Abstract
As the second most destructive disease in soybean (Glycine max (L.) Merr), Phytophthora root or stem-rot (PRR) of soybean, which was caused by Phytophthora sojae (P. sojae), could lead to a 10–40% or complete yield loss annually. Researches were needed to perform to identify the P. sojae-resistant germplasm and to better understand the genetic basis of P. sojae resistance in soybean. A total of 225 diverse soybean cultivars and 109 recombinant inbred lines (RILs) derived from crossing ‘DongnongL-28’ (resistant to P. sojae race 1) and ‘Hefeng 25’ (susceptible to P. sojae race 1) were used to evaluate P. sojae race 1 resistance. These 225 soybean cultivars were sequenced using the Specific Locus Amplified Fragment Sequencing (SLAF-seq) approach, and 28,722 single-nucleotide polymorphisms (SNPs) were obtained from the mapping of resistant loci through genome-wide association. Eight quantitative trait nucleotides (QTNs) were associated with resistance to P. sojae race 1. The QTN on Chr.03 was colocalised according to linkage mapping of the RILs. A total of 18 candidate genes were predicted in the flanking region of the colocalised locus on Chr.03. There were stress response-related motifs, such as cis-acting regulatory elements involved in salicylic acid or MeJA responsiveness, in the 1-kb upstream region of sixteen genes. Quantitative RT-PCR showed that the Glyma.03G033700 was induced by P. sojae race 1. Association analysis for Rps loci showed that Glyma.03G033700 and Glyma.03G033800 were the candidates near peak SNP. The identified loci along with the candidate genes could be valuable for studying the molecular mechanisms underlying soybean resistance to P. sojae race 1 and breeding resistant varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytophthora sojae (P. sojae), a soil-borne oomycete pathogen, causes Phytophthora root or stem-rot (PRR) of soybean (Glycine max (L.) Merr) at different growth stages of soybean (Schmitthenner 1985). As the second most destructive disease in soybean, the yield losses caused by PRR are 10–40% or complete yield loss (Li and Ma 1999; Zhang et al. 2013a), and the economic loss is $10–20 billion per year (Tyler 2007). In China, PRR was first found in Heilongjiang Province in 1991 (Shen and Su 1991) and has been continuously found in other soybean production areas. Although new races and virulence biotypes have continuously emerged in China (Huang et al. 2016), P. sojae race 1 is still the most prevalent strain in China, especially in Northeast China.
Soybean resistance to P. sojae is controlled by two mechanisms, partial (or tolerance) and complete resistance (Sugimoto et al. 2012). Presently, the analysis of P. sojae resistance mechanisms heavily relies on linkage analysis derived from population mapping of parents with significant phenotypic differences. Partial resistance to P. sojae is regulated by a complex QTL-mediated resistance network. More than 70 QTLs have been found to lead to partial resistance to P. sojae through linkage analysis. Of these QTLs, 15 have been identified in eight separate populations of the PRR-resistant cultivar ‘Conrad’ (Burnham et al. 2003a, b; Han et al. 2008; Li et al. 2010; Tucker et al. 2010; Wang et al. 2010, 2012a, b; Weng et al. 2007; Wu et al. 2011a, b). These QTLs are mainly distributed on chromosome 2 (Chr.02), Chr.13, Chr.18 and Chr.19. For complete resistance, the first single-dominant Rps gene (Rps1k) was identified in 1957. Currently, nearly 30 Rps genes or alleles on nine different soybean chromosomes (Chr.) have been identified from various resistance sources based on linkage analysis and genetic mapping. Of them, Chr.03 contained the most Rps genes/alleles, including Rps1 (five alleles Rps1a, Rps1b, Rps1c, Rps1d, and Rps1k), Rps7, Rps9, RpsYD25, RpsYD29, RpsUN1, RpsQ, RpsHC18, RpsWY, RpsHN and RpsX (Demirbas et al. 2001; Weng et al. 2001; Gao et al. 2005; Fan et al. 2009; Sugimoto et al. 2011; Sun et al. 2011; Wu et al. 2011a; Lin et al. 2013; Zhang et al. 2013b, 2014; Li et al. 2017; Zhong et al. 2017; Cheng et al. 2017; Niu et al. 2017; Zhong et al. 2019). Additionally, Rps2 and RpsUN2 were located on Chr.16 (Demirbas et al. 2001; Lin et al. 2013). Rps3 (three alleles Rps3a, Rps3b, and Rps3c) and RpsSN10, which was either linked to or allelic with Rps8, were identified on Chr.13 (Demirbas et al. 2001; Sandhu et al. 2005; Gordon et al. 2006; Yu et al. 2010; Burnham et al. 2003a). Rps4, Rps5, Rps6 and RpsJS were found on Chr.18 (Demirbas et al. 2001; Sandhu et al. 2004; Sun et al. 2014a, b). RpsYB30, RpsZS18, RpsSu, Rps10 and Rps11 were located on chromosome 19, 2, 10, 17 and 7, respectively (Zhu et al. 2007; Yao et al. 2010; Wu et al. 2011b; Zhang et al. 2013a; Ping et al. 2016). In recent years, the P. sojae strain has become increasingly complicated due to mutations and outcrossings among different P. sojae strains (Grau et al. 2004). The emergence of new virulent pathotypes of P. sojae often overcomes Rps gene-mediated resistance in commercial cultivars (Grau et al. 2004). Normally, Rps genes provide P. sojae resistance for only 8–15 years. Thus, researchers must constantly seek for and identify some new Rps genes. Recently, linkage analysis has been used in discovering QTLs for the resistance to P. sojae; however, due to the lack of the repeatedly reported loci, most of them were still difficult to directly utilise in molecular-assisted selection (MAS).
Genome-wide association analysis (GWAS), an alternative to linkage analysis, has begun to be used to effectively analyse the genetic basis of resistance to the disease (Sun et al. 2014a; Schneider et al. 2016; Huang et al. 2016). Sun et al. (2014b) detected 4 association signals that led to partial resistance via 214 accessions and 495 SSR markers. Schneider et al. (2016) found 16 QTNs that led to partial resistance based on 1395 Korean samples and 495 SSR markers. High-throughput sequencing technologies have further promoted the application of GWAS for studying P. sojae resistance. Huang et al. (2016) identified 14 association signals from 11 P. sojae isolates through genome-wide association analysis (GWAS) based on 224 germplasm accessions and 1645 single-nucleotide polymorphisms (SNPs) and reverified Rps1b, Rps1c, Rps4, Rps7 and novel resistance genes. This study only found a few novel QTNs that were different from the QTLs identified by the bi-parental mapping strategy due to lower marker density. By early 2019, no studies had been conducted to identify the QTNs underlying P. sojae race 1 resistance based on high-throughput technology.
In the present study, GWAS and linkage mapping of resistance to P. sojae race 1 based on high-resolution SNP maps generated from genome resequencing were performed. The aim of this study was to identify loci associated with P. sojae race 1 resistance and to screen candidate genes located in QTN regions by a qRT-PCR approach and Rps loci-based association analysis.
Materials and methods
Plant materials
Two mapping populations were used in this study. One was a natural population consisting of 225 diverse soybean accessions to represent the genetic diversity inside and outside China. These germplasms covered the regions of China (from 19 degree to 48 degree of north latitude and from 74 degree to 128 degree of east longitude, Table S1). Additionally, 24 soybean cultivars from outside of China were also included. Among the 225 germplasms, soybean cultivars with good and poor resistance to P. sojae race 1 were involved, including ‘DongongL-28’ (a highly resistant cultivar) and ‘Hefeng 25’ (a highly susceptible cultivar). The cross population, consisting of 109 F5:15 RILs, was derived from crossing ‘Hefeng 25’ and ‘DongongL-28’.
Phenotypic evaluation of soybean resistance to P. sojae race 1
P. sojae race 1 resistance of all the tested plant materials was evaluated through the hypocotyl wound infestation technique described by Burnham et al. (2003a). P. sojae race 1 was isolated from infected soybean plants in 2017. A total of 30 seedlings of each tested material were incubated in a mist chamber at 25 + 2 °C, with 100% relative humidity and a 14-h photoperiod at a light intensity of 350 μmol m−2 s−1. All tested materials were evaluated 5 days after inoculation. A line was classified as resistant if 70% of the seedlings were alive and susceptible if 30% of the seedlings were alive. A line was classified as resistant if more than 70% of the seedlings were alive and susceptible if less than 30% of the seedlings were alive. Evaluation of all lines was repeated three times.
SNP genotyping data collection
Leaf genomic DNA of the association panel was isolated via the CTAB method and then reduced-sequencing via the SLAF-seq methodology (Sun et al. 2013). The reference genome Williams 82 (www. phetozome.net) was pre-analysed, and two restriction digestion enzymes, MseI (EC 3.1.21.4) and HaeIII (EC 3.1.21.4), were selected to produce evenly distributed sequencing tags in unique genomic regions of soybean. For each tested sample, more than 50,000 sequencing tags (300–500 bp in length) were obtained, which were further used to define sequencing libraries. The combination of the barcode method and Illumina Genome Analyser II system (Illumina Inc., San Diego, CA, USA) was utilised to obtain 45-bp sequence reads at both ends of the sequencing tags from each sample library. Alignments between raw paired-end reads and the reference genome Williams 82 (www.phetozome.net) via the Short Oligonucleotide Alignment Program 2 (SOAP2) software were conducted. Raw reads in the same genomic position were used to define SLAF tags. A total of 60 thousand high-quality SLAF tags were obtained from each of the 225 genotypes. In SNP calling, the minor allele frequency (MAF) threshold was set at 0.05. If the depth of the minor allele was larger than 1/3 of the total sample depth, the genotype was regarded as heterozygous.
For linkage analysis of a population, raw sequencing data were generated from the Illumina HiSeq2500 system. The soybean reference genome (Glycine max Wm82.a2. v1) was used for read mapping of two parents using SOAPaligner. The sequencing depth and coverage relative to the reference genome were calculated based on the mapping results. A consistent sequence of each parent was obtained using SOAPsnp software. The reads of the RIL population were aligned with the parental genome sequences using SOAPaligner software. For each sample, only the reads of unique mapping positions were considered. If a mismatch happened between a certain single read and one of the two parents, the read was considered to be from the genome of the other parent; a similar process was used for all the unique mapping reads. Chi-square tests were conducted for all SNPs to detect segregation distortion. Markers with a segregation distortion test p < 0.001 or that contained an abnormal base were filtered out. Markers were deleted if more than 10% of individuals had missing genotypes. For construction of the linkage map, the genetic distance between bin markers was calculated using the Kosambi mapping function implemented in the QTL IciMaping v3.0 software.
Population structure evaluation and linkage disequilibrium (LD) analysis
The population structure of the tested natural population was defined by the principle component analysis (PCA) approach using GAPIT software (Lipka et al. 2012). The SNP threshold value (MAF > 0.1 and missing data < 10%) and r2 (squared allele frequency correlations) were used to define LD between pairs of SNPs based on the TASSEL version 3.0 software (Bradbury et al. 2007). In contrast to the GWAS, missing SNP genotypes were not imputed with the major allele before LD analysis. The parameters of the programme included the MAF (> 0.05) and the integrity of each SNP (> 80%).
Genome-wide association analysis
Association signals of resistance to P. sojae race 1 were identified based on 28,722 SNPs from 225 tested accessions using the Compressed Mixed Linear Model (CMLM) in GAPIT (Lipka et al. 2012) and the FARMCPU method (Liu et al. 2016). p values were calculated using the Bonferroni method at 0.05 (≤ 1.74 × 10−6) (Holm 1979). The p values were set as the threshold to determine whether significant association signals existed.
QTL mapping based on linkage analysis
QTL analysis was performed using the CIM method implemented with QTL IciMaping v3.0 using stepwise regression for cofactor selection. The LOD score threshold was determined by the results of 1000 permutations for each trait. The percentage of phenotypic variance and additive effect explained by a QTL for a trait were also estimated.
Prediction of candidate genes controlling resistance to P. sojae race 1
Candidate genes located in the 200-kb genomic region of each peak SNP were classified and annotated underlying the soybean reference genome (http://www.soybase.org).
Quantitative RT-PCR assay
Two lines, ‘Williams 82’ (resistant to P. sojae race 1) and ‘Hefeng25’ (susceptible to P. sojae race 1), from the association panel were selected to investigate their expression pattern. The same method described in the resistance evaluation section was used to inoculate the above two lines of P. sojae race 1. The control groups of each line were exposed to the same cultural conditions except for inoculation. Stems of each line from the inoculation group and the control group were collected at 0 h, 4 h, 8 h, 12 h, 24 h, 36 h and 48 h after inoculation. Total mRNA of each sampled root was isolated using the GenElute™ Total RNA Purification Kit. The expression level of candidate genes was determined by quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) analysis. Real-time RT-PCR amplifications were performed using a real-time RT-PCR kit according to the manufacturer’s instructions (Takara, Japan) on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, USA). Approximately 1 μg of total RNA was used for each reverse transcription according to the protocol of the ReverTra Ace qPCR RT Kit (TOYOBO Co., Ltd., Japan). Each amplification reaction was performed with 1 μL of the resulting cDNA first-strand cDNA synthesis solution, 1.5 μL of each primer (10 μM), 10 μL of SYBR Green PCR Master Mix and 7.5 μL RNase free ddH2O in a total reaction of 20 μL. The PCR cycling conditions were as follows: 95 °C for 5 s, 60 °C for 20 s, 72 °C for 20 s for 40 cycles and 60 °C for 1 min. The relative mRNA level of each candidate gene was evaluated against the soybean actin4 (GmACTIN) (GenBank Accession Number AF049106) reference gene. Three biological duplications and three technical repetitions of each gene were conducted by qRT-PCR. The sequences of the primer pairs used to amplify the candidate genes are presented in Table S2.
Association analysis for Rps loci on Chr.03
For eleven resistant lines and twenty-two susceptible lines, genome resequencing with a 10-fold depth was conducted on an Illumina HiSeq 2500 sequencer. Paired-end resequencing reads were mapped to the soybean reference genome with BWA (Version: 0.6.1-r104) (Zhou et al. 2015) using the default parameters. SAMtools4 (Version: 0.1.18) software was used to convert the mapping results into the BAM format and to filter unmapped and non-unique reads. Duplicated reads were filtered with the Picard package (picard.sourceforge.net, Version: 1.87) (Zhou et al. 2015). The BEDtools (Version: 2.17.0) (Zhou et al. 2015) coverage Bed software was used to compute the coverage of the sequence alignments. A sequence was defined as absent if its coverage was lower than 90% and present if its coverage was greater than 90% (Zhou et al. 2015).
SNP detection was performed using the Genome Analysis Toolkit (GATK, version 2.4–7-g5e89f01) (Zhou et al. 2015) and SAMtools. Only SNPs detected by both methods were analysed further. SNPs with allele frequencies lower than 20% in the population were discarded. SNP annotation was performed according to the soybean genome (Williams 82 assembly v2.1) using the package ANNOVAR (Version: 2013-08-23) (Zhou et al. 2015). The sequence variations of the genomic region of Rps loci on Chr.03 was collected based on the SNP genotyping data. Association analysis was conducted with the same method to GWAS as described above.
Results
Phenotype characteristic of P. sojae race 1 resistance in RILs and the natural population
The mean, coefficient of variation, skewness and kurtosis for resistance to P. sojae race 1 of the association and linkage analysis panels are shown (Table S3 and Fig. 1). The association panel consisted of 225 accessions with resistance to P. sojae race 1 (ranging from 0 to 100%), and an average disease loss percentage of 51.74% was calculated (Table S3). The linkage panel comprised 109 RIL lines, which varied from 0 to 100 (with an average disease loss percentage of 38.71%). The coefficients of variation in the association and linkage panels were 0.61 and 0.91, respectively. Normal distributions without any significant skewness or kurtosis for the association and linkage panels were observed (Table S3).
Genotyping and characteristic analysis of the association and linkage panels
For the association panel, a total of 28,722 SNPs was identified through SLAF sequencing (minor allele frequency ≥ 0.05 and missing data ≤3%), which were distributed across all 20 chromosomes of the soybean genome. These SNP markers covered 947 Mbp, which accounted for approximately 99.79% of the entire soybean genome (Fig. S1A). The number of SNPs was different among the 20 chromosomes, and the mean SNP per chromosome was 1436 (ranging from 747 in Chr.11 to 2335 in Chr.18). The average marker density was approximately one SNP per 33.04 kb (Fig. S2A).
For the linkage mapping population, a total of 5221 bins markers along the 20 chromosomes were identified based on resequencing (Fig. S1B), with their physical coordinates referenced to the soybean reference genome. Chr.18 and Chr.12 also had the most and fewest bin markers, respectively. The total genetic length of this bin map was approximately 3152.3 cM, with a mean interval between markers of 0.60 cM (an average physical bin length of 181.41 Kb). The longest and shortest distances in this map were 193.67.24 cM (Chr18) and 115.17 cM (Chr.11), respectively, which included 381 and 165 bin markers, respectively.
Genome-wide association and linkage mapping of P. sojae race 1 resistance
Principal component and kinship analyses of the association panel were conducted using 28,722 SNPs, and the results showed that the first three principle components (PCs) could explain 16.19% of the total genetic variation (Fig. S2C) and that the relatedness among the tested accessions was low (Fig. S2B). In this study, the LD decay distance was 212 Kb (Fig. S2D). For association analysis, the compressed Mixed Linear Model (CMLM) and Fixed and random model Circulating Probability Unification (FarmCPU) were utilised to identify association signals for P. sojae race 1. A total of eight QTNs were associated with P. sojae race 1 resistance (Fig. 2A, B and Table 1), which were distributed on 5 chromosomes: Chr.03, Chr.07, Chr.14, Chr.15 and Chr.17. Among them, four QTNs were overlapped or located near the genomic region of the known QTL underlying P. sojae race 1 resistance, and the four another QTNs were novel for P. sojae race 1 resistance (Table 1). Rprr-3-2 and Rprr-17-2 were detected by two mapping methods, CMLM and FarmCPU. The LD pattern among the SNPs from the genomic region of association signals on Chr.03 and Chr.17 was evaluated. Rprr-3-1 and Rprr-3-2 were not in the same LD block. Also, Rprr-17-1, Rprr-17-2 and Rprr-17-3 were not in the same LD block (Fig. S3), indicating that these loci might be independent with each other. The allele effects of all the identified resistance QTNs were analysed, and the results indicated that different alleles for each identified QTN significantly affected P. sojae race 1 resistance of tested samples (Table 1). These identified QTNs and their alleles were valuable for MAS of cultivars with excellent resistance to P. sojae race 1.
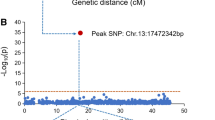
GWAS and QTL mapping results. (A) Manhattan plot of association mapping of soybean resistance to P. sojae race 1 based on the CMLM method. (B) Manhattan plot of association mapping of soybean resistance to P. sojae race 1 based on the FARMCPU method. (C) QTL mapping of soybean resistance to P. sojae race 1 by the CIM method, and the candidate genes in the colocation region of the Chr03 locus by GWAS and linkage mapping
For linkage analysis, two QTL (Qprr3-1 and Qprr3-2) intervals underlying P. sojae race 1 resistance were detected that both mapped on Chr.03 (Fig. 2C). These two QTLs explained 5.87% and 56.7% of the phenotypic variation (Table 2), which was derived from ‘DongongL-28’. QTL Qprr3-2 and QTN Rprr-3-2 colocated by linkage mapping and GWAS.
Analysis of the candidate genes providing resistance to P. sojae race 1 on Chr.03
For association analysis, 84 genes were found in the 200-kbp flanking region of eight QTNs (Table S4). Of these identified genes, four had no functional annotations/known domains or were derived from unknown functional families. Another 80 genes were classified into leucine-rich repeat protein kinase family proteins, LRR and NB-ARC domain-containing disease resistance proteins, hormone metabolism, secondary metabolism and signalling (Table S4). Many of these identified genes have been shown to be involved in plant disease resistance (Jiang et al. 2018; Marie-Cécile et al. 2014). Jiang et al. (2018) reported a nucleotide-binding site leucine-rich repeat gene from tomato that conferred resistance to Phytophthora sojae in tomato. This gene has the same domain as Glyma.03G034200, located near Rprr-3-1 of Chr.03. Marie-Cécile et al. (2014) found a downy mildew resistance gene, PDLP1, and Glyma.07G225800, which derived from the same gene family. Other genes were novel for Phytophthora sojae resistance.
The resistance loci on Chr.03 were identified by both linkage mapping and GWAS. Hence, the eighteen genes in the 200-Kb flanking region of the QTLs Qprr3-2 and QTNs Rprr-3-1 and Rprr-3-2 on Chr.03 were considered candidate genes for further analysis. The 1.5-kb upstream sequences of the eighteen genes were predicted for cis-acting elements. Cis-acting regulatory elements involved in methyl jasmonate responsiveness, salicylic acid responsiveness and/or defence and stress responsiveness were found in the upstream region of the sixteen genes (Table S5). Quantitative RT-PCR of the sixteen candidate genes was performed based on the results of the cis-acting element prediction. Of the sixteen genes, Glyma.03G033700, which encodes a C2H2-like zinc finger protein, was upregulated by P. sojae race 1 in the resistant line ‘Williams 82’ at 4 h post inoculation. Glyma.03G033800, which encodes a cell wall β-expansin protein, was downregulated by P. sojae race 1 in the resistant line ‘Williams 82’ at 4–12 h post inoculation (Fig. 3).
Expression patterns of the candidate genes. The mRNA levels of each candidate gene were analysed in resistant soybean accession ‘Williams 82’ and susceptible soybean accession ‘Hefeng25’. Infected and uninfected samples were collected at 0, 4, 8, 12, 24, 36 and 48 h post inoculation from isolated P. sojae race 1 and empty carrot agar medium. The GmACTIN (GenBank Accession Number AF049106) gene was used as an internal reference. The values are the mean ± SD of three biological replicates
A total of 379 SNPs were identified in the genomic region of Rps loci on Chr.03 (Fig. 4A). One SNP, Chr.03:3930474, was significantly associated with soybean resistance to P. sojae race 1. Two genes closest to Chr.03:3930474 are Glyma.03G033800 and Glyma.03G033700. (Fig. 4B). Combined with the result of quantitative RT-PCR assay, two genes, Glyma.03G033700 and Glyma.03G033800, were identified as the possible causal genes at the loci on Chr.03 of P. sojae race 1 resistance in soybean. These genes should be further studied to determine the mechanism of P. sojae resistance and their application in MAS.
Physical map and association analysis underlying P. sojae resistance of Rps loci on Chr.03. (A) Physical map of Rps loci on Chr.03 reported by the present study and previous studies. The marks with red colour indicate loci detected by the present study. The marks with blue colour indicate loci detected by previous studies. (B) Association analysis based on 379 SNP within the Rps loci (Chr.03: 2.9 Mb–9.9 Mb) generated from 33 soybean germplasms through genome resequencing. The X-axis is the physical position of SNP, and the Y-axis indicates the significance level of each SNP. The blue dotted line indicates the threshold of -Log10(p) value for the association signals
Discussion
Breeding new soybean cultivars with resistance is the most effective method in controlling P. sojae in the field. Resistance to P. sojae is controlled by two mechanisms, partial (or tolerance) and Rps-mediated complete resistance (Sugimoto et al. 2012). No single strategy can completely control P. sojae. Partial resistance offers a high degree of protection by delaying or limiting the damage caused by P. sojae to the soybean plant, even under low or moderate disease pressure (Dorrance et al. 2003; Tooley and Grau 1982). However, partial resistance cannot provide a level of control adequate to maintain profitable yields. Rps-mediated complete resistance is not durable due to the selection pressure that is exerted by the diverse virulence pathotypes of P. sojae. Although Rps-mediated complete resistance does not control all strains of P. sojae, Rps genes or alleles are still helpful in managing specific P. sojae strains. P. sojae race 1 is the most prevalent strain found in Northeast China, but other complicated P. sojae populations have been found in this region as well. Thus, long-term management of P. sojae in Northeast China may require the integration of multiple disease management strategies, such as breeding cultivars with both partial resistance and appropriate single gene resistance combined with tillage, rotation, soil drainage and seed treatments (Dorrance et al. 2003; Han et al. 2017). In this study, 225 accessions, including landraces or elite cultivars, were examined for resistance to P. sojae race 1, most of which were a key parent or the main cultivated cultivars in Northeast China. The results indicated that only a few of the tested samples had a high level of partial resistance to P. sojae race 1 (Table S1 and Fig. 1), some of which also were partially resistant to P. sojae isolated from Northeast China (data not shown). These accessions with resistance or partial resistance may be useful as sources of resistance for breeding new varieties adapted to Northeast China.
To date, few resistance QTLs have been identified based on different mapping populations using limited specific materials (www.soybase.org); most of these identified QTLs are specific to the genetic background. Therefore, the novel QTL/gene of the cultivar from Northeast China still needs to be identified for P. sojae MAS. In this study, 8 QTNs and two QTLs were identified to be associated with P. sojae race 1 through association and linkage analyses. Qprr3-2, a QTN identified from GWAS, has a similar genomic region to that of Rprr-3-2 from the resistance source ‘DongnongL-28’. Among these identified QTNs, four QTNs overlapped with or were near the known resistance QTL (Tables 1 and 2). Another four QTNs were novel for P. sojae resistance. Presently, only a few P. sojae race 1 resistance genes have been identified and cloned. GWAS could offer valuable information to identify and validate candidate genes of P. sojae resistance, especially in the relatively smaller LD block (average length 150–200 kbp) of soybean. Among the identified candidate genes, Glyma.03G033700 and Glyma.03G033800 were determined being the potential candidates response to P. sojae race 1 in soybean through Rps loci-based association analysis and a quantitative RT-PCR assay. Although Rprr-3-2 and Qprr3-2 have similar genomic regions as a cluster of Rps loci such as RpsYD29 (Zhang et al. 2013b), RpsHN (Niu et al. 2017) and RpsUN1 (Lin et al. 2013), etc. (Fig. 4A), the resistance donor of the soybean line and the candidate genes were different from those found in our study. However, the specific mechanism of the two candidate genes identified in this study still needs to be further analysed.
The soybean germplasm has been shown to be a valuable reservoir of disease resistance (Athow et al. 1986; Dorrance and Schmitthenner 2000; Kyle et al. 1998), which can be used to improve P. sojae resistance in soybean. In this study, two resistance lines (Ken14 and DN1068) exhibited high and partial resistance to P. sojae race 1, and these two lines had high yields and were adapted to Northeast China. Hence, they are of great value to transfer high resistance levels to other cultivars in Northeastern China through MAS.
References
Athow KL, Laviolette FA, Hahn ACL, Ploper LD (1986) Genes for resistance to Phytophthora megasperma f. sp. glycinea in PI 273483D, PI 64747, PI 274212, PI 82312N, and PI 340046. Soybean Genet Newslett 13(13):119–131
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23(19):2633–2635
Burnham KD, Dorrance AE, Francis DM, Fioritto RJ, Martin SKS (2003a) Rps8, a new locus in soybean for resistance to Phytophthora sojae. Crop Sci 43:101–105
Burnham KD, Dorrance AE, VanToai TT, Martin SKS (2003b) Quantitative trait loci for partial resistance to Phytophthora sojae in soybean. Crop Sci 43:1610–1617
Cheng YB, Ma QB, Ren HL, Xia QJ, Song EL, Tan ZY, Li SX, Zhang GY, Nian H (2017) Fine mapping of a Phytophthora-resistance gene RpsWY in soybean (Glycine max L.) by high-throughput genome-wide sequencing. Theor Appl Genet 130:1041–1051
Demirbas A, Rector BG, Lohnes DG, Fioritto RJ, Graef GL, Cregan PB, Shoemaker RC, Specht JE (2001) Simple sequence repeat markers linked to the soybean Rps genes for Phytophthora resistance. Crop Sci 44:1220–1227
Dorrance AE, Schmitthenner AF (2000) New sources of resistance to Phytophthora sojae in the soybean plant introductions. Plant Dis 84(12):1303–1308
Dorrance AE, McClure SA, St. Martin SK (2003) Effect of partial resistance on Phytophthora root and stem rot incidence and yield of soybeans in Ohio. Plant Dis 87:308–312
Fan AY, Wang XM, Fang XP, Wu XF, Zhu ZD (2009) Molecular identification of Phytophthora resistance gene in soybean cultivar Yudou 25. Acta Agron Sin 35:1844–1850
Gao HY, Narayanan NN, Ellison L, Bhattacharyya MK (2005) Two classes of highly similar coiled coil-nucleotide binding-leucine rich repeat genes isolated from the Rps1-k locus encode Phytophthora resistance in soybean. Mol Plant-Microbe Interact 18:1035–1045
Gordon SG, Martin SKS, Dorrance AE (2006) Rps8 maps to a resistance gene rich region on soybean molecular linkage group F. Crop Sci 46:168–173
Grau CR, Dorrance AE, Bond J, Russin J (2004) Fungal diseases. Soybeans. In: Boerma HR, Specht JE (eds) Improvement, production and uses, 3rd edn. Agronomy Monogr, American Society of Agron, Madison, pp 679–763
Han YP, Teng WL, Yu KF, Poysa V, Anderson T, Qiu LJ, Lightfoot DA, Li WB (2008) Mapping QTL tolerance to Phytophthora root rot in soybean using microsatellite and RAPD/SCAR derived markers. Euphytica 162:231–239
Han Y, Zhang Y, Wu D, Zhao X, Teng W, Li D, Li W (2017) Identification of novel quantitative trait loci associated with tolerance to phytophthora root rot in the soybean cultivar Hefeng 25 using two recombinant inbred line populations. Can J Plant Sci 97:1–8
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6(2):65–70
Huang J, Guo N, Li YH, Sun JT, Hu GJ, Zhang HP, Li YF, Zhang X, Zhao JM, Xing H, Qiu LJ (2016) Phenotypic evaluation and genetic dissection of resistance to Phytophthora sojae in the Chinese soybean mini core collection. BMC Genet 17:85
Jiang N, Cui J, Meng J, Luan YS (2018) A tomato nucleotide binding sites-leucine-rich repeat gene is positively involved in plant resistance to Phytophthora infestans. Phytopathology 108:980–987. https://doi.org/10.1094/PHYTO-12-17-0389-R
Kyle DE, Nickell CD, Nelson RL, Pedersen WL (1998) Response of soybean accessions from provinces in southern China to Phytophthora sojae. Plant Dis 82(5):555–559
Li BY, Ma SM (1999) The occurrence of soybean Phytophthora root rot and its control. Chin J Oil Crop Sci 21:47-50 (in Chinese with English abstract)
Li XP, Han YP, Teng WL, Zhang SZ, Yu KF, Poysa V, Anderson T, Ding JJ, Li WB (2010) Pyramided QTL underlying tolerance to Phytophthora root rot in mega-environments from soybean cultivars ‘Conrad’ and ‘Hefeng 25’. Theor Appl Genet 121:651–658
Li Y, Sun S, Zhong C, Wang X, Wu X, Zhu Z (2017) Genetic mapping and development of co-segregating markers of RpsQ, which provides resistance to in soybean. Theor Appl Genet 130:1223–1233
Lin F, Zhao MX, Ping JQ, Johnson A, Zhang B, Abney TS, Hughes TJ, Ma JX (2013) Molecular mapping of two genes conferring resistance to Phytophthora sojae in a soybean landrace PI 567139B. Theor Appl Genet 126:2177–2185
Lipka AE, Tian F, Wang Q, Peiffer J, Li M, Bradbury PJ, Gore MA, Buckler ES, Zhang Z (2012) GAPIT: genome association and prediction integrated tool. Bioinformatics 28(18):2397–2399
Liu X, Huang M, Fan B, Buckler ES, Zhang Z (2016) Iterative usage of fixed and random effect models for powerful and efficient genome-wide association studies. PLoS Genet 12(2):e1005767
Marie-Cécile C, Lennart W, Jan S, Kim F, Sophie P, Alexandra J, Silke R, Jonathan J, Christine F (2014) The plasmodesmal protein PDLP1 localises to haustoria-associated membranes during downy mildew infection and regulates callose deposition. PLoS Pathog 10(11):e1004496
Niu JP, Guo N, Sun JT, Li LH, Cao YC, Li SG, Huang JL, Zhao JM, Zhao TJ, Xing H (2017) RpsHN fine mapping of a resistance gene that controls using recombinant inbred lines and secondary populations. Front Plant Sci 8:538
Ping JQ, Fitzgerald JC, Zhang CB, Lin F, Bai YH, Wang DC, Aggarwal R, Rehman M, Crasta O, Ma JX (2016) Identification and molecular mapping of Rps11, a novel gene conferring resistance to Phytophthora sojae in soybean. Theor Appl Genet 129:445–451
Sandhu D, Gao HY, Cianzio S, Bhattacharyya MK (2004) Deletion of a disease resistance nucleotide-binding-site leucine-rich-repeatlike sequence is associated with the loss of the Phytophthora resistance gene Rps4 in soybean. Genetics 168:2157–2167
Sandhu D, Schallock KG, Rivera-Velez N, Lundeen P, Cianzio S, Bhattacharyya MK (2005) Soybean phytophthora resistance gene Rps8 maps closely to the Rps3 region. J Hered 96:536–541
Schmitthenner AF (1985) Problems and progress in control of Phytophthora root rot of soybean. Plant Dis 69:362–368
Schneider R, Rolling W, Song Q, Cregan P, Dorrance AE, McHale LK (2016) Genome-wide association mapping of partial resistance to Phytophthora sojae in soybean plant introductions from the Republic of Korea. BMC Genomics 17:607
Shen CY, Su YC (1991) Discovery and preliminary studies of Phytophthora megasperma on soybean in China. Acta Phytopathol Sin 21: 298 (in Chinese with English abstract)
Sugimoto T, Yoshida S, Kaga A, Hajika M, Watanabe K, Aino M, Tatsuda K, Yamamoto R, Matoh T, Walker DR (2011) Genetic analysis and identification of DNA markers linked to a novel Phytophthora sojae resistance gene in the Japanese soybean cultivar Waseshiroge. Euphytica 182:133–145
Sugimoto T, Kato M, Yoshida S, Matsumoto I, Kobayashi T, Kaga A, Hajika M, Yamamoto R, Watanabe K, Aino M, Matoh T, Walker DR, Biggs AR, Ishimoto M (2012) Pathogenic diversity of Phytophthora sojae and breeding strategies to develop Phytophthora- resistant soybeans. Breed Sci 61:511–522
Sun S, Wu XL, Zhao JM, Wang YC, Tang QH, Yu DY, Gai JY, Xing H (2011) Characterization and mapping of RpsYu25, a novel resistance gene to Phytophthora sojae. Plant Breed 130:139–143
Sun X, Liu D, Zhang X, Li W, Liu H, Hong W, Jiang C, Guan N, Ma C, Zeng H, Xu C, Song J, Huang L, Wang C, Shi J, Wang R, Zheng X, Lu C, Wang X, Zheng H (2013) SLAF-seq: an efficient method of large-scale de novo SNP discovery and genotyping using high-throughput sequencing. PLoS One 8(3):e58700
Sun J, Guo N, Lei J, Li L, Hu G, Xing H (2014a) Association mapping for partial resistance to Phytophthora sojae in soybean (Glycine max (L.) Merr.). J Genet 93:355–363
Sun JT, Li LH, Zhao JM, Huang J, Yan Q, Xing H, Guo N (2014b) Genetic analysis and fine mapping of RpsJS, a novel resistance gene to Phytophthora sojae in soybean [Glycine max (L.) Merr]. Theor Appl Genet 127:913–919
Tooley P, Grau C (1982) Identification and quantitative characterization of rate-reducing resistance to Phytophthora megasperma f. sp. glycinea in soybean seedlings. Phytopathology 72:727–733
Tucker DM, Saghai Maroof MA, Mideros S, Skoneczka JA, Nabati DA, Buss GR, Hoeschele I, Tyler BM, Martin SKST, Dorrance AE (2010) Mapping quantitative trait loci for partial resistance to Phytophthora sojae in a soybean interspecific cross. Crop Sci 50:628–635
Tyler BM (2007) Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol Plant Pathol 8(1):1–8
Wang HH, Waller LC, Tripathy S, Martin SKS, Zhou LC, Krampis K, Tucker DM, Mao YC, Hoeschele I, Maroof MAS, Tyler BM, Dorrance AE (2010) Analysis of genes underlying soybean quantitative trait loci conferring partial resistance to Phytophthora sojae. Plant Gen 3:23–40
Wang H, St. Martin SK, Dorrance AE (2012a) Comparison of phenotypic methods and yield contributions of quantitative trait loci for partial resistance to in soybean. Crop Sci 52(2):609–622
Wang H, Wijeratne A, Wijeratne S, Lee S, Taylor CG, St MS, McHale L, Dorrance AE (2012b) Dissection of two soybean QTL conferring partial resistance to Phytophthora sojae through sequence and gene expression analysis. BMC Genomics 13:428
Weng C, Yu K, Anderson TR, Poysa V (2001) Mapping genes conferring resistance to Phytophthora root rot of soybean, Rps1a and Rps7. J Hered 92(5):442–446
Weng CR, Yu KF, Anderson TR, Poysa V (2007) A quantitative trait locus influencing tolerance to Phytophthora root rot in the soybean cultivar ‘Conrad’. Euphytica 158:81–86
Wu XL, Zhang BQ, Sun S, Zhao JM, Yang F, Guo N, Gai JY, Xing H (2011a) Identification, genetic analysis and mapping of resistance to Phytophthora sojae of Pm28 in soybean. Agric Sci China 10(10):1506–1511
Wu XL, Zhou B, Sun S, Zhao JM, Chen SY, Gai JY, Xing H (2011b) Genetic analysis and mapping of resistance to Phytophthora sojae of Pm14 in soybean. Sci Agric Sin 44(3):456–460
Wu X, Zhou B, Zhao J, Guo N, Zhang B, Yang F (2011c) Identification of quantitative trait loci for partial resistance to phytophthora sojae in soybean. Plant Breeding 130(2): 144–149
Yao HY, Wang XM, Wu XF, Xiao YN, Zhu ZD (2010) Molecular mapping of Phytophthora resistance gene in soybean cultivar Zaoshu 18. J Plant Genet Res 11(2):213–217 (in Chinese with English abstract)
Yu AL, Xu PF, Wang JS, Zhang SZ, Wu JJ, Li WB, Chen WY, Li NH, Fan SJ, Wang X, Jiang LY (2010) Genetic analysis and SSR mapping of gene resistance to Phytophthora sojae race 1 in soybean cv Suinong 10. Chin J Oil Crop Sci 32(4):462–466 (in Chinese with English abstract)
Zhang JQ, Xia CJ, Duan CX, Sun SL, Wang XM, Wu XF, Zhu ZD (2013a) Identification and candidate gene analysis of a novel Phytophthora resistance gene Rps10 in a Chinese soybean cultivar. PLoS One 8(7):e69799
Zhang JQ, Xia CJ, Wang XM, Duan CX, Sun SL, Wu XF, Zhu ZD (2013b) Genetic characterization and fine mapping of the novel Phytophthora resistance gene in a Chinese soybean cultivar. Theor Appl Genet 126:1555–1561
Zhang ZN, Hao JJ, Yuan JZ, Song QJ, Hyten DL, Cregan PB, Zhang GR, Gu CH, Li M, Wang DC (2014) Phytophthora root rot resistance in soyean E00003. Crop Sci 54:492–499
Zhong C, Sun S, Li Y, Duan C, Zhu Z (2017) Next-generation sequencing to identify candidate genes and develop diagnostic markers for a novel Phytophthora resistance gene, RpsHC18, in soybean. Theor Appl Genet 131:525–538
Zhong C, Li YP, Sun SL, Duan CX, Zhu ZD (2019) Phytophthora sojae genetic mapping and molecular characterization of a broad-spectrum resistance gene in Chinese soybean. Int J Mol Sci 20(8):1809
Zhou Z, Jiang Y, Wang Z, Gou Z, Lyu J, Li W, Yu Y, Shu L, Zhao Y, Ma Y (2015) Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat Biotechnol 33:408–414
Zhu ZD, Huo YL, Wang XM, Huang JB, Wu XF (2007) Molecular identification of a novel Phytophthora resistance gene in soybean. Acta Agron Sin 33(1):154–157
Acknowledgements
This study was financially supported by the Heilongjiang Provincial Project (GJ2018GJ0098, GX17B002, JC2018007 and C2018016), the Chinese National Natural Science Foundation (31671717, 31471517), the National Key R & D Project (2016YFD0100304, 2017YFD0101302, and 2017YFD0101306-05), the National Project (2014BAD22B01, 2016ZX08004001-007), the Youth Leading Talent Project of the Ministry of Science and Technology in China (2015RA228), the National Ten-thousand Talents Program, Postdoctoral Fund in Heilongjiang Province (LBH-Z15017, LBH-Q17015), the national project (CARS-04-PS04), the ‘Youth Innovation Talent’ Project of the general undergraduate universities in Heilongjiang province (UNPYSCT-2016145), and the ‘Academic Backbone’ Project of Northeast Agricultural University (17XG22).
Author information
Authors and Affiliations
Contributions
XZ, DFB and WW conceived the study and contributed to population development. CJZ and YJ contributed to genotyping. HPJ contributed to phenotypic evaluation. LJQ, YPH and WBL contributed to the experimental design and writing of the paper. All authors contributed to and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors have adhered to the ethical responsibilities outlined by Molecuar Breeding.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, X., Bao, D., Wang, W. et al. Loci and candidate gene identification for soybean resistance to Phytophthora root rot race 1 in combination with association and linkage mapping. Mol Breeding 40, 100 (2020). https://doi.org/10.1007/s11032-020-01179-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-020-01179-9