Abstract
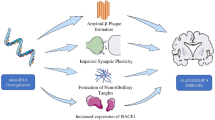
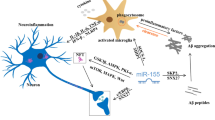
MicroRNAs (miRNAs) are considered among the most reliable biomarkers to diagnose and predict Alzheimer’s disease (AD), due to their regulatory nature. The main goal of this study was to evaluate the expression of miR4422 and miR3714, as the main regulators of GSAP and BACE1 expression, in AD patients compared with healthy subjects. Twenty patients with a mild to moderate AD (58–71 years old) and 15 healthy subjects (58–73 years old) participated in this study. The expression levels of miR4422 and miR3714 as the target genes and 5S rRNA and miRlet7a-5p as the reference genes were measured in the two groups. To compare the expression between the case and the control groups, the t test or the Wilcoxon test was used, based on the data distribution patterns. The efficiencies of amplification of the miR4422, miR3714, 5S rRNA, and miRlet7a-5p genes all were in the acceptable range. The mean miR4422-5S rRNA dCt value was significantly different between the two groups (p = 0.018). The relative fold change of the expression was 0.43. The mean miR4422-miRlet7a-5p dCt value (p = 0.41), the mean miR3714-5S rRNA dCt value (p = 0.10), and the mean miR3714-miRlet7a-5p dCt value (p = 0.063) were not significantly different between the two groups. We indicated that miR4422 could be a reliable biomarker for Alzheimer’s diagnosis. It seems that the reduced expression of miR4422 that targets GSAP and BACE1 expression can lead to an increase in the formation of Aβ plaque.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD), as the most common cause of dementia, slowly destroys memory, cognitive skills, and, eventually, the ability to carry out the simplest tasks (Madadi & Mehdizadeh, 2014). Currently, the global prevalence of AD is estimated to be about 24 million and is expected to quadruple by 2050, which imposes considerable costs on society (Reitz & Mayeux, 2014). This issue puts the importance of an effective treatment in attention, which, in turn, emphasizes demands for accurate and early diagnosis of the disease.
Since 1984, the key diagnostic criteria of AD have been the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS–ADRDA) criteria. These criteria consider both clinical and neuropathological signs and symptoms and classify the pattern of the disease into three categories: “possible,” “probable,” and “definite (McKhann et al., 1984).
However, advances in cellular and molecular biology revealed that previous diagnostic criteria should be reviewed in primary respects. Today, the AD spectrum is known to be broader than what was previously thought and is going to include molecular biomarkers. Biomarkers are described as naturally occurring molecules, genes, or characteristics by which a particular pathological or physiological process can be identified. Biomarkers are expected to increase the diagnostic sensitivity and specificity and provide the possibility of diagnosing AD before the onset of clinical symptoms.
Considering previous research, a set of cerebrospinal fluid (CSF) biomarkers including beta-amyloid (Aβ) levels, the intraneuronal inclusion of the microtubules, and phosphorylated tau (Borroni et al., 2002) collectively increases the diagnosis sensitivity to > 95% and the specificity to > 85% (Blennow et al., 2010). However, lumbar punctures for CSF acquisition are intrusive and invasive, and have several side effects such as severe backache, nausea, and weakness in the elderly. These challenges attracted researchers to peripheral circulating biomarkers.
Koyama et al. showed the plasma Aβ as a highly statistical and clinically significant predictor of dementia and cognitive impairment (Koyama et al., 2012). Experimental evidence still supports the hypothesis that an imbalance between production and clearance of extracellular concentration of Aβ42 is a central initiating factor in AD (Selkoe & Hardy, 2016). It has been found that the dysregulation of expression of beta-secretase 1 (BACE1) and gamma-secretase activating protein (GSAP) genes may exacerbate the deposition of Aβ42. BACE1 gene product is responsible for the first step of amyloid precursor protein (APP) processing and GSAP is the main regulator of gamma-secretase activity, which cuts the transmembrane domain of the APP to form the Aβ. Therefore, deficient expression of the regulators of the expression of these two genes may also be involved in the pathophysiology of the disease.
miRNAs are a class of small (~ 22-nucleotide) non-coding ribonucleic acid molecules that downregulate the expression of more than 60% of all known genes in a post-transcriptional RNA silencing process (Ha & Kim, 2014). miRNAs may cleave, destabilize, or disrupt the translation of the target mRNAs (Bartel, 2009). Recent evidence suggests that the dysregulation of some miRNA expression in peripheral blood may be considered as potential biomarkers for the diagnosis of AD (Snyder et al., 2014).
So far, several miRNAs have been identified to regulate the expression of BACE1 genes. For example, Hebert et al. (Hébert et al., 2008) found that the miR-29a/b-1 cluster is significantly decreased in AD patients, which, in turn, leads to abnormally high BACE1 protein. Xie et al. (Xie et al., 2017) demonstrated that miR-9 plays a central role in regulating the BACE1 expression via downregulation of cAMP response element–binding protein (CREB) and Gong et al. (Gong et al., 2017) confirmed that miR-15b represses BACE1 expression in patients with sporadic Alzheimer’s disease. Furthermore, He et al. (Cheng et al., 2010) suggested that the downregulation of GSAP expression through a synthetic small RNA may reduce the production of Aβ.
The main goal of this study was to evaluate the expression of miR4422 (GSAP- and BACE1-associated miRNA) and miR3714 (BACE1-associated miRNA), found through bioinformatics-based approaches, in AD patients compared with healthy subjects.
Materials and Methods
Participants
In this cross-sectional study, 20 Alzheimer’s cases, aged 58 to 71 years old, were selected using a convenience sampling method. All patients were examined by an experienced neurologist and AD was diagnosed using the NINCDS–ADRDA criteria (Dubois et al., 2007). Their mild to moderate sporadic AD was confirmed based on the Mini–Mental State Examination (MMSE) (mean score = 18.72 ± 0.78). None of the patients had a history of any other neurological or mental disorder, diabetes, severe infectious disease, stroke, and familial history of AD. Fifteen healthy subjects with the same age range of patients (58 to 73 years) and MMSE mean score of 29.0 ± 1.0 were also included in the control group. According to their reports and evaluation of medical history, healthy subjects were not suffering from any neurological or psychiatric disorder. All the participants were male and none of them was addicted to nicotine, drugs, or alcohol. All subjects participated in the study with their own or their first-degree relatives’ informed consent. All stages of the study were conducted under the Ethics Committee of the Iran University of Medical Sciences (IR.IUMS.REC 1394.94-02-87-26052).
Finding the Target miRNAs
Using the miRDB database, we tried to find the most significant miRNAs targeting the BACE1 and GSAP genes. miRDB is an online tool for miRNA target prediction based on a rich database, MirTarget, which holds information about thousands of miRNA-target interactions from high-throughput experiments (Wong & Wang, 2015). In this database, by entering the name of the gene, all associated miRNAs are found and ranked based on the similarity score. These scores are assigned by the new computational target prediction algorithm. The higher the score, the more confidence we have in this prediction.
RNA Extraction and Quantitative Real-time PCR Amplification
Five milliliters of peripheral blood was collected from each participant in an EDTA tube to prevent coagulation and placed in an ice bucket. Blood samples were immediately centrifuged and serum was isolated. Serum miRNAs were extracted from each sample using miRCURY RNA Isolation Kit – Biofluids (Exiqon, Denmark) based on the manufacturer’s protocol. cDNAs were synthesized using Universal cDNA Synthesis Kit (Exiqon, Denmark) based on the manufacturer’s protocol. For this purpose, each of the template miRNA samples was adjusted to a concentration of 5 ng/μL using nuclease-free water. The reverse transcriptase working solution was prepared by mixing 2 μL of the 5× reaction buffer, 4.5 μL of the nuclease-free water, 1 μL of the enzyme mix, 0.5 μL of the RNA spike-ins, and 2 μL of the template total miRNA, to a final volume of 10 μL. The mixture was incubated for 60 min at 42 °C. The reverse transcriptase was heat-inactivated at 95 °C for 5 min and then stored at 4 °C. cDNA templates were diluted for the real-time PCR reactions by nuclease-free water. Four microliters of the diluted cDNA template, 1 μL of the LNA PCR primer sets (Exiqon, Denmark), and 5 μL of the PCR master mix were mixed to a final volume of 10 μL. Real-time PCR amplification was performed as follows: 95 °C, 10 min for polymerase activation/denaturation; 45 amplification cycles at 95 °C, 10 s, 60 °C, 1 min; and at the ramp-rate of 1.6 °C/s6 optical read. The quantitative real-time PCR amplification was performed using the LightCycler®96 PCR system, Roche, Germany. Moreover, seven serial dilutions of cDNA template known to express the gene of interest were prepared for separate real-time reactions to determine the amplification efficiency.
Data Analysis
The Shapiro–Wilk test was used for normality to detect all departures from the normality distribution. In order to compare the data between the case and the control groups. If the distribution of data was normal, the t test was used, and if the distribution was not normal, then the Wilcoxon test was used. To calculate the efficiency of amplification, a regression line was generated by calculating the Ct standard curve data points and the slope of the line (m) was used to estimate the efficiency according to:
Ct is defined as the number of cycles required for the fluorescent signal to cross the threshold. The 2−dCt value was used to analyze the relative gene expression. It requires the assignment of one or more housekeeping genes, which are assumed to be uniformly and constantly expressed in all samples, as well as one or more reference samples. The expression of other samples is then compared with that of the reference sample. All analyses were performed using R studio version 1.1.456.
Results
Demographic Data
The mean age of the case group was 64.45 ± 3.48 and the mean age of the control group was 64.86 ± 4.67 (Fig. 1). The Shapiro–Wilk test showed that the distribution of age data was normal (W = 0.96439, p = 0.3084). The independent t test showed no significant difference between the two groups in terms of age (t = − 0.28642, df = 25.687, p = 0.7769).
miRNA Selection and Efficiencies of Amplification
We found miR3714 which targets BACE1 gene and miR4422 which targets GSAP and BACE1 genes as the most significant miRNAs and their expression was compared between the two groups based on the two housekeeping genes, 5S rRNA and miRlet7a-5p (LNA PCR primer set, Exiqon, Denmark). Since only small RNAs were extracted, quantification was not possible by Nanodrop or spectrophotometry. Plotting the Ct values along with corresponding concentrations in a logarithmic scale and generating a linear regression curve through the data points are shown which leads the slope of the trend line to be − 3.39, − 3.38, − 3.51, and − 3.57 for the miR4422, miR3714, 5S rRNA, and miRlet7a-5p genes, respectively Fig. 2. The efficiency of amplification was obtained 97%, 98%, 92%, and 90% for the amplification of the miR4422, miR3714, 5S rRNA, and miRlet7a-5p genes, respectively. All amplification efficiencies were in the acceptable range (90–110%).
The standard curves generated by plotting the Ct values along with the corresponding concentrations on a logarithmic scale. The straight lines indicated the linear regression models with the slopes equal to − 3.39, − 3.38, − 3.51, and − 3.57 for the miR4422, miR3714, 5S rRNA, and miRlet7a-5p genes, respectively
The Mean Ct Values of the Expression of Target and Housekeeping miRNAs
The mean Ct values of miR4422, miR3714, 5S rRNA, and miRlet7a-5p were 42.36 ± 3.32, 41.54 ± 1.98, 21.89 ± 21.89, and 34.73 ± 3.08 for the case group and 40.76 ± 2.31, 41.73 ± 1.75, 23.89 ± 3.12, and 33.00 ± 2.99 for the control group, respectively (Fig. 3).
The Normalized Ct Values of the Expression of Target miRNAs
When the expression of miR4422 gene was normalized by the expression of 5S rRNA gene, the mean dCt values for the case and the control groups were obtained 20.47 ± 3.69 and 16.86 ± 4.51, respectively (Fig. 4). The miR4422-5S rRNA dCt values were distributed normally (W = 0.94002, p = 0.056). Independent t test analysis showed that the mean miR4422-5S rRNA dCt values were significantly different between the two groups (t = 2.5283, df = 26.636, p = 0.018).
The mean dCt values of the expression of miR4422 gene normalized by the expression of miRlet7a-5p gene were 6.48 ± 5.15 and 7.76 ± 4.24, for the case and the control groups, respectively (Fig. 4). The miR4422-miRlet7a-5p dCt values were not distributed normally (W = 0.92721, p = 0.02) and the Wilcoxon rank-sum test showed that the mean miR4422-miRlet7a-5p dCt values are not significantly different between the two groups (W = 125, p = 0.41).
The mean dCt values of the expression of miR3714 gene normalized by the expression of 5S rRNA gene were 19.65 ± 3.46 and 17.84 ± 3.00, for the case and the control groups, respectively (Fig. 4). The miR3714-5S rRNA dCt values were distributed normally (W = 0.987, p = 0.95) and the independent t test showed that the mean miR3714-5S rRNA dCt values were not significantly different between the two groups (t = 1.65, df = 32.23, p = 0.10).
Finally, the mean dCt values of the expression of miR3714 gene normalized by the expression of miRlet7a-5p gene were 6.81 ± 3.36 and 8.73 ± 3.07, for the case and the control groups, respectively (Fig. 4). The miR3714-miRlet7a-5p dCt values were not distributed normally (W = 0.845, p < 0.001) and the Wilcoxon rank-sum test showed that the mean miR3714-miRlet7a-5p dCt values were not significantly different between the two groups (W = 92, p = 0.063).
In order to better illustrate the fold change of the expression of target genes in the case group, relative to the control group, 2−dCt values were also calculated for each target gene. Then, the mean values for the control group were considered equal to 1 and the values for the case group were also changed in the same proportion.The fold changes of the expression of miR4422 and miR3714 mRNA levels in the case group were 0.43 and 0.51 relative to the 5S rRNA expression, and 0.67 and 0.75 relative to the miRlet7a-5p expression (Fig. 5).
Discussion
By delving into the area of molecular biology, researchers’ attempts to find specific biomarkers to help Alzheimer’s precise and early diagnosis have entered a new phase. Due to the problems and risks associated with the examination of CSF biomarkers, peripheral blood–based biomarkers were taken into consideration, and when it turned out that the expression of most genes in the brain is consistent with their expression in the peripheral tissues, peripheral expression alterations were considered as reliable AD biomarkers.
In the meantime, since miRNAs regulate the expression of many other genes, they have been the focus of attention. Circulating miRNAs has been proposed as being applicable in diagnostics as other biomarkers for the diseases. Their expression usually exhibits detectable alterations under unusual physiological and pathological conditions. Moreover, circulating microRNAs still have been suggested as reliable predictors for the prognosis of different diseases in practical clinical applications (Wang et al., 2018; Tang et al., 2017).
Furthermore, developing miRNA therapeutics for neurological diseases has been a promising opportunity in recent years. This stems from the very small size of miRNAs, which makes them amenable for manipulation and synthesis in laboratory conditions (Greenberg & Soreq, 2014). On the other hand, through the regulation of numerous targets of the same pathway, each miRNA may affect an entire pathway and, for this reason, low doses of therapeutic miRNAs or targeting only one miRNA could have extensive impacts (Rupaimoole & Slack, 2017). Recent discoveries, originated from scientists’ efforts over decades, have uncovered roles of miRNAs as potential diagnostic biomarkers and therapeutic targets in AD.
It has been more than 20 years since it was first proposed that the deposition of amyloid β-peptide in brain tissue may cause neurodegeneration in AD (Hardy & Selkoe, 2002). One leading theory argued that the Aβ plaques lead to AD because of direct toxicity to the adjacent neurons and other theories suggested that amyloid extraneuronal preplaque oligomers trigger the intraneuronal death signal (Carter & Lippa, 2001). In any case, the formation of the Aβ plaques is significantly associated with the onset and severity of the disease, and any biomarker that could indicate the formation of these plaques can have a diagnostic and research value.
In this study, using the miRDB database, we found miR4422 as the most significant miRNA which targets the BACE1 and GSAP genes. Studies have shown that the irregular overexpression of both BACE1 and GSAP genes can contribute to the formation of amyloid plaques. Hampel et al. argued that BACE1 activity is increased in the brain tissue of patients with sporadic AD (Hampel & Shen, 2009). In a postmortem study in 2010, Coulson et al. demonstrated that BACE1 mRNA levels were remarkably elevated in medial temporal and superior parietal gyri of AD patients, compared with healthy controls (Coulson et al., 2010). Furthermore, more recently, Manzine et al. indicated higher levels of BACE1 mRNA in plasma of AD patients and proposed this finding as an aid diagnostic tool along with other diagnostic tests (Manzine et al., 2016).
Reduced GSAP expression in neural cells is demonstrated to decrease Aβ generation (Hussain et al., 2012). Perez et al. investigated that the neocortical 98-kDa transcript of GSAP is significantly increased in AD patients and is correlated with cognitive deficits (Perez et al., 2017) and Satoh et al. provided evidence that GSAP level is elevated in postmortem brain of AD patients (Satoh et al., 2011). Moreover, pharmacological inhibition of GSAP is suggested as an efficient Aβ-lowering therapeutic strategy (Chu et al., 2016).
On the other hand, studies have also shown alterations in miRNAs expression profile and the effect on BACE1 and GSAP genes in AD brain tissue. Hébert et al. suggested that the miR-29a/b-1 cluster expression has a significant negative relationship with BACE1 mRNA levels in sporadic AD patients (Hebert et al., 2008). In vitro experiments have confirmed that miR-29b-1 and miR-29a inhibit the expression of the BACE1 (Deng et al., 2014). Additionally, studies have recently found an inverse correlation between miR-324-3p expression and GSAP mRNA level (Srivastava et al., 2017; Hajjari et al., 2017). Following previous studies, our study addresses the role of the miR4422 in regulating the expression of BACE1 and GSAP genes in Alzheimer’s disease.
Conclusions
As a result, it seems that reducing the activity of the BACE1 and the GSAP genes, in any way, including inhibiting their expression through miRNAs, can reduce the formation of amyloid plaques. Therefore, reduced expression of miRNAs that target these two genes can also lead to an increase in the formation of Aβ plaques. Our study revealed a significantly decreased level of miR4422 in AD patients in comparison with healthy controls. Obviously, confirmation of our findings in future studies and on larger populations can raise the expression of miR4422 gene as a reliable biomarker for AD diagnosis.
References
Bartel DP (2009) MicroRNA target recognition and regulatory functions. Cell 136(2):215–233. https://doi.org/10.1016/j.cell.2009.01.002
Blennow K, Hampel H, Weiner M, Zetterberg H (2010) Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 6:131–144
Borroni B, Colciaghi F, Corsini P, Akkawi N, Rozzini L, Del Zotto E, Talarico G, Cattabeni F, Lenzi GL, Di Luca M, Padovani A (2002) Early stages of probable Alzheimer disease are associated with changes in platelet amyloid precursor protein forms. Neurol Sci : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 23(5):207–210. https://doi.org/10.1007/s100720200042
Carter J, Lippa CF (2001) Beta-amyloid, neuronal death and Alzheimer’s disease. Curr Mol Med 1(6):733–737
Cheng S, Li L, He S, Liu J, Sun Y, He M, Grasing K, Premont RT, Suo WZ (2010) GRK5 deficiency accelerates β-amyloid accumulation in Tg2576 mice via impaired cholinergic activity. J Biol Chem 285(53):41541–41548
Chu J, Wisniewski T, Praticò D (2016) GATA1-mediated transcriptional regulation of the γ-secretase activating protein increases Aβ formation in down syndrome. Ann Neurol 79(1):138–143. https://doi.org/10.1002/ana.24540
Coulson DT, Beyer N, Quinn JG, Brockbank S, Hellemans J, Irvine GB, Ravid R, Johnston JA (2010) BACE1 mRNA expression in Alzheimer’s disease postmortem brain tissue. Journal of Alzheimer’s disease : JAD 22(4):1111–1122. https://doi.org/10.3233/jad-2010-101254
Deng Y, Ding Y, Hou D (2014) Research status of the regulation of miRNA on BACE1. Int J Neurosci 124(7):474–477. https://doi.org/10.3109/00207454.2013.858249
Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6(8):734–746
Gong G, An F, Wang Y, Bian M, Yu LJ, Wei C (2017) miR-15b represses BACE1 expression in sporadic Alzheimer’s disease. Oncotarget 8(53):91551–91557. https://doi.org/10.18632/oncotarget.21177
Greenberg DS, Soreq H (2014) MicroRNA therapeutics in neurological disease. Curr Pharm Des 20(38):6022–6027
Ha M, Kim VN (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15(8):509–524. https://doi.org/10.1038/nrm3838
Hajjari SN, Mehdizadeh M, Sadigh-Eteghad S, Shanehbandi D, Teimourian S, Baradaran B (2017) Secretases-related miRNAs in Alzheimer’s disease: new approach for biomarker discovery. Neurol Sci : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 38(11):1921–1926. https://doi.org/10.1007/s10072-017-3086-3
Hampel H, Shen Y (2009) Beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) as a biological candidate marker of Alzheimer’s disease. Scand J Clin Lab Invest 69(1):8–12. https://doi.org/10.1080/00365510701864610
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353
Hébert SS, Horré K, Nicolaï L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B (2008) Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. Proc Natl Acad Sci 105(17):6415–6420. https://doi.org/10.1073/pnas.0710263105
Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B (2008) Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A 105(17):6415–6420. https://doi.org/10.1073/pnas.0710263105
Hussain I, Fabrègue J, Anderes L, Ousson S, Borlat F, Eligert V, Berger S, Dimitrov M, Alattia J-R, Fraering PC (2012) The role of γ-secretase activating protein, GSAP and imatinib in the regulation of γ-secretase activity and amyloid-β generation. J Biol Chem 288(4):2521–2531 jbc-M112
Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F (2012) Plasma amyloid-beta as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol 69(7):824–831. https://doi.org/10.1001/archneurol.2011.1841
Madadi S, Mehdizadeh M (2014) Alzheimer diseases. Avicenna Journal of Neuro Psych Physiology 1 (2)
Manzine PR, Souza MS, Cominetti MR (2016) BACE1 levels are increased in plasma of Alzheimer’s disease patients compared with matched cognitively healthy controls. Personalized medicine 13(6):531–540
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7):939–944
Perez SE, Nadeem M, Malek-Ahmadi MH, He B, Mufson EJ (2017) Frontal cortex and hippocampal gamma-secretase activating protein levels in prodromal Alzheimer disease. Neurodegener Dis 17(6):235–241. https://doi.org/10.1159/000477937
Reitz C, Mayeux R (2014) Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88(4):640–651. https://doi.org/10.1016/j.bcp.2013.12.024
Rupaimoole R, Slack FJ (2017) MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16(3):203–222. https://doi.org/10.1038/nrd.2016.246
Satoh J, Tabunoki H, Ishida T, Saito Y, Arima K (2011) Immunohistochemical characterization of γ-secretase activating protein expression in Alzheimer’s disease brains. Neuropathol Appl Neurobiol 38(2):132–141. https://doi.org/10.1111/j.1365-2990.2011.01206.x
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8(6):595–608. https://doi.org/10.15252/emmm.201606210
Snyder HM, Carrillo MC, Grodstein F, Henriksen K, Jeromin A, Lovestone S, Mielke MM, O’Bryant S, Sarasa M, Sjøgren M, Soares H, Teeling J, Trushina E, Ward M, West T, Bain LJ, Shineman DW, Weiner M, Fillit HM (2014) Developing novel blood-based biomarkers for Alzheimer’s disease. Alzheimer’s Dement : the journal of the Alzheimer’s Association 10(1):109–114. https://doi.org/10.1016/j.jalz.2013.10.007
Srivastava PK, Roncon P, Lukasiuk K, Gorter JA, Aronica E, Pitkänen A, Petretto E, Johnson MR, Simonato M (2017) Meta-analysis of microRNAs dysregulated in the hippocampal dentate gyrus of animal models of epilepsy. eNeuro 4(6):ENEURO.0152-0117.2017. https://doi.org/10.1523/ENEURO.0152-17.2017
Tang Y, Zhao S, Wang J, Li D, Ren Q, Tang Y (2017) Plasma miR-122 as a potential diagnostic and prognostic indicator in human glioma. Neurol Sci : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 38(6):1087–1092. https://doi.org/10.1007/s10072-017-2912-y
Wang H, Peng R, Wang J, Qin Z, Xue L (2018) Circulating microRNAs as potential cancer biomarkers: the advantage and disadvantage. Clin Epigenetics 10:59. https://doi.org/10.1186/s13148-018-0492-1
Wong N, Wang X (2015) miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res 43(Database issue):D146–D152. https://doi.org/10.1093/nar/gku1104
Xie H, Zhao Y, Zhou Y, Liu L, Liu Y, Wang D, Zhang S, Yang M (2017) MiR-9 regulates the expression of BACE1 in dementia induced by chronic brain hypoperfusion in rats. Cell Physiol Biochem : international journal of experimental cellular physiology, biochemistry, and pharmacology 42(3):1213–1226. https://doi.org/10.1159/000478919
Funding
This work was supported by the Medical Research Council of Iran University of Medical Sciences (IUMS) (grant number 26052) and the Cognitive Sciences and Technologies Council (COGC) of Iran research grant 4023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All subjects participated in the study with their own or their first-degree relatives’ informed consent. All stages of the study were conducted under the Ethics Committee of the Iran University of Medical Sciences (IR.IUMS.REC 1394.94-02-87-26052).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hajjri, S.N., Sadigh-Eteghad, S., Mehrpour, M. et al. Beta-Amyloid-Dependent miRNAs as Circulating Biomarkers in Alzheimer’s Disease: a Preliminary Report. J Mol Neurosci 70, 871–877 (2020). https://doi.org/10.1007/s12031-020-01511-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01511-0