Abstract
Alzheimer’s disease (AD) is the most common type of dementia. The evolution and aggregation of amyloid beta (β) oligomers is linked to insulin resistance in AD, which is also the major characteristic of type 2 diabetes (T2D). Being physically inactive can contribute to the development of AD and/or T2D. Aerobic exercise training (AET), a type of physical exercise, can be useful in preventing or treating the negative outcomes of AD and T2D. AD, T2D and AET can regulate the expression of microRNAs (miRNAs). Here, we review some of the changes in miRNAs expression regulated by AET, AD and T2D. MiRNAs play an important role in the gene regulation of key signaling pathways in both pathologies, AD and T2D. MiRNA dysregulation is evident in AD and has been associated with several neuropathological alterations, such as the development of a reactive gliosis. Expression of miRNAs are associated with many pathophysiological mechanisms involved in T2D like insulin synthesis, insulin resistance, glucose intolerance, hyperglycemia, intracellular signaling, and lipid profile. AET regulates miRNAs levels. We identified 5 miRNAs (miR-21, miR-29a/b, miR-103, miR-107, and miR-195) that regulate gene expression and are modulated by AET on AD and T2D. The identified miRNAs are potential targets to treat the symptoms of AD and T2D. Thus, AET is a non-pharmacological tool that can be used to prevent and fight the negative outcomes in AD and T2D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer´s disease (AD) is the most common neurodegenerative disease (EMA 2018). Six million Americans were living with AD in 2020 and it is expected that in 2050 we will have approximately 13.8 million people living with AD worldwide (Physicians 2020). AD is also a type of dementia where patients usually present difficulty forming new memories. The dementia has been clinically attributed to the cell death that results from a large number of insoluble amyloid fibrils. These amyloid fibrils may be present in a vast number of tissues (muscle, bones, etc.) and can cause damage to peripheral tissues and the brain (Gong et al. 2003). Aggregations of amyloid beta (Aβ) oligomers, strong central nervous system (CNS) neurotoxins, are thought to be responsible for cellular damage, and has been associated with the development of insulin resistance and cognitive decline in AD (Dias et al. 2020).
The main feature of type 2 diabetes (T2D) is also insulin resistance (De Sousa et al. 2021b). This chronic metabolic disorder affects over 200 million individuals globally, and it is projected that this may rise to 400 million individuals with diabetes by 2030 (IDF 2015). T2D is characterized by the presence of hyperglycemia and insulin resistance, with or without insulin deficiency (De Sousa et al. 2021b). The presence of cognitive decline in T2D is supported by a higher risk of developing neurodegenerative diseases (De Sousa et al. 2021e), especially AD (Wang et al. 2018a). Physical inactivity can contribute to the development of AD and/or T2D (Snel et al. 2012). For this reason, physical exercise is a non-pharmacological recommendation for patients with diabetes (De Sousa 2018; Ranasinghe et al. 2018), AD (Alkadhi and Dao 2018; De Sousa et al. 2021d), and many others pathological conditions (Pedersen and Saltin 2015; De Sousa et al. 2020b, 2021a; Eskandari et al. 2020; Cavalcante et al. 2021).
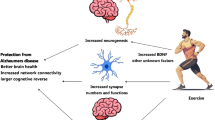
Aerobic exercise training (AET), a type of physical exercise, can induce marked physiological adaptations, such as increased production of Irisin (De Sousa et al. 2021c). AET can also regulate microRNAs (miRNAs), which play an important role in the regulation of signaling pathways that will interfere in different pathologies (Caria et al. 2018). Examining the effects of AET in AD and T2D may help to explain mechanisms of insulin resistance, inflammation and metabolic dysregulation in neurodegenerative disorders. A recent systematic review suggested that large-scale, robust controlled randomized clinical trials should be performed to evaluate if physical exercise would contribute to improve cognitive function in T2D patients (Zhao et al. 2018), what we could suggest also to be better addressed in AD. The identification of how AET influences the regulation of miRNAs in T2D and AD may also identify molecular targets for pharmacological interventions. Here, we have performed a narrative review to try to link some of these mechanisms. This study evaluated the potential novel effects of AET on AD and T2D.
Classic mechanisms of AD
AD is the most common cause of dementia and related neurodegenerative disorders (Alzheimer´s Association 2010). Aging is the greatest risk factor for AD, but the development of the pathophysiology is not a normal part of aging. The amyloid cascade hypothesis based on the role of Aβ peptide has been the major point investigated in the last 30 years (Folch et al. 2019). However, medicines developed that had as main target β-secretase 1 (BACE1), which is the major beta secretase for the generation of β-peptides, have failed in clinical trials (Hawkes 2017; Egan et al. 2018).
The amyloid hypothesis consists in the cleavage of amyloid precursor protein (APP) by β- and gamma-secretase what will lead to an increased number of cytotoxic residues, which will form oligomers that will cause neuron damage (De Sousa et al. 2020a). Inflammation, oxidative stress and insulin resistance can be also seen in the brain of AD patients and animal models (Zhao et al. 2004; Lee et al. 2009). Another hypothesis for the development of AD is Tau hyperphosphorylation (Gratuze et al. 2018). Synaptic dysfunction is related to accumulation of hyperphosphorylated tau protein in AD (Smith et al. 2015). The third mostly studied hypothesis in AD reveals the existence of cholinergic neurons loss and nicotinic acetylcholine receptors (nAChRs) reduction throughout the brain (Magdesian et al. 2005).
Nevertheless, all listed hypothesis can be found in AD patients and in animal models suggesting multiple harmful effects of this disease to the brain. However, it seems that insulin resistance, a common feature between AD and T2D, develops a pivotal role between Aβ and tau pathologies (Mullins et al. 2017; Rad et al. 2018).
Classic mechanisms of T2D
Several processes are associated with T2D and the main known mechanisms linked to this disease are: hyperglycemia, insulin resistance, hyperinsulinemia, hyperlipidemia, increase in reactive oxygen species, inflammation, fibrosis and apoptosis (Roden and Shulman 2019).
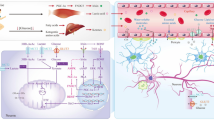
State of overnutrition generates metabolic imbalance, promoting activation of the renin–angiotensin–aldosterone system, which stimulates the mechanistic of rapamycin (mTOR)-S6 kinase 1 (S6K1), inhibiting insulin signaling on IRS-1 and IRS-2, decreasing the activation of via PI3K-AKT. Another described molecular mechanism that leads to the inactivation of the PI3K-AKT signaling pathway and insulin resistance is the phosphorylation of serine residues from IRS-1 or IRS-2 that attenuates glucose uptake by this signaling pathway (Jia et al. 2016).
These long-term metabolic and molecular changes will promote dysfunction of some cell organelles, such as: mitochondria, generating mitochondrial dysfunction; in the endoplasmic reticulum, leading to endoplasmic reticulum stress and impaired calcium handling (Jia et al. 2016); and in the cell nucleus and DNA, due to epigenetic alterations linked to changes in DNA methylation, histone acetylation and deacetylation (Kang et al. 2016), modified expression of miRNAs, long-non coding RNA, among many other non-coding RNAs (Raciti et al. 2015), resulting in remodeling and dysfunction of specific organs such as the brain (Biessels and Despa 2018), heart (Dillmann 2019), blood vessels (Shi and Vanhoutte 2017), adipose tissue (Roden and Shulman 2019), kidneys (Assayag et al. 2017) and gut (Hashimoto et al. 2020).
However, a simple session of aerobic exercise activates the AMP kinase, which in turn induces the translocation of GLUT4 to the cell surface, increasing glucose uptake (Musi et al. 2001), demonstrating that exercise is an important tool in combating insulin resistance, T2D and AD (Improta-Caria et al. 2020).
AET features
Physical exercise is considered an excellent non-pharmacological strategy to prevent and help treat AD (De la Rosa et al. 2020) and T2D (Sampath Kumar et al. 2019). AET more specifically is the type of exercise that is widely studied in the literature and shows several positive effects on the human body (Hillman et al. 2008). AET improves cell function of the innate and adaptive immune system (Improta-Caria et al. 2021), improves the function of endocrine hormones (Hackney and Lane 2015), promotes changes in the morphology and function of several organs, especially the brain (Colcombe et al. 2006), heart (Schüttler et al. 2019) and blood vessels (Hurley et al. 2019), which are very affected organs in both AD and T2D.
In recent years, the effects of AET on molecular mechanisms have been investigated, mainly mechanisms associated with miRNAs in both healthy (Baggish et al. 2011; Nielsen et al. 2014) and diseased individuals (Fernandes et al. 2012; Gomes et al. 2017; Improta-Caria and Aras 2021). However, the molecular mechanisms regulated by AET-induced miRNAs in AD and T2D are poorly studied.
MiRNAs: links between AET, T2D and AD
MiRNAs are small non-coding single-stranded RNAs, having usually 22 nucleotides, that play a role in post-transcriptional mechanisms of the regulation of gene expression (Ha and Kim 2014). MiRNAs are involved with obesity (Iacomino et al. 2016) and adipocyte differentiation and proliferation, and target PPAR´s during the process (Tyagi et al. 2019). They also influence cardiovascular inflammation (Nemecz et al. 2016), T2D (Baroukh et al. 2007), AD (Liu et al. 2014c), and AET (Silva et al. 2017). MiRNAs regulate up to 60% of the protein-coding genes in the human genome (Muljo et al. 2010). A portion of our genome generates functional small RNAs that will not be translated into protein, but that will instead play a very important role in regulating gene expression (Caria et al. 2018). Moreover, miRNA expression profiles are evidently able to identify different types of cancers, however the role of miRNAs in cell biology or organisms remains unclear. In order to understand the roles of miRNAs, it is necessary to systematically identify the targets they regulate.
Expression of miRNAs are associated with many pathophysiological mechanisms involved in T2D (insulin synthesis, insulin resistance, glucose intolerance, hyperglycemia, intracellular signaling, and lipid profile) (Caria et al. 2018). MiRNA regulation is related to several comorbidities and complications of T2D, such as impaired angiogenesis, micro- and macrovascular damage (Stępień et al. 2018). There is a strong possibility that miR-126 is involved in the pathogenesis of micro- and macrovascular complications of T2D (Caria et al. 2018). A number of miRNAs have been identified as regulators of insulin transcription and translation at higher levels of blood glucose, such as miR-124, miR-107, miR-30a, and miR-30d (Baroukh et al. 2007; Tang et al. 2009; Aaltonen et al. 2010). Downregulation of miR-484, miR-690, and miR-296 was observed in mice models of T2D, and is related to the inhibition of insulin transcription (Tang et al. 2009). Insulin secretion is also regulated by a few miRNAs, including miR-375 and miR-9 (Poy et al. 2004; Joglekar et al. 2009). The regulation of the molecular mechanisms involved in T2D patients seems to be regulated by mir375, miR-101 and miR-802 (Kong et al. 2011; Higuchi et al. 2015). These are a few examples only of how miRNAs are crucial in several pathophysiological roles in T2D and associated comorbidities and conditions (Table 1).
MiRNA dysregulation is also evident in AD and has been associated with several neuropathological alterations (Table 2), including altered expressions of species that are known to be involved in AD pathology, including microglia and astrocytes (Shaik et al. 2018). MiR-132/212 was reported to be down-regulated in the frontal cortex in mild cognitive decline (Smith et al. 2015). MiRNA 153 is also downregulated in the AD brain and it is associated with higher expressions and mutations of APP (Long et al. 2012). MiR-195 is suggested to be downregulated in the AD brain leading to increased production of Aβ40 and Aβ42, the strongest cytotoxic forms of the peptide, contributing to a greater formation of pathogenic amyloid plaques (Shaik et al. 2018). Future studies must investigate drugs that can target dysregulation of miRNAs in T2D and AD. Nevertheless, the investigation of alternative signaling pathways and mechanisms, including the changes induced in miRNAs by AET is necessary (Table 3). Identifying the changes caused by AET hold potential for the development of novel therapies for the treatment of T2D and AD.
AET induces changes in miRNAs in both T2D and AD
AET is capable of improving insulin resistance and dyslipidemia (De Sousa 2018). Cellular homeostasis is markedly affected by a single exercise session and in response to chronic exercise training, which induce marked changes in the circulating miRNA profile (Caria et al. 2018). AET promotes positive effects in miRNA-mediated gene regulation among healthy participants, but clinical studies focusing on people with T2D and AD have not been well-explored to date (Muljo et al. 2010; Caria et al. 2018; Shaik et al. 2018).
The identification of the miRNAs regulated by AET in people with T2D and/or AD is important in order to understand the molecular alterations in signaling pathways, proteins, enzymes and interleukins. These findings are crucial for the development of new therapies, drug-related or not, in order to prevent or combat TD2M and AD.
Circulating miRNAs overlaps between AD, T2D and AET
Here, we show what is currently known about identified circulating miRNAs in AD, T2D and AET, and the common miRNAs to all three on a Venn diagram (Fig. 1). We identified 7 circulating miRNAs deregulated and associated with AD and T2D, 76 circulating miRNAs deregulated in AD and AET and 11 circulating miRNAs deregulated in T2D and AET. In particular, we identified 26 circulating miRNAs deregulated in the 3 situations, they are: miR-532, let-7i, miR-144, miR-140, miR-30a, miR-375, miR-222, miR-30d, miR-125b, miR-126, miR-21, miR-142, miR-34a, miR-20b, miR-146a, miR-148a, miR-15a, miR-23a, miR-766, miR-210, miR-195, miR-130a, miR-424, miR-23b, miR-29a and miR-191. After an analysis of the expression pattern of these 26 miRNAs, it was found that 2 miRNAs (miR-23a and miR-532) showed an expression pattern different from the pattern in the AET. Both miR-23a and miR-532 are downregulated in disease and upregulated in AET.
Inflammation is a very common situation in AD and T2D and NF-κB is an overexpressed transcriptional factor in this situation. The NF-κB p-65 subunit binds to miR-23a promoter and decreases its expression (Rathore et al. 2012), favoring an increase in the inflammatory profile. Dysregulation of miR-23a has also been associated with dyslipidemia (Karolina et al. 2012). On the other hand, AET increases the expression of miR-23a, suggesting that AET can decrease the inflammatory process and attenuate dyslipidemia through the regulation of this miRNA in AD and T2D.
Under conditions of inflammation, miR-532 is downregulated and this miRNA targets the proapoptotic gene BCL2 antagonist/killer 1 (BAK1). Thus, BAK1 is overexpressed, elevating the inflammatory profile and promoting apoptosis (Chen et al. 2020). In contrast, AET increases miR-532 expression, suggesting that it may be a molecular mechanism induced by AET to reduce BAK1 expression and subsequently decrease inflammation and apoptosis in AD and T2D. These remarkable data are further strong evidence that scientific research should be driven to investigate the changes induced in miRNA´s by AET in T2D and AD. Such identification will also facilitate finding possible targets for new therapies.
Conclusions
AET is a non-pharmacological tool that can prevent and be used as a therapy in T2D and AD, helping to avoid memory loss and several pro-inflammatory mechanisms in both diseases. We suggest that investigating molecular mechanisms of the actions of AET on molecular pathways and the regulation of miRNAs will not only provide all known benefits of how better prescribe physical exercise, but will also illuminate new targets to the ultimate aim that is to find a cure to these diseases. AET will at the very least likely diminish the suffering of patients through the development of new and effective therapies. AET and physical exercise in general is a therapy itself.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aaltonen S, Ortega-Alonso A, Kujala UM, Kaprio J (2010) A longitudinal study on genetic and environmental influences on leisure time physical activity in the finnish twin cohort. Twin Res Hum Genet 13:475–481. https://doi.org/10.1375/twin.13.5.475
Al-Kafaji G, Al-Mahroos G, Alsayed NA et al (2015) Peripheral blood microRNA-15a is a potential biomarker for type 2 diabetes mellitus and pre-diabetes. Mol Med Rep 12:7485–7490. https://doi.org/10.3892/MMR.2015.4416
Al-Muhtaresh HA, Salem AH, Al-Kafaji G (2019) Upregulation of circulating cardiomyocyte-enriched miR-1 and miR-133 associate with the risk of coronary artery disease in type 2 diabetes patients and serve as potential biomarkers. J Cardiovasc Transl Res 12:347–357. https://doi.org/10.1007/S12265-018-9857-2
Alexandrov PN, Dua P, Hill JM et al (2012) MicroRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF). Int J Biochem Mol Biol 3:365–373
Alkadhi KA, Dao AT (2018) Exercise decreases BACE and APP levels in the hippocampus of a rat model of Alzheimer’s disease. Mol Cell Neurosci 86:25–29. https://doi.org/10.1016/j.mcn.2017.11.008
Alzheimer’s Association (2010) Alzheimer's disease facts and figures. Alzheimers Dement 6(2):158–194. https://doi.org/10.1016/j.jalz.2010.01.009
Amin KN, Umapathy D, Anandharaj A, et al (2020) miR-23c regulates wound healing by targeting stromal cell-derived factor-1α (SDF-1α/CXCL12) among patients with diabetic foot ulcer. Microvasc Res 127.https://doi.org/10.1016/J.MVR.2019.103924
Amr KS, Abdelmawgoud H, Ali ZY et al (2018) Potential value of circulating microRNA-126 and microRNA-210 as biomarkers for type 2 diabetes with coronary artery disease. Br J Biomed Sci 75:82–87. https://doi.org/10.1080/09674845.2017.1402404
Aoi W, Ichikawa H, Mune K et al (2013) Muscle-enriched microRNA miR-486 decreases in circulation in response to exercise in young men. Front Physiol 4:80. https://doi.org/10.3389/fphys.2013.00080
Assayag EB, Eldor R, Korczyn AD et al (2017) Type 2 diabetes mellitus and impaired renal function are associated with brain alterations and poststroke cognitive decline. Stroke 48:2368–2374. https://doi.org/10.1161/STROKEAHA.117.017709
Baggish AL, Hale A, Weiner RB et al (2011) Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol 589:3983–3994. https://doi.org/10.1113/jphysiol.2011.213363
Baggish AL, Park J, Min P-K et al (2014) Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J Appl Physiol 116:522–531. https://doi.org/10.1152/japplphysiol.01141.2013
Balasubramanyam M, Aravind S, Gokulakrishnan K et al (2011) Impaired miR-146a expression links subclinical inflammation and insulin resistance in Type 2 diabetes. Mol Cell Biochem 351:197–205. https://doi.org/10.1007/S11010-011-0727-3
Baldeón LR, Weigelt K, De Wit H et al (2014) Decreased serum level of miR-146a as sign of chronic inflammation in type 2 diabetic patients. PLoS ONE 9:1–16. https://doi.org/10.1371/journal.pone.0115209
Banzet S, Chennaoui M, Girard O et al (2013) Changes in circulating microRNAs levels with exercise modality. J Appl Physiol 115:1237–1244. https://doi.org/10.1152/japplphysiol.00075.2013
Barber JL, Zellars KN, Barringhaus KG et al (2019) The effects of regular exercise on circulating cardiovascular-related microRNAs. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-019-43978-x
Baroukh N, Ravier MA, Loder MK et al (2007) MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cell lines. J Biol Chem 282:19575–19588. https://doi.org/10.1074/jbc.M611841200
Bekris LM, Lutz F, Montine TJ et al (2013) MicroRNA in Alzheimer’s disease: an exploratory study in brain, cerebrospinal fluid and plasma. Biomarkers 18:455–466. https://doi.org/10.3109/1354750X.2013.814073
Bhatnagar S, Chertkow H, Schipper HM et al (2014) Increased microRNA-34c abundance in Alzheimer’s disease circulating blood plasma. Front Mol Neurosci 7:1–11. https://doi.org/10.3389/fnmol.2014.00002
Biessels GJ, Despa F (2018) Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 14:591–604. https://doi.org/10.1038/S41574-018-0048-7
Brennan E, Wang B, McClelland A et al (2017) Protective effect of let-7 miRNA family in regulating inflammation in diabetes-associated atherosclerosis. Diabetes 66:2266–2277. https://doi.org/10.2337/db16-1405
Caria ACI, Nonaka CKV, Pereira CS et al (2018) Exercise training-induced changes in microRNAs: beneficial regulatory effects in hypertension, type 2 diabetes, and obesity. Int J Mol Sci 19:1–36. https://doi.org/10.3390/ijms19113608
Cavalcante BRR, Improta-caria AC, de Melo VH, De Sousa RAL (2021) Exercise-linked consequences on epilepsy. Epilepsy Behav 121:1–6. https://doi.org/10.1016/j.yebeh.2021.108079
Chang WS, Wang YH, Zhu XT, Wu CJ (2017) Genome-Wide Profiling of miRNA and mRNA Expression in Alzheimer’s Disease. Med Sci Monit 23:2721–2731. https://doi.org/10.12659/MSM.905064
Chen FX, Shen Y, Liu Y et al (2020) Inflammation-dependent downregulation of miR-532-3p mediates apoptotic signaling in human sarcopenia through targeting BAK1. Int J Biol Sci 16:1481–1494. https://doi.org/10.7150/ijbs.41641
Cheng L, Doecke JD, Sharples RA et al (2015) Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol Psychiatry 20:1188–1196. https://doi.org/10.1038/mp.2014.127
Clauss S, Wakili R, Hildebrand B et al (2016) MicroRNAs as biomarkers for acute atrial remodeling in marathon runners (The miRathon study – a sub-study of the Munich marathon study). PLoS ONE 11:e0148599. https://doi.org/10.1371/journal.pone.0148599
Colcombe SJ, Erickson KI, Scalf PE et al (2006) Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 61:1166–1170. https://doi.org/10.1093/GERONA/61.11.1166
Cosín-Tomás M, Antonell A, Lladó A et al (2017) Plasma miR-34a-5p and miR-545-3p as early biomarkers of Alzheimer’s disease: potential and limitations. Mol Neurobiol 54:5550–5562. https://doi.org/10.1007/s12035-016-0088-8
Cui SF, Wang C, Yin X et al (2016) Similar responses of circulating microRNAs to acute high-intensity interval exercise and vigorous-intensity continuous exercise. Front Physiol 7:102. https://doi.org/10.3389/fphys.2016.00102
Dangla-Valls A, Molinuevo JL, Altirriba J et al (2017) CSF microRNA profiling in Alzheimer’s disease: a Screening and validation study. Mol Neurobiol 54:6647–6654. https://doi.org/10.1007/s12035-016-0106-x
de Gonzalo-Calvo D, Dávalos A, Montero A et al (2015) Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J Appl Physiol 119:124–134. https://doi.org/10.1152/japplphysiol.00077.2015
de Gonzalo-Calvo D, van der Meer RW, Rijzewijk LJ et al (2017) Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Sci Rep 7:47. https://doi.org/10.1038/s41598-017-00070-6
de Gonzalo-Calvo D, Dávalos A, Fernández-Sanjurjo M et al (2018) Circulating microRNAs as emerging cardiac biomarkers responsive to acute exercise. Int J Cardiol 264:130–136. https://doi.org/10.1016/j.ijcard.2018.02.092
De la Rosa A, Olaso-Gonzalez G, Arc-Chagnaud C et al (2020) Physical exercise in the prevention and treatment of Alzheimer’s disease. J Sport Heal Sci 9:394–404. https://doi.org/10.1016/J.JSHS.2020.01.004
De Sousa RAL (2018) Brief report of the effects of the aerobic, resistance, and high-intensity interval training in type 2 diabetes mellitus individuals. Int J Diabetes Dev Ctries 38:138–145. https://doi.org/10.1007/s13410-017-0582-1
De Sousa RAL, Harmer AR, Freitas DA et al (2020a) An update on potential links between type 2 diabetes mellitus and Alzheimer’s disease. Mol Biol Rep 47:6347–6356. https://doi.org/10.1007/s11033-020-05693-z
De Sousa RAL, Peixoto MFD, Leite HR et al (2020b) Neurological consequences of exercise during prenatal Zika virus exposure to mice pups. Int J Neurosci. https://doi.org/10.1080/00207454.2020.1860970
De Sousa RAL, Improta-Caria AC, Aras-Júnior R et al (2021a) Physical exercise effects on the brain during COVID-19 pandemic: links between mental and cardiovascular health. Neurol Sci 42:1325–1334
De Sousa RAL, Improta-Caria AC, Cassilhas RC (2021b) Effects of physical exercise on memory in type 2 diabetes: a brief review. Metab Brain Dis 1–5.https://doi.org/10.1007/s11011-021-00752-1
De Sousa RAL, Improta-Caria AC, de F Souza BS (2021c) Exercise-linked Irisin: consequences on mental and cardiovascular health in type 2 diabetes. Int J MolSci 22:2199. https://doi.org/10.3390/IJMS22042199
De Sousa RAL, Rodrigues CM, Mendes BF et al (2021d) Physical exercise protocols in animal models of Alzheimer ’ s disease : a systematic review. Metab Brain Dis 36:85–95. https://doi.org/10.1007/s11011-020-00633-z
De Sousa RAL, Santos LG, Lopes PM et al (2021e) Physical exercise consequences on memory in obesity : a systematic review. Obes Rev 1–10.https://doi.org/10.1111/obr.13298
Denham J, Prestes PR (2016) Muscle-enriched microRNAs isolated from whole blood are regulated by exercise and are potential biomarkers of cardiorespiratory fitness. Front Genet 7:196. https://doi.org/10.3389/fgene.2016.00196
Denk J, Boelmans K, Siegismund C et al (2015) MicroRNA profiling of CSF reveals potential biomarkers to detect Alzheimer’s disease. PLoS One 10.https://doi.org/10.1371/journal.pone.0126423
Denk J, Oberhauser F, Kornhuber J et al (2018) Specific serum and CSF microRNA profiles distinguish sporadic behavioural variant of frontotemporal dementia compared with Alzheimer patients and cognitively healthy controls. PLoS One 13.https://doi.org/10.1371/JOURNAL.PONE.0197329
Derkow K, Rössling R, Schipke C et al (2018) Distinct expression of the neurotoxic microRNA family let-7 in the cerebrospinal fluid of patients with Alzheimer’s disease. PLoS One 13.https://doi.org/10.1371/JOURNAL.PONE.0200602
Dias RG, Silva MSM, Duarte NE et al (2015) PBMCs express a transcriptome signature predictor of oxygen uptake responsiveness to endurance exercise training in men. Physiol Genomics 47:13–23. https://doi.org/10.1152/physiolgenomics.00072.2014
Dias IR, de Santos C S, Magalhães COD et al (2020) Does calorie restriction improve cognition? IBRO Reports 9:37–45. https://doi.org/10.1016/j.ibror.2020.05.001
Dillmann WH (2019) Diabetic cardiomyopathy. Circ Res 124:1160–1162. https://doi.org/10.1161/CIRCRESAHA.118.314665
Dong H, Li J, Huang L et al (2015) Serum microRNA profiles serve as novel biomarkers for the diagnosis of Alzheimer’s disease.https://doi.org/10.1155/2015/625659
Du W, Lei C, Dong Y (2021) MicroRNA-149 is downregulated in Alzheimer’s disease and inhibits β-amyloid accumulation and ameliorates neuronal viability through targeting BACE1. Genet Mol Biol 44:1–8. https://doi.org/10.1590/1678-4685-GMB-2020-0064
Egan MF, Kost J, Tariot PN et al (2018) Randomized Trial of Verubecestat for Mild-to-Moderate Alzheimer’s Disease. N Engl J Med 378:1691–1703. https://doi.org/10.1056/NEJMoa1706441
EMA (2018) Guideline on the clinical investigation of medicines for the treatment of Alzheimer’s disease. Eur Med Agency 44:1–36
Eskandari M, Asghari H, Saghebjoo M, Kazemi T (2020) Short duration moderate resistance training reduces blood pressure and plasma TNF-α in hypertensive men: the importance role of upper and lower body training. Sci Sport. https://doi.org/10.1016/j.scispo.2019.12.005
Fernandes T, Magalhães FC, Roque FR, et al (2012) Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: role of microRNAs-16, -21, and -126. Hypertens (Dallas, Tex 1979) 59:513–20. https://doi.org/10.1161/HYPERTENSIONAHA.111.185801
Fernández-Sanjurjo M, Úbeda N, Fernández-García B et al (2020) Exercise dose affects the circulating microRNA profile in response to acute endurance exercise in male amateur runners. Scand J Med Sci Sports 30:1896–1907. https://doi.org/10.1111/SMS.13759
Folch J, Olloquequi J, Ettcheto M et al (2019) The involvement of peripheral and brain insulin resistance in late onset Alzheimer’s dementia. Front Aging Neurosci 11:1–16. https://doi.org/10.3389/fnagi.2019.00236
Frigerio CS, Lau P, Salta E et al (2013) Reduced expression of hsa-miR-27a-3p in CSF of patients with Alzheimer disease. Neurology 81:2103–2106. https://doi.org/10.1212/01.wnl.0000437306.37850.22
Galimberti D, Villa C, Fenoglio C et al (2014) Circulating miRNAs as potential biomarkers in alzheimer’s disease. J Alzheimer’s Dis 42:1261–1267. https://doi.org/10.3233/JAD-140756
Gámez-Valero A, Campdelacreu J, Vilas D et al (2019) Exploratory study on microRNA profiles from plasma-derived extracellular vesicles in Alzheimer’s disease and dementia with Lewy bodies. Transl Neurodegener 8.https://doi.org/10.1186/S40035-019-0169-5
García-Jacobo RE, Uresti-Rivera EE, Portales-Pérez DP et al (2019) Circulating miR-146a, miR-34a and miR-375 in type 2 diabetes patients, pre-diabetic and normal-glycaemic individuals in relation to β-cell function, insulin resistance and metabolic parameters. Clin Exp Pharmacol Physiol 46:1092–1100. https://doi.org/10.1111/1440-1681.13147
Geekiyanage H, Jicha GA, Nelson PT, Chan C (2012) Blood serum miRNA: non-invasive biomarkers for Alzheimer’s disease. Exp Neurol 235:491–496. https://doi.org/10.1016/j.expneurol.2011.11.026
Goldberg M, Islam MR, Kerimoglu C et al (2021) Exercise as a model to identify microRNAs linked to human cognition: a role for microRNA-409 and microRNA-501. Transl Psychiatry 11.https://doi.org/10.1038/S41398-021-01627-W
Gomes CPC, Oliveira-Jr GP, Madrid B et al (2014) Circulating miR-1, miR-133a, and miR-206 levels are increased after a half-marathon run. Biomarkers 19:585–589. https://doi.org/10.3109/1354750X.2014.952663
Gomes JL, Fernandes T, Soci UP et al (2017) Obesity Downregulates MicroRNA-126 Inducing Capillary Rarefaction in Skeletal Muscle: Effects of Aerobic Exercise Training. Oxid Med Cell Longev 2017:2415246. https://doi.org/10.1155/2017/2415246
Gong Y, Chang L, Viola KL et al (2003) Alzheimer’s disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci U S A 100:10417–10422. https://doi.org/10.1073/pnas.1834302100
Gratuze M, Joly-Amado A, Vieau D et al (2018) Mutual relationship between tau and central insulin signalling: consequences for ad and tauopathies? Neuroendocrinology 107:181–195. https://doi.org/10.1159/000487641
Guedes JR, Santana I, Cunha C et al (2016) MicroRNA deregulation and chemotaxis and phagocytosis impairment in Alzheimer’s disease. Alzheimer’s Dement Diagn Assess Dis Monit 3:7–17. https://doi.org/10.1016/j.dadm.2015.11.004
Gui YX, Liu H, Zhang LS et al (2015) Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 6:37043–37053. https://doi.org/10.18632/oncotarget.6158
Guo R, Fan G, Zhang J et al (2017) A 9-microRNA signature in serum serves as a noninvasive biomarker in early diagnosis of Alzheimer’s disease. J Alzheimers Dis 60:1365–1377. https://doi.org/10.3233/JAD-170343
Ha M, Kim VN (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15:509–524
Hackney AC, Lane AR (2015) Exercise and the regulation of endocrine hormones. Prog Mol Biol Transl Sci 135:293–311. https://doi.org/10.1016/BS.PMBTS.2015.07.001
Hajjri SN, Sadigh-Eteghad S, Mehrpour M et al (2020) Beta-amyloid-dependent miRNAs as circulating biomarkers in Alzheimer’s disease: a preliminary report. J Mol Neurosci 70:871–877. https://doi.org/10.1007/S12031-020-01511-0
Hara N, Kikuchi M, Miyashita A et al (2017) Serum microRNA miR-501-3p as a potential biomarker related to the progression of Alzheimer’s disease. Acta Neuropathol Commun 5:10. https://doi.org/10.1186/S40478-017-0414-Z
Hashimoto Y, Hamaguchi M, Kaji A et al (2020) Intake of sucrose affects gut dysbiosis in patients with type 2 diabetes. J Diabetes Investig 11:1623–1634. https://doi.org/10.1111/JDI.13293
Hawkes N (2017) Merck ends trial of potential Alzheimer’s drug verubecestat. BMJ 356:j845. https://doi.org/10.1136/bmj.j845
Hicks SD, Jacob P, Middleton FA et al (2018) Translational physiology: distance running alters peripheral microRNAs implicated in metabolism, fluid balance, and myosin regulation in a sex-specific manner. Physiol Genomics 50:658. https://doi.org/10.1152/PHYSIOLGENOMICS.00035.2018
Higuchi C, Nakatsuka A, Eguchi J et al (2015) Identification of circulating miR-101, miR-375 and miR-802 as biomarkers for type 2 diabetes. Metabolism 64:489–497. https://doi.org/10.1016/j.metabol.2014.12.003
Hillman CH, Erickson KI, Kramer AF (2008) Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci 9:58–65. https://doi.org/10.1038/NRN2298
Hurley DM, Williams ER, Cross JM et al (2019) Aerobic exercise improves microvascular function in older adults. Med Sci Sports Exerc 51:773–781. https://doi.org/10.1249/MSS.0000000000001854
Iacomino G, Russo P, Stillitano I et al (2016) Circulating microRNAs are deregulated in overweight/obese children: preliminary results of the I. Family study. Genes Nutr 11:3–11. https://doi.org/10.1186/s12263-016-0525-3
IDF (2015) International Diabetes Federation. Diabetes Atlas, Seventh Ed
Improta-Caria AC, Aras R (2021) Treinamento com Exercício Físico e Doença de Chagas: Função Potencial dos MicroRNAs. Arq Bras Cardiol 117:132–141. https://doi.org/10.36660/abc.20200330
Improta-Caria AC, Nonaka CKV, Cavalcante BRR et al (2020) Modulation of microRNAs as a potential molecular mechanism involved in the beneficial actions of physical exercise in Alzheimer disease. Int J Mol Sci 21:1–35. https://doi.org/10.3390/ijms21144977
Improta-Caria AC, Soci ÚPR, Pinho CS et al (2021) Physical exercise and immune system: perspectives on the COVID-19 pandemic. Rev Assoc Med Bras 67:102–107. https://doi.org/10.1590/1806-9282.67.SUPPL1.20200673
Jain G, Stuendl A, Rao P, et al (2019) A combined miRNA-piRNA signature to detect Alzheimer’s disease. Transl Psychiatry 9.https://doi.org/10.1038/S41398-019-0579-2
Jia G, DeMarco VG, Sowers JR (2016) Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 12:144–153. https://doi.org/10.1038/nrendo.2015.216
Jia LH, Liu YN (2016) Downregulated serum miR-223 servers as biomarker in Alzheimer’s disease. Cell Biochem Funct 34:233–237. https://doi.org/10.1002/CBF.3184
Jiao Y, Zhu M, Mao X et al (2015) MicroRNA-130a expression is decreased in Xinjiang Uygur patients with type 2 diabetes mellitus. Am J Transl Res 7:1984–1991
Jiménez-Lucena R, Camargo A, Alcalá-Diaz JF, et al (2018) A plasma circulating miRNAs profile predicts type 2 diabetes mellitus and prediabetes: from the CORDIOPREV study. Exp Mol Med 50.https://doi.org/10.1038/s12276-018-0194-y
Jin Y, Tu Q, Liu M (2018) MicroRNA-125b regulates Alzheimer’s disease through SphK1 regulation. Mol Med Rep 18:2373–2380. https://doi.org/10.3892/mmr.2018.9156
Joglekar MV, Joglekar VM, Hardikar AA (2009) Expression of islet-specific microRNAs during human pancreatic development. Gene Expr Patterns 9:109–113. https://doi.org/10.1016/j.gep.2008.10.001
Kang S, Tsai LTY, Rosen ED (2016) Nuclear Mechanisms of Insulin Resistance. Trends Cell Biol 26:341. https://doi.org/10.1016/J.TCB.2016.01.002
Karolina DS, Armugam A, Tavintharan S et al (2011) MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS ONE 6:e22839. https://doi.org/10.1371/journal.pone.0022839
Karolina DS, Tavintharan S, Armugam A et al (2012) Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab 97:E2271–E2276. https://doi.org/10.1210/jc.2012-1996
Kern F, Ludwig N, Backes C et al (2019) Systematic Assessment of Blood-Borne MicroRNAs Highlights Molecular Profiles of Endurance Sport and Carbohydrate Uptake. Cells 8.https://doi.org/10.3390/CELLS8091045
Kiko T, Nakagawa K, Tsuduki T et al (2014) MicroRNAs in plasma and cerebrospinal fluid as potential markers for Alzheimer’s disease. J Alzheimer’s Dis 39:253–259. https://doi.org/10.3233/JAD-130932
Kong L, Zhu J, Han W et al (2011) Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol 48:61–69. https://doi.org/10.1007/s00592-010-0226-0
Kumar P, Dezso Z, MacKenzie C, et al (2013) Circulating miRNA Biomarkers for Alzheimer’s Disease. PLoS One 8.https://doi.org/10.1371/journal.pone.0069807
Kumar S, Vijayan M, Reddy PH (2017) MicroRNA-455-3p as a potential peripheral biomarker for Alzheimer’s disease. Hum Mol Genet 26:3808–3822. https://doi.org/10.1093/hmg/ddx267
La Sala L, Mrakic-Sposta S, Tagliabue E et al (2019) Circulating microRNA-21 is an early predictor of ROS-mediated damage in subjects with high risk of developing diabetes and in drug-naïve T2D. Cardiovasc Diabetol 18.https://doi.org/10.1186/S12933-019-0824-2
Lee C-C, Kuo Y-M, Huang C-C, Hsu K-S (2009) Insulin rescues amyloid beta-induced impairment of hippocampal long-term potentiation. Neurobiol Aging 30:377–387. https://doi.org/10.1016/j.neurobiolaging.2007.06.014
Leidinger P, Backes C, Deutscher S et al (2013) A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biol 14:R78. https://doi.org/10.1186/gb-2013-14-7-r78
Li W, Li X, Xin X et al (2016) MicroRNA-613 regulates the expression of brain-derived neurotrophic factor in Alzheimer’s disease. Biosci Trends 10:372–377. https://doi.org/10.5582/bst.2016.01127
Li Y, Yao M, Zhou Q, et al (2018) Dynamic regulation of circulating microRNAs during acute exercise and long-term exercise training in basketball athletes. Front Physiol 9.https://doi.org/10.3389/fphys.2018.00282
Li X, Tang Y, Jia Z et al (2020) Decreased expression of miR-24 in peripheral plasma of type 2 diabetes mellitus patients associated with diabetic foot ulcer. Wound Repair Regen 28:728–738. https://doi.org/10.1111/WRR.12850
Lin CJ, Lan YM, Ou MQ et al (2019) Expression of miR-217 and HIF-1α/VEGF pathway in patients with diabetic foot ulcer and its effect on angiogenesis of diabetic foot ulcer rats. J Endocrinol Invest 42:1307–1317. https://doi.org/10.1007/S40618-019-01053-2
Liu CG, Song J, Zhang YQ, Wang PC (2014a) MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer’s disease. Mol Med Rep 10:2395–2400. https://doi.org/10.3892/mmr.2014.2484
Liu CG, Wang JL, Li L et al (2014b) MicroRNA-135a and -200b, potential Biomarkers for Alzheimer’s disease, regulate β secretase and amyloid precursor protein. Brain Res 1583:55–64. https://doi.org/10.1016/j.brainres.2014.04.026
Liu CG, Wang JL, Li L, Wang PC (2014c) MicroRNA-384 regulates both amyloid precursor protein and β-secretase expression and is a potential biomarker for Alzheimer’s disease. Int J Mol Med 34:160–166. https://doi.org/10.3892/ijmm.2014.1780
Liu Y, Gao G, Yang C et al (2014d) The role of circulating microRNA-126 (miR-126): a novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int J Mol Sci 15:10567–10577. https://doi.org/10.3390/ijms150610567
Liu X, Xiao J, Zhu H et al (2015) miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab 21:584–595. https://doi.org/10.1016/j.cmet.2015.02.014
Liu Y, He X, Li Y, Wang T (2018) Cerebrospinal fluid CD4+ T lymphocyte-derived miRNA-let-7b can enhances the diagnostic performance of Alzheimer’s disease biomarkers. Biochem Biophys Res Commun 495:1144–1150. https://doi.org/10.1016/J.BBRC.2017.11.122
Long JM, Ray B, Lahiri DK (2012) MicroRNA-153 physiologically inhibits expression of amyloid-β precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J Biol Chem 287:31298–31310. https://doi.org/10.1074/jbc.M112.366336
Lugli G, Cohen AM, Bennett DA, et al (2015) Plasma exosomal miRNAs in persons with and without Alzheimer disease: Altered expression and prospects for biomarkers. PLoS One 10.https://doi.org/10.1371/journal.pone.0139233
Lusardi TA, Phillips JI, Wiedrick JT et al (2017) MicroRNAs in human cerebrospinal fluid as biomarkers for Alzheimer’s disease. J Alzheimer’s Dis 55:1223–1233. https://doi.org/10.3233/JAD-160835
Magdesian MH, Nery A, Martins AHB et al (2005) Peptide blockers of the inhibition of neuronal nicotinic acetylcholine receptors by amyloid beta. J Biol Chem 280:31085–31090. https://doi.org/10.1074/jbc.M502406200
Manzine PR, Pelucchi S, Horst MA et al (2018) MicroRNA 221 Targets ADAM10 mRNA and is Downregulated in Alzheimer’s Disease. J Alzheimer’s Dis 61:113–123. https://doi.org/10.3233/JAD-170592
McKeever PM, Schneider R, Taghdiri F et al (2018) MicroRNA expression levels are altered in the cerebrospinal fluid of patients with young-onset Alzheimer’s disease. Mol Neurobiol 5512(55):8826–8841. https://doi.org/10.1007/S12035-018-1032-X
Min P-K, Park J, Isaacs S et al (2016) Influence of statins on distinct circulating microRNAs during prolonged aerobic exercise. J Appl Physiol 120:711–720. https://doi.org/10.1152/japplphysiol.00654.2015
Mooren FC, Viereck J, Krüger K, Thum T (2014) Circulating microRNAs as potential biomarkers of aerobic exercise capacity. Am J Physiol Heart Circ Physiol 306:H557–H563. https://doi.org/10.1152/ajpheart.00711.2013
Muljo SA, Kanellopoulou C, Aravind L (2010) MicroRNA Targeting in Mammalian Genomes: Genes and Mechanisms. Wiley Interdiscip Rev Syst Biol Med 1–20. https://doi.org/10.1016/j.neuroimage.2013.08.045.The
Müller M, Kuiperij HB, Claassen JA et al (2014) MicroRNAs in Alzheimer’s disease: differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol Aging 35:152–158. https://doi.org/10.1016/j.neurobiolaging.2013.07.005
Müller M, Jäkel L, Bruinsma IB et al (2016) MicroRNA-29a is a candidate biomarker for Alzheimer’s disease in cell-free cerebrospinal fluid. Mol Neurobiol 53:2894–2899. https://doi.org/10.1007/s12035-015-9156-8
Mullins RJ, Diehl TC, Chia CW, Kapogiannis D (2017) Insulin resistance as a link between amyloid-beta and tau pathologies in Alzheimer’s disease. Front Aging Neurosci 9:1–16. https://doi.org/10.3389/fnagi.2017.00118
Musi N, Fujii N, Hirshman MF et al (2001) AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes 50:921–927. https://doi.org/10.2337/DIABETES.50.5.921
Nagaraj S, Laskowska-Kaszub K, Dębski KJ et al (2017) Profile of 6 microRNA in blood plasma distinguish early stage Alzheimer’s disease patients from non-demented subjects. Oncotarget 8:16122–16143. https://doi.org/10.18632/ONCOTARGET.15109
Nemecz M, Alexandru N, Tanko G, Georgescu A (2016) Role of microRNA in endothelial dysfunction and hypertension. Curr Hypertens Rep 18:87. https://doi.org/10.1007/s11906-016-0696-8
Nielsen S, Åkerström T, Rinnov A et al (2014) The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS ONE 9:e87308. https://doi.org/10.1371/journal.pone.0087308
Nunez Lopez YO, Garufi G, Seyhan AA (2017) Altered levels of circulating cytokines and microRNAs in lean and obese individuals with prediabetes and type 2 diabetes. Mol Biosyst 13:106–121. https://doi.org/10.1039/c6mb00596a
Olivieri F, Spazzafumo L, Bonafè M et al (2015) MiR-21–5p and miR-126a-3p levels in plasma and circulating angiogenic cells: relationship with type 2 diabetes complications. Oncotarget 6:35372–35382. https://doi.org/10.18632/oncotarget.6164
Ortega FJ, Mercader JM, Moreno-Navarrete JM et al (2014) Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care 37:1375–1383. https://doi.org/10.2337/dc13-1847
Pedersen BK, Saltin B (2015) Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sport 25:1–72. https://doi.org/10.1111/sms.12581
Pescador N, Pérez-Barba M, Ibarra JM et al (2013) Serum circulating microRNA profiling for identification of potential type 2 diabetes and obesity biomarkers. PLoS ONE 8:e77251. https://doi.org/10.1371/journal.pone.0077251
Physicians PC (2020) 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement 16:391–460. https://doi.org/10.1002/alz.12068
Poy MN, Eliasson L, Krutzfeldt J et al (2004) A pancreatic islet-specific microRNA regulates insulin secretion MicroRNAs (miRNAs) constitute a growing class of non-coding RNAs that are thought to regulate gene expression by translational repression. Lett to Nat 432:2–6
Prabu P, Rome S, Sathishkumar C et al (2015) Circulating MiRNAs of “Asian Indian Phenotype” identified in subjects with impaired glucose tolerance and patients with type 2 diabetes. PLoS ONE 10:e0128372. https://doi.org/10.1371/journal.pone.0128372
Raciti GA, Longo M, Parrillo L et al (2015) Understanding type 2 diabetes: from genetics to epigenetics. Acta Diabetol 52:821–827. https://doi.org/10.1007/S00592-015-0741-0
Rad SK, Arya A, Karimian H et al (2018) Mechanism involved in insulin resistance via accumulation of β-amyloid and neurofibrillary tangles: link between type 2 diabetes and alzheimer’s disease. Drug Des Devel Ther 12:3999–4021. https://doi.org/10.2147/DDDT.S173970
Radom-Aizik S, Zaldivar F, Oliver S et al (2010) Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes. J Appl Physiol 109:252–261. https://doi.org/10.1152/japplphysiol.01291.2009
Radom-Aizik S, Zaldivar F, Leu SY et al (2012) Effects of exercise on microRNA expression in young males peripheral blood mononuclear cells. Clin Transl Sci 5:32–38. https://doi.org/10.1111/j.1752-8062.2011.00384.x
Radom-Aizik S, Zaldivar F, Haddad F, Cooper DM (2013) Impact of brief exercise on peripheral blood NK cell gene and microRNA expression in young adults. J Appl Physiol 114:628–636. https://doi.org/10.1152/japplphysiol.01341.2012
Radom-Aizik S, Zaldivar FP, Haddad F, Cooper DM (2014) Impact of brief exercise on circulating monocyte gene and microRNA expression: implications for atherosclerotic vascular disease. Brain Behav Immun 39:121–129. https://doi.org/10.1016/j.bbi.2014.01.003
Ranasinghe C, Hills AP, Constantine GR et al (2018) Study protocol: a randomised controlled trial of supervised resistance training versus aerobic training in Sri Lankan adults with type 2 diabetes mellitus: SL-DART study. BMC Public Health 18:1–10. https://doi.org/10.1186/s12889-018-5069-6
Rathore MG, Saumet A, Rossi JF et al (2012) The NF-κB member p65 controls glutamine metabolism through miR-23a. Int J Biochem Cell Biol 44:1448–1456. https://doi.org/10.1016/J.BIOCEL.2012.05.011
Ren RJ, Zhang YF, Dammer EB et al (2016) Peripheral blood microRNA expression profiles in Alzheimer’s disease: screening, validation, association with clinical phenotype and implications for molecular mechanism. Mol Neurobiol 53:5772–5781. https://doi.org/10.1007/s12035-015-9484-8
Riancho J, Vázquez-Higuera JL, Pozueta A et al (2017) MicroRNA profile in patients with Alzheimer’s disease: analysis of miR-9-5p and miR-598 in raw and exosome enriched cerebrospinal fluid samples. J Alzheimers Dis 57:483–491. https://doi.org/10.3233/JAD-161179
Roden M, Shulman GI (2019) The integrative biology of type 2 diabetes. Nature 576:51–60. https://doi.org/10.1038/S41586-019-1797-8
Sampath Kumar A, Maiya AG, Shastry BA et al (2019) Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Phys Rehabil Med 62:98–103. https://doi.org/10.1016/J.REHAB.2018.11.001
Sangalli E, Tagliabue E, La SL et al (2020) Circulating MicroRNA-15a associates with retinal damage in patients with early stage type 2 diabetes. Front Endocrinol (lausanne) 11:254. https://doi.org/10.3389/FENDO.2020.00254/BIBTEX
Sapp RM, Chesney CA, Eagan LE et al (2020) Changes in circulating microRNA and arterial stiffness following high-intensity interval and moderate intensity continuous exercise. Physiol Rep 8:14431. https://doi.org/10.14814/phy2.14431
Satoh JI, Kino Y, Niida S (2015) MicroRNA-Seq data analysis pipeline to identify blood biomarkers for alzheimer’s disease from public data. Biomark Insights 2015:21–31. https://doi.org/10.4137/BMI.S25132
Sawada S, Kon M, Wada S et al (2013) Profiling of circulating MicroRNAs after a bout of acute resistance exercise in humans. PLoS ONE 8:e70823. https://doi.org/10.1371/journal.pone.0070823
Schipper HM, Maes OC, Chertkow HM, Wang E (2007) MicroRNA Expression in Alzheimer Blood Mononuclear Cells. Gene Regul Syst Bio 1:GRSB.S361. https://doi.org/10.4137/grsb.s361
Schüttler D, Clauss S, Weckbach LT, Brunner S (2019) Molecular mechanisms of cardiac remodeling and regeneration in physical exercise. Cells 8.https://doi.org/10.3390/CELLS8101128
Seyhan AA, Nunez Lopez YO, Xie H et al (2016) Pancreas-enriched miRNAs are altered in the circulation of subjects with diabetes: a pilot cross-sectional study. Sci Rep 6:1–15. https://doi.org/10.1038/srep31479
Shaik MM, Tamargo IA, Abubakar MB et al (2018) The role of microRNAs in Alzheimer’s disease and their therapeutic potentials. Genes (Basel) 9.https://doi.org/10.3390/genes9040174
Shi Y, Vanhoutte PM (2017) Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes 9:434–449. https://doi.org/10.1111/1753-0407.12521
Silva GJJ, Bye A, el Azzouzi H, Wisløff U (2017) MicroRNAs as important regulators of exercise adaptation. Prog Cardiovasc Dis 60:130–151. https://doi.org/10.1016/j.pcad.2017.06.003
Smith PY, Hernandez-Rapp J, Jolivette F et al (2015) MiR-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo. Hum Mol Genet 24:6721–6735. https://doi.org/10.1093/hmg/ddv377
Snel M, Gastaldelli A, Ouwens DM et al (2012) Effects of adding exercise to a 16-week very low-calorie diet in obese, insulin-dependent type 2 diabetes mellitus patients. J Clin Endocrinol Metab 97:2512–2520. https://doi.org/10.1210/jc.2011-3178
Souza VC, Morais GS, Henriques AD et al (2020) Whole-blood levels of microRNA-9 are decreased in patients with late-onset Alzheimer disease. Am J Alzheimers Dis Other Demen 35.https://doi.org/10.1177/1533317520911573
Stępień EŁ, Durak-Kozica M, Kamińska A et al (2018) Circulating ectosomes: determination of angiogenic microRNAs in type 2 diabetes. Theranostics 8:3874–3890. https://doi.org/10.7150/thno.23334
Sun L, Li X, Li G et al (2017) Actinidia chinensis Planch. Improves the indices of antioxidant and anti-inflammation status of type 2 diabetes mellitus by activating keap1 and Nrf2 via the upregulation of MicroRNA-424. Oxid Med Cell Longev 2017. https://doi.org/10.1155/2017/7038789
Tan L, Yu JT, Liu QY et al (2014a) Circulating miR-125b as a biomarker of Alzheimer’s disease. J Neurol Sci 336:52–56. https://doi.org/10.1016/j.jns.2013.10.002
Tan L, Yu JT, Tan MS et al (2014b) Genome-wide serum microRNA expression profiling identifies serum biomarkers for Alzheimer’s disease. J Alzheimer’s Dis 40:1017–1027. https://doi.org/10.3233/JAD-132144
Tang X, Muniappan L, Tang G, Ozcan S (2009) Identification of glucose-regulated miRNAs from pancreatic beta cells reveals a role for miR-30d in insulin transcription. RNA 15:287–293. https://doi.org/10.1261/rna.1211209
Tyagi S, Gupta P, Saini AS et al (2019) The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res 2:551–560. https://doi.org/10.4103/2231-4040.90879
Uhlemann M, Möbius-Winkler S, Fikenzer S et al (2014) Circulating microRNA-126 increases after different forms of endurance exercise in healthy adults. Eur J Prev Cardiol 21:484–491. https://doi.org/10.1177/2047487312467902
Van Craenenbroeck AH, Ledeganck KJ, Van Ackeren K et al (2015) Exercise training in cardiovascular disease: mechanisms and outcomes: plasma levels of microRNA in chronic kidney disease: patterns in acute and chronic exercise. Am J Physiol - Hear Circ Physiol 309:H2008. https://doi.org/10.1152/AJPHEART.00346.2015
Van Harten AC, Mulders J, Scheltens P et al (2015) Differential expression of microRNA in cerebrospinal fluid as a potential novel biomarker for Alzheimer’s disease. J Alzheimer’s Dis 47:243–252. https://doi.org/10.3233/JAD-140075
Wang T, Chen K, Li H et al (2015) The feasibility of utilizing plasma MiRNA107 and BACE1 messenger RNA gene expression for clinical diagnosis of amnestic mild cognitive impairment. J Clin Psychiatry 76:135–141. https://doi.org/10.4088/JCP.13M08812
Wang F, Shang Y, Zhang R, et al (2018a) A SIRT1 agonist reduces cognitive decline in type 2 diabetic rats through antioxidative and anti‑inflammatory mechanisms. Mol Med Rep 1–9.https://doi.org/10.3892/mmr.2018.9699
Wang Z, Qin W, Wei CB et al (2018b) The microRNA-1908 up-regulation in the peripheral blood cells impairs amyloid clearance by targeting ApoE. Int J Geriatr Psychiatry 33:980–986. https://doi.org/10.1002/gps.4881
Wang L, Liu J, Wang Q et al (2019) MicroRNA-200a-3p mediates neuroprotection in Alzheimer-related deficits and attenuates amyloid-beta overproduction and tau hyperphosphorylation via coregulating BACE1 and PRKACB. Front Pharmacol 10.https://doi.org/10.3389/fphar.2019.00806
Wang J, Chen C, Zhang Y (2020) An investigation of microRNA-103 and microRNA-107 as potential blood-based biomarkers for disease risk and progression of Alzheimer’s disease. J Clin Lab Anal 34.https://doi.org/10.1002/JCLA.23006
Westerberg E, Molin CJ, Lindblad I et al (2017) Physical exercise in myasthenia gravis is safe and improves neuromuscular parameters and physical performance-based measures: a pilot study. Muscle Nerve 56:207–214. https://doi.org/10.1002/mus.25493
Witvrouwen I, Gevaert AB, Possemiers N et al (2021a) Plasma-derived microRNAs are influenced by acute and chronic exercise in patients with heart failure with reduced ejection fraction. Front Physiol 12https://doi.org/10.3389/FPHYS.2021.736494
Witvrouwen I, Gevaert AB, Possemiers N et al (2021b) Circulating microRNA as predictors for exercise response in heart failure with reduced ejection fraction. Eur J Prev Cardiol. https://doi.org/10.1093/EURJPC/ZWAA142
Wu Q, Ye X, Xiong Y, et al (2016) The protective role of microRNA-200c in Alzheimer’s disease pathologies is induced by beta amyloid-triggered endoplasmic reticulum stress. Front Mol Neurosci 9.https://doi.org/10.3389/fnmol.2016.00140
Wu Y, Xu J, Xu J et al (2017) Lower serum levels of miR-29c-3p and miR-19b-3p as biomarkers for Alzheimer’s disease. Tohoku J Exp Med 242:129–136. https://doi.org/10.1620/tjem.242.129
Wu T, Xie D, Zhao X et al (2021) Enhanced expression of miR-34c in Peripheral Plasma Associated with Diabetic Foot Ulcer in Type 2 Diabetes Patients. Diabetes Metab Syndr Obes 14:4263–4273. https://doi.org/10.2147/DMSO.S326066
Xie B, Liu Z, Jiang L et al (2017) Increased Serum miR-206 Level Predicts Conversion from Amnestic Mild Cognitive Impairment to Alzheimer’s Disease: A 5-Year Follow-up Study. J Alzheimers Dis 55:509–520. https://doi.org/10.3233/JAD-160468
Xiong Y, Chen L, Yan C et al (2020) Circulating exosomal miR-20b-5p inhibition restores Wnt9b signaling and reverses diabetes-associated impaired wound healing. Small 16.https://doi.org/10.1002/SMLL.201904044
Yan S, Wang T, Huang S et al (2016) Differential expression of microRNAs in plasma of patients with prediabetes and newly diagnosed type 2 diabetes. Acta Diabetol 53:693–702. https://doi.org/10.1007/s00592-016-0837-1
Yang Z, Chen H, Si H et al (2014) Serum miR-23a, a potential biomarker for diagnosis of pre-diabetes and type 2 diabetes. Acta Diabetol 51:823–831. https://doi.org/10.1007/s00592-014-0617-8
Yang G, Song Y, Zhou X et al (2015) MicroRNA-29c targets β-site amyloid precursor protein-cleaving enzyme 1 and has a neuroprotective role in vitro and in vivo. Mol Med Rep 12:3081–3088. https://doi.org/10.3892/mmr.2015.3728
Yang ZM, Chen LH, Hong M et al (2017) Serum microRNA profiling and bioinformatics analysis of patients with type 2 diabetes mellitus in a Chinese population. Mol Med Rep 15:2143–2153. https://doi.org/10.3892/mmr.2017.6239
Yang TT, Liu CG, Gao SC et al (2018) The serum exosome derived microRNA-135a, -193b, and -384 were potential Alzheimer’s disease biomarkers. Biomed Environ Sci 31:87–96. https://doi.org/10.3967/BES2018.011
Yang Q, Zhao Q, Yin Y (2019) miR‑133b is a potential diagnostic biomarker for Alzheimer’s disease and has a neuroprotective role. Exp Ther Med 2711–2718.https://doi.org/10.3892/etm.2019.7855
Yllmaz ŞG, Erdal ME, Özge AA, Sungur MA (2016) Can peripheral microRNA expression data serve as epigenomic (Upstream) biomarkers of Alzheimer’s disease? Omi A J Integr Biol 20:456–461. https://doi.org/10.1089/omi.2016.0099
Zampetaki A, Kiechl S, Drozdov I et al (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 107:810–817. https://doi.org/10.1161/CIRCRESAHA.110.226357
Zeinali F, Aghaei Zarch SM, VahidiMehrjardi MY et al (2020) Effects of synbiotic supplementation on gut microbiome, serum level of TNF-α, and expression of microRNA-126 and microRNA-146a in patients with type 2 diabetes mellitus: study protocol for a double-blind controlled randomized clinical trial. Trials 21:1–9. https://doi.org/10.1186/S13063-020-04236-Y/FIGURES/2
Zeng Q, Zou L, Qian L et al (2017) Expression of microRNA-222 in serum of patients with Alzheimer’s disease. Mol Med Rep 16:5575–5579. https://doi.org/10.3892/MMR.2017.7301
Zhang J, Wang R (2021) Deregulated lncRNA MAGI2-AS3 in Alzheimer’s disease attenuates amyloid-β induced neurotoxicity and neuroinflammation by sponging miR-374b-5p. Exp Gerontol 144.https://doi.org/10.1016/J.EXGER.2020.111180
Zhang B, Wang A, Xia C et al (2015a) A single nucleotide polymorphism in primary-microRNA-146a reduces the expression of mature microRNA-146a in patients with Alzheimer’s disease and is associated with the pathogenesis of Alzheimer’s disease. Mol Med Rep 12:4037–4042. https://doi.org/10.3892/mmr.2015.3968
Zhang T, Li L, Shang Q et al (2015b) Circulating miR-126 is a potential biomarker to predict the onset of type 2 diabetes mellitus in susceptible individuals. Biochem Biophys Res Commun 463:60–63. https://doi.org/10.1016/j.bbrc.2015.05.017
Zhang Y, Lv X, Liu C et al (2016a) MiR-214-3p attenuates cognition defects via the inhibition of autophagy in SAMP8 mouse model of sporadic Alzheimer’s disease. Neurotoxicology 56:139–149. https://doi.org/10.1016/j.neuro.2016.07.004
Zhang Y, Xing H, Guo S et al (2016b) microRNA-135b has a neuroprotective role via targeting of β-site APP-cleaving enzyme 1. Exp Ther Med 12:809–814. https://doi.org/10.3892/etm.2016.3366
Zhang T, Brinkley TE, Liu K, et al (2017) Circulating MiRNAs as biomarkers of gait speed responses to aerobic exercise training in obese older adults. Aging (Albany NY) 9:900–913. https://doi.org/10.18632/aging.101199
Zhao RR, O’Sullivan AJ, Fiatarone Singh MA (2018) Exercise or physical activity and cognitive function in adults with type 2 diabetes, insulin resistance or impaired glucose tolerance: a systematic review. Eur Rev Aging Phys Act 15
Zhao W, Chen H, Quon MJ, Alkon DL (2004) Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol 490:71–81. https://doi.org/10.1016/j.ejphar.2004.02.045
Zhou X, Chen Y, Mok KY et al (2019) Non-coding variability at the APOE locus contributes to the Alzheimer’s risk. Nat Commun 10:1–16. https://doi.org/10.1038/s41467-019-10945-z
Acknowledgements
We are thankful to CAPES.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Brazil—Finance Code 001.
Author information
Authors and Affiliations
Contributions
Ricardo Augusto Leoni De Sousa: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Roles/Writing—original draft; Writing—review & editing. Alex Cleber Improta-Caria: Data curation; Formal analysis; Investigation; Supervision; Validation; Visualization; Roles/Writing—original draft; Writing—review & editing.
Corresponding author
Ethics declarations
Compliance with ethical standards
Not applicable.
Consent for publication
Not applicable.
Declaration of competing interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Sousa, R.A.L., Improta-Caria, A.C. Regulation of microRNAs in Alzheimer´s disease, type 2 diabetes, and aerobic exercise training. Metab Brain Dis 37, 559–580 (2022). https://doi.org/10.1007/s11011-022-00903-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-00903-y