar-pestle and then centrifuged at 12,000 rpm for 15 min at 4 °C. 400 µL of supernatant was mixed with 400 µL of 10 mM potassium phosphate buffer (pH 7.0) and 800 µL of 1M potassium iodide (KI) in a 2 mL eppendorf tube. All mixtures were gently mixed and measured for absorbance at 390 nM; H2O2 production was determined with standard Hydrogen peroxide. Standard curve was prepared for absorbance against concentration of H2O2 in solution.
Lipid peroxidation
Extent of lipid peroxidation was determined according to [37] by estimating the malondialdehyde (MDA) produced by the thiobarbituric acid (TBA) reaction. 200 mg of cell suspension culture were homogenized in 1 mL of TBA (0.25% w/v) solution in 10% TCA with the help of chilled mortar and pestle. Homogenized mixture was kept at 95 °C for 30 min and then quickly cooled on ice to stop reaction. The homogenate was centrifuged at 12,000 rpm for 15 min at 4 °C and absorbance of the supernatant measured at 532 nm for MDA-TBA complex. Non-specific absorbance was corrected by subtracting the absorbance at 600 nm, and the amount of MDA-TBA concentration determined by its molar extinction coefficient (155 mM−1 cm−1).
Trypan blue staining of suspension cells
The viability of suspension cells was determined by the trypan blue staining method [38]. 0.4% trypan blue solution was prepared (40 mg of trypan blue dissolved in 10 mL lactic acid, 10 mL phenol pH 7.5–8.0, 10 mL glycerol, 10 mL of distilled water) and used for staining of suspension cells. 100 µl of suspension culture treated with equal volume of trypan blue solution was mixed uniformly and kept at room temperature for 5 min. After incubation, 20 µl of dye treated suspension cells were placed on microscopic glass slide and visualized under bright field microscope.
Statistical analysis
Three biological replicates were considered for each experiment and presented as mean ± SD. For statistical analysis one-way ANOVA, with Dunnett as a post-test, was calculated using Graph Pad prism, version 5.01 (GraphPad Software, San Diego, CA). The statistical significance of differences between control and treated samples was at different P-values, and are reported in figure legends.
Results and discussion
Callus development and kinetics of biomass accumulation in cell suspension culture
All steps, starting from plant explants till the suspension culture development, are shown in Fig. 2. Plants were successfully grown in tissue culture bottles and the chopped root explants (0.5–1 cm) aseptically transferred onto root callus induction media. After 15 days of transfer, callus formation started to appear around the root surface and cut root ends. After 4 weeks, the calli were transferred on fresh callus induction media plate for more proliferation and appeared fragile creamish in color. Maximum percentage of callus induction was induced in combination with 2,4-Dichlorophenoxyacetic acid (2 mg/L) and Kinetin (0.2 mg/L) from root explants [33]. This creamish color callus was maintained on the same composition of semi-solid media for the further experiments.
Different steps for callus and cell suspension culture development from root of W. somnifera (a) 5 weeks old plantlets grown under controlled conditioned on ½ MS media in tissue culture bottle at 25 ± 2 °C (b) aseptically transfer of chopped root explant on callus induction media for 4 weeks (c) after 4 weeks callus was formed round root and cut end (d) well grown creamish color fragile callus after 30 days of subculture on callus induction media (e) suspension culture in 250 mL Erlenmeyer flask after 30 days inoculation of fragile callus, grown under dark condition at 100 rpm and 25 ± 2 °C
Approximately 500 mg of fragile, creamish color callus was inoculated in suspension culture media and kept on rotatory shaker at 100 rpm at 25 ± 2 °C for 30 days. From the first batch of suspension culture, 10 mL suspension cell was inoculated into Erlenmeyer flask (250 mL) containing freshly prepared 40 mL suspension media. After inoculation from 10th days at every 5 day of interval for 30 days, 10 mL of suspension culture was taken for fresh cell biomass estimation. Cell biomass increased with time, it was 0.9, 1.18, 1.77, 2.23 and 2.33 g/10 mL suspension culture at 10, 15, 20, 25 and 30 days respectively, which could be an equivalent of ‘exponential growth phase’ (Fig. 4a). Fresh cell biomass did not change significantly in the period from 25 to 30 days of subculture; here we considered 30th day for measurement of maximum cell growth (23–25% w/v FCB). Sivanadhan and his co-worker found exponential growth of root suspension culture for the period of 7–28 days, maximum growth reaching on the 28th day (14.72 g FCB/30 mL) [18].
Viability of suspension cells, prior to elicitor treatment was determined by staining the cells with trypan blue and observed under bright field microscope (Fig. 4b). Generally trypan blue staining differentiates live and dead cells in suspension culture [38]. In a viable cell trypan blue is not absorbed; however, it traverses the membrane in a dead cell. It enters the dead cell through plasma membrane and stains complete cytoplasm in blue color. Because of negatively charged trypan blue, enters damaged cell membrane and gives intense blue color to cytoplasm, while viable cells maintains the membrane integrity hence do not take dye and remained unstained [39]. Arrow in Figure No 4(b), indicates actively growing, banana shaped cells of W. somnifera root suspension cells. Similar to this observation, previous reports on cell suspension culture of strawberry and tobacco suspension cells have shown round and elongated cells [40, 41].
Impact of elicitors on biomass accumulation
Mycelial extract and culture filtrate elicitor were prepared from W. somnifera native endophytic fungus A. terreus grown in modified Czapek Dox media in static conditions for 10 days at 30 °C (Fig. 3). Earlier reports highlighted dose dependent application of (3.3, 6, and 10% v/v) culture filtrate of endophytic fungi to induce the biomass and sanguinarine production from P. somniferum suspension culture [24]. In this study, 5% (v/v) culture filtrate of native endophyte A. terreus and only 1% (w/v) of their mycelia extract were used. Elicitors were applied during exponential growth stage in 28 day old suspension culture, and fresh cell biomass was approximately 23.8–25% w/v FCB in all culture shake flask. Fresh cell biomass after treatment with culture filtrate at 6H, 12H, 24H and 48H of culture filtrate was determined to be 2.5 ± 0.059, 2.55 ± 0.012, 2.62 ± 0.080 and 2.65 ± 0.082 gm FCB/10 mL suspension culture, respectively. However, in mycelia extract treatment, cell biomass was found to be 2.47 ± 0.079, 2.5 ± 0.07, 2.55 ± 0.12, and 2.61 ± 0.062 g/10 mL suspension culture at 6H, 12H, 24H and 48H of treatment, respectively. In case of abiotic elicitor SA also did not show any significant change in cell biomass even at 6H (2.38 ± 0.047 g/10 mL) and 48H (2.38 ± 0.082 g/10 mL), and it remained the same (Fig. 4). Hence, it was imperative that the application of mycelia extract or culture filtrate did not show any significant change on cell growth.
Growth kinetic of suspension culture, microscopy of suspension cell culture and effect of different elicitor on cell biomass a growth kinetic of suspension culture after subculture at 10th, 15th, 20th, 25th and 30th days, b bright field microscopic view of actively growing suspension cell before subjecting for treatments. Arrow indicates actively growing root suspension cells (transparent and banana shaped) of W. somnifera. c Effect of different elicitors on fresh cell biomass at different time point 6H, 12H, 24H and 48H post treatment. Cell stained with trypan blue. Scale bar represented at 100 µm
Secondary metabolite accumulation in suspension cell culture started after the 7th day of subculture, and synthesized profusely as cell growth entered the exponential stage [42]. The application of endophytic fungus P. indica cell homogenate (3% v/v) for 7 days to W. somnifera suspension culture on 15th day significantly enhanced biomass (15.2 ± 0.56 g/L) and withanolide accumulations (4.9 ± 0.23 mg/L) [21]. Addition of sea weed extract of Sargassum wightii and Gracilaria edulis in shoot suspension culture of W. somnifera resulted in significantly enhanced cell biomass and withanolide production [43]. Exposure of suspension culture after 5 weeks for 24 h to 40% weed extract of G. edulis and 50% of S. wightii significantly evoked biomass production up to 62.4 g FW and 56.80 g FW respectively [43]. Verma and her group applied fungal elicitor from endophytic fungi, T. harzianum culture filtrate, at the dose of 3.3% v/v, and induced maximum biomass accumulation (GI = 293.50 ± 14.82) as well as highest sanguinarine production (0.090 ± 0.008% dry wt.) [24]. However, 10% culture filtrate of C. globosum and A. niveoglaucus showed maximum growth index of 202.00 m ± 25.40 and 187.50 ± 2.80, respectively, but showed lesser sanguinarine accumulation in 5.0 L bioreactor [24]. In the present study, none of the elicitors showed significant changes in suspension cell biomass accumulation between the exposure times 6–48 H; the possible reason behind this might be that the endophytic fungi neither secreted any growth promoting exogenous elicitors in culture filtrate nor was any component present in mycelia extract to induce the cell suspension growth within treatment point.
Effect of elicitors on withanolides production in suspension cell culture
Withania somnifera roots are known to be a prominent source for the production of withanolide A, so we examined the effect of abiotic elicitor SA (0.1 mM), and biotic elicitor culture filtrate and mycelia extract on withanolide A production in suspension culture at various times point (6H, 12H, 24H and 48H) in shake flask. SA and Me-JA are well known for hormone production in plant under abiotic or biotic stress conditions, although its external application enhances secondary metabolite accumulation and resistance against the environmental stress [44,45,46]. All the three elicitors significantly induce withanolide A content at each time point compared to control (Fig. 5). In SA treatment, withanolide A content was found to be enhanced by 1.34 fold (4.31 ± 0.45 µg/g FCB), 2.10 fold (7.56 ± 0.1 µg/g FCB), 2.94 fold (8.31 ± 0.20 µg/g FCB), and 1.79 fold (5.39 ± 0.13 µg/g FCB), irrespective of 6H, 12H, 24H and 48H of treatment respectively, which was significantly higher than the control (3.06 ± 0.21 µg/g FCB) (Fig. 5a–d). A maximum value of 8.31 ± 0.20 µg/g FCB withanolide A was observed in SA treatment at 12H, which thereafter decreased with time up to 48H. Treatment of 40 day old W. somnifera hairy root culture with SA for 4 h significantly enhanced the withanolide A (132.44 mg/g DW; 58-fold higher), withanone (84.35 mg/g DW; 46-fold higher), and withaferin A (70.72 mg/g DW; 42-fold higher) content compared to control [16]. In culture filtrate treatment, enhanced production of withanolide A content by 3.81 fold (12.20 ± 0.52 µg/g FCB), 2.22 fold (8.02 ± 0.23 µg/g FCB), 2.28 fold (6.43 ± 0.30 µg/g FCB) and 1.66 fold (4.99 ± 0.12 µg/g FCB) corresponding to time 6H, 12H, 24H and 48H of treatment respectively, was observed, compared to control (3.06 ± 0.21 µg/g FCB) (Fig. 5a–d). Culture filtrate drastically induced withanolide A accumulation at early stages of treatment (6H), followed by decline in the content with increasing time onward. Elicitor derived from the mycelial component of A. terreus was employed parallel to other elicitors under same conditions. At various time points likewise, 6H, 12H, 24H, and 24H, increase in withanolide A content by 2.62 fold (8.38 ± 0.42 µg/g FCB), 2.25 fold (8.13 ± 0.13 µg/g FCB), 3.65 fold (10.29 ± 0.45 µg/g FCB) and 3.21 fold (9.63 ± 0.33 µg/g FCB) respectively, was achieved, compared to control (3.06 ± 0.21 µg/g FCB) (Fig. 5a–d). Mycelial extract treatment showed higher withanolide A content at 24H (10.29 ± 0.45 µg/g FCB), which then reduced marginally at 48H (9.63 ± 0.33 µg/g FCB), but was higher compared to 6H or 12H of the same treatments (8.38 ± 0.42 µg/g FCB). Interestingly, the results obtained after treatment of mycelial extract and culture filtrate with regard to withanolide A showed greater elicitation potential as compared to abiotic elicitor, however, in control sample, content remained approximately constant.
Effect of elicitor treatments on withanolide A accumulation in cell suspension culture at different time point a withanolide A content per gm of cell biomass at 6H of post elicitors treatment, b withanolide A content per gm of cell biomass at 12H of post elicitors treatment, c withanolide A content per gm of cell biomass at 24H of post elicitors treatment, d withanolide A content per gm of cell biomass at 48H of post elicitors treatment. Error bars represent standard deviation of three biological replicates. Asterisks indicate a significant difference from the control (Student’s t test; *P < 0.05, **P < 0.01)
In an earlier study, different elicitors such as cadmium chloride, aluminium chloride, and chitosan, and precursors such as cholesterol, mevalonic acid and squalene were applied to induce the withanolide accumulation in root suspension culture at different time points (0–72 H) [18]. Within 4H of exposure with chitosan (100 mg/L), there was a substantial enhancement in major and minor withanolides such as withanolide A (14.76 mg/g DW), withanolides B (9.34 mg/g DW), withaferin A (6.68 mg/g DW), withanone (12.27 mg/g DW), 12 deoxywithanstramonolide (4.62 mg/g DW), withanosides IV (2.48 mg/g DW), and V (3.62 mg/g DW) [18]. Abiotic elicitor aluminium chloride (10 mg/L) and Cadmium chloride (15 mg/L) exposed for 4H elicitation resulted in enhanced withanolides production without causing any significant changes in biomass accumulation. Although when 48H was employed with their precursor such as mevalonic acid (200 µM), cholesterol (200 µM) and squalene (6 mM), a reduction in the biomass accumulation was seen, but withnaolide accumulation was significantly higher than control.
Kumar and co-workers (2012) reported that inoculation of 3% (v/v) P. indica culture filtrate (filter-sterilized) in Linum album hairy roots culture for 48H led to 1.4 fold increase in biomass and 3.5–4.5 fold increase in secondary metabolite [47]. Successively, the same group revealed elicitation potential of P. indica, and compared known elicitors like methyl jasmonate (Me-JA), chitin and chitosan in the suspension cultures of Lantana camara [48]. So, the application of Me-JA at 62.5 µM and 2.5% (v/v) culture filtrate of P. indica enhanced ursolic acid (3.5-fold), oleanolic acid (5.6-fold), and betulinic acid (7.8-fold) production to similar levels [48]. Similarly, inoculation of P. indica cell homogenate to W. somnifera hairy root culture led to biomass and secondary metabolite induction [49]. The treatment of Tessaria absinthioides suspension culture with elicitor preparations of Verticillum sp., Monodyctis cataneae, Acremonium sp., and Aspergillus niger substantially enhanced the sesquiterpene tessaric acid (TA) production by 281%, 197%, 149%, and 139% respectively compared to the untreated control [50].
Novelty of our work is in application of native, endophytic fungi (Aspergillus terreus) of W. somnifera as an elicitor for synthesis of withanolide A in root suspension cells. Previous researcher have explored the application of biotic elicitors from random sources such as A. alternata, F. solani, V. dahlia and most of the cases these eliciting organisms were not endophytes [22]. Few cases of non-native and non-specific endophytes such as P. indica has also been used as biotic elicitors for induction of secondary metabolites in hairy root and shoot suspension culture [21, 22]. In this studies, instead of non-native fungal elicitors, we deployed crop specific functional endophyte of W. somnifera [51], for secondary metabolite production in root suspension cultures of W. somnifera. We hypothesized, to use potential crop specific endophyte Aspergillus terreus, which is indeed native to W. somnifera plant, as a biotic elicitor during suspension cell culture for synthesis of withanolide A. Thus, this could be our novel approach so far and application of functional endophyte of medicinal plant which assists in in planta synthesis of bioactive compound and also acts as a potential biotic elicitor during suspension culture system is an added advantage. Hence it will have an edge over conventional approaches, in terms of its industrial acceptability – since the biotic elicitors used, is from inherent endophyte of plant system and a non-pathogen of the host plant. To substantiate, we report for the first time, culture filtrate and mycelia extract of innate, functional endophytic fungus (Aspergillus terreus 2aWF) of W. somnifera has enhanced maximum withanolide A induction from less treatment duration in root suspension cells. A. terreus 2aWF culture filtrate, at 6H induction time resulted in maximum withanolide A (3.81 times) while with mycelia extract at early 6H yielded 2.6 times higher further maintained upto 48H (3.2–3.6 times) over the untreated culture cells, which is better than non-native P. indica biotic elicitor in shoot suspension culture (1.8 times after 7 days exposure to elicitor) [22].
Effect of elicitors on withanolide biosynthetic pathway genes
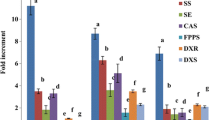
Elicitors that enhance withanolide A content also modulate withanolide biosynthetic pathways genes. Therefore, we examined the gene expression profile in elicitor treated and untreated cell suspension cultures after 48H, and quantified few selected withanolide biosynthetic pathway genes, viz. HMGR, DXR, FPPS, SQS, SQE, CAS, SMT1, STE1 and CYP710A1, which play an important role in withanolide biosynthesis [31, 52]. However, the complete pathway of withanolides biosynthesis still remains elusive and is challenging research problem [53]. HMGR and DXR are upstream genes for MVA and MEP pathway for the formation of downstream common precursor isopentenyl diphosphate (IPP). In the treatment of SA, culture filtrate and mycelia extract, HMGR expression levels increased significantly by 8.3, 2.6 and 6.5 fold respectively, compared to control (Fig. 6a). The higher expression levels of HMGR, DXS, DXR, FPPS, SQS, SQE and CAS genes were observed following application of 3% cell homogenate, 3% culture filtrate, and fungal disc of P. indica in W. somnifera suspension culture or callus treatment [21]. However, DXR expression level was 4, 5.8 and 12 fold higher in SA, culture filtrate, and mycelia extract treatment respectively (Fig. 6b). From IPP onward, FPPS gene was observed to have enhanced 4.3, 5.9 and 8.8 fold by SA, culture filtrate and mycelia extract treatment respectively (Fig. 6c). Squalene formed by gene SQS, which were increased by 3.2, 6.4 and 2.7 fold in corresponding to SA, culture filtrate and mycelia extract treatment (Fig. 6d). Upon treatment with seaweed extract of G. edulis (50%) and S. wightii (60%) to W. somnifera, hairy root culture for 48H exposure significantly induced the expression levels of HMGR, FPPS, SQE and SQS gene [54]. 2, 3-oxidosqualene is formed through squalene precursor that is a multi-branch point for other metabolites such as β-amyrin, lupeol, lanosterol and cycloartenol, which proceeded in presence of SQE gene. The treatment of SA, culture filtrate, and mycelia extract profoundly induced the SQE expression by 6.4, 7.3 and 8.6 fold higher level relative to the untreated cells (Fig. 6e). In the biosynthesis of cycloartenol, CAS gene was observed to be upregulated by 2.9, 2.1 and 2.7 fold in SA, culture filtrate and mycelia extract treatment respectively (Fig. 6f). It was recently shown that applied biotic elicitor, apart from P. indica, such as V. dahliae, A. alternata and F. solani fungal culture filtrate or cell homogenate, also induced the expression levels of HMGR, DXS, DXR, FPPS, SQS, SQE and CAS gene in shake flask [22]. In all the three treatments SMT1 expression level was enhanced by approximately 2.7 fold, which was similar to the values in SA, culture filtrate and mycelia extract treatment as compared with the control suspension cell (Fig. 6g). Further, the dehydrating gene STE1 was quantified and was found to increase by 2.9, 3.1 and 2.4 fold in SA, culture filtrate and mycelia extract treatment respectively (Fig. 6h). Last gene in this study, CYP710A1 in stigmasterol biosynthesis was seen to increase by 2.4, 3.4 and 7.1 higher SA, culture filtrate and mycelia extract treatment respectively, over control suspension cell (Fig. 6i). The employment of 15 µM Me-JA for 4H to W. somnifera hairy root increased expression levels of the SQS (6.58 fold), SQE (5.38 fold), CAS (5.7 fold), SMT-1 (6.9 fold), ODM (8.24 fold) and SDS (9.78 fold) [49]. However, with treatment with P. indica cell homogenate (3%) to hairy root for 48H, the expression levels of some important genes of withanolides pathway like SQS (2.87 fold), SQE (3.25 fold), CAS (3.08 fold), ODM (4.42 fold), SMT1(4.81 fold) and CYP710A1 (5.024 fold) were significantly higher as compared to the control [49].
Effect of different elicitor on withanolide biosynthesis genes studied at 48H of treatment. At each time point withanolide A content was higher in elicitor treated than control, so only at 48H treated suspension cell taken for withanolides biosynthesis pathway genes expression level quantification. Real-time qPCR analysis showing relative expression levels of upstream and downstream withanolides pathway genes aHMGR, bDXR, cFPPS, dSQS, eSQE, fCAS, gSMT1, hSTE1, iCYP710A1. Expression levels of these genes were normalized to 18S rRNA and are represented in comparison with control. Data are mean ± SD (n = 3 biological replicates) and y axis represents relative quantity (RQ). RQ was calculated using the equation; RQ = 2− ΔΔCt. Error bars represent standard deviation. Asterisks indicate a significant difference from the control (Student’s t test; **P < 0.01)
The external application of universal abiotic elicitor SA (2 mM) to leaf of W. somnifera for 6H exposure was observed to differentially elicit important MEP pathway genes such as WsDXS and WsDXR than control [55]. Otherwise if W. somnifera seedling roots dipped for 4H in SA (2 mM) that also enhanced transcript expression level of WsCAS by 2.49 fold [28]. In another experiment, lower concentration of SA (0.1 mM) exposed for 24H and 48H also influenced to induce the accumulation of P450s A-type monooxygenases WsCYP98A and WsCYP76A transcript upto 2.5 and threefold, respectively [34].
The amendment of known abiotic elicitor SA and crude biotic elicitor extracted from endophytic fungi A. terreus differentially regulated the withanolide biosynthetic pathway genes for withanolides accumulation. Here, we have noticed that native endophyte A. terreus in the form of culture filtrate or their mycelia extract could act as elicitor molecules to induce secondary metabolites in suspension culture.
Effect of elicitor on hydrogen peroxide accumulation
Salicylic acid is a well-known hormone that induces hydrogen peroxide formation and secondary metabolites within plants, and induces systemic resistance against biotic stress [56, 57]. In this study, we examined the effect of SA as well as culture filtrate and mycelia extract on hydrogen peroxide production by fresh cell biomass at 48H in all elicitor treated suspension culture. In SA treated suspension culture, hydrogen peroxide production (115 ± 4.40 nM/g FCB) was enhanced by 20% as compared to control (96.5 ± 2.17 nM/g FCB). However, in culture filtrate and mycelia, extract treated suspension culture were produced by 42% (137.5 ± 3.62 nM/g FCB) and 27% (122.8 ± 1.25 nM/g FCB) higher, respectively, than untreated suspension culture 96.5 ± 1.25 nM/g FCB (Fig. 7a). In the present study, we found that not only SA elicited H2O2 production, culture filtrate and mycelia extract also significantly induced the accumulation of H2O2 within suspension up to 48H of treatment.
Effect of elicitor treatments on hydrogen peroxide and MDA production in treated suspension culture at 48H of treatment. a hydrogen peroxide production per gm of cell biomass in control and treated sample, b MDA production per gm of cell biomass in control and treated sample. Asterisks indicate a significant difference from the control (Student’s t test; *P < 0.05 **P < 0.01)
Arabidopsis leaves were treated with 1 and 5 mM SA for 8H enhanced H2O2 levels by 59% and 194%, respectively, compared to control leaves. However, when SA (5 mM) is treated along with 10 mM H2O2 for 8H, it resulted in enhanced in vivo H2O2 levels by 367% compared to control leaves [56]. Also, the application of SA in suspension culture of Salvia miltiorrhiza showed significantly enhanced rosmarinic acid (RA) production, H2O2 production, as well as phenylalanine ammonia-lyase (PAL) activity [58]. Moreover, the treatment of SA to tobacco suspension culture cell (Nicotiana tabacum, cell line BY-2) triggered different type of reactive oxygen species (ROS) like superoxide, hydroxyl radical and hydrogen peroxide [59]. The accumulation of hydrogen peroxide triggered MDA production in BY-2 cell-line, which was indirectly induced by SA [59].
Lipid peroxidation
The peroxidation of lipids is considered as the most damaging process known to occur in every living organism. During lipid peroxidation (LPO), products are formed from polyunsaturated precursors that include small hydrocarbon fragments such as ketones, malondialdehyde (MDA), etc., and compounds related to them [60]. Generally, all kinds of abiotic and biotic stresses lead to reactive oxygen species generation, which inferred to oxidation of unsaturated membrane lipids called lipid peroxidation. Here, we estimated the lipid peroxidation by indirect method to the quantification of MDA production at 48H treatment. MDA content was significantly increased during application of SA, culture filtrate and mycelia extract to suspension cell culture (Fig. 7b). The MDA amount was 0.28 ± 0.014, 0.30 ± 0.04 and 0.25 ± 0.007 µM/gm FCB in SA, culture filtrate and mycelia extract treated respectively than control (0.21 ± 0.007 µM/gm FCB).
Previous studies reported the application of SA to BY-2 suspension culture leading to the accumulation of MDA through induction of hydrogen peroxide [59]. Toxic effect of aluminum salt has been studied on soybean cell suspension cultures, which showed induction of lipid peroxidation, leading to MDA accumulation [61]. The osmotic stresses are also known to induce MDA production in Palm embryonic suspension culture. Palm embryogenic suspension culture cell were subjected to osmotic stress with Polyethylene glycol 6000 (0–20%). Raising PEG levels in suspension culture induces malondialdehyde (MDA) concentration, and was the highest at 10% PEG, and thereafter, MDA content decreased with increasing the PEG concentration [62]. Beside to known SA, culture filtrate and mycelia extract of A. terreus significantly induced the MDA content at 48H of treatment.
Conclusions
In this study, we studied the ability of innate endophyte of W. somnifera, Aspergillus terreus 2aWF to act as biotic elicitor, and significantly induce withnaolide A accumulation in root cell suspension cultures. 5% of culture filtrate and only 1% mycelia extract could serve as an elicitor for maximum induction of withanolide A. This is the first report on exploration of W. somnifera plant’s native fungal endophyte culture filtrate and mycelia extract as an elicitor in their host cell suspension culture. Apart from conventional methods, a sustainable step of recruiting native endophytes of important medicinal plants as elicitors to induce secondary metabolites in suspension cultures must potentially be unveiled.
References
Raghavan A, Shah ZA (2015) Withania somnifera: a pre-clinical study on neuroregenerative therapy for stroke. Neural Regen Res 10(2):183–185
Sachdeva H, Sehgal R, Kaur S (2013) Studies on the protective and immunomodulatory efficacy of Withania somnifera along with cisplatin against experimental visceral leishmaniasis. Parasitol Res 112(6):2269–2280
Antony ML, Lee J, Hahm ER, Kim SH, Marcus AI, Kumari V, Ji X, Yang Z, Vowell CL, Wipf P, Uechi GT, Yates NA, Romero G, Sarkar SN, Singh SV (2013) Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of beta-tubulin. J Biol Chem 289(3):1852–1865
Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazon J (2009) Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 14(7):2373–2393
Li AX, Sun M, Li X (2017) Withaferin-A induces apoptosis in osteosarcoma U2OS cell line via generation of ROS and disruption of mitochondrial membrane potential. Eur Rev Med Pharmacol Sci 21(6):1368–1374
Yu Y, Katiyar SP, Sundar D, Kaul Z, Miyako E, Zhang Z, Kaul SC, Reddel RR, Wadhwa R (2017) Withaferin-A kills cancer cells with and without telomerase: chemical, computational and experimental evidences. Cell Death Dis 8(4):e2755
Zhang QZ, Guo YD, Li HM, Wang RZ, Guo SG, Du YF (2017) Protection against cerebral infarction by Withaferin A involves inhibition of neuronal apoptosis, activation of PI3K/Akt signaling pathway, and reduced intimal hyperplasia via inhibition of VSMC migration and matrix metalloproteinases. Advances in Medical Sciences 62(1):186–192
Singh P, Guleri R, Angurala A, Kaur K, Kaul SC, Wadhwa R, Pati PK (2017) Addressing challenges to enhance the bioactives of Withania somnifera through organ, tissue, and cell culture based approaches. Biomed Res Int 2017:3278494
Ciddi V (2006) Withaferin A from cell cultures of Withania somnifera. Indian J Pharm Sci 68(4):490–492
Sharada M, Ahuja A, Suri KA, Vij SP, Khajuria RK, Verma V, Kumar A (2006) Withanolide production by in vitro cultures of Withania somnifera and its association with differentiation. Biol Plant 51(1):161–164
Sabir F, Sangwan NS, Chaurasiya ND, Misra LN, Sangwan RS (2008) In vitro withanolide production by Withania somnifera L. cultures. Z Naturforsch C 63(5–6):409–413
Baldi A, Singh D, Dixit VK (2008) Dual elicitation for improved production of withaferin A by cell suspension cultures of Withania somnifera. Appl Biochem Biotechnol 151(2–3):556–564
Nagella P, Murthy HN (2010) Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresour Technol 101(17):6735–6739
Nagella P, Murthy HN (2011) Effects of macro elements and nitrogen source on biomass accumulation and withanolide—a production from cell suspension cultures of Withania somnifera (L.). Plant Cell Tissue Organ Cult 104(1):119–124
Sivanandhan G, Arun M, Mayavan S, Rajesh M, Mariashibu TS, Manickavasagam M, Selvaraj N, Ganapathi A (2012) Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.). Ind Crops Prod 37(1):124–129
Sivanandhan G, Dev GK, Jeyaraj M, Rajesh M, Arjunan A, Muthuselvam M, Manickavasagam M, Selvaraj N, Ganapathi A (2013) Increased production of withanolide A, withanone, and withaferinA in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Cult 114(1):121–129
Sivanandhan G, Dev GK, Jeyaraj M, Rajesh M, Muthuselvam M, Selvaraj N, Manickavasagam M, Ganapathi A (2013) A promising approach on biomass accumulation and withanolides production in cell suspension culture of Withania somnifera (L.) Dunal. Protoplasma 250(4):885–898
Sivanandhan G, Selvaraj N, Ganapathi A, Manickavasagam M (2014) Enhanced biosynthesis of withanolides by elicitation and precursor feeding in cell suspension culture of Withania somnifera (L.) Dunal in shake-flask culture and bioreactor. PLoS ONE 9:e104005
Marero LM, Jin JH, Shin JH, Lee HJ, Chung IS, Lee HJ (1997) Effect of fungal elicitation on indirubin production from a suspension culture of Polygonum tinctorium. Enzyme Microb Technol 21(2):97–101
Namdeo A, Patil S, Fulzele DP (2002) Influence of fungal elicitors on production of ajmalicine by cell cultures of Catharanthus roseus. Biotechnol Prog 18(1):159–162
Ahlawat S, Saxena P, Ali A, Abdin MZ (2016) Piriformospora indica elicitation of withaferin A biosynthesis and biomass accumulation in cell suspension cultures of Withania somnifera. Symbiosis 69(1):37–46
Ahlawat S, Saxena P, Ali A, Khan S, Abdin MZ (2017) Comparative study of withanolide production and the related transcriptional responses of biosynthetic genes in fungi elicited cell suspension culture of Withania somnifera in shake flask and bioreactor. Plant Physiol Biochem 114:19–28
Verma P, Khan SA, Mathur AK, Shanker K, Kalra A (2014) Fungal endophytes enhanced the growth and production kinetics of Vinca minor hairy roots and cell suspensions grown in bioreactor. Plant Cell Tissue Organ Cult 118(2):257–268
Verma P, Khan SA, Mathur AK, Ghosh S, Shanker K, Kalra A (2014) Improved sanguinarine production via biotic and abiotic elicitations and precursor feeding in cell suspensions of latex-less variety of Papaver somniferum with their gene expression studies and upscaling in bioreactor. Protoplasma 251(6):1359–1371
Senthil K, Jayakodi M, Thirugnanasambantham P, Lee SC, Duraisamy P, Purushotham PM, Rajasekaran K, Nancy Charles S, Mariam Roy I, Nagappan AK, Kim GS, Lee YS, Natesan S, Min TS, Yang TJ (2015) Transcriptome analysis reveals in vitro cultured Withania somnifera leaf and root tissues as a promising source for targeted withanolide biosynthesis. BMC Genom 16:14
Singh S, Pal S, Shanker K, Chanotiya CS, Gupta MM, Dwivedi UN, Shasany AK (2014) Sterol partitioning by HMGR and DXR for routing intermediates toward withanolide biosynthesis. Physiol Plant 152(4):617–633
Jadaun JS, Sangwan NS, Narnoliya LK, Singh N, Bansal S, Mishra B, Sangwan RS (2016) Over-expression of DXS gene enhances terpenoidal secondary metabolite accumulation in rose-scented geranium and Withania somnifera: active involvement of plastid isoprenogenic pathway in their biosynthesis. Physiol Plant 159(4):381–400. https://doi.org/10.1111/ppl.12507
Mishra S, Bansal S, Mishra B, Sangwan RS, Asha, Jadaun JS, Sangwan NS (2016) RNAi and homologous over-expression based functional approaches reveal triterpenoid synthase gene-cycloartenol synthase is involved in downstream withanolide biosynthesis in Withania somnifera. PLoS One 11:e0149691
Grover A, Samuel G, Bisaria VS, Sundar D (2013) Enhanced withanolide production by overexpression of squalene synthase in Withania somnifera. J Biosci Bioeng 115(6):680–685. https://doi.org/10.1016/j.jbiosc.2012.12.011
Patel N, Patel P, Kendurkar SV, Thulasiram HV, Khan BM (2015) Overexpression of squalene synthase in Withania somnifera leads to enhanced withanolide biosynthesis. Plant Cell Tissue Organ Cult 122(9):409–420
Singh AK, Dwivedi V, Rai A, Pal S, Reddy SG, Rao DK, Shasany AK, Nagegowda DA (2015) Virus-induced gene silencing of Withania somnifera squalene synthase negatively regulates sterol and defence-related genes resulting in reduced withanolides and biotic stress tolerance. Plant Biotechnol J 13(9):1287–1299. https://doi.org/10.1111/pbi.12347
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Rani G, Grover IS (1999) In vitro callus induction and regeneration studies in Withania somnifera. Plant Cell Tissue Organ Cult 57(1):23–27
Rana S, Bhat WW, Dhar N, Pandith SA, Razdan S, Vishwakarma R, Lattoo SK (2014) Molecular characterization of two A-type P450s, WsCYP98A and WsCYP76A from Withania somnifera (L.) Dunal: expression analysis and withanolide accumulation in response to exogenous elicitations. BMC Biotechnol 14:89
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Junglee S, Urban L, Sallanon H, Lopez-Lauri F (2014) Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am J Analyt Chem 5(11):730–736. https://doi.org/10.4236/ajac.2014.511081
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Fernández-Bautista N, Domínguez-Núñez JA, Moreno MC, Berrocal-Lobo M (2016) Plant tissue trypan blue staining during phytopathogen infection. Bio-protocol 6(24): e2078
Tamagnone L, Merida A, Stacey N, Plaskitt K, Parr A, Chang CF, Lynn D, Dow JM, Roberts K, Martin C (1998) Inhibition of phenolic acid metabolism results in precocious cell death and altered cell morphology in leaves of transgenic tobacco plants. Plant Cell 10(11):1801–1816
Steward N, Martin R, Engasser JM, Goergen JL (1999) A new methodology for plant cell viability assessment using intracellular esterase activity. Plant Cell Report 19(16):171–176
Forni C, Frattarelli A, Damiano C (1999) Different size, shape and growth behaviour of cells in suspension cultures of strawberry (Fragaria x ananassa Duch.). Plant Biosyst 133(2):205–212
Ouyang J, Wang XD, Zhao B, Wang YC (2005) Enhanced production of phenylethanoid glycosides by precursor feeding to cell culture of Cistanche deserticola. Process Biochem 40(11):3480–3484
Sivanandhan G, Selvaraj N, Ganapathi A, Manickavasagam M (2014) Improved production of withanolides in shoot suspension culture of Withania somnifera (L.) Dunal by seaweed extracts. Plant Cell Tissue Organ Cult 119(1):221–225
Yi GE, Robin AH, Yang K, Park JI, Hwang BH, Nou IS (2016) Exogenous methyl jasmonate and salicylic acid induce subspecies-specific patterns of glucosinolate accumulation and gene expression in Brassica oleracea L. Molecules 21:1417
Zang YX, Ge JL, Huang LH, Gao F, Lv XS, Zheng WW, Hong SB, Zhu ZJ (2015) Leaf and root glucosinolate profiles of Chinese cabbage (Brassica rapa ssp. pekinensis) as a systemic response to methyl jasmonate and salicylic acid elicitation. J Zhejiang Univ Sci B 16(8):696–708
Shahzad R, Waqas M, Khan AL, Hamayun M, Kang SM, Lee IJ (2015) Foliar application of methyl jasmonate induced physio-hormonal changes in Pisum sativum under diverse temperature regimes. Plant Physiol Biochem 96:406–416
Kumar V, Rajauria G, Sahai V, Bisaria VS (2012) Culture filtrate of root endophytic fungus Piriformospora indica promotes the growth and lignan production of Linum album hairy root cultures. Process Biochem 47(6):901–907
Kumar P, Chaturvedi R, Sundar D, Bisaria VS (2016) Piriformospora indica enhances the production of pentacyclic triterpenoids in Lantana camara L. suspension cultures. Plant Cell Tissue Organ Cult 125(1):23–29
Saxena P, Ahlawat A, Ali A, Abdin MZ (2016) Gene expression analysis of the withanolide biosynthetic pathway in hairy root cultures of Withania somnifera elicited with methyl jasmonate and the fungus Piriformospora indica. Symbiosis 71(2):143–154
Sanz MK, Hernandez XE, Tonn CE, Guerreiro E (2000) Enhancement of tessaric acid production in Tessaria absinthioides cell suspension cultures. Plant Cell Rep 19(8):821–824
Kushwaha RK, Singh S, Pandey SS, Kalra A, Vivek Babu CS (2019) Fungal endophytes attune withanolide biosynthesis in Withania somnifera, prime to enhanced withanolide A content in leaves and roots. World J Microbiol Biotechnol 35(2):20. https://doi.org/10.1007/s11274-019-2593-1
Singh G, Tiwari M, Singh SP, Singh S, Trivedi PK, Misra P (2016) Silencing of sterol glycosyltransferases modulates the withanolide biosynthesis and leads to compromised basal immunity of Withania somnifera. Sci Rep 6:25562. https://doi.org/10.1038/srep25562
Srivastava S, Sanchita, Singh R, Srivastava G, Sharma A (2018) Comparative study of withanolide biosynthesis-related miRNAs in root and leaf tissues of Withania somnifera. Appl Biochem Biotechnol 185(4):1145–1159
Sivanandhan G, Arunachalam C, Selvaraj N, Sulaiman AA, Lim YP, Ganapathi A (2015) Expression of important pathway genes involved in withanolides biosynthesis in hairy root culture of Withania somnifera upon treatment with Gracilaria edulis and Sargassum wightii. Plant Physiol Biochem 91:61–64
Gupta P, Agarwal AV, Akhtar N, Sangwan RS, Singh SP, Trivedi PK (2012) Cloning and characterization of 2-C-methyl-d-erythritol-4-phosphate pathway genes for isoprenoid biosynthesis from Indian ginseng, Withania somnifera. Protoplasma 250(1):285–295
Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB (1997) Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol 115(1):137–149
Leon J, Lawton MA, Raskin I (1995) Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiol 108(4):1673–1678
Hao W, Guo H, Zhang J, Hu G, Yao Y, Dong J (2014) Hydrogen peroxide is involved in salicylic acid-elicited rosmarinic acid production in Salvia miltiorrhiza cell cultures. Sci World J 2014:843764
Kawano T, Muto S (2000) Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco cell suspension culture. J Exp Bot 51(135):685–693
Garg N, Manchanda G (2009) ROS generation in plants: Boon or bane? Plant Biosyst 143(1):81–96
Rath I, Barz W (2000) The role of lipid peroxidation in aluminium toxicity in soybean cell suspension cultures. Z Naturforsch B 55(11–12):957–964
Helaly MN, El-Hosieny HAR, El-Sarkassy NM, Fuller MP (2017) Growth, lipid peroxidation, organic solutes, and anti-oxidative enzyme content in drought-stressed date palm embryogenic callus suspension induced by polyethylene glycol. In Vitro Cell Dev Biol Plant 53(2):133–141
Acknowledgements
This work was supported by grant NWP BSC0117 (XII 5 Year Plan Network Project) from the Council of Scientific and Industrial Research (CSIR), India. Sincere thanks to Director, CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow, India for his encouragement. R. K. Kushwaha greatly acknowledges, ICMR for financial assistance in the form of fellowship and contingency grant for research activity. SSP acknowledges CSIR, India for financial assistance in the form of Senior Research Associateship (SRA). C. S. Vivek Babu and R. K. Kushwaha greatly acknowledges Dr. Dinesh A. Nagegowda for providing withanolides standards and primers and Dr. D. K. Venkata Rao for sharing his lab facilities.
Author information
Authors and Affiliations
Contributions
RKK performed all the experiments and CSV analyzed and prepared the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
CIMAP Publication Communication Number: CIMAP/PUB/2018/39.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kushwaha, R.K., Singh, S., Pandey, S.S. et al. Innate endophytic fungus, Aspergillus terreus as biotic elicitor of withanolide A in root cell suspension cultures of Withania somnifera. Mol Biol Rep 46, 1895–1908 (2019). https://doi.org/10.1007/s11033-019-04641-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04641-w