Abstract
Serotonin exerts a significant role in the mammalian central nervous system embryogenesis and brain ontogeny. Therefore, we investigate the effect of perinatal fluoxetine (FLX), a selective serotonin reuptake inhibitor, administration on the behavioral expression of adult male Swiss mice. For this purpose, two groups (n = 6 each, and ~ 35 g) of pregnant female Swiss mice were mated. Their offspring were treated with FLX (10 mg/Kg, s.c.) from postnatal day (PND) 5 to 15. At PND 16, one male puppy of each litter was euthanized, and the hippocampus was dissected for RNA analysis. At 70 days of life, the male offspring underwent a behavioral assessment in the open field, object recognition task, light-dark box, tail suspension and rotarod test. According to our results, the programmed animals had a decrease in TPH2, 5HT1a, SERT, BDNF, and LMX1B expression. Also, it was observed less time of immobility in tail suspension test and higher grooming time in the open field test. In the light-dark box test, the FLX-treated offspring had less time in the light side than control. We also observed a low cognitive performance in the object recognition task and poor motor skill learning in the rotarod test. These findings suggest that programming with FLX during the neonatal period alters a hippocampal serotonergic system, promoting anxiety and antidepressant behavior in adults, as well as a low mnemonic capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serotonin (5-HT) is a neurotransmitter involved in the control of diverse physiological functions, such as modulating vascular tone, gastrointestinal motility, and platelet function. Also, the dysfunction of the 5-HT system is involved in the pathophysiology of mood disturbance (Mohammad-Zadeh et al. 2008). Depression and anxiety disorders are mental illnesses that affect a large number of individuals (Nestler and Hyman 2010), and are one of the leading reasons for death and disability globally (Smith 2014). The causal mechanisms of patients with both depression and anxiety disorders might be overlapped (Kessler et al. 1996). However, the clinical treatment of depression and anxiety disorders is limited due to the gaps in knowledge of the etiology of these diseases (Mahar et al. 2014). To bridge these gaps, animal models of anxiety and depression, termed anxiety-like and depression-like behaviors, have been widely used to clarify the mechanisms related to the pathogenesis of these diseases (Nestler and Hyman 2010).
Exposure to different factors during key periods, such as the perinatal period, has long-lasting effects and may participate in the pathogenesis of several mood disorders (Brunton 2015). Because of 5-HT is involved in the formation of brain networks during ontogenetic development, any changes in serotoninergic neurotransmission during this period may produce harmful effects that persist until adulthood (Lesch and Waider 2012). Drugs that upregulate 5-HT neurotransmission are commonly used in the treatment of depression among these are selective serotonin reuptake inhibitors (SSRIs). However, the use of these drugs during pregnancy can increase the risk of mood disorders in the offspring of humans and rodents (Gemmel et al. 2018). Furthermore, exposure to SSRI through lactation reduces the production of serotonin in adult mice (Maciag et al. 2006). Other studies showed that prenatal exposure to fluoxetine (FLX), an antidepressant drug, can decrease the social play in rat pups, such an effect can persist to adulthood (Olivier et al. 2011). The administration of FLX or other antidepressants during early life also can decrease the social interaction in juvenile male and female rats (Rodriguez-Porcel et al. 2011).
However, FLX was the first SSRI, with recognized safety, its side effects may increase the risk of anxiety (Perez-Caballero et al. 2014). In rats, acute FLX administration increases the extracellular 5-HT level in the raphe nuclei and frontal cortex (Bel and Artigas 1996), while chronic administration increases 5-HT in several brain nuclei (Invernizzi et al. 1996; Kreiss and Lucki 1995; Rutter et al. 1994)Preclinical studies demonstrated that chronic FLX decreases the depression-like behaviors in the forced swim test (Detke et al. 1995) and reverses the depression-like behaviors caused by chronic stress (Grippo et al. 2006) or olfactory bulb lesion (Machado et al. 2012). In addition to decreasing depression-like behaviors, the acute administration of FLX increases the anxiety-like behavior in rats, while the effects of its chronic administration were inconclusive (Perez-Caballero et al. 2014).
The related mechanisms involved in behavioral changes induced by early-life FLX administration are not well established. In this study, we assessed the effects of chronic FLX administration during early development on the expression of mRNA that encodes proteins related to the serotoninergic system and neurogenesis in the midbrain and hypothalamus. Additionally, we followed the impact of this treatment on memory, anxiety-like, and depression-like behaviors in adult mice.
Material and methods
Swiss Webster mice of 60 days of age (~35 g) derived from the Federal Rural University of Rio de Janeiro colony were used in this study. After an acclimatization period of 15 days, the mice were housed in plastic cages (30 × 19 × 13 cm) and mated together with a ratio of one female to one male. Day 1 of pregnancy was determined by the presence of spermatozoa in a vaginal smear.
After birth, male’s and female’s offspring were equally divided into two groups with a maximum of 10. Then, the lactating dams and their offspring were separated into two groups. The offspring of the treated group was received FLX (10 mg/Kg, s.c.), whereas the offspring of the control group was received saline 0.9% only from postnatal day (PND) 5 to 15. FLX dose was chosen per studies of Galindo et al. (2015). At PND 16, one male offspring of each litter was euthanized, and its hippocampus region was dissected from the whole brain under the cold plate and kept at −70 °C for RNA analysis.
At PND 21, two male puppies of each litter were weaned to six animals per plastic cage (35 cm × 50 cm × 35 cm). The remaining offspring and the dams were euthanized. At 70 days of life, a behavioral assessment in the open field, object recognition task, light-dark box, tail suspension test, and rotarod were recorded (Fig. 1). All animals used in this work were housed at a controlled temperature (20 ± 2 °C) with daily exposure to a 12 h light-dark cycle and free access to water and commercial rodent diet. In all cases, the rats were anesthetized by an injection of thiopental (90 mg/Kg, i.p.) and euthanized by decapitation.
Schematic representation of the experimental design. After birth, male’s and female’s offspring were equally divided into two groups with a maximum of 10. Then, the lactating dams and their offspring were separated into two groups. The offspring of the treated group was received FLX (10 mg/Kg, s.c.), whereas the offspring of the control group was received saline 0.9% only from postnatal day (PND) 5 to 15. At PND16, one male offspring of each litter was euthanized, and the hippocampus was for RNA analysis. By 70 days of life, a behavioral assessment in the open field, object recognition task, light-dark box, tail suspension test, and rotarod were recorded
Ethics committee
This investigation was carried out according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No.85–23, revised 1996) and was approved by the institutional animal welfare committee pertinent Brazilian legislation under Protocols number: 23083.012282/2017.
Behavioral tests
At PND 70, the offspring underwent a battery of tests, including open field, dark-light box, tail suspension, and rotarod tests. The tests were performed at one-day interval, and the order of tests within the battery was determined according to the progressive degree of invasiveness.
Except for the object recognition test, all testing was performed between 7 and 11 a.m. During each test, the experimenter remained outside the testing room. Each test was recorded, and behavior parameters were analyzed by at least two observers.
Open field test
Each mouse was placed individually in the center of a white acrylic cage (30 cm × 30 cm × 15 cm) and allowed to explore the cage for five minutes. During this time, number of squares crossed, number of rearing (standing on hind legs with paws pressed against the wall of the arena), time of grooming, time in the center zone, center distance (the distance traveled in the center of the arena) and center ratio (center distance to total distance ratio) were assessed. At the end of testing, the number of fecal pellets was also counted, and the arena was cleaned with a 10% ethanol solution. In this test, locomotor activity is indicated by the total distance traveled in the apparatus, while the vertical activity is assigned by the number of rearing. Concerning defecation, this parameter appeared, under some circumstances, to represent an emotional behavior. Lastly, anxiety-like responses were linked to time in the center zone and center ratio, whereas grooming time indicates higher stress responsiveness.
Object recognition task
We used the open-field apparatus as the context to perform this experimental protocol. That way, each animal was introduced in the apparatus in the absence of objects or another behavioral stimulus for 5 min, for just 1 day. On the day after the end of the habituation period, the animals were subjected to a training session for memory acquisition. Two cell culture flasks filled with sand (A1 and A2) were placed at opposite corners of the apparatus used for the test. Thus, each animal was positioned individually in the arena center, and the familiarization session was stopped when there has been a 20 s exploration of both objects and when a 10 min period is over. After 6 h, the animals were exposed again to the test context for object recognition. In this step used to assess the ability of them to retain information, it was used as a familiar object (A3) and a new object (B), a tower of Lego bricks. As in the training session, the retention test also lasted 5 min (Leger et al. 2013).
It is noteworthy that between each animal tested, the apparatus and the objects were properly sanitized with alcohol 70% to counteract any olfactory clue. Moreover, the exploration was only considered when the animals put the nose at up to 2 cm towards objects. Any other kind of physical contact, such as to lean, or climb over objects, was not considered as exploration. The basic measurements were the time spent by rats in exploring each object during the retention test. From this basic value, several variables could be calculated (see Table 1). The variable e is the total time spent investigating both objects during the retention test. The d1 index depicts the absolute difference in exploitation between the new and the familiar objects. The d2 index is a relative measure of discrimination corrected by the level of exploration in the retention test (e), and the d3 index shows the proportion of e devoted to the novel object (Akkerman et al. 2012).
Light-dark box test
The animals were individually placed in an acrylic cage (45 cm × 27 cm × 27 cm) unequally divided into two chambers by a black partition containing a small opening. Two-thirds of this chamber was illuminated (400 lx), and the remaining section was closed and dark. Mice were placed inside the dark side and allowed to freely move between the two chambers for 5 min. During this time, the time spent on the light side, number of transitions and latency to first entry into the light side was recorded. In this test, these parameters are associated with anxiety-like behavior.
Tail suspension test
In this protocol, the mice were suspended 100 cm above the stand by adhesive tape placed approximately 1 cm from the tip of the tail. The test was videotaped for five minutes. During this period, the time of immobility and latency to the first immobility episode were evaluated. The immobility assumes a low resilience, and consequently, a high level of depression-like behavior.
Rotarod test
The rotarod test was performed by placing a mouse on a rotating drum and measuring the time each animal was able to maintain its balance while walking on top of the rod. Mice underwent 4 trials of up to five minutes, and the inter-trial interval was 30–40 min. The speed of the rotarod was 10 rpm, and the height from the ground was 50 cm. Some animals were attached to the rotarod axis. The latency to the first fall was recorded for each trial, and the animals could fall three times. Some mice were attached to the rotating axis as they began to fall and rode completely around the rod. For these animals, the latency to the first fall was still considered. In this protocol, coordination and motor skill learning were evaluated (Shiotsuki et al. 2010).
RNA analysis
Total RNA was extracted using a standard method (TRIzol reagent; Invitrogen, Carlsbad, CA, USA). The RT-PCR analyses were carried out using 1 μg of total RNA extracted from the hippocampi of PND 16 male pups using a Superscript III kit (Invitrogen).
Real-time RT-PCR analyses were performed in a fluorescent temperature cycler (Applied Biosystems 7500; Life Technologies Co., Carlsbad, CA, USA) according to the recommendations of the manufacturer. Briefly, after initial incubation at 50 °C for 2 min and 95 °C for 10 min, reactions were cycled 40 times using the following parameters for all genes studied: 95 °C for 15 s, 60 °C for 30 s and 72 °C for 45 s. SYBR Green (Applied BioSystems, Foster City, CA, USA) fluorescence was detected at the end of each cycle to monitor the amount of PCR product formed during that cycle. We used genes that coded proteins related to the serotonergic system (Tph2, Sert, 5HT1a receptor, Lmx1b) and neuroplasticity (BDNF, brain-derived neurotrophic factor). Primers used for the amplification of cDNAs of interest were synthesized by Extend Biotecnologia Ltda. The forward and reverse primers’ sequences are listed in Table 2.
We determined relative mRNA levels (2-ΔΔCt) by comparing the PCR cycle threshold (Ct) between groups, after correcting for the internal control β-actin (Schmittgen and Livak 2008). Assays were repeated two times, and the data were merged after normalization.
Statistical analysis
All results are presented as the means ± SE. The assumption of normal data distribution was assessed with the Shapiro-Wilk test. If the data did pass the normality test, parametric comparisons were performed. In this case, between-group comparisons were analyzed with the Student’s unpaired T-test. on the other hand, the Mann-Whitney test was used to compare data without normal distribution. Grubbs’ test was used for detecting outliers. Cohen’s d analysis was used to evaluate the effect sizes between the groups, which is the difference between means divided by the standard deviation. In this measure, effect sizes were interpreted as small (0.2 < d < 0.5), moderate (0.5 < d < 0.8) and large (d > 0.8). Differences were considered statistically significant when p < 0.05. GraphPad Prism 5 statistical software (La Jolla, CA, USA) was used for all statistical analyses.
Results
RNA analysis
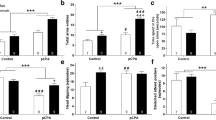
In the hippocampus, animals programmed with FLX 10 mg/kg had infra-regulation of TPH2 (0.07 ± 0.007 vs. 1.07 ± 0.19, p < 0.001), 5HT1a (0.08 ± 0.007 vs. 1.02 ± 0.17, p < 0.001), SERT (0.35 ± 0.08 vs. 1.09 ± 0.18, p = 0.004), BDNF (0.19 ± 0.02 vs. 1, 07 ± 0.19, p = 0.001) and Lmx1b (0.03 ± 0.01 vs. 1.12 ± 0.25, p = 0.001) compared to controls (Fig. 2). In Table 3, according to Cohen’s d analysis, prenatal treatment with FLX induced a strong effect in all genes studied.
Behavioral analysis
In the open field test, it was shown that the FLX group had an increase in the total time of grooming (7.62 ± 1.47 s vs. 3.0 ± 0.97 s, p = 0.01). In anxiety-related parameters, there was a tendency to reduce center zone time (p = 0.07), and center ratio (p = 0.07). The total number of squares crossed, rearing, and fecal pellets were not significantly different (Fig. 3).
The behavioral parameters of the open field test box test in 70 PND offspring treated with T4 200μg/kg during perinatal period. In this protocol, it was demonstrated difference in grooming time, which is related to stress response. □ represent control group and ■, FLX-treated group (10 mg/kg); *P < .05, n = 10
In the object recognition task, we found that neonatal FLX treatment did not alter the total time of exploration (p = 0.24). However, this treatment decreased d1 (3.22 ± 2.52 s vs. 13.44 ± 2.83 s, p = 0.01), d2 (0.149 ± 0.04 s vs. 0.32 ± 0.04 s, p = 0.01), and d3 (54.2 ± 3.76% vs. 66.6 ± 2.02%, p = 0.01) indexes (Fig. 4).
We also recorded interesting data in the light-dark box paradigm (Fig. 5). In this test, we observed that neonatal treatment with FLX promoted a significant increase in latency to light (147.5 ± 36.7 s vs. 17.5 ± 1.83 s, p = 0.006). Moreover, the programmed group had shorter time in the light side (36.6 ± 10.40 s vs. 75.7 ± 6.57 s, p = 0.008) and a decrease in number of transitions (8.01 ± 2.31 vs. 17.5 ± 1.41, p = 0.004). However, there was no statistical difference in the number of SAP (p = 0.21).
Regarding tail suspension test (Fig. 6), we observed that neonatal FLX-induced a reduction in depression-like behavior characterized by shorter immobility time (77.6 ± 7.25 s vs. 112.1 ± 12.24 s, p = 0.02). In rotarod test, we noticed a significant effect of treatment [F (1, 68) = 5.45; p = 0.02] and trials [F (3, 68) = 11.58; p < 0.001] (Fig. 7). However, no statistical differences were reported in the interaction between these variables [F (3, 68) = 1.01; p = 0.39].
Induction of locomotor activity in rotarod test in 74 PND offspring treated with saline or FLX 10 mg/kg during perinatal period. In this protocol, it was observed a poor motor learning skill FLX-treated group. White circles represent control group and black circles, FLX-treated group (10 mg/kg); **P < .05, n = 1
Concerning Cohen’s d analysis, FLX neonatal treatment induced a strong effect on some behavioral parameters (Table 4), such as time in center zone (d = 0.88), center ratio (d = 0.86), and grooming time (d = 1.28) in the open field test. In object recognition task, a strong effect was observed in d1 (d = 1.27), d2 (d = 1.43), and d3 (d = 1.30) indexes. Regarding light-dark box paradigm, strong effect was observed in latency to light (d = 1.58), transitions (d = 1.60), and time in light side (d = 1.46). In tail suspension test, a strong effect was also demonstrated in immobility time (d = 1.21). In the other parameters not mentioned, medium or weak effects were observed.
Discussion
In the current study, we found that FLX treatment initiates significant alterations in several genes associated with the hippocampal serotonergic system. Moreover, this treatment induces psychobiological programming in the adult offspring characterized by anxyogenesis, low mnemonic performance, poor motor learning and antidepressant behavior.
To explain the recorded transcriptional impacts in our work, we should highlight the role of the LMX1b. This transcriptional factor is essential for the development and maturation of dopaminergic and serotonergic systems. Besides, it is also involved in the embryogenesis of the kidney and skeletal system (Ding et al. 2003). Deletions or mutations of this gene are associated with hereditary osteo-onychodysplasia, open-angle glaucoma, and renal dysplasia (Dai et al. 2009). In mice, the deletion of this gene affects all serotonergic neurons and other cell types. In general, these animals deceased during the perinatal period (Zhao et al. 2006).
Based on such assumptions, an elegant study was performed by Song and colleagues (Song et al. 2011), who used an animal model with conditional deletion of LMX1b only in neurons that express Pet1a, which is a gene exclusively expressed in neurons of the central serotonin (5-HT) system. When these animals are treated with tamoxifen in adulthood, the expression of LMX1b is silenced. In this condition, there is a reduction in 5-HT levels when compared to the control group. There is also a downregulation in the TPH2 and SERT of the dorsal raphe nucleus. However, no changes in the levels of dopamine and norepinephrine, or the expression of aromatic L-amino acid decarboxylase and Pet1a were observed during the experimental period (Song et al. 2011). From a behavioral point of view, the conditional inactivation of the Lmx1b factor, specifically in serotonergic neurons, results in increased aversive memory in the context-conditioned fear test (Dai et al. 2008).
BDNF is known to modulate neuronal plasticity and plays a vital role in the regulation of neurotransmission, neuronal regeneration and survival. Although BDNF and 5-HT seem to be part of distinct signaling systems, these two substances interact with each other to regulate neurogenesis and synaptic plasticity, especially in the neural circuits involved in depression and anxiety (Martinowich and Lu 2008). In animal models and human polymorphisms, which are associated with increased central 5-HT bioavailability, there is a reduction in the expression of the mRNA encoding BDNF precursor (Homberg et al. 2014). In our study, we found a reduction in BDNF expression in the hippocampus of FLX treated offspring. Although these data are opposed to the results obtained with chronic SSRI treatment in adulthood, we observed that the reciprocal modulation between these two molecules involves different transcription factors during the development phase, such as Arnt2, CaRF, CREB, NFkB, and Npas4 (Guidotti et al. 2012; Luoni et al. 2013). The elevation in 5-HT levels-induced deficient BDNF transcription could be attributed to the reduction in the expression of Npas4 in SERT knockout mice (Guidotti et al. 2012).
5-HT1a knockout mice also showed a reduction in the expression of BDNF and the TrKB receptor phosphorylation of the ventral hippocampus (Wu et al. 2012). Moreover, in the cell culture of mesencephalic raphe serotonergic neurons, adding 5-HT to the medium can increase the BDNF gene expression and the number of cells that express serotonergic markers. This variation was dose-dependent. Also, this effect has been pharmacologically proven to be initiated by the 5-HT1a receptor and by the activation of the TrKB receptor (Galter and Unsicker 2000). Also, in another in vitro study, it was found that 18 h of exposure to BDNF was enough to almost double the number of serotonergic neurons and axonal growth. This marked effect was associated with the elevation in the expression of the genes encoding TPH2, SERT, and 5-HT1a receptors (Rumajogee et al. 2002). Thus, we can suggest the existence of an autocrine or paracrine loop may be mediated by BDNF to regulate the serotonergic phenotype. This hypothesis, together with the Lmx1b infra-regulation, could help to understand the recorded transcriptional differences in our study. Also, the present data raises some relevant questions regarding the mechanisms by which the fluctuation in 5-HT levels can modulate per se the expression of genes related to its neurotransmission.
Few studies addressed the effects of FLX on the serotonergic system during the postnatal period. Due to clinical and translational relevance, most of the previous data is related to the prenatal period through maternal treatment. Although this phase is morphologically important, we believe that the postnatal treatment window has greater physiological relevance, especially from a neurobehavioral aspect. During the first two weeks after delivery, the brain has high plasticity. Based on this premise, changes in the bioavailability of neurotransmitters can promote the impairment in CNS development and, therefore, predispose behavioral changes in adulthood (Kepser and Homberg 2015). The period between 7 and 20 PND in rodents corresponds approximately to the late stages of fetal development and the first two to three years of life in humans (Semple et al. 2013). In this phase, there is an increase in brain mass, as well as in the maturation of monoaminergic neurotransmission (Hansson et al. 1998).
FLX treatment from PND 2 to PND 21 stimulates anxiety-like behavior in the open field test, and the elevation in plus-maze, as well as increased depression-like behavior, was observed in the forced swim test. Such changes have been completely reversed by ketanserin, a selective 5-HT2a receptor antagonist (Sarkar et al. 2016). Similarly, treated mice offspring with citalopram, clomipramine, and FLX from PND 4 to PND 21 increases the anxiety-like behavior in different experimental paradigms in adulthood (Ansorge et al. 2008). More recently, the perinatal FLX also decreased the social behavior in mice. In this study, it is suggested that reduced monoamine oxidase A expression might be associated with SSRI exposure and behavioral deficits symptomatic of autism (Bond et al. 2020). To explain behavioral changes in our study, we should emphasize that SERT knockout mice have infra-regulation for BDNF and the transcription factor Npas (Guidotti et al. 2012). Both molecules are elaborated in the development and maturation of the GABAergic system in hippocampal regions and the prefrontal cortex (Lin et al. 2008; Sakata et al. 2009). SERT knockout mice also present important changes in the GABAergic system, characterized by a reduction in the expression of Vgat, Gad 67, γ2 subunit of the GABAA receptor, and proteins of calcium-binding specific to subgroups of GABAergic neurons (Guidotti et al. 2012). Thus, the neonatal FLX could alter the GABAergic system in limbic and cortical regions of mice offspring and, consequently, increased the anxiety-like behavior. Changes in the GABAergic system could also explain the reduction in the cognitive performance in these animals.
In our study, we also observed low mnemonic performance and antidepressant behavior. Karpova and her group verified that neonatal treatment with FLX caused an antidepressant effect in adult mice, and this behavioral pattern was reversed by the treatment with FLX in adulthood (Karpova et al. 2009). Regarding cognitive status, Sprowles and coauthors showed that perinatal exposure to SSRI impaired the learning and memory in the water maze and passive avoidance tests (Sprowles et al. 2017). This behavioral impairment could be explained by the work of Donovan et al. (2019), who reported that the Lmx1b/Pet1 regulatory cascade is mandatory for the 5-HT axon arborization gene and protocadherin-alphac2 during postnatal development of forebrain axons. As protocadherin proteins are important in the late-stage maturation of serotonergic projections (Katori et al. 2009), we infer that perinatal FLX may compromise hippocampal circuits, that related to memory and antidepressant behavior. We cannot forget that the FLX treatment also seems to disrupt BDNF pathways in the present study, which could also be related to such behavioral responses. Further studies will be necessary to explain the impacts of SSRI treatment, especially in mnemonic performance.
Regarding the induction of locomotor activity in rotarod, we showed that the FLX-treated offspring also had low motor skill learning. Similar results were obtained by Lee and Lee (2012), who verified a low motor performance in adolescent rats exposed to FLX during the neonatal period. This variation could be attributed to the impairment in the dendritic structure of striatal and cortical neurons due to FLX treatment.
Overall, although many questions remain about the mechanisms through which perinatal SSRI treatment produces behavioral changes during adulthood, our results provide new information concerning a little more molecular mechanisms of the early-life SSRI exposure-induced affective disorders and cognitive impairment.
References
Akkerman S, Blokland A, Reneerkens O, van Goethem NP, Bollen E, Gijselaers HJM, Lieben CKJ, Steinbusch HWM, Prickaerts J (2012) Object recognition testing: methodological considerations on exploration and discrimination measures. Behav Brain Res 232:335–347. https://doi.org/10.1016/j.bbr.2012.03.022
Ansorge MS, Morelli E, Gingrich JA (2008) Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci 28:199–207. https://doi.org/10.1523/JNEUROSCI.3973-07.2008
Bel, N., & Artigas, F. (1996). In vivo effects of the simultaneous blockade of serotonin and norepinephrine transporters on serotonergic function. Microdialysis studies. The Journal of Pharmacology and Experimental Therapeutics, 278(3), 1064–72. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8819487
Bond CM, Johnson JC, Chaudhary V, et al. (2020) Perinatal fluoxetine exposure results in social deficits and reduced monoamine oxidase gene expression in mice. Brain Res. https://doi.org/10.1016/j.brainres.2019.06.001
Brunton PJ (2015) Programming the brain and behaviour by early-life stress: a focus on neuroactive steroids. J Neuroendocrinol 27(6):468–480. https://doi.org/10.1111/jne.12265
Dai JX, Han HL, Tian M, Cao J, Xiu JB, Song NN, Huang Y, Xu TL, Ding YQ, Xu L (2008) Enhanced contextual fear memory in central serotonin-deficient mice. Proc Natl Acad Sci U S A 105:11981–11986. https://doi.org/10.1073/pnas.0801329105
Dai JX, Johnson RL, Ding YQ (2009) Manifold functions of the nail-patella syndrome gene Lmx1b in vertebrate development. Development Growth and Differentiation 51:241–250. https://doi.org/10.1111/j.1440-169X.2008.01083.x
Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121(1):66–72. https://doi.org/10.1007/bf02245592
Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF (2003) Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci 6:933–938. https://doi.org/10.1038/nn1104
Donovan LJ, Spencer WC, Kitt MM, Eastman BA, Lobur KJ, Jiao K, Silver J, Deneris ES (2019) Lmx1b is required at multiple stages to build expansive serotonergic axon architectures. ELife. 8. https://doi.org/10.7554/eLife.48788
Galindo, L. C. M., Barros, Da M. L. D., Pinheiro, I. L., Santana, R. V. de C., Matos, R. J. B. De, Leandro, C. G., … Manhães De Castro R. (2015). Neonatal serotonin reuptake inhibition reduces hypercaloric diet effects on fat mass and hypothalamic gene expression in adult rats. Int J Dev Neurosci https://doi.org/10.1016/j.ijdevneu.2015.07.004, 46, 76, 81
Galter D, Unsicker K (2000) Sequential activation of the 5-Ht1(a) serotonin receptor and TrkB induces the serotonergic neuronal phenotype. Mol Cell Neurosci 15:446–455. https://doi.org/10.1006/mcne.2000.0841
Gemmel M, Bögi E, Ragan C, Hazlett M, Dubovicky M, van den Hove DL, Oberlander TF, Charlier TD, Pawluski JL (2018) Perinatal selective serotonin reuptake inhibitor medication (SSRI) effects on social behaviors, neurodevelopment and the epigenome. Neurosci Biobehav Rev 85:102–116. https://doi.org/10.1016/j.neubiorev.2017.04.023
Grippo AJ, Beltz TG, Weiss RM, Johnson AK (2006) The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry 59(4):309–316. https://doi.org/10.1016/j.biopsych.2005.07.010
Guidotti G, Calabrese F, Auletta F, Olivier J, Racagni G, Homberg J, Riva MA (2012) Developmental influence of the serotonin transporter on the expression of Npas4 and GABAergic markers: modulation by antidepressant treatment. Neuropsychopharmacology. 37:746–758. https://doi.org/10.1038/npp.2011.252
Hansson SR, Mezey É, Hoffman BJ (1998) Serotonin transporter messenger RNA in the developing rat brain: early expression in serotonergic neurons and transient expression in non- serotonergic neurons. Neuroscience. 83:1185–1201. https://doi.org/10.1016/S0306-4522(97)00444-2
Homberg JR, Molteni R, Calabrese F, Riva MA (2014) The serotonin-BDNF duo: developmental implications for the vulnerability to psychopathology. Neurosci Biobehav Rev 43:35–47. https://doi.org/10.1016/j.neubiorev.2014.03.012
Invernizzi R, Bramante M, Samanin R (1996) Role of 5-HT1A receptors in the effects of acute chronic fluoxetine on extracellular serotonin in the frontal cortex. Pharmacol Biochem Behav 54(1):143–147. https://doi.org/10.1016/0091-3057(95)02159-0
Karpova NN, Lindholm J, Pruunsild P, Timmusk T, Castrén E (2009) Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur Neuropsychopharmacol 19:97–108. https://doi.org/10.1016/j.euroneuro.2008.09.002
Katori S, Hamada S, Noguchi Y, Fukuda E, Yamamoto T, Yamamoto H, Hasegawa S, Yagi T (2009) Protocadherin-α family is required for serotonergic projections to appropriately innervate target brain areas. J Neurosci 29:9137–9147. https://doi.org/10.1523/JNEUROSCI.5478-08.2009
Kepser LJ, Homberg JR (2015) The neurodevelopmental effects of serotonin: a behavioural perspective. Behav Brain Res 277:3–13. https://doi.org/10.1016/j.bbr.2014.05.022
Kessler, R. C., Nelson, C. B., McGonagle, K. A., Liu, J., Swartz, M., & Blazer, D. G. (1996). Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. The British Journal of Psychiatry. Supplement, (30), 17–30. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8864145
Kreiss, D. S., & Lucki, I. (1995). Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. The Journal of Pharmacology and Experimental Therapeutics, 274(2), 866–76. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7636750
Lee LJ, Lee LJH (2012) Neonatal fluoxetine exposure alters motor performances of adolescent rats. Dev Neurobiol. https://doi.org/10.1002/dneu.20942
Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T (2013) Object recognition test in mice. Nat Protoc 8:2531–2537. https://doi.org/10.1038/nprot.2013.155
Lesch K-P, Waider J (2012) Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 76(1):175–191. https://doi.org/10.1016/j.neuron.2012.09.013
Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME (2008) Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 455:1198–1204. https://doi.org/10.1038/nature07319
Luoni A, Hulsken S, Cazzaniga G, Racagni G, Homberg JR, Riva MA (2013) Behavioural and neuroplastic properties of chronic lurasidone treatment in serotonin transporter knockout rats. Int J Neuropsychopharmacol 16:1319–1330. https://doi.org/10.1017/S1461145712001332
Machado DG, Cunha MP, Neis VB, Balen GO, Colla A, Grando J, Brocardo PS, Bettio LEB, Capra JC, Rodrigues ALS (2012) Fluoxetine reverses depressive-like behaviors and increases hippocampal acetylcholinesterase activity induced by olfactory bulbectomy. Pharmacol Biochem Behav 103(2):220–229. https://doi.org/10.1016/j.pbb.2012.08.024
Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RCS, Paul IA (2006) Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology 31(1):47–57. https://doi.org/10.1038/sj.npp.1300823
Mahar I, Bambico FR, Mechawar N, Nobrega JN (2014) Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev 38:173–192. https://doi.org/10.1016/j.neubiorev.2013.11.009
Martinowich K, Lu B (2008) Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 33:73–83. https://doi.org/10.1038/sj.npp.1301571
Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM (2008) Serotonin: a review. J Vet Pharmacol Ther 31(3):187–199. https://doi.org/10.1111/j.1365-2885.2008.00944.x
Nestler EJ, Hyman SE (2010) Animal models of neuropsychiatric disorders. Nat Neurosci 13(10):1161–1169. https://doi.org/10.1038/nn.2647
Olivier JDA, Vallès A, van Heesch F, Afrasiab-Middelman A, Roelofs JJPM, Jonkers M, Peeters EJ, Korte-Bouws GAH, Dederen JP, Kiliaan AJ, Martens GJ, Schubert D, Homberg JR (2011) Fluoxetine administration to pregnant rats increases anxiety-related behavior in the offspring. Psychopharmacology 217(3):419–432. https://doi.org/10.1007/s00213-011-2299-z
Perez-Caballero L, Torres-Sanchez S, Bravo L, Mico JA, Berrocoso E (2014) Fluoxetine: a case history of its discovery and preclinical development. Expert Opin Drug Discovery 9(5):567–578. https://doi.org/10.1517/17460441.2014.907790
Rodriguez-Porcel, F., Green, D., Khatri, N., Harris, S. S., May, W. L., Lin, R. C. S., & Paul, I. A. (2011). Neonatal exposure of rats to antidepressants affects behavioral reactions to novelty and social interactions in a manner analogous to autistic spectrum disorders. Anatomical Record (Hoboken, N.J. : 2007), 294(10), 1726–35. https://doi.org/10.1002/ar.21402
Rumajogee P, Madeira A, Vergé D, Hamon M, Miquel MC (2002) Up-regulation of the neuronal serotoninergic phenotype in vitro: BDNF and cAMP share Trk B-dependent mechanisms. J Neurochem 83:1525–1528. https://doi.org/10.1046/j.1471-4159.2002.01264.x
Rutter JJ, Gundlah C, Auerbach SB (1994) Increase in extracellular serotonin produced by uptake inhibitors is enhanced after chronic treatment with fluoxetine. Neurosci Lett 171(1–2):183–186. https://doi.org/10.1016/0304-3940(94)90635-1
Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, Shen L, Lu B (2009) Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A 106:5942–5947. https://doi.org/10.1073/pnas.0811431106
Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ (2016) Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci 39:763–781. https://doi.org/10.1016/j.tins.2016.09.002
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3(6):1101–1108. https://doi.org/10.1038/nprot.2008.73
Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ (2013) Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106-107:1–16. https://doi.org/10.1016/j.pneurobio.2013.04.001
Shiotsuki H, Yoshimi K, Shimo Y, Funayama M, Takamatsu Y, Ikeda K, Takahashi R, Kitazawa S, Hattori N (2010) A rotarod test for evaluation of motor skill learning. J Neurosci Methods 189:180–185. https://doi.org/10.1016/j.jneumeth.2010.03.026
Smith K (2014) Mental health: a world of depression. Nature 515(7526):181–181. https://doi.org/10.1038/515180a
Song NN, Xiu JB, Huang Y, Chen JY, Zhang L, Gutknecht L, Lesch KP, Li H, Ding YQ (2011) Adult raphe-specific deletion of Lmx1B leads to central serotonin deficiency. PLoS One 6:e15998. https://doi.org/10.1371/journal.pone.0015998
Sprowles JLN, Hufgard JR, Gutierrez A, Bailey RA, Jablonski SA, Williams MT, Vorhees CV (2017) Differential effects of perinatal exposure to antidepressants on learning and memory, acoustic startle, anxiety, and open-field activity in Sprague-Dawley rats. Int J Dev Neurosci 61:92–111. https://doi.org/10.1016/j.ijdevneu.2017.06.004
Wu YC, Hill RA, Klug M, Van Den Buuse M (2012) Sex-specific and region-specific changes in BDNF-TrkB signalling in the hippocampus of 5-HT1A receptor and BDNF single and double mutant mice. Brain Res 1452:10–17. https://doi.org/10.1016/j.brainres.2012.03.011
Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW IV et al (2006) Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci 26:12781–12788. https://doi.org/10.1523/JNEUROSCI.4143-06.2006
Acknowledgements
We thank the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq) and Coordination of Superior Level Staff Improvement (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES) for the fellowship granted to graduate students present in this project, the Fundação de Amparo à Pesquisa do Estado de São Paulo to Janaina Sena de Souza (FAPESP, São Paulo, Brazil, processes #2017/07053-3 and #2018/22763-0) and Gisele Giannocco (FAPESP, São Paulo, Brazil, process #2017/23169-1). We also thank Antonio Vicente C. L. da Costa and Ipojucan Pereira de Souza, employers of Federal Rural University of Rio de Janeiro (UFRuralRJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laureano-Melo, R., Dos-Santos, R.C., da Conceição, R.R. et al. Perinatal fluoxetine treatment promotes long-term behavioral changes in adult mice. Metab Brain Dis 35, 1341–1351 (2020). https://doi.org/10.1007/s11011-020-00606-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00606-2