Abstract
Neurological disease comprises a series of disorders featuring brain dysfunction and neuronal cell death. Among the factors contributing to neuronal death, excitotoxicity induced by excitatory amino acids, such as glutamate, plays a critical role. However, the mechanisms about how the excitatory amino acids induce neuronal death remain elucidated. In this study, we investigated the role of HIF-1α (hypoxia inducible factor-1α) and RTP801 in cell apoptosis induced by quinolinic acid (QUIN), a glutamatergic agonist, in PC12 cells. We found that QUIN at 5 μM increased the expression of HIF-1α significantly with a peak at 24 h. After the treatment with QUIN (5–20 μM) for 24 h, the cells exhibited decreased viability and cell apoptosis with a concomitant increased expression of apoptosis related proteins. QUIN treatment also induced the generation of intracellular reactive oxygen species and RTP801 up-regulation in a HIF-1α-dependent manner that were inhibited by 2-methoxyestradiol, a HIF-1α inhibitor. Importantly, HIF-1 or RTP801 invalidation by siRNA rescued the cell apoptosis induced by QUIN or cobalt chloride, a chemical inducer of HIF-1. Taken together, these findings support the concept that neurotoxicity induced by QUIN is associated with HIF-1-dependent RTP801 activation and provide insight into the potential of RTP801 inhibitor in treatment of neurological disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Excitotoxicity, the sustained activation of receptors for excitatory amino acids, plays an important role in the pathogenesis of various chronic neurodegenerative disorders, such as Parkinson’s disease (PD) and Huntington’s disease (HD). The excessive stimulation of glutamate receptors, mostly the N-methyl-D-aspartate (NMDA) subtypes, increases intracellular Ca2+ levels and triggers events for the initiation of deadly cascades (Mattson 2007; Szydlowska and Tymianski 2010). In addition, the biological changes have been featured in animal models of neuronal excitotoxicity, such as generation of reactive oxygen species (ROS), lipid peroxidation, inhibition of cellular respiration and elevated inflammation (Barger et al. 2007; Maalouf et al. 2007).
Quinolinic acid (QUIN), a selective NMDA receptor agonist, is a metabolite of tryptophan along the kynurenine pathway (Lugo-Huitron et al. 2013). In neurons, sustained NMDA receptor activation by QUIN induces Ca2+ influx and subsequent activation of proteases, phospholipase and endonucleases, resulting in neuronal death (Ryu et al. 2005; Essa et al. 2013). QUIN also induced the autophagy of neuronal cells, which can be blocked by NMDA antagonist MK801 (Braidy et al. 2014). In glial cells, QUIN enhances the expression and secretion of potent chemokines and proinflammatory cytokines, such as interleukin-1β, monocyte chemoatractant protein-1 and interferon-γ (Guillemin et al. 2005), leading to marked inflammatory responses in brain. In vivo studies have shown that intrastriatal administration of QUIN in rodents produces similar biochemical changes to that in patients with HD (Pierozan et al. 2014). In patients with neurodegenerative disorders, the content of QUIN in brain is also elevated (Stoy et al. 2005). These results suggest the role of QUIN in the pathology of neurodegenerative disorders, while the mechanisms by which QUIN induces neuronal degeneration and loss still remain elucidated.
Increasing evidences show that excitotoxins induce neuronal apoptosis, leading to neurodegeneration (Mehta et al. 2013). Among the factors involved in neuronal apoptosis, hypoxia inducible factor-1 (HIF-1), a transcription factor that mediates fundamental cellular responses to hypoxia, regulates the adaptive response of neuronal cells to different stresses (Semenza 2000, 2010). HIF-1 is composed of two subunits, an oxygen-regulated HIF-1α and a constitutively expressed HIF-1β subunit. During hypoxia, HIF-1α is stabilized, dimerizes with HIF-1β and translocates to the nucleus to bind to hypoxia responsive elements (Semenza 2010). Thereafter, multiple target genes of HIF-1 will be activated, including glycolytic enzymes, growth factor and proapoptic proteins (Zaman et al. 1999; Meijer et al. 2012; Ferrer et al. 2014). Under ischemic conditions, HIF-1α is significantly up-regulated in neuronal cells and exerts regulatory effects on the tissue injury (Helton et al. 2005; Baranova et al. 2007). Since HIF-1α is involved in both protective signals and detrimental changes, the role of HIF-1α in neuronal survival remains controversial.
Among the target genes of HIF-1 involved in regulation of cell survival, RTP801 (REDD1/DITT4) functions as a suppressor of mammalian target of rapamycin (mTOR), resulting in the inhibition of mTOR activity following modest hypoxia or energy deprivation (Brugarolas et al. 2004). In response to hypoxia, RTP801 is highly induced in Drosophila and mammalian cells (Reiling and Hafen 2004; DeYoung et al. 2008). Overexpression of RTP801 potently inhibits the activity of mTOR, whereas genetic deletion of RTP801 impairs the downregulation of mTOR activity in hypoxia (DeYoung et al. 2008). Moreover, RTP801 has been shown to function in regulation of PI3K/Akt signaling and formation of intracellular reactive oxygen species (ROS) (Regazzetti et al. 2010), implying multiple roles of RTP801 in cellular physiological process.
In this study, we investigated the effects of QUIN on cultured rat adrenal pheochromocytoma PC12 cells. We demonstrated that QUIN induces HIF-1α accumulation and activation, leading to the activation of RTP801 and cell apoptosis. The results indicate that inhibition of HIF-1α or RTP801 may be a potential neuroprotective strategy to counteract the toxicity of QUIN and an efficient therapy for neurodegenerative disease.
Results
QUIN induced PC12 cell injury, ROS generation and HIF-1α up-regulation
We first determined the cell viability of PC12 cells by MTT assay after the treatment with different concentrations of QUIN for 24 h. As shown in Fig. 1a, QUIN (2.5–20 μM) reduced cell viability in a concentration-dependent manner. In addition, administration of QUIN at 2.5 ~ 10 μM for 2 h significantly induced intracellular ROS generation (Fig. 1b).
QUIN decreased cell viability, increased ROS generation and HIF-1α expression in PC12 cells. a The treatment with QUIN for 24 h reduced the cell viability determined by MTT reduction assay. b ROS generation was significantly induced in PC12 cells by QUIN. c Western blotting analysis showed the increased expression of HIF-1α in PC12 cells treated with QUIN. Data are expressed as mean ± SD; n = 8 wells for cell viability and ROS generation, n = 4 for HIF-1α expression; *P < 0.05, **P < 0.01 compared with control
It has been reported that after the stimulation by ischemia or glutamate, the stabilization of HIF-1α is significantly induced in neurons, leading to the cell death (Helton et al. 2005). Thus, to characterize the expression of HIF-1α in response to QUIN, a time course of induction curve was performed. PC12 cells were treated with QUIN for increased periods of time, and HIF-1α expression was detected by immunoblotting. As shown in Fig. 1c, HIF-1α was weakly expressed in PC12 cells under normal conditions. An increase in HIF-1α protein level was detected after a 0.5-h incubation with QUIN at 5 μM, the level peaked at 24 h and declined to basal level at 72 h.
Pharmacological Inhibition of HIF-1α protected PC12 cells against QUIN-induced cell injury
Employing 2-methoxyestradiol (2-Me), a HIF-1α inhibitor, we assessed the cell viability and HIF-1 expression. As shown in Fig. 2, 2-Me at 10 μM significantly protected PC12 cells against the injury induced by QUIN treatment at 5 μM for 24 h, while 2-Me at 10 μM alone did not affect the cell viability (data not shown).
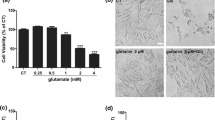
A HIF-1α inhibitor 2-Me protected the PC12 cells from cell injury induced by QUIN. a Representative micrographs showed the morphological changes in PC12 cells at 24 h after QUIN treatment. Scale bar = 20 μm. b The pretreatment with 2-Me attenuated cell injury induced by QUIN treatment for 24 h. c and d The pretreatment with 2-Me protected against cell death detected by LDH activity measurement and cell counting. Data are expressed as mean ± SD; n = 8; *P < 0.01 compared with control, #P < 0.01 compared with 5 μM QUIN
During the neuronal death induced by neurotoxin, apoptotic cell death accounted for a large part of neuronal death. Thus, we determined the cell apoptosis induced by QUIN and the effect of HIF-1α inhibitor on cell apoptosis. Using Hoechst 33258 staining, we found that PC12 cells underwent apoptosis after the treatment with QUIN for 24 h, showing condensed nuclei and enhanced blue fluorescence (Fig. 3a). The apoptosis was also blocked by 2-Me at 10 μM.
2-Me attenuated cell apoptosis induced by QUIN. a Representative micrographs showed the apoptotic cells at 24 h after QUIN administration with or without 2-Me. Arrows indicate apoptotic cells exhibiting condensed nuclei with strong bright Hoechst 33258 staining. Scale bar = 20 μm. b 2-Me decreased Bax expression and increased Bcl-2 expression, resulting in the reduced ratio of Bax and Bcl-2. Data are expressed as mean ± SD; n = 4; *P < 0.01 compared with control, #P < 0.01 compared with 5 μM QUIN
The apoptosis regulator proteins, such as B-cell lymphoma 2 (Bcl-2) and bcl-2-like protein 4 (Bax), are involved in the modulation of cell apoptosis. Bcl-2 possesses an anti-apoptotic activity, while Bax is a pro-apoptotic protein and the increased expression ratio of Bax and Bcl-2 indicates the execution of apoptosis (Rahmani et al. 2013). After the QUIN treatment at 5 ~ 10 μM for 24 h, the expression ratio of Bax/Bcl-2 was increased, which was blocked by the administration of 2-Me at 10 μM (Fig. 3b).
Treatment with 2-Me decreased blocked HIF-1α up-regulation and nucleic accumulation
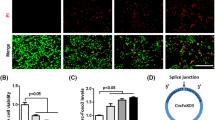
To confirm that the protective effect of 2-Me is due to HIF-1α inhibition, PC12 cells were treated with QUIN at 5 μM for 24 h with or without 2-Me. As shown in Fig. 4a, 10 μM of 2-Me blocked the HIF-1α up-regulation after QUIN treatment. Upon activation, HIF-1α is transferred into nuclei and functions as a potent transcriptional factor, initiating the expression of its target genes (Semenza 2000). By immunostaining with HIF-1α antibody, we found that HIF-1α was weakly expressed in cytosol under normal conditions. While after the treatment with QUIN, HIF-1α protein expression was markedly induced and translocated into the nuclei (Fig. 4b). Accordingly, 2-Me blocked the nuclear accumulation of HIF-1α.
2-Me inhibited HIF-1α activation and up-regulation induced by QUIN. a Treatment with 2-Me blocked HIF-1α up-regulation. Data are expressed as mean ± SD; n = 4; *P < 0.01 compared with control, #P < 0.01 compared with 5 μM QUIN only. b Representative micrographs showed that 2-Me inhibited the nucleic accumulation of HIF-1α induced by QUIN. Scale Bar = 20 μm
Knockdown of HIF-1α blocked the induction of RTP801
Given the biological importance of RTP801 in cell apoptosis and the fact that RTP801 is a downstream target of HIF-1α, we speculated that HIF-1α/RTP801 signaling might play a role in PC12 cell injury. In order to efficiently inhibit HIF-1α and RTP801 expression, PC12 cells were transfected with siRNA directed against HIF-1α and RTP801. As shown in Fig. 5a, HIF-1α siRNA significantly inhibited HIF-1α and RTP801 induction by QUIN. However, knockdown of RTP801 only blocked RTP801 up-regulation but did not affect HIF-1α expression. Likewise, quantitative PCR analysis showed that HIF-1α siRNA abolished the up-regulation of both HIF-1α and RTP801 mRNA, and RTP801 siRNA only inhibited the induction of RTP801 (Fig. 5b).
Silencing of HIF-1α or RTP801 rescued PC12 cell death induced by QUIN. a Western blotting analysis demonstrated that HIF-1α or RTP801 siRNA blocked the up-regulation of HIF-1α and RTP801 at 24 h post-QUIN challenge. Results are representative of 3 separate experiments. b Quantitative PCR analysis showed that HIF-1α or RTP801 siRNA inhibited HIF-1α or RTP801 up-regulation. Scr1: HIF-1α scrambled RNA, scr 2: RTP801 scrambled RNA. c and d Silencing of HIF-1α or RTP801 protected the PC12 cells against cell injury. Data are expressed as mean ± SD; n = 4; *P < 0.01 compared with control, #P < 0.01 compared with QUIN only
Knockdown of HIF-1-dependent RTP801 rescued QUIN-induced cell injury
Previous studies have showed that RTP801 is activated following HIF-1α activation, resulting in the inhibition of mTOR signaling (Horak et al. 2010). Subsequently, the activation of RTP801 leads to a series of biochemical processes, such as cell apoptosis, cell growth arrest, and autophagy (Schwarzer et al. 2005; Ben Sahra et al. 2011). Thus, we determined the effect of HIF-1α or RTP801 siRNA on cell death induced by QUIN. As shown in Fig. 5c and d, the apoptotic cell death induced by QUIN was rescued by either HIF-1α or RTP801 siRNA (Fig. 6a). In addition, employing HIF-1α and RTP801 siRNA, the expression ratio of Bax/Bcl-2 was reduced (Fig. 6b). To confirm the involvement of HIF-1α/RTP801 signaling, PC12 cells were treated with cobalt chloride, a chemical inducer of HIF-1. As shown in Fig. 7a, b and c, the apoptotic cell death induced by cobalt chloride was attenuated by HIF-1α or RTP801 siRNA. Exposure of the cells to cobalt chloride for 4 h induced significant elevation in HIF-1α and RTP801 protein levels, which were blocked by HIF-1α or RTP801 siRNA respectively (Fig. 7d).
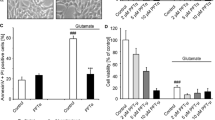
Silencing of HIF-1α or RTP801 blocked cell apoptosis in the PC12 cells after QUIN treatment. a Hoechst 33258 staining assay demonstrated that HIF-1α or RTP801 siRNA inhibited the cell apoptosis. b Knockdown of HIF-1α or RTP801 decreased the expression ratio of Bax and Bcl-2. Data are expressed as mean ± SD; n = 4; *P < 0.01 compared with control, #P < 0.01 compared with the respective scrambled RNA
Knockdown of HIF-1α or RTP801 attenuated cell apoptosis induced by cobalt chloride. a and b The treatment with HIF-1α or RTP801 siRNA attenuated cell death induced by cobalt chloride. c Cell apoptosis was ameliorated by knockdown of HIF-1α or RTP801 expression. d After 4-h incubation of cobalt chloride, the expression of HIF-1α and RTP801 was markedly increased. HIF-1α or RTP801 siRNA blocked the up-regulation of HIF-1α and RTP801 respectively. Data are expressed as mean ± SD; n = 4; *P < 0.01 compared with control, #P < 0.01 compared with the respective scrambled RNA
Discussion
In this study, we found that QUIN at 5 ~ 20 μM significantly induced cell apoptosis and HIF-1α up-regulation in PC12 cells. Pharmacological inhibition of HIF-1α by 2-Me blocked the nuclear accumulation of HIF-1α and the cell injury. In addition, RNA invalidation of HIF-1α by siRNA blocked RTP801 expression; both HIF-1α and RTP801 siRNA protected the cells against apoptosis, suggesting that the HIF-1α-dependent RTP801 activation is involved in the cell injury. These results are consistent with the previous results that QUIN induces neuronal apoptosis in vivo (Nakai et al. 1999; Colin-Gonzalez et al. 2013) and confirmed the role of RTP801 in neuronal apoptosis.
In addition to persistent stimulation of NMDA receptor, elevated ROS generation also contributes to the excitotoxicity by QUIN. In the presence of oxygen, QUIN produces the accumulation of metal ions, such as Cu2+ and Fe2+, and therefore increases the formation of superoxide anion, hydrogen peroxide and hydroxyl radical (Colin-Gonzalez et al. 2013). Thereafter, increased ROS formation leads to the DNA injury, lipid peroxidation and damage of biomembrane structure and neuronal cell apoptosis (Duong et al. 2008). Recently, it has been reported that ROS can also induce the activation of HIF-1α (Yuan et al. 2011) and modulate neuronal apoptosis (Agrawal et al. 2011). In neuronal cells, ischemic-like insult induces ROS generation, HIF-1α activation and cell apoptosis, which can be reversed by antioxidants (Rayner et al. 2006; Duong et al. 2008). Consistent with the previous studies, our study showed that PC12 cells exhibited elevated ROS level and HIF-1α expression after QUIN treatment, which was accompanied by cell apoptosis, implying the role of ROS/HIF-1α pathway in neuronal death.
A series of studies have been performed to elucidate the molecular mechanisms by which HIF-1α regulates neuronal survival and suggest that HIF-1α may possess opposite effects in different conditions. For example, inhibition of HIF-1α protects cortical neurons against ischemic insult (Lin et al. 2013; Cheng et al. 2014) and activation of HIF-1α contributes to neuronal apoptosis (Jiang et al. 2012), indicating the pro-apoptotic character of HIF-1α. In severe hypoxic conditions, HIF-1 activation potentiates p53 signaling and leads to neuronal apoptosis (Fan et al. 2009). While other studies show the protective effects of HIF-1 activation on neuronal death. Inhibition of HIF-1 abolishes the beneficial effects of the neuroprotective agent (Lopez-Hernandez et al. 2015) and enhances the toxicity of neurotoxins (Jeong and Park 2012), whereas induction of HIF-1 protects against neuronal death (Seo et al. 2010; Du et al. 2011). HIF-1 may also up-regulate the expression of growth factors and exert anti-apoptotic effects (Piret et al. 2002). Our results showed that HIF-1α expression was increased after the treatment with QUIN from 6 h with a peak at 24 h and the nucleic accumulation of HIF-1α was induced. Both the upregulation and nucleic accumulation of HIF-1α were blocked by 2-Me. These results support the detrimental role of HIF-1α in neuronal survival and suggest that pharmacological inhibition of HIF-1α may be a useful therapy for neuronal excitotoxicity.
Several signaling pathways are involved in QUIN-induced cell death, including nuclear factor-like 2 (Colin-Gonzalez et al. 2014), peroxisome proliferator activated receptor-γ (Mishra et al. 2014), and histone deacetylase (Mishra et al. 2014). In this study, we found that RTP801 was involved in mediating the QUIN-induced toxicity, demonstrated by protection against cell death by the knockdown of RTP801 expression. RTP801 has been reported to be related to ROS generation and DNA damage. In an animal model of cerebral ischemia, RTP801 expression is increased in the infarct region (Wu et al. 2011). In patients with PD and HD, the increased levels of RTP801 are also detected in neurons (Malagelada et al. 2006; Martin-Flores et al. 2015). Recently, RTP801 has been identified as a negative regulator of Schwann cell myelination and knockdown of RTP801 produced more myelinated segments (Noseda et al. 2013). Additionally, the up-regulation of RTP801 is shown in animal models of non-neuronal injury, such as cardiac ischemic injury and acute cigarette smoke–induced lung injury (Yoshida et al. 2010). Taken together, these results identify RTP801 as a positive regulator of neuronal death.
In addition, we found that silencing of HIF-1α expression by siRNA reduced RTP801 expression and protected cells against cell injury induced by QUIN, indicating the involvement of HIF-1α/RTP801 signaling in neuronal death. To confirm the role of HIF-1α/RTP801 signaling in cell death, the cells were treated with cobalt chloride. Similar to QUIN treatment, cobalt chloride induced the up-regulation of HIF-1 and RTP801 and cell apoptosis that was rescued by knockdown of HIF-1α or RTP801. In agreement with our results, knockdown of RTP801 exhibits neuroprotection in the animal models of HD and ischemic injury (Shoshani et al. 2002; Martin-Flores et al. 2015). Inhibition of RTP801 by rapamycin or siRNA also protects against neuronal death in experimental models of PD (Malagelada et al. 2010). These results confirm that inhibition of RTP801 may be useful in the treatment of ischemic disease or neurodegenerative disease. Importantly, we found that HIF-1α siRNA blocked the expressions of HIF-1α and RTP801, while RTP801 siRNA cannot block HIF-1α up-regulation, showing that HIF-1α is the upstream signal of RTP801.
In summary, our studies demonstrate that QUIN induced intracellular ROS generation and HIF-1α activation in rat PC12 cells, leading to HIF-1α-dependent RTP801 activation and cell apoptosis in rat PC12 cells. Blockade of HIF-1α or RTP801 expression prevents QUIN-induced death. Hence, RTP801 is a downstream effector of HIF-1α and pharmacological inhibition of RTP801 might be a potential approach for the treatment of neurodegenerative disease.
Experimental procedures
Cell culture
Rat pheochromocytoma cells (PC12 cells) were purchased from Institute of Cell Biology, Chinese Academy of Science (Shanghai, China). The cells were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM, Gibco Life Technologies, USA) supplemented with 10 % horse serum, 5 % fetal bovine serum, penicillin (100 000U/L) and streptomycin (100 mg/L; Sigma-Aldrich Chemical Co., MO, USA) and maintained in a humidified atmosphere at 37 °C. The cells were differentiated with 50 ng/ml nerve growth factor (NGF, #0005017, Harlan Laboratories Inc, USA) in DMEM with 1 % fetal bovine serum for 9 days. Thereafter, the cells were washed with DMEM at 24 h before experiments and cultured in DMEM with 1 % fetal bovine serum. In the experiments involving treatment with drugs, cells were pre-treated for 30 min with drug or vehicle. The stock solutions of cobalt chloride (200 mM; Sigma-Aldrich Chemical Co., USA) and 2-methoxyestradiol (20 mM; Sigma-Aldrich Chemical Co., USA) were prepared before each treatment.
Cell death determination
Cell injury was examined by the determination of lactate dehydrogenase (LDH) level in culture medium. Fifty microliters of culture supernatants were collected from each well and LDH activity was measured by a LDH assay kit (Roche, USA) according to the manufacturer’s instruction.
Cell viability assay
Cells were plated at 5 × 104/ml in 96-well plates. After 24 h, cells were treated with QUIN and the agents. After the treatment, 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, USA) was added to each well to reach a final concentration of 0.5 mg/ml. After incubation at 37 °C for 4 h, the medium was removed and 100 μl dimethyl sulfoxide was added to each well. The absorbance at 490 nm was measured with a microplate reader (Elx800, Bio-Tek instrument, USA). Results were expressed as the percentage of control. In another series, at the end of the treatment, the cells were trypsinized and resuspended in the medium mixed with 0.4 % trypan blue (1:1, Sigma-Aldrich, USA). The trypan blue negative cells were counted using a haemocytometer.
Intracellular reactive oxygen species determination
The measurement of intracellular reactive oxygen species (ROS) was based on the oxidation of 2′,7′-dichloro-dihydrofluorescein diacetate (H2DCFDA, Sigma-Aldrich, USA) to an intracellular fluorescent product. Briefly, after the treatment with QUIN for 2 h, PC12 cells grown on 96-well plates were washed with Hank’s solution and incubated with 50 μM H2DCFDA in Hank’s solution for 40 min. Thereafter, the cells were washed and the fluorescence was measured at excitation of 485 nm and emission of 530 nm on a platereader (Varioskan Flash, ThermoFisher Scientific Inc, USA).
Cell apoptosis assay
Cells grown on coverslips were washed with PBS and then stained with Hoechst 33258 at 10 mg/L for 10 min at 37 °C. thereafter, the cells were observed under a fluorescent microscope (Olympus BX41, Japan). The apoptotic cells were determined as condensed or fragmented nuclei with strong bright fluorescence. At least 10000 cells were counted in more than 4 fields in each coverslip. The apoptotic cells were expressed as percentage of total cells.
Immunocytochemistry for the detection of HIF-1α
Cells seeded on coverslips were fixed with ice-cold methanol for 5 min and incubated in 5 % normal goat serum for 1 h at room temperature. Then the cells were incubated with mouse monoclonal anti-HIF-1α antibody (1:100, Novus, USA) at 4 °C overnight. After washing with PBS, the coverslips were incubated with Alexa Fluor 488-conjugated secondary antibody (1:600, Jackson ImmunoResearch Laboratories, USA). The nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole). Finally, the labeled cells were observed with a fluorescent microscopt (BX-41, Olympus, Japan).
Quantitative PCR
Total RNA was isolated using an RNeasy Mini kit including DNase I digestion (Qiagen, USA). The reverse transcription reaction was carried out with a High Capacity cDNA Archived Kit (Applied Biosystems, USA) according to the manufacturer’s protocol. Subsequently, the real-time PCR analysis was performed with a sequence detection system (ABI Prism 7000; Applied Biosystems, USA). Amplification of specific PCR products was detected using the SYBR Green PCR Master Mix (Applied Biosystems, USA) according to the manufacturer’s protocol. The following primers were used for analysis: rat HIF-1α, 5′-CCACAGGACAGTACAGGAG-3′ and 5′-TCAAGTCGTGCTGAATAATC; rat RTP801, 5′-GCTCTGGACCCCAGTCTAGT-3′ and 5′-GGGACAGTCCTTCAGTCCTT-3′; rat cyclophilin 5′-CCCACCGTGTTCTTCGACAT-3′ and 5′-TGCAAACAGCTCGAAGCAGA-3′. The gene expression was normalized to cyclophilin.
Western blotting analysis
The cells were washed twice with ice-cold PBS and then lysed in cell lysis buffer (Cell Signaling Technology, USA) containing 1 μM phenylmethysulfonyl fluoride (Sigma-Aldrich, USA) at 4 °C. Then the homogenates were centrifuged at 10,000×g for 30 min at 4 °C. The protein samples were separated by a 10 % SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane (Millipore, USA). The blot was blocked with 5 % non-fat milk and incubated with an anti-HIF-1α antibody (1:500, Novus Biologicals, USA), an anti-RTP801 antibody (1:1000, Thermo Fisher Scientific Inc., USA), an anti-Bax antibody (1:2000, Cell Signaling Technology, USA), an anti-B-cell lymphoma 2 antibody (Bcl-2, 1:2000, Cell Signaling Technology, USA) and an anti-beta-actin antibody (β-actin, 1:2000, Cell Signaling Technology, USA) at 4 °C. Overnight primary antibody incubation was followed by incubation with a horseradish-conjugated secondary antibody (1:5000, Jackson ImmunoResearch Laboratories, USA) and enhanced chemiluminescence reagents (Pierce Biotechnolog, USA). Blots were exposed on an X-ray film. The results of protein expression are normalized to β-actin.
siRNA treatment of cells
Cells were grown to 60 % confluence in 24 well plate before transfection and 20 pmol of duplex siRNA (Santa Cruz Biotechnology Inc, USA) were diluted in 200 μl of Opti-Mem I (Invitrogen life Technologies, USA). In parallel, 2 μl of Oligofectamine (Invitrogen life Technologies, USA) were added to 200 μl of Opti-Mem I and incubated at room temperature for 10 min. Then the indicated duplex siRNA oligonucleotide solution was added to the Oligofectamine/Opti-Mem I mixture, incubated at room temperature for 20 min. After rinse with Opti-Mem I to remove any residual serum, the cells were incubated with the siRNA complexes in serum-free conditions for 4 h at 37 °C in a 5 % CO2 incubator. Serum was then added back to the medium, and cells were incubated for an additional 48 h before beginning an experiment.
Data analysis
Differences between groups were examined for statistical significance using one-way analysis of variance (ANOVA) followed by Dunnett’s Multiple Comparison. P < 0.05 denoted the presence of a statistically significant difference.
References
Agrawal M, Kumar V, Kashyap MP, Khanna VK, Randhawa GS, Pant AB (2011) Ischemic insult induced apoptotic changes in PC12 cells: protection by transresveratrol. Eur J Pharmacol 666:5–11
Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC (2007) Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci 27:6320–6332
Barger SW, Goodwin ME, Porter MM, Beggs ML (2007) Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem 101:1205–1213
Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti JF, Giorgetti-Peraldi S, Bost F (2011) Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 71:4366–4372
Braidy N, Brew BJ, Inestrosa NC, Chung R, Sachdev P, Guillemin GJ (2014) Changes in cathepsin D and beclin-1 mRNA and protein expression by the excitotoxin quinolinic acid in human astrocytes and neurons. Metab Brain Dis 29:873–883
Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG Jr (2004) Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev 18:2893–2904
Cheng YL, Park JS, Manzanero S, Choi Y, Baik SH, Okun E, Gelderblom M, Fann DY, Magnus T, Launikonis BS, Mattson MP, Sobey CG, Jo DG, Arumugam TV (2014) Evidence that collaboration between HIF-1alpha and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol Dis 62:286–295
Colin-Gonzalez AL, Orozco-Ibarra M, Chanez-Cardenas ME, Rangel-Lopez E, Santamaria A, Pedraza-Chaverri J, Barrera-Oviedo D, Maldonado PD (2013) Heme oxygenase-1 (HO-1) upregulation delays morphological and oxidative damage induced in an excitotoxic/pro-oxidant model in the rat striatum. Neuroscience 231:91–101
Colin-Gonzalez AL, Luna-Lopez A, Konigsberg M, Ali SF, Pedraza-Chaverri J, Santamaria A (2014) Early modulation of the transcription factor Nrf2 in rodent striatal slices by quinolinic acid, a toxic metabolite of the kynurenine pathway. Neuroscience 260:130–139
DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW (2008) Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev 22:239–251
Du F, Wu XM, Gong Q, He X, Ke Y (2011) Hyperthermia conditioned astrocyte-cultured medium protects neurons from ischemic injury by the up-regulation of HIF-1 alpha and the increased anti-apoptotic ability. Eur J Pharmacol 666:19–25
Duong TT, Antao S, Ellis NA, Myers SJ, Witting PK (2008) Supplementation with a synthetic polyphenol limits oxidative stress and enhances neuronal cell viability in response to hypoxia-re-oxygenation injury. Brain Res 1219:8–18
Essa MM, Braidy N, Vijayan KR, Subash S, Guillemin GJ (2013) Excitotoxicity in the pathogenesis of autism. Neurotox Res 23:393–400
Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F (2009) The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev 62:99–108
Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN, Reginato MJ (2014) O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell 54:820–831
Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM (2005) Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol 31:395–404
Helton R, Cui J, Scheel JR, Ellison JA, Ames C, Gibson C, Blouw B, Ouyang L, Dragatsis I, Zeitlin S, Johnson RS, Lipton SA, Barlow C (2005) Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci 25:4099–4107
Horak P, Crawford AR, Vadysirisack DD, Nash ZM, DeYoung MP, Sgroi D, Ellisen LW (2010) Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc Natl Acad Sci U S A 107:4675–4680
Jeong JK, Park SY (2012) Transcriptional regulation of specific protein 1 (SP1) by hypoxia-inducible factor 1 alpha (HIF-1alpha) leads to PRNP expression and neuroprotection from toxic prion peptide. Biochem Biophys Res Commun 429:93–98
Jiang H, Huang Y, Xu H, Sun Y, Han N, Li QF (2012) Hypoxia inducible factor-1alpha is involved in the neurodegeneration induced by isoflurane in the brain of neonatal rats. J Neurochem 120:453–460
Lin C, Wu CJ, Wei IH, Tsai MH, Chang NW, Yang TT, Kuo YM (2013) Chronic treadmill running protects hippocampal neurons from hypobaric hypoxia-induced apoptosis in rats. Neuroscience 231:216–224
Lopez-Hernandez B, Cena V, Posadas I (2015) The endoplasmic reticulum stress and the HIF-1 signalling pathways are involved in the neuronal damage caused by chemical hypoxia. Br J Pharmacol
Lugo-Huitron R, Ugalde Muniz P, Pineda B, Pedraza-Chaverri J, Rios C, Perez-de la Cruz V (2013) Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev 2013:104024
Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM (2007) Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 145:256–264
Malagelada C, Ryu EJ, Biswas SC, Jackson-Lewis V, Greene LA (2006) RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson’s disease by a mechanism involving mammalian target of rapamycin inactivation. J Neurosci 26:9996–10005
Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA (2010) Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci 30:1166–1175
Martin-Flores N, Romani-Aumedes J, Rue L, Canal M, Sanders P, Straccia M, Allen ND, Alberch J, Canals JM, Perez-Navarro E, Malagelada C (2015) RTP801 is involved in mutant huntingtin-induced cell death. Mol Neurobiol
Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6:337–350
Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL (2013) Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698:6–18
Meijer TW, Kaanders JH, Span PN, Bussink J (2012) Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res 18:5585–5594
Mishra J, Chaudhary T, Kumar A (2014) Rosiglitazone synergizes the neuroprotective effects of valproic acid against quinolinic acid-induced neurotoxicity in rats: targeting PPARgamma and HDAC pathways. Neurotox Res 26:130–151
Nakai M, Qin ZH, Wang Y, Chase TN (1999) Free radical scavenger OPC-14117 attenuates quinolinic acid-induced NF-kappaB activation and apoptosis in rat striatum. Brain Res Mol Brain Res 64:59–68
Noseda R, Belin S, Piguet F, Vaccari I, Scarlino S, Brambilla P, Martinelli Boneschi F, Feltri ML, Wrabetz L, Quattrini A, Feinstein E, Huganir RL, Bolino A (2013) DDIT4/REDD1/RTP801 is a novel negative regulator of Schwann cell myelination. J Neurosci 33:15295–15305
Pierozan P, Fernandes CG, Dutra MF, Pandolfo P, Ferreira F, de Lima BO, Porciuncula L, Wajner M, Pessoa-Pureur R (2014) Biochemical, histopathological and behavioral alterations caused by intrastriatal administration of quinolic acid to young rats. FEBS J 281:2061–2073
Piret JP, Mottet D, Raes M, Michiels C (2002) Is HIF-1alpha a pro- or an anti-apoptotic protein? Biochem Pharmacol 64:889–892
Rahmani M, Aust MM, Attkisson E, Williams DC Jr, Ferreira-Gonzalez A, Grant S (2013) Dual inhibition of Bcl-2 and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in human myeloid leukemia cells through a GSK3- and Bim-dependent mechanism. Cancer Res 73:1340–1351
Rayner BS, Duong TT, Myers SJ, Witting PK (2006) Protective effect of a synthetic anti-oxidant on neuronal cell apoptosis resulting from experimental hypoxia re-oxygenation injury. J Neurochem 97:211–221
Regazzetti C, Bost F, Le Marchand-Brustel Y, Tanti JF, Giorgetti-Peraldi S (2010) Insulin induces REDD1 expression through hypoxia-inducible factor 1 activation in adipocytes. J Biol Chem 285:5157–5164
Reiling JH, Hafen E (2004) The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev 18:2879–2892
Ryu JK, Choi HB, McLarnon JG (2005) Peripheral benzodiazepine receptor ligand PK11195 reduces microglial activation and neuronal death in quinolinic acid-injected rat striatum. Neurobiol Dis 20:550–561
Schwarzer R, Tondera D, Arnold W, Giese K, Klippel A, Kaufmann J (2005) REDD1 integrates hypoxia-mediated survival signaling downstream of phosphatidylinositol 3-kinase. Oncogene 24:1138–1149
Semenza GL (2000) HIF-1 and human disease: one highly involved factor. Genes Dev 14:1983–1991
Semenza GL (2010) HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20:51–56
Seo JS, Seol JW, Moon MH, Jeong JK, Lee YJ, Park SY (2010) Hypoxia protects neuronal cells from human prion protein fragment-induced apoptosis. J Neurochem 112:715–722
Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, Kalinski H, Kamer I, Rozen A, Mor O, Keshet E, Leshkowitz D, Einat P, Skaliter R, Feinstein E (2002) Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol Cell Biol 22:2283–2293
Stoy N, Mackay GM, Forrest CM, Christofides J, Egerton M, Stone TW, Darlington LG (2005) Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J Neurochem 93:611–623
Szydlowska K, Tymianski M (2010) Calcium, ischemia and excitotoxicity. Cell Calcium 47:122–129
Wu XM, Qian ZM, Zhu L, Du F, Yung WH, Gong Q, Ke Y (2011) Neuroprotective effect of ligustilide against ischaemia-reperfusion injury via up-regulation of erythropoietin and down-regulation of RTP801. Br J Pharmacol 164:332–343
Yoshida T, Mett I, Bhunia AK, Bowman J, Perez M, Zhang L, Gandjeva A, Zhen L, Chukwueke U, Mao T, Richter A, Brown E, Ashush H, Notkin N, Gelfand A, Thimmulappa RK, Rangasamy T, Sussan T, Cosgrove G, Mouded M, Shapiro SD, Petrache I, Biswal S, Feinstein E, Tuder RM (2010) Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat Med 16:767–773
Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR (2011) Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol 226:2925–2933
Zaman K, Ryu H, Hall D, O’Donovan K, Lin KI, Miller MP, Marquis JC, Baraban JM, Semenza GL, Ratan RR (1999) Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci 19:9821–9830
Acknowledgments
This research was supported by the fund of the National Natural Science Foundation of China (81300059 and 81400911), Natural Science Foundation of Jiangsu Province (bk20140573), China Postdoctoral Science Foundation (2014M560408) and Postdoctoral Science Foundation of Jiangsu Province (1402172C).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Rights and permissions
About this article
Cite this article
Huang, X., Yang, K., Zhang, Y. et al. Quinolinic acid induces cell apoptosis in PC12 cells through HIF-1-dependent RTP801 activation. Metab Brain Dis 31, 435–444 (2016). https://doi.org/10.1007/s11011-015-9782-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9782-x