Abstract
Fruit set and fruit development can be limited due to ineffective pollination in off-season crops of Cucurbita pepo. To avoid this problem, parthenocarpy, the natural or artificial fruit development without fertilization, is required. The application of synthetic growth regulators is a common practice for inducing stimulative parthenocarpy in zucchini cultivars, but this method increases production costs and may cause other fruit defects. The disadvantages associated with this can be overcome through the use of vegetative parthenocarpic cultivars, which allow fruit set without any external stimuli. Three zucchini cultivars have been studied and differences have been found in parthenocarpic fruit development. Ethylene release of unpollinated fruit has corroborated the parthenocarpic fruit development. Vegetative parthenocarpy was observed in the Whitaker cultivar. Furthermore, the involvement of the auxin signalling pathway in controlling fruit set and parthenocarpy have been studied. Transcriptome analysis of auxin signalling genes, CpARF8, CpIAA9 and CpTIR1, have shown tissue-specific expression and have revealed a decrease in the expression levels of these genes in pollinated fruits after the fertilization signal, indicating their role in the transition from ovary to fruit. Nevertheless, it has also been shown that expression of these genes can be different between parthenocarpic cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cucurbita pepo represents one of the main species of vegetables crops in terms of human consumption. Zucchini, the most economically valuable morphotype of this species, is harvested as immature a few days after fruit set, when the fruit reaches 20 to 25 cm in length (Paris 2000). Pollination and fruit set trigger fruit development, becoming an essential process of crop yield. Ineffective pollination/fertilization, produced by low or high temperatures or inadequate humidity, causes fruit yield decline as a result of a decrease of fruit set (Picken 1984; Nepi and Pacini 1993; Nepi et al. 2010). Vegetative or stimulative parthenocarpy occurs when the ovary of a flower develops into a fruit without fertilization (Durner 2013), and is applied to avoid this problem. The application of synthetic growth regulators is a common practice for inducing stimulative parthenocarpy in zucchini cultivars (Gustafson 1942) and is specially required to adapt cultivars to winter conditions in greenhouses (off-season crops), when pollinating insects are less active. This practice increases production costs and may cause fruit defects (Vivian-Smith and Koltunow 1999; Fu et al. 2008; De Jong et al. 2009a, b). The drawbacks associated with the application of synthetic growth regulators can be overcome through the use of parthenocarpic cultivars as with Cucumis sativus (De Ponti and Garretsen 1976). However, vegetative parthenocarpy is still of limited use in zucchini, producing low yields in field crops.
Most genes involved in fruit set have been related to growth regulators of fruit development as auxins, gibberellins and cytokinins (Ozga and Reinecke 2003; Srivastava and Handa 2005; De Jong et al. 2009a, b). In addition, molecular biology studies have determined that parthenocarpy could be controlled by a single gene, such as a transcriptional factor or receptor of the phytohormone signalling pathways (Goetz et al. 2006, 2007; Martí et al. 2007; Serrani et al. 2010; Fuentes et al. 2012). This is the case of two important auxin-responsive gene families such as ARF (Auxin Response Factor) and Aux/IAA that have been related to the development of parthenocarpic fruit in Arabidopsis thaliana and Solanum lycopersicum (Kumar et al. 2011).
Sequence analyses of the highly conserved domains of ARFs and Aux/IAA families have shown how small changes in the protein domains can transform the normal fruit set into parthenocarpic fruit development. A typical ARF protein contains a conserved N-terminal B3-like DNA-binding domain (DBD) that includes an ARF family-specific domain (referred to as AUX_RESP domain), a variable middle region (MR) and a C-terminal Aux/IAA domain (CTD). The amino acid composition of the variable middle region is critical in determining the function of ARFs (Tiwari et al. 2003, 2004; Guilfoyle and Hagen 2007). MR rich in glutamine (Q-rich) are activation domains, whereas those rich in proline, serine and threonine (PST-rich) are repressor domains (Wu et al. 2014). On the other hand, Aux/IAA proteins generally have four characteristic domains. Domain I contains a functionally characterized transcriptional repressor motif while domain II interacts with a component of the ubiquitin-proteasome protein degradation pathway, which is essential for auxin signalling. Domains III and IV act as C-terminal dimerization domains, mediating homodimerization and heterodimerization among Aux/IAA family members and dimerization with similar domains found in ARFs (Tiwari et al. 2004; Guilfoyle and Hagen 2012).
Involvement of the auxin signalling pathway in controlling fruit initiation has been studied in S. lycopersicum and A. thaliana. Two components of the auxin signalling pathway have been implicated in repressing fruit set in the absence of fertilization, auxin response factor 8 (ARF8) and the Aux/IAA protein IAA9, that can directly block transcription of fruit initiation genes (Vivian-Smith et al. 2001; Wang et al. 2005; Goetz et al. 2006). After pollination and fertilization, auxins act binding to their receptor TIR1 (Transport Inhibitor Response 1) promoting degradation of Aux/IAA9 via the ubiquitin-proteasome system (Goetz et al. 2007). In the absence of IAA9, ARF8 can stimulate expression of early response genes, initiating fruit development (Goetz et al. 2006). Recessive mutations in Arabidopsis ARF8 (mutant fwf, fruit without fertilization) and introduction of Arabidopsis ARF8 with point mutations in the ARF8 gene in tomato induced parthenocarpy in these species (Goetz et al. 2006, 2007). On the other hand, downregulation of the Aux/IAA9 gene (Wang et al. 2005) or one single-base deletion mutant in S. lycopersicum (Mazzucato et al. 2015) resulted in parthenocarpic fruit development. As ARF8 and IAA9 genes, TIR1 has also been involved in fruit development without pollination/fertilization in C. sativus (Cui et al. 2014) and S. lycopersicum (Ren et al. 2011).
Several research programmes have focused on evaluating the parthenocarpic potential of groups of C. pepo cultivars but not on determination of the function of these genes during fruit development in this species (Durham 1925; Nitsch et al. 1952; Martínez et al. 2014). The first cultivar described as vegetative parthenocarpic was Whitaker, and most recently, three commercialized hybrids cultivars, Cavili, Parthenon and Argo, have displayed parthenocarpic potential and a characteristic ethylene profile during early fruit development related to parthenocarpic fruit set (Martínez et al. 2014). However, there are no studies aimed at identifying how auxins and auxin signalling genes as ARF8, IAA9 and TIR1 control fruit development in zucchini. In this work, the significance of these genes to induce fruit development and parthenocarpy in this species will be determined by a genomic and transcriptomic analysis in different zucchini cultivars. The expected results could be valuable to define the possibilities of integrating these genes in zucchini breeding programmes assisted by specific molecular tools, and to elucidate the role of these genes in controlling fruit set in this species.
Materials and methods
Plants, growing conditions and treatments
Plants of the parthenocarpic Cavili (Numhens® Bayer CropScience), the parthenocarpic Whitaker (New York State Agriculture Experiment Station, New York, USA) and the non-parthenocarpic Cucurbita pepo subsp. pepo L. Zucchini Group ‘MUCU-16’ (Cucurbita core collection of the Cucurbits Breeding Group from the COMAV-UPV) were grown under the same conditions in a greenhouse at the IFAPA research centre in Almeria (Spain), following standard local commercial practises for both plant nutrition and pest and disease control. Three treatments were applied on the three cultivars: pollination, non-pollination and synthetic auxins. In all cases, female flowers were protected with paper bags before anthesis to prevent the transfer of pollen by insects. Only female flowers were selected for treatments. Paper bags were removed in anthesis early in the morning and female flowers were self-pollinated by hand for pollination or treated with 0.5 ml of 0.8% of synthetic auxins “fruitone” (0.45% 1-naphthalene acetic acid, 1.2% 1-naphthaleneacetamide) for auxin treatment (Martínez et al. 2013). In non-pollination treatment, paper bags were removed after anthesis to claim that ovaries were not pollinated.
Fruit growth parameters and ethylene production
Fruit growth parameters were compared among pollinated, non-pollinated and auxin treated ovaries/fruits in each cultivar. Fruit length, fruit diameter (measured on apex), placental diameter and pericarp thickness were measured on twelve ovaries/fruits collected from anthesis to 5 days post anthesis (DPA). To determinate ethylene production, fruits were harvested at the same DPA, and ethylene production was measured on four replicates of three ovaries/fruits in each one. For this purpose, three ovaries/fruits at the same developmental stage were enclosed in sealed containers for 24 h, and the produced ethylene was determined by gas chromatography. The production of ethylene of each sample was repeated four times in a VARIAN 3900 gas chromatograph equipped with a flame ionization detector (FID).
Searching for target gene sequences and phylogenetic analysis
To find the sequences of the genes ARF8, IAA9, TIR1 in C. pepo, we initially surveyed the Cucurbita pepo COMAV transcriptome v3 (http://cucurbigene.net/db/transcriptome_v3/) through Nucleotide BLAST using the CDS sequences of Arabidopsis, tomato, cucumber and melon as queries. All sequences identified in the Cucurbita pepo transcriptome were used as query in the BLASTN searches against the NCBI database to verify the Cucurbita pepo CDS for each gene. The protein sequences were obtained from Cucurbita pepo COMAV transcriptome v3. All peptide sequences were used as a query in the BLAST searches on NCBI’s CDD tool to find their potential functional domains (Marchler-Bauer et al. 2015). The Pfam 29.0 database was used to confirm the presence of conserved domains under an E-value level of 1.0 (http://pfam.sanger.ac.uk/). Multiple sequence alignments were carried out in Clustal X v2.0 (Larkin et al. 2007). Pairwise comparisons were made using Geneious R8 v8.0.4 using the protein sequences alignment. A Neighbour-Joining tree was constructed by using the algorithm based on the Tamura-Nei model available in the MEGA 6.0 program (Tamura et al. 2013) with 1000 bootstrap replications to assess the robustness of the tree. The phylogenetic tree was constructed with the protein sequences collected from the NCBI database and CpARF8, CpIAA9 and CpTIR1 peptides.
Gene expression analysis
Young stems and pericarp of three ovaries/fruits from pre-anthesis to 7 DPA, were frozen in liquid nitrogen and stored at −80 °C prior to RNA extraction. To determine the gene expression in all treatments in the three cultivars, two replications per sample were performed. Each replication was the result of an independent extraction of total RNA from the sample.
Total RNA was extracted using the TRIzol® reagent (Ambion®) according to manufacturer’s instructions. RNA concentration and purity were determined with a Nanodrop 200 Spectrophotometer (Eppendorf, Hamburg, Germany), which calculated absorbance at 260 nm. Only RNA samples with 260/280 ratios between 1.9 and 2.1 and 260/230 ratios of >2.0 were used for cDNA synthesis. cDNA was synthesized from 1 μg of total RNA for each sample using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems®) with the use of the random primers according to the manufacturer’s instructions. Prior to cDNA synthesis, genomic DNA was efficiently removed with DNAse I Amplification Grade (Invitrogen™). A positive control was included to test for contaminating genomic DNA. Specific primer pairs for qPCR amplification of target genes were designed with PRIMER3 software (Untergasser et al. 2012) using sequences references in the Cucurbigene database (Table 1). qPCR was performed using the LightCycler system (Lightcycler 1.5, Roche).
All reactions were performed using the SYBR Green master mix (Roche) following the procedure described by the manufacturer. PCR conditions were as follows: pre-incubation at 95 °C for 5 min followed by 40 cycles, each consisting of 10 s at 95 °C, 10 s at 60 °C and 20 s at 72 °C. Finally, a dissociation analysis of the PCR products was performed by running a gradient from 65 to 95 °C to confirm the presence of a single PCR product. Two technical replicates were run for each sample, with a negative control using water as template. Expression levels of the target genes were calculated using the advanced relative quantification model with efficiency correction, multiple reference gene normalization and the use of error propagation rules (Hellemans et al. 2007). Based on previous results (Obrero et al. 2011 ), two genes (CpPP2A and CpEF1α) were selected as reference genes to normalize. The qPCR product for each target gene was cloned into the pGEM-T vector (Promega, Madison, WI, USA), and sequenced (University of Almeria, Spain). The sequence of the amplification product for each primer pair was compared with the amplicon selected in the Cucurbigene database (Table 1) using the BioEdit programme v7.2.5.
Statistical analysis
Simple analysis of variance (ANOVA) at p < 0.05 was performed by the Statistix v.9 software, and each two means were compared with the method of Tukey’s honest significance test for fruit growth parameters and ethylene production. To apply these statistical techniques, the variables were required to follow a normal distribution. Exploratory data analysis of gene expression data (multi-dimensional scaling plot, cluster dendogram and hierarchical clustering) was done with R 3.2.5 to visualize and summarize the overall quality of the data and identify general patterns. Prior to this analysis, data were log transformed.
Results
Differences of early fruit development in zucchini
Fruit development has been evaluated in three cultivars, a non-parthenocarpic cultivar, MUCU-16, and two parthenocarpic cultivars, Cavili and Whitaker. There were substantial differences in harvested fruits from anthesis to 5 DPA, comparing pollinated respect to unpollinated fruit. Morphological and histological changes are clearly differentiated in the non-parthenocarpic cultivar. Fruits differed in shape and placental tissue, observed by sectioning the style in both treatments (Fig. 1). Pollinated fruit of MUCU-16 showed a cylindrical-elongated shape with dark green colour skin without changes in the stylar, similar to the other cultivars (Fig. 1a). The placental tissue was translucent light green along those stages, shiny and with small developing seeds (Fig. 1b). In contrast, unpollinated fruit suffered the aborting process from 3 DPA, characterized by the flattening of fruit walls and apex deformity (Fig. 1c). Moreover, placental and mesocarp tissue displayed a process of senescence that produced dark tissue and the absence of developing seeds (Fig. 1d).
Morphological changes occurring during early zucchini fruit development. a Pollinated fruit of the non-parthenocarpic cultivar MUCU-16 from anthesis until 5 DPA. b Section of the style of pollinated fruit of the non-parthenocarpic cultivar MUCU-16 from anthesis until 5DPA. c Unpollinated fruit of the non-parthenocarpic cultivar MUCU-16 from anthesis until 5 DPA. d Section of the stylar region of unpollinated fruit of the non-parthenocarpic cultivar MUCU-16 from anthesis until 5 DPA
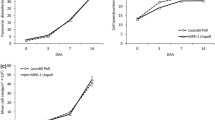
Fruit growth changed under different treatments. Pollinated fruit of the non-parthenocarpic cultivar MUCU-16 showed an exponential phase of growth from 3 DPA, with significant differences in fruit length and placental diameter, in respect to unpollinated and auxin treated fruit. Unpollinated fruit delayed its growth and did not reach the exponential phase observed in pollinated fruit (Fig. 2a). The application of synthetic auxins did not promote the same effect in fruit growth as pollination in this cultivar, showing a growth slope similar to unpollinated, although with slightly significant differences in the later stages. In consequence, pollinated fruit reached the commercial size (≥ 20 cm of fruit length) but auxin treated fruit only reached 15 cm after the same number of growth days (Fig. 2a).
Fruit growth of zucchini fruit. Increase of fruit length and placental diameter as a function of days post anthesis (DPA) in unpollinated fruit (UF), pollinated fruit (PF) and auxin treated fruit (AF) in the cultivars a MUCU-16, b Cavili and c Whitaker. Values correspond to means (n = 12). Error bars indicate the SE and asterisks indicate a significant difference from the mean of each treatment in the same DPA (*P < 0.05)
Changes during the early fruit development were also observed in the parthenocarpic cultivars. In contrast to MUCU-16, unpollinated fruit of Cavili rapidly increased in size after 3 DPA, showing no significant differences in length with pollinated fruit (Fig. 2b). Surprisingly, the application of synthetic auxins did not produce an intermediate growth between pollination and non-pollination in fruits of this cultivar. Auxin treated fruits displayed the lowest length at 4 DPA (Fig. 2b). Regarding placental diameter, in pollinated fruit it was higher than in unpollinated and auxin treated fruit from 3 DPA (Fig. 2b). The absence of positive growth signals such as pollination or synthetic auxins did not produce changes in fruit length and placental diameter in Whitaker. All fruits of this genotype grew similarly regardless of the treatment applied (Fig. 2c).
The internal development was analysed through fruit diameter and pericarp thickness (Fig. S1). In the non-parthenocarpic variety, fruit diameter showed that there were great differences between treatments at 5 DPA. At this stage, fruit diameter reached approximately 30 mm in unpollination treatment, 55 mm in pollination and 34 mm in auxin treatment. In the case of parthenocarpic cultivars, fruit diameter of Cavili also displayed changes depending on the treatment applied, showing the highest value in pollinated fruit. Conversely, fruit diameter of Whitaker maintained similar growth between treatments, not suffering changes by the application of auxins or pollination.
Determination of fruit ethylene pattern
Fruit development has been associated with a distinctive pattern of ethylene release in zucchini (Martínez et al. 2014). In our case, in the non-parthenocarpic cultivar (Fig. 3a), unpollinated fruit showed a peak in ethylene production at 3 DPA (7.63 nl/gFW), and production remained at high levels (4.9 nl/gFW) from this developmental stage with significant differences between treatments. In contrast, a decrease in ethylene production was observed in pollinated fruit and auxin treated fruit of this cultivar from 3 DPA. Pollinated fruit showed ten times less ethylene production with respect to unpollinated fruit, and the application of synthetic auxins produced two times less ethylene. The application of this phytohormone produced an intermediate level of ethylene between non-pollinated and pollinated fruit.
Evolution of ethylene production during early fruit development in zucchini fruits. Ethylene production as a function of days post anthesis (DPA) in unpollinated fruit (UF), pollinated fruit (PF) and auxin treated fruit (AF) in the cultivars a MUCU-16, b Cavili and, c Whitaker. Values correspond to means (n = 4). Error bars indicate the SE and asterisks indicate a significant difference from the mean of each treatment in the same DPA (*P < 0.05)
Ethylene production in the parthenocarpic cultivars remained low during fruit development from 3 DPA in all treatments (Fig. 3b and c). Levels ranging 0.3–1.5 nl/gFW were observed in both cultivars. In Cavili, auxin treated fruit decreased its ethylene production from 1 DPA, pollinated fruit decreased at 2 DPA, and unpollinated fruit declined at 3 DPA (Fig. 3b). In Whitaker, auxin treated fruit decreased its ethylene production at 1 DPA and pollinated and unpollinated fruit at 2 DPA. All fruits from these genotypes showed similar levels of ethylene, without significant differences at 3 DPA regardless of the treatment applied (Fig. 3c).
Identification and sequence analysis of CpARF8, CpIAA9 and CpTIR1
Extensive searches were carried out in the C. pepo transcriptome (https://cucurbigene.upv.es/), using all previously reported coding sequences for these genes in Arabidopsis, tomato, cucumber and melon as BLAST queries (Table 1). The sequence of target genes in zucchini were selected from thirteen sequences for CpARF8, six sequences for the CpIAA9 gene and four sequences for CpTIR1 according to their homology with cucumber and melon in the Cucurbita pepo transcriptome.
To study homology in selected sequences, pairwise sequence comparisons were carried out. Proteins of the gene TIR1 showed a high identity level (more than 70%) with respect to C. pepo, even in phylogenetically distant species such as Arabidopsis thaliana and S. lycopersicum (Fig. 4a). Homology analysis of CpARF8 protein also showed high identity between proteins (67–93%), being Cucurbita spp. proteins were more similar. The most dissimilar protein between species was IAA9, with a pairwise homology between species approximately 50–87% (Fig. 4a). An unrooted tree was constructed from an alignment of their protein sequences by the N-J method (Fig. 4b). In order to analyse the phylogenetic relationship between Arabidopsis, tomato (S. lycopersicum L.), cucumber (C. sativus), melon (Cucumis melo) and zucchini of ARF8, IAA9 and TIR1 genes, the proteins were clustered into three major groups corresponding to each gene family. Protein sequences of CpARF8, CpIAA9 and CpTIR1 grouped within a clade that comprises the protein sequences of C. sativus and C. melo (Fig. 4b).
Pairwise comparison and phylogenetic tree of ARF8 family members, IAA9 family members and TIR1 family members. a Pairwise comparison of the selected sequences in the NCBI database and the protein sequences of Cucurbigenes for each gene family. b Tree ofARF8 proteins, IAA9 proteins and TIR1 proteins of Arabidopsis (At) tomato (Sl), cucumber (Cs), melon (Cm) and zucchini (Cp). The unrooted tree was generated using the Clustal X v.2.0 program and the N-J method using MEGA 6.0. c Schematic representation of the sequence motifs found within ARF8 family members, IAA9 family members and TIR1 family members using NCBI’s CDD tool
The sequences obtained were analysed through NCBI’s CDD tool (Marchler-Bauer et al. 2015), and the Pfam 29.0 database to check their corresponding conserved domains. The predicted protein CpARF8 displayed the characteristic domains of the family gene, the plant-specific B3 DNA binding domain (pfam02362) between 127 and 228 aa, the conserved region of auxin-responsive transcription factor ARF (pfam06507) between 260 and 337 aa and Aux/IAA domain (pfam02309) between 719 and 810 aa. The middle region (MR) of the CpARF8 comprised the region between amino acids 338 and 718 and was characterized by 34 prolines, 50 serines and 45 glutamines. The latter was distributed in glutamine repeats. On the other hand, analysis of these conserved domains in the CpIAA9 protein showed the Aux/IAA domain (pfam02309) between 67 and 345 aa, also observed in CpARF8. In the conserved domain analysis, CpTIR1 protein showed structural motives leucine rich repeats and a F-box (pfam12937) between 7 and 44 aa (Fig. 4c).
Expression analysis of CpARF8, CpIAA9 and CpTIR1
Transcript levels of CpARF8, CpIAA9 and CpTIR1 were measured by qPCR from pre-anthesis to 7 DPA. The results were averaged between two biological replicates and analysed by dimensional scaling plot, cluster dendogram, and hierarchical clustering (Fig. S2).
CpARF8, CpIAA9 and CpTIR1 were detected in young leaves, immature ovaries (floral stage of preanthesis), ovaries (floral stage of anthesis) and during early fruit development (1 to 7 DPA) in each cultivar. Transcript levels of CpARF8 and CpIAA9 were lower in leaves compared to ovary/fruit, in contrast to CpTIR1 that showed higher expression levels in leaf (data not shown). Both floral stages maintained similar expression levels that changed after anthesis independent of the treatment applied (Fig. 5). In the non-parthenocarpic cultivar MUCU-16 (Fig. 5a), expression levels of these genes when the fruit was pollinated and throughout its development, did not exceed the amount of mRNA found in floral stages. CpARF8 showed similar levels to floral stages at 1 and 3 DPA, but between 1.6–1.8 fold lower at 5 and 7 DPA. CpIAA9 also showed a downregulation of 1.5-fold at 1 DPA, but expression levels were similar to floral stages from 3 DPA. The most significant changes in gene expression after pollination appeared in CpTIR1 which was downregulated up to 2.1-fold during fruit development with respect to the anthesis. However, these genes showed a general increase of transcript levels in unpollinated and auxin treated fruit of this cultivar with respect to the pollination treatment, with a steady accumulation along fruit development. CpARF8 and CpIAA9 were up-regulated at 5 and 7 DPA, and CpTIR1 displayed an increase of up to two fold with respect to levels found in pollinated fruit.
Relative expression levels of ARF8, IAA9 and TIR1 during early fruit development in zucchini. Expression was analysed from pre-anthesis (−1 DPA) until seven days post anthesis (7 DPA) in unpollinated fruit (UF), pollinated fruit (PF) and auxin treated fruit (AF) in each cultivar. Expression was normalized with two genes CpEFα1 and CpPP2A. Normalized values of relative expression are given as averages of two biological replicates. Bars indicate SE
Expression profiles of CpARF8, CpIAA9 and CpTIR1 differed between parthenocarpic cultivars. In Cavili cultivar (Fig. 5b), CpARF8 expression showed slight changes along fruit development with respect to floral stages; meanwhile, CpIAA9 constantly increased its levels up to four fold, from 3 DPA under the different treatments. CpTIR1 showed an up-regulation of over 1.3-fold with respect to preanthesis and anthesis, regardless of 1 DPA. At this stage, unpollinated fruit showed an increase of relative RNA expression amount, unobserved in auxin treated and pollinated fruit. In the Whitaker cultivar (Fig. 5c), these genes showed similar expression patterns, regardless of 1 DPA. At this stage, CpARF8 and CpIAA9 decreased their expression in pollinated fruit, unobserved in CpTIR1. From 3 to 5 DPA, the three genes displayed a decrease of expression in unpollinated fruits, a slight increase in auxin treated fruit and similar expression in pollinated fruit.
Discussion
Analysis of the development changes during fruit growth in zucchini
In general, fruit set and exponential growth are phases are clearly essential for early development of all fruits. The early fruit development is well characterized in other cucurbitaceous such as cucumber, and comprises phases of cell division and expansion that occur in the first days post anthesis in cucumber (Ando et al. 2012). However, there have been few detailed studies of the fruit development and these phases in C. pepo, essential for understanding the aborting process and the parthenocarpic fruit development in this species. In this work, it has been observed that in the non-parthenocarpic cultivar MUCU-16, the fruit decreased its growth without pollination or application of synthetic auxins, showing a deformed apex and brown necrotic placental tissue (Fig. 1). These morphological changes observed were signs of the aborting process in zucchini. The deformation of the apex might indicate a mismatch of the phases of cell division and cell expansion of the fruit walls (Ando et al. 2012). The senescent placental tissue might be due to an absence of developing seeds that synthesized high levels of plant growth hormones to induce the growth (Gillaspy et al. 1993). In contrast, the fruit received positive signals from pollination that stimulated its growth (Fig. 2a). Two phases have been distinguished in the early fruit development in zucchini, the fertilization phase and the signalling phase. The phase of fertilization was observed until 2 DPA, when the fruit grew more slowly. At this phase, the pollen tube reached the ovule and the fertilization occurred (Nepi and Pacini 1993). After this first phase, the signalling phase started. The fertilized ovules became developing seeds, which emitted signals that produced the exponential increase of fruit length and placental tissue (Gillaspy et al. 1993), as observed in pollinated fruit from 3 DPA in C. pepo (Fig. 1a).
On the other hand, the application of synthetic auxins also induced fruit growth in unpollinated fruit of the non-parthenocarpic cultivar (Fig. 2a) as in other horticultural plants (Serrani et al. 2008), suggesting that these synthetic hormones could replace the signals provided by pollination. However, the fruit length reached was lower than pollinated fruit. This supports the hypothesis that the addition of synthetic hormones does not initiate the same processes as natural pollination (Mapelli 1981; Wang et al. 2005; Vriezen et al. 2008).
Unpollinated fruit of both parthenocarpic cultivars grew and reached commercial size in the greenhouses. In Cavili cultivar, fruit growth changed depending on the treatment (Fig. 2b). Pollinated fruit reached the highest length and placental diameter in contrast to fruit treated with synthetic auxins that displayed the lowest length, also observed in fruits of gynoecious parthenocarpic cucumber treated with synthetic indole-3-acetic acid (Hikosaka and Sugiyama 2015). However, morphological changes that occurred during early fruit development were not affected by treatment (synthetic auxins and pollination) in Whitaker (Fig. 2c). This cultivar could be considered vegetative parthenocarpic and could be used for future genetic studies of parthenocarpy in zucchini.
Vegetative parthenocarpic fruit development is correlated with a decrease of ethylene biosynthesis
Ethylene has long been known to be involved in different phases of fruit development (Pandolfini 2009). This hormone plays a key role during fruit set, coordinating ovary growth and flower abscission after pollination (Balbi and Lomax 2003; Pandolfini 2009).
Recently, low levels of ethylene biosynthesis from 3 DPA were associated with parthenocarpic fruit development in selected cultivars of zucchini, including Cavili (Martínez et al. 2014). Whitaker has also displayed this particular pattern, but it declines in ethylene biosynthesis from 2 DPA (Figs. 2c and 3). This is similar to the pattern found in pollinated fruit of non-parthenocarpic cultivar. Although synthetic auxins or pollination was applied in fruit at anthesis, ethylene patterns did not change in these cultivars from 3 DPA. Application of several phytohormones promotes auxin accumulation in the ovary that could repress ethylene biosynthesis (Kim et al. 1992; Martínez et al. 2013). This auxin accumulation had already been associated with parthenocarpy fruit set in cucumber (Kim et al. 1992), and in a similar way, parthenocarpy could be produced by an increase in the internal levels of auxin in the ovary in zucchini.
In contrast to parthenocarpic varieties, unpollinated fruit of the non-parthenocarpic cultivar MUCU-16 showed high levels of ethylene at 3 DPA that decreases when the fruit is pollinated or treated with synthetic auxins (Fig. 3). Ethylene levels in auxin treated fruit did not decrease as in pollinated fruit suggesting that the addition of this growth regulator does not produce the same effect as pollination on the fruit (Mapelli 1981; Wang et al. 2005; Vriezen et al. 2008). Pollinated fruit and auxin treated fruit in this cultivar showed reduction of ethylene peak at 3 DPA similar to parthenocarpic cultivars, further evidencing that this could be promoted by the same stimuli, an accumulation of auxins in the zucchini ovary, preventing significant ethylene increase found on 3 DPA in unpollinated fruit in this cultivar (Fig. 3). This accumulation might be caused by non-senescent and viable ovules found in the fruit that are required to establish the parthenocarpic response in a similar way as Arabidopsis, where the number of viable ovules in the pistil is correlated with the size of parthenocarpic fruit (Carbonell-Bejerano et al. 2011).
Ethylene’s involvement in ovule senescence supports this previous evidence. Analysis of the expression of ethylene biosynthesis genes suggests that ethylene is synthesized in ovules at the onset of ovule senescence in unfertilised pistils of Arabidopsis (Carbonell-Bejerano et al. 2011). Probably, ovule senescence found in unpollinated zucchini fruit (Fig. 1) could induce ethylene biosynthesis at 3 DPA that would repress fruit growth.
These findings could demonstrate the antagonist link between ethylene and auxin during the transition from ovary to fruit in C. pepo. These important plant growth regulators have complex interactions in growth and developmental processes (Muday et al. 2012). Auxin is known to regulate specific genes involved in ethylene biosynthesis as ACS genes (Abel and Theologis 1996) and ethylene influences many features of auxin-dependent seedling growth in Arabidopsis (Muday et al. 2012). Additional insights are required to understand the signalling networks that regulate these synergistic and antagonist activities of ethylene and auxin in controlling tissue-specific growth and developmental responses. Low transcriptional level of ACS genes has been found in fruit treated with synthetic auxins in a non-parthenocarpic cultivar of zucchini (Martínez et al. 2013), more evidence of antagonist crosstalk of these regulators during fruit development in this species.
The expression of identified auxin signalling genes regulated the fruit set in Cucurbita pepo
Fruit development is closely related to auxin response genes as ARFs, Aux/IAAs, and TIRs encoded by multigene families (De Jong et al. 2009a; Pandolfini 2009; Serrani et al. 2008). The availability of genomic resources such as ESTs (http://cucurbigene.net/db/transcriptome_v3/) has provided a new opportunity to look for the members of these families in zucchini. Thus far, ARF genes and IAA genes have been identified in maize, tomato, grape and cucumber (Kumar et al. 2011; Wan et al. 2014; Wu et al. 2014; Xing et al. 2011). In zucchini, these genes have not been identified yet, and their function is unknown. Here, identification of CpARF8, CpIAA9 and CpTIR1 was performed in the zucchini coding sequences.
Sequence analysis of the ARF8, IAA9 and TIR1 zucchini proteins indicated that they contained the conserved domains identified for each protein family, which were the same found in S. lycopersicum (Kumar et al. 2011, Ren et al. 2011) and C. sativus (Cui et al. 2014, Wu et al. 2014). Phylogenetic analysis revealed the close relationship between ARF8 and IAA9 genes (Fig. 4). These proteins have been previously related in a complex that represses fruit initiation genes until the signal of fertilization in Arabidopsis. ARF8 is a repressor of fruit development, because it acts by enhancing the IAA9 repressive function (Goetz et al. 2006). Moreover, the middle region of CpARF8 showed a similar percentage of serines, threonines and prolines to other vegetables species, which seems to confirm the conservation of its function in zucchini.
Expression of CpARF8, CpIAA9 and CpTIR1 varied considerably between leaves and ovary/fruit in C. pepo. CpARF8 and CpIAA9 were mostly expressed in zucchini ovary implying that they might have important functions in fruit development, similar to other Cucurbitaceous (Wu et al. 2014). Since transcript abundance in a particular organ is an important factor in elucidating the function, CpTIR1 might have more important functions during the vegetative growth of zucchini plant because it is highly expressed in leaves. This tissue-specific expression was also observed in diverse species as C. sativus (Wu et al. 2014; Cui et al. 2014) and tomato (Kumar et al. 2011), suggesting a higher conservation of gene function.
Although several studies of genome wide expression of auxin related genes have recently published in maize, tomato, grape and cucumber (Kumar et al. 2011; Wan et al. 2014; Wu et al. 2014; Xing et al. 2011), there are no studies yet that link their expression during fruit development to parthenocarpic fruit growth in C. pepo. In the non-parthenocarpic cultivar, changes observed in the expression of these genes in pollinated fruit after the signal of fertilization are consistent with the important role of CpARF8, CpIAA9 and CpTIR1 in the transition from ovary to fruit, as occurred in C. sativus (Wu et al. 2014; Cui et al. 2014) and S. lycopersicum (Kumar et al. 2011; Ren et al. 2011). There is a general downregulation of these genes only under pollination treatment, showing a low quantity of RNA in contrast to unpollinated and auxin treated fruit. This could weaken their repressor action and allow fruit development (Goetz et al. 2007), showing similar regulation as in tomato, where IAA9 knock-out genotypes and downregulation of this gene showed a strong tendency to develop fruit without pollination (Wang et al. 2005; Mazzucato et al. 2015). In the absence of pollination signal, these genes were up-regulated implying that they might be functioning during the whole development of this organ, repressing fruit formation (Fig. 5). Moreover, exogenous auxins and unpollination treatments promoted similar expression patterns, but auxin treated fruit grew and unpollinated fruit showed the aborting process. Differences in the expression patterns between pollinated fruit and auxin treated fruit corroborate that the addition of synthetic growth regulators does not have the same effect as pollination (Vriezen et al. 2008). In this case, gene transcription does not seem to be regulated by auxin per se (Ulmasov et al. 1999a, b). These genes were included previously in a proposed model of fruit development activated in the presence of the fertilization cue. After pollination and fertilization occurs, auxin acts binding to its receptor TIR1 that indirectly regulated ARF8 activity by promoting turnover of Aux/IAA9 (Goetz et al. 2007). Our data suggested the presence of this pathway related to the fertilization cue in C. pepo. Fruit growth observed in auxin treatment could indicate the activation of alternative pathways, which might imply other members of these gene families that induce parthenocarpy by exogenous growth regulators (Kumar et al. 2011; Wu et al. 2014).
In contrast, regulation of fruit development in parthenocarpic cultivars varied considerably. Firstly, pollination did not promote a clear downregulation of these genes as in the case of the non-parthenocarpic cultivar. This treatment maintained and also increased RNA amount with respect to floral stages, as it is shown clearly for CpIAA9. These differences observed suggest an alternative pathway in fruit formation promoted by pollination in parthenocarpic cultivars. In Cavili, treatments did not vary greatly the effect observed in each stage of fruit development, increasing expression of CpIAA9 and, to a lesser extent, the expression of CpTIR1. CpIAA9 seems to be a key parthenocarpy related gene in Cavili, as reported in previous studies carried out in tomato (Wang et al. 2005; Mazzucato et al. 2015). On the other hand, expression of CpARF8 was maintained in this cultivar. Additionally, it seems that fruit formation is less dependent on differences in the regulation of these genes, depending more on the stage of fruit development. However, gene expression is affected by treatments in Whitaker cultivar. Pollination treatment presented a fluctuating pattern and was different for unpollination and auxin treatments, which displayed the same pattern. These changes in gene expression had no effect on fruit development in this cultivar, in contrast to Cavili cultivar (Figs. 2 and 5), observed mainly from 3 DPA. These differences in gene expression during parthenocarpic fruit development between Whitaker and Cavili relate these genes to the control of fruit development in Whitaker, which could promote the vegetative parthenocarpy.
Conclusion
The morphological-histological characterization carried out initially in zucchini allows distinguishing essential phases of early fruit development, including an exponential growth phase observed during pollination and parthenocarpic fruit development. Differences between both parthenocarpic cultivars analysed give evidence of different regulation of fruit formation. In Whitaker cultivar, a more homogenous fruit development was observed independent of the treatment applied, and pollination has no effect on ethylene profile. In contrast, fruit development changes under the different treatments in Cavili cultivar, and pollinated and unpollinated fruit showed different ethylene patterns. This data supports the vegetative parthenocarpy for Whitaker; meanwhile the parthenocarpy of Cavili, as other cultivars, is more dependent of treatments applied (pollination and auxin treatment).
Transcriptome analyses of CpARF8, CpIAA9 and CpTIR1 have revealed that they show tissue-specific expression, maintaining the structure and function showed in other species. These key auxin signalling genes showed a specific level of quantified mRNA in pre-anthesis and anthesis that changed after the fertilization cue, supporting their role in the preparation of the ovary to become fruit in zucchini. Pollination in the non-parthenocarpic cultivar MUCU-16 promotes downregulation of these genes and allows fruit development. In contrast, non-pollination and synthetic auxins promote up-regulation of these genes in this cultivar, which corroborate that the addition of synthetic growth regulators does not have the same effect as pollination. On the other hand, differences in expression data observed between the two parthenocarpic cultivars showed the complexity of the signalling process of fruit formation. The fruit of Cavili show gene expression more dependent on the stage of development. Although further experiments such as the induction of mutations in CpIAA9 are necessary, previous (Wang et al. 2005; Mazzucato et al. 2015) and present data suggest that this gene could be an important key for studying parthenocarpy. However, Whitaker shows differences in these patterns in response to different treatments, suggesting that other complementary pathways could regulate fruit formation. All these morphological, physiological and molecular evidence should be considered in breeding programmes to develop future parthenocarpic varieties, one of the main demands of zucchini crops.
Abbreviations
- Aux/IAA:
-
Auxin/indole-3-acetic acid
- ARF:
-
Auxin Response Factor
- AuxRE:
-
Auxin Response Element
- BLAST:
-
Basic Local Alignment Search Tool
- CDD:
-
Conserved Domain Database
- CDS:
-
Coding Sequence
- DPA:
-
Day Post Anthesis
- N-J:
-
Neighbour Joining
References
Abel S, Theologis A (1996) Early genes and Auxin action. Plant Physiol 111:9–17
Ando K, Carr KM, Grumet R (2012) Transcriptome analyses of early cucumber fruit growth identifies distinct gene modules associated with phases of development. BMC Genomics 13:518. doi:10.1186/1471-2164-13-51
Balbi V, Lomax TL (2003) Regulation of early tomato fruit development by the Diageotropica gene. Plant Physiol 131:186–197
Carbonell-Bejerano P, Urbez C, Granell A, Carbonell J, Perez-Amador MA (2011) Ethylene is involved in pistil fate by modulating the onset of ovule senescence and the GA-mediated fruit set in Arabidopsis. BMC Plant Biol 11:8. doi:10.1186/1471-2229-11-84
Cui L, Zhang T, Li J, Lou Q, Chen J (2014) Cloning and expression analysis of Cs-TIR1/AFB2: the fruit development-related genes of cucumber (Cucumis sativus L.) Acta Physiol Plant 36:139–149
De Jong M, Mariani C, Vriezen WH (2009a) The role of auxin and gibberellin in tomato fruit set. J Exp Bot 60:1523–1532
De Jong M, Wolters-Arts J, Feron R, Mariani C, Vriezen WH (2009b) The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J 57:160–170
De Ponti OMB, Garretsen F (1976) Inheritance of parthenocarpy in pickling cucumbers (Cucumis sativus L.) and linkage with other characters. Euphytica 25:633–642
Durham GB (1925) Has parthenogenesis been confused with hermaphroditism in Cucurbita? Amer Nat 59:283–294
Durner EF (2013) Physiology of growth in specific organs: flowers, fruits and seeds. In: CABI Publishing (ed). Principles of horticulture physiology, 1st edn. Oxfordshire, pp 57–83
Fu FQ, Mao WH, Yu JQ, Zhou YH, Asami T, Yu JQ (2008) A role of brassinosteroids in early fruit development in cucumber. J Exp Bot 59:2299–2308
Fuentes S, Ljung K, Sorefan K, Alvey E, Harberd NP, Ostergaard L (2012) Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell 24:3982–3996
Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5:1439–1451
Goetz M, Vivian-Smith A, Johnson SD, Koltunow AM (2006) AUXIN RESPONSE FACTOR8 is a negative regulator of fruit initiation in Arabidopsis. Plant Cell 18:1873–1886
Goetz M, Hooper LC, Johnson SD, Rodrigues JC, Vivian-Smith A, Koltunow AM (2007) Expression of aberrant forms of AUXIN RESPONSE FACTOR8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiol 145:351–366
Guilfoyle TJ, Hagen G (2007) Auxin response factors. Curr Opin Plant Biol 10:453–460
Guilfoyle TJ, Hagen G (2012) Getting a grasp on domain III/IV responsible for Auxin response factor-IAA protein interactions. Plant Sci 190:82–88
Gustafson FG (1942) Parthenocarpy: natural and artificial. Bot Rev 8:599–654
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19. doi:10.1186/gb-2007-8-2-r19
Hikosaka S, Sugiyama N (2015) Effects of exogenous plant growth regulators on yield, fruit growth, and concentration of endogenous hormones in Gynoecious Parthenocarpic cucumber (Cucumis sativus L.) Hortic J 84:342–349
Kim IS, Okubo H, Fujieda K (1992) Endogenous levels of IAA in relation to parthenocarpy in cucumber (Cucumis sativus L.) Sci Hortic 52:1–8
Kumar R, Akhilesh KT, Sharma AK (2011) Genome-wide analysis of auxin response factor (ARF) gene family from tomato and analysis of their role in flower and fruit development. Mol Gen Genomics 285:245–260
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm I, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Mapelli S (1981) Changes in cytokinin in the fruits of parthenocarpic and normal tomatoes. Plant Sci Lett 22:227–233
Marchler-Bauer A, Bo Y, Han L et al (2015) CDD: NCBI’s conserved domain database. Nucleic Acids Res 43(D):222–226
Martí C, Orzáez D, Ellul P, Moreno V, Carbonell J, Granell A (2007) Silencing of induces facultative parthenocarpy in tomato fruits. Plant J 52(5):865–876
Martínez C, Manzano S, Megías Z, Garrido D, Picó B, Jamilena M (2013) Involvement of ethylene biosynthesisand signalling in fruit set and early fruit development in zucchini squash (Cucurbita pepo L.) BMC Plant Biol. doi:10.1186/1471-2229-13-139
Martínez C, Manzano S, Megias Z, Garrido D, Picó Sirvent MB, Jamilena M (2014) Sources of parthenocarpy for zucchini breeding: relationship with ethylene production and sensitivity. Euphytica 200:349–362
Mazzucato A, Cellini F, Bouzayen M, Zouine M, Mila I, Minoia S, Petrozza A, Picarella ME, Ruiu F, Carreiro F (2015) A TILLING allele of the tomato Aux/IAA9 gene offers new insights into fruit set mechanisms and perspectives for breeding seedless tomatoes. Mol Breed 35:22
Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17:181–195
Nepi M, Pacini E (1993) Pollination, pollen viability and pistil receptivity in Cucurbita pepo. Ann Bot 72:527–536
Nepi M, Cresti L, Guarnieri M, Pacini E (2010) Effect of relative humidity on water content, viability and carbohydrate profile of Petunia hybrid and Cucurbita pepo pollen. Plant Syst Evol 284:57–64
Nitsch JP, Kurtz EB Jr, Liverman JL, Went FW (1952) The development of sex expression in cucurbit flowers. Am Jo Bot 39:32–43
Obrero A, Die JV, Román B, Gómez P, Nadal S, González-Verdejo CI (2011) Selection of reference genes for gene expression studies in zucchini (Cucurbita pepo) using qPCR. J Agric Food Chem 59:5402–5411
Ozga JA, Reinecke DM (2003) Hormonal interactions in fruit development. J Plant Growth Regul 22:73–81
Pandolfini T (2009) Seedless fruit production by hormonal regulation of fruit set. Nutrients 1:168–177
Paris HS (2000) History of the cultivar-groups of Cucurbita pepo. In: Janick J (ed) Hort Rev 25 (2001):71–170. John Wiley, New York
Picken AJF (1984) A review of pollination and fruit-set in the tomato (Lycopersicon esculentum Mill.) J Hortic Sci 59:1–13
Ren Z, Li Z, Miao Q, Yang Y, Deng W, Hao Y (2011) The auxin receptor homologue in Solanum lycopersicum stimulates tomato fruit set and leaf morphogenesis. J Exp Bot 68:2815–2826
Serrani JC, Ruiz-Rivero O, Fos M, García-Martínez JL (2008) Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J 56:922–934
Serrani JC, Carrera E, Ruiz-Rivero O, Gallego-Giraldo L, Pereira-Peres E, García-Martinez JL (2010) Inhibition of auxin transport from the ovary apical shoot induces parthenocarpic fruit-set in tomato mediated by gibberellins. Plant Physiol 153:851–862
Srivastava A, Handa AK (2005) Hormonal regulation of tomato fruit development: a molecular perspective. J Plant Growth Regul 24:67–82
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Tiwari SB, Hagen G, Guilfoyle T (2003) The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 15:2533–2543
Tiwari SB, Hagen G, Guilfoyle TJ (2004) Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16:533–543
Ulmasov T, Hagen G, Guilfoyle TJ (1999a) Activation and repression of transcription by Auxin-response factors. Proc Natl Acad Sci U S A 96:5844–5849
Ulmasov T, Hagen G, Guilfoyle TJ (1999b) Dimerization and DNAbinding of Auxin response factors. Plant J 19:309–319
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3- new capabilities and interfaces. Nucleic Acids Res 40:e115
Vivian-Smith A, Koltunow AM (1999) Genetic analysis of growth regulator-induced parthenocarpy in Arabidopsis. Plant Physiol 121:437–451
Vivian-Smith A, Luo M, Chaudhury A, Koltunow A (2001) Fruit development is actively restricted in the absence of fertilization in Arabidopsis. Development 128:2321–2331
Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C (2008) Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol 177:60–76
Wan S, Li W, Zhu Y, Liu Z, Huang W, Zhan J (2014) Genome-wide identification, characterization and expression analysis of the auxin response factor gene family in Vitis vinifera. Plant Cell Rep 33:1365–1375
Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latché A, Pech JC, Bouzayen M (2005) The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17:2676–2692
Wu J, Liu S, Guan X, Chen L, He Y, Wang J, Lu G (2014) Genome-wide identification and transcriptional profiling analysis of auxin response-related gene families in cucumber. BMC Res Notes. doi:10.1186/1756-0500-7-218
Xing H, Pudake RN, Guo G, Xing G, Hu Z, Zhang Y, Sun Q, Ni Z (2011) Genome-wide identification and expression profiling of auxin response factor (ARF) gene family in maize. BMC Genomics. doi:10.1186/1471-2164-12-1
Acknowledgments
This work was supported by the project RTA2014-00078 from the Spanish Institute of Agronomy Research INIA (Instituto Nacional de Investigación y Tecnología Agraría y Alimentaria). TPV is supported by a FPI scholarship from RTA2011-00044-C02-01/02 project of INIA (Instituto Nacional de Investigación y Tecnología). The authors thank Dr. Robinson for a supply of seeds of Whitaker cultivar, Dr. Maria Leticia Ruiz for her help in pairwise comparison and phylogenetic analysis of the sequences and Nicholas Davies for his help in grammatical revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pomares-Viciana, T., Die, J., Del Río-Celestino, M. et al. Auxin signalling regulation during induced and parthenocarpic fruit set in zucchini. Mol Breeding 37, 56 (2017). https://doi.org/10.1007/s11032-017-0661-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-017-0661-5