Abstract
Endophytes have been reported from all plant species from different parts of tissue including root, stem and leaves. Here we report, three fungal endophytes, Aspergillus terreus strain 2aWF (2aWF), Penicillium oxalicum strain 5aWF (5aWF), and Sarocladium kiliense strain 10aWF (10aWF) from Withania somnifera, which could enhance withanolides content in leaf and root. Upon treatment with the above endophytes to 4 weeks old plants in field conditions, W. somnifera elicited withanolide A content (97 to 100%) in leaves without considerable changes in withaferin A content. Furthermore, withanolide A content in roots of 5aWF and 10aWF endophyte treated W. somnifera plants increased up to 52% and 65% respectively. Incidentally, expression profile of withanolide and sterol biosynthetic pathway genes HMGR, DXR, FPPS, SQS, SQE, CAS, SMT1, STE1 and CYP710A1 were significantly upregulated in 2aWF, 5aWF and 10aWF fungal endophyte treated plants. Besides, modulation of withanolide biosynthetic pathway genes, fungal endophytes also induce a host resistant related gene, NPR1 resulting in 2, 4 and 16 fold expression levels in 2aWF, 10aWF and 5aWF endophyte treatments respectively, compared to control plants. Overall, our results illustrate that application of native-fungal endophytes 2aWF (96.60%), 5aWF (95%) and 10aWF (147%) enhances plant biomass in addition to withanolide content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are embedded with numerous microorganisms both in aerial and underground parts, which could be beneficial or harmful to plant health. Some fungi or bacteria that reside in plant tissues at inter or intracellular spaces without causing any disease symptoms are called endophytes (Schulz and Boyle 2005). Endophytes maintain good harmony inside the host tissues and help in plant growth, biomass yield (Oteino et al. 2015) and increase resistance of the plant to tolerate biotic and abiotic stresses (Rodriguez et al. 2008; Lata et al. 2018). Besides, endophytes bring about increase in plant biomass owing to the secretion of phytohormones like gibberellins (GAs) or indole-3-acetic acid (IAA) (Khan et al. 2012; Hoffman et al. 2013) or via supplying nutrients or modulating plant growth regulators within the plant cell (Oteino et al. 2015; Hardoim et al. 2008).Traditionally only medicinal plants have been considered as main sources of natural pharmaceutical compounds. However, recently endophytes have been recognized as an alternative source of plant secondary metabolites or similar compounds such as azadirachtin, podophyllotoxin, deoxypodophyllotoxin, paclitaxel, vinblastine, vincristine, camptothecin and analogue for the host plants (Kumar et al. 2013; Kusari et al. 2012). Thus, endophytes are considered as “A Treasure House of Bioactive Compounds of Medicinal Importance” (Gouda et al. 2016). Incidentally, fungal endophytes of W. somnifera have been recognized a source of antibacterial and antifungal against plant pathogen and immunomodulatory compounds (Kumar et al. 2013; Qadri et al. 2013; Salini et al. 2014).
Withania somnifera (L.) has traditionally been used for antitumor, cardio protective, anxiolytic-antidepressant, adaptogen, immunomodulatory, Alzheimer’s disease, neurodegenerative disorders and also used in radiation therapy application (Hosny and Farouk 2012; Raghavan and Shah 2015). It contains a class of steroidal lactones called as “Withanolides” these include the withanolide A, withaferin A, withanolide D, withanone and as many as 43 molecules (Trivedi et al. 2016), which are pharmaceutically active compounds and have been used as novel medicines (Mirjalili et al. 2009). Owing to global demand of withanolides, various attempts have been made to increase the production of withanolides both in vivo and in vitro conditions. Under in- vitro conditions, tissue culture techniques have been reported demonstrating enhanced production of withanolides through hairy root culture and cell suspension culture under controlled conditions (Sivanandhan et al. 2013, 2014). Moreover, lack of complete withanolide biosynthesis pathway makes an impediment to transgenic approach. To some extent, the transcriptome analysis of W. somnifera leaf and root has opened a window for putative pathways for withanolides biosynthesis (Fig. 1) (Dhar et al. 2015). In this pathway, phytosterol is a prominent precursor for all types of withanolides and their derivatives; the silencing or over expression of any methylerythritol 4-phosphate (MEP)/mevalonate(MVA)/intermediate pathway genes directly affects phytosterols constituent, and eventually has an impact on withanolides content in leaves (Singh et al. 2014). The overexpression of WsCAS or GrDXS gene in transgenic W.somnifera exhibits higher withanolide content in leaves as compared to control plants (Jadaun et al. 2016; Mishra et al. 2016). Also, the overexpression of downstream pathway gene squalene synthase (SQS) in W. somnifera leads to enhanced withanolide biosynthesis in roots, stem and leaves (Grover et al. 2013; Patel et al. 2015). On the other hand, the transient suppression of squalene synthase gene in W. somnifera resulted in decreased phytosterol and withanolide content in leaves and increased plant susceptibility against the pathogens (Singh et al. 2015). Withanolides can be converted into their numerous derivatives through glycosylation, methylation, hydroxylation and others modifications (Singh et al. 2016). The metabolic engineering of withanolide synthesis pathway and phytosterol concentration, affects withnaolide content in the W. somnifera leaves (Saema et al. 2015).For instance silencing of sterol glycosyltransferases (SGTs) in W. somnifera increases the phytosterol and withanolides in leaves, in return compromising with enhanced plant growth and synthesis of sterol derivatives (Singh et al. 2016; Saema et al. 2015). However, over expression of WsSGT in tobacco plants, increases both phytosterol and glycosylated phytosterol in leaves, and consequently increases the immunity against biotic and abiotic stress (Pandey et al. 2014). Beside genetic manipulation, the treatment of W. somnifera with phytohormones like salicylic acid and methyl jasmonate also enhances withanolide content in the leaves (Jadaun et al. 2016; Mishra et al. 2016; Singh et al. 2015).
Schematic representation of withanolides biosynthetic pathway in W. somnifera. Solid arrows indicate single-step reactions, dashed arrows indicate several steps, dotted arrows represent unidentified steps, and the double arrow show the exchange between cytosolic and plastid compartments AACT acetyl-CoA thiolase, BAS β-Amyrin synthase, CAS Cycloartenol Synthase, CDP-ME 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol, CDP-MEK 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase, CDP-MEP 2-phospho-4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol, CDP-MES 2-C-methyl-d-erythritol4-phosphate cytidylyl transferase, CECI Cycloeucalenol cycloisomerase, CYP710A1 C-22 Sterol desaturase, DMAPP dimethylallyl diphosphate, DOXP 1-deoxy-d-xylulose 5-phosphate, DWF1 Delta-24 sterol reductase, DWF5 Sterol delta-7 reductase, DXR 1-deoxy-d-xylulose 5-phosphate reductoisomerase, DXS 1-deoxy-d-xylulose-5-phosphate synthase, FK Delta 14-sterol reductase, FPP Farnesyl diphosphate, FPPS FPP synthase, GPP Geranylgeranyl diphosphate, GPPS Geranyl diphosphate Synthase, HDR 4-hydroxy-3-methylbut-2-enyldiphosphate reductase, HDS 4-hydroxy-3-methylbut-2-enyldiphosphate synthase, HMBPP 4-hydroxy-3-methylbut-2-enyl diphosphate reductase, HMG-CoA 3-hydroxy-3-methylglutaryl-CoA, HMGR HMG-CoA reductase, HMGS HMG-CoA synthase, HYDI C-7,8 sterol isomerase, IPP isopentenyl diphosphate, IPPI isopentenyl diphosphate isomerase, LAS Lanosterol synthase, LS Lupeol synthase, MDD mevalonate diphosphosphate decarboxylase, MECP 2-C-methyl-d-erythritol 2,4-cyclodiphosphate, MECPS 2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase, MEP 2-C-methyl-d-erythritol 4-phosphate, MK Mevalonate kinase, MTs Methyl transferase, MVA Mevalonate, MVA-5-P 5-phosphomevalonate, MVAPP mevalonate pyrophosphate, ODM Obtusifoliol 14-demethylase, 3-PGAL glyceraldehyde 3-phosphate, PMK phosphomevalonate kinase, SGT Sterol glycosyl transferase, SMO1 Sterol-4α-methyl oxidase 1, SMO2 Sterol-4α-methyl oxidase 2, SMT1 Sterol methyltransferase 1, SQ Squalene, SQE Squalene monooxygenase/epoxidase, SQS Squalene Synthase, STE1 C-5 sterol desaturase
Major challenge of the twenty-first century is environmental friendly and sustainable agriculture practices (The National Academies Press 2010). ). Endophytes generally modulate plant growth and development (Hardoim et al. 2008). Burkholderia phytofirmans strain PsJN, reported as endophyte and plant growth promoting rhizobacteria (PGPR) enhances chilling tolerance of grapevine plants (Ait Barka et al. 2006). However, mechanism of metabolite secretion by endophyte and its systemic colonization during diseases management at plant-endophyte interface remains elusive (Pandey et al. 2016a). Endophytes could also be used as inoculum to produce secondary metabolites, extracellular enzymes and plant hormone like substances that are active biochemical components in the positive interactions with plants (Khan et al. 2012; Hoffman et al. 2013; Gouda et al. 2016).
Hitherto, limited attempts have been made on endophyte-mediated enhancement for in-planta withanolide biosynthesis. However, library of native, functional and crop specific endophytic strains needs to be investigated for site-specific and region specific deed to consider them as economical and viable approaches for medicinal plants cultivation with enhanced yields of therapeutically important metabolites. Considering the above aspects, here we report functional role of three inherent, fungal endophytes Aspergillus terreus strain 2aWF(MH196570), Penicillium oxalicum strain 5aWF(MH196571) and Sarocladium kiliense strain 10aWF(MF800957) which could enhance plant biomass, withanolide A content in leaves and their possible interaction during withanolide biosynthesis in host plant.
Materials and methods
Endophyte source
Leaf and root sample of Poshita variety of W. somnifera Dunal (L) were collected from the field of Central Institute of Medicinal and Aromatic Plants, Lucknow (INDIA). Leaves and roots were washed in running tap water; further, surface of plant parts was sterilized with 50% sodium hypochlorite solution for 5 min followed by four times washing with autoclaved distilled water. This was followed by sterilization with 70% ethanol, 30 s for leaf and 90 s for root and further washed five times with autoclaved distilled water. Surface sterilization efficiency was confirmed by spreading 100 µL of finally washed water onto PDA plates [potato dextrose agar; containing potato (200 g/L), dextrose (20 g/L), and agar (15 g/L), pH 6.0] and kept at 28 °C for one week. Following surface sterilization, leaves/roots were chopped into pieces and aseptically transferred to PDA medium plate supplemented with streptomycin (100 µg/mL) (to inhibit endophytic bacterial growth). These plates were incubated at 28 °C and colonies emerging from sides of leaves/root were sub-cultured on fresh PDA plates, glycerol stocks were made, and stored at − 80 °C for future use.
Evaluation of fungal endophytes of W. somnifera under field conditions
W. somnifera Dunal (var. Poshita) seeds were obtained from the National Gene Bank for Medicinal and Aromatic Plants, CSIR-CIMAP, Lucknow and field experiments with fungal endophytes were conducted at CSIR-CIMAP, Research Center located at Bangalore, Karnataka, India. W. somnifera Dunal (var. Poshita) nurseries were developed in autoclaved soil filled polythene bags under natural photoperiod and temperature conditions in poly house. 4 weeks old plantlets were used for transplantation in research farm of CSIR-CIMAP, Research Centre, Bangalore. The dimension of each treatment bed was 3 m x3m (length x width) comprising 4 plants in 4 rows. Plant to plant distance was 50 cm, and a distance of 50 cm from edge to plant was maintained. Spore suspension (1 × 108 spore/mL−1) of individual fungal endophyte prepared in phosphate buffered saline (PBS) (NaCl 8 g L−1, KCl 0.2 g L−1, Na2HPO4 1.44 g L−1, K2HPO4 0.24 g L−1; pH 7.40) was used to inoculate W. somnifera plants under field conditions. W. somnifera plants treated with only PBS solution, served as untreated control plants. Based on performance of all 11 endophytes, we have selected three fungal endophytes Aspergillus terreus MH196570 (2aWF), Penicillium oxalicum MH196571 (5aWF) and Sarocladium kiliense MF800957 (10aWF) for further independent field evaluations. The treatments were imposed in experimental plots of dimension (3 m × 3 m) with 4 replications in red-sandy and loamy soil with pH 6.2. The 4 week old nursery of W. somnifera plants were transplanted at a spacing of 45 cm × 45 cm (row to plant 60 cm and plant to plant 45 cm) at a depth of 10–12 cm. There were four rows in each plot. Each plot was separated with 50 cm guard ridge to lessen the effect of adjoining plots. Each bed contained 16 plants (12 peripheral and 4 central plants). Central plants were considered as non-peripheral plants from each bed for taking growth observations. Each treatment bed having 4 rows with 16 plants were treated with 10 mL endophytic culture per plant. Plant growth parameters including plant height (measured from soil surface to tip of the plant) and number of branches were recorded at the time interval of 30 days after post inoculation till harvesting. Harvesting was carried out at 150 days after transplanting. Plants were manually uprooted with proper care without damaging the root system. Fresh and dry root and shoot weights were recorded and subjected for withanolide analysis. The leaf samples from above treatments were subjected for real-time qPCR analysis to understand the involvement of selected endophytes in withanolide biosynthesis.
Analysis of plant height, biomass, seed yield and photosynthetic pigments
Plants heights were measured from each treatment after 150 days of endophytic fungi inoculation. After harvesting fresh shoot and root, total seed yield was recorded from individual treatment plots. In order to measure their dry weight, shoots and roots of respective treatments were dried in shade at room temperature. Photosynthetic pigments were measured in W. somnifera under field conditions after 150 days of endophyte inoculation. The uniform area of endophyte treated W. somnifera leaves was taken with 6 mm diameter cork borer, followed by incubation in methanol for overnight at 4 °C. Absorbance was taken at 666 nm, 653 nm and 470 nm for three biological and three technical replicates. Chlorophyll and carotenoids were estimated (Lichtenthaler and Wellburn 1971). Chlorophyll a = (15.65 A666−7.34 A653), Chlorophyll b = (27.05 A653−11.21 A666) and Carotenoids = [1000 A470−(2.86xChl b−129.2xChl a)].
HPLC analysis of withanolides from root and leaves of W. somnifera
Withanolides analyses were carried out from both endophyte treated and control W. somnifera plants after 150 days post inoculation (dpi). Leaf and root withanolides were extracted using ethyl acetate overnight at room temperature. Ethyl acetate extract was concentrated in vacuum drier (V-AL mode) for 20 min at 30 °C. Extracted samples were analyzed by high performance liquid chromatography (HPLC) [Shimadzu 10AVP; Shimadzu, Kyoto, Japan] containing Phenomenex Luna C-18 (250 mm × 4.6 mm, 5 µ) column]. 20 µL methanol reconstituted samples were manually injected into HPLC; mobile phase comprised 0.1 M of ammonium acetate (CH3COONH4) dissolved in 18 MΩ MQ water (solvent A) and acetonitrile (solvent B). Gradient programming was carried out initially with solvent system at 95% of A, then shifted to 55% A and 20% A at 18.0 and 25.0 min respectively. A total of 20% A was maintained for the next 10 min, increased to 55% A at 35 min, followed by 95% at 40 min which was maintained till the run time reached 45 min. The flow rate was set at 1.5 mL/min with UV detector wavelength of 227 nm. Peaks of withaferin A and withanolide A were identified by comparing retention time of reference standards.
Real-time-quantitative PCR (RT-qPCR) analysis of endophyte treated W. somnifera plants
Total RNA was isolated from leaves of endophyte treated W. somnifera plants (150 dpi) using RNA isolation kit (Sigma-Aldrich) and gDNA contamination removed by treatment with RNase-free DNase I (Ambion). Total RNA quantity and quality was analyzed using spectrophotometer (Eppendorfbiospectrometer) at OD 260/280. First-strand cDNA was synthesized from 2 µg of total RNA using the ReadyScript® cDNA Synthesis Kit (Sigma-Aldrich) according to the manufacturer’s instructions. Primers used for the real-time-quantitative PCR (RT-qPCR) were followed as designed by Singh et al. 2015 (Suppl. Table S3). RT-qPCR was carried out using SYBR® Green JumpStartTMTaqReadyMix™ (Sigma-Aldrich).PCR reactions were optimized as 10 min at 95 °C, followed by 40 cycles of denaturation at 95 °C for 15 s each and annealing/extension at 60 °C for 1 min each. Fluorescent signal intensities were recorded and analyzed by StepOnePlus™ Real-Time PCR System (Applied Biosystems). Specificity of RT-qPCR was evaluated by subjecting all amplicons to a melt-curve analysis using dissociation method (Applied Biosystems). The threshold cycle (Ct) for each gene was normalized against the Ct for 18S gene, which was used as the constitutive reference transcript. Mean Ct values were calculated from technical triplicates, and relative levels of the transcripts of different withanolides biosynthetic genes in endophyte-inoculated plants were compared with uninoculated control plant (calibrator) using the relative quantification 2−ΔΔCt method (Livak and Schmittgen 2001).
Determination of colonization of endophytes in W. somnifera plants
Colonization of three endophytes 2aWF, 5aWF and 10aWF were examined in leaf and root tissue after 60 days post inoculation in W. somnifera plants in pot experiments under controlled conditions. Endophytes were selected for colonization analysis based on their in planta enhancement ability of withanolides under field conditions. Surface sterilized leaf and root tissues were thoroughly ground in autoclaved distilled water under aseptic conditions using autoclaved mortar-pestle, and serial diluted samples were inoculated on Rose Bengal Chloramphenicol Agar with three replicates each. The plates were incubated for 3–5 days at 28 °C. After incubation, total numbers of viable colonies were counted and colony characteristic were recorded. The re-isolated endophytic fungal colonies, thus obtained were confirmed based on their colony characteristic, microscopic observations of mycelium and conidial structures, followed by internal transcribed spacer (ITS) sequencing analysis.
Fungal genomic DNA isolation and amplification of (ITS) fragments
Fungal genomic DNA was isolated according to protocol Möller et al. (1992) with slight modifications. Pure culture of individual fungi was grown on PDA plate at 28 °C for 4–5 days and mycelia were removed from agar surface and ground in liquid nitrogen using mortar and pestle. 500 µL TES extraction buffer (100 mMTris, pH 8.0, 10 mM EDTA, 2% SDS) was added to ground mycelia and incubated for 60 min at 60 °C. Then 140 µL of 5 M NaCl (final conc. 1.4 M NaCl) along with 1/10 volume (65 µL) of 10% cetyltrimethyl ammonium bromide (CTAB) was added and incubated for 10 min at 65 °C. 700 µL of chloroform: isoamylalcohol (24:1) was added and gently mixed, followed by incubation at 0 °C for 30 min. The samples were further centrifuged at 12,000 rpm at 4 °C for 10 min, 500 µL isopropanol added to the supernatant, and kept on ice for 15–30 min. The samples were again centrifuged at 12,000 rpm at 4 °C for 10 min followed by washing twice with cold 70% ethanol. Pellet obtained was air dried at room temperature and dissolved with 50 µL DNase free water. Internal transcribed spacer ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS 4 (5′-TCCTCCGCTTATTGATATGC-3′) were used as primers for PCR amplifications. PCR reaction mixture (25 µL) comprised 2.51 µL PCR buffer, 1 µL each of 10 µM ITS1 and ITS4, 0.5 µL mixed 10 mMdNTPs, 0.2 µL of 5 U/µL of Taq DNA polymerase and 18.8 µL autoclaved MilliQ water. The PCR thermo cycling conditions were maintained as first denaturation step at 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 7 min (EppendorfMastercycler nexus GX2). PCR products were separated on 0.8% agarose gel and amplicons excised from agarose gel using Nucleo-pore PCR Clean-up Gel Extraction kit (Genetix Biotech Asia Pvt. Ltd., India) according to manufacturer’s instructions prior to sequencing. Sequencing of purified ITS amplicons was carried out at Amnion Biosciences Pvt. Ltd., Bengaluru, India. ITS sequences were analyzed using BLAST, version 2.5 (National Center for Biotechnology Information databases) and identical neighbor sequence (E value = 0) were downloaded. The ITS sequence of 2aWF, 5aWF and 10aWF isolates were aligned with reference sequences using ClustalW alignment tool (MEGA6). A phylogenetic tree was prepared using the 1000 bootstrapped neighbor-joining tree method (MEGA6).
Phosphatase, pectinase and cellulase activity of fungal endophytes
Qualitative assay of phosphate solublization activity of endophytic fungi, 2aWF, 5aWF and 10aWF were carried out on Pikovskaya’s agar medium (Pikovskaya 1948) supplemented with bromophenol blue (0.025 g/L). Fungal endophytic culture from PDA plates (6 mm diameter agar plug) were inoculated on Pikovskaya’s agar plate and kept at 28 °C for 1 week. Phosphatase activity of fungi was inferred by transparent zone around mycelia. Pectin degrading ability of endophytic fungi was also qualitatively analyzed on mineral salt agar medium, containing 10% citrus pectin as carbon source. Pectin utilization was detected by flooding the culture plates with freshly prepared iodine–potassium iodide solution (iodine-1.0 g, potassium iodide-5.0 g in 330 mL distilled water), light yellowish zone was formed due to pectin degradation (Hankin et al. 1971). Cellulose hydrolysis activity was qualitatively tested on Czapek-Dox medium agar plates containing 1% carboxy- methyl cellulose as carbon source (Teather and Wood 1982). 1 week old fungus cultures (6 mm diameter agar plug) were transferred on Czapek-Dox medium plate and kept at 28 °C for 3 days. After 3 days plates are incubated for 18 h at 50 °C which is the optimum temperature for cellulase activity. Thereafter, 5 mL of 1% Congo-Red staining solution was added to the plates and kept on shaker at 50 rev/min for 15 min. The Congo-Red staining solution was then discarded, 10 mL of 1 N NaOH was added to the plates and shaken again at 50 rev/min for 15 min. Finally, 1 N NaOH was discarded and the stained plates were analyzed for detection of yellow zones around the fungal colonies.
Statistical analysis
The number of biological replicates considered for each experiment is represented in the figure legends. Calculations of mean, standard deviations or standard errors for each data were used for statistical evaluation using social science statistic online software. One way ANOVA analysis with Dunnett as a post-test was calculated by using Graph Pad prism, version 5.01 (GraphPad Software, San Diego, CA). The statistical significance of differences between control and treated samples were represented at different P value.
Results
Endophyte treatments enhanced plant height, biomass and seed yield of W. somnifera
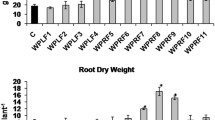
Total 11 endophytic fungi isolated from leaves and roots of W. somnifera Dunal (var. Poshita) were evaluated for their ability to enhance withanolides in planta under field conditions. Post endophytic treatments W. somnifera plants after 30 days, showed no significant difference in plant height in all treatments, whereas with increasing time, upto 150 days, difference was observed. Fungal endophytes effect on plant height, biomass and withanolide content of W.somnifera at 150 days post transplantation were observed (Fig. 2a–e). At 150 days post inoculation (dpi), the plant height was significantly increased (15–57%) in most of endophyte treatments except 6WF, 8WF and 9WF as compared with control (Fig. 2a). Fresh shoot weight was significantly increased in all treatment (41–153%) over uninoculated W.somnifera plants while weight of fresh root was significantly higher (58–169%) in 2aWF, 5aWF, 6WF, 7WF, 9WF, 10aWF, 11WF and 12aWF comparedto control plants (Fig. 2b). Dry shoot weight also showed significant enhancement in most endophyte treated plants, except in 3WF (Fig. 2c). Similarly, endophyte treated plants showed significant increase in dry root weight except 3WF and 8WF as compared to control plants (Fig. 2c). Overall 1WF, 2aWF, 5aWF, 7WF, 10aWF, 11WF and 12aWF endophytic fungi were performed substantially well, in increasing plant height and biomass of W. somnifera under field conditions. On the basis of overall performance, endophytes 2aWF, 5aWF and 10aWF were used for independent field evaluations. Further experiment with the 2aWF, 5aWF and 10aWF were carried out similar to the previous field dimension 3 m × 3 m during same season. Plant height was 40–45% higher in 2aWF and 5aWF treatments, while there was no significant difference observed in 10aWF treatment as compared to control in independent beds (Fig. 3a). Fresh shoot weights in 2aWF, 5aWF and 10aWF endophyte treated plants increased by 66 to 127%; similarly fresh root weight increased from 98 to 183% as compared with untreated plants (Fig. 3b). After shade drying, indeed the dry shoot weight followed the same trend and it was 90–300% higher in endophyte treated plants as compared to control plants. Dry root weight was 97 to 178% higher in endophyte treated than control plants (Fig. 3c). Flowering started after 60 days, mature berry fruits were collected after harvesting and kept for drying in shed at room temperature. Total seed yield was significantly higher (82–120%) in 2aWF, 5aWF and 10aWF endophytes treatment as compared to untreated plants (Fig. 3d). After 150 days of 2aWF, 5aWF and 10aWF endophyte treatment, W. somnifera leaf samples were collected for photosynthetic pigment estimation. Photosynthesis mainly depends on chlorophyll and carotenoid pigments distribution within leaf and is a reflection of plant health parameters. Here, we measured the photosynthetic pigment per unit area in 2aWF, 5aWF and 10aWF endophyte treated and untreated plants. There was no significant change observed in chlorophyll a, chlorophyll b, and carotenoid pigment in endophyte treated plant as compared to control plants (Fig. 4a–c).
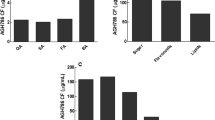
Performance of all isolated endophytic fungi under field conditions on plant biomass and withanolides content in root and leaf tissue at 150 days post inoculation (dpi) (a) height of plant prior to harvesting at 150 dpi (b) fresh shoot and root weight harvesting at 150 dpi (c) dry shoot and root weight after shed dry till to constant weight measurement (d) relative percentage of WFA and WLA content in leaves of treated and untreated plants (e) relative percentage of WLA content in roots of untreated and treated plant. Vertical bars indicate S.D. of mean (n = 3). *Represent significant at P < 0.1, **represent significant at p < 0.05, ***represent significant at p < 0.01. WFA Withaferin A, WLA Withanolide A
Performance of endophytes 2aWF, 5aWF and 10aWF on height, biomass and seed yield of plant at 150 days of post inoculation (a) height of plant estimated before harvesting (b) fresh shoot and root weight measured at post harvesting (c) dry shoot and root estimated after shed dry till to constant weight (d) dry seed weight estimated after processing of dry berry fruit from treated and untreated plant. Each treatment was followed with three biological replicated. Error bars represent standard deviation of three replicated. Asterisks indicate a significant difference from the control (Student’s t-test; *p < 0.05, **p < 0.01, ***p < 0.001)
Effect of endophytes 2aWF, 5aWF and 10aWF on photosynthetic pigment in leaves at 150 days post inoculation. a chlorophyll a per unit leaf area in control and treated plant leaves, b chlorophyll b per unit leaf area in control and treated plant leaves, c carotenoid per unit leaf area in control and treated plant leaves. Each treatment was followed with three biological replicated. Error bars represent standard deviation of three replicated
Identification of endophytes, molecular characterization and phylogenetic analysis
Eleven endophytic fungi were isolated from leaves and roots of W. somnifera Dunal (var. Poshita). Three endophytic fungi were isolated from, leaf tissue (1WF, 2aWF and 3WF) and eight endophytic fungi from the root tissue (5aWF, 6WF, 7WF, 8WF, 9WF, 10aWF, 11WF and 12aWF). Based on their in planta withanolides enhancement ability under field conditions, three promising endophytic fungi 2aWF, 5aWF and 10aWF were subjected for ITS sequencing analysis and identified as Aspergillus terreus strain 2aWF (MH196570), Penicillium oxalicum strain 5aWF (MH196571), Sarocladium kiliense strain 10aWF (MF800957) respectively (Suppl. Fig. S1, Suppl. Table S1). Phylogenetic relationship between ITS nucleotide sequences of the endophytes were compared with sequences retrieved from the NCBI database (Fig. 5a–c). Phylogenetic analysis revealed that 2aWF, 5aWF and 10aWF have maximum similarity with Aspergillus terreus strain AKF2 (Accession No. KJ685810.1), Penicillium oxalicum strain DI16-113 (Accession No. LT558935.1) and Sarocladium kiliense isolate TO3 (Accession No. MF682447.1) respectively (Fig. 5a–c).
Phylogenetic relationship between W. somnifera endophytic fungi (2aWF, 5aWF and 10aWF) and representative species based homology of ITS sequences. The reference sequences are indicated by GenBank accession number followed by species name. The evolutionary history was inferred using the Neighbor-Joining method based on the Tamura–Nei model with 1000 bootstrap replications. a Aspergillus terreus strain (2aWF), b Penicillium oxalicum strain (5aWF) and c Sarocladium kiliense strain (10aWF)
Withanolide content in leaves and roots of endophyte treated W. somnifera plants
Withanolides quantification was carried out after 150 days of 2aWF, 5aWF and 10aWF treatments W. somnifera leaf and root tissue using HPLC (Figs. 6, 7). Chromatograms of withaferin A and withanolide A from leaves of control and endophyte treated W. somnifera plants are depicted in Fig. 6c–f. Withaferin A, prominent steroidal lactone in leaves of W. somnifera was not affected by 2aWF, 5aWF and 10aWF endophytes treatments (Fig. 6g). Withanolide A is a signature compound of roots and its content is lesser in leaves of W. somnifera. However, upon treatment of 2aWF, 5aWF and 10aWF endophytes, withanolide A content in leaves of W.somnifera (209.5%, 197.6% and 197.1% respectively) increased drastically as compared to control (Fig. 6g).Similarly, withanolide A content in treated and untreated roots of endophyte treated W. somnifera plants were quantified using HPLC (Fig. 7b–e). Withanolide A content of roots were significantly enhanced in 5aWF (52%) and 10aWF (65%) endophyte treated plants, where as in 2aWF treated W. somnifera plants remained same as control (Fig. 7f).
HPLC chromatogram and withanolides content in leaves of endophyte treated (2aWF, 5aWF and 10aWF) and untreated W. somnifera plants at 150 days post inoculation. a chromatogram of standard withaferin A, b chromatogram of standard withanolide A, c chromatogram of untreated plant leaves, d chromatogram of 2aWFtreated plant leaves, e chromatogram of 5aWFtreated leaves, f chromatogram of 10aWF treated plant leaves, g relative percentage of withaferin A and withanolide A content in leaves of treated and untreated plant. HPLC analysis for each sample was carried out in triplicate. Error bars represent standard deviation. Asterisks indicate a significant difference from the control (Student’s t-test; ***p < 0.001). WLA Withanolide A, WFA Withaferin A
HPLC chromatogram and withanolide A content in roots of endophyte treated (2aWF, 5aWF and 10aWF) and untreated W. somnifera plants at 150 days post inoculation. a Chromatogram of standard withanolide A, b chromatogram of untreated plant roots, c chromatogram of 2aWF treated plant roots, d chromatogram of 5aWF treated plant roots, e chromatogram of 10aWF treated plant roots, f relative percentage of withanolide A content in untreated and treated plant roots. HPLC analysis for each sample was carried out in triplicate. Error bars represent standard deviation. Asterisks indicate a significant difference from the control (Student’s t-test; ***p < 0.001). WLA Withanolide A
Real-time-quantitative PCR (RT-qPCR) analysis of withanolides biosynthetic genes
To understand, the role of 2aWF, 5aWF and 10aWF fungal endophytes during withanolide biosynthesis, expression of pathway genes were quantified through real-time quantitative PCR using leaves of W. somnifera after 150 dpi. In addition, the expression of HMGR, DXR, FPPS, SQS, SQE, CAS, SMT1, STE1 and CYP710A1 was quantified, which all are known to play a key role in withanolide biosynthesis. HMGR and DXR are key genes in MVA and MEP pathway respectively, in production of common precursor isopentenyl diphosphate (IPP). Upon treatment with endophytes 2aWF, 5aWF and 10aWF HMGR expression level increased 1.7–2.2 folds as compared to untreated plants of W. somnifera (Fig. 8a). DXR expression was not affected by endophyte 5aWF, while 2aWF and 10aWF enhanced 1.5–4.5 fold expression level compared to control plants (Fig. 8b). Besides, expression level of FPPS was 2.2–6.8 fold higher in 2aWF, 5aWF and 10aWF endophyte treatments as when compared with untreated plants (Fig. 8c). Downstream pathway genes of withanolide biosynthesis, SQS gene was not affected by treatment with endophyte 10aWF, however, 2aWF and 5aWF increased 1.7 to 2.1 times higher than untreated (Fig. 8d). 2, 3-oxidosqualene is a precursor for various pathways (Fig. 1), which is formed from preceding squalene in the presence of SQE gene. Endophytes 2aWF, 5aWF and 10aWF were profoundly induced the SQE expression level by 5.0 to 6.6 fold higher relative to untreated plant leaves (Fig. 8e). Expression of CAS gene was upregulated 1.2 to 2.1 fold with2aWF, 5aWF and 10aWF endophyte treatment, implicating their role in cycloartenol biosynthesis (Fig. 8f). SMT1 expression level in 2aWF and 5aWF treated plants was enhanced 1.2 and 2.6 fold respectively, however, 4.2 fold higher expression was noticed in 10aWFtreated plants as compared with control (Fig. 8g). Dehydrating enzyme STE1, increased by 1.4 to 2.5 fold in the presence of endophytes 2aWF and 5aWF; similarly in case of 10aWF, 7.4 fold higher expression level was observed than control leaves (Fig. 8h). CYP710A1 expression level was 3.0, 4.4 and 1.4 fold higher in 2aWF, 5aWF and 10aWF respectively, than the control leaves (Fig. 8i). Overall, the withanolides biosynthetic pathways genes were observed to be highly upregulated during 2aWF, 5aWF and 10aWF endophyte treatments as compared to untreated control W. somnifera plants. The nonexpressor of pathogenesis-related gene 1 (NPR1) is key gene involved in regulation of plant disease resistance. It induces pathogenesis-related (PR) genes against broad spectrum pathogen, both in systemic acquired resistance (SAR) and induced systemic resistance (ISR). Besides, the modulation of withanolides biosynthetic pathway genes by 2aWFand 10aWF endophyte, it also enhance the expression of NPR1 gene significantly upto 2–4 fold respectively. However, 5aWF endophyte treated plant showed maximum induction of NPR1 expression level (16.0 fold) as compared with untreated plant (Fig. 9).
Influence of fungal endophytes (2aWF, 5aWF and 10aWF) on withanolide biosynthetic pathway genes. Real-time qPCR analysis showing relative expression levels of upstream and downstream withanolides pathway genes in endophyte treated leaves at 150 days of post inoculation. a HMGR, b DXR, c FPPS, d SQS, e SQE, f CAS, g SMT1, h STE1, i CYP710A1. Expression levels of these genes were normalized to 18S rRNA and are represented in comparison with control. Data are mean ± SD (n = 3 biological replicates) and y axis represents relative quantity (RQ). RQ was calculated using the equation; RQ = 2 −ΔΔCt. Error bars represent standard deviation. Asterisks indicate a significant difference from the control (Student’s t-test; *p < 0.001, **p < 0.0001)
Effect of endophytes (2aWF, 5aWF and 10aWF) on the induction of systemic acquired resistant gene, NPR1. Real-time qPCR analysis showing relative expression levels of induced systemic acquired gene NPR1 in endophyte treated W. somnifera leaves at 150 days of post inoculation. Expression levels of this gene was normalized to 18S rRNA and are represented in comparison with control. Data are mean ± SD (n = 3 biological replicates) and y axis represents relative quantity (RQ). RQ was calculated using the equation; RQ = 2−ΔΔCt. Error bars represent standard deviation. Asterisks indicate a significant difference from the control (Student’s t-test; **p < 0.0001)
Colonization of endophytic fungi within W. somnifera plants
The colonization of endophytic fungi was confirmed by reisolation of inoculated endophytic fungi to W. somnifera which was grown in autoclaved soil in pots under controlled condition. After 60 days of 2aWF, 5aWF and 10aWF endophyte treatment to W. somnifera plants, root and leaf tissue were examined for presence of respective endophyte in plant system. 2aWF endophytic fungi were noticed in both roots and leaves but maximum colonization was observed in root as compared to leaves (Table 1). However, 5aWF and 10aWF fungi were restricted to root tissue. Notably, none of the above endophytes were detected either in leaf or root tissue of control W. somnifera plants (Table 1).
Phosphatase, pectinase and cellulolytic activities of endophytes
We examined the phosphatase, pectinase and cellulolytic activity of 2aWF, 5aWF and 10aWF endophytes, qualitatively on solid media. Prominent activity of cellulase and pectinase were showed by 2aWF and 5aWF while these activities were absent in 10aWF (Suppl. Table S2). On solid Pikovskaya’s media, 5aWF revealed significant phosphate solubilizing activity whereas reduced activity was observed in case of 10aWF, while phosphatase activity was totally absent in 2aWF. (Supp. Table S2).
Discussion
The aim of the present study was to understand the role of functional endophytes from leaf and root tissue of W. somnifera and further to explore their potential interaction in modulating biosynthesis of withanolides and biomass of plant as well. In present study, upon primary screening with all 11 fungal endophytes under both pot and field conditions we have selected Aspergillus terreus strain 2aWF, Penicillium oxalicum strain 5aWF and Sarocladium kiliense strain 10aWF for further field evaluation and experimentation. Endophtyes from W. somnifera have been previously reported from Madhya Pradesh, Kerala, Western Himalayas (Indian) and Pakistan (Tenguria and Khan 2015; Ngamau et al. 2014; Qadri et al. 2013). Endophytes were identified and characterized from different medicinal and non-medicinal plant, while some have been used as bioinoculants (Ngamau et al. 2014).
Nutrient availability to plant is an important factor for increasing plant height, biomass and yield. Earlier studies recognized endophytic microorganisms in nitrogen fixation and phosphate solublization activity, which exert their beneficial effects to the host plants (Khan et al. 2015).The treatment of plant strengthening root endophyte P. indica in Spilanthes calva and Withania somnifera enhanced growth and net primary productivity plants (Rai et al. 2001; Franken 2012).The endophytes of Papaver somnifera and Catharanthus roseus have been recognized as plant growth promoters and inducers of secondary metabolites in planta (Pandey et al. 2016a, b). Here, we have noticed 2aWF and 5aWF were substantially increased the plant height by 40–45% than untreated, control plants, while in 10aWF treatment remained unchanged. However, the fresh biomass was significantly higher (66–127%) in endophyte treatments than untreated, control plant. Interestingly, total seed yield was significantly higher (82–120%) in all endophyte treated plants compared to control. Similar kind of observations were noticed with treatment of endophyte Paecilomyces formosus LHL10, which showed significantly improved the shoot growth, fresh weight as well as chlorophyll content of rice seedlings (Khan et al. 2012) and treatment of P. indica endophyte on barley plant enhanced fresh biomass 1.65 times and 11% grain yield as compared to control (Oteino et al. 2015).
Imperative aim of the present study was to find functional endophytes which could induce valuable secondary metabolite, withanolides in planta besides increasing plant biomass. Withaferin A is one of the major withanolide present in leaves while withanolide A is in limiting content. The application of 2aWF, 5aWF and 10aWF endophytes drastically induced withanolide A (97–100%) content without significant alteration in the levels of withaferin A in endophyte treated leaves of W. somnifera. The applications of nitrogen source or elicitor to the plants have been reported to induce withanolides accumulation in leaves (Pal et al. 2016). Foliar spray of salicylic acid (SA) and methyl jasmonate (MeJA) has been previously reported to induce accumulation of withanolides content in the leaves of W. somnifera (Jadaun et al. 2016; Mishra et al. 2016; Rana et al. 2014). In case of root, withanolide A showed significant increase upto 52–65% in 5aWF and 10aWF treated plants, while with 2aWF, there was no significant change in levels of withanolide A with respect to control. This is the first report on examining the application of native, fungal endophytes to induce withanolide A content in the leaves and root of W. somnifera. Similar observations were reported in Catharanthus roseus and poppy plants that endophytes could induce secondary metabolite accumulation in plant system without causing any disease to host plant (Pandey et al. 2016a, b). Although the exact mechanism is not known, it could be possible that the endophytic fungi act as biotic elicitor or secretes any abiotic component inside the plant, which trigger withanolides accumulation. The increase in levels of withanolide A in leaves or root might be due to differential regulation of withanolide biosynthetic pathway genes by treatment of 2aWF, 5aWF and 10aWF endophytes and with respect to withaferin A levels, it could have reached the threshold levels of withaferin A to withanolide A.
Biosynthesis of phytosterols are highly conserved in all plants and their significance have been understood by suppression or overexpression of particular genes such as CAS (Babiychuk et al. 2008; Mishra et al. 2016), SQE (Han et al. 2009), SQS (Seo et al. 2005; Singh et al. 2015), DXS (Jadaun et al. 2016), HMGR and STE1 (Van Deenen et al. 2011; Holmberg et al. 2003), FPPS (Closa et al. 2010), HMGR and DXR (Singh et al. 2014).The overexpression of MVA upstream pathway WsHMGRgene significantly increased phytosterol such as cycloartenol, sitosterol, stigmasterol and campesterol compared to overexpression WsDXR transgenic tobacco plant (Singh et al. 2014).The overexpression of GrDXS, a MEP pathway gene in both Pelargonium graveolens and W. somnifera caused accumulation of phytosterols in leaves (Jadaun et al. 2016). The co-expression of HMGR along with STE1 in tobacco plant enhanced the carbon flux toward phytosterol accumulation, which could be responsible for withanolides biosynthesis in W. somnifera (Holmberg et al. 2003). Independent application of phytosterol or overexpression of withanolide pathways genes, WsHMGR/WsDXR affected withanolides accumulation in W. somnifera leaves (Singh et al. 2014). However, inoculation with native fungal endophytes (2aWF, 5aWF and 10aWF) have also enhanced the expression of both WsHMGR and WsDXR by 1.7–2.2 fold and 1.5–4.4 fold respectively, in planta and resulted into higher withanolide A accumulation in leaves. FPPS is very important for isoprenoids that are precursors for phytosterol and brassinosteroid and is also crucial for development of plant growth. Earlier studies have proved that the complete loss of FPPS results into growth arrest at early stages of plant growth (Closa et al. 2010). Nonetheless, in fungal endophytes (2aWF, 5aWF and 10aWF) treated plants, FPPS expression was significantly upregulated as compared to control.
Accumulation of phytosterol and triterpenes directly depend on the expression level of SQS in Eleutherococcus senticosus (Seo et al. 2005). The transient suppression of SQS gene in W. somnifera significantly decreased the phytosterol and withanolide content in leaves, as a result reduced expression of defence related genes and plants became more susceptible to pathogens (Singh et al. 2015). The fungal endophyte treatments (2aWF and 5aWF) on W. somnifera, upregulated the expression level of SQS (1.7–2.1 fold), leading to enhanced withanolide A content as compared to control. Similarly, transient suppression of SQE gene directly affected the synthesis of 2,3-oxidosqualene, a multi branch point precursor that is important for phytosterol and ginsenoside in Panax ginseng (Han et al. 2009).Considering the importance of SQE gene expression, in this report we have made an attempt to analyze the expression level of this gene in endophyte treated plants. Interestingly, endophytes (2aWF, 5aWF and 10aWF) have triggered the expression levels of SQE gene in W. somnifera as compared to control plants.
Cycloartenol formed after cyclization of 2, 3-oxidosqualene is mediated by CAS, which is the key precursor in the biosynthesis of phytosterols as well as withanolides through a series of desaturation, hydroxylation, epoxidations, cyclization, chain elongation, and glycosylation steps (Mishra et al. 2016). Transgenic plant of W. somnifera with overexpressed CAS gene displayed substantial enhancement of withanolide accumulation in leaves (Mishra et al. 2016). Here, fungal endophyte treatments enhanced the withanolide A content in leaves but transcript level of CAS was noticeably increased in 5aWF (1.3 fold) and 10aWF (2.1 fold), while in 2aWF treated plants, expression levels remained same as untreated plants. Analysis of downstream pathway gene, STE1 which converts episterol to 5-dehydrosterol, has revealed 1.4, 2.5 and 7.4 fold enhancement in 2aWF, 5aWF and 10aWF treatments, respectively. CYP710A1 is responsible for conversion of sitosterol to stigmasterol and the concentration of stigmasterol, has directly affected withanolides content in W. somnifera leaves (Singh et al. 2014). The expression level of WsCYP isoforms were significantly upregulated by abiotic elicitors like salicylic acid (SA) and methyl jasmonate (MeJA) for improved withanolide accumulation in W.somnifera leaves (Rana et al. 2014). Interestingly, fungal endophyte (2aWF, 5aWF and 10aWF) treatments have triggered the expression levels of CYP710A1 gene, consequently enhanced the withanolide content in W. somnifera as compared to control plants. Fundamentally, plant-endophyte interactions modulate secondary metabolism in Echinacea purpurea, opium poppy and C. roseus (Maggini et al. 2017; Pandey et al. 2016a, b). Hence, we are reporting functional benefits and trade off of fungal endophytes (2aWF, 5aWF and 10aWF) for enhanced accumulation of withanolides through modulation of biosynthetic pathways of steroidal lactones in W. somnifera (Fig. 10).
Schematic representation of differential regulation of withanolides biosynthetic pathway genes by functional fungal endophytes 2aWF, 5aWF and 10aWF. Expression level of different genes of withanolide biosynthesis in presence of 2aWF Aspergillus terreus strain 2aWF, 5aWF Penicillium oxalicum strain 5aWF, 10aWF Sarocladium kiliense strain 10aWF were represented in white to dark color filled circles. Intensity of grey to dark color in circles indicate upregulation of specific gene and white colored circles indicate expression of uninoculated, control plants. Solid arrows indicate single-step reactions, dashed arrows indicate several steps and dotted arrows represent unidentified steps. Enzyme acronyms: HMGR 3-hydroxy-3-methylglutaryl-CoA reductase, DXR 1-deoxy-d-xylulose-5-phosphate reductoisomerase, FPPS Farnesyl diphosphate synthase, CAS Cycloartenol synthase, SQS Squalene synthase, SQE squalene epoxidase, SMT1 Sterol methyltransferase, STE1 C-5 sterol desaturase, CYP710A1 C-22 Sterol desaturase
In addition, endophytes are also known to induce the pathogenesis-related (PR) genes in plants as defense strategy (Dutt et al. 2015). W. somnifera seed priming with endophytic bacteria Bacillus amyloliquefaciens and Pseudomonas fluorescens individually and in combination demonstrated enhanced germination rate, reduced plant mortality (71.40%) and enhance physiological performance under pathogen A. alternata stress (Mishra et al. 2018a,b). Root endophyte P. indica induces resistance against the Arabidopsis powdery mildew Golovinomyces orontii via expression of ISR gene, NPR1 (Molitor and Kogel 2009). It is believed that ISR is induced in Arabidopsis by jasmonic acid (JA) and ethylene (ET) hormone via the expression of NPR1 gene (Leon-Reyes et al. 2009). Similar kind of observation was noticed with, the treatment of endophyte Fusarium oxysporum and Fusarium solani, in tomato plants, where the plants developed resistance against nematode Meloidogyne incognita and foliar pathogen, respectively (Gao et al. 2010; El-Fattah Adnan Dababat and Alexander Sikora 2007). Hence, our observation with 2aWF, 5aWF and 10aWF endophytes treatment to W. somnifera could mimic avirulent pathogens to induce systemic acquired resistance within plants and consequently result ~ 16 fold increased expression level of NPR1 gene.
Colonization of isolated endophytic fungi (2aWF, 5aWF and 10aWF) in W. somnifera revealed that endophytes inhabit within the host plant without causing any disease symptoms. Root endophytic fungi (5aWF and 10aWF) were colonized only in roots, while leaf endophyte 2aWF inhabited in leaf after 60 days of post inoculation.
In this study, it was found that endophyte treatments have not significantly affected photosynthetic pigments in W. somnifera. The applications of native endophytes, in opium poppy, have shown to enhance the photosynthetic pigment and CO2 assimilation (Pandey et al. 2016a).The2aWF and 5aWF endophytes have displayed in vitro cellulose and pectin degradation. In evolutionary changes, many latent pathogenic fungi have modified into symbiont endophytes (Carroll 1988), and acquired the ability to degrade the cell wall component and cementing material pectin in medicinal plant Asclepias sinaica (Fouda et al. 2015). In addition, phosphorus acquisition mediated through endophytic microbes like Colletotrichum tofieldiae (Hiruma et al. 2016) and Serendipita indica in Arabidopsis thaliana for enhanced plant growth under phosphate limiting condition have been well documented. In this study, phosphate solublization activity was noticed in 5aWF followed by 10aW and was totally absent in 2aWF. Thus above findings associate, reasonable plant growth promoting activities of endophytic fungi (2aWF, 5aWF and 10aWF) of W. somnifera.
Conclusions
We conclude, fungal endophytes Aspergillus terreus strain 2aWF (MH196570), Penicillium oxalicum strain 5aWF (MH196571) and Sarocladium kiliense strain 2aWF (MF800957) induce positive stress on secondary metabolite production of W. somnifera without causing any deficiencies on plant health. These fungal endophytes enhance plant height, biomass, and withanolide A content in leaves and roots as well. Further, 2aWF, 5aWF and 10aWF endophytes differentially regulate withanolide biosynthetic pathway genes and eventually lead to substantial enhancement of withanolide A content in leaves and roots. However, the exact molecular mechanism of their interaction or genetic basis of secondary metabolite enhancement still needs to be investigated. Thus, these functional fungal endophytes could be used as sustainable tool to develop effective bioinoculants either independently or in combination with available biofertilzers to enhance the secondary metabolite contents in W. somnifera plants.
References
Ait Barka E, Nowak J, Clement C (2006) Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl Environ Microbiol 72:7246–7252. https://doi.org/10.1128/AEM.01047-06
Babiychuk E, Bouvier-Nave P, Compagnon V, Suzuki M, Muranaka T, Van Montagu M, Kushnir S, Schaller H (2008) Albinism and cell viability in cycloartenol synthase deficient Arabidopsis. Plant Signal Behav 3:978–980
Carroll G (1988) Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecology 69:2–8. https://doi.org/10.2307/1943154
Closa M, Vranova E, Bortolotti C, Bigler L, Arro M, Ferrer A, Gruissem W (2010) The Arabidopsis thaliana FPP synthase isozymes have overlapping and specific functions in isoprenoid biosynthesis, and complete loss of FPP synthase activity causes early developmental arrest. Plant J 63:512–525. https://doi.org/10.1111/j.1365-313X.2010.04253.x
Dhar N, Razdan S, Rana S, Bhat WW, Vishwakarma R, Lattoo SK (2015) A decade of molecular understanding of Withanolide biosynthesis and in vitro studies in Withania somnifera (L.) Dunal: prospects and perspectives for pathway engineering. Front Plant Sci 27:1031. https://doi.org/10.3389/fpls.2015.01031
Dutt M, Barthe G, Irey M, Grosser J (2015) Transgenic citrus expressing an arabidopsis NPR1 gene exhibit enhanced resistance against Huanglongbing (HLB; Citrus Greening). PLoS ONE 11:e0137134. https://doi.org/10.1371/journal.pone.0147657
El-Fattah Adnan Dababat A, Alexander Sikora R (2007) Induced resistance by the mutualistic endophyte, Fusarium oxysporum strain 162, toward Meloidogyne incognita on tomato. J Biol Sci Tech 17:969–975
Fouda AH, Hassan SE, Eid AM, Ewais EE (2015) Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.). Ann Agric Sci 60:95–104
Franken P (2012) The plant strengthening root endophyte Piriformospora indica: potential application and the biology behind. Appl Microbiol Biotechnol 96:1455–1464. https://doi.org/10.1007/s00253-012-4506-1
Gao FK, Dai CC, Liu XZ (2010) Mechanisms of fungal endophytes in plant protection against pathogens. Afr J Microbiol Res 4:1346–1351
Gouda S, Das G, Sen SK, Shin HS, Patra JK (2016) Endophytes: a treasure house of bioactive compounds of medicinal importance. Front Microbiol 7:1538. https://doi.org/10.3389/fmicb.2016.01538
Grover A, Samuel G, Bisaria VS, Sundar D (2013) Enhanced withanolide production by overexpression of squalene synthase in Withania somnifera. J Biosci Bioeng 115:680–685. https://doi.org/10.1016/j.jbiosc.2012.12.011
Han JY, In JG, Kwon YS, Choi YE (2009) Regulation of ginsenoside and phytosterol biosynthesis by RNA interferences of squalene epoxidase gene in Panax ginseng. Phytochemistry 71:36–46. https://doi.org/10.1016/j.phytochem.2009.09.031
Hankin L, Zucker M, Sands DC (1971) Improved solid medium for the detection and enumeration of pectolytic bacteria. Appl Microbiol 22:205–209
Hardoim PR, van Overbeek LS, Elsas JD (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16:463–471. https://doi.org/10.1016/j.tim.2008.07.008
Hiruma K, Gerlach N, Sacristan S, Nakano RT, Hacquard S, Kracher B, Neumann U, Ramirez D, Bucher M, O’Connell RJ, Schulze-Lefert P (2016) Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 165:464–474. https://doi.org/10.1016/j.cell.2016.02.028
Hoffman MT, Gunatilaka MK, Wijeratne K, Gunatilaka L, Arnold AE (2013) Endohyphal bacterium enhances production of indole-3-acetic acid by a foliar fungal endophyte. PLoS ONE 8:e73132. https://doi.org/10.1371/journal.pone.0073132
Holmberg N, Harker M, Wallace AD, Clayton JC, Gibbard CL, Safford R (2003) Co-expression of N-terminal truncated 3-hydroxy-3-methylglutaryl CoA reductase and C24-sterol methyltransferase type 1 in transgenic tobacco enhances carbon flux towards end-product sterols. Plant J 36:12–20
Hosny MH, Farouk HH (2012) Protective effect of Withania somnifera against radiation-induced hepatotoxicity in rats. Ecotoxicol Environ Saf 80:14–19. https://doi.org/10.1016/j.ecoenv.2012.02.003
Jadaun JS, Sangwan NS, Narnoliya LK, Singh N, Bansal S, Mishra S, Sangwan RS (2016) Over-expression of DXS gene enhances terpenoidal secondary metabolite accumulation in rose-scented geranium and Withania somnifera: active involvement of plastid isoprenogenic pathway in their biosynthesis. Physiol Plantarum 59:381–400. https://doi.org/10.1111/ppl.12507
Khan R, Shahzad S, Choudhary MI, Khan SA, Ahmad A (2010) Communities of endophytic fungi in medicinal plant Withania somnifera. Pak J Bot 42:1281–1287
Khan AL, Hamayun M, Kang SM, Kim YH, Jung HY, Lee JH, Lee IJ (2012) Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC Microbiol 12:3. https://doi.org/10.1186/1471-2180-12-3
Khan AR, Ullah I, Waqas M, Shahzad R, Hong SJ, Park GS, Jung BK, Lee IJ, Shin JH (2015) Plant growth-promoting potential of endophytic fungi isolated from Solanum nigrum leaves. World J Microbiol Biotechnol 31:1461–1466. https://doi.org/10.1007/s11274-015-1888-0
Kumar A, Patil D, Rajamohanan PR, Ahmad A (2012) Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS ONE 8:e71805. https://doi.org/10.1371/journal.pone.0071805
Kumar S, Kaushik N, Proksch P (2013) Identification of antifungal principle in the solvent extract of an endophytic fungus Chaetomium globosum from Withania somnifera. Springerplus 2:37. https://doi.org/10.1186/2193-1801-2-37
Kusari S, Verma VC, Lamshoeft M, Spiteller M (2012) An endophytic fungus from Azadirachta indica A. Juss. that produces azadirachtin. World J Microbiol Biotechnol 28:1287–1294. https://doi.org/10.1007/s11274-011-0876-2
Lata R, Chowdhury S, Gond SK, White JF Jr (2018) Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol 66:268–276. https://doi.org/10.1111/lam.12855
Leon-Reyes A, Spoel SH, De Lange ES, Abe H, Kobayashi M, Tsuda S, Millenaar FF, Welschen RA, Ritsema T, Pieterse CM (2009) Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol 149:1797–1809. https://doi.org/10.1104/pp.108.133926
Lichtenthaler HK, Wellburn AR (1971) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆Ct method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Maggini V, De LM, Mengoni A, Gallo ER, Miceli E, Reidel RVB, Biffi S, Pistelli L, Fani R, Firenzuoli F, Bogani P (2017) Plant-endophytes interaction influences the secondary metabolism in Echinacea purpurea (L.) Moench: an in vitro model. Sci Rep 7:16924. https://doi.org/10.1038/s41598-017-17110-w
Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazon J (2009) Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 14:2373–2393. https://doi.org/10.3390/molecules14072373
Mishra S, Bansal S, Mishra B, Sangwan RS, Asha, Jadaun JS, Sangwan NS (2016) RNAi and homologous over-expression based functional approaches reveal triterpenoid synthase gene-cycloartenol synthase is involved in downstream withanolide biosynthesis in Withania somnifera. PLoS ONE 11: e0149691. https://doi.org/10.1371/journal.pone.0149691
Mishra A, Singh SP, Mahfooz S, Singh SP, Bhattacharya A, Mishra N, Nautiyal CS (2018a) Endophyte-mediated modulation of defense-responsive genes and systemic resistance in Withania somnifera (L.) Dunal under Alternaria alternata stress. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02845-17
Mishra A, Singh SP, Mahfooz S, Bhattacharya A, Mishra N, Shirke PA, Nautiyal CS (2018b) Bacterial endophytes modulates the withanolide biosynthetic pathway and physiological performance in Withania somnifera under biotic stress. Microbiol Res 212–213:17–28
Molitor A, Kogel KH (2009) Induced resistance triggered by Piriformospora indica. Plant Signal Behav 4:215–216
Möller EM, Bahnweg G, Sandermann H, Geiger HH (1992) A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res 20:6115–6116
Ngamau CN, Matiru VN, Tani A, Muthuri CW (2014) Potential use of endophytic bacteria as biofertilizer for sustainable banana (Musa spp.) production. Afr J Hort Sci 8:1–11
Oteino N, Lally RD, Kiwanuka S, Lloyd A, Ryan D, Germaine KJ, Dowling DN (2015) Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol 6:745. https://doi.org/10.3389/fmicb.2015.00745
Pal S, Yadav AK, Singh AK, Rastogi S, Gupta MM, Verma RK, Nagegowda DA, Pal A, Shasany AK (2016) Nitrogen treatment enhances sterols and withaferin A through transcriptional activation of jasmonate pathway, WRKY transcription factors, and biosynthesis genes in Withania somnifera (L.) Dunal. Protoplasma 254:389–399 https://doi.org/10.1007/s00709-016-0959-x
Pandey V, Niranjan A, Atri N, Chandrashekhar K, Mishra MK, Trivedi PK, Misra P (2014) WsSGTL1 gene from Withania somnifera, modulates glycosylation profile, antioxidant system and confers biotic and salt stress tolerance in transgenic tobacco. Planta 239:1217–1231. https://doi.org/10.1007/s00425-014-2046-x
Pandey SS, Singh S, Babu CS, Shanker K, Srivastava NK, Kalra A (2016a) Endophytes of opium poppy differentially modulate host plant productivity and genes for the biosynthetic pathway of benzylisoquinoline alkaloids. Planta 243:1097–1114. https://doi.org/10.1007/s00425-016-2467-9
Pandey SS, Singh S, Babu CS, Shanker K, Srivastava NK, Shukla AK, Kalra A (2016b) Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci Rep 6:26583. https://doi.org/10.1038/srep26583
Patel N, Patel P, Kendurkar SV, Thulasiram HV, Khan BM (2015) Overexpression of squalene synthase in Withania somnifera leads to enhanced withanolide biosynthesis. Plant Cell Tissue Organ Cult 122:409–420
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Microbiologiya 17:362–370
Qadri M, Johri S, Shah BA, Khajuria A, Sidiq T, Lattoo SK, Abdin MZ, Riyaz-Ul-Hassan S (2013) Identification and bioactive potential of endophytic fungi isolated from selected plants of the Western Himalayas. Springerplus 2:8. https://doi.org/10.1186/2193-1801-2-8
Raghavan A, Shah ZA (2015) Withania somnifera: a pre-clinical study on neuroregenerative therapy for stroke. Neural Regen Res 10:183–185. https://doi.org/10.4103/1673-5374.152362
Rai M, Acharya D, Singh A, Varma A (2001) Positive growth responses of the medicinal plants Spilanthes calva and Withania somnifera to inoculation by Piriformospora indica in a field trial. Mycorrhiza 11:123–128. https://doi.org/10.1007/s005720100115
Rana S, Bhat WW, Dhar N, Pandith SA, Razdan S, Vishwakarma R, Lattoo SK (2014) Molecular characterization of two A-type P450s, WsCYP98A and WsCYP76A from Withania somnifera (L.) Dunal: expression analysis and withanolide accumulation in response to exogenous elicitations. BMC Biotechnol 14:89. https://doi.org/10.1186/s12896-014-0089-5
Rodriguez RJ, Henson J, Van VE, Hoy M, Wright L, Beckwith F, Kim YO, Redman RS (2008) Stress tolerance in plants via habitat-adapted symbiosis. ISME J 2:404–416. https://doi.org/10.1038/ismej.2007.106
Saema S, ur Rahman L, Niranjan A, Ahmad IZ, Misra P (2015) RNAi-mediated gene silencing of WsSGTL1 in W. somnifera affects growth and glycosylation pattern. Plant Signal Behav 10:e1078064. https://doi.org/10.1080/15592324.2015
Salini TS, Dibu D, Shabanamol S, Sharrel R, Jisha MS (2014) Antimicrobial and immunomodulatory potential of endophytic fungus fusarium solani isolated from Withania somnifera. WJPR 10:879–890
Schulz B, Boyle C (2005) The endophytic continuum. Mycol Res 109:661–686
Seo JW, Jeong JH, Shin CG, Lo SC, Han SS, Yu KW, Harada E, Han JY, Choi YE (2005) Overexpression of squalene synthase in Eleutherococcus senticosus increases phytosterol and triterpene accumulation. Phytochemistry 66:869–877. https://doi.org/10.1016/j.phytochem.2005.02.016
Singh S, Pal S, Shanker K, Chanotiya CS, Gupta MM, Dwivedi UN, Shasany AK (2014) Sterol partitioning by HMGR and DXR for routing intermediates toward withanolide biosynthesis. Physiol Plant 152:617–633. https://doi.org/10.1111/ppl.12213
Singh AK, Dwivedi V, Rai A, Pal S, Reddy SG, Rao DK, Shasany AK, Nagegowda DA (2015) Virus-induced gene silencing of Withania somnifera squalene synthase negatively regulates sterol and defence-related genes resulting in reduced withanolides and biotic stress tolerance. Plant Biotechnol J 13:1287–1299. https://doi.org/10.1111/pbi.12347
Singh G, Tiwari M, Singh SP, Singh S, Trivedi PK, Misra P (2016) Silencing of sterol glycosyltransferases modulates the withanolide biosynthesis and leads to compromised basal immunity of Withania somnifera. Sci Rep 6:25562. https://doi.org/10.1038/srep25562
Sivanandhan G, Dev GK, Jeyaraj M, Rajesh M, Arjunan A, Muthuselvam M, Manickavasagam M, Selvaraj N, Ganapathi A (2013) Increased production of withanolide A, withanone, and withaferin A in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Cult 114:121–129
Sivanandhan G, Selvaraj N, Ganapathi A, Manickavasagam M (2014) Improved production of withanolides in shoot suspension cultureof Withania somnifera (L.) Dunal by seaweed extracts. Plant Cell Tissue Organ Cult 119:221–225
Teather RM, Wood PJ (1982) Use of Congo redpolysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43:777–780
Tenguria RK, Khan NF (2015) Biodiversity of endophytic fungi in Withania Somnifera leaves of panchmarhi biosphere reserve, Madhya Pradesh. JIPBS 2:222–228
Toward Sustainable Agricultural Systems in the 21st Century (2010) The National Academies Press, 500 Fifth Street, NW, Washington, DC, pp 624–6242
Trivedi MK, Panda P, Sethi KK, Jana S (2016) Metabolite profiling of Withania somnifera roots hydroalcoholic extract using LC-MS, GC-MS and NMR spectroscopy. Chem Biodivers. https://doi.org/10.1002/cbdv.201600280
Van Deenen N, Bachmann AL, Schmidt T, Schaller H, Sand J, Prufer D, Schulze GC (2011) Molecular cloning of mevalonate pathway genes from Taraxacum brevicorniculatum and functional characterisation of the key enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Mol Biol Rep 39:4337–4349. https://doi.org/10.1007/s11033-011-1221-4
Acknowledgements
This work was supported by NWP BSC0117 (XII Five Year Plan Network Project) from the Council of Scientific and Industrial Research (CSIR), India. Authors express sincere thanks to the Director, CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow, India for his support and encouragement. RKK acknowledges Indian Council of Medical Research (ICMR), India for financial assistance in the form of fellowship and contingency grant for research activity. CSV and RKK greatly acknowledges Dr. Dinesh A Nagegowda for providing withanolides standards & primers and Dr. D.K. Venkata Rao for sharing his lab facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
CIMAP Publication Communication Number: CIMAP/PUB/2018/4.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kushwaha, R.K., Singh, S., Pandey, S.S. et al. Fungal endophytes attune withanolide biosynthesis in Withania somnifera, prime to enhanced withanolide A content in leaves and roots. World J Microbiol Biotechnol 35, 20 (2019). https://doi.org/10.1007/s11274-019-2593-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2593-1