Abstract
Key message
Association and linkage mapping techniques were used to identify and verify single nucleotide polymorphisms (SNPs) associated with Sclerotinia sclerotiorum resistance. A novel resistant gene, GmGST , was cloned and shown to be involved in soybean resistance to SSR.
Abstract
Sclerotinia stem rot (SSR), caused by the fungus Sclerotinia sclerotiorum, is one of the most devastating diseases in soybean (Glycine max (Linn.) Merr.) However, the genetic architecture underlying soybean resistance to SSR is poorly understood, despite several mapping and gene mining studies. In the present study, the identification of quantitative trait loci (QTLs) involved in the resistance to S. sclerotiorum was conducted in two segregating populations: an association population that consisted of 261 diverse soybean germplasms, and the MH population, derived from a cross between a partially resistant cultivar (Maple arrow) and a susceptible cultivar (Hefeng25). Three and five genomic regions affecting resistance were detected by genome-wide association study to control the lesion length of stems (LLS) and the death rate of seedling (DRS), respectively. Four QTLs were detected to underlie LLS, and one QTL controlled DRS after SSR infection. A major locus on chromosome (Chr.) 13 (qDRS13-1), which affected both DRS and LLS, was detected in both the natural population and the MH population. GmGST, encoding a glutathione S-transferase, was cloned as a candidate gene in qDRS13-1. GmGST was upregulated by the induction of the partially resistant cultivar Maple arrow. Transgenic experiments showed that the overexpression of GmGST in soybean increased resistance to S. sclerotiorum and the content of soluble pigment in stems of soybean. The results increase our understanding of the genetic architecture of soybean resistance to SSR and provide a framework for the future marker-assisted breeding of resistant soybean cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sclerotinia stem rot (SSR) or white mold of soybean [Glycine max (L.) Merr.] is caused by the Sclerotinia sclerotiorum (Lib) de Bary, which is especially severe in particularly humid and cool (< 25 °C) weather (Moellers et al. 2017; Rousseau 2004). The frequency of SSR outbreaks had increased since 1978, spreading over the entire world resulting in yield losses of up to 94% in severe SSR outbreak seasons in the major growing areas such as Canada, China and the USA (Boland and Hall 1986; Cline and Jacobsen 1983; Wang et al. 2019). For every 10% increase in SSR incidence, soybean yield is reduced by 170–330 kg/ha (Peltier et al. 2012). Although chemical (Briard et al. 1997; Mueller et al. 2004) and biological (Bastien et al. 2012) methods as well as some agronomic cultural practices such as crop rotation, reduced tillage and wide row spacing (Kurle et al. 2001; Mila and Yang 2008; Mueller et al. 2002) have been utilized to control SSR, the use of resistant cultivars is the most effective management method (Kurle et al. 2001). The SSR-resistant mechanisms in soybean cultivars include physiological resistance and escape or avoidance resistance (Coyne et al. 1974; Kim and Diers 2000; Rousseau 2004). Although complete resistance has not been reported in soybean, partial resistance or incomplete resistance has been proven to be effective in increasing the resistance of soybean cultivars to SSR (Zhao et al. 2015). Hence, screening resistant cultivars with partial resistance and subsequent breeding utilization are the objectives of some breeding programs (Grau 1982).
Inheritance of partial resistance in soybean is complicated and is controlled by major and minor genes/quantitative trait loci (QTLs) and is easily affected by the environment and genotype and their interaction (Zhao et al. 2015). The advance of DNA markers has allowed the detection of QTL underlying partial resistance to SSR. To date, more than 100 QTLs distributed on 18 of the 20 soybean chromosomes, based on linkage mapping strategy and the bi-parent population, have been reported in the SoyBase databank (David et al. 2010), most of which are not observed in another population due to the limited resolution and accuracy of these QTLs or genomic regions with higher linkage disequilibrium (LD). Hence, these identified SSR QTLs are difficult to directly utilize in molecular-assisted breeding (MAS). Compared with traditional linkage mapping, genome-wide association studies (GWAS) are an alternative method to detect SSR QTLs. GWAS, based on the natural population, have been shown to have the potential to dissect the genetic basis of complex traits in crops, which have more extensive recombination events and shorter LD segments, and therefore increased resolution and accuracy of marker–phenotype associations (Li et al. 2015). Recently, the high-throughput sequencing method was applied in GWAS, which has further accelerated the development of GWAS. GWAS of SSR in soybean have been conducted. Bastien et al. identified four SSR QTLs in chromosome 01 (Chr. 01), Chr. 15, Chr. 19 and Chr. 20 through 130 lines and 7864 single nucleotide polymorphisms (SNPs) (Bastien et al. 2014). Moellers et al. found 58 significant main-effect loci and 24 significant epistatic interactions associated with partial resistances of SSR based on 466 soybean accessions and a SoySNP50K BeadChip, respectively (Moellers et al. 2017). Zhao et al. identified five SNPs that were significantly associated with SSR resistance in 330 soybean samples and 25,179 SNPs based on stem pigmentation after treatment with oxaloacetic acid (Zhao et al. 2015). Sun et al. reported seven SNPs and 46 SNPs related to partial SSR resistance using GWAS via CMLM and mrMLM methods, respectively (Sun et al. 2020). However, these studies on QTL and GWA mapping for SSR resistance lacked mutual verification. Up to now, few studies combining results of GWAS and linkage mapping have been reported for SSR resistance, except for the research of Zhao et al. (2015). There were also few reports about SSR resistance genes obtained by map-based cloning.
To address the knowledge gap, the present investigation aimed to analyze the genetic architecture of soybean resistance to SSR using both GWAS and linkage analysis based on several datasets: 24,619 SNPs from 261 diverse soybean germplasms; a high-density genetic map generated from 5,221 bins; and 149 RILs. The study also aimed to identify the beneficial alleles of each associated SNP loci to provide valuable markers for marker-assisted selection (MAS) and to identify and verify potential candidate genes that might be associated with soybean resistance to SSR.
Materials and methods
Plant materials and phenotypic evaluation
Two mapping populations were used in the present study. A natural population consisting of 261 accessions representing the genetic diversity inside and outside China collected from the Chinese National Soybean GeneBank (CNSGB) was used for the GWAS (Han et al. 2016). Among the 261 soybean accessions, 238 germplasms covered the regions of China from 22 to 48° N and from 74 to 128° E and other 23 germplasms were collected representing the genetic diversity of non-Chinese regions (Table S1). Soybean cultivars with high and low resistance to S. sclerotiorum were included in the panel of 261 germplasms, including Maple Arrow (a partially resistant cultivar), and Hefeng 25 (a susceptible cultivar) (Li et al. 2009a; Zhao et al. 2015). The MH population, consisting of 149 F5:20 RILs, was derived from the cross of Hefeng 25 and Maple Arrow and was used for QTL mapping.
The inoculated plant materials were germinated in a greenhouse (27 ± 1 °C) with a 16-h day length according to the ‘cut stem method’ described by Kull et al. (2003). The experiment was designed as a randomized complete block with three replications. The main stems of 5-week-old plants were inoculated by S. sclerotiorum grown on PDA culture medium and incubated in a mist chamber (relative humidity > 80%) for 24 h and were transferred to an adjacent greenhouse (25 ± 1 °C). After 14 days, the lesion length (LL) on the main stem was measured. The death rate of the seedlings (DRS) of each accession was calculated 14 days post-inoculation according to the following formula: DRS = Nd × 100/Na; Nd is the number of dead plants, Na is the total number of inoculated plants.
Genotyping and genetic feature analysis of the association population
The CTAB method was used to isolate the genomic DNA of each tested accession. For the association panel, specific locus-amplified fragment sequencing (SLAF-seq) methodology was conducted for genotyping (Sun et al. 2013). The alignment between raw paired-end reads and the soybean reference genome was conducted using Short Oligonucleotide Alignment Program 2 (SOAP2) software (Li et al. 2009b). The raw reads in the same genomic position were used to define the SLAF groups. The SNPs were defined based on MAF ≥ 0.05. The genotype was regarded as heterozygous when the depth of the minor allele/the total depth of the sample ≥ 1/3.
The principal component analysis (PCA) approach was used to analyze the population structure of the tested population using TASSEL version 3.0 (Bradbury et al. 2007). LD between pairs of SNPs was defined based on SNP (MAF > 0.05 and missing data < 10%), and r2 (squared allele frequency correlations) using the software TASSEL version 3.0 (Bradbury et al. 2007). In contrast to the GWAS, missing SNP genotypes were not attributed to the major allele before LD analysis. Parameters in the program included MAF (≥ 0.05) and the integrity of each SNP (≥ 80%).
Association mapping
The association signals of resistance to SSR were identified using 24,619 SNPs from 261 tested accessions. GWAS was conducted using restricted two-stage multi-locus genome-wide association analysis (RTM-GWAS) (He et al. 2017). The Bonferroni method at α ≤ 0.05 (p ≤ 2.03 × 10−6) was utilized to calculate the p value, which was set as the threshold to declare whether significant association signals existed.
RI population genotyping and linkage map construction
For the MH population, DNA sequencing libraries of the two parents (Maple arrow and Hefeng25) and each line of the RI population were constructed and sequenced on an Illumina HiSeq2500 sequencing platform following the manufacturer's instructions. The sequencing reads for the RI population and the parental lines were aligned to the soybean reference genome (assembly Glycine_max_v2.1) (Schmutz et al. 2010) using the SOAP2 program (Li et al. 2009b). GATK (McKenna et al. 2010) was used to identify polymorphic SNPs between the RI population and the parental lines. Co-segregating SNPs were separated into bins, and a bin map was constructed based on the recombinant breakpoints of the MH population with HighMap (Liu et al. 2014).
Linkage analyses were performed using IciMapping v4.1 (Meng et al. 2015); putative QTLs were identified based on an LOD threshold of 2.5. Genetic distances among markers were calculated using the Kosambi mapping function (Kosambi 1944). Linkage groups were discriminated at a log-likelihood threshold of 3.0.
Quantitative real-time PCR (qRT-PCR)
For candidate gene identification, the dynamic changes in the expression of nine candidate genes were analyzed by qRT-PCR assay after inoculation with S. sclerotiorum. Primers of the candidate genes are listed in Table S2. Stems were collected from the SSR-susceptible line Hefeng25 and the SSR-partially resistant line Maple arrow at the first trifoliate stage at 0, 4, 8, 12, and 24 h post-inoculation (hpi). Three replicate samples were collected per line for RNA extraction.
In addition, the expression of the candidate gene GmGST was assessed in various plant tissues with two aims stated below.
First, to compare tissue-specific expression patterns, plant tissues including stems, leaves, petioles, terminal buds, cotyledons, hypocotyls, flower, roots, seed, and root nodules were collected from Hefeng25 and Maple arrow (three samples collected per tissue).
Second, to determine the abundance of GmGST in wild-type Hefeng25/Maple arrow and in the GmGST-overexpressing transgenic Hefeng25/Maple arrow leaves were collected at the third trifoliate stage.
The primer pair qGmGST (F/R) was used in the above two experiments (Table S2). Plants were grown in a greenhouse under a 16-h light/8-h dark photoperiod at 25 ± 1 °C until sampling.
Total RNA was isolated from different tissues using RNAprep Pure Plant kits (DP432, Tiangen). First-strand cDNA was synthesized from total RNA using TIANScript RT kits (KR104, Tiangen). qRT-PCRs were performed on an ABI 7500 Fast platform using SuperReal PreMix Plus (SYBR Green) kits (FP205, Tiangen). Each qRT-PCR (20 μl) included 2 μl cDNA, 10 μl 2 × SuperReal PreMix Plus, 0.4 μl 50 × ROX Reference Dye∆, 5 μl of each forward and reverse primer, and 6.6 μl ddH2O2. The qRT-PCR amplification conditions were 95 °C for 2 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 30 s. Three technical replicates were performed per sample, and the relative levels of transcript abundance were calculated using the 2−∆∆CT method (Livak and Schmittgen 2001). The housekeeping gene GmActin4 (GenBank accession no. AF049106) was used as the internal standard.
GmGST cloning and vector construction
The full-length cDNA sequence of GmGST was amplified from the stem of line ‘Maple arrow’ using RT-PCR. RT-PCRs were performed using the KOD One PCR Master Mix (Code No. KMM-201; Toyobo (Shanghai) Biotech Co. Ltd., China), following the manufacturer's instructions. The primers used were GmGST-F and GmGST-R, which were designed based on sequences flanking GmGST in the Phytozome database (Goodstein et al. 2012), with the 5’ ends modified to include Bgl II and BstE II restriction sites (Table S2). The qRT-PCR cycling conditions were as follows: 5 min at 94 °C; 35 cycles of 30 s at 94 °C, 30 s at 60 °C, and 45 s at 72 °C; and a final 10 min at 72 °C. The purified PCR products were ligated into the pGM-T vector (VK207, Tiangen). Positive clones expressing the correct sequence were further inserted into the pCAMBIA3301 vector using double digestion and ligation. The bar gene was used as a selection marker in the pCAMBIA3301 vector. Two expression vectors (35S:GmGST or 35S:bar) were constructed for transformation using the recombinant pCAMBIA3301 plasmid.
To construct a plasmid vector expressing Cas9 and sgRNA of GmGST, two vectors were used. First, the sgRNA of GmGST was designed using the web-based tool CRISPR-P (http://cbi.hzau.edu.cn/crispr/) (Lei et al. 2014) and then synthesized (BOSHI Biology, Harbin, China). A pUC19 vector with a soybean U6-10 promoter (Sun et al. 2015) was used as a template to conduct two independent PCRs to generate GmU6-10-sgRNA (primer pair GmU6-10-F/sgRNA-GST-R) and the sgRNA-scaffold (primer pair sgRNA-GST-F/scaffold-R). The third PCR was conducted using the PCR products from the two reactions above as templates to generate the GmU6-10-sgRNA-scaffold. Then, the GmU6-10-sgRNA-scaffold was cloned into the binary vector with a codon-optimized Cas9 gene for dicotyledons driven by the CaMV 35S promoter by in-fusion ligation reaction (Clontech 5 × In-HD Enzyme Premix, Takara, Japan) to generate the gene-editing vector pCas9-sgGmGST. All the primers for the vector construction are listed in Table S2.
Plant transformation
To verify that GmGST expression was associated with the resistance of soybean to SSR, the GmGST gene was overexpressed and edited. For gene overexpression, Hefeng25 and Maple arrow were selected as the recipients of genetic transformation. For gene editing, Maple arrow was used as a target.
To develop transgenic soybean, the 35S:GmGST recombinant plasmid and pCas9-sgGmGST plasmid were transformed into Agrobacterium tumefaciens strain EHA105, followed by stable transformation into the cotyledon nodes of soybean cultivars Hefeng25 and Maple arrow according to Paz et al. (2004). Transgenic soybean plants were grown in a greenhouse under a 16-h light/8-h dark photoperiod at 25 ± 1 °C. For overexpression detection, the expression of GmGST in leaves of T2 transgenic soybean plants was verified using PCR amplification (3301-bar-F/R), western blotting, and qRT-PCR (qGmGST-F/R) (Table S2). For GmGST-mutant identification, the DNA fragment containing sgRNA was sequenced with primer pair mtseq-F/R (Table S2).
Evaluation of transgenic plant resistance to Sclerotinia stem rot
Effects of S. sclerotiorum were tested on the GmGST-overexpressed soybean and mutant plants using an excised leaf inoculation method (Kull et al. 2003) with minor modification. Briefly, the fully expanded leaves of 2-week-old soybeans were cut from the stem and then placed in a labeled, moistened paper towel. An 8-mm2 plug with mycelium was placed on one side of the middle trifoliolate leaf between the main leaf vein and the leaf edge and gently pressed to ensure good contact with the leaf surface. After 48 h, the diameter of lesions on inoculated leaves was measured during the following 5 days. To test the effects of GmGST expression on the content of soluble pigments in soybean stems, the pigment content in transgenic plants was measured using the method described by Wegulo et al. (1998a).
Subcellular localization of GmGST
The full-length coding region of GmGST was inserted into the pCAMBIA1302 vector under the control of the 35S promoter to generate a GFP-fused GmGST vector (35S:GmGST-GFP). This recombinant vector and the control vector (35S:GFP) were transfected into separate groups of tobacco mesophyll cells (Šlndelářová and Šlndelář 1991). The transfected cells were examined and imaged under a confocal laser scanning microscope (DMi8, Leica, China). The primer sequences GmGST-GFP-F and GmGST-GFP-R were used for subcellular localization (Table S2).
Results
Phenotyping and statistical analysis for soybean resistance to Sclerotinia stem rot
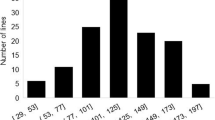
The distribution of the lesion length of stems (LLS) after inoculation by S. sclerotiorum in RILs was an approximate normal distribution and ranged from 3 to 14 cm with mean of 6.83 cm ± 2.45 cm, and that in natural population was similar in distribution to the RI population. The phenotypic value of LLS ranged from 1.26 cm to 17.8 cm with a mean of 6.08 cm ± 2.50 cm in the natural population. The coefficients of variation of lesion length were 0.36 in the RI population and 0.41 in the association population.
The distribution of the death rate of seedlings (DRS) after inoculation by S. sclerotiorum in RILs was skewed and ranged from 0 to 1 with a mean of 0.86 ± 0.22, and that in the natural population was also skewed and ranged from 0.2 to 1 with a mean of 0.80 ± 0.20. The coefficients of variation of DRS were 0.26 in the RI population and 0.25 in the association population. The two traits reflecting soybean resistance to SSR showed that the resistance was quantitative in terms of genetics, and no immune type was found (Fig. S1, Table S3).
Bin map construction and QTL mapping for SSR resistance
Genome resequencing was conducted to genotype the parental lines (Maple arrow and Hefeng25) and the 149 RILs in the mapping population. For Maple arrow, 31.58 GB of raw data was generated, and 78.8% of the reads were successfully aligned to the soybean reference genome with an average depth of 32.27-fold; for Hefeng25, 29.81 GB of raw data was generated, and 78.3% of the reads were successfully aligned to the soybean reference genome with an average depth of 29.81-fold (Table S4). Across all RILs, 962.54 GB of raw data was generated, with an average sequencing depth of 6.12-fold (Table S4). A total of 319 million SNPs were identified among the 149 RILs; all SNP sites in the RILs were integrated as recombination bin units. Finally, a genetic linkage map with 5221 bin markers was constructed across the 20 chromosomes (Table S5). The total length of the bin map was 6307.03 centimorgans (cM), with a mean interval between markers of 1.21 cM (Table S5, Fig. S2).
Based on linkage mapping, four QTLs on Chr. 03, Chr. 08, Chr. 12 and Chr. 15 were significantly associated with the LLS in soybean RILs (Table 1). Each QTL explained 1.06 to 13.17% of the phenotypic variation (Table 1). qLLS15-1 flanked with markers Block3520 and Block3519 is a major QTL with the largest effect with respect to genetic contribution. Only one, but still a major QTL, was detected on Chr. 13 to underlie the death rate of seedlings and explained 21.14% of the phenotypic variation. The beneficial allele of the two major QTLs was from resistant parent ‘Maple Arrow’.
SNP-based genotyping in natural population and GWA mapping for resistance to SSR
A total of 50,000 high-quality SLAF from each of the 261 genotypes in the association panel were obtained, containing 24,619 SNPs (MAF ≥ 0.05) (Han et al. 2016). The mean genomic distance between SNP tags was approximately 37.9 Kbp (Fig. S3A). The mean linkage disequilibrium (LD) across all tested soybean accessions was 202 kbp (Fig. S3B). GWAS based on 24,619 SNPs showed that five and three quantitative trait nucleotides (QTNs) were significantly associated with LLS and DRS, respectively (Table 2). No pleiotropic loci were found to control both of the traits in the natural population, indicating that the two traits might be controlled by different genes.
One major QTL mapped to Chr. 13, between the interval of Block2994 and Block2997 (named qDRS13-1), was associated with LLS and DRS in the RI population, and the locus controlled DRS in both the linkage analysis (R2 = 21%; Fig. 1a) and the GWAS (Chr13-17,472,342; −Log10(P) = 34.70; Fig. 1b). Thus, it was tentatively identified qDRS13-1 as a stable, widely adaptable locus associated with SSR resistance in soybean.
QTL and association mapping results of the major resistance locus on Chr. 13. a QTL mapping result of Chr. 13. The red dotted line indicates a threshold of LOD value above 2.5. b Association mapping result of Chr. 13. The red dotted line indicates a threshold of −log10(P) value above. c Candidate genes in the flanking region of peak SNP associated with soybean resistance to SSR
Candidate gene mining in a major locus on Chr. 13
In the interval of qDRS13-1 and the linkage genetic region of QTN (Chr13-17,472,342), nine coding genes were predicted (Table S6). In order to identify the candidate gene for the target locus on Chr. 13, the expression patterns of the nine coding genes were analyzed in the parental lines Maple arrow (partially resistant to SSR) and Hefeng25 (susceptible to SSR) after infection with S. sclerotiorum by qRT-PCR. The expressions of six of the nine genes were detected except for those of Glyma.13G073000, Glyma.13G073100 and Glyma.13G073300. Of the detected genes, the expression patterns of Glyma.13G072700, Glyma.13G072900 and Glyma.13G073400 shared a similar tendency in Maple arrow and Hefeng25 after inoculation. However, the basic expression abundance was different between the two parents. Glyma.13G072600 and Glyma.13G072800 were slightly unregulated in Maple arrow at 4 hpi, but the difference in the expression abundance between the two parents was not obvious. With respect to Glyma.13G072300, the expression was significantly unregulated in Maple arrow compared to that in Hefeng25 at 4 and 8 hpi, indicating that the gene might be induced by infection with S. sclerotiorum and was differentially expressed in different cultivars with different sensitivity resistance (Fig. 2). Thus, Glyma.13G072300, encoding Glutathione S-transferase (GST) C-terminal-like/translation elongation factor EF1B and named GmGST, was considered the candidate gene of qDRS13-1 for a further study.
Patterns of GmGST transcript expression and location of the GmGST protein
The results of qRT-PCR showed that the GmGST gene were expressed in all the tested tissues or organs but differed in expression abundance between resistant and susceptible lines. Overall, the abundance of expression in tested samples from the disease-resistant line Maple arrow was higher than that from disease-susceptible line Hefeng25 except for the cotyledons. In the hypocotyl especially, the GmGST transcript level was significantly higher in Maple arrow than in Hefeng25 (Fig. 3a).
The expression pattern of GmGST and GST protein localization. a The expression pattern of GmGST in different soybean tissues. R: Root; H: Hypocotyl; C: Cotyledon; S: Stem; Vc: Euphyllia; V1: The first compound leaf; P: Petiole; RN: Root nodule. b The expression pattern of GmGST after treatment with SSR. c Subcellular localization of the GmGST protein
After inoculation with S. sclerotiorum, GmGST in the stems was significantly upregulated in Maple arrow and slightly upregulated in Hefeng25 from 4 to 24 hpi (Fig. 3b). However, the increased duration of the GmGST expression level in Maple arrow was longer than that in Hefeng25. These results suggested that GmGST was regulated by S. sclerotiorum infection and that this gene was differentially expressed between SSR-resistant and SSR-susceptible soybean lines. It is speculated that the difference in the GmGST expression level might cause the differentiation in soybean response to S. sclerotiorum.
The green fluorescent protein (GFP) and the GmGST-GFP fusion protein were expressed both under the control of the 35S promoter in separate tobacco mesophyll cells. In cells carrying 35S:GFP and 35S:GmGST-GFP, the GFP signal was dispersed throughout the cell, indicating that GmGST is a cytoskeletal protein with nuclear localization (Fig. 3c).
Expression of GmGST influenced soybean resistance to SSR and soluble pigment content in stem
GmGST was overexpressed in the SSR partially resistant line Maple arrow and SSR-susceptible line Hefeng25 to generate GmGST-ox Maple arrow and GmGST-ox Hefeng25. Two and three independent T2 lines of GmGST-ox Maple arrow and GmGST-ox Hefeng25 were generated, respectively (Fig. S4 A-C). At 3 days post-inoculation (dpi) with S. sclerotiorum, the leaves of both the non-transgenic Maple arrow and Hefeng25 plants were infected and exhibited lesions. As shown in Fig. 4a, the leaves of the susceptible line Hefeng25 detached and exhibited larger water-soaked lesions than those of the partially resistant line Maple arrow. Although the GmGST-ox Hefeng25 transgenic plants exhibited lesions, the lesion areas were significantly smaller than those of non-transgenic Hefeng25. The GmGST-ox Maple arrow transgenic plants at 3 dpi exhibited slight necrosis, and the lesion area was significantly smaller than that of non-transgenic Maple arrow. These results indicated that constitutive expression of GmGST not only enhances the resistance of susceptible soybean lines but also increases the resistance of partially resistant soybean lines to S. sclerotiorum.
The effect of the expression of the GmGST gene on SSR resistance-related traits. a Phenotyping of leaves from GmGST over-expressed Hefeng25 and Maple arrow and their wild-type plants after SSR infection for 3 days. b sgRNA mutation detection in soybean hair roots. c Phenotyping of leaves from GmGST-mutant Maple arrow and its wild-type plants after SSR infection for 3 days. d The soluble pigment content of over-expressed Hefeng25 and Maple arrow, GmGST-mutant Maple arrow and their wild-type plants. ** indicates significance at p < 0.01
Two sgRNAs that targeted GmGST were designed. The targeted DNA mutations were detected in soybean hairy roots. There was a base deletion in the sgRNA-1 target region at the sgRNA-1 site, which was identified in six of the ten independent transgenic hairy roots, with a mutation frequency of 60% (Fig. 4b). A total of three transgenic lines that had a mutation at the GmGST-sgRNA-1 site were created. The leaves of wild-type Maple arrow plants and GmGST-mutant (GmGST-mt) plants were inoculated with S. sclerotiorum.
The leaves of GmGST-mt plants exhibited obvious, large, water-soaked lesions at 3 dpi (Fig. 4c). In contrast, the leaves of wild-type Maple arrow showed only a slight lesion, and the lesion was significantly smaller than those of the GmGST-mt plants (P < 0.01). These results indicated that the resistance of GmGST-mt plants to SSR was weakened.
To verify whether the GmGST gene influences the soluble pigment content in soybean stems, the soluble pigment contents of the stems of Hefeng25-ox, Maple arrow-ox, GmGST-mt Maple arrow were compared. The soluble pigment contents in the stems of the GmGST-ox line were significantly higher than those in the wild type (Fig. 4d). The average soluble pigment content of Hefeng25-ox plants was 2.33 times higher than that of Hefeng25 wild-type plants, with an increase of 133.3%. The average soluble pigment content of Maple arrow-ox plants was 1.75 times higher than that of Maple arrow wild-type plants, with an increase of 75%. Correspondingly, the soluble pigment contents in the stems of the GmGST-mt line were significantly lower than those in the wild type (a decrease of 69.15%; Fig. 4d).
Discussion
Soybean resistance to SSR is complex in terms of the pathogenic mechanism and resistance pathway and is related to the scope of the quantitative traits (Sun et al. 2020; Vuong et al. 2015). Five QTLs and eight QTNs underlying soybean resistance to SSR were identified. Remarkably, the major locus, qDRS13-1, underlying the seedling death rate after S. sclerotiorum inoculation was detected by linkage mapping and GWAS. It was also reported a major locus overlapping with qDRS13-1 and found it controlled an SSR resistance-related trait, the soluble pigment content in soybean stems under oxalic acid treatment, which can be detected by two types of mapping populations (Zhao et al. 2015). The results from the present study and the previous study reported by Zhao et al. (2015) supported the deduction that qDRS13-1 should be a stable QTL related to soybean resistance to SSR.
Increasing evidence has shown that the levels of transcription and enzymatic activity of plant glutathione S-transferases (GST) are regulated by pathogen invasion (Dudler et al. 1991; Hahn and Strittmatter 1994; Liao et al. 2014). For example, a GST gene, HaGSTp1, cloned from in sunflower (Helianthus annuus L.) was induced by S. sclerotiorum and oxalic acid, overexpression of the HaGSTp1 gene enhanced S. sclerotiorum and oxalic acid resistance in tobacco (Ma et al. 2018a, b). Similarly, a soybean GST gene, GmGST, as one of candidate genes in qDRS13-1 locus, was screened by analyzing expression abundance in the present study. Our results showed that GmGST was upregulated in disease-resistant variety Maple arrow after S. sclerotiorum infection. Hence, GST gene might be the causal gene of qDRS13-1 that involved in the resistance of soybean to the infection of S. sclerotiorum.
In soybean, a variation in expression patterns was found among tau, phi, lambda, and TCHQD class GSTs, whereas all of the EF1Bγ, DHAR, theta, and zeta GSTs were expressed in all tissues of the terminal bud, unifoliate leaves, cotyledons, hypocotyl, roots, pods, stems, trifoliate leaves and flowers (Liu et al. 2015). Consistently, our results showed that GmGST gene was expressed in all tested tissues in the present study (Fig. 3a). Remarkably, the transcript abundance of GmGST was higher in resistant line Maple arrow than in susceptible line Hefeng25 in almost all the tested tissues except roots and cotyledons. According to the gene annotation, GmGST belongs to GSTs, i.e., the EF1Bγ class. Previously, it was shown that the loss of eukaryotic elongation factor 1 (eEF1Bγ) from the yeast Saccharomyces cerevisiae resulted in resistance to oxidative stress (Esposito and Kinzy 2010). Our result showed that overexpression of GmGST increased the soybean resistance to S. sclerotiorum and the resistance to S. sclerotiorum decreased in GmGST gene-mutant plants. GSTs are involved in the detoxification of xenobiotic and endobiotic compounds by conjugating glutathione (GSH) to various hydrophobic and electrophilic substrates (Messner et al. 2003). All three GST genes of the EF1Bγ class showed GSH-conjugating activity with CDNB and DCNB in Populus (Liu et al. 2015). Hence, these results suggest that the GST genes of the EF1Bγ class might have detoxification and antioxidant functions, and GmGST might be involved in the reaction against S. sclerotiorum as a GST, i.e., the EF1Bγ class. The soluble pigment content in the stem, based on the oxalic acid reaction, was found to be positively correlated with SSR resistance of soybeans under both controlled environmental and field conditions (Li et al. 2009a; Wegulo et al. 1998a). As one of the soluble pigment components, anthocyanins were reported to be involved in the resistance to plant diseases. For example, anthocyanin extracts from sugarcane cultivars had an inhibitory effect on conidial germination (Padmanaban et al. 2000). GSTs is involved in a late step of anthocyanin biosynthesis in plants (Alfenito et al. 1998). Our results showed that overexpression of GmGST significantly increased the soluble pigment content in soybean stems after exogenous oxalic acid treatment and the soluble pigment content significantly decreased in the stem of GmGST-mutant plants. These results suggest that the GmGST gene might play a role in SSR resistance of soybean partially by regulating the anthocyanin content in stems. However, the mechanism of GmGST-mediated soybean resistance to SSR still needs further study.
References
Alfenito MR, Souer E, Goodman CD, Buell R, Mol J, Koes R, Walbot V (1998) Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell 10:1135–1149
Bastien M, Huynh TT, Giroux G, Iquira E, Belzile F (2012) A reproducible assay for measuring partial resistance to Sclerotinia sclerotiorum in soybean. Can J Plant Ence 92:279–288
Bastien M, Sonah H, Belzile F (2014) Genome wide association mapping of sclerotinia sclerotiorum resistance in soybean with a genotyping-by-sequencing approach. The Plant Genome 7:1–13
Boland GJ, Hall R (1986) Growth room evaluation of soybean cultivars for resistance to Sclerotinta sclerotiorum. Can J Plant Sci 66:559–564
Bradbury P, Zhang Z, Kroon D, Casstevens T, Y, Buckler E, (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23:2633–2635
Briard M, Dutertre M, Brygoo Y (1997) Du nouveau du côté des Sclerotinia. Phytoma 490:1519
Cline MN, Jacobsen BJ (1983) Methods for evaluating soybean cultivars for resistance to Sclerotinia sclerotiorum. Plant Dis 67:784–786
Coyne DP, Steadman JR, Anderson FN (1974) Effect of modified plant architecture of great northern dry bean varieties (Phaseolus vulgaris) on white mold severity, and components of yield. Plant Dis Rep 58:379–382
David G, Nelson RT, Cannon SB, Shoemaker RC (2010) Soybase, the usda-ars soybean genetics and genomics database. Nucl Acids Res 38(suppl_1):D843–D846
Dudler R, Hertig C, Rebmann G, Bull J, Mauch F (1991) Pathogen-induced genes in wheat. In: Hennecke H, Verma DPS (eds) Advances in molecular genetics of plant-microbe interactions, vol. 1. Current plant science and biotechnology in agriculture, vol 10. Springer, Dordrecht
Esposito AM, Kinzy TG (2010) The eukaryotic translation elongation Factor 1Bgamma has a non-guanine nucleotide exchange factor role in protein metabolism. J Biol Chem 285:37995–38004
Goodstein DM, Shu S, Russell H, Rochak N, Hayes RD, Joni F, Therese M, William D, Uffe H, Nicholas P (2012) Phytozome: a comparative platform for green plant genomics. Nucl Acids Res 40:1178–1186
Grau CR (1982) Resistance of soybean cultivars to Sclerotinia sclerotiorum. Plant Dis 66:506–508
Hahn K, Strittmatter G (1994) Pathogen-defence gene prp1-1 from potato encodes an auxin-responsive glutathione S-transferase. Eur J Biochem 226(2):619–626
Han Y, Zhao X, Liu D, Li Y, Lightfoot DA, Yang Z, Zhao L, Zhou G, Wang Z, Huang L, Zhang Z, Qiu L, Zheng H, Li W (2016) Domestication footprints anchor genomic regions of agronomic importance in soybeans. New Phytol 209:871–884
He J, Meng S, Zhao T, Xing G, Gai J (2017) An innovative procedure of genome-wide association analysis fits studies on germplasm population and plant breeding. Tagtheoretical Appl Geneticstheoretische Und Angewandte Genetik 130:2327
Kim HS, Diers BW (2000) Inheritance of partial resistance to sclerotinia stem rot in soybean. Crop Ence 40:5561
Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugen 12
Kull LS, Vuong TD, Powers KS, Eskridge KM, Steadman JR, Hartman GL (2003) Evaluation of resistance screening methods for Sclerotinia stem rot of soybean and dry bean. Plant Dis 87:1471–1476
Kurle JE, Grau CR, Oplinger ES, Mengistu A (2001) Tillage, crop sequence, and cultivar effects on sclerotinia stem rot incidence and yield in soybean. Agron J 93:973–982
Lei Y, Lu L, Liu HY, Li S, Xing F, Chen LL (2014) CRISPR-P: a web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol Plant 7:1494–1496
Liao W, Ji L, Wang J, Chen Z, Ye M, Ma H, An X (2014) Identification of glutathione S-transferase genes responding to pathogen infestation in Populus tomentosa. Funct Integr Genomics 14:517–529
Li D, Sun M, Han Y, Teng W, Li W (2009a) Identification of QTL underlying soluble pigment content in soybean stems related to resistance to soybean white mold (Sclerotinia sclerotiorum). Euphytica 172:49–57
Li R, Yu C, Li Y, Lam T-W, Yiu S-M, Kristiansen K, Wang J (2009b) SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25:1966–1967
Li Y-h, Reif J, Ma Y-s, Hong H-l, Liu Z-x (2015) Targeted association mapping demonstrating the complex molecular genetics of fatty acid formation in soybean. BMC Genom 16:841
Liu D, Ma C, Hong W, Huang L, Liu M, Liu H, Zeng H, Deng D, Xin H, Song J, Xu C, Sun X, Hou X, Wang X, Zheng H (2014) Construction and analysis of high-density linkage map using high-throughput sequencing data. PLoS ONE 9:e98855
Liu HJ, Tang ZX, Han XM, Yang ZL, Zhang FM, Yang HL, Liu YJ, Zeng QY (2015) Divergence in enzymatic activities in the soybean GST supergene family provides new insight into the evolutionary dynamics of whole-genome duplicates. Mol Biol Evol 32:2844–2859
Livak KJ, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 25:402–408
Ma L, Zhang Y, Meng Q, Shi F, Jia L, Li Y (2018a) Enhancement of and oxalic acid resistance in tobacco by a novel pathogen-induced gst gene from sunflower. Crop Sci 58(3):1318–1327
Ma L, Zhang Y, Meng Q, Shi F, Liu J, Li Y (2018b) Molecular cloning, identification of GSTs family in sunflower and their regulatory roles in biotic and abiotic stress. World J Microbiol Biotechnol 34(8):109
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly MJGr, (2010) The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genom Res 20:1297–1303
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. The Crop J 3:269–283
Messner B., Thulke O., Schäffner A.R. (2003) Arabidopsis glucosyltransferases with activities toward both endogenous and xenobiotic substrates. Planta 217:138–146
Mila AL, Yang XB (2008) Effects of fluctuating soil temperature and water potential on sclerotia germination and apothecial production of sclerotinia sclerotiorum. Plant Dis 92:78–82
Moellers TC, Singh A, Zhang J, Brungardt J, Kabbage M, Mueller DS, Grau CR, Ranjan A, Smith DL, Chowda-Reddy RV (2017) Main and epistatic loci studies in soybean for Sclerotinia sclerotiorum resistance reveal multiple modes of resistance in multi-environments. Sci Rep 7:3554
Mueller DS, Bradley CA, Grau CR, Gaska JM, Kurle JE, Pedersen WL (2004) Application of thiophanate-methyl at different host growth stages for management of sclerotinia stem rot in soybean. Crop Prot 23:983–988
Mueller DS, Dorrance AE, Derksen RC, Ozkan E, Pedersen WL (2002) Efficacy of fungicides on sclerotinia sclerotiorum and their potential for control of sclerotinia stem rot on soybean. Plant Dis 86:26–31
Padmanaban P, Viswanathan R, Mohanraj D, Section PP, Institute SB, Coimbatore I (2000) Possible involvement of anthocyanin compounds in resistance of sugarcane against red rot. Indian Phytopathol 53:311–313
Paz MM, Shou HX, Guo ZB, Zhang ZY, Banerjee AK, Wang K (2004) Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica 136:167–179
Peltier AJ, Bradley CA, Chilvers MI, Malvick DK, Mueller DS, Wise KA, Esker PD (2012) Biology, yield loss, and control of sclerotinia stem rot of soybean. J Int Pest Manag 3(2):B1–B7
Rousseau G, Huynh Thanh T, Dostaler D, Rioux S (2004) Greenhouse and field assessments of resistance in soybean inoculated with sclerotia, mycelium, and ascospores of Sclerotinia sclerotiorum. Can J Plant Ence 84:615
Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng JJn (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Šlndelářová M, Šlndelář L (1991) Subcellular localization of glucose-6-phosphate dehydrogenase in tobacco mesophyll protoplasts. Biol Plant 33:150–155
Sun M, Jing Y, Zhao X, Teng W, Qiu L, Zheng H, Li W, Han Y (2020) Genome-wide association study of partial resistance to sclerotinia stem rot of cultivated soybean based on the detached leaf method. PLoS ONE 15:e0233366
Sun X, Hu Z, Chen R, Jiang Q, Song G, Zhang H, Xi Y (2015) Targeted mutagenesis in soybean using the CRISPR-Cas9 system. Rep 5:10342
Sun X, Liu D, Zhang X, Li W, Liu H, Hong W, Jiang C, Guan N, Ma C, Zeng H, Xu C, Song J, Huang L, Wang C, Shi J, Wang R, Zheng X, Lu C, Wang X, Zheng H (2013) SLAF-seq: an efficient method of large-scale de novo SNP discovery and genotyping using high-throughput sequencing. PLoS ONE 8:e58700
Vuong TD, Diers BW, Hartman GL (2015) Identification of QTL for resistance to sclerotinia stem rot in soybean plant introduction 194639. Crop Ence 48:2209–2214
Wang Z, Ma LY, Cao J, Li YL, Ding LN, Zhu KM, Yang YH, Tan XL (2019) Recent Advances in Mechanisms s of Plant Defense to Sclerotinia sclerotiorum. Front Plant Sci 10:1314
Wegulo SN, Yang XB, Martinson CA (1998a) Soybean cultivar responses to Sclerotinia sclerotiorum in field and controlled environment studies. Plant Dis 82:1264–1270
Zhao X, Han Y, Li Y, Liu D, Sun M, Zhao Y, Lv C, Li D, Yang Z, Huang L (2015) Loci and candidate gene identification for resistance to Sclerotinia sclerotiorum in soybean (Glycine max L. Merr.) via association and linkage maps. Plant J Cell Mol Biol 82:245–255
Acknowledgements
This study was conducted in the Key Laboratory of Soybean Biology of the Chinese Education Ministry, Soybean Research & Development Center (CARS), and the Key Laboratory of Northeastern Soybean Biology and Breeding/Genetics of the Chinese Agriculture Ministry and was financially supported by the Chinese National Natural Science Foundation (31871650, 31671717, 31471517, 31971967), National Key R & D Project (2016YFD0100304, 2017YFD0101306, 2017YFD0101302), Heilongjiang Provincial Project (JC2018007, GX17B002, C2018016, GJ2018GJ0098),the National Project (2014BAD22B01, 2016ZX08004001-007), the Youth Leading Talent Project of the Ministry of Science and Technology in China (2015RA228), The National Ten-thousand Talents Program, Postdoctoral Fund in Heilongjiang Province (LBH-Z15017), The national project (CARS-04-PS04). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
YPH and XZ designed and supervised the research; JNZ, WJL and YTZ conducted the experiment and analyzed the data; WS, HPJ, YHZ, JYZ and WLT conducted the phenotype identification and transgenic work. XZ, JNZ and LJQ wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors have adhered to the ethical responsibilities outlined by Theoretical and Applied Genetics.
Additional information
Communicated by Volker Hahn.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jianan, Z., Li, W., Zhang, Y. et al. Identification of glutathione transferase gene associated with partial resistance to Sclerotinia stem rot of soybean using genome-wide association and linkage mapping. Theor Appl Genet 134, 2699–2709 (2021). https://doi.org/10.1007/s00122-021-03855-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-021-03855-6