Abstract
The serotoninergic system plays an important role in the ontogeny of the mammalian central nervous system, and changes in serotonin production during development may lead to permanent changes in brain cytoarchitecture and function. The present study investigated the programming effects of neonatal serotonin depletion on behavior and molecular components of the serotoninergic system in adult male and female rats. Subcutaneous para-chlorophenylalanine (pCPA) administration (100 mg kg−1) was performed daily on postnatal days 8–16 to deplete brain serotonin content. During adulthood, elevated plus-maze, open field, social interaction, forced swimming, and food, saline, and sucrose intake tests were performed. Relative expression of serotonin neurotransmission components in several brain areas was determined by qPCR. Additionally, serotonin immunofluorescence and neuropeptide mRNA expression were assessed in dorsal raphe (DRN) and paraventricular (PVN) nuclei, respectively. Rat performance in behavioral tests demonstrated a general increase in locomotor activity and active escape behavior as well as decreased anxiety-like behavior after neonatal brain serotonin depletion. The behavioral programming effects due to neonatal serotonin depletion were more pronounced in females than males. At the gene expression level, the mRNA of Tph1 and Tph2 were lower in DRN while Htr2c was higher in the amygdala of pCPA-treated males, while Htr1a, Htr2c, Oxt, Avp, Crh, and Trh were not different in any treatments or sex in PVN. The results indicate that neonatal serotonin depletion has long-term consequences on locomotion and anxiety-like behavior associated with long-lasting molecular changes in the brain serotoninergic system in adult rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serotonin (or 5-hydroxytriptamine, 5-HT) producing neurons express the enzyme tryptophan hydroxylase (TPH), responsible for the first step and the rate-limiting of 5-HT biosynthesis [1]. These neurons also express the 5-HT transporter (SERT), which is responsible for the reuptake of 5-HT into the neurons, and the inhibitory 5-HT type 1 A (5-HT1A) autoreceptors [2]. Additionally, 5-HT1A, as well as 5-HT2C, among others, are postsynaptic heteroreceptors widely distributed in the brain [2, 3] and they are related to mood and cognition control [4]. The dorsal raphe nucleus (DRN) is the main source of 5-HTergic neurons and it is known that DRN neurons project to the striatum, amygdala, prefrontal cortex, septum, hippocampus, and hypothalamus [2], which may justify the ability of 5-HTergic neurotransmission to modulate a wide variety of behavioral and homeostatic functions, including emotional states and responses [5, 6], motor activity [3], food intake [7], and sodium appetite [8].
There is extensive evidence showing that early adverse events can affect the 5-HTergic system in mammals. The deficiency in the human 5-HTergic system during development has been linked with adult anxiety [5], depression [6], and antisocial behavior [9] and disturbances in the 5-HTergic system by adverse early life experiences may increase the vulnerability to psychiatric disorders [10]. Additionally, adult monkeys that experienced maternal rejection during infancy show deficiencies in the 5-HTergic system components [11]. In rats, maternal separation enhanced the inhibitory effect of citalopram (a selective 5-HT reuptake inhibitor) on the 5-HTergic neuron firing rate [12], and increased mRNA expression of SERT (Slc6a4) [13] and Tph2 [14] in the DRN in adulthood. Contact with unrelated pups by juvenile female rats reduced anxiety and augmented the mRNA Tph2 expression in DRN later in life [15]. Altogether, these results show that early adverse events can affect the 5-HTergic system in mammals, the mechanisms by which changes in neonatal 5-HTergic neurotransmission modulate behavioral responses in adulthood are far from being completely understood.
In the present study, we sought to further examine the role of 5-HT during the neonatal period. Taking into account that 5-HT plays an important organizational role in the development of the mammalian central nervous system [16] and that this neurotransmitter is involved in the regulation of a large number of behaviors [3, 5,6,7,8], we postulate that the brain 5-HT levels reduction during the neonatal period may exert long-lasting behavioral effect. This study was also conceived to detect long-lasting alteration of gene expression of some 5-HTergic components, since neonatal brain 5-HT level disturbances have been associated with cellular [17] neurochemical [18], and molecular [19] changes. To induce neonatal 5-HT depletion, we employed the irreversible TPH inhibitor para-chlorophenylalanine (pCPA), which affects responsiveness to noxious stimuli [20], sexual behavior [21,22,23,24], anxiety-like behavior [23], and locomotor activity [20, 22] later in life, in addition to reducing the number and complexity of fetal cortical interneurons [25]. We administered pCPA between postnatal days 8 and 16 because this is a critical period in which 5-HTergic nerve terminals have rapid proliferation [26] and Tph2 expression levels as well as TPH2 enzymatic activity increase, reaching a peak on postnatal day 22 and then decrease by 40% between postnatal days 22 and 61 [27]. Additionally, in rats, there is an essential sexual dimorphism on DRN mRNA Tph2 expression [28] and 5-HT levels [29] throughout development. Thus, developmental 5-HT depletion could impact animals in a sex-dependent manner and we have, therefore, studied the effects of neonatal pCPA treatment on both male and female animals.
Materials and Methods
Animals and Ethics Statement
Animal handling and experimental procedures were performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health [30] and in compliance with pertinent Brazilian regulations (Law 11,794, Decree 6899) as well as approval by the Ethics Committee on Animal Use of Federal University of São Paulo (CEUA/UNIFESP), under protocol #6751130919 (ID 009317). Rats weighing 200–320 g were group-housed under controlled temperature (23 ± 2 °C) and light conditions (12:12 h light/dark cycle; lights on at 6:00 a.m.) with free access to standard food pellets and filtered water.
Neonatal Brain 5-HT Depletion
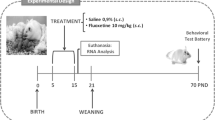
Neonatal brain 5-HT depletion was performed as reported by Farabollini et al. [23]. Briefly, litters of nine dams, born on the same day, were randomly selected. The day of birth was designated as postnatal day 0. On postnatal day 1, litters were randomly culled to 8–10 pups per dam (equal sex ratio, as near as possible). From each litter, half of the animals of each sex were randomly assigned to one of two experimental conditions: vehicle treatment (control, n = 40, 18 males and 22 females), receiving 0.1 mL/10 g BW of isotonic saline (0.15 M); or para-chlorophenylalanine methylester treatment (pCPA, Sigma-Aldrich, St Louis, MO, USA, n = 44, 25 males and 19 females), receiving, 100 mg kg−1 dissolved in 0.1 mL/10 g of BW isotonic saline solution. Both groups received daily subcutaneous injections from postnatal days 8 to 16. The litters were weaned at 21 days and then housed in groups of 4 or 5 animals of the same sex, treatment, and age. All evaluations were performed between 60 and 72 days of age.
General Experimental Procedures
Behavioral studies were performed in a quiet room between 7 and 11 p.m. (lights off at 6 p.m.) starting at postnatal day 60. In order to minimize the use of animals and to comply with “three Rs” (replacement, reduction, and refinement) [31], each animal was submitted to a battery of behavioral tests. The behavioral test sequence ranged from the least to the most potentially stressful as follows: elevated plus-maze and open field (exposition to a new and potentially threatening environment, day 60), social interaction (exposition to a new environment with an unfamiliar rat, day 62), forced swimming training and test (physical and psychological stress, days 64–65), and metabolic cage (social isolation, days 65–72). The evaluations in the metabolic cages were performed in the following sequence: adaptation (days 65–67), basal food and water intake (day 68), hypertonic saline (day 70), and sucrose preference tests (day 72). At the end of the experiments (day 72), all animals were submitted to euthanasia by a high dose of sodium thiopental (100 mg kg−1, i.p.) followed by decapitation. For behavioral analyses, 22 control (9 male and 13 female) and 24 pCPA (14 male and 10 female) rats were used. Investigators were blinded to the experimental group for all data analysis.
Another set of rats (to avoid interference from behavioral tests on gene expression [32]) was euthanized by decapitation on postnatal day 70. Each brain was rapidly removed, frozen on dry ice, and stored at − 80 °C until dissection and RNA extraction to evaluate target gene expression levels by quantitative real-time PCR (qPCR). For this study, 12 control (6 male and 6 female) and 12 pCPA (6 male and 6 female) rats were used.
On postnatal day 70, a third set of rats (not exposed to behavioral tests) was deeply anesthetized with sodium thiopental (100 mg kg−1, i.p.) and transcardially perfused with 80 mL of heparinized 0.15 M saline solution followed by 300 mL of 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.2). The brains were removed, post-fixed in the same perfusion solution overnight at 4 °C, and then stored in 30% sucrose in 0.1 M PB solution at 4 °C. The brains were used for immunofluorescence analysis. For this study, 6 control (3 male and 3 female) and 8 pCPA (5 male and 3 female) rats were used.
Elevated Plus-Maze Test
The elevated plus-maze consists of two opposite open arms (50 × 10 cm) crossed with two opposite enclosed arms (40 cm high wall). The arms are connected to a central square (10 × 10 cm), giving the apparatus a plus sign appearance. The maze was elevated 50 cm above the floor in a dimly lit room. Rats were individually placed in the central square of the plus-maze facing an enclosed arm. During the next 5 min, the time spent and numbers of entries into open and closed arms were recorded. An arm entry was scored when all four limbs were inside the arm. The elevated plus-maze is a validated test for anxiety-like behavior in rodents, in which an increased exploration of open arms indicates decreases in anxiety status. Total entries into open and closed arms and ethological parameters (i.e., stretched attend posture, rearing and head dipping) were evaluated to support the assessment of motor activity and emotional reactivity, respectively [33].
Open-Field Test
The open-field test evaluates exploratory and motor activity and anxiety-like behavior [34]. The rats were individually placed in a white acrylic square cage (80 × 80 × 30 cm) divided into 25 equal quadrants. In this paradigm, they were allowed to freely explore the apparatus for 5 min. During this time, the following parameters were scored: ambulation (by quantifying the overall number of quadrants explored by the rats), central quadrant exploration, immobility time, and grooming episodes.
Social Interaction Test
The social interaction arena consisted of a square acrylic box (60 × 60 × 30 cm) with a solid floor placed in a dimly illuminated room. The rats were paired based on body mass and placed in the test area for 10 min. The time spent by the experimental rat in active social interaction with an unfamiliar rat of the same sex was scored. Active social interactions were characterized by sniffing, following, grooming, kicking, boxing, and crawling over or under the partner [35]. Four male and four female rats were used as interaction partners.
Forced Swimming Test
The forced swimming test has been extensively used to investigate the antidepressant effects of drugs in rodents [36]. Briefly, rats were placed individually in acrylic cylinder (25 cm diameter and 30 cm deep) containing water at 25 °C with no chance of escaping or touching the bottom of the cylinder. Twenty-four hours before the experiment, the rats were submitted to a 15 min forced swimming session in order to recognize and adapt to the scheduled test. The next day, they were submitted to a valid forced swimming test for 5 min. Latency to and bouts of immobility were scored.
Metabolic Cage Measurements
Animals were placed in metabolic cages for 48 h to adapt, and then, three consecutive ingestive assessments were performed with 48-h intervals: (1) basal food and water intake; (2) hypertonic 0.3 M NaCl preference test; and (3) 2% sucrose preference tests. Each assessment lasted 24 h with standard laboratory chow and filtered water available ad libitum. Hypertonic saline and sucrose preference tests were carried out using a two-bottle free choice paradigm: water and 0.3 M NaCl or water and 2% sucrose available ad libitum for rats in graduated volumetric tubes. All measures were expressed per 100 g of body mass.
Brain Micropunch, RNA Extraction, and qPCR
First, the prefrontal cortex was dissected as an oblique slice cut with a scalpel from the bregma to the anterior pole at a 45° angle. Then, the remaining frozen brain was sliced into 60 μm coronal sections using a cryostat (Leica Microsystems CM1850 Cryostat; Wetzlar, Germany). On the basis of plates from the Paxinos and Watson [37] rat brain atlas, a 1 mm diameter micropunch needle was used to collect the PVN (from bregma − 1.32 to bregma − 2.04), basolateral amygdala (BLA; − 2.04 to − 3.60), and DRN (− 6.84 to − 8.04) from the slices. Thereafter, slices were stained with toluidine blue (0.1% v/v), and the punch location was confirmed by light microscopy, as previously described [38]. The brains with incorrect micropunch of the target brain nuclei were not used in the study. Tissue from different structures was stored in QIAzol Lysis Reagent (QIAgen, Crawley, UK) at − 80 C.
Total RNA was extracted from brain nuclei samples by combining QIAzol Lysis Reagent with RNeasy kit protocols (QIAgen) as directed by the manufacturer. Samples were treated with DNAse (amplification grade; Thermo Fisher Scientific, Waltham, MA, USA) then submitted to reverse transcription with 250 to 1000 ng of total RNA using the Quantitec Reverse Transcription Kit (QIAgen). Quantitative PCR was carried out in triplicate using TaqMan™ qPCR Master Mix (Applied Biosystems, Foster City, CA, USA) in a QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific). For each of the following genes, qPCR was carried out using Applied Biosystems gene expression probes (Foster City, CA, USA): Tph1 (Probe ID Rn01476867_m1), Tph2 (Probe ID Rn00598017_m1), Htr1a (Probe ID Rn00561409_s1), Htr2c (Probe ID Rn00562748_m1), Slc6a4 (Probe ID Rn00564737_m1), Avp (Probe ID Rn00566449_m1), Oxt (Probe ID Rn00564446_g1), Crh (Probe ID Rn01462137_m1), Trh (Probe ID Rn00564880_m1). As housekeeping (endogenous control), the β-actin gene (Actb, Probe ID 4351319) was used, since Actb mean cycle threshold (Ct) values were not different between groups in any of the target brain areas. The differences between the samples were determined through calculation of the relative expression between the genes of interest and the housekeeping gene applying the 2−∆∆Ct (∆∆Ct = ∆Ctunknown − ∆Ctcontrol) method described by Livak and Schmittgen [39]. The relative gene expression is presented as fold changes from control.
Immunofluorescence
Brains were sectioned using a cryostat into 30 μm thick coronal slices containing the DRN as described by Paxinos and Watson [37]. Sections were stored in antifreeze (3:3:1 glycerol:ethyleneglycol:0.1 M PB) at − 20 °C until use for immunofluorescence technique. Immediately before immunofluorescence procedures, free-floating sections were washed in 0.01 M PB and then incubated with 10% normal goat serum (NGS, Vector Laboratories, Burlingame, CA) and 0.3% Triton X-100 in 0.1 M PB for 2 h to block sites of nonspecific binding and to permeabilize the slices, respectively, and subsequently were incubated for 48 h at 4 °C with rabbit polyclonal anti-5-HT antibody (1:5000; ImmunoStar Cat# 20080, RRID:AB_572263, Hudson, WI, USA) in 0.1 M PB containing 2% NGS. Then, the slices were washed in 0.01 M PB and incubated for 2 h at room temperature in donkey Alexa Fluor 488-conjugated anti-rabbit IgG antibody (1:500; Jackson ImmunoResearch Labs Cat# 711-545-152, RRID:AB_2313584, West Grove, PA) in 0.1 M PB containing 5% normal goat serum, and washed in 0.01 M PB. Finally, sections were mounted on glass slides, air-dried and covered with Fluoromount (Sigma-Aldrich). Samples in which primary or secondary antibodies were excluded were processed in parallel and used as negative control. Images at 3-μm intervals (Z-sectioning) and optical stacks of seven images were acquired using a confocal microscope (Leica TCS SP8,). From each stack, the image with the highest fluorescence level was selected and only that image was used for quantification. For each animal, images at × 10 showing the entire DRN and at × 40 showing the dorsal part (DRD) and ventral part (DRV) of this nucleus were assayed. Three brain sections at − 8.04 mm from bregma were quantified from each animal. Immunofluorescence images were digitized and analyzed using the Fiji software (National Institutes of Health, Bethesda, MD, RRID:SCR_002285; http://fiji.sc/Fiji). Investigators were blinded to the experimental grouping while taking photomicrographs and performing image analysis. Average 5-HT immunoreactivity in the area of interest in each section was calculated maintaining the gain, laser power, and offset constant. Results were expressed as percentage of change with respect to the male control group.
Experimental Design and Statistical Analyses
All values were expressed as means ± SEM. Normality of the data was assessed with the Shapiro–Wilk test. When variables were not normally distributed, values were rank-transformed before statistical analysis according to Hora and Conover [40]. Outliers were identified and eliminated by the Grubbs’ method. Comparisons among groups were performed using two-way analysis of variance (ANOVA) (factors: treatment and sex) followed by Newman-Keuls test for multiple comparisons. For more information about specific statistical analyses, please see each figure legend. The statistical analyses were performed using the GraphPad Prism software (version 8, San Diego, USA; RRID:SCR_002798). In all cases, an effect was determined to be significant if the p value was ≤ 0.05. In addition, to estimate the effect size, eta squared (η2) was calculated as the sum of squares of a factor or interaction divided by the total sum of squares.
Results
Elevated Plus-Maze Test
Two-way ANOVA showed significant main effects of sex [F(1,30) = 21.98, p = 5.6 × 10−5, η2 = 0.2258] and pCPA treatment [F(1,30) = 41.76, p = 3.9 × 10−7, η2 = 0.4290] for open arms entries but no significant interaction between the factors (Fig. 1a). Females entered more into open arms than males and pCPA-treated rats entered more than controls. Similarly, total arm entries (entries into open arms + closed arms) were affected by sex [F(1,30) = 48.90, p = 9.0 × 10−8, η2 = 0.3219] and by neonatal pCPA treatment [F(1,30) = 60.06, p = 1.2 × 10−8, η2 = 0.3953], with a significant interaction between them [F(1,30) = 12.95, p = 0.0011, η2 = 0.0853] (Fig. 1b). The Newman-Keuls post hoc test showed that pCPA females had a higher number of total entries than pCPA males (p = 0.0001) and control females (p = 0.0001). Also, control females and pCPA males had a higher number of total entries than control males (p = 0.0229 and p = 0.0170; respectively). Time spent in open arms was significantly increased by neonatal pCPA treatment [F(1,30) = 10.58, p = 0.0028, η2 = 0.2385] (Fig. 1c). Overall, these results suggest that neonatal 5-HT depletion leads to increased locomotor activity and decreased anxiety in adult animals. Additionally, pCPA treatment affected female rats to a greater extent than males.
Effects of brain serotonin depletion during neonatal period in male and female adult rats on a number of entries into open arms, b total number of entries (entries into open arms plus entries into closed arms), c time spent in the open arms, d number of rearing episodes, e number of head dipping episodes, and f number of stretching attend postures during 5 min of evaluation in the elevated plus-maze apparatus. Values are mean ± SEM. The number of rats per group is indicated in each bar. Data were analyzed by a two-way ANOVA followed by the Newman-Keuls post hoc test. The values of percentages of time spent in the open arms were transformed to ranks before ANOVA. **p < 0.01 and ***p < 0.001 compared to control groups; +p < 0.05, ++p < 0.01, and +++p < 0.001 compared to the respective male group; #p < 0.05, ##p < 0.01, and ###p < 0.001 compared to the respective control group
For rearing counts, ANOVA showed a significant main effect of pCPA treatment, reducing rearing episodes, as well as an interaction between sex and pCPA treatment [F(1,30) = 16.32, p = 0.0003, η2 = 0.3016 and F(1,30) = 6.14, p = 0.0190, η2 = 0.1135; respectively]. The Newman-Keuls post hoc test revealed that pCPA males performed less rearing than control males (p = 0.0005) and that pCPA females (p = 0.0125) (Fig. 1d). Regarding head dipping episodes, ANOVA showed that females perform this behavior more than males [F(1,30) = 6.17, p = 0.0188, η2 = 0.1331] and also showed a significant interaction between sex and treatment [F(1,30) = 6.17, p = 0.0188, η2 = 0.1331]. In the control group, females performed more head dips than males (p = 0.0075), while pCPA increased head dips only in male rats (p = 0.0094) (Fig. 1e). Stretched attend posture episodes were significantly lower in pCPA treated rats compared to control [F(1,30) = 59.87, p = 1.2 × 10−8, η2 = 0.6605] (Fig. 1f). These results indicate that 5-HT depletion appears to affect risk assessment and increase impulsivity in adult animals.
Open-Field Test
The sex significantly affected the number of central quadrant crossing [F(1,39) = 7.04, p = 0.0115, η2 = 0.1525] (Fig. 2a): females crossed more central quadrants than males. Regarding the total number of quadrants explored, ANOVA showed a significant main effect of both sex and treatment [F(1,39) = 19.61, p = 7.5 × 10−5, η2 = 0.2807 and F(1,39) = 8.824, p = 0.0051, η2 = 0.1263; respectively], pCPA-treated females explored more total quadrants in relation to males and pCPA-treated rats showed more ambulation than controls (Fig. 2b). Females spent less time immobile in the open field than males [F(1,39) = 17.709, p = 0.0001, η2 = 0.2872], while the pCPA treatment did not change the immobility time (Fig. 2c). For grooming, we found a significant effect of sex [F(1,39) = 18.10, p = 0.0001, η2 = 0.2366] and interaction between sex and pCPA treatment [F(1,39) = 16.91, p = 0.0002, η2 = 0.2211], with pCPA-treated male rats showing more grooming episodes in relation to control males (p = 0.0002) and pCPA-treated females (p = 0.0002) (Fig. 2d). The performance in the open-field test showed an increase in the locomotor activity of pCPA-pretreated rats, and also, a less anxious profile of female rats.
Effects of brain serotonin depletion during the neonatal period in male and female adult rats on a number of central quadrants crossed, b ambulation (total number of quadrants crossed), c inactivity time, and d number of grooming episodes during 5 min of evaluation in the open-field apparatus. Values are mean ± SEM. The number of rats per group is indicated in each bar. Data were analyzed by a two-way ANOVA followed by the Newman-Keuls post hoc test. The values of total quadrants crossed; inactivity time and grooming were transformed to ranks before ANOVA. **p < 0.01 compared to control groups; +++p < 0.001 compared to the respective male group; ###p < 0.001 compared to the respective control group
Social Interaction and Forced Swimming Tests
Data from the social interaction test showed that neither neonatal pCPA treatment nor sex affected the parameters assessed (Fig. 3a). Two-way ANOVA showed that the time that rats remained immobile in forced swimming test differed between sexes [F(1,39) = 7.049, p = 0.0114, η2 = 0.1311], with females spending more time immobile than males. Additionally, neonatal pCPA treatment decreased the immobility time in relation to control treated rats [F(1,39) = 7.613, p = 0.0088, η2 = 0.1416] (Fig. 3b). Escape latency in this test was not affected by sex or neonatal pCPA treatment (Fig. 3c). These results, together with those obtained in the plus-maze and open-field tests, suggest that the neonatal 5-HT depletion increases rats’ locomotion in different contexts (more or less stressful) during adulthood. Also, males and females seem to have differences in coping strategies.
Effects of brain serotonin depletion during the neonatal period in male and female adult rats on a time spent by the rat pair in active social interaction (sniffing, following, grooming, kicking, boxing, and crawling over or under the partner) during 10 min in the social interaction box, b immobility time during 5 min in the forced swimming test, and c latency to immobility in the forced swimming test. Values are mean ± SEM. The number of rats per group is indicated in each bar. Data were analyzed by a two-way ANOVA. The values of social interaction time and immobility time were transformed to ranks before ANOVA. **p < 0.01 compared to control groups
Body Mass and Intake Behaviors
Animals were weighed at postnatal day 63. As we expected, females were lighter than males [F(1,42) = 163.2, p < 10−15, η2 = 0.7578]. Additionally, neonatal pCPA treatment reduced body mass [F(1,42) = 9.54, p = 0.0036, η2 = 0.0443]. Neither food nor water intake was different across sex or pCPA treatment. Significant sex effects regarding hypertonic saline and water intake as well as saline preference were found [F(1,28) = 18.27, p = 0.0002, η2 = 0.3468, F(1,28) = 6.80, p = 0.0145, η2 = 0.1831, and F(1,28) = 6.70, p = 0.0151, η2 = 0.1542; respectively], with females showing higher values than males in all parameters. Additionally, there was a significant interaction between sex and pCPA treatment regarding saline intake and preference: F(1,28) = 5.80, p = 0.0229, η2 = 0.1100 and F(1,28) = 7.91, p = 0.0089, η2 = 0.1819; respectively. Females previously treated with pCPA exhibited the highest levels of saline solution intake (Newman-Keuls: p = 0.0005 vs. pCPA males and p = 0.0320 vs. control females), resulting in increased preference for hypertonic NaCl (Newman-Keuls: p = 0.0037 vs. pCPA males and p = 0.0339 vs. control females). Sucrose consumption and preference were higher in females [F(1,28) = 51.69, p = 8.0 × 10−8, η2 = 0.6157 and F(1,28) = 8.13, p = 0.0081, η2 = 0.1876; respectively]. The pCPA treatment induced an increase in water intake and reduced sucrose preference [F(1,28) = 6.00, p = 0.0209, η2 = 0.1705 and F(1,28) = 6.68, p = 0.0153, η2 = 0.1541; respectively]. See Table 1 for more details about ANOVA F and p values. In short, neonatal 5-HT depletion reduced body mass in both sexes, had no effect on food intake, and increased saline preference in adult female rats. In addition, females showed an increase in sucrose preference, which resulted in an increase in sucrose intake.
Gene Expression in the DRN
Neonatal pCPA treatment significantly decreased Tph1 and Tph2 mRNA expression [F(1,17) = 16.09, p = 0.0009, η2 = 0.3848 and F(1,17) = 7.63, p = 0.0133, η2 = 0.2129; respectively]. We also found a significant interaction between pCPA treatment and sex [F(1,17) = 4.98, p = 0.0393, η2 = 0.1192 and F(1,17) = 9.82, p = 0.0061, η2 = 0.2741; respectively] in mRNA expression of these enzymes in the DRN (Fig. 4a and b). According to the Newman-Keuls post hoc test, pCPA males and control females had less expression of Tph1 than control males (p = 0.0021 and p = 0.0092). Also, pCPA males and control females showed less relative expression of Tph2 than control males (p = 0.0034 and p = 0.0190; respectively). Therefore, the decrease in the mRNA expression of the 5-HT synthesis enzyme could result in a drop in 5-HT levels in pCPA-treated animals. The mRNA for the Htr1a receptor and 5-HT transporter Slc6a4 was not affected by sex or neonatal pCPA treatment (Fig. 4c and d).
Effects of brain serotonin depletion during the neonatal period in male and female adult rats on relative mRNA expression of a tryptophan hydroxylase type 1 (Tph1), b tryptophan hydroxylase type 2 (Tph2), c serotonin receptor type 1A (Htr1a), and d serotonin transporter (Slc6a4) in dorsal raphe nucleus. Values are mean ± SEM. The number of rats per group is indicated in each bar. Data were analyzed by a two-way ANOVA followed by the Newman-Keuls post hoc test. *p < 0.05 and ***p < 0.001 compared to control groups; +p < 0.05 and ++p < 0.01 compared to the respective male group; ##p < 0.01 compared to the respective control group
5-HT Immunofluorescence in the DRN
A representative image of the location of the DRD and DRV is shown at Fig. 5a. There was a significant interaction between sex and pCPA treatment in DRD and DRV subnuclei [F(1,10) = 7.98, p = 0.0180, η2 = 0.3894 and F(1,10) = 10.21, p = 0.0096, η2 = 0.4641; respectively]. The Newman-Keuls post hoc test showed that the immunofluorescence signal was lower in the female control group compared to the male control group in both subnuclei (p = 0.0477 and p = 0.0399; respectively) (Fig. 5b–e).
Effects of brain serotonin depletion during the neonatal period in male and female adult rats on serotonin (5-HT) immunofluorescence in dorsal raphe nucleus (DRN). a Drawing of a coronal section of rat brain at bregma − 8.04 mm, and representative micro-photograph at × 10 of DRN. b Relative serotonin levels and c representative microphotograph at × 40 of control, male and female, and pCPA, male and female brain slices in the dorsal part (DRD) of DRN. d Relative serotonin levels and e representative microphotograph at × 40 of control, male and female, and pCPA, male and female brain slices in the ventral part (DRV) of DRV. Values are mean ± SEM. The number of rats per group is indicated in each bar. Data were analyzed by a two-way ANOVA followed by the Newman-Keuls post hoc test. +p < 0.05 compared to the respective male group
Gene Expression in BLA, PFC, and PVN
Neither sex nor pCPA treatment changed Htr1a mRNA expression in the BLA (Fig. 6a). Regarding Htr2c mRNA expression in the BLA, we found a significant main effect of pCPA treatment [F(1,14) = 9.21, p = 0.0089, η2 = 0.1630] and a significant interaction between sex and pCPA treatment [F(1,14) = 28.73, p = 0.0001, η2 = 0.5082]. The Newman-Keuls post hoc test showed that pCPA males and control females had higher Htr2c expression than control males (p = 0.0004 and p = 0.0005; respectively) (Fig. 6b). Like BLA, the Htr1a mRNA expression was not affected by pCPA or sex in PFC (Fig. 6c). When considering Htr2c mRNA expression in PFC, there was a marginally significant interaction between the factors [F(1,14) = 4.57, p = 0.0506, η2 = 0.1913], with control females showing higher expression than control males (p = 0.0440; Fig. 6d). In short, neonatal 5-HT depletion seems to affect gene expression of serotonin receptors differentially according to brain region, sex, and receptor type.
Effects of brain serotonin depletion during the neonatal period in male and female adult rats on relative mRNA expression of serotonin receptor type 1A (Htr1a) at a basolateral amygdala (BLA), c prefrontal cortex (PFC), and e paraventricular nucleus (PVN); as well as relative mRNA expression of serotonin receptor type 2C (Htr2c) at b BLA, d PFC, and f PVN. Values are mean ± SEM. The number of rats per group is indicated in each bar. Data were analyzed by a two-way ANOVA followed by the Newman-Keuls post hoc test. **p < 0.01 compared to control groups; +++p < 0.001 compared to the respective male group; ###p < 0.001 compared to the respective control group
Of particular interest are the projections from the DRN to the hypothalamic paraventricular nucleus (PVN) and mRNA expression of 5-HT receptors in this nucleus [38, 41], which enables interaction between the 5-HTergic system and the neuroendocrine regulation of behavioral responses. However, in our study, ANOVA showed no effect of pCPA treatment on the expression of Htr1a [F(1,16) = 4.24, p = 0.0561, η2 = 0.2068] and Htr2c mRNA in PVN (Fig. 6e and f; respectively). Relative mRNA expression of Avp, Oxt, Crh, and Trh in PVN were not affected by sex, neonatal pCPA treatment or interaction (Fig. 7).
Effects of brain serotonin depletion during the neonatal period in male and female adult rats on relative mRNA expression of a arginine vasopressin (Avp), b oxytocin (Oxt), c corticotrophin-release hormone (Crh), and d thyrotropin-release hormone (Trh) in paraventricular nucleus. Values are mean ± SEM. The number of rats per group is indicated in each bar. Data were analyzed by a two-way ANOVA. The values of Avp and Oxt were transformed to ranks before ANOVA
Discussion
The present study reveals the consequences of 5-HT depletion during the neonatal period on the behavioral expression in adult rats and further investigates the possible causes and mechanisms, to shed light on this behavioral programming.
The pCPA-induced 5-HT depletion in the neonatal period increased exploration in the open-field test, indicating a degree of locomotor hyperactivity. These results are consistent with those obtained in the plus-maze test, in which a higher number of total arm entries was observed in 5-HT depleted groups, indicating an increase of locomotor activity. These data corroborate findings of other studies demonstrating that treatment with pCPA during the prenatal or neonatal periods produces hyperlocomotion in adulthood [20, 42]. We also observed that 5-HT depleted male rats had fewer rearing episodes, suggesting a decrease in vertical exploration of pCPA-treated males. Additionally, neonatal 5-HT depleted rats appeared to be less anxious than their controls, since they entered and spent more time in the open arms. Our data from the plus-maze test are comparable with those obtained by Farabollini et al. [23] and Wilson et al. [22]. We also observed a decrease in stretched attend posture episodes in pCPA-treated animals. The drop in the occurrence of this action has been associated with a reduction of anxiety levels, but according to Cruz et al. [33], it is also influenced by other factors related to decision making and risk assessment. Last, there was an increase in grooming episodes in 5-HT depleted male rats in the open-field test. Grooming has been used as an index of displacement [33] because grooming episodes increase in novel situations [43] as well indicating habituation to stressful contexts (i.e., behavior induced by arousal [44]). Considering that neonatal 5-HT depletion reduced anxiety-like behavior in our study, the increased grooming episodes observed in 5-HT depleted males could reflect faster habituation to the novel situation (the open-field box). On the whole, performance in plus-maze and open-field tests showed that neonatal 5-HT depletion resulted in an adult phenotype characterized by general locomotor activation and decreased anxiety. Also, 5-HT depletion appeared to affect risk assessment and increase impulsivity in adult animals. Data from the social interaction test showed that neonatal 5-HT depletion did not change the parameters evaluated among the experimental groups. Our results are in accordance with evidence reported by Farabollini et al. [23], who also did not observe behavioral alterations in a different social interaction model. In the forced swimming test, neonatal 5-HT depletion decreased the immobility time, probably as a consequence of hyperlocomotion of these animals. Other authors have observed an increase in the immobility time in the forced swimming test in male and female adult rats pretreated with pCPA between gestational days 14–17 [42], demonstrating the fundamental importance of the moment at which the 5-HT is depleted.
To better understand these behavioral programming effects induced by neonatal brain 5-HT depletion, we have evaluated gene expression related to 5-HTergic signaling in the brain. We found that neonatal 5-HT depletion induced no changes in mRNA expression of the Htr1a or Slc6a4 transporter in the DRN on both male and female animals, suggesting that the shut-off process of these neurons remained unchanged in our model. On the other hand, neonatal 5-HT depletion significantly decreased both Tph1 and Tph2 expression in the DRN. The promoter region of these genes contains several scattered CpG sites, which methylation can affect its expression [45]. In fact, stress was associated with an increased methylation in Tph1 and Tph2 promoter region in rats, resulting in a decreased expression of these genes in the brain [46]. In this regard, it was demonstrated that the 5′ untranslated region of Tph2 gene mediates its transcriptional repression [47]. Thus, neonatal brain 5-HT deficit may lead to increase in Tph1 and Tph2 gene methylation, causing a long-lasting reduction in their mRNA expression in adulthood. Since TPH is the key enzyme that limits the rate of 5-HT synthesis [1], a decrease in its gene expression and potentially in its levels likely lead to a reduction of 5-HT production in the DRN. To verify this hypothesis, we quantified the levels of 5-HT in DRD and DRV subnuclei by immunofluorescence. Although not statistically significant, the neonatal 5-HT depletion led to reductions of 35% and 41% in 5-HT levels in the DRD and DRV, respectively, but only in male rats, which showed a decline in Tph1 and Tph2 expression.
Ren et al. [48] reported that neurons of DRD and DRV exhibit different projections and behavioral modulation. Thus, the decrease in adulthood 5-HT levels in both DRD and DRV subnuclei of DRN might be involved in the behavioral changes observed after neonatal pCPA treatment. Naslund et al. [49] have previously demonstrated that a batch of Wistar rats expressing anxiolytic-like behavior have lower expression of the TPH2 at both mRNA and protein levels in raphe nuclei, associated with reduced levels of 5-HT in the amygdala. Therefore, neonatal pCPA treatment not only programs the Tph1 and Tph2 expression but also reduces DRN 5-HT content, potentially affecting 5-HTergic neurotransmission in different brain areas, such as the amygdala, with an impact on locomotor activity and anxiolytic-like behavior at adulthood.
Brain 5-HT level manipulations were demonstrated to change mRNA expression of 5-HTergic receptors in adult rats. Barbon et al. [50] found downregulation of Htr2c mRNA expression in the prefrontal and frontal cortex in young adult rats treated with a selective 5-HT reuptake inhibitor, whereas pCPA treatment of adult rats produced an upregulation of Htr2c expression in the striatum [51]. Thus, neonatal 5-HT depletion may produce long-lasting changes in the expression of 5-HT receptors in brain areas receiving projections from the raphe. In fact, neonatal 5-HT depletion markedly increased mRNA expression of Htr2c in the BLA of males. 5-HT2C receptor alterations have been associated with the development of psychopathologies [10] and, considering the fundamental importance of the interaction between genetic and environmental factors for the development of this kind of disease, we can speculate that the regulation of 5-HT2C mRNA and protein expression could depend on epigenetic and transcriptional regulatory mechanisms [52]. In fact, 5-HT2C receptor is encoded by a complex transcription unit, which includes the coding region and an extended 5′ untranslated region containing two introns and three exons, which host miRNAs. These miRNAs can bind to mRNA, and therefore, block translation, causing RNA decay or cleavage, or chromatin silencing [53]. On the other hand, Tang et al. [54] found that the levels of histone acetylation associated with Htr2c promoter are correlated with its gene expression levels. These epigenetic mechanisms are especially sensitive to early life experiences [45]; therefore, we can assume that one or more of them may be responsible for the changes induced by neonatal 5-HT depletion observed in our model.
The 5-HT2C receptor has been associated with the regulation of anxiety, depression, and locomotion [3]. Thus, higher 5-HT2C receptor mRNA expression in the BLA observed in our study could be related to the lower anxiety levels exhibited by the 5-HT-depleted rats. It is well established that amygdala activation, particularly the basolateral subdivision, increases anxiety-like behavior [55]. However, BLA pyramidal neurons are under control of local inhibitory interneurons, mostly GABAergic, [56] which express and respond to the excitatory 5-HT2C receptor [57]. Therefore, the increase of Htr2c expression in BLA observed in our study could lead to an increase of 5-HT2c receptors, which in turn would inhibit BLA through the activation of GABAergic neurons, contributing to the reduced anxiolytic-like behavior observed in adult rats submitted to 5-HT depletion during development. Prominently, control females showed higher Htr2c receptor levels in BLA than males, corresponding to the lower anxiety levels and the higher locomotion observed in the behavioral tests. There is evidence of sex differences in the expression of this receptor in the hippocampus of mice, with females showing higher levels of Htr2c expression than males [58]. On the other hand, these receptors are also involved in metabolic regulation. In fact, Wan et al. [59] demonstrated that risperidone (a selective 5-HT2C antagonist) induced body mass gain, glucose intolerance, and hypertriglyceridemia, among other effects, and the infusion of a non-selective 5-HTergic antagonist into the basolateral amygdala resulted in hyperphagia in female rats [60]. Therefore, the increase of Htr2c expression could play a role in the body mass loss caused by neonatal 5-HT depletion in our study.
The 5-HTergic system has also been associated with food [7], water [61], and salt [8] intake control. Neonatal 5-HT depletion reduced body mass in both sexes, with no effect on food intake in adulthood. Thus, pCPA-induced 5-HT depletion might have changed food intake and/or metabolic function during the neonatal treatment, programming the body mass gain during development, without affecting the food intake control in adulthood. The hypothalamic PVN is involved in several autonomic and neuroendocrine modulations such as hydromineral regulation, energy balance, and stress response [38, 62]. Additionally, the hypothalamus is known to be sexually dimorphic in relation to the 5-HTergic system during development, since the concentration of 5-HT is higher in the neonatal hypothalamus of 12-day-old females than males [63]. There is evidence linking postsynaptic 5-HT1A receptors with anxiety reduction and locomotor activation [64]. Although we found no changes in Htr1a expression in the BLA and PFC, 5-HT depletion tended to increase Htr1a levels in PVN. Besides the apparent increase in Htr1a in the PVN, we found no changes in the expression of the neuropeptides Avp, Oxt, Crh, and Trh. Similarly, Mirochnik et al. [65] reported no differences in the expression of vasoactive intestinal polypeptide or AVP in the supraoptic neurons of adult rats prenatally treated with pCPA.
Furthermore, we found some behavioral sexual dimorphism. Female rats showed higher exploratory activity and anxious profile to a lesser extent compared to males. Sex differences in locomotor activity were previously observed in rats [42, 66]. Also, Kokras et al. [66] found a higher number of center entries by female rats in basal conditions, which indicated lower anxiety levels, similarly to what we found. On the other hand, female rats spent more time immobile in the forced swimming test, suggesting differences in coping strategies (passive or active coping behavior) or learning and memory. Our results are in accordance with previous findings that have systematically demonstrated longer immobilization time in female rats [66, 67]. Sexual behavioral dimorphism might be a consequence of circulating sexual hormone levels since gonadectomy in adult rats eliminates these sex differences [66]. In accordance with other authors [28], we demonstrated that both Tph1 and Tph2 mRNA expression and 5-HT immunostaining in the DRN were lower in females than in males. Similarly, Rubinow et al. [68] observed lower 5-HT synthesis in women than men, explaining, at least in part, the women’s greater susceptibility to anxiety and/or depressive disorders than men [69]. Control females also showed increased Htr2c mRNA expression in the BLA compared to control male rats. In fact, it was previously demonstrated that brain expression of both Tph2 [70, 71] and Htr2c [70,71,72] is modulated by sexual steroids Thus, it is possible that a reduced DRN Tph2 expression and 5-HT content in female rats are associated with a more anxiolytic-like behavior when compared to males. In fact, Naslund et al. [73] demonstrated an interaction between baseline anxiety and gonadal state on Tph2 expression in male rats, suggesting that androgens may contribute to inter-individual differences in anxiety-like behavior due to its interaction with 5-HTergic neurotransmission. Additionally, we observed that females showed higher intake and preference for hypertonic saline only with neonatal 5-HT depletion. These results are in accordance with the well-known inhibitory 5-HT role on sodium appetite, as evidenced by induction of a 0.3 M saline-solution intake after a DRN lesion [74]. Similar to the other assessed behaviors, neonatal 5-HT depletion had a stronger impact on females. We also observed a sexually dimorphic response regarding the consumption of a palatable sweet solution, with higher sucrose preference and intake by female rats. The sex difference of sucrose intake was previously described by other authors [75]. Moreover, Clarke and Ossenkopp [76] demonstrated that in rats there are sex differences in taste responsiveness to sweet solutions, probably related to estrogen levels, since taste responsiveness changes throughout the estrous cycle.
Notably, neonatal 5-HT depletion affected locomotion and anxiety-like behavior in a sex-specific way (i.e., 5-HT depletion increased ambulation mainly in females and reduced head dipping only in males). In association, we found that the mRNA for Tph1 and Tph2 were reduced in DRN, while the Htr2c was increased in the BLA by pCPA treatment, interestingly only in males. Sex differences in 5-HT system are manifested from early ontogeny, with levels of 5-HT and 5-HIAA being higher in female than male rats during the first 12–14 postnatal days [21, 29]. Therefore, one possibility to explain our results is that the higher 5-HT levels of females at this developmental stage make them more vulnerable to its depletion. Further studies are required to identify the mechanism involved in the dimorphic effect of neonatal 5-HT depletion. It is important to point that we have used intact, randomly cycling female rats. Likewise, we cannot discard the influence of different estrous cycle stages on molecular and behavior responses, as reported by other studies [71, 72].
In summary, our results corroborate the hypothesis that brain 5-HT depletion during the neonatal period could lead to a plastic remodeling of the 5-HTergic system [23]. This plasticity includes Tph1/2 expression and 5-HT levels reduction in the dorsal raphe nucleus and a concomitant upregulation of postsynaptic htr2c gene expression in the basolateral amygdala, a raphe projection area. These gene expression changes are correlated with behavioral alterations such as locomotor activation and anxiolytic-like profile. In view of the marked sexual dimorphism in behavior and gene expression, it is necessary to consider both sexes to study and interpret the modulation and/or implications of the 5-HTergic system.
Data Availability
Primary data are available from the authors on request.
References
Walther DJ, Bader M (2003) A unique central tryptophan hydroxylase isoform. Biochem Pharmacol 66(9):1673–1680. https://doi.org/10.1016/s0006-2952(03)00556-2
Olivier B (2015) Serotonin: a never-ending story. Eur J Pharmacol 753:2–18. https://doi.org/10.1016/j.ejphar.2014.10.031
Nebuka M, Ohmura Y, Izawa S, Bouchekioua Y, Nishitani N, Yoshida T, Yoshioka M (2020) Behavioral characteristics of 5-HT2C receptor knockout mice: locomotor activity, anxiety-, and fear memory-related behaviors. Behav Brain Res 379:112394. https://doi.org/10.1016/j.bbr.2019.112394
Ogren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekstrom JC, Svenningsson P, Meister B, Kehr J et al (2008) The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res 195(1):54–77. https://doi.org/10.1016/j.bbr.2008.02.023
Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR et al (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274(5292):1527–1531. https://doi.org/10.1126/science.274.5292.1527
Miller JM, Kinnally EL, Ogden RT, Oquendo MA, Mann JJ, Parsey RV (2009) Reported childhood abuse is associated with low serotonin transporter binding in vivo in major depressive disorder. Synapse 63(7):565–573. https://doi.org/10.1002/syn.20637
Medeiros MA, Costa-e-Sousa RH, Olivares EL, Cortes WS, Reis LC (2005) A reassessment of the role of serotonergic system in the control of feeding behavior. An Acad Bras Cienc 77(1):103–111. https://doi.org/10.1590/s0001-37652005000100008
Reis LC (2007) Role of the serotoninergic system in the sodium appetite control. An Acad Bras Cienc 79(2):261–283. https://doi.org/10.1590/s0001-37652007000200009
Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R (2002) Role of genotype in the cycle of violence in maltreated children. Science 297(5582):851–854. https://doi.org/10.1126/science.1072290
Chagraoui A, Thibaut F, Skiba M, Thuillez C, Bourin M (2016) 5-HT2C receptors in psychiatric disorders: a review. Prog Neuro-Psychopharmacol Biol Psychiatry 66:120–135. https://doi.org/10.1016/j.pnpbp.2015.12.006
Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, Innis RB (2006) Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J Neurosci 26(17):4638–4643. https://doi.org/10.1523/JNEUROSCI.5199-05.2006
Arborelius L, Hawks BW, Owens MJ, Plotsky PM, Nemeroff CB (2004) Increased responsiveness of presumed 5-HT cells to citalopram in adult rats subjected to prolonged maternal separation relative to brief separation. Psychopharmacology 176(3–4):248–255. https://doi.org/10.1007/s00213-004-1883-x
Gardner KL, Hale MW, Lightman SL, Plotsky PM, Lowry CA (2009) Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression. Brain Res 1305:47–63. https://doi.org/10.1016/j.brainres.2009.09.065
Gardner KL, Hale MW, Oldfield S, Lightman SL, Plotsky PM, Lowry CA (2009) Adverse experience during early life and adulthood interact to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience 163(4):991–1001. https://doi.org/10.1016/j.neuroscience.2009.07.055
Harding KM, Lonstein JS (2016) Extensive juvenile “babysitting” facilitates later adult maternal responsiveness, decreases anxiety, and increases dorsal raphe tryptophan hydroxylase-2 expression in female laboratory rats. Dev Psychobiol 58(4):492–508. https://doi.org/10.1002/dev.21392
Lesch KP, Waider J (2012) Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 76(1):175–191. https://doi.org/10.1016/j.neuron.2012.09.013
Frazer S, Otomo K, Dayer A (2015) Early-life serotonin dysregulation affects the migration and positioning of cortical interneuron subtypes. Transl Psychiatry 5:e644. https://doi.org/10.1038/tp.2015.147
Bhanja S, Mohanakumar KP (2010) Early-life treatment of antiserotonin antibodies alters sensitivity to serotonin receptors, nociceptive stimulus and serotonin metabolism in adult rats. Int J Dev Neurosci 28(4):317–324. https://doi.org/10.1016/j.ijdevneu.2010.02.007
Sachs BD, Rodriguiz RM, Tran HL, Iyer A, Wetsel WC, Caron MG (2015) Serotonin deficiency alters susceptibility to the long-term consequences of adverse early life experience. Psychoneuroendocrinology 53:69–81. https://doi.org/10.1016/j.psyneuen.2014.12.019
Adlard BP, Smart JL (1974) Some aspects of the behavior of young and adult rats treated with p-chlorophenylalanine in infancy. Dev Psychobiol 7(2):135–144. https://doi.org/10.1002/dev.420070206
Wilson CA, Pearson JR, Hunter AJ, Tuohy PA, Payne AP (1986) The effect of neonatal manipulation of hypothalamic serotonin levels on sexual activity in the adult rat. Pharmacol Biochem Behav 24(5):1175–1183. https://doi.org/10.1016/0091-3057(86)90167-x
Wilson CA, Gonzalez I, Farabollini F (1992) Behavioural effects in adulthood of neonatal manipulation of brain serotonin levels in normal and androgenized females. Pharmacol Biochem Behav 41(1):91–98. https://doi.org/10.1016/0091-3057(92)90065-n
Farabollini F, Hole DR, Wilson CA (1988) Behavioral effects in adulthood of serotonin depletion by P-chlorophenylalanine given neonatally to male rats. Int J Neurosci 41(3–4):187–199. https://doi.org/10.3109/00207458808990725
Keleta YB, Lumia AR, Anderson GM, McGinnis MY (2007) Behavioral effects of pubertal anabolic androgenic steroid exposure in male rats with low serotonin. Brain Res 1132(1):129–138. https://doi.org/10.1016/j.brainres.2006.10.097
Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG (2007) Embryonic depletion of serotonin affects cortical development. Eur J Neurosci 26(2):331–344. https://doi.org/10.1111/j.1460-9568.2007.05661.x
Tissari AH (1975) Pharmacological and ultrastructural maturation of serotonergic synapses during ontogeny. Med Biol 53(1):1–14
Rind HB, Russo AF, Whittemore SR (2000) Developmental regulation of tryptophan hydroxylase messenger RNA expression and enzyme activity in the raphe and its target fields. Neuroscience 101(3):665–677. https://doi.org/10.1016/s0306-4522(00)00402-4
Lukkes JL, Norman KJ, Meda S, Andersen SL (2016) Sex differences in the ontogeny of CRF receptors during adolescent development in the dorsal raphe nucleus and ventral tegmental area. Synapse 70(3):125–132. https://doi.org/10.1002/syn.21882
Giulian D, Pohorecky LA, McEwen BS (1973) Effects of gonadal steroids upon brain 5-hydroxytryptamine levels in the neonatal rat. Endocrinology 93(6):1329–1335. https://doi.org/10.1210/endo-93-6-1329
NIH (2011) Guide for the care and use of laboratory animals. vol Institute of Laboratory Animal Resources, Commission on Life Sciences. National Research Council, 8th edn. National Institute of Health, Washington, DC
Russell WMS, Burch RL (1959) The principles of humane experimental technique, 6th edn. Methuen & Co LTD, London
Zhang J, Xue M, Mei Y, Li Z, Ceng Z, Li Y, Zhang Y, Li N et al (2020) Co-expression network of mRNAs and lncRNAs regulated by stress-linked behavioral assays. Psychopharmacology 237(2):571–582. https://doi.org/10.1007/s00213-019-05390-1
Cruz AP, Frei F, Graeff FG (1994) Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav 49(1):171–176. https://doi.org/10.1016/0091-3057(94)90472-3
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463(1–3):3–33. https://doi.org/10.1016/s0014-2999(03)01272-x
Cheeta S, Kenny PJ, File SE (2000) Hippocampal and septal injections of nicotine and 8-OH-DPAT distinguish among different animal tests of anxiety. Prog Neuro-Psychopharmacol Biol Psychiatry 24(7):1053–1067. https://doi.org/10.1016/s0278-5846(00)00129-9
Pollak DD, Rey CE, Monje FJ (2010) Rodent models in depression research: classical strategies and new directions. Ann Med 42(4):252–264. https://doi.org/10.3109/07853891003769957
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Elsevier Academic Pess, San Diego
Dutra SG, Paterson A, Monteiro LR, Greenwood MP, Greenwood M, Amaral LS, Melo MR, Colombari DS et al (2020) Physiological and transcriptomic changes in the hypothalamic-neurohypophysial system after 24 hours of furosemide-induced sodium depletion. Neuroendocrinology. https://doi.org/10.1159/000505997
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Hora SC, Conover WJ (1984) The F statistic in the two-way layout with rank–score transformed data. J Am Stat Assoc 79(387):6–673. https://doi.org/10.1080/01621459.1984.10478095
Lopez JF, Akil H, Watson SJ (1999) Neural circuits mediating stress. Biol Psychiatry 46(11):1461–1471. https://doi.org/10.1016/s0006-3223(99)00266-8
Vataeva LA, Kudrin VS, Vershinina EA, Mosin VM, Tiul'kova EI, Otellin VA (2007) Behavioral alteration in the adult rats prenatally exposed to para-chlorophenylalanine. Brain Res 1169:9–16. https://doi.org/10.1016/j.brainres.2007.06.056
Bindra D, Spinner N (1958) Response to different degrees of novelty: the incidence of various activities. J Exp Anal Behav 1(4):341–350. https://doi.org/10.1901/jeab.1958.1-341
Spruijt BM, van Hooff JA, Gispen WH (1992) Ethology and neurobiology of grooming behavior. Physiol Rev 72(3):825–852. https://doi.org/10.1152/physrev.1992.72.3.825
Chen GL, Miller GM (2012) Advances in tryptophan hydroxylase-2 gene expression regulation: new insights into serotonin-stress interaction and clinical implications. Am J Med Genet B Neuropsychiatr Genet 159B(2):152–171. https://doi.org/10.1002/ajmg.b.32023
Chen Y, Xu H, Zhu M, Liu K, Lin B, Luo R, Chen C, Li M (2017) Stress inhibits tryptophan hydroxylase expression in a rat model of depression. Oncotarget 8(38):63247–63257. https://doi.org/10.18632/oncotarget.18780
Patel PD, Bochar DA, Turner DL, Meng F, Mueller HM, Pontrello CG (2007) Regulation of tryptophan hydroxylase-2 gene expression by a bipartite RE-1 silencer of transcription/neuron restrictive silencing factor (REST/NRSF) binding motif. J Biol Chem 282(37):26717–26724. https://doi.org/10.1074/jbc.M705120200
Ren J, Friedmann D, Xiong J, Liu CD, Ferguson BR, Weerakkody T, DeLoach KE, Ran C et al (2018) Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell 175(2):472–487 e420. https://doi.org/10.1016/j.cell.2018.07.043
Naslund J, Studer E, Pettersson R, Hagsater M, Nilsson S, Nissbrandt H, Eriksson E (2015) Differences in anxiety-like behavior within a batch of Wistar rats are associated with differences in serotonergic transmission, enhanced by acute SRI administration, and abolished by serotonin depletion. Int J Neuropsychopharmacol 18(8). https://doi.org/10.1093/ijnp/pyv018
Barbon A, Orlandi C, La Via L, Caracciolo L, Tardito D, Musazzi L, Mallei A, Gennarelli M et al (2011) Antidepressant treatments change 5-HT2C receptor mRNA expression in rat prefrontal/frontal cortex and hippocampus. Neuropsychobiology 63(3):160–168. https://doi.org/10.1159/000321593
Naslund J, Studer E, Nilsson S, Eriksson E (2020) Expression of 22 serotonin-related genes in rat brain after sub-acute serotonin depletion or reuptake inhibition. Acta Neuropsychiatr:1–7. https://doi.org/10.1017/neu.2020.9
Srivastav S, Walitza S, Grunblatt E (2018) Emerging role of miRNA in attention deficit hyperactivity disorder: a systematic review. Atten Defic Hyperact Disord 10(1):49–63. https://doi.org/10.1007/s12402-017-0232-y
Zhang Z, Falaleeva M, Agranat-Tamir L, Pages A, Eyras E, Sperling J, Sperling R, Stamm S (2013) The 5′ untranslated region of the serotonin receptor 2C pre-mRNA generates miRNAs and is expressed in non-neuronal cells. Exp Brain Res 230(4):387–394. https://doi.org/10.1007/s00221-013-3458-8
Tang B, Dean B, Thomas EA (2011) Disease- and age-related changes in histone acetylation at gene promoters in psychiatric disorders. Transl Psychiatry 1:e64. https://doi.org/10.1038/tp.2011.61
Wang DV, Wang F, Liu J, Zhang L, Wang Z, Lin L (2011) Neurons in the amygdala with response-selectivity for anxiety in two ethologically based tests. PLoS One 6(4):e18739. https://doi.org/10.1371/journal.pone.0018739
Rainnie DG, Asprodini EK, Shinnick-Gallagher P (1991) Inhibitory transmission in the basolateral amygdala. J Neurophysiol 66(3):999–1009. https://doi.org/10.1152/jn.1991.66.3.999
Sun YN, Li LB, Zhang QJ, Hui YP, Wang Y, Zhang L, Chen L, Han LN et al (2013) The response of juxtacellular labeled GABA interneurons in the basolateral amygdaloid nucleus anterior part to 5-HT(2)A/(2)C receptor activation is decreased in rats with 6-hydroxydopamine lesions. Neuropharmacology 73:404–414. https://doi.org/10.1016/j.neuropharm.2013.06.021
Zajac MS, Renoir T, Perreau VM, Li S, Adams W, van den Buuse M, Hannan AJ (2018) Short-term environmental stimulation spatiotemporally modulates specific serotonin receptor gene expression and behavioral pharmacology in a sexually dimorphic manner in Huntington’s disease transgenic mice. Front Mol Neurosci 11:433. https://doi.org/10.3389/fnmol.2018.00433
Wan XQ, Zeng F, Huang XF, Yang HQ, Wang L, Shi YC, Zhang ZH, Lin S (2019) Risperidone stimulates food intake and induces body weight gain via the hypothalamic arcuate nucleus 5-HT2c receptor-NPY pathway. CNS Neurosci Ther 26:558–566. https://doi.org/10.1111/cns.13281
Parker GC, Bishop C, Coscina DV (2002) Estrous cycle and food availability affect feeding induced by amygdala 5-HT receptor blockade. Pharmacol Biochem Behav 71(4):701–707. https://doi.org/10.1016/s0091-3057(01)00668-2
Takahashi M, Tanaka J (2016) Serotonin release in the subfornical organ area induced by sodium and water intake in the rat. Physiol Behav 164(Pt A):123–128. https://doi.org/10.1016/j.physbeh.2016.04.037
Sivukhina EV, Jirikowski GF (2016) Magnocellular hypothalamic system and its interaction with the hypothalamo-pituitary-adrenal axis. Steroids 111:21–28. https://doi.org/10.1016/j.steroids.2016.01.008
Gladue BA, Humphrys RR, Debold JF, Clemens LG (1977) Ontogeny of biogenic amine systems and modification of indole levels upon adult sexual behavior in the rat. Pharmacol Biochem Behav 7(3):253–258. https://doi.org/10.1016/0091-3057(77)90142-3
Kusserow H, Davies B, Hortnagl H, Voigt I, Stroh T, Bert B, Deng DR, Fink H et al (2004) Reduced anxiety-related behaviour in transgenic mice overexpressing serotonin 1A receptors. Brain Res Mol Brain Res 129(1–2):104–116. https://doi.org/10.1016/j.molbrainres.2004.06.028
Mirochnik V, Bosler O, Tillet Y, Calas A, Ugrumov M (2005) Long-lasting effects of serotonin deficiency on differentiating peptidergic neurons in the rat suprachiasmatic nucleus. Int J Dev Neurosci 23(1):85–91. https://doi.org/10.1016/j.ijdevneu.2004.07.021
Kokras N, Pastromas N, Papasava D, de Bournonville C, Cornil CA, Dalla C (2018) Sex differences in behavioral and neurochemical effects of gonadectomy and aromatase inhibition in rats. Psychoneuroendocrinology 87:93–107. https://doi.org/10.1016/j.psyneuen.2017.10.007
Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C (2015) Forced swim test: What about females? Neuropharmacology 99:408–421. https://doi.org/10.1016/j.neuropharm.2015.03.016
Rubinow DR, Schmidt PJ, Roca CA (1998) Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry 44(9):839–850. https://doi.org/10.1016/s0006-3223(98)00162-0
Altemus M, Sarvaiya N, Neill Epperson C (2014) Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 35(3):320–330. https://doi.org/10.1016/j.yfrne.2014.05.004
Bethea CL, Coleman K, Phu K, Reddy AP, Phu A (2014) Relationships between androgens, serotonin gene expression and innervation in male macaques. Neuroscience 274:341–356. https://doi.org/10.1016/j.neuroscience.2014.05.056
Charoenphandhu J, Teerapornpuntakit J, Nuntapornsak A, Krishnamra N, Charoenphandhu N (2011) Anxiety-like behaviors and expression of SERT and TPH in the dorsal raphe of estrogen- and fluoxetine-treated ovariectomized rats. Pharmacol Biochem Behav 98(4):503–510. https://doi.org/10.1016/j.pbb.2011.02.023
Zhou W, Cunningham KA, Thomas ML (2002) Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res 100(1–2):75–83. https://doi.org/10.1016/s0169-328x(02)00134-1
Naslund J, Studer E, Johansson E, Eriksson E (2016) Effects of gonadectomy and serotonin depletion on inter-individual differences in anxiety-like behaviour in male Wistar rats. Behav Brain Res 308:160–165. https://doi.org/10.1016/j.bbr.2016.04.015
Cavalcante-Lima HR, Badaue-Passos D Jr, de-Lucca W Jr, Lima HR, Costa-e-Sousa RH, Olivares EL, Cedraz-Mercez PL, Reis RO et al (2005) Chronic excitotoxic lesion of the dorsal raphe nucleus induces sodium appetite. Braz J Med Biol Res 38(11):1669–1675. https://doi.org/10.1590/s0100-879x2005001100015
Pitychoutis PM, Nakamura K, Tsonis PA, Papadopoulou-Daifoti Z (2009) Neurochemical and behavioral alterations in an inflammatory model of depression: sex differences exposed. Neuroscience 159(4):1216–1232. https://doi.org/10.1016/j.neuroscience.2009.01.072
Clarke SN, Ossenkopp KP (1998) Taste reactivity responses in rats: influence of sex and the estrous cycle. Am J Phys 274(3):R718–R724. https://doi.org/10.1152/ajpregu.1998.274.3.R718
Acknowledgments
The authors are grateful to MSc. Gabriel Esquitini for the excellent technical assistance.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was partially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). RR was supported by grant 2016/17968-6, Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP). LCR was supported by grant of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) 308893/2018-2 and 010239/2016-2. DL was supported by grant 423854/2018-6, CNPq. CENG was supported by Associação Fundo de Apoio à Pesquisa (AFIP). ASM and VT were supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) under the program CAPES-Print (Process number 88887.374200/2019-00).
Author information
Authors and Affiliations
Contributions
Conceptualization: LCR and ASM; methodology; VT, RM, DL, ASM, and LCR; formal analysis and investigation: VT, EVL, RM, QSRC, RCDS, VF, DL, CENG, RR, and ASM; writing—original draft preparation: VT and ASM; writing—review and editing: all authors; funding acquisition: LCR and ASM; resources: CENG, RR, LCR, and ASM; supervision: LCR and ASM.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Animal handling and experimental procedures were performed in compliance with pertinent Brazilian regulations (Law 11,794, Decree 6899) as well as approval by the Ethics Committee on Animal Use of Federal University of São Paulo (CEUA/UNIFESP), under protocol #6751130919 (ID 009317).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trujillo, V., Valentim-Lima, E., Mencalha, R. et al. Neonatal Serotonin Depletion Induces Hyperactivity and Anxiolytic-like Sex-Dependent Effects in Adult Rats. Mol Neurobiol 58, 1036–1051 (2021). https://doi.org/10.1007/s12035-020-02181-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02181-0