Abstract
Petunia hybrida and Cucurbita pepo pollen was exposed to 30 and 75% relative humidity (RH). Water content, viability and carbohydrate content (glucose, fructose, sucrose and starch) were measured at fixed intervals over 6 h. Water content of C. pepo pollen decreased drastically at both RHs, while P. hybrida pollen dehydrated slightly at RH 30% and hydrated at RH 75%. The pollen of the two species also showed very different sensitivity to dehydration. P. hybrida pollen was resistant to desiccation, with viability remaining around 80% throughout the experiment, whereas C. pepo was very sensitive to desiccation, showing an abrupt decrease in viability when its water content reached about 13% (fresh weight basis) at both RHs. Carbohydrate content was also different in the two species. Sucrose content increased soon after dehydration of P. hybrida pollen at RH 30%, whereas it remained almost constant in C. pepo pollen at the same RH. The results are discussed in the framework of basic pollen physiology and of plant reproductive strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Living organisms depend on water. Water regulates their biological reactions, serves as a fluid medium and stabilizes the structure of macromolecules. Although heavy water loss from living organisms may have deleterious effects and may lead to death (Alper and Oliver 2002), some organisms can survive removal of a large proportion of their cellular water (Potts 1994; Kleins et al. 1999; Buitink 2000; Alper and Oliver 2002). Well-known examples are prokaryotes (Potts 1994), so-called resurrection plants (Wolkers et al. 1998), seeds (Leopold et al. 1994) and pollen (Buitink et al. 1996). Pollen grains are generally dispersed in partially dehydrated state, namely with a water content (WC) varying between 5 and 20% (Stanley and Linskens 1974). In this state they can survive in the external environment, and their viability remains high at least for a number of days. The cytoplasm of this type of pollen is stained by the Periodic Acid-Schiff (PAS) reaction and has a high sucrose content (Speranza et al. 1997). Exceptions to this general rule include pollen of certain species that has about 50% WC at shedding and is known as partially hydrated pollen (Nepi et al. 2001). Once the anther opens, the latter type of pollen loses water quickly, and its viability decreases sharply in a few hours (Gay et al. 1987; Nepi and Pacini 1993). Partially hydrated pollen shares cytological and physiological features, such as large size, pore apertures, very fast germination, very high viability at shedding, weakly PAS-positive cytoplasm and low sucrose content (Speranza et al. 1997; Nepi et al. 2001).
This paper deals with the effects of different environmental conditions on water content, viability and carbohydrate content of partially dehydrated and partially hydrated pollen to test pollen capacity to adjust to changing environmental conditions over a period (up to 6 h) coherent with that of natural pollen presentation rather than longer artificial pollen storage. P. hybrida and C. pepo were chosen as representatives of the two hydration types on the basis of pollen morphology (Franchi et al. 2002). Besides morphology and water content, the two pollen species differ in starch and sucrose content: mature Petunia pollen is sucrose rich and starchless, whereas that of C. pepo is starchy but poor in sucrose (see Table 1).
Materials and methods
Six plants of C. pepo (cv Tondo di Nizza) and eight of P. hybrida (cv Surfinia Violet) were grown in pots in a climatic chamber with the following settings: daylight for 16 h, day temperature 20°C, night temperature 18°C and RH 60%. When plants started to flower, pollen samples were collected. C. pepo pollen was collected from five newly open flowers (anthesis only lasts 6 h: Nepi and Pacini 1993) chosen randomly among the plants. Petunia pollen was collected from 50 flowers in the first 4 days of anthesis (which lasts for 7–8 days, personal observation) randomly chosen among the plants. Samples were immediately used for the experiments.
Pollen water content
Air flows at two RHs (30 and 75%) were created inside the chamber of an analytical balance. The laboratory compressed air system was used to create the flow and a saturated salt solution to achieve the appropriate RH. The air flow (1.5 l/min) passed through a plastic box containing a saturated solution of CaCl2·6H2O for RH 30% and NaCl for RH 75% (Winston and Bates 1960) into the chamber of the analytical balance where a hygrometer monitored RH. A sample of pollen was distributed uniformly on a slide and placed on the plate of the balance; another sample of pollen was deposited uniformly on a second slide off the plate. The weight of pollen samples was recorded at fixed time intervals (30, 60, 90, 120, 150, 180, 240, 300 and 360 min). At the end of the experiment the weighed sample was dried in an oven at 85°C for 40 h (the time required to reach constant weight), and water percentage was calculated as [fw − dw/fw] × 100, where fw is fresh weight and dw dry weight. The experiment was repeated three times, and values were indicated as mean ± SD. All experiments were performed at room temperature (T = 20–22°C).
Pollen viability
During the same time intervals, a sample of pollen was obtained from the second slide for FCR (fluorochrome reaction), which was carried out according to Heslop-Harrison and Heslop-Harrison (1970). The FCR tests two pollen features: integrity of the plasma membrane of the vegetative cell and presence of an active esterase (Shivanna and Heslop-Harrison 1981).
A few drops of FDA 0.5% in acetone were added to: (1) 3 ml 20% sucrose solution in the case of C. pepo; (2) 3 ml 30% sucrose solution in the case of Petunia. The different sucrose concentrations have been found to be the optimum for these two species (Dafni 1992). Two drops of this solution were added to the pollen. After 10 min, the slide was observed by UV microscope to detect fluorescent and non-fluorescent pollen grains. The viability of at least 100 pollen grains was checked in triplicate at each time interval, and values were indicated as mean ± SD.
Sugar and starch analysis
Sugars (glucose, fructose and sucrose) were determined in Petunia and Cucurbita pollen samples (30 mg) exposed to 30 and 75% RH for the same time intervals as in the previous experiments. Pollen samples were placed in 80% methanol solution (v/v) and homogenized for 3 min using a PRO200 homogenizer equipped with a 7 mm × 120 mm generator to completely break down the pollen grains. Samples were observed by microscope to check that pollen was completely ruptured. Each suspension was then transferred to a 1.8-ml Eppendorf tube, dried under vacuum (Speedvac, Savant Instruments) and stored at −80°C. Frozen samples were resuspended in 0.5 ml de-ionized water, placed in a vortex apparatus and centrifuged in an ALC 4,224 centrifuge at 11,000 rpm for 8 min. One hundred microliters of the supernatant were withdrawn for sugar analysis by enzymatic test (Boehringer Mannheim Sucrose/d-Glucose/d-Fructose enzymatic test, cat. no. 716 260). The remaining liquid and the pellet were pretreated for starch testing. Pretreatment was necessary to convert starch to a soluble form and was carried out by adding 200 μl DMSO (dimethylsulphoxide) and 50 μl HCl 8 M. Samples for starch analysis were incubated for 30 min at 60°C; 50 μl NaOH 8 M was then added, and a final dilution to 1 ml was made with citrate buffer (pH 4). Following a second 8-min centrifuge cycle at 11,000 rpm, a 100-μl aliquot of supernatant was withdrawn for starch analysis using another enzymatic test (Boehringer Mannheim enzymatic test, cat. no. 207 748).
All the analyses were performed in triplicate with a Varian (Cary) UV-visible spectrophotometer at 365-nm wavelength. Sugar and starch concentrations were expressed on dry weight basis and indicated as mean ± SD.
Pollen cytology
Mature pollen of C. pepo and P. hybrida was collected from recently opened flowers. Pollen samples were fixed in 5% glutaraldehyde in phosphate buffer at pH 6.9, dehydrated in an ethyl alcohol series and embedded in Technovit 7100 (Heraeus Kulzer GmbH).
To detect total insoluble polysaccharides, the embedded material was cut into semithin sections (2–5 μm) and stained with:
-
1.
PAS for total insoluble polysaccharides after blocking free aldehyde groups (O’Brien and McCully 1981);
-
2.
IKI (iodine-potassium iodide) for starch (Johansen 1940).
Colored sections were observed with an Axio Imager Z1 light microsope (Zeiss) equipped with a digital camera (Zeiss Axiocam MRM).
For SEM observation, critical point drying was performed; then the specimens were gold-coated in a sputter coater (Edwards S150A) and observed with a Philips XL 20 scanning electron microscope.
Results
Pollen morphology and cytology
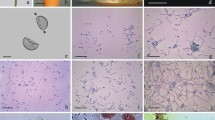
Pollen of C. pepo is spheroidal, very large (180–200 μm in diameter), polyporate with echinate ornamentation and coated with abundant pollenkitt (Fig. 1a). The cytoplasm is weakly stained by PAS and is rich in starch grains (Fig. 1b, c).
Pollen of Cucurbita pepo and Petunia hybrida. C. pepo pollen is spheroidal, very large (180–200 μm in diameter), polyporate with echinate ornamentation and coated with abundant pollenkitt (a). The cytoplasm is stained weakly by PAS (b) and is rich in starch grains (c, IKI staining). P. hybrida pollen is prolate, medium sized (major diameter 40–45 μm), tri-colpate with striate ornamentations and coated with very little pollenkitt (d). The cytoplasm is stained by PAS (e), but contains very few starch grains (f, IKI staining). a–c Bar = 50 μm; d–f bar = 10 μm
Pollen of P. hybrida is prolate, medium sized (major diameter 40–45 μm), tri-colpate with striate ornamentations and coated with very little pollenkitt (Fig. 1d). The cytoplasm is stained by PAS but contains very few starch grains (Fig. 1e, f).
Pollen water content and viability
Cucurbita pepo pollen collected from just opened anthers has a 43.7 ± 6.7% WC (Table 1). Great water loss occurred in the first 90 min of exposure to RH 30% (Fig. 2), when WC decreased to 8.5 ± 1.4%. In the following hours it stabilized around 4%. Pollen viability remained over 80% in the first 60 min of exposure. A sudden drastic decrease in viability was observed between 60 and 90 min. At 60 min WC was 13.2 ± 1.9%. The decrease in WC and viability showed the same pattern when pollen was exposed to RH 75% (Fig. 2), but WC stabilized around 10% after 180 min, and the dramatic decrease in viability occurred between 120 and 150 min. At that time, WC was 13.3 ± 2.2% (Fig. 2).
Water content and viability of Cucurbita pepo pollen at RH 30 and 75%. The water content of fresh pollen (time 0) was 43.7%. Great water loss occurred in the first 90 min of exposure to RH 30%. Pollen viability remained over 80% in the first 60 min of exposure. A sudden and drastic decrease in viability was observed between 60 and 90 min. The decrease in WC and viability showed the same pattern when pollen was exposed to RH 75%, but WC stabilized around 10% after 180 min, and the dramatic decrease of viability occurred between 120 and 150 min
The WC of fresh pollen of Petunia was 10.1 ± 1.5% (Table 1). At RH 30% WC decreased to 6.2 ± 0.9% in 6 h (Fig. 3). Viability remained high (>80%) (Fig. 3). When pollen of Petunia was exposed to RH 75%, it re-hydrated, reaching a maximum WC of 15.2 ± 0.9% after 30 min, followed by a slight decrease to 14.6 ± 0.9% and stabilization (Fig. 3).
Water content and viability of Petunia hybrida pollen at RH 30 and 75%. Water content of fresh pollen (time 0) was 10.1%. At RH 30%, the WC decreased to 6.2% in 6 h. Viability remained above 80%. At RH 75%, the pollen re-hydrated, reaching a maximum of 15.2% WC after 30 min; then there was a slight decrease to 14.6% followed by stabilization
Carbohydrate content
Cucurbita pollen contained a modest amount of glucose, fructose and sucrose (Table 1), which remained almost constant when pollen was kept at RH 30% (Fig. 4). The same happened at RH 75% (data not shown). The starch content of untreated C. pepo pollen was 10.88 ± 1.3 μg/mg (Table 1) and remained more or less constant during exposure to RH 30% (Fig. 4) and RH 75%.
The sugar content of Petunia pollen was much higher (Table 1) and varied with RH (Fig. 5). When pollen was kept at RH 30% sucrose content increased in the first 30 min, then decreased and increased again after 360 min. Fructose and glucose showed similar patterns that were opposite to that of sucrose: they decreased in the first 30 min, increased to 120 min and then decreased again. At RH 75% the sugar content varied in a smaller range and showed an opposite pattern to that at RH 30%. There was no apparent stoichiometric relation between sucrose and fructose–glucose variations.
Discussion
Water content and pollen viability
Having a WC of 43.7%, C. pepo pollen is classified as partially hydrated according to Nepi et al. (2001). It is known to be dehydration sensitive (Kerhoas et al. 1986; Gay et al. 1987; Nepi and Pacini 1993), losing water readily at low (30%) and moderately high (75%) relative humidities: the lower the relative humidity, the higher the rate of water loss. The present experiment showed 50% water loss after 30 min at RH 30% and after 60 min at RH 75%. No apparent protection against water loss was provided by the abundant layer of pollenkitt on the pollen surface (Nepi and Pacini 1993).
The water content varied with the negative power of time at both relative humidities (WC = At −1.094 and WC = At −0.768 for RH 30 and 75%, respectively, where A is the intercept on the y axis, i.e., water content of fresh pollen, and t is the time of exposure; see Fig. 2). This means that the highest rate of water loss occurs in the first minutes of exposure, causing a rapid decrease in water potential. The same has been observed in maize pollen, where water potential was found to be related to the negative power of water content (Aylor 2003). A similar relationship has been reported between pollen water content and exposure time with a combination of temperatures and RHs, although a negative exponential curve had the best fit (Fonseca and Westgate 2005).
Pollen viability showed a different relationship. After an initial slight decrease, there was an abrupt decrease at both relative humidities, followed by a further slight decrease. It is interesting that pollen viability fell off sharply below a WC of about 13% at RH 30 and 75% (13.2 and 13.3%, respectively), indicating a critical threshold around this WC. FCR showed that the plasma membrane, and probably also other cell membranes, broke down below this critical value, causing cell death. Kerhoas et al. (1987) found that maize pollen can withstand drying down to a WC of 28%, when 60–80% of grains show a negative FCR; at a WC of 13–15% all grains were negative. A critical value of maize pollen water content of 28.7%, below which viability dropped to zero, was also calculated more recently by Fonseca and Westgate (2005). These similarities between maize and C. pepo pollen are not surprising if we consider that the two pollens are partially hydrated at anthesis with 57–58% WC (Kerhoas et al. 1987; Fonseca and Westgate 2005) and contain starch as carbohydrate reserve (Franchi et al. 1996). The partially hydrated pollen of Sorghum bicolor is also sensitive to rapid dehydration, completely losing germinability after exposure to RH 50% for 15–30 min (Lansac et al. 1994). On the other hand, there are examples of partially hydrated pollen that sustains strong dehydration. Pollen of Schlumbergera truncata (Cactaceae) has 38% WC at anthesis (partially hydrated according to Nepi et al. 2001), but tolerates desiccation to 4% WC (Boyle 2001).
Freshly dehisced anthers may buffer pollen from desiccation, reducing the rate of water loss and prolonging pollen viability, as suggested also for maize pollen by Fonseca and Westgate (2005). This may be significant for C. pepo, where the position of the anthers inside a short corolla tube and their abundant connective tissue ensure a high RH around the pollen.
Since Petunia pollen has a WC of 10.1%, it is classified as partially dehydrated (Nepi et al. 2001), and its cytological features (medium-sized tricolpate pollen, PAS-positive cytoplasm and absence of starch) confirm this character. It was much more stable under the conditions tested in the present study and showed a completely different pattern than Cucurubita pollen. At 30% RH, it was more resistant to water loss than Cucurbita pollen: only slight dehydration occurred after 360 min. On the other hand, sudden slight hydration occurred after 15 min at RH 75% followed by stabilization. Under both conditions, the changes in water content did not affect pollen viability that was generally over 80%. With regard to other species with partially dehydrated pollen, viability (according to FCR) and germination of Nicotiana tabacum pollen did not change after 6 h at RH 95% and room temperature, whereas at 38°C the same treatment did not affect FCR but delayed germination and tube growth (Shivanna and Cresti 1989). Cistus incanus and Myrtus communis pollen showed great loss of viability at high relative humidity (100%) combined with high temperature (30–40°C) (Aronne 1999). These results suggest that moderate but prolonged hydration of Petunia pollen may also alter pollen germination, especially at high temperature, which was not tested in our study.
Response of sugars to changes in water content
Carbohydrates, water content, viability and longevity (maintenance of viability) of pollen appear to be related. According to Speranza et al. (1997), partially dehydrated pollen has a high sucrose content, low levels of starch and good longevity. Sucrose may impart desiccation tolerance mainly in two ways: (1) it participates in the formation of intracellular glasses in anhydrobiotes, such as seeds and pollen, slowing down the reactions and changes in structure and chemical composition typical of aging (Buitink et al. 1998; Buitink et al. 2000); (2) it protects membranes (Hoekstra et al. 1989; Pacini 2000; Buitink and Leprince 2004) because it can replace water at hydrogen-bonding sites, preserving native protein structure and spacing between phospholipids and reducing plasma membrane damage (Buitink and Leprince 2004 and reference therein). Importantly, sucrose is the major (around 95%) intrinsic soluble sugar in pollen (Buitink et al. 1996). Hoekstra et al. (1989, 1992) found sucrose contents of 5 and 23% (dry weight) in Zea mays pollen and Typha latifolia pollen, respectively; these pollens also differ in desiccation tolerance, the former being tolerant and the latter intolerant (Buitink et al. 1996). The sucrose contents of desiccation-intolerant C. pepo pollen (3.2 ± 0.3 μg/g) and desiccation-tolerant P. hybrida pollen (156.7 ± 16.1 μg/mg) found by us are in line with previous findings. The fundamental function of sucrose in protecting from dehydration was also evident from our experiments with Petunia pollen: when dehydration occurred, the first response (in the first 30 min) was an increase in sucrose (from 156.7 to 200.5 μg/mg), followed by a return to initial values. The same response was obtained exposing Trachycarpus fortunei pollen to RH 20% (Guarnieri et al. 2006) and Helleborus pollen to low temperature (Vesprini et al. 2002). This is not surprising because the real stress produced by low RH and low temperature is a decrease in water availability due to evaporation in the former and to freezing in the latter case. It is also of interest that an initial increase in sucrose due to lower water availability is followed by restoration of the quantity of this sugar (see Guarnieri et al. 2006; Vesprini et al. 2002). This suggests that some form of homeostasis maintains a constant sucrose pool after disturbance, in this case dehydration. Sucrose metabolism, largely based on the enzymes sucrose synthase and invertase, regulates hexose levels, modulating the hexose-based sugar signals that coordinate metabolic activities (Kock 2004; Rolland and Sheen 2005).
Sucrose content seemed more sensitive to dehydration than hydration, at least under our experimental conditions. In fact, hydration of Petunia pollen at RH 75% only slightly affected sucrose content, though the absolute water exchange was very similar to that occurring during dehydration at RH 30% (4.4 and 4% average, respectively).
Conclusions
Cucurbita pepo and P. hybrida pollen are physiologically very different, the first being partially hydrated and desiccation intolerant and the second partially dehydrated and desiccation tolerant (Nepi et al. 2001). The very low water content and high sucrose content of Petunia pollen may suggest the presence of a glassy state (Buitink and Leprince 2004). Pollen must be programmed to reach this particular state. In partially dehydrated pollen, hydrolysis of starch during the last phases of development (Pacini 1996) may prepare pollen to attain the glassy state after dehydration. Starch hydrolysis in the last phases of pollen development generates high levels of sucrose that allow formation of the glassy state and protection of the plasma membrane during and after dehydration. When starch hydrolysis does not occur, as in C. pepo, pollen is much more sensitive to desiccation. Because desiccation tolerance is closely related to pollen viability and longevity, it has a strong influence on reproductive strategy and pollination (Dafni and Firmage 2000). Species with dehydration-tolerant pollen may wait at length for pollination to occur. On the contrary, species with pollen sensitive to dehydration rely on rapid transport of pollen to a compatible stigma; otherwise, it does not germinate and fertilize the ovules. C. pepo is a good example of this: it is pollinated by bees, and the maximum production of floral nectar occurs very early in the morning in summer when the relative humidity is highest and water loss therefore reduced (Nepi and Pacini 1993). Most of the pollen is removed 2 h after flower opening, when pollen viability is still above 80% (Nepi and Pacini 1993).
References
Alper P, Oliver ML (2002) Drying without dying. In: Black M, Pritchard HW (eds) Desiccation tolerance in plants. CAB International, Wallingford, pp 3–43
Aronne G (1999) Effects of relative humidity and temperature stress on pollen viability of Cistus incanus and Myrtus communis. Grana 38:364–367
Aylor DE (2003) Rate of dehydration of corn (Zea mays L.) pollen in the air. J Exp Bot 54:2307–2312
Boyle TM (2001) Environmental control of moisture content and viability in Schlumbergera truncate (Cactaceae) pollen. J Amer Soc Hort Sci 126:625–630
Buitink J (2000) Biological glasses: nature’s way to preserve life. PhD thesis. Wageningen Agricultural University, Wageningen
Buitink J, Leprince O (2004) Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology 48:215–228
Buitink J, Walters-Vertucci C, Hoekstra FA, Leprince O (1996) Calorimetric properties of dehydrating pollen: analysis of desiccation tolerant and intolerant species. Plant Physiol 111:235–242
Buitink J, Walters C, Hoekstra FA, Crane J (1998) Storage behavior of Typha latifolia pollen at low water contents: an interpretation on the basis of water activity and glass concepts. Physiol Plant 103:145–153
Buitink J, Leprince O, Hemminga MA, Hoekstra FA (2000) Molecular mobility in the cytoplasm: an approach to describe and predict lifespan of dry germplasm. PNAS U.S.A. 97:2385–2390
Dafni A (1992) Pollination ecology: a practical approach. Oxford University Press, Oxford
Dafni A, Firmage D (2000) Pollen viability and longevity: practical, ecological and evolutionary implications. In: Dafni A, Hesse M, Pacini E (eds) Pollen and pollination. Springer, Wien, pp 113–132
Fonseca AE, Westgate ME (2005) Relationship between desiccation and viability of maize pollen. Field Crop Res 94:114–125
Franchi GG, Bellani L, Nepi M, Pacini E (1996) Types of carbohydrate reserves in pollen: localization, systematic distribution and ecophysiological significance. Flora 191:143–159
Franchi GG, Nepi M, Dafni A, Pacini E (2002) Partially hydrated pollen: taxonomic distribution, ecological and evolutionary significance. Plant Syst Evol 234:211–227
Gay C, Kerhoas C, Dumas C (1987) Quality of a stress-sensitive Cucurbita pepo pollen. Planta 171:82–87
Guarnieri M, Speranza A, Nepi M, Artese D, Pacini E (2006) Ripe pollen carbohydrate changes in Trachycarpus fortunei: the effect of relative humidity. Sexual Plant Reprod 19:117–124
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol 45:25–36
Hoekstra FA, Crowe LM, Crowe JH (1989) Differential desiccation sensitivity of corn and Pennisetum pollen linked to their sucrose contents. Plant Cell Environ 12:83–91
Hoekstra FA, Crowe JH, Crowe LM, van Roekel T, Vermeer E (1992) Do phospholipids and sucrose determine membrane phase transitions in dehydrating species? Plant Cell Environ 15:601–606
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Kerhoas C, Gay CG, Duplan JC, Dumas C (1986) Water content evolution in Cucurbita pepo during ageing: a NMR study. In: Mulcahy DL, Bergamini Mulcahy G, Ottaviano E (eds) Biotechnology and ecology of pollen. Springer, New York, pp 502–505
Kerhoas C, Gay CG, Dumas C (1987) A multidisciplinary approach to the study of the plasma membrane of Zea mays pollen during controlled dehydration. Planta 171:1–10
Kleins M, Elster R-C, Rodrigo M-J, Blervacq A-S, Salamini F, Bartels D (1999) Isolation and expression analysis of two stress-responsive sucrose-synthase genes from the resurrection plant Craterostigma plantagineum (Hochst.). Planta 209:13–24
Kock K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Bio 7:235–246
Lansac AR, Sullivan CY, Johnson BE, Lee KW (1994) Viability of the pollen of Sorghum [Sorghum bicolor (L.) Moench]. Ann Bot 74:27–33
Leopold AC, Sun WQ, Bernal-Lugo I (1994) The glassy state in seeds: analysis and function. Seed Sci Res 4:267–274
Nepi M, Pacini E (1993) Pollination, pollen viability and pistil receptivity in Cucurbita pepo. Ann Bot 72:527–536
Nepi M, Franchi GG, Pacini E (2001) Pollen hydration status at dispersal: cytophysiological features and strategies. Protoplasma 216:171–180
O’Brien TP, McCully ME (1981) The study of plant structure–principles and selected methods. Termacarphi Pty, Melbourne
Pacini E (1996) Types and meaning of pollen carbohydrate reserves. Sexual Plant Reprod 9:362–366
Pacini E (2000) From anther and pollen ripening to pollen presentation. Plant Sys Evol 222:19–43
Potts M (1994) Desiccation tolerance of prokaryotes. Microbiol Rev 58:755–805
Rolland F, Sheen J (2005) Sugar sensing and signalling networks in plants. Biochem Soc T 33(part 1):269–271
Shivanna KR, Cresti M (1989) Effects of high humidity and temperature stress on pollen membrane integrity and pollen vigour in Nicotiana tabacum. Sexual Plant Reprod 2:137–141
Shivanna KR, Heslop-Harrison J (1981) Membrane state and pollen viability. Ann Bot 47:759–770
Speranza GE, Calzoni CL, Pacini E (1997) Occurrence of monosaccharides and polysaccharide reserves in mature pollen grains. Sex Plant Reprod 10:110–115
Stanley RG, Linskens HF (1974) Pollen: biology, biochemistry, management. Springer, New York
Vesprini JL, Nepi M, Cresti L, Guarnieri M, Pacini E (2002) Changes in cytoplasmic carbohydrate content during Helleborus pollen presentation. Grana 41:16–20
Winston PW, Bates DH (1960) Saturated salt solutions for the control of humidity in biological research. Ecology 41:232–237
Wolkers WF, Oldenhof H, Alberrda M, Hoekstra FA (1998) A Fourier transform infrared study of sugar glasses: application to anhydrobiotic higher plant cells. Biochim Biophys Acta 1379:83–96
Acknowledgments
This research was funded by PAR (Piano di Ateneo per la Ricerca, University of Siena).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nepi, M., Cresti, L., Guarnieri, M. et al. Effect of relative humidity on water content, viability and carbohydrate profile of Petunia hybrida and Cucurbita pepo pollen. Plant Syst Evol 284, 57–64 (2010). https://doi.org/10.1007/s00606-009-0237-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-009-0237-x