Abstract
Several animal studies have showed the beneficial effects of physical exercise (PE) on brain function and health. Alzheimer’s Disease (AD) is the most common type of dementia, characterized by the presence of aggregated extracellular amyloid-beta (Aβ) and neurofibrillary tangles, with progressive cognitive decline. Therapeutic approaches such as PE showed to be effective in halting AD progression. Here, we present a systematic review about PE and AD. The search was carried out using the PubMed and LILACS databases. The following keywords were used: Alzheimer; PE; animal model. All found studies adopted aerobic exercise training as the PE protocol (100%). We identified running on treadmill as the most commonly used PE routine (62.5%). The duration of each session, intensity, frequency, and period of training most used were 60 min/day (62.5%), moderate intensity (87.5%), 5 days/week (62.5%), and 4 (37.5%) or 12 (37.5%) weeks, respectively. The AD animal models most used were the Tg APP/PS1ΔE9 (25%), models based on i.c.v. infusion of AβOs (25%) and streptozotocin (25%). All protocols used rodents to their experiments (100%), but mice were the most common (62.5%). Finally, the main results presented in all studies were capable to reduce significantly AD consequences, such as reducing Aβ or pro-inflammatory proteins levels (100%). The lack of resistance training protocols in animal models of AD indicates a huge gap that should be investigated in future studies. We suggest that PE protocols must be adapted according to the specie, lineage and life span of the animal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s Disease (AD) is the most common age-dependent neurodegenerative disorder (Physicians 2020). AD is characterized by an aggregated of extracellular amyloid-beta (Aβ) fibrils, which form plaques, and hyperphosphorylated tau, which are known as neurofibrillary tangles (Querfurth and Laferla 2010; Dubois et al. 2010). AD is also characterized by memory loss and impairment of cognitive function, leading patients to death in 8 years on average (Querfurth and Laferla 2010; Ferreira and Klein 2011). Aβ oligomers (AβOs), a common feature of AD, aggregate due to a mechanism that involves amyloid precursor protein (APP), which is found mainly in the synapses of neurons, and favors their growth and accumulation through a self-propagating process that seems to be mediated by inflammation (Füger et al. 2017). AβOs formation is completely dependent on the participation of beta-secretase 1 (BACE-1), which can be found mainly in neurons (Egan et al. 2018). The cytotoxic form of AβOs are the ones with 40 or 42 amino acids (Aβ40-Aβ42) (Brito-Moreira et al. 2017). Due to the impossibility of performing cellular and molecular studies in living human brain tissue, several different animal models have been developed to study AD interventions and treatments (Lourenco et al. 2013; Forny-germano et al. 2014).
Several pharmacological and non-pharmacological treatments, such as physical exercise (PE), have been tested in animal models of AD (Batista et al. 2018; Tapia-Rojas et al. 2016). Researchers have questioned if healthy diets, PE, and better life style can delay the progression of dementia (George and Hemachandra Reddy 2019). There is no doubt that dietary composition affects cognition in mouse models of AD (Kadish et al. 2016). It has been reported that a paleolithic diet with and without combined aerobic and resistance exercise increases functional brain responses and hippocampal volume (Stomby et al. 2017). However, PE is the cheapest and healthiest non-pharmacological treatment that shows benefits when used in AD animal models (Kachur et al. 2017; Shaik et al. 2018). The effects of PE on brain health and AD are thought to reach the molecular level (neurogenesis, angiogenesis, and synaptogenesis) by neurotrophins and growth factors, such as the brain-derived neurotrophic factor (BDNF), the insulin-like growth factor 1 (IGF-1), hormones and second messengers (Cassilhas et al. 2016; Oskarsson et al. 2015). However, these different benefits provided by PE practicing are fully dependent on the type of exercise and its variables, such as duration, intensity, and frequency (Wisloff et al. 2007; Roden 2012). The most common types of PE referred to scientific literature are aerobic exercise training (AET) and resistance exercise training (RET) (de Sousa 2018; Soares and de Sousa 2013). AET improves metabolism and cardiovascular functions, while RET is supposed to improve strength, bone density, and to inhibit the consequences of aging (Soares and de Sousa 2013). The purpose of this review is to highlight the best protocols of PE for AD animal models, considering the type of PE and the major training variables manipulation (duration, intensity, and frequency). We also intend to develop a framework to guide researchers on the choice of PE protocols seen in AD animal models and suggest the replication of these protocols for humans.
Alzheimer’s disease: General review and new perspectives
AD is one of the most expensive and devastating disease worldwide affecting the brain, leading to an impairment in cognitive function and memory loss (Physicians 2020; Ferreira et al. 2018). AD is considered a progressive dementia with neuronal death and the presence of 2 main microscopic neuropathological hallmarks: extracellular amyloid plaques and intracellular neurofibrillary tangles (Dubois et al. 2010). The Aβ plaques usually develop firstly in the hippocampus, and then spread to other brain areas, such as the temporal and frontal lobes, what could explain partially the deficits of encoding memories, thinking, and decision making in AD patients (Braak and Braak 1991).
Recent evidence reveals that human brain is an insulin-sensitive organ and share similar physiology presenting most of the molecular mechanisms of other mammals (Bomfim et al. 2012; Talbot et al. 2012; Craft and Watson 2004). Although the presence of plaques and tangles are considered the main characteristic in scientific literature, a growing body of evidence supports that inflammation and insulin resistance are also important features in animal models of AD (Lacor et al. 2007; De Felice et al. 2009). AβOs seems to mediate an inflammation process, mainly through gliosis activation, that would lead to insulin resistance, synapse loss, and memory impairment in animal models of AD (Lourenco et al. 2013). Astrocytes and microglial activation trigger inflammatory pathways that lead to insulin resistance in AD (Soreq et al. 2017; Bhat et al. 2012). Another hallmark in animal models of AD is the interference of reactive gliosis in synaptic function (Kotilinek et al. 2002; De Felice and Ferreira 2014). AD neurons present a significant change in spines size, shape, density, and reduction of the dendritic tree resulting in structural changes that impair neuronal function and memory (Camandola and Mattson 2017). Understanding the cellular and molecular mechanisms of synapses dysfunction and memory loss in AD is a public health challenge, and the development of animal models represents an important tool to understand this pathophysiology.
Physical exercise: Medicine to prevent Alzheimer’s disease
Epidemiological studies showed that physical activity (PA) reduces the risk of AD, and low levels of PA is responsible for about 13% of all AD cases (Scarmeas et al. 2009; Scarmeas et al. 2011). On the other hand, a 25% increase in PA could potentially prevent almost 1 million cases of AD worldwide (Barnes and Yaffe 2011). It is well-known that PA and/or PE are crucial for maintaining healthy body and brain (Cassilhas et al. 2016; Di Liegro et al. 2020). The PA can be considered as a consistent routine of body movement, such as gardening or swiping the floor, that burns calories in higher levels than a rest condition. PE includes planned and structured PA that aims to enhance muscular tone or endurance capacity (Garber et al. 2011). Despite PA and PE present different concepts, their outcomes frequently achieve a similar overall benefit (Di Liegro et al. 2020; Garber et al. 2011; Pedersen and Saltin 2015). The effects of PA and PE reduce the risk of cardiovascular diseases, obesity, type 2 diabetes, AD, and other diseases and chronic conditions (Di Liegro et al. 2020; Pedersen and Saltin 2015). However, we did not find studies comparing PA and PE, and whether they have any effects with advanced stages of the disease condition. Nevertheless, PE has a greater level of complexity than PA since it includes different types of exercise, such as AET and RET, which are prescribed based on different variables, such as volume, frequency, intensity, and duration (Garber et al. 2011). AET is characterized by the execution of exercises with higher utilization and transport of oxygen, predominant recruitment of red fibers, also known as type I fibers, or fibers of slow contraction (Garber et al. 2011; Qaisar et al. 2016). On the other hand, RET is characterized by the execution of exercises against external resistance, which might be the individual’s body mass, air resistance, or elastic resistance, with predominant recruitment of white fibers, also known as type I fibers, or fibers of rapid contraction (Qaisar et al. 2016).
According to the World Health Organization (WHO) recommendations, older adults should perform RET at a minimum weekly frequency of 2 days (World Health Organization 2017). Yet, AET, should be performed for at least 150 min at moderate intensity, or for 75 min at high intensities, at least 5x weekly (World Health Organization 2017; Haskell et al. 2007). Usually, mammals present similar physiologies, but they are not equal at all, and life span differs to most of the mammals (Dutta and Sengupta 2016). Different PE protocols in animal models have been developed to study their benefits on pathological hallmarks of AD. Here, we present a systematic review about PE protocols in animal models of AD. We also suggest the best PE strategies based on the animal models of AD found in this study.
Methodology
This is a systematic literature review. The adopted criteria followed the scientific recommendations: definition of the proposal, identification, and screening of studies in the scientific databases, eligibility of the studies, inclusion of the studies, analysis, and reporting the results. The search was carried out using the PubMed and LILACS databases in April 2020, and the following keywords/descriptors were used according to Medical Sub-Headings (MeSH) guidelines: Alzheimer; PE; animal model. All terms were combined using the operator “AND” in order to appear all keywords/descriptors in the manuscripts.
Evaluation, selection and analysis of the studies
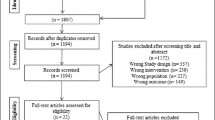
We followed a schedule to analyze all studies as described: (a) looked for keywords/descriptors in the title of the manuscripts; (b) read the abstracts; (c) read the methodology applied; (d) full reading of the articles extracting information of interest such as the article title, authors’ names and year of the publication, AD model used, PE protocol, main results found. We used as inclusion criteria studies: written in English, published in the last 5 years between April-2015 and April-2020; containing or referring to the keywords/descriptors in the title; addressing animal models, which performed PE. It was adopted as exclusion criteria review articles and studies: published in other languages rather than English; not having the keywords/descriptors in the title; not published in the last 5 years; using PA instead of PE; addressing human models of PE; using PE combined with any type of medicines or others non-pharmacological treatments in the animal model; and for not using proper AD models, such as models that do not lead to AβOs and/or hyperphophorylated tau accumulation. Literature review studies, course completion papers, dissertations, theses, and annals abstracts were also excluded. The search resulted in the identification of 111 studies. After applying the eligibility criteria, 90 studies were excluded. Then, after reading the remaining 21 studies we excluded 2 review studies; 6 studies that used PA instead of PE; 3 studies that used PE combined with any type of medicines or others non-pharmacological treatments; 1 study that was published firstly in 2014; and 1 study in which the model used did not lead to Aβ and/or hyperphophorylated tau accumulation; the hallmark of AD. Finally, we found 8 eligible studies to report their results (Fig. 1).
Study flowchart. Search at PubMed and LILACS revealed 111 studies, 90 were excluded after reading their titles and abstracts. Thirteen studies matched to the exclusion criteria (2 review, 6 that used PA instead of PE; 3 used PE combined with non-pharmacological treatments or medicines; 1 was published in 2014; and 1 did not correspond to an animal model of AD). Thus, just 8 studies were selected and included in the report
Results
The main results found in this systematic review indicated the usage of AET as the main PE intervention in animal models of AD (Table 1).
Discussion
All studies found adopted AET as the PE protocol (100%) (Lourenco et al. 2019; Wu et al. 2018; Lu et al. 2017; Haskins et al. 2016; Moore et al. 2016; Koo et al. 2017; Zhang et al. 2018; Alkadhi and Dao 2018). We identified running (62.5%) swimming (25%) and spinning wheels (12.5%) as the PE protocols performed in AD animal models, with the running on treadmill being the most used (62.5%). The duration of each session, intensity, frequency, and period of training most used were 60 min/day (62.5%), moderate intensity (87.5%), 5 days/week (62.5%), and 4 (37.5%) or 12 (37.5%) weeks, respectively (Lourenco et al. 2019; Wu et al. 2018; Moore et al. 2016; Koo et al. 2017). There was just one study that analyzed a low-intensity or high-intensity PE protocol (12,5%) (Moore et al. 2016). The most used AD models were the Tg APP/PS1ΔE9 (25%) and the models based on i.c.v. injection of AβOs (25%) or streptozotocin (25%) (Lourenco et al. 2019; Wu et al. 2018; Lu et al. 2017; Zhang et al. 2018; Alkadhi and Dao 2018). All animals in the selected studies were rodents (rats or mice), but mice were the most commonly used (62.5%) (Lourenco et al. 2019; Haskins et al. 2016; Moore et al. 2016; Koo et al. 2017; Zhang et al. 2018). Finally, the main results presented in all studies (100%) were capable to reduce significantly AD consequences, such as reducing AβOs or pro-inflammatory proteins levels or inhibiting cognitive decline or memory loss (Lourenco et al. 2019; Wu et al. 2018; Lu et al. 2017; Haskins et al. 2016; Moore et al. 2016; Koo et al. 2017; Zhang et al. 2018; Alkadhi and Dao 2018).
Irisin, a myokine recently discovered, is produced in response to PE with an important function in the CNS (Jedrychowski et al. 2015; Farshbaf et al. 2015). FNDC5 is a type I transmembrane protein regulated by peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α). The residue of its cleavage, Irisin, induces some benefits in mice, such as the browning of the white adipose tissue and the protection against insulin resistance, a hallmark of AD (Lourenco et al. 2019; Boström et al. 2012). Irisin has shown to be extremely important to inhibit cognitive decline in different animal models of AD (Lourenco et al. 2019).
Physical exercise, Irisin and animal models of Alzheimer’s disease
PE induces a variety of beneficial effects in the brain, such as the enhancement in the blood flow to the hippocampi (brain areas related to cognitive functions and memories), the increase of synapses, plasticity, neurogenesis of dentate gyrus (the major neurogenesis area in hippocampi), and changes in the morphology of the dendrites and dendritic spines (Wrann et al. 2013). PE increases brain-derived neurotrophic factor (BDNF), which acts through PGC-1α/FNDC5/Irisin pathway leading to several effects on brain development, such as neuronal cell survival, differentiation, migration, proliferation, synaptogenesis, and plasticity (Wrann et al. 2013).
A recent study evaluated the effects of PE on the PGC-1α/FNDC5/Irisin pathway using different models of AD in mice (Lourenco et al. 2019). AD was induced in C57BL/6 or Swiss mice through i.c.v. injection of amyloid-beta oligomers, which are responsible for building the amyloid plaques in the brain of mammals. Amyloid plaques contribute to a disruption of synaptic plasticity and the development of insulin resistance leading to cognitive decline and memory loss in AD. Genetically modified models of AD in mice were also used in this study, such as the APP/PS1 Δ E9 and APP/PS1 M146. Swiss mice performed a swimming training for 60 min, 5 days/week, during 5 weeks (the first week was considered to be the familiarization period). C57BL/6 mice swam during 20 min, 3 weeks in groups of 4 because they are less resistant to long periods of exercising, according to the authors comments. APP/PS1ΔE9 mice were subjected to daily swimming sessions for 1 week. C57BL/6 and APP/PS1ΔE9 received intraperitoneal injections of anti-FNDC5 antibody or nonspecific IgG (5 μg). Compared to the untrained animals, the exercised Swiss mice, C57BL/6 and APP/PS1ΔE9 presented lower cognitive and memory deficits when evaluated in the novel object recognition task. Exercised C57BL/6 and APP/PS1ΔE9 also presented higher synaptic plasticity when submitted to long term potentiation recordings. Interestingly, when the exercised animals received the anti-FNDC5 antibody the beneficial effects of exercise in memory decline, synaptic plasticity were abolished. These results unveil the need of protocol adaptation according to the animal species or lineage. Besides the actual importance of Irisin in AD this was the only study evaluating the effects of irisin in AD model.
Irisin is also related to an improved sensitization of the insulin receptors (Gizaw et al. 2017). The brain is an insulin-sensitive organ, and insulin signaling is impaired in the brains of AD experimental models and AD patients (Ferreira et al. 2018). Insulin resistance is considered to be one of the main features of AD (Ferreira et al. 2018).
Insulin resistance, physical exercise and animal models of Alzheimer’s disease
Insulin resistance involves molecular alterations in the phosphoidilinositol-3- kinase (PI3K) pathway, such as reduced activity of the kinase linked to insulin receptor (IR) and reduced phosphorylation levels of the substrates 1 and 2 of the IR (IRS-1 and IRS-2) in tyrosine residues, which are the IRS related to the management of glucose metabolism (Lourenco et al. 2013; de Sousa 2018). PE is a non-pharmacological and useful tool to prevent or treat the development and consequences of AD in many animal models (Lourenco et al. 2019; Wu et al. 2018; Lu et al. 2017; Haskins et al. 2016; Moore et al. 2016; Koo et al. 2017; Zhang et al. 2018; Alkadhi and Dao 2018).
None of the selected studies presented in Table 1 analyzed insulin hormone, receptors or substrates, but all of them evaluated inflammatory proteins and/or Aβ burden (Lourenco et al. 2019; Wu et al. 2018; Lu et al. 2017; Haskins et al. 2016; Moore et al. 2016; Koo et al. 2017; Zhang et al. 2018; Alkadhi and Dao 2018). It is well established that impaired PI3K signaling is associated with cognitive decline and memory loss in animal models of AD (Lourenco et al. 2013; Batista et al. 2018; De Felice et al. 2009; Figueiredo et al. 2013). Another important information is that insulin resistance in AD is mediated by inflammation through activation of microglia and astrocytes, increased levels of oxidative stress, APP, BACE-1 and Aβ proteins. (Lourenco et al. 2019; Wu et al. 2018; Lu et al. 2017; Haskins et al. 2016; Moore et al. 2016; Koo et al. 2017; Zhang et al. 2018; Alkadhi and Dao 2018).
Inflammation induces AβOs and hyperphosphorylated tau accumulation (Tapia-Rojas et al. 2016). The capacity of pro-inflammatory cytokines to induce cognitive decline and memory loss seems to be crucial for modulating learning and memory (Sousa et al. 2019).
Inflammation, physical exercise and animal models of Alzheimer’s disease
It was evaluated the quantitativity presence of AβOs in 7 of the 8 studies, and it was showed a reduction of it in all of them (Lourenco et al. 2019; Wu et al. 2018; Lu et al. 2017; Moore et al. 2016; Koo et al. 2017; Zhang et al. 2018; Alkadhi and Dao 2018). The study that did not evaluate the presence of AβOs used a triple Tg mice of AD, which is well established in scientific literature to form AβOs (Haskins et al. 2016). It is well known that PE can be used as a medicine to treat several inflammatory processes in different physiological states, pathologies and conditions (Pedersen and Saltin 2015; Melo et al. 2019; Freitas et al. 2019). Nevertheless, how PE acts at the cellular and molecular levels may change depending on the disease, and thus PE protocols need to be adapted according to the specie and lineage of the AD animal model (Lourenco et al. 2019; Improta Caria et al. 2018).
Our results showed that treadmill running was the most prevalent modality in the studies with AD animal models and revealed to be an effective modality of exercise to inhibit AD features and inflammation (Moore et al. 2016; Koo et al. 2017; Zhang et al. 2018; Alkadhi and Dao 2018). A study conducted by Zhang et al. (2018) revealed that long-term AET on treadmill diminished AβOs load and increased neuronal density. They also showed a reduction of gliosis in the APP/PS1ΔE9 mice. AET revealed to be an effective therapeutic method in AD, modulating different signaling pathways, Aβ, neuronal density, and astrocyte levels. Another study revealed that PE prevented the increase of Aβ, APP, and BACE-1 proteins (Alkadhi and Dao 2018). Koo et al. (2017) also showed that AET attenuated cognitive deficits by reducing the levels of BACE-1 and Aβ load through SIRT-1 regulation. Finally, Moore et al. showed in Tg 2576 mice that, compared with low intensity running, high-intensity running promotes reduction of oxidative stress and Aβ40 levels in hippocampi and cortex, areas of the brain related with memory (Moore et al. 2016).
Wu et al. 2018 evaluated rats that performed a swimming protocol for 4 weeks, and thereafter, they received an i.c.v. injection of streptozotocin to evaluate inflammation, oxidative stress, and Aβ burden (Wu et al. 2018). The prior exercise prevents Aβ aggregation, inflammation, and oxidative stress. Another study also using i.c.v. injection of streptozotocin evaluated the memory deficits, Aβ aggregation, and inflammation in rats that ran on a treadmill, 5 days/week for 4 weeks (Lu et al. 2017). The authors also found that the PE protocol applied reduced all AD features analyzed. Another study with i.c.v. injection of streptozotocin to develop AD also found similar positive results using a running protocol (Lu et al. 2017).
Haskins et al. used spinning wheels as the PE protocol (Haskins et al. 2016). Tg triple mice performed the protocol at moderate intensity once or 3 times a week and it was found an improvement on monocyte chemotactic protein-1 (MCP-1) and expressed and secreted T cells. The exercise performed 3 times a week was effective in restoring the expression of inflammatory brain cytokines, delaying the development of AD. These data together indicate that moderate or high-intensity PE protocols are capable of reducing and/or revert the negative consequences of the AD in animal models. Thus, PE can manage the pro-inflammatory mechanisms that mediate the cognitive ability and memory in AD experimental models.
Inflammation and animal models of Alzheimer’s disease
It is known that inflammation plays a crucial role in AD (De Felice and Ferreira 2014). Although there are a few animal models that do not mimic AD at all, even when presenting severe inflammation (Özbeyli et al. 2017; Neves et al. 2016). For example, Özbeyli et al. performed a surgical technique and induced ovariectomy in rats identifying cognitive decline, which was prevented by PE. The authors classified the ovariectomy procedure as an AD-like model, but there was not identification of AβOs and/or hyperphophorylated tau accumulation, which are hallmarks in AD (Özbeyli et al. 2017). Severe inflammation process, such as sepsis, also may lead to temporary cognitive decline (Neves et al. 2016). It does not mean that sepsis or ovariectomy are animal models of AD. It is necessary the identification of plaques and tangles, cognitive decline and/or memory loss, inflammation and/or insulin resistance in order to classify the cognitive decline as AD (Querfurth and Laferla 2010).
Tg APP/PS1ΔE9 and i.c.v. injection of AβOs, or streptozotocin, are the most used animal models of AD which mimic the features of this disease, such as Aβ and inflammation (Lourenco et al. 2019; Wu et al. 2018; Lu et al. 2017; Zhang et al. 2018; Alkadhi and Dao 2018). I.c.v. injection of AβOs demands an excellent know-how of preparing Aβ derived diffusible ligands (ADDLs) through manipulation of high performance liquid chromatography (HPLC) system and a high level skill to operate the stereotaxic equipment. This model shows some AD features for a short period. There are no studies identifying these features 30 days after the injection. The i.c.v. injection of streptozotocin is not expensive and demands just knowledge of how to inoculate the substance by stereotaxis. Streptozotocin is also cheaper than ADDLs. Although this model does not mimic many features of AD, such as microtubules dysfunction that leads to the development of hyperphosphorylated tau (Mahmoud et al. 2016; De Felice et al. 2014). Tg models is more expensive than other AD models, but it is worthy. For example, Tg APP/PS1ΔE9 mimics the AD pathology with similar features to the human disease (Moore et al. 2016; Ferreira and Gralle 2007). Because of the genetic mutations, Tg models present permanently the AD features, being mice, the main animals of these models. Another possibility that should be investigated is the use of different animal models, such as macaques, to study PE protocols in AD.
Many factors need to be analyzed before prescribing PE training in animal models. Several PE protocols varying in frequency, intensity, and duration have been used. These variables are not usually taken into consideration when the results are interpreted.
Physical exercise training protocols in animal models of Alzheimer’s disease
The variety of AET protocols in different AD models reveals that there is not a common sense in the scientific literature about the ideal frequency, intensity, and duration that should be used. Although we identified studies with similar frequency, intensity, and duration. Nevertheless, these similar parameters of PE protocols were not performed to the same AD models. The lack of a gold standard protocol and the use of different animal models make it difficult the choice of the best PE.
We found a higher prevalence of using mice instead of rats to prescribe PE protocols as medicine in animal models of AD (Lourenco et al. 2019; Haskins et al. 2016; Moore et al. 2016; Koo et al. 2017; Zhang et al. 2018). Even identifying mouse as the main model of AD used, there was not a conclusion about the more adequate PE protocol for these animals. For example, Zhang et al. (2018) used Tg APP/PS1ΔE9 mice running 6 days/week during 20 weeks. On the other hand, Lourenco et al. (2019) used Tg APP/PS1ΔE9 mice which swan for 20 min, during 5 days/week, for 3 weeks. Even presenting completely different AET protocols, both studies ameliorated AD at the Tg APP/PS1ΔE9 mice (Lourenco et al. 2019; Zhang et al. 2018). Another study described a protocol where the Tg2575 mice had to run 60 min/day, 5 days/week for 12 weeks, totalizing 3 months of training (Moore et al. 2016).
Finally, there was a PE protocol described by Lourenco et al. (2019) that used only 3 weeks of training, which was the shortest protocol applied in the Tg APP/PS1ΔE9 mice. The frequency of the AET lasted just for 20 min in every single session to the Tg APP/PS1ΔE9 mice. Nevertheless, this might be the most suitable PE protocol to be used in Tg models of AD. Thus, short and long-term exercise protocols described in this review inhibited cognitive decline and memory loss in AD. The short and long-term PE have many physiological benefits that can contribute to avoid the development of dementia (Fig. 2).
It was not mentioned in any of the PE protocols of the animal models of AD presented factors such as: the training periodization, the variation of different cycles of physical training (macrocycle, mesocycle and microcycle) and their respective intensities and volumes, the shock and regenerative training period. Certainly, these factors must be used when prescribing PE training protocols for humans (Haskell et al. 2007; Forbes et al. 2015), so are not they necessary to others mammals?
Links between physical exercise, inflammation, type 2 diabetes and Alzheimer’s disease
We have reported until now about the potential mechanisms linking PE, inflammation and insulin resistance in AD (Lourenco et al. 2019; Wu et al. 2018; Lu et al. 2017; Haskins et al. 2016; Moore et al. 2016; Koo et al. 2017; Zhang et al. 2018; Alkadhi and Dao 2018). However, it is necessary to discuss about their association with type 2 diabetes (T2D), because these pathologies share some common mechanisms (Biessels and Reagan 2015; Folch et al. 2019). There is a hypothesis that AD could be a type 3 diabetes due to these similarities (Folch et al. 2019). Inflammation and insulin resistance are reported to be common features in AD and T2D (Ferreira et al. 2018; De Felice and Ferreira 2014). Thus, it is important to have good life habits to inhibit the progression or the development of these common features in AD and T2D (De Sousa et al. 2020). The regular practice of PE is a possible, cheap and non-pharmacological approach to achieve better life habits.
The main role of insulin and its receptors in different brain regions is mainly associated to the sustaining of synaptogenesis and neuronal plasticity (Folch et al. 2019). Peripheral insulin resistance contributes to β cell death in the pancreas, and the development of T2D-related comorbidities, which can be prevented or inhibited by the regular practice of PE (de Sousa 2018). Changes in glucose metabolism in both pathologies occur mainly by alterations on IRS-1 and IRS-2, which are mediated by the activation of inflammatory mechanisms (De Sousa et al. 2020). It seems that unhealthy life habits will trigger inflammation and insulin resistance leading to cognitive decline in AD and T2D, which can be prevented or treated by PE.
Conclusions
The most suitable animal model of AD in studies with PE is mice. Tg APP/PS1ΔE9 was identified as the best model of AD since it presents all features of the disease permanently. AET was the only type of PE protocol found in the studies using animal models of AD. Short and long-term exercise protocols were capable to prevent cognitive decline and memory loss in AD. It seems that short and long-term PE are able to counteract AD-related pathology in rodents. Nevertheless, the lack of RET protocols in animal models of AD indicates a huge gap that should be investigated in future studies. The AET protocol most used was running on treadmill, 60 min/day, 5 days/week for 4 or 12 weeks at moderate intensity. We recommend the use in animal models of less duration and frequency of the PE protocol, such as 50 min/day, 3 days/week for no more than 2 weeks. We also advice the progression of PE protocol (increasing the intensity variables and decreasing the volume variables) every 2 days to the animal models. Finally, we suggest that PE protocols must be adapted according to the specie, lineage and life span of the animal.
References
Alkadhi KA, Dao AT (2018) Exercise decreases BACE and APP levels in the hippocampus of a rat model of Alzheimer’s disease. Mol Cell Neurosci 86:25–29. https://doi.org/10.1016/j.mcn.2017.11.008
Barnes DE, Yaffe K (2011) The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 10:S1474–S4422. https://doi.org/10.1093/milmed/166.4.331
Batista AF, Frony-Germano L, Clarke JR, Silva NMLE, Brito-Moreira J, Boehnke SE, Winterborn A, Coe BC, Lablans A, Vital JF, Marques SA, Martinez AM, Gralle M, Holscher C, Klein WL, Houzel J-C, Ferreira ST, Munoz DP, De Felice FG (2018) The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J. Pathol. 245:85–100. https://doi.org/10.1002/path.5056
Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C (2012) Astrocyte senescence as a component of Alzheimer’s disease. PLoS One 7:1–10. https://doi.org/10.1371/journal.pone.0045069
Biessels GJ, Reagan LP (2015) Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci 16:660–671. https://doi.org/10.1038/nrn4019
Bomfim TR, Forny-germano L, Sathler LB, Brito-moreira J, Houzel J, Decker H, Silverman MA, Kazi H, Melo HM, Mcclean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomers. J Clin Invest 122:1339–1353. https://doi.org/10.1172/JCI57256DS1
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Zingaretti MC, Vind BF, Tu H, Cinti S, Gygi SP, Spiegelman BM (2012) A PGC1a dependent myokine that derives browning of white fat and thermogenesis. Nature 481:463–468. https://doi.org/10.1038/nature10777.A
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. https://doi.org/10.1109/ICINIS.2015.10
Brito-Moreira J, Lourenco MV, Oliveira MM, Ribeiro FC, Ledo JH, Diniz LP, Vital JFS, Magdesian MH, Melo HM, Barros-Aragão F, De Souza JM, Alves-Leon SV, Gomes FCA, Clarke JR, Figueiredo CP, De Felice FG, Ferreira ST (2017) Interaction of amyloid-β (Aβ) oligomers with neurexin 2α and neuroligin 1 mediates synapse damage and memory loss in mice. J Biol Chem 292:7327–7337. https://doi.org/10.1074/jbc.M116.761189
Camandola S, Mattson M (2017) Brain metabolism in health , aging , and neurodegeneration. EMBO J. 36:1–19. https://doi.org/10.15252/embj.201695810
Cassilhas RC, Tufik S, De Mello MT (2016) Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol Life Sci 73:975–983. https://doi.org/10.1007/s00018-015-2102-0
Craft S, Watson GS (2004) Review insulin and neurodegenerative disease : shared and specific mechanisms. Lancet Neurol 3:169–178
De Felice FG, Ferreira ST (2014) Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 63:2262–2272. https://doi.org/10.2337/db13-1954
De Felice FG, Vieira M, Bomfim T, Decker H, Velasco P, Lambert M, Viola K, Zhao W-W, Ferreira S, Klein W (2009) Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A 106:1971–1976. https://doi.org/10.1073/pnas.0809158106
De Felice FG, Lourenco MV, Ferreira ST (2014) How does brain insulin resistance develop in Alzheimer’s disease? Alzheimers Dement 10:S26–S32. https://doi.org/10.1016/j.jalz.2013.12.004
De Sousa RAL, Harmer AR, Freitas DA, Mendonça VA, Lacerda ACR, Leite HR (2020) An update on potential links between type 2 diabetes mellitus and Alzheimer’s disease. Mol Biol Rep 47:6347–6356. https://doi.org/10.1007/s11033-020-05693-z
Di Liegro CM, Schiera G, Proia P, Di Liegro I (2020) Physical activity and brain health. Genes (Basel) 10:1–40. https://doi.org/10.3389/fphys.2019.01550
Dubois B, Feldman HH, Jacova C, Cummings JL, DeKosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza LC, Stern Y, Visser PJ, Scheltens P (2010) Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 9:1118–1127. https://doi.org/10.1016/S1474-4422(10)70223-4
Dutta S, Sengupta P (2016) Men and mice: relating their ages. Life Sci 152:244–248. https://doi.org/10.1016/j.lfs.2015.10.025
Egan MF, Kost J, Tariot PN, Aisen PS, Cummings JL, Vellas B, Sur C, Mukai Y, Voss T, Furtek C, Mahoney E, Harper Mozley L, Vandenberghe R, Mo Y, Michelson D (2018) Randomized trial of Verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 378:1691–1703. https://doi.org/10.1056/NEJMoa1706441
Farshbaf MJ, Ghaedi K, Megraw TL (2015) Does PGC1 a / FNDC5 / BDNF elicit the beneficial effects of exercise on neurodegenerative disorders? NeuroMolecular Med. https://doi.org/10.1007/s12017-015-8370-x
Ferreira ST, Gralle M (2007) Structure and functions of the human amyloid precursor protein : the whole is more than the sum of its parts. Prog Neurobiol 82:11–32. https://doi.org/10.1016/j.pneurobio.2007.02.001
Ferreira ST, Klein WL (2011) The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol. Learn. Mem. 96:529–543. https://doi.org/10.1016/j.nlm.2011.08.003
Ferreira LSS, Fernandes CS, Vieira MNN, De-Felice FG (2018) Insulin resistance in Alzheimer’s disease. Front Neurosci 12:1–11. https://doi.org/10.1016/j.trsl.2016.12.005
Figueiredo CP, Clarke JR, Ledo JH, Ribeiro FC, Costa CV, Melo HM, Mota-Sales AP, Saraiva LM, Klein WL, Sebollela A, De Felice FG, Ferreira ST (2013) Memantine rescues transient cognitive impairment caused by high-molecular-weight aβ oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J. Neurosci. 33:9626–9634. https://doi.org/10.1523/JNEUROSCI.0482-13.2013
Folch J, Olloquequi J, Ettcheto M, Busquets O, Sánchez-López E, Cano A, Espinosa-Jiménez T, García ML, Beas-Zarate C, Casadesús G, Bulló M, Auladell C, Camins A (2019) The involvement of peripheral and brain insulin resistance in late onset Alzheimer’s dementia. Front Aging Neurosci 11:1–16. https://doi.org/10.3389/fnagi.2019.00236
Forbes D, Thiesen EJ, Blake CM, Forbes S (2015) Exercise programs for people with dementia. Cochrane Database Syst. Rev. 132:1–12. https://doi.org/10.1590/1516-3180.20141323T2
Forny-germano L, Lyra NM, Batista F, Brito-moreira J, Gralle XM, Boehnke SE, Coe BC, Lablans A, Marques SA, Martinez AMB, Klein WL, Houzel XJ, Ferreira ST, Munoz DP, De Felice FG (2014) Alzheimer ’ s disease-like pathology induced by amyloid-  oligomers in nonhuman Primates. Neurobiol Dis 34:13629–13643. https://doi.org/10.1523/JNEUROSCI.1353-14.2014
Freitas D, Rocha-vieira E, de Sousa RAL, Alvarenga B, Rocha-gomes A, Garcia B, Cassilhas RC, Mendonça VA, Camargos ACR, Lacerda AC, Leite HR (2019) High-intensity interval training improves cerebellar antioxidant capacity without affecting cognitive functions in rats. Behav Brain Res 376:1–7. https://doi.org/10.1016/j.bbr.2019.112181
Füger P, Hefendehl JK, Veeraraghavalu K, Wendeln AC, Schlosser C, Obermüller U, Wegenast-Braun BM, Neher JJ, Martus P, Kohsaka S, Thunemann M, Feil R, Sisodia SS, Skodras A, Jucker M (2017) Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat Neurosci 20:1371–1376. https://doi.org/10.1038/nn.4631
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP (2011) Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43:1334–1359. https://doi.org/10.1249/MSS.0b013e318213fefb
George EK, Hemachandra Reddy P (2019) Can healthy diets, regular exercise, and better lifestyle delay the progression of dementia in elderly individuals? J Alzheimers Dis 72:S37–S58. https://doi.org/10.3233/JAD-190232
Gizaw M, Anandakumar P, Debela T (2017) A review on the role of Irisin in insulin resistance and type 2 diabetes mellitus. Aust J Pharm 20:235–242
Haskell WL, Lee I-M, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116:1081–1093. https://doi.org/10.1161/CIRCULATIONAHA.107.185649
Haskins M, Jones TE, Lu Q, Bareiss SK (2016) Early alterations in blood and brain RANTES and MCP-1 expression and the effect of exercise frequency in the 3xTg-AD mouse model of Alzheimer’s disease. Neurosci Lett 610:165–170. https://doi.org/10.1016/j.neulet.2015.11.002
Improta Caria AC, Nonaka CKV, Pereira CS, Soares MBP, Macambira SG, de Freitas Souza BS (2018) Exercise training-induced changes in microRNAs: beneficial regulatory effects in hypertension, type 2 diabetes, and obesity. Int. J. Mol. Sci. 19:1–36. https://doi.org/10.3390/ijms19113608
Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM (2015) Detection and quantitation of circulating human Irisin by tandem mass spectrometry mark. Cell Metab 22:734–740. https://doi.org/10.1016/j.physbeh.2017.03.040
Kachur S, Chongthammakun V, Lavie CJ, De Schutter A, Arena R, Milani RV, Franklin BA (2017) Impact of cardiac rehabilitation and exercise training programs in coronary heart disease. Prog Cardiovasc Dis 60:103–114. https://doi.org/10.1016/j.pcad.2017.07.002
Kadish I, Kumar A, Beitnere U, Jennings E, McGilberry W, van Groen T (2016) Dietary composition affects the development of cognitive deficits in WT and Tg AD model mice. Exp Gerontol 86:39–49. https://doi.org/10.1016/j.exger.2016.05.003
Koo JH, Kang EB, Oh YS, Yang DS, Cho JY (2017) Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer’s disease. Exp Neurol 288:142–152. https://doi.org/10.1016/j.expneurol.2016.11.014
Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH (2002) Reversible memory loss in a mouse transgenic model of Alzheimer ’ s disease. J Neurosci 22:6331–6335
Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL (2007) Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci 27:796–807. https://doi.org/10.1523/JNEUROSCI.3501-06.2007
Lourenco MV, Clarke JR, Frozza RL, Bomfim TR, Forny-Germano L, Batista AF, Sathler LB, Brito-Moreira J, Amaral OB, Silva CA, Freitas-Correa L, Espírito-Santo S, Campello-Costa P, Houzel J-C, Klein WL, Holscher C, Carvalheira JB, Silva AM, Velloso LA, Munoz DP, Ferreira ST, De Felice FG (2013) TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s β-amyloid oligomers in mice and monkeys. Cell Metab. 18:831–843. https://doi.org/10.1016/j.cmet.2013.11.002
Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, Gonçalves RA, Clarke JR, Beckman D, Staniszevski A, Berman H, Guerra LA, Forny-Germano L, Meier S, Wilcock DM, De Souza JM, Alves-Leon SV, Prado VF, Prado MAM, Abisambra JF, Troval-Moll F, Mattos P, Arancio O, Ferreira ST, De Felice FG (2019) Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 25:165–175. https://doi.org/10.1038/s41591-018-0275-4
Lu Y, Dong Y, Tucker D, Wang R, Ahmed ME, Brann D, Zhang Q (2017) Treadmill exercise exerts streptozotocin- induced rat model of sporadic Alzheimer’s disease. J Alzheimers Dis 56:1469–1484. https://doi.org/10.1158/1940-6207.CAPR-14-0359.Nrf2-dependent
Mahmoud R, Wainwright SR, Galea LAM (2016) Sex hormones and adult hippocampal neurogenesis: regulation, implications, and potential mechanisms. Front Neuroendocrinol 41:129–152. https://doi.org/10.1016/j.yfrne.2016.03.002
Melo CS, Rocha-Vieira E, Freitas DA, Soares BA, Rocha-Gomes A, Riul TR, Mendonça VA, Lacerda ACR, Camargos ACR, Carvalho LED, De Sousa RAL, Leite HR (2019) A single session of high-intensity interval exercise increases antioxidants defenses in the hippocampus of Wistar rats. Physiol Behav 211:112675. https://doi.org/10.1016/j.physbeh.2019.112675
Moore KM, Girens RE, Larson SK, Jones MR, Restivo JL, Holtzman DM, Cirrito JR, Yuede CM, Zimmerman SD, Timson BF (2016) A spectrum of exercise training reduces soluble Aβ in a dose-dependent manner in a mouse model of Alzheimer’s disease. Neurobiol Dis 85:218–224. https://doi.org/10.1016/j.nbd.2015.11.004
Neves FS, Marques PT, Aragão FB, Nunes JB, Venancio AM, Cozachenco D, Frozza RL, Passos GF, Costa R, De Oliveira J, Engel DF, De Bem AF, Benjamim CF, De Felice FG, Ferreira ST, Clarke JR, Figueiredo CP, Clarke JR (2016) Brain-defective insulin signaling is associated to late cognitive impairment in post-septic mice. Mol Neurobiol 55:1–10. https://doi.org/10.1007/s12035-016-0307-3
Oskarsson ME, Paulsson JF, Schultz SW, Ingelsson M, Westermark P, Westermark GT (2015) In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and alzheimer disease. Am. J. Pathol. 185:834–846. https://doi.org/10.1016/j.ajpath.2014.11.016
Özbeyli D, Sarı G, Özkan N, Karademir B, Yüksel M, Çilingir Kaya ÖT, Kasımay Çakır Ö (2017) Protective effects of different exercise modalities in an Alzheimer’s disease-like model. Behav Brain Res 328:159–177. https://doi.org/10.1016/j.bbr.2017.03.044
Pedersen BK, Saltin B (2015) Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sport 25:1–72. https://doi.org/10.1111/sms.12581
Physicians PC (2020) 2020 Alzheimer’s disease facts and figures. Alzheimers Dement 16:391–460. https://doi.org/10.1002/alz.12068
Qaisar R, Bhaskaran S, Van Remmen H (2016) Muscle fiber type diversification during exercise and regeneration. Free Radic Biol Med 98:56–67. https://doi.org/10.1016/j.freeradbiomed.2016.03.025
Querfurth HW, Laferla FM (2010) Alzheimer’s disease. N Engl J Med 362:329–344
Roden M (2012) Exercise in type 2 diabetes: to resist or to endure? Diabetologia. 55:1235–1239. https://doi.org/10.1007/s00125-012-2513-5
Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y (2009) Physical activity, diet, and risk of Alzheimer disease. JAMA – J Am Med Assoc 302:627–637. https://doi.org/10.1038/jid.2014.371
Scarmeas N, Luchsinger JA, Brickman AM, Cosentino S, Schupf N, Xin-Tang M, Gu Y, Stern Y (2011) Physical activity and alzheimer disease course. Am J Geriatr Psychiatry 19:471–481. https://doi.org/10.1097/JGP.0b013e3181eb00a9
Shaik MM, Tamargo IA, Abubakar MB, Kamal MA, Greig NH, Gan SH (2018) The role of microRNAs in Alzheimer’s disease and their therapeutic potentials. Genes (Basel) 9. https://doi.org/10.3390/genes9040174
Soares FHR, de Sousa MBC (2013) Different types of physical activity on inflammatory biomarkers in women with or without metabolic disorders: a systematic review. Women Health 53:298–316. https://doi.org/10.1080/03630242.2013.782940
Soreq L, Rose J, Soreq E, Hardy J, Trabzuni D, Cookson MR, Smith C, Ryten M, Patani R, Ule J (2017) Major shifts in glial regional identity are a transcriptional Hallmark of human brain aging. Cell Rep 18:557–570. https://doi.org/10.1016/j.celrep.2016.12.011
de Sousa RAL (2018) Brief report of the effects of the aerobic , resistance , and high-intensity interval training in type 2 diabetes mellitus individuals diabetes mellitus. Int J Diabetes Dev Ctries 38:138–145. https://doi.org/10.1007/s13410-017-0582-1
Sousa RAL, Freitas DA, Leite HR (2019) Cross-talk between obesity and central nervous system: role in cognitive function. Interv. Obes. Diabetes 3:7–9. https://doi.org/10.31031/IOD.2019.03.000551
Stomby A, Otten J, Ryberg M, Nyberg L, Olsson T, Boraxbekk CJ (2017) A paleolithic diet with and without combined aerobic and resistance exercise increases functional brain responses and hippocampal volume in subjects with type 2 diabetes. Front Aging Neurosci 9:1–10. https://doi.org/10.3389/fnagi.2017.00391
Talbot K, Wang H, Kazi H, Han L, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE (2012) Demonstrated brain insulin resistance in alzheimer’s disease patients is assocaited with IGF-1 resisitance, IRS-1 dysregulation, and cogntive decline. J. Clin. Invest. 122:1316–1338. https://doi.org/10.1172/JCI59903DS1
Tapia-Rojas C, Aranguiz F, Varela-Nallar L, Inestrosa NC (2016) Voluntary running attenuates memory loss, decreases Neuropathological changes and induces neurogenesis in a mouse model of Alzheimer’s disease. Brain Pathol 26:62–74. https://doi.org/10.1111/bpa.12255
Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjærpe T (2007) Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 115:3086–3094. https://doi.org/10.1161/CIRCULATIONAHA.106.675041
World Health Organization (2017) Physical activity and older adults. Glob Strateg Diet Phys Act Heal
Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM (2013) Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 18:649–659. https://doi.org/10.1016/j.cmet.2013.09.008
Wu C, Yang L, Tucker D, Dong Y, Zhu L, Duan R, Liu TC-Y, Zhang Q (2018) Beneficial effects of exercise pretreatment in a sporadic Alzheimer’s rat model. Med Sci Sports Exerc 50:945–956. https://doi.org/10.1016/j.physbeh.2017.03.040
Zhang J, Guo Y, Wang Y, Song L, Zhang R, Du Y (2018) Long-term treadmill exercise attenuates Aβ burdens and astrocyte activation in APP/PS1 mouse model of Alzheimer’s disease. Neurosci Lett 666:70–77. https://doi.org/10.1016/j.neulet.2017.12.025
Acknowledgments
We are thankful to Coordenação de Pessoal de Ensino Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
RAL, CMR and BFM review and wrote the manuscript; RALS, CMR and BFM performed the literature research; RALS, CMR, BFM, ACIC, MFDP and RCC analyzed and critically discussed the data and reviewed the manuscript; RALS, CMR, BFM, ACIC, MFDP, RCC supervised the review. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Sousa, R.A.L., Rodrigues, C.M., Mendes, B.F. et al. Physical exercise protocols in animal models of Alzheimer’s disease: a systematic review. Metab Brain Dis 36, 85–95 (2021). https://doi.org/10.1007/s11011-020-00633-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00633-z