Abstract
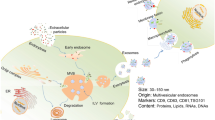
Posterior ocular disease, a disease that accounts for 55% of all ocular diseases, can contribute to permanent vision loss if left without treatment. Due to the special structure of the eye, various obstacles make it difficult for drugs to reach lesions in the posterior ocular segment. Therefore, the development of highly permeable targeted drugs and delivery systems is particularly important. Exosomes are a class of extracellular vesicles at 30–150 nm, which are secreted by various cells, tissues, and body fluids. They carry various signaling molecules, thus endowing them with certain physiological functions. In this review, we describe the ocular barriers and the biogenesis, isolation, and engineering of exosomes, as exosomes not only have pharmacological effects but also are good nanocarriers with targeted properties. Moreover, their biocompatibility and immunogenicity are better than synthetic nanocarriers. Most importantly, they may have the ability to pass through the blood–eye barrier. Thus, they may be developed as both targeted nano-drugs and nano-delivery vehicles for the treatment of posterior ocular diseases. We focus on the current status and potential application of exosomes as targeted nano-drugs and nano-delivery vehicles in posterior ocular diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The eye is one of the most precious organs of the human body, and it is a unique sensory organ in both anatomy and physiology [1]. The eye is usually divided into the anterior and posterior segments by lens interface. The anterior segment contains the cornea, conjunctiva, pupil, aqueous humor, iris, ciliary body, and lens, taking up about one-third of the eye. And the posterior segment comprises about two-thirds of the eye, including the sclera, choroid, retina, Bruch membrane, vitreous, and optic nerve [2, 3]. At least 2.2 billion people worldwide are visually impaired or blind, according to the World Health Organization. Of all ophthalmic diseases, 55% are posterior ocular diseases, which can lead to permanent vision loss if left without treatment. Posterior eye diseases mainly include age-related macular degeneration (AMD), diabetic retinopathy (DR), uveitis, diabetic macular edema (DME), cytomegalovirus retinitis (CMV), and retinitis pigmentosa (RP) [4,5,6,7]. Among them, AMD and DR are considered to be the leading causes of irreversible visual impairment. At present, the conventional treatment of the posterior ocular diseases are vitreous injection and implants [8]. Vascular endothelial growth factor (VEGF) inhibitors such as bevacizumab and ranibizumab are commonly used for the treatment of AMD by vitreous injection. The frequent vitreous injection can cause some risks such as endophthalmitis and retinal detachment, leading to poor patient compliance [9]. Implants overcome many disadvantages of intravitreal injection. However, the risk of surgical procedures and drug deposition may lead to adverse effects [10]. Topical administration can avoid the risk of vitreous injections and implants, which is the simplest and most commonly used method.
Due to the special structure of the eye, there are various barriers for the local drug delivery. Various physiological structures and tear turnover result in low drug bioavailability, poor tissue permeability, systemic side effects, and other problems. Traditional delivery systems, such as suspensions, eye drops, and ointments, are rarely regarded as very effective dosage forms for the treatment of posterior ocular diseases [11]. The development of nanotechnology makes it possible to overcome these obstacles. Due to their nanoscale size, nano-delivery systems facilitate drugs through tightly connected cells, thereby enhancing drug permeability and increasing drug bioavailability [12]. Nanomedicines can have the ability to target lesion sites through certain modification materials, which increase drug concentration in the local area [13]. In recent years, many promising nano-preparations have been developed and investigated for drug delivery to the ocular posterior segment, such as liposomes, lipid nanoparticles, polymer nanoparticles, nanoemulsions, nanomicelles, and dendrimers [4, 14]. Various synthetic nanocarriers have been extensively researched and have shown potential therapeutic effects. However, these synthetic nanomaterials can induce unanticipated immune responses and interaction with protein, which may lead to toxicity and therapeutic failure [15,16,17,18].
Exosomes, the endogenous nanocarriers, avoid the problems mentioned above. They have the potential to interoperate with receptor cells, specifically, and penetrate tissues while protecting internal cargo from disintegration [19,20,21]. Recent studies have established that exosomes are able to carry small molecules such as drugs and biomolecules within cells [22]. Compared to synthetic nanocarriers, exosomes isolated from the patient’s cells have higher biocompatibility, lower toxicity, and lower immunogenicity [23, 24]. More importantly, exosomes can cross the blood–brain barrier (BBB) [25]. Blood–eye barrier and the BBB have a similar neuroepithelial origin, microstructure, and vascular system and function [26]. The BBB and blood–eye barriers are formed by polarized endothelial cells and epithelial cells and have similar transport proteins [27, 28]. The evidence reveals its potential to penetrate the blood–eye barrier. Exosomes are vesicles of 30–150 nm [29]. A particle size of 20–200 nm is deemed to be the appropriate particle size to pass through the ocular barrier: less than 20 nm is easily cleared by the blood and lymphatic circulation, and more than 200 nm will decrease the permeability [14, 30]. It is obvious that exosomes are within this particle size range. In addition, exosomes carry certain molecules during their synthesis, making them therapeutic.
This review describes the current challenges in delivering drugs to the back of the eye based on the physiological barriers of the eye and summarizes the biogenesis, isolation, and engineering of exosomes. In addition, the review is to discuss the potential opportunities of exosomes including their therapeutic effects and as delivery vehicles in the treatment of posterior eye diseases.
Obstruction of drug administration in posterior ocular disease
Local administration mainly delivers drugs to the posterior ocular tissue through the following three pathways: (i) corneal pathway: The drugs pass through the cornea, into the aqueous humor, lens/iris, vitreous, and eventually into the retina; (ii) conjunctiva-sclera pathway: the drugs pass through the conjunctiva, sclera and then enter the choroid and retina; (iii) other pathways: The drugs penetrate to the systemic cycle by nasolacrimal drainage/corneal capillaries and then reach the retina through the blood–retinal barrier (BRB) [31, 32]. However, various physiological barriers of the eye can lead to low drug bioavailability and difficulty in reaching the lesion site. We briefly discuss the challenges of drug delivery to fundus lesion sites (Fig. 1).
Tear film barrier
The tear film is the outermost membrane of the eye, which is the first barrier. The tear film with a thickness of 3 μm consists of three layers: the outermost layer is a lipid membrane, the middle layer is composed of water and protein, and the mucin layer is at the base [33]. Therefore, the hydrophilic/lipophilic nature of the drugs affects their ability to penetrate the tear film barrier. Meanwhile, mucins are electronegative, so the electrical charges between the drug molecules and carriers determine how the drugs interact with the eye surface [34]. Tear film turnover (1–2 μL·min−1) reduces drug retention time on the corneal surface, which is the main factor limiting the local retention time [35]. Human tear volume is about 7 μL, and the volume of eye drops can reach 50 μL. Although the capsule tail can temporarily stay at 30 μL, most of the drugs through nasolacrimal drainage enter directly into the systemic circulation, resulting in low drug bioavailability [36]. Therefore, the physicochemical properties of drug molecules and their carriers, as well as their interactions with the tear, determine whether the drugs can cross the tear membrane barrier and enter the subsequent tissues.
Cornea barrier
The cornea is the second major barrier for drug penetration, which is a transparent avascular connective tissue, consisting of 5 layers, respectively, epithelium, stroma, endothelium, Bowman membrane, and Descemet membrane [37]. The surface of the cornea is lined with closely connected hydrophobic epithelial cells, which prevents hydrophilic substances such as tears and macromolecular substances from entering the intercellular space [38]. The stroma accounts for approximately 90% of the corneal thickness and is made up of collagen fibrils and glycosaminoglycans. The highly hydrated structure of the stroma is a rate-limiting barrier to the penetration of lipophilic drug molecules [39]. Thus, the drugs entry into the corneal barrier depends on their hydrophilicity/hydrophobicity and molecular weight. In addition, the drug delivery systems with both hydrophobic and hydrophilic structures are more likely to enter the eye through the corneal pathway.
Conjunctiva barrier
The conjunctiva is a thin and translucent vasiform mucus membrane that surrounds the cornea. The conjunctiva is divided into the epithelium, the basement membrane, and the lamina propria [40]. The epithelium consists of goblet cells and lamellar cells that are closely connected. It operates as a barrier for the transport of hydrophilic substances by the side of the cell. One of its main functions is to help maintain the tear film [41]. Lamina propria is a loose layer of fibrous vascular connective tissue [42]. The conjunctiva surface is larger than the cornea, its permeability is 2–30 times higher than the cornea, and its absorbable diameter is less than 300 nm [43]. Therefore, the conjunctiva absorbs more drugs than the cornea. However, the conjunctiva still absorbs very few drugs. The conjunctiva contains abundant lymphoid tissue, which is involved in the immune defense system of the ocular surface and resists the entry of drugs [40].
Len barrier
The lens is a double convex and transparent tissue, held suspended by the suspensory ligaments between the iris and vitreous chamber [44]. It is the only refractive substrate of the eyeball with accommodative capacity [45]. Hence, it is a major instrument for vision formation.
Sclera barrier
The sclera, which is composed of non-keratinized laminated squamous epithelium and goblet cells, is covered by conjunctiva. Microscopically, the sclera is made up of dense fibers of different diameters that are interlaced with each other. Collagen fibers and proteoglycans in the interfibrous matrix of the sclera allow hydrophilic substances to diffuse through the scleral tissue [46]. The sclera is highly permeable, and its penetration primarily depends on the molecular radius of the drugs rather than the lipophilicity of the drugs. Moreover, the permeability of the sclera does not significantly decrease with age [43]. The sclera has a large surface area. Few proteolytic enzymes or protein-binding sites can degrade or isolate drugs, which facilitates drug transport [47].
Choroid barrier
The choroid is a flexible layer of blood vessels adjacent to the sclera. Its main function is to provide nutrition to the retina [48]. Moreover, choroidal clearance is based on the high plasma flow and the porous leaky structures of choroidal capillaries that prevents the transport of macromolecular drugs [49, 50].
Blood–eye barrier
The blood–eye barrier consists of a blood–aqueous barrier (BAB) located in the anterior uveal membrane and a BRB located in the retro-ocular region. It has three key functions: maintaining tissue and fluid components, producing aqueous humor, and preventing pathogens from entering the eye [7]. BAB and BRB, due to their tight connections between cells, not only limit the invasion of pathogens to protect the retina but also limit the circulation of drugs from the body to the eye and from the eye to the body [51].
The BAB is composed of the compact capillary endothelium of the iris and ciliary epithelium [52]. Tight junctions act as gatekeepers for paracellular transport, restricting the selective diffusion of ions and small solutes through the spaces between adjacent cells [53]. Drug transporters in the iris and ciliary body not only reduce the penetration of drugs into the aqueous humor but participate in the elimination of aqueous humor drugs, thereby reducing the bioavailability of drugs [54].
The BRB is made up of two successive monolayer cells with unique spatial orientation and construction. The retinal pigment epithelium cells (RPEC) are the main constituent of the outer BRB (oBRB), and the retinal microvascular endothelial cells (RMEC) are the core component of the inner BRB (iBRB) [55]. They have tight intercellular connections and thus form a powerful barrier for drug penetration [56]. However, the retina has selective permeability [57]. The tight connections of cells in iBRB limit the penetration of hydrophilic drugs into the retina, whereas hydrophobic drugs penetrate more freely. And the permeability of the drugs through the oBRB decreases as the molecular radius increases [58, 59]. In general, lipophilic drugs and lower molecular weight drugs have higher permeability in BRB.
To sum up, the corneal route is the primary route of local drug delivery to the eye. The conjunctiva-sclera local administration route is short [7], so it is considered to be the main route of local administration. In addition, due to the presence of the blood–eye barrier, drugs are limited to reaching the retinal region on account of nasolacrimal drainage and capillary penetration. In order to achieve therapeutic intraocular concentrations, high doses of drugs are required. As a result, drugs are distributed and accumulated in the tissues of the body, causing side effects. Although it is difficult to treat ocular diseases with topical eye drops, it is still the safest, most convenient, and highly compliant way. Therefore, ocular preparation technology with strong permeability, good retention, and even targeted release is urgently needed.
Exosome-a lipid bilayer nanostructure
Exosomes are extracellular vesicles (EVs) of 30–150 nm and have a lipid bilayer structure, which contains many important bioactive molecules such as proteins, enzymes, and nucleic acids (mRNA, miRNA, DNA) in the interior and surface [29, 60, 61]. Some specific physiological functions such as therapeutic effects and targeting properties are given by these active molecules. Their nano-lipid bilayer structure allows them to cross ocular barriers. Therefore, exosomes have the potential as drugs and carriers in posterior ocular diseases [62]. Recently, researchers are not only limited to studying natural exosomes as drugs and carriers. In order to give exosomes more functions, engineered exosomes have aroused the great interest of researchers.
Exosome biogenesis
Exosomes are a sort of EVs, the phenomenon of sheep reticulocytes secreting nanovesicles during maturation was detected as early as the 1980s after these vesicles became known as exosomes [63, 64]. Exosome biogenesis is a successive cytological process depending on endosomes (Fig. 2). Firstly, cytoplasmic membrane endocytosis can form early endosomes. As early endosomes mature, RNA, DNA, and proteins are encapsulated in the cytoplasm to form late endosomes [65]. Then late endosomes form multivesicular bodies (MVBs), which include intraluminal vesicles (ILVs) that are created by budding inward through the membrane of late endosomes [66, 67]. The optimal mechanism for MVBs and ILVs formation is driven by the endoplasmic sorting complex required for transport (ESCRT) [68]. Subsequently, MVBs with the highest cholesterol content merge with the cytoplasmic membrane to expel ILVs out of the cell to form exosomes, which are consistent with the cholesterol-rich exosomes [69]. Another avenue for MVBs is to fuse with lysosomes and are later degraded, which is the main fate of MVBs [70, 71].
Biogenesis and secretion of exosomes. Exosome formation originates from early endosomes formed by endocytosis in the cytoplasmic membrane. The membranes of late endosomes buss inward to form ILVs and convert them into MVBs. After MVBs merge with the cytoplasmic membrane to expel ILVs out of the cell to form exosomes
Natural exosomes
Exosomes are secreted by a variety of cells, including epithelial cells, mesenchymal stem cells, dendritic cells, neurons, reticulocytes, cancer cells, B and T cells, and astrocytes, under normal or pathological conditions [72,73,74,75,76]. They are widely present in plasma [77], urine [78], milk [79], saliva [80], nasal secretion [81], amniotic fluid [82], and cerebrospinal fluid [83]. Exosomes are promising natural therapeutic agents, due to the variety of biomolecules in the synthesis process.
A variety of methods have been used to isolate exosomes. The most common method of isolation is ultracentrifugation, which is based on the difference in density and size between exosomes and impurities [84]. The methods of representative ultracentrifugation for exosomes are differential ultracentrifugation and density gradient ultracentrifugation [85]. Ultracentrifugation is able to collect a large number of exosomes [86]. Polymer-based precipitation separation is also commonly used to isolate exosomes [87]. The highly hydrophilic polymers associated with water molecules around exosomes to form a hydrophobic microenvironment, which causes the exosomes to precipitate. Both ultrafiltration and size exclusion chromatography are dependent on the size difference between exosomes and other components in the sample [88]. In addition to the strategy of isolating exosomes based on their sizes, densities, and other physical properties. There are other methods for isolating exosomes, such as immunoaffinity capture through interaction between antibodies and some specific proteins, lipids, and polysaccharides on the surface of exosomes [89]. Each method has its advantages and disadvantages (Table 1). The combined application of multiple methods has been widely studied and applied to adapt to mass production. Moreover, there are no established standards for the isolation and purification of exosomes, which is also an important research direction for researchers in the future.
Engineered exosomes
Due to the limited tissue and cell-specific targeted properties, there are many challenges when considering natural exosomes as therapeutic drugs and delivery carriers, so it is necessary to modify exosomes according to physiological requirements. At this time, engineered exosomes emerged to meet specific therapeutic purposes. Engineered exosomes adopt two modification strategies, namely cargo engineering and surface engineering (Fig. 3).
Engineering exosomes via cargo engineering and surface engineering. Cargo engineering encapsulates proteins, DNA, RNA and other therapeutic drugs into exosomes via different methods. Suface engineering modifies different targeting molecules to give exosomes stronger targeting properties by genetic engineering and chemical modification
Cargo engineering
Cargo engineering refers to the loading of therapeutic drugs into exosomes, including pre-loading and post-loading. Pre-loading is the loading of drugs before exosome separation, and post-loading is the loading of drugs after separation [90].
Pre-loading uses therapeutic drugs to treat donor cells so that the cells secrete drug-laden exosomes. It is mainly through the co-incubation with the donor cells to secrete the corresponding exosomes. This approach aims to cause the donor cells to accumulate therapeutic compounds and secrete exosomes that contain the therapeutic compounds. Due to its non-targeting property, the yield of this method is low [91]. Another approach is gene editing, which uses genes to modify parental cells so they overexpress therapeutic RNA, proteins and peptides, and then load them into exosomes [92].
Post-loading is the loading of therapeutic molecules such as DNA, RNA, proteins, and small molecules directly into exosomes. For the post-loading of exosomes, passive and active-loading techniques are usually used. Passive-loading involves the co-incubation of drugs and exosomes, with the drugs diffusing into the exosomes along a concentration gradient. This passive-loading strategy is based on the concentration gradient and the hydrophobicity of the cargos, as hydrophobic drugs may interact with the lipid bilayer membrane of the vesicles. This method usually has a low loading capacity [93]. Active-loading allows the drugs to diffuse into the exosomes by temporarily disrupting the exosomal membrane through external forces. Active-loading mainly includes ultrasound, extrusion, electroporation, freeze–thaw cycles, and permeabilization [94]. Compared to passive-loading, active-loading capacity can be increased by a multiple of approximately 11 [95].
Surface engineering
Apart from achieving specific efficacy by loading drugs, exosomes can also be engineered. Although exosomes are natural carriers, surface modifications still be easily performed. The modified strategies can be divided into two types, genetic engineering and chemical modification [93]. Genetic engineering makes proteins or peptides fuse with membrane proteins on the surface of the exosomes by co-incubation. Genetic engineering has its intrinsic drawbacks, such as the complexity of the operation and the limited range of proteins that can be applied [96]. Chemical modification refers to modifying a diversity of chemical ligands or functional molecules onto the surface of exosomes by covalent linkages or non-covalent interactions [96, 97]. In addition, other methods such as electrostatic interactions, ligand-receptor interactions, and aptamer-based surface modification were performed by anchoring CP05 peptides that have been used for surface modification of exosomes [98]. All of the methods mentioned above are used to empower exosomes with targeting functions by modifying specific molecules onto the surface of the exosomes.
As mentioned above, exosome is an endogenous substance with targetability and adequate security. Exosomes are endowed with some therapeutic effects because they carry signaling molecules during synthesis. And exosomes are good drug carriers due to their lipid bilayer structure and their ability to cross multiple physical barriers. Hence, exosomes are promising drug carriers for delivering various drugs to the posterior segment of the eye through the ocular barriers [39]. Meanwhile, exosomes themselves are easy to be modified to achieve stronger targeting.
Application of exosomes in posterior ocular disease
Many studies have demonstrated that exosomes play significant roles in the treatment of many diseases, including cancer [99], cardiovascular diseases [100, 101], neurodegenerative diseases [102], and tissue injury [103, 104]. And some studies based on exosomes to treat diseases are already in clinical trials, for example, cancer and COVID-19 pneumonia [105]. In a prospective clinical trial of dry eye disease, miR-204-containing exosomes as eye drops notably alleviate GVHD-associated dry eye disease by suppressing inflammation and improving epithelial recovery. This study suggests that exosomes as eye drops are feasible and efficacious in treating GVHD-associated dry eye disease [106]. The eye, due to its unique sensitivity and multiple barriers to postocular administration, requires a highly specific, penetrative, and non-toxic therapeutic strategy. Exosomes are a potential “cell-free” therapy that is suitable for the treatment of posterior ocular diseases due to their ability to cross barriers, migrate to the targets, and their safety is also confirmed [107]. Meanwhile, exosomes are ideal vehicles because of their higher biocompatibility and lower immunogenicity. In addition, the exosome membrane prevents the degradation of their molecular contents before arriving at the target cells [62]. In this section, we will discuss application of exosome in posterior ocular disease, including them as therapeutic agents and carriers.
Exosomes as targeted drugs to treat posterior ocular disease
Due to the variety of biological information they carry, the function of exosomes is mainly determined by the substances they carry [108]. Exosomes provide a novel perspective and potential therapeutic approach for treating posterior ocular diseases.
Exosomes for the treatment of AMD
AMD is a progressive degenerative disease of the macula, the region of the retina in charge of vision and color. It is divided into dry AMD and wet AMD, and wet AMD is the leaking AMD, which is characterized by abnormal growth of new blood vessels invading the retinal pigment epithelium (RPE) from the choroidal layer [109, 110]. In recent years, the use of exosomes to treat AMD has received more and more attention from researchers.
Hajrasouliha et al. [111] injected of RAC-Exos by extro-ocular and exosomes started to appear in the neural retina at 15 min, gradually increased after 30 min, and then became more diffuse throughout the neural retina. This injection method alleviates the side effects of intravitreal injection, such as intraocular inflammation, and the exosomes still reach the target tissue. Because exosomes have the ability to migrate to target tissues and target cells. RAC-Exos mainly targets macrophages and vascular endothelial cells, and inhibits angiogenesis by reducing the release of inflammatory and angiogenic factors [112, 113]. RAC-Exos contained known anti-angiogenic proteins, such as endothelial inhibitors and PEDF, which may be operative in the inhibition of laser-induced choroidal neovascularization (CNV) in mice. RPE-Exos do not have this ability. But subretinal injection of RPE-Exos can diminish the apoptosis of photoreceptors and inhibit the expression level of inflammatory cytokines, which can also achieve the effect of retinal protection [114]. Microglia-derived exosomes can also inhibit the expression of angiogenic factors such as VEGF and reduce retinal angiogenesis, thereby reducing visual damage [115]. During subretinal fibrosis secondary to neovascular AMD, RPE cells lose their characteristic epithelial morphology and function and then transform into myofibroblasts, which is known as epithelial-mesenchymal transformation (EMT). Another study demonstrated that the intravitreal injection of human umbilical cord MSC-derived exosomes (HUCMSC-Exos) effectively ameliorated laser-induced CNV and subretinal fibrosis via the suppression of EMT process [116]. In addition, it has been reported that intravitreal injection of MSC-derived exosomes (MSC-Exos) can ameliorate retinal laser damage by reducing damage and inhibiting apoptosis and inflammatory responses [117].
In summary, exosomes play a therapeutic role at various stages of AMD through anti-inflammatory, anti-angiogenesis, anti-apoptosis, and inhibition of fibrosis. Many studies have shown that exosomes play a similar role in the treatment of diseases such as cancer and stroke and it is proven to have good therapeutic responses [118, 119]. The effectiveness of exosomes in the treatment of AMD is supported by these evidences.
Exosomes for the treatment of DR
DR is a usual complication of diabetes mellitus and a primary reason for blindness and visual impairment in mid-aged and elderly people, affecting their quality of life severely [120]. It is characterized by progressive changes in the retinal microvasculature, leading to increased vascular permeability, pathological intraocular hyperplasia, inflammation, angiogenesis, and retinal ischemia. Consistent with AMD, anti-inflammation and anti-angiogenesis are also ways to treat DR. At the same time, DR can cause retinal neuropathy. Photoreceptor cells are a class of cells in the neural retina that play an important role in the retina [121, 122]. Recently, the research on exosomes in the treatment of DR gradually has been increasing.
The study demonstrated that vitreous injection of MSC-derived exosomes (MSC-Exos) reduced IL-B, IL-18, and caspase-1 levels after hyperglycemic stimulation. Further studies showed that miR-126 in MCS-Exos down-regulated the expression of high mobility group box 1 (HMGB1) protein and its downstream inflammatory factors [123]. Thereinto, HMGB1 is a danger-associated protein pattern receptor which can sense high glucose as a stressor and HMGB1is a key player in retinal inflammation in DR [124]. Since retinal ischemia was a common underlying mechanism in DR. Vitreous administration of MCS-Exos can reduce the severity of retinal ischemia and neovascularization, and immunogenicity is not detected when exosomes are administered to mice with normal immune function [125]. The other study revealed that injection of 293 T cell-derived exosomes (293 T-Exos) into the vitreous fluid of ischemic eyes reduced the apoptosis of retinal cells and 293 T-Exos mainly ingested by retinal neurons and ganglion cells [126]. Therefore, 293 T-Exos have the potential to treat retinal diseases. Dongyan Pan et al. also found that exosomes may exert neuroprotective effects by promoting the survival of retinal ganglion cells (RGCs) and activation of glial cells through the administration of HUCMSC-Exos in a mouse optic nerve crush model [127].
Exosomes may improve the occurrence and development of DR through anti-inflammatory, improvement of retinal ischemia and neovascularization, and protection of optic nerve. Meanwhile, exosomes did not show immunogenicity, which is a very appealing novel non-cellular therapeutic approach that warrants further exploration in ophthalmology.
Exosomes for the treatment of autoimmune uveitis
Autoimmune uveitis is a disease characterized by intraocular inflammation, which can lead to visual impairment and even blindness if not diagnosed and treated appropriately [128]. Conventional treatment is the local or systemic use of corticosteroids and immunosuppressive agents, which are highly efficacious, but can be associated with serious systemic side effects [129]. New therapeutic approaches for attenuation of autoimmune uveitis are urgently needed.
Lingling Bai et al. [130] proved that MSC-Exos efficiently alleviated experimental autoimmune uveitis (EAU), established murine model of autoimmune uveitis, indicating their potential therapeutic use in the treatment of this disease. Both clinical and histological analysis revealed that periocular injection of MSC-Exos significantly improved EAU, and protected retinal function in experimental rats. Subsequently, the proportion of Gr-1+, CD161+, CD68+, and CD4+ cells in the inflamed retina decreased. CCL2 and CCL21 chemokines are involved in chemotaxis of inflammatory cells in the injured eyes. But MSC-Exos suppressed effects of CCL2 and CCL21 chemokines. Mice treated with exosomes that contain IL-35 (i35-Exos) by retro-orbital injection showed only mild EAU compared to control mice (PBS). The results of optical coherence tomography (OCT) analysis revealed substantial accumulation of inflammatory cells in vitreous and optic nerve head of control untreated eyes compared to mice treated with i35-Exos. Meanwhile, reduction of Th17 cells in eyes of mice treated with i35-Exos but not to control mouse eyes. Th17 cells are closely related to the production of EAU [131]. In another study, circulating exosomes were isolated from the blood of rats with EAU (EAU-Exos) induced by immunization with IRBP R16 peptide. However, EAU-Exos selectively suppressed the immune response of R16-specific T cells in vitro. Afterward, naive Lewis rats were pre-inoculated with EAU-Exos and these rats were induced to recur EAU, the results showed that EAU-Exos could reduce the frequency and severity of EAU recurrence. Therefore, the use of autologous circulating exosomes as a vaccine has the potential to inhibit the recurrence of autoimmune uveitis [132]. Results obtained above strongly suggest that exosomes efficiently suppress inflammatory response in inflamed retina, should be further explored as novel therapeutic agents for the treatment of human autoimmune uveitis.
To sum up, exosomes have shown outstanding therapeutic effects as a cell-free therapy in ophthalmology and their safety and targeting ability have been affirmed. In addition to the above major retinal pathologies, exosomes have also demonstrated positive therapeutic effects in foundational studies of other retinal diseases such as DME [133]. Due to the sensitive physiology of the eye, the development of topical formulations that can be administered as eye drops without the risk of injection needs to be further explored.
Exosomes as targeted delivery vehicles to treat posterior ocular disease
There are different nanocarriers with both natural and synthetic origins that have been developed for the treatment of a wide variety of diseases. These nanocarriers present different matrix compositions, highlighting liposomes, nanoparticles, nanomicelles, or dendrimers [134]. As mentioned above, exosomes have a lipid bilayer nanostructure secreted by various cells [22, 29]. Compared with synthetic nanocarriers, exosomes have lower immunogenicity and higher biocompatibility. Exosomes represent a promising nanomedicine strategy mainly due to their ability penetrate the most difficult barriers to penetrate, including the BBB [25]. Moreover, exosomes are easily modified to achieve the desired function. They have intrinsic targeting and promising physiological features to be used as a nanocarrier for delivering therapeutic molecules to the posterior segment of the eye [135]. The therapeutic molecules including various types of nucleic acids, proteins, and small-molecule drugs can be loaded into the exosomes as cargo to treat posterior ocular diseases.
Delivery of nucleic acid
A variety of miRNAs have been shown to play important roles in inflammation regulation, angiogenesis, tissue repair and regeneration. Exosomes can transport miRNAs to target sites through barriers, where they are taken up by cells and subsequently regulate the receptor cells. The phototoxin N-methyl-Nnitrosourea (MNU) was used to establish a photoreceptor-specific injury model in mice. The study discovered mesenchymal stem cell transplantation (MSCT) counteracted photoreceptor apoptosis and alleviated retinal morphological and functional degeneration in a mouse model. Interestingly, effects of MSCT were inhibited after blockade of exosomal generation. Therefore, it is speculated that exosomes alleviate and inhibit the damage of photoreceptors. By studying the potential mechanisms of exosomes, the researchers identified that miR-21 critically maintained photoreceptor viability against MNU injury by targeting programmed cell death 4 (Pdcd4) and was transferred from MSC-derived exosomes in vivo for functional regulation [136]. Pakravan et al. [137] showed that miR-100 was enriched in bone marrow MSC-Exos and transferred by exosomes to inhibit angiogenesis by downregulating VEGF in breast cancer cells in vitro. Anti-VEGF therapy plays an important role in abnormal neovascularization of ocular posterior segment diseases such as AMD and DR. This study provides a direction for exosomes to transport miRNAs to targeted VEGF in the posterior segment of the eye to inhibit angiogenesis.
Therefore, it is possible to use exosomes to deliver nucleic acid to treat posterior ocular diseases, especially with their unique miRNA cargo. Many studies showed the role of miRNA in the function and survival of different retinal cells such as photoreceptors or Müller glias, and they are related to many diseases [138]. Here, exosome-delivered miRNA has been widely studied in the treatment of various diseases.
Delivery of proteins
Besides the use of nucleic acid in the treatment of posterior ocular diseases, exosomes can be used to deliver small proteins and peptides. These proteins and peptides with properties including anti-angiogenesis and anti-inflammatory can be explored. Xue Dong et al. [139] constructed an EXO-linked peptide (EXOKV11) to inhibit pathological retinal angiogenesis. EXOKV11 and KV11 were injected by retro-orbital method. The results showed that the signal of EXOKV11-injected group was stronger than that of KV11-injected group and signals were still detectable in the retina 12 h after injection, which demonstrated that EXOKV11 was delivered into the eye more efficiently than KV11 and the EXOKV11 had high stability. In the oxygen-induced retinopathy (OIR) mouse model, EXOKV11 showed a stronger inhibitory effect on neovascularization and endothelial cell (EC) proliferation. In the mouse OIR model, increased VEGF secretion caused by hypoxia is the major cause of neovascularization and vascular leakage. However, EXOKV11 effectually suppressed VEGF-induced vascular leakage in mouse model by retro-orbital injection. In addition, bevacizumab, a very commonly used VEGF antibody against AMD, has been found to be partially taken up by RPE cells after intravitreal injection and then re-released by exosomes to produce therapeutic effects [140]. Vitreous injection is commonly used in clinical administration of bevacizumab, but there are many side effects in this way. The above studies suggest that it is feasible to use exosomes as the carrier of bevacizumab in a less invasive way such as retro-orbital injection.
It revealed that investigators could develop exosomes encapsulating some proteins such VEGF inhibitors for ocular delivery in a less invasive way to reduce the adverse effects of vitreous injections and enhance patient compliance. Meanwhile, the development of topical non-invasive formulations that can be administered as eye drops needs to be further explored. There have been studies about intranasal delivery of protein-based drugs cross the BBB by exosomes in Parkinson’s, which offers strong implications for the various drugs to passage through the ocular barriers into the posterior ocular segment.
Delivery of chemical drugs
Due to the specific physiology of the eye, targeted delivery of small molecules to fundus lesion sites remains challenging. The ocular biocompatibility of systemically administered drugs is still poor, and topical drops are insufficient to achieve therapeutic concentrations of drugs in the posterior ocular lesion site [141]. Exosomes can not only encapsulate hydrophobic drugs but also hydrophilic drugs due to the fact that exosomes have a hydrophobic lipid membrane and a hydrophilic core [142, 143]. Curcumin interferes with the progression of AMD by inhibiting oxidative stress, inflammation, and angiogenesis [144]. The hydrophobicity of curcumin leads to its low bioavailability in vivo. However, encapsulation of exosomes improved the solubility and stability of curcumin, thus boosting its anti-inflammatory properties [145]. Surface-functionalized MSC-Exos with curcumin were able to distribute in ischemic brain tissue [146]. Owing to its poor pharmacokinetics, peroxidase has difficulty crossing the BBB, but its distribution in the brain has been observed by intranasal and injectable administration via exosomal delivery [147]. Since the BBB is the most difficult barrier to penetrate and the blood–eye barrier is similar to the BBB, so it is possible to deliver drugs into the blood–eye barrier via exosomes.
Exosomes have a great potential for drug delivery because of their good tissue targeting, good biocompatibility, and membrane permeability. Along this pathway, we can go further to explore its application in the novel topical drug delivery systems to delivery various drugs for the treatment of the posterior ocular diseases.
Conclusions
Diseases of the posterior segment of the eye, including AMD and DR, are major threats to vision. Although most of these diseases can be avoided, eye diseases are still a crucial study considering the proliferation of electronic products. The current review summarizes the various barriers of the eye and exosome generation, and more importantly, the potential applications of exosomes in the treatment of posterior ocular diseases. It highlights and summarizes the results of recent and past studies and acknowledges the great potential of exosomes for targeting the posterior segment of the eye, including therapeutic drugs and delivery vehicles. The increasing attention towards the exosomes is highly praiseworthy, considering their abilities contacted with specificity towards a lot of diseases that affect the human physiological system. As therapeutic molecules, exosomes can modulate posterior ocular diseases through the contents they carry. As carriers, exosomes are expected to be able to cross multiple barriers to reach the ocular lesion in a less invasive or even non-invasive way. And exosomes have good biocompatibility and immunogenicity. This potential can be seen in diseases of the posterior ocular segment, as well as in difficult diseases such as cancer and cardiovascular disease, where exosomes can play a huge role and have immeasurable promise.
Data availability
No data was used for the research described in the article.
References
Juliana FR, Kesse S, Boakye-Yiadom KO, Veroniaina H, Wang HH, Sun MH (2019) Promising approach in the treatment of glaucoma using nanotechnology and nanomedicine-based systems. Molecules. https://doi.org/10.3390/molecules24203805
Joseph RR, Venkatraman SS (2017) Drug delivery to the eye: what benefits do nanocarriers offer? Nanomedicine 12:683–702. https://doi.org/10.2217/nnm-2016-0379
Nayak K, Misra M (2018) A review on recent drug delivery systems for posterior segment of eye. Biomed Pharmacother 107:1564–1582. https://doi.org/10.1016/j.biopha.2018.08.138
Wang R, Gao Y, Liu AC, Zhai GX (2021) A review of nanocarrier-mediated drug delivery systems for posterior segment eye disease: challenges analysis and recent advances. J Drug Target 29:687–702. https://doi.org/10.1080/1061186x.2021.1878366
Naidorf-Rosenblatt H, Landau-Part D, Moisseiev J, Alhalel A, Huna-Baron R, Skaat A, Pilus S, Levi L, Leshno A (2022) Ocular surface temperature differences in retinal vascular diseases. Retina-J Retinal Vitreous Dis 42:152–158. https://doi.org/10.1097/IAE.0000000000003278
Abdulla D, Ali Y, Menezo V, Taylor SRJ (2022) The use of sustained release intravitreal steroid implants in non-infectious uveitis affecting the posterior segment of the eye. Ophthalmol Therapy 11:479–487. https://doi.org/10.1007/s40123-022-00456-4
Moisseiev E, Loewenstein A (2017) Drug delivery to the posterior segment of the eye. Dev Ophthalmol 58:87–101. https://doi.org/10.1159/000455276
Weng YH, Liu J, Jin SB, Guo WS, Liang XJ, Hua ZB (2017) Nanotechnology-based strategies for treatment of ocular disease. Acta Pharma Sinica B 7:281–291. https://doi.org/10.1016/j.apsb.2016.09.001
del Amo EM, Rimpela AK, Heikkinen E, Kari OK, Ramsay E, Lajunen T, Schmitt M, Pelkonen L, Bhattacharya M, Richardson D, Subrizi A, Turunen T, Reinisalo M, Itkonen J, Toropainen E, Casteleijn M, Kidron H, Antopolsky M, Vellonen KS, Ruponen M, Urtti A (2017) Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res 57:134–185. https://doi.org/10.1016/j.preteyeres.2016.12.001
Yasin MN, Svirskis D, Seyfoddin A, Rupenthal ID (2014) Implants for drug delivery to the posterior segment of the eye: a focus on stimuli-responsive and tunable release systems. J Control Release 196:208–221. https://doi.org/10.1016/j.jconrel.2014.09.030
Qamar Z, Qizilbash FF, Iqubal MK, Ali A, Narang JK, Ali J, Baboota S (2019) Nano-based drug delivery system: recent strategies for the treatment of ocular disease and future perspective. Recent Pat Drug Deliv Formul 13:246–254. https://doi.org/10.2174/1872211314666191224115211
Srinivasarao DA, Lohiya G, Katti DS (2019) Fundamentals, challenges, and nanomedicine-based solutions for ocular diseases. Wiley Interdiscip Rev-Nanomed Nanobiotechnol. https://doi.org/10.1002/wnan.1548
Xu TT, Xu XY, Gu Y, Fang L, Cao F (2018) Functional intercalated nanocomposites with chitosan-glutathione-glycylsarcosine and layered double hydroxides for topical ocular drug delivery. Int J Nanomed 13:917–937. https://doi.org/10.2147/ijn.S148104
Bonilla L, Espina M, Severino P, Cano A, Ettcheto M, Camins A, Garcia ML, Souto EB, Sanchez-Lopez E (2022) Lipid nanoparticles for the posterior eye segment. Pharmaceutics. https://doi.org/10.3390/pharmaceutics14010090
Vashisht M, Rani P, Onteru SK, Singh D (2017) Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in vitro. Appl Biochem Biotechnol 183:993–1007. https://doi.org/10.1007/s12010-017-2478-4
van den Boorn JG, Schlee M, Coch C, Hartmann G (2011) SiRNA delivery with exosome nanoparticles. Nat Biotechnol 29:325–326. https://doi.org/10.1038/nbt.1830
Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA (2013) Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol 8:137–143. https://doi.org/10.1038/nnano.2012.237
Stewart JM, Keselowsky BG (2017) Combinatorial drug delivery approaches for immunomodulation. Adv Drug Deliv Rev 114:161–174. https://doi.org/10.1016/j.addr.2017.05.013
Liao W, Du Y, Zhang CH, Pan FW, Yao Y, Zhang T, Peng Q (2019) Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater 86:1–14. https://doi.org/10.1016/j.actbio.2018.12.045
Record M, Subra C, Silvente-Poirot S, Poirot M (2011) Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol 81:1171–1182. https://doi.org/10.1016/j.bcp.2011.02.011
Yu B, Li XR, Zhang XM (2020) Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World J Stem Cells 12:178–187. https://doi.org/10.4252/wjsc.v12.i3.178
Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Djonov V, Arsenijevic N, Volarevic V (2018) Therapeutic potential of mesenchymal stem cell-derived exosomes in the treatment of eye diseases. Adv Exp Med Biol 1089:47–57. https://doi.org/10.1007/5584_2018_219
Zhu XH, Badawi M, Pomeroy S, Sutaria DS, Xie ZL, Baek A, Jiang JM, Elgamal OA, Mo XK, La Perle K, Chalmers J, Schmittgen TD, Phelps MA (2017) Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracellular Vesicles. https://doi.org/10.1080/20013078.2017.1324730
Quah BJC, O’Neill HC (2005) The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol Dis 35:94–110. https://doi.org/10.1016/j.bcmd.2005.05.002
Zheng MN, Huang M, Ma XY, Chen HZ, Gao XL (2019) Harnessing exosomes for the development of brain drug delivery systems. Bioconjug Chem 30:994–1005. https://doi.org/10.1021/acs.bioconjchem.9b00085
Manfré L, Midiri M, Giuffré G, Mangiameli A, Cardella G, Ponte F, De Maria M, Lagalla R (1997) Blood-ocular barrier damage: use of contrast-enhanced MRI. Eur Radiol 7:110–114. https://doi.org/10.1007/s003300050121
Desalvo MK, Mayer N, Mayer F, Bainton RJ (2011) Physiologic and anatomic characterization of the brain surface glia barrier of drosophila. Glia 59:1322–1340. https://doi.org/10.1002/glia.21147
Cattelotte J, Andre P, Ouellet M, Bourasset F, Scherrmann JM, Cisternino S (2008) In situ mouse carotid perfusion model: glucose and cholesterol transport in the eye and brain. J Cereb Blood Flow Metab 28:1449–1459. https://doi.org/10.1038/jcbfm.2008.34
Huang CJ, Quinn D, Sadovsky Y, Suresh S, Hsia KJ (2017) Formation and size distribution of self-assembled vesicles. Proc Natl Acad Sci USA 114:2910–2915. https://doi.org/10.1073/pnas.1702065114
Amrite AC, Edelhauser HF, Singh SR, Kompella UB (2008) Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis 14:150–160
Tsai CH, Wang PY, Lin IC, Huang H, Liu GS, Tseng CL (2018) Ocular drug delivery: role of degradable polymeric nanocarriers for ophthalmic application. Int J Mol Sci. https://doi.org/10.3390/ijms19092830
Boddu SH, Gupta H, Patel S (2014) Drug delivery to the back of the eye following topical administration: an update on research and patenting activity. Recent Pat Drug Deliv Formul 8:27–36. https://doi.org/10.2174/1872211308666140130093301
Glasgow BJ (2020) Evidence for phospholipids on the surface of human tears. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.61.14.19
Fernandes AR, Sanchez-Lopez E, Santini A, Santos Td, Garcia ML, Silva AM, Souto EB (2021) Mono- and dicationic DABCO/quinuclidine composed nanomaterials for the loading of steroidal drug: 32 factorial design and physicochemical characterization. Nanomaterials 11:2758. https://doi.org/10.3390/nano11102758
Ruponen M, Urtti A (2015) Undefined role of mucus as a barrier in ocular drug delivery. Eur J Pharm Biopharm 96:442–446. https://doi.org/10.1016/j.ejpb.2015.02.032
Awwad S, Ahmed A, Sharma G, Heng JS, Khaw PT, Brocchini S, Lockwood A (2017) Principles of pharmacology in the eye. Br J Pharmacol 174:4205–4223. https://doi.org/10.1111/bph.14024
DelMonte DW, Kim T (2011) Anatomy and physiology of the cornea. J Cataract Refract Surg 37:588–598. https://doi.org/10.1016/j.jcrs.2010.12.037
Sridhar MS (2018) Anatomy of cornea and ocular surface. Indian J Ophthalmol 66:190–194. https://doi.org/10.4103/ijo.IJO_646_17
Zhang YQ, Zhang WJ, Liu W, Hu XJ, Zhou GD, Cui L, Cao Y (2008) Tissue engineering of corneal stromal layer with dermal fibroblasts: phenotypic and functional switch of differentiated cells in cornea. Tissue Eng Part A 14:295–303. https://doi.org/10.1089/tea.2007.0200
Makuloluwa AK, Hamill KJ, Rauz S, Bosworth L, Haneef A, Romano V, Williams RL, Dartt DA, Kaye SB (2021) The conjunctival extracellular matrix, related disorders and development of substrates for conjunctival restoration. Ocul Surf. https://doi.org/10.1016/j.jtos.2021.05.011
Gipson IK (2016) Goblet cells of the conjunctiva: a review of recent findings. Prog Retin Eye Res 54:49–63. https://doi.org/10.1016/j.preteyeres.2016.04.005
Forrester JV, Dick AD, McMenamin PG, Roberts F, Pearlman E (2016) The eye: basic sciences in practice, 4th edn. Elsevier
Wang YY, Xu XY, Gu Y, Cheng YJ, Cao F (2018) Recent advance of nanoparticle-based topical drug delivery to the posterior segment of the eye. Expert Opin Drug Deliv 15:687–701. https://doi.org/10.1080/17425247.2018.1496080
Chen W, Tan X, Chen X (2017) Anatomy and physiology of the crystalline lens. In: Liu Y (ed) Pediatric lens diseases. Springer, Singapore, pp 21–28
Augusteyn RC (2010) On the growth and internal structure of the human lens. Exp Eye Res 90:643–654. https://doi.org/10.1016/j.exer.2010.01.013
Atta G, Tempfer H, Kaser-Eichberger A, Guo YW, Schroedl F, Traweger A, Heindl LM (2020) The lymphangiogenic and hemangiogenic privilege of the human sclera. Ann Anatomy-Anatomischer Anzeiger. https://doi.org/10.1016/j.aanat.2020.151485
Mahaling B, Katti DS (2016) Physicochemical properties of core-shell type nanoparticles govern their spatiotemporal biodistribution in the eye. Nanomed-Nanotechnol Biol Med 12:2149–2160. https://doi.org/10.1016/j.nano.2016.05.017
Hancock SE, Wan CR, Fisher NE, Andino RV, Ciulla TA (2021) Biomechanics of suprachoroidal drug delivery: from benchtop to clinical investigation in ocular therapies. Expert Opin Drug Deliv 18:777–788. https://doi.org/10.1080/17425247.2021.1867532
Thrimawithana TR, Young S, Bunt CR, Green C, Alany RG (2011) Drug delivery to the posterior segment of the eye. Drug Discov Today 16:270–277. https://doi.org/10.1016/j.drudis.2010.12.004
Kim SH, Lutz RJ, Wang NS, Robinson MR (2007) Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res 39:244–254. https://doi.org/10.1159/000108117
Velagaleti PR, Buonarati MH (2014) Challenges and strategies in drug residue measurement (bioanalysis) of ocular tissues. Methods Pharmacol Toxicol. https://doi.org/10.1007/7653_2013_6
Grassiri B, Zambito Y, Bernkop-Schnurch A (2021) Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv Colloid Interface Sci. https://doi.org/10.1016/j.cis.2020.102342
Coca-Prados M (2014) The blood-aqueous barrier in health and disease. J Glaucoma 23:S36–S38. https://doi.org/10.1097/ijg.0000000000000107
Lee J, Pelis RM (2016) drug transport by the blood-aqueous humor barrier of the eye. Drug Metab Dispos 44:1675–1681. https://doi.org/10.1124/dmd.116.069369
Yemanyi F, Bora K, Blomfield AK, Wang Z, Chen J (2021) Wnt signaling in inner blood-retinal barrier maintenance. Int J Mol Sci. https://doi.org/10.3390/ijms222111877
Spaide RF, Klancnik JM Jr, Cooney MJ (2015) Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 133:45–50. https://doi.org/10.1001/jamaophthalmol.2014.3616
Pitkänen L, Ranta VP, Moilanen H, Urtti A (2005) Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest Ophthalmol Vis Sci 46:641–646. https://doi.org/10.1167/iovs.04-1051
Xu HZ, Le YZ (2011) Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci 52:2160–2164. https://doi.org/10.1167/iovs.10-6518
Díaz-Coránguez M, Ramos C, Antonetti DA (2017) The inner blood-retinal barrier: cellular basis and development. Vision Res 139:123–137. https://doi.org/10.1016/j.visres.2017.05.009
Harrell CR, Simovic Markovic B, Fellabaum C, Arsenijevic A, Djonov V, Arsenijevic N, Volarevic V (2018) Therapeutic potential of mesenchymal stem cell-derived exosomes in the treatment of eye diseases. Adv Exp Med Biol 1089:47–57. https://doi.org/10.1007/5584_2018_219
Gurung S, Perocheau D, Touramanidou L, Baruteau J (2021) The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal 19:47. https://doi.org/10.1186/s12964-021-00730-1
Mathieu M, Martin-Jaular L, Lavieu G, Théry C (2019) Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21:9–17. https://doi.org/10.1038/s41556-018-0250-9
Harding C, Heuser J, Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97:329–339. https://doi.org/10.1083/jcb.97.2.329
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation: association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262:9412–9420. https://doi.org/10.1016/S0021-9258(18)48095-7
Lin H, Chen H, Zhao X, Ding T, Wang Y, Chen Z, Tian Y, Zhang P, Shen Y (2022) Advances of exosomes in periodontitis treatment. J Transl Med 20:279. https://doi.org/10.1186/s12967-022-03487-4
Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH (2019) Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. https://doi.org/10.3390/cells8040307
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science. https://doi.org/10.1126/science.aau6977
Hanson PI, Cashikar A (2012) Multivesicular body morphogenesis. Annu Rev Cell Dev Biol 28:337–362. https://doi.org/10.1146/annurev-cellbio-092910-154152
Skotland T, Sandvig K, Llorente A (2017) Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res 66:30–41. https://doi.org/10.1016/j.plipres.2017.03.001
Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9:19. https://doi.org/10.1186/s13578-019-0282-2
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326
Nasser MI, Masood M, Adlat S, Gang D, Zhu S, Li G, Li N, Chen J, Zhu P (2021) Mesenchymal stem cell-derived exosome microRNA as therapy for cardiac ischemic injury. Biomed Pharmacother 143:112118. https://doi.org/10.1016/j.biopha.2021.112118
Yang Y, Yuan L, Du X, Zhou K, Qin L, Wang L, Yang M, Wu M, Zheng Z, Xiang Y, Qu X, Liu H, Qin X, Liu C (2021) Involvement of epithelia-derived exosomes in chronic respiratory diseases. Biomed Pharmacother 143:112189. https://doi.org/10.1016/j.biopha.2021.112189
Ma D, Zhang QY, Rong HM, Zhai K, Tong ZH (2022) Proteomic profiling and functional analysis of B cell-derived exosomes upon pneumocystis infection. J Immunol Res 2022:5187166. https://doi.org/10.1155/2022/5187166
Chen SW, Zhu SQ, Pei X, Qiu BQ, Xiong D, Long X, Lin K, Lu F, Xu JJ, Wu YB (2021) Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol Cancer 20:144. https://doi.org/10.1186/s12943-021-01448-x
Wu X, Xu X, Xiang Y, Fan D, An Q, Yue G, Jin Z, Ding J, Hu Y, Du Q, Xu J, Xie R (2022) Exosome-mediated effects and applications in inflammatory diseases of the digestive system. Eur J Med Res 27:163. https://doi.org/10.1186/s40001-022-00792-y
Zhang X, Xu Y, Ma L, Yu K, Niu Y, Xu X, Shi Y, Guo S, Xue X, Wang Y, Qiu S, Cui J, Wang H, Tian X, Miao Y, Meng F, Qiao Y, Yu Y, Wang J (2022) Essential roles of exosome and circRNA_101093 on ferroptosis desensitization in lung adenocarcinoma. Cancer Commun 42:287–313. https://doi.org/10.1002/cac2.12275
Wang L, Wang J, Wang Z, Zhou J, Zhang Y (2021) Higher urine exosomal miR-193a is associated with a higher probability of primary focal segmental glomerulosclerosis and an increased risk of poor prognosis among children with nephrotic syndrome. Front Cell Dev Biol 9:727370. https://doi.org/10.3389/fcell.2021.727370
Bae IS, Kim SH (2021) Milk exosome-derived microRNA-2478 suppresses melanogenesis through the Akt-GSK3β pathway. Cells. https://doi.org/10.3390/cells10112848
Hofmann L, Abou Kors T, Ezić J, Niesler B, Röth R, Ludwig S, Laban S, Schuler PJ, Hoffmann TK, Brunner C, Medyany V, Theodoraki MN (2022) Comparison of plasma- and saliva-derived exosomal miRNA profiles reveals diagnostic potential in head and neck cancer. Front Cell Dev Biol 10:971596. https://doi.org/10.3389/fcell.2022.971596
Crenshaw BJ, Sims B, Matthews QL (2018) Biological function of exosomes as diagnostic markers and therapeutic delivery vehicles in carcinogenesis and infectious diseases. Nanomedicines. https://doi.org/10.5772/intechopen.80225
Yang H, Yang S, Shen H, Wu S, Ruan J, Lyu G (2021) Construction of the amniotic fluid-derived exosomal ceRNA network associated with ventricular septal defect. Genomics 113:4293–4302. https://doi.org/10.1016/j.ygeno.2021.11.003
Thakur A, Parra DC, Motallebnejad P, Brocchi M, Chen HJ (2022) Exosomes: small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact Mater 10:281–294. https://doi.org/10.1016/j.bioactmat.2021.08.029
Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, Du L (2021) Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol 9:811971. https://doi.org/10.3389/fbioe.2021.811971
Lin S, Yu Z, Chen D, Wang Z, Miao J, Li Q, Zhang D, Song J, Cui D (2020) Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small 16:e1903916. https://doi.org/10.1002/smll.201903916
Zhang MD, Jin K, Gao L, Zhang ZK, Li F, Zhou FF, Zhang L (2018) Methods and technologies for exosome isolation and characterization. Small Methods. https://doi.org/10.1002/smtd.201800021
Cao F, Gao Y, Chu Q, Wu Q, Zhao L, Lan T, Zhao L (2019) Proteomics comparison of exosomes from serum and plasma between ultracentrifugation and polymer-based precipitation kit methods. Electrophoresis 40:3092–3098. https://doi.org/10.1002/elps.201900295
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P (2020) Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed 15:6917–6934. https://doi.org/10.2147/ijn.S264498
Liu WZ, Ma ZJ, Kang XW (2022) Current status and outlook of advances in exosome isolation. Anal Bioanal Chem 414:7123–7141. https://doi.org/10.1007/s00216-022-04253-7
Wang J, Chen D, Ho EA (2021) Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release 329:894–906. https://doi.org/10.1016/j.jconrel.2020.10.020
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D (2017) Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 38:754–763. https://doi.org/10.1038/aps.2017.12
Weng Z, Zhang B, Wu C, Yu F, Han B, Li B, Li L (2021) Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol 14:136. https://doi.org/10.1186/s13045-021-01141-y
Liang Y, Duan L, Lu J, Xia J (2021) Engineering exosomes for targeted drug delivery. Theranostics 11:3183–3195. https://doi.org/10.7150/thno.52570
Butreddy A, Kommineni N, Dudhipala N (2021) Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: insights from drug delivery to clinical perspectives. Nanomaterials. https://doi.org/10.3390/nano11061481
Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM (2015) Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 205:35–44. https://doi.org/10.1016/j.jconrel.2014.11.029
Lin Y, Lu Y, Li X (2020) Biological characteristics of exosomes and genetically engineered exosomes for the targeted delivery of therapeutic agents. J Drug Target 28:129–141. https://doi.org/10.1080/1061186x.2019.1641508
Kučuk N, Primožič M, Knez Ž, Leitgeb M (2021) Exosomes engineering and their roles as therapy delivery tools, therapeutic targets, and biomarkers. Int J Mol Sci. https://doi.org/10.3390/ijms22179543
Salunkhe S, Dheeraj BM, Chitkara D, Mittal A (2020) Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J Control Release 326:599–614. https://doi.org/10.1016/j.jconrel.2020.07.042
Paskeh MDA, Entezari M, Mirzaei S, Zabolian A, Saleki H, Naghdi MJ, Sabet S, Khoshbakht MA, Hashemi M, Hushmandi K, Sethi G, Zarrabi A, Kumar AP, Tan SC, Papadakis M, Alexiou A, Islam MA, Mostafavi E, Ashrafizadeh M (2022) Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol 15:83. https://doi.org/10.1186/s13045-022-01305-4
Han C, Yang J, Sun J, Qin G (2022) Extracellular vesicles in cardiovascular disease: biological functions and therapeutic implications. Pharmacol Ther 233:108025. https://doi.org/10.1016/j.pharmthera.2021.108025
Sun J, Shen H, Shao L, Teng X, Chen Y, Liu X, Yang Z, Shen Z (2020) HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther 11:373. https://doi.org/10.1186/s13287-020-01881-7
Cano A, Muñoz-Morales Á, Sánchez-López E, Ettcheto M, Souto EB, Camins A, Boada M, Ruíz A (2023) Exosomes-based nanomedicine for neurodegenerative diseases: current insights and future challenges. Pharmaceutics. https://doi.org/10.3390/pharmaceutics15010298
Fan L, Liu C, Chen X, Zheng L, Zou Y, Wen H, Guan P, Lu F, Luo Y, Tan G, Yu P, Chen D, Deng C, Sun Y, Zhou L, Ning C (2022) Exosomes-loaded electroconductive hydrogel synergistically promotes tissue repair after spinal cord injury via immunoregulation and enhancement of myelinated axon growth. Adv Sci 9:e2105586. https://doi.org/10.1002/advs.202105586
Song Y, Wang B, Zhu X, Hu J, Sun J, Xuan J, Ge Z (2021) Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol 37:51–64. https://doi.org/10.1007/s10565-020-09530-8
Santos P, Almeida F (2021) Exosome-based vaccines: history, current state, and clinical trials. Front Immunol 12:711565. https://doi.org/10.3389/fimmu.2021.711565
Zhou T, He C, Lai P, Yang Z, Liu Y, Xu H, Lin X, Ni B, Ju R, Yi W, Liang L, Pei D, Egwuagu CE, Liu X (2022) miR-204-containing exosomes ameliorate GVHD-associated dry eye disease. Sci Adv 8:eabj9617. https://doi.org/10.1126/sciadv.abj9617
Mathew B, Torres LA, Gamboa Acha L, Tran S, Liu A, Patel R, Chennakesavalu M, Aneesh A, Huang CC, Feinstein DL, Mehraeen S, Ravindran S, Roth S (2021) Uptake and distribution of administered bone marrow mesenchymal stem cell extracellular vesicles in retina. Cells. https://doi.org/10.3390/cells10040730
Zhang F, Guo J, Zhang Z, Qian Y, Wang G, Duan M, Zhao H, Yang Z, Jiang X (2022) Mesenchymal stem cell-derived exosome: a tumor regulator and carrier for targeted tumor therapy. Cancer Lett 526:29–40. https://doi.org/10.1016/j.canlet.2021.11.015
van Lookeren CM, LeCouter J, Yaspan BL, Ye W (2014) Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol 232:151–164. https://doi.org/10.1002/path.4266
Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K (2016) Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci 73:1765–1786. https://doi.org/10.1007/s00018-016-2147-8
Hajrasouliha AR, Jiang G, Lu Q, Lu H, Kaplan HJ, Zhang HG, Shao H (2013) Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J Biol Chem 288:28058–28067. https://doi.org/10.1074/jbc.M113.470765
Liu Z, Mao X, Yang Q, Zhang X, Xu J, Ma Q, Zhou Y, Da Q, Cai Y, Sopeyin A, Dong Z, Hong M, Caldwell RB, Sodhi A, Huo Y (2022) Suppression of myeloid PFKFB3-driven glycolysis protects mice from choroidal neovascularization. Br J Pharmacol 179:5109–5131. https://doi.org/10.1111/bph.15925
Rohlenova K, Goveia J, García-Caballero M, Subramanian A, Kalucka J, Treps L, Falkenberg KD, de Rooij L, Zheng Y, Lin L, Sokol L, Teuwen LA, Geldhof V, Taverna F, Pircher A, Conradi LC, Khan S, Stegen S, Panovska D, De Smet F, Staal FJT, McLaughlin RJ, Vinckier S, Van Bergen T, Ectors N, De Haes P, Wang J, Bolund L, Schoonjans L, Karakach TK, Yang H, Carmeliet G, Liu Y, Thienpont B, Dewerchin M, Eelen G, Li X, Luo Y, Carmeliet P (2020) Single-cell RNA sequencing maps endothelial metabolic plasticity in pathological angiogenesis. Cell Metab 31:862-877.e14. https://doi.org/10.1016/j.cmet.2020.03.009
Wang Y, Zhang Q, Yang G, Wei Y, Li M, Du E, Li H, Song Z, Tao Y (2021) RPE-derived exosomes rescue the photoreceptors during retina degeneration: an intraocular approach to deliver exosomes into the subretinal space. Drug Deliv 28:218–228. https://doi.org/10.1080/10717544.2020.1870584
Xu W, Wu Y, Hu Z, Sun L, Dou G, Zhang Z, Wang H, Guo C, Wang Y (2019) Exosomes from microglia attenuate photoreceptor injury and neovascularization in an animal model of retinopathy of prematurity. Mol Ther Nucleic Acids 16:778–790. https://doi.org/10.1016/j.omtn.2019.04.029
Li D, Zhang J, Liu Z, Gong Y, Zheng Z (2021) Human umbilical cord mesenchymal stem cell-derived exosomal miR-27b attenuates subretinal fibrosis via suppressing epithelial-mesenchymal transition by targeting HOXC6. Stem Cell Res Ther 12:24. https://doi.org/10.1186/s13287-020-02064-0
Yu B, Shao H, Su C, Jiang Y, Chen X, Bai L, Zhang Y, Li Q, Zhang X, Li X (2016) Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci Rep 6:34562. https://doi.org/10.1038/srep34562
Cun Y, Jin Y, Wu D, Zhou L, Zhang C, Zhang S, Yang X, Zuhong W, Zhang P (2022) Exosome in crosstalk between inflammation and angiogenesis: a potential therapeutic strategy for stroke. Mediators Inflamm 2022:7006281. https://doi.org/10.1155/2022/7006281
Ansari MA, Thiruvengadam M, Venkidasamy B, Alomary MN, Salawi A, Chung IM, Shariati MA, Rebezov M (2022) Exosome-based nanomedicine for cancer treatment by targeting inflammatory pathways: current status and future perspectives. Semin Cancer Biol 86:678–696. https://doi.org/10.1016/j.semcancer.2022.04.005
Li Q, Pang L, Yang W, Liu X, Su G, Dong Y (2018) Long non-coding RNA of myocardial infarction associated transcript (LncRNA-MIAT) promotes diabetic retinopathy by upregulating transforming growth factor-β1 (TGF-β1) signaling. Med Sci Monit 24:9497–9503. https://doi.org/10.12659/msm.911787
Li EH, Huang QZ, Li GC, Xiang ZY, Zhang X (2017) Effects of miRNA-200b on the development of diabetic retinopathy by targeting VEGFA gene. Biosci Rep. https://doi.org/10.1042/bsr20160572
Pardue MT, Allen RS (2018) Neuroprotective strategies for retinal disease. Prog Retin Eye Res 65:50–76. https://doi.org/10.1016/j.preteyeres.2018.02.002
Zhang W, Wang Y, Kong Y (2019) Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Invest Ophthalmol Vis Sci 60:294–303. https://doi.org/10.1167/iovs.18-25617
Steinle JJ (2020) Role of HMGB1 signaling in the inflammatory process in diabetic retinopathy. Cell Signal 73:109687. https://doi.org/10.1016/j.cellsig.2020.109687
Moisseiev E, Anderson JD, Oltjen S, Goswami M, Zawadzki RJ, Nolta JA, Park SS (2017) Protective effect of intravitreal administration of exosomes derived from mesenchymal stem cells on retinal ischemia. Curr Eye Res 42:1358–1367. https://doi.org/10.1080/02713683.2017.1319491
Yan B, Gao L, Huang Y, Wang X, Lang X, Yan F, Meng B, Sun X, Li G, Wang Y (2020) Exosomes derived from BDNF-expressing 293T attenuate ischemic retinal injury in vitro and in vivo. Aging. https://doi.org/10.18632/aging.202245
Pan D, Chang X, Xu M, Zhang M, Zhang S, Wang Y, Luo X, Xu J, Yang X, Sun X (2019) UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J Chem Neuroanat 96:134–139. https://doi.org/10.1016/j.jchemneu.2019.01.006
Prete M, Dammacco R, Fatone MC, Racanelli V (2016) Autoimmune uveitis: clinical, pathogenetic, and therapeutic features. Clin Exp Med 16:125–136. https://doi.org/10.1007/s10238-015-0345-6
Servat JJ, Mears KA, Black EH, Huang JJ (2012) Biological agents for the treatment of uveitis. Expert Opin Biol Ther 12:311–328. https://doi.org/10.1517/14712598.2012.658366
Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, Yu B, Chen X, Li X, Zhang X (2017) Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep 7:4323. https://doi.org/10.1038/s41598-017-04559-y
Kang M, Choi JK, Jittayasothorn Y, Egwuagu CE (2020) Interleukin 35-producing exosomes suppress neuroinflammation and autoimmune uveitis. Front Immunol 11:1051. https://doi.org/10.3389/fimmu.2020.01051
Jiang G, Yun J, Kaplan HJ, Zhao Y, Sun D, Shao H (2021) Vaccination with circulating exosomes in autoimmune uveitis prevents recurrent intraocular inflammation. Clin Exp Ophthalmol 49:1069–1077. https://doi.org/10.1111/ceo.13990
Jiang L, Cao H, Deng T, Yang M, Meng T, Yang H, Luo X (2021) Serum exosomal miR-377-3p inhibits retinal pigment epithelium proliferation and offers a biomarker for diabetic macular edema. J Int Med Res 49:3000605211002975. https://doi.org/10.1177/03000605211002975
Riau AK, Ong HS, Yam GHF, Mehta JS (2019) Sustained delivery system for stem cell-derived exosomes. Front Pharmacol 10:1368. https://doi.org/10.3389/fphar.2019.01368
Chaput N, Théry C (2011) Exosomes: immune properties and potential clinical implementations. Semin Immunopathol 33:419–440. https://doi.org/10.1007/s00281-010-0233-9
Deng CL, Hu CB, Ling ST, Zhao N, Bao LH, Zhou F, Xiong YC, Chen T, Sui BD, Yu XR, Hu CH (2021) Photoreceptor protection by mesenchymal stem cell transplantation identifies exosomal MiR-21 as a therapeutic for retinal degeneration. Cell Death Differ 28:1041–1061. https://doi.org/10.1038/s41418-020-00636-4
Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N, Javan M (2017) MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr) 40:457–470. https://doi.org/10.1007/s13402-017-0335-7
Rajool Dezfuly A, Safaee A, Salehi H (2021) Therapeutic effects of mesenchymal stem cells-derived extracellular vesicles’ miRNAs on retinal regeneration: a review. Stem Cell Res Ther 12:530. https://doi.org/10.1186/s13287-021-02588-z
Dong X, Lei Y, Yu Z, Wang T, Liu Y, Han G, Zhang X, Li Y, Song Y, Xu H, Du M, Yin H, Wang X, Yan H (2021) Exosome-mediated delivery of an anti-angiogenic peptide inhibits pathological retinal angiogenesis. Theranostics 11:5107–5126. https://doi.org/10.7150/thno.54755
Aboul Naga SH, Dithmer M, Chitadze G, Kabelitz D, Lucius R, Roider J, Klettner A (2015) Intracellular pathways following uptake of bevacizumab in RPE cells. Exp Eye Res 131:29–41. https://doi.org/10.1016/j.exer.2014.12.010
Radhakrishnan K, Sonali N, Moreno M, Nirmal J, Fernandez AA, Venkatraman S, Agrawal R (2017) Protein delivery to the back of the eye: barriers, carriers and stability of anti-VEGF proteins. Drug Discov Today 22:416–423. https://doi.org/10.1016/j.drudis.2016.10.015
Sanghani A, Andriesei P, Kafetzis KN, Tagalakis AD, Yu-Wai-Man C (2022) Advances in exosome therapies in ophthalmology-from bench to clinical trial. Acta Ophthalmol 100:243–252. https://doi.org/10.1111/aos.14932
Hood JL (2016) Post isolation modification of exosomes for nanomedicine applications. Nanomedicine 11:1745–1756. https://doi.org/10.2217/nnm-2016-0102
Vallée A (2022) Curcumin and Wnt/β-catenin signaling in exudative age-related macular degeneration (review). Int J Mol Med. https://doi.org/10.3892/ijmm.2022.5135
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18:1606–1614. https://doi.org/10.1038/mt.2010.105
Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J (2018) Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150:137–149. https://doi.org/10.1016/j.biomaterials.2017.10.012
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release 207:18–30. https://doi.org/10.1016/j.jconrel.2015.03.033
Kimiz-Gebologlu I, Oncel SS (2022) Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release 347:533–543. https://doi.org/10.1016/j.jconrel.2022.05.027
Acknowledgements
We thank our many colleagues who have contributed to our studies on this research.
Funding
This project was funded by National Natural Science Foundation of China (82074276), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202002), Science and Technology Program of Tianjin (22ZYJDSS00100), Innovation Research Project of Chinese People's Armed Police Force (ZZKY20222419), and Program for Science and Technology of Logistics University of Chinese People’s Armed Police Force (WHJ202301).
Author information
Authors and Affiliations
Contributions
XP: Conceptualization, Design, Writing—original draft preparation & editing. TZ: Writing-editing. RL and XJ: Writing-review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
No animals was used for the research described in the article.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, X., Zhang, T., Liu, R. et al. Potential in exosome-based targeted nano-drugs and delivery vehicles for posterior ocular disease treatment: from barriers to therapeutic application. Mol Cell Biochem 479, 1319–1333 (2024). https://doi.org/10.1007/s11010-023-04798-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04798-w